Submitted:
26 June 2023
Posted:
26 June 2023
You are already at the latest version
Abstract
Keywords:
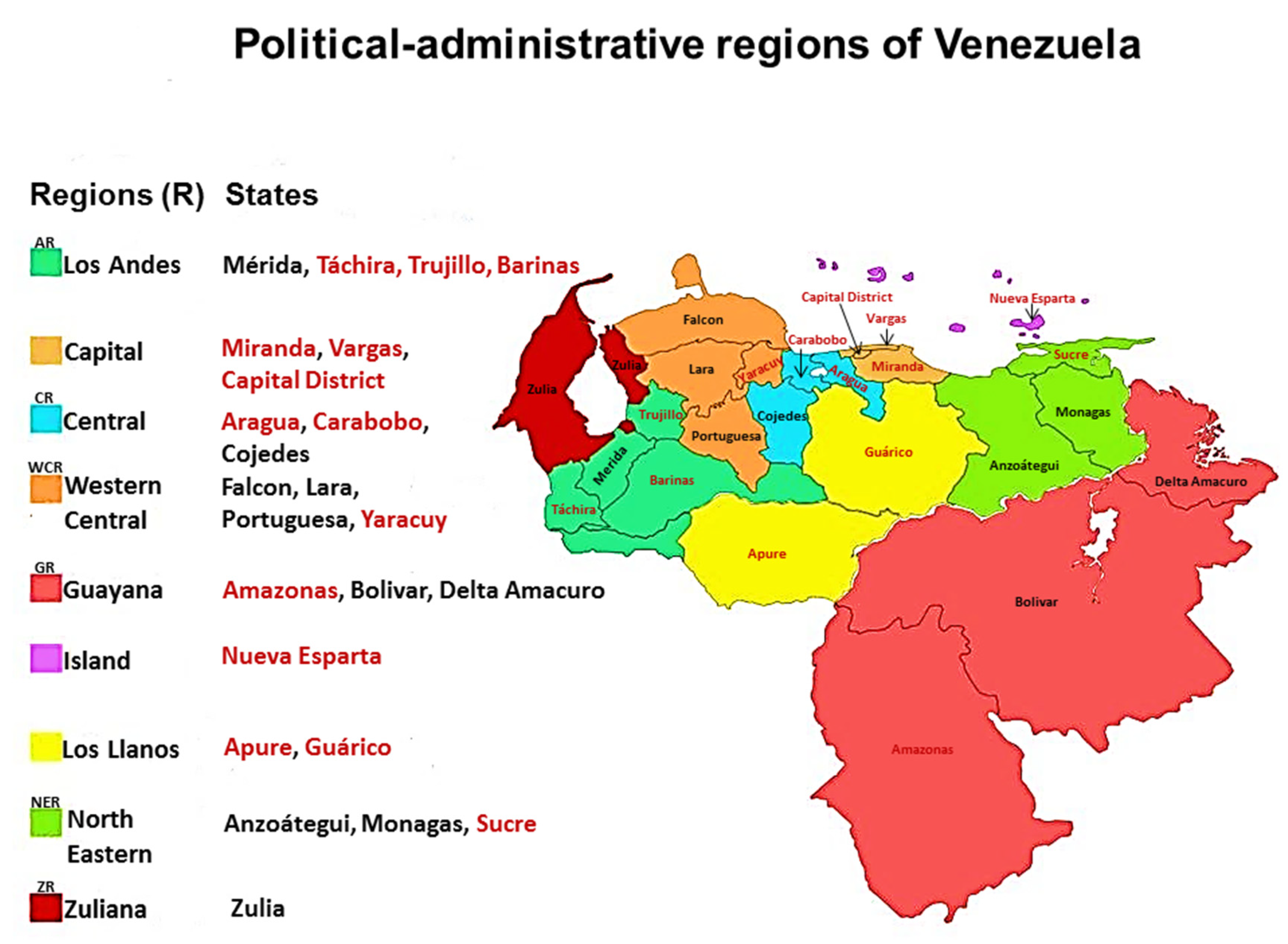
1. Introduction
2. Genera and species of Botryosphaeriales identified with morphological descriptions in Venezuela
| Fungi | Host | Place | Reference |
|---|---|---|---|
| Diplodia Fr. | Ceiba pentandra (L.) Gaertn-old leaves | Buena vista, Lara state | [39] |
| Diplodi a sp. | Cassia L.- root | - | [40] |
| Diplodia ochromae Pat. | Ochroma lagopus Sw. -trunk | - | [40] |
| Diplodia mutila Fr. Apud Mont. |
Pinus caribaea morelet var. hondurensis (Barr. and Golf.)- blue stain on wood |
Chaguaramas, Anzoátegui state | [36] |
| Dothiorella Sacc. | Delonix regia (Bojer ex Hook) Raf. -branches | El Tocuyo, Lara state | [39] |
| Dothiorella sp. | Psidium guajava L.-fruit rot | Merida and Zulia states | [33] |
| Dothiorella dothidea (= Botryosphaeria dothidea) | Prunus pérsica (L.) Batsch- brown rot of fruits | El Arenal, Merida state | [31] |
| Fusicoccum Corda | Echinodorus berteroi (Spreng) Fassett-leaves | Guanare, Portuguesa state | [39] |
| Lasiodiplodia theobromae (Pat.) Griffon & Maubl. | Pachystachys lutea Nees-branches | Barquisimeto, Lara state | [39] |
| L. theobromae | Anacardium occidentale L- terminal branch death | Barquisimeto, Lara state | [39] |
| L. theobromae | Mangifera indica L-branches and seeds | La Calzada de Páez, Barinas state | [39] |
| L. theobromae | Annona reticulata L.-old leaves | Barquisimeto, Lara state | [39] |
| L. theobromae | Catharanthus roseus (L.) G. Don-Leaves | Barquisimeto, Lara state | [39] |
| L. theobromae | Crescentia cujete L.- branches and leaves | Wide distribution in Venezuela | [39] |
| L. theobromae | Citrullus lanatus (Thunb.) Matsum. & Nakai- fruits rot and branches | La Miel, Lara state | [39] |
| L. theobromae | Juniperus lucayana Britton- twigs | Barquisimeto, Lara state | [39] |
| L. theobromae | Curatella Americana L.-old leaves | La Calzada de Páez, Barinas state | [39] |
| L. theobromae | Codiaeum variegatum (L.) Blume var. pictum (Lodd.) Muell | - | [39] |
| L. theobromae | Hura crepitans L.-old leaves | La Calzada de Páez, Barinas state | [39] |
| L. theobromae | Manihot esculenta Crantz-branches | Urachiche, Yaracuy state | [39] |
| Lasiodiplodia theobromae | Duranta repens L.-branches | Ureña, Táchira | [39] |
| L. theobromae | Arachis hypogaea L.-root | Buría Londres, Lara state | [39] |
| L. theobromae | Phaseolus lunatus L.-branches | Sabana de Parra, Yaracuy state | [39] |
| L. theobromae | Sansevieria trifasciata Prain.-old leaves | Barquisimeto, Lara state | [39] |
| L. theobromae | Cedrela odorata L.-branches | Chivacoa, Yaracuy state | [39] |
| L. theobromae | Cecropia peltata L.-branches | Chivacoa, Yaracuy | [39] |
| L. theobromae | Ficus pumila L.-old leaves and galls on the leaves | Barquisimeto, Lara state | [39] |
| L. theobromae | Maxillaria Ruiz & Pavon-old leaves | Duaca, Lara | [39] |
| L. theobromae | Passiflora edulis Sims. form flavicarpa Degener | El Eneal, Lara | [39] |
| L. theobromae | Salix babylonica L.- black root rot | Barquisimeto, Lara state | [39] |
| L. theobromae | Pachystachys lutea Nees-branches | Barquisimeto, Lara state | [39] |
| L. theobromae | Cajanus indicus Spreng.-branches | Lara state | [39] |
| L. theobromae | Duranta repens L.-branches | Tachira state | [39] |
| L. theobromae | Theobroma cacao L. | - | [40] |
| L. theobromae | Vinca rosea L.-leaf and branch | Lara state | [39] |
| L. theobromae | Persea Americana Mill.-fruits | Yaracuy state | [41] |
| L. theobromae | Citrus latifolia Tanaka-fruits | Yaracuy state | [41] |
| L. theobromae | Citrus sinensis (L.) Osbeck-fruits | Yaracuy state | [41] |
|
Lasiodiplodia theobromae |
C. sinensis-Lesion and Gummosis on the branches | Caño Amarillo, Tachira state | [30] |
| L. theobromae |
Citrus aurantiifolia-Lesion and Gummosis on the branches |
Caño Amarillo, Tachira state | [30] |
| L. theobromae |
Passiflora edulis Sims f. flavicarpa-Dieback on the branches |
South of Maracaibo Lake, Zulia and Merida states |
[32] |
| L. theobromae | Pinus caribaea var. hondurensis-blue stain on wood | Uverito plantation and Uverito sawmill, Monagas state | [123] |
| L. theobromae |
Azadirachta indica A. Juss-blue stain on wood |
Cojedes state | [37] |
| L. theobromae | Pinus oocarpa Schiede ex Schltdl | Merida state | [37] |
| L. theobromae | Mangifera indica-branches dieback | Maracay (INIA-CENIAP), Aragua state | [42] |
| Microdiplodia buddleiae Gucevicz | Opuntia caracasana Salm.-spot leaves | Humocaro Bajo, Lara state | [39] |
| Macrophomina phaseolina (Tassi) Goidanich | Begonia sp.- spot on the leaf | Barquisimeto, Lara state | [39] |
| Macrophomina phaseolina | Calendula officinalis L.-stem and inflorescence | Barinas state | [39] |
| M. phaseolina | Ipomoea batata (L.) Lam.-stolons at the roots | Siquisique, Lara state | [39] |
| M. phaseolina | Phaseolus vulgaris L.-stem and basal rot | Moroturo, Lara state | [39] |
| M. phaseolina | Glycine Willd. | - | [40] |
| M. phaseolina | Gossypium L. | - | [40] |
| M. phaseolina | Ipomoea L. | - | [40] |
| M. phaseolina | Nicotiana L. | - | [40] |
| M. phaseolina | Phaseolus L. | - | [40] |
| M. phaseolina | Psidium guajava L.-Fruits | - | [40] |
| M. phaseolina | Solanum melongena L. | - | [40] |
| M. phaseolina | Vigna Savi | - | [40] |
| Neofusicoccum mangiferae (Syd. & P. Syd.) Crous, Slippers & A.J.L. Phillips | Mangifera indica- death of branches | Maracay (INIA-CENIAP), Aragua state | [42] |
| Neofusicoccum parvum (Pennycook & Samuels) Crous, Slippers & A.J.L. Phillips | Mangifera indica- death of branches | Maracay (INIA-CENIAP), Aragua state | [42] |
| Sphaeropsis Sacc. | Cecropia peltata L.-branch and trunk knots | Reserva Forestal de Ticoporo, Mirí, Barinas state | [39] |
| Sphaeropsis sp. | Phthirusa paniculata (Kunth) J.F.Macbr.-leaf | Lara state | [39] |
| Sphaeropsis palmarum Cooke | Cocos nucifera L.-old leaves | Cumanacoa, Sucre state | [39] |
| Sphaeropsis sapinea (Fr.) Dyko & B. Sutton | Pinus caribaea Morelet- Chlorosis in the needles and discoloration lesions on the stem | Nirgua, Yaracuy state | [35] |
| S. sapinea | Pinus oocarpa Schiede-blue stain on wood | Andes region (1600 meters above sea level), Merida state |
[37] |
| Sphaeropsis sapinea | Pinus caribaea var. hondurensis-Shoot blight, dieback and canker on trunks, branches, and roots (Plantations), and death at the tips of the needles (seedlings in nurseries) | Uverito (Monagas state), and Coloradito y Los Hachos (Anzoátegui state) | [34] |
| Sphaeropsis tumefaciens Hedges | Citrus L.-gall | - | [40] |
| Botryosphaeria festucae (Lib.) Arx & E. Müll | Zea mays L.-bract, leaf, and seed | - | [40] |
| Botryosphaeria dothidea (Moug. ex Fr.) Ces. & De Not | Compositae-stem | Aragua state | [40] |
| Botryosphaeria ribis Grossenb. & Duggar | Rosa canina L.-branch | Lara state | [39] |
| Genera | Conidia | Conidiomata | Conidiophores | Conidiogenesis cells | Paraphyses |
|---|---|---|---|---|---|
| Cophinforma Doilom, J.K. Liu & K.D. Hyde | Hyaline, thin walled, unicellular, aseptate, rarely becoming septate, mostly fusoid to ellipsoidal. Most conidia longer than 30 μm | Material pycnidial, superficial, multilocular, dark brown to black, eustromatic | Absent | Enteroblastic, hyaline, cylindrical | Absent |
| Diplodia Fr. | Initially hyaline, aseptate, thick-walled, becoming 1–septate only rarely becoming 2-septate, pale transluscent brown after discharge from the pycnidia. Some species the conidia become pigmented while still enclosed in the conidioma and these species the conidia rarely become septate. | Pycnidial, ostiolate, formed in uni- or multiloculate stromata | When present: hyaline,simple, occasionally septate, rarely branched, cylindrical, | Holoblastic, hyaline, cylindrical | Absent |
| Dothiorella Sacc. | Initially hyaline, becoming dark brown and one-euseptate within the pycnidial cavity, ellipsoid to ovoid, thick-walled, externally smooth, or striate, internally verruculose | Stromatic, ostiolate, individual or in loose clusters of up to 10 conidiomata, immersed, breaking through the bark when mature. |
Absent | Holoblastic, hyaline, smooth-walled, cylindrical | Absent |
| Lasiodiplodia Ellis & Everh. | Hyaline when young, later becoming medianly 1-euseptate, dark brown with longitudinal striations, thick-walled, oblong to ellipsoid, straight, broadly rounded at the apex, base truncate | Stromatic, immersed or superficial, separate, or aggregated and confluent, globose, dark brown, uni- or multilocular | Often reduced to conidiogenous cells, if present hyaline, simple, sometimes septate, rarely branched | Holoblastic, hyaline, smooth, cylindrical to subobpyriform, discrete, determinate, or indeterminate | Present |
| Macrophomina Petr. | Aseptate, obtuse at each end, straight, cylindrical to fusiform, thin-walled, smooth, guttulate, enclosed in mucoid sheath. Immature conidia hyaline, mature conidia becoming medium to dark brown. | Pycnidial, stromatic, dark brown to black, solitary, or gregarious | Reduced to conidiogenous cells | Enteroblastic, phialidic, determinate, discrete, lageniform to doliiform, hyaline, smooth, with wide aperture and minute collarette, formed from the inner cells of the pycnidial wall, enclosed in mucoid sheath |
Absent |
| Neofusicoccum Crous, Slippers & A.J.L. Phillips | Mostly fusoid to ellipsoidal, hyaline. | Stromatic, pycnidial, solitary or aggregated, often occurring within the same stroma as the ascomata, walls composed of dark brown | When present hyaline, cylindrical, branched at the base, smooth, 0–1 septate | Enteroblastic, integrated, hyaline, smooth, cylindrical | Absent |
| Pseudofusicoccumaceae Tao Yang & Crous, Pseudofusicoccum Mohali, Slippers & M.J. Wingf. | Conidia are more cylindrical than in Noefusicoccum species and surrounded by a persistent mucous sheath, hyaline. | Large, superficial, unilocular or multilocular locule | Reduced to conidiogenous cells | Holoblastic, smooth, cylindrical to subcylindrical, hyaline |
Present or absent |
3. DNA Sequence-based identification of Botryosphaeriales in Venezuela
3.1. Phylogenetic analysis
4. Taxonomy, diversity and distribution of a new genus, new species and reports found in Venezuela and other regions of the world
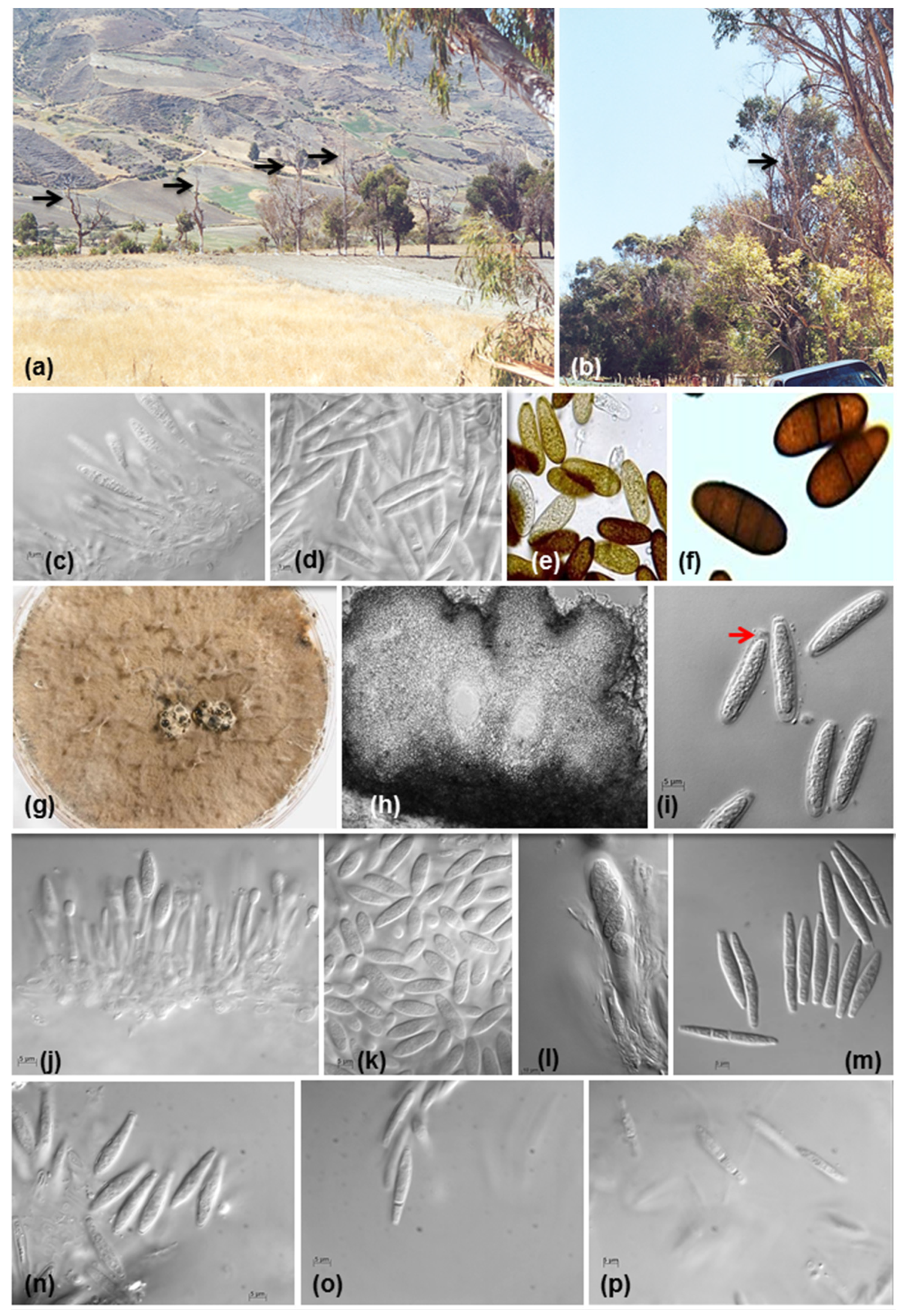
5. Symptoms associated with species from Botryosphaeriales in Venezuela
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wingfield, M.J.; Brockerhoff, E.G.; Wingfield, B.D.; Slippers, B. Planted forest health: The need for a global strategy. Science 2015, 349, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Batista, E.; Lopes, A.; Alves, A. What Do We Know about Botryosphaeriaceae? An Overview of a Worldwide Cured Dataset. Forests 2021, 12, 313. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Slippers, B.; Hurley,B. P.; Coutinho, T.A.; Wingfield, B.D.; Roux, J. Eucalypt pests and diseases: growing threats to plantation productivity. Southern For. 2008, 70, 139–144. [Google Scholar] [CrossRef]
- Windfield, M.J. Daniel McAlpine Memorial Lecture. Increasing threat of disease to exotic plantation forests in the southern hemisphere: lessons from cryphonectria canker. Australs. Plant Pathol. 2003, 32, 133–139. [Google Scholar] [CrossRef]
- Branco, M.; Brockerhoff, E.G.; Castagneyrol, B.; Orazio, C.; Jactel, H. Host range expansion of native insects to exotic trees increases with area of introduction and the presence of congeneric native trees. J. Appl. Ecol. 2015, 52, 69–77. [Google Scholar] [CrossRef]
- Karlman, M.; Hansson, P.; Witzell, J. Scleroderris canker on lodgepole pine introduced in northern Sweden. Can. J. For. Res. 1994, 24, 1948–1959. [Google Scholar] [CrossRef]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology, and impact. Fungal Biol. Rev. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Alves,A. ; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef]
- Michailides, T.J.; Morgan, D.P. Spore release by Botryosphaeria dothidea in pistachio orchards and disease control by altering the trajectory angle of sprinklers. Phytopathology 1993, 83, 145–152. [Google Scholar] [CrossRef]
- Zlatković, M.; Keča, N.; Wingfield, M.J.; Jami, F.; Slippers, B. New and unexpected host associations for Diplodia sapinea in the Western Balkans. For. Pathol. 2017, 47, e12328. [Google Scholar] [CrossRef]
- Burgess, T.I.; Sakalidis, M.L.; Hardy, G.E.S.J. Gene flow of the canker pathogen Botryosphaeria australis between Eucalyptus globulus plantations and native eucalypt forests in Western Australia. Austral Ecol. 2006, 31, 559–566. [Google Scholar] [CrossRef]
- Pavlic, D.; Slippers, B.; Coutinho, T.A.; Wingfield, M.J. Botryosphaeriaceae occurring on native Syzygium cordatum in South Africa and their potential threat to Eucalyptus. Plant Pathol. 2007, 56, 624–636. [Google Scholar] [CrossRef]
- Stanosz, G.R.; Smith, D.R.; Leisso, R. Diplodia shoot blight and asymptomatic persistence of Diplodia pinea on or in stems of jack pine nursery seedlings. Forest Pathol. 2007, 37, 145–154. [Google Scholar] [CrossRef]
- Sakalidis, M.L.; Hardy, G.E.S.J.; Burgess, T.I. Class III endophytes, clandestine movement amongst hosts and habitats and their potential for disease; a focus on Neofusicoccum australe. Australasian Plant Pathol. 2011, 40, 510–521. [Google Scholar] [CrossRef]
- Begoude, B.A.D.; Slippers, B.; Perez, G.; Wingfield, M.J.; Roux, J. High gene flow and outcrossing within populations of two cryptic fungal pathogens on a native and non-native host in Cameroon. Fungal Biol. 2012, 116, 343–353. [Google Scholar] [CrossRef]
- Moral, J.; Morgan, D.; Trapero, A.; Michailides, T.J. Ecology and Epidemiology of Diseases of Nut Crops and Olives Caused by Botryosphaeriaceae Fungi in California and Spain. Plant Dis. 2019, 103, 1809–1827. [Google Scholar] [CrossRef]
- Jacobs, K.A.; Rehner, S.A. ; Rehner, S.A. Comparison of cultural and morphological characters and ITS sequences in anamorphs of Botryosphaeria and related taxa. Mycologia 1998, 90, 601–610. [Google Scholar] [CrossRef]
- Denman, S.; Crous, P.W.; Taylor, J.E.; Kang, J.Ch.; Pascoe, I.; Wingfield, M.J. An overview of the taxonomic history of Botryosphaeria, and a re-evaluation of its anamorphs based on morphology and ITS rDNA phylogeny. Stud. Mycol. 2000, 45, 129–140. Available online: https://wwwstudiesinmycologyorg/sim/Sim45/content/pdf/129-140.pdf.
- Pavlic, D.; Slippers, B.; Couthino, T.A.; Wingfield, M.J. Multiple gene genealogies and phenotypic data reveal cryptic species of the Botryosphaeriaceae: A case study on the Neofusicoccum parvum/N. ribis complex. Mol. Phylogenet. Evol. 2009, 51, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Stanosz, G.R. ; Stanosz, G.R. Molecular and morphological differentiation of Botryosphaeria dothidea (anamorph Fusicoccum aesculi) from some other fungi with Fusicoccum anamorphs. Mycologia 2001, 93, 505–515. [Google Scholar] [CrossRef]
- Zhou, S.; Stanosz, G.R. Relationships among Botryosphaeria species and associated anamorphic fungi inferred from the analysis of ITS and 5.8S rDNA sequences. Mycologia 2001, 93, 516–527. [Google Scholar] [CrossRef]
- Zhou, S.; Smith, D.R.; Stanosz, G.R. Differentiation of Botryosphaeria species and related anamorphic fungi using Inter Simple or Short Sequence repeat (ISSR) fingerprinting. Mycol. Res. 2001, 105, 919–926. [Google Scholar] [CrossRef]
- Slippers, B.; Crous, P.W.; Jami, F.; Groenewald, J.Z.; Wingfield, M.J. Diversity in the Botryosphaeriales: Looking back, looking forward. Fungal Biol. 2017, 121, 307–321. [Google Scholar] [CrossRef]
- Crous, P.W.; Slippers, B.; Wingfield, M.J.; Rheeder, J.; Marasas, W.F.O.; Philips, A.J.L.; Alves, A.; Burgess, T.; Barber, P.; Groenewald, J.Z. Phylogenetic lineages in the Botryosphaeriaceae. Stud. Mycol. 2006, 55, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Slippers, B.; Boissin, E.; Phillips, A.J.L.; Groenewald, J.Z.; Wingfield, M.J.; Postma, A.; Burgess, T.; Crous, P.W. Phylogenetic lineages in the Botryosphaeriales: A systematic and evolutionary framework. Stud. Mycol. 2013, 76, 31–49. [Google Scholar] [CrossRef]
- Yang, T.; Groenewald, J.Z.; Cheewangkoon, R.; Jami, F.; Abdollahzadeh, J.; Lombard, L.; Crous, P.W. Families, genera, and species of Botryosphaeriales. Fungal Biol. 2017, 121, 322–346. [Google Scholar] [CrossRef]
- Wikee, S.; Lombard, L.; Nakashima, C.; Motohashi, K.; Chukeatirote, E.; Cheewangkoon, R.; Mckenzie, E.; Hyde, K.; Crous, P. A phylogenetic re-evaluation of Phyllosticta (Botryosphaeriales). Stud. Mycol. 2013, 76, 1–29. [Google Scholar] [CrossRef]
- Minnis, A.M.; Kennedy, A.H.; Grenier, D.B.; Palm, M.E.; Rossman, A.Y. Phylogeny and taxonomic revision of the Planistromellaceae including its coelomycetous anamorphs: contributions towards a monograph of the genus Kellermania. Persoonia 2012, 29, 11–28. [Google Scholar] [CrossRef]
- Wyka, S.A.; Broders, K.D. The new family Septorioideaceae, within the Botryosphaeriales and Septorioides strobi as a new species associated with needle defoliation of Pinus strobus in the United States. Fungal Biol. 2016, 8, 1030–1040. [Google Scholar] [CrossRef]
- Cedeño, L.; Palacios-Prü, E.; Palacios-Prü, E. ; Identificación de Botryodiplodia theobromae como la causa de las lesiones y gomosis en cítricos. Fitopatol. Venez. 1992, 5, 10–13. [Google Scholar]
- Cedeño, L.; Mohali, S.R.; Carrero, C. Primer reporte en Venezuela de Dothiorella dothidea como la causa de la podredumbre marrón en frutos del duraznero. Fitopatol. Venez. 1994, 7, 34–36. [Google Scholar]
- Cedeño, L.; Carrero, C.; Mohali, S.; Palacios-Pru, E.; Quintero, K. Muerte regresiva en parchita causada por Lasiodiplodia theobromae en Venezuela. Fitopatol. Venez. 1995, 8, 7–10. [Google Scholar]
- Cedeño, L.; Carrero, C.; Santos, R.; Quintero, K. Podredumbre marrón en frutos del guayabo causada por Dothiorella, fase conidial Botryosphaeria dothidea, en los estados Mérida y Zulia. Fitopatol. Venez. 1998, 11, 16–23. [Google Scholar]
- Cedeño, L.; Carrero, C.; Franco, W.; Torres-Lezama, A. Sphaeropsis sapinea asociado con quema del cogollo, muerte regresiva y cáncer en troncos, ramas y raíces del Pino Caribe en Venezuela. Interciencia 2001, 26, 210–215. Available online: https://www.redalyc.org/articulo.oa?id=33905606.
- Mohali, S. First Report in Venezuela of Sphaeropsis sapinea, causal agent of the blue stain on Caribbean pine. Fitopatol. Venez. 1997, 10, 23. [Google Scholar]
- Mohali, S.R.; Encinas, O. Association of Diplodia mutila with blue stain of Caribbean pine in Venezuela. For. Pathol. 2001, 31, 187–189. [Google Scholar] [CrossRef]
- Mohali, S.R.; Encinas, O.; Mora, N. Manchado azul en madera de Pinus oocarpa y Azadirachta indica en Venezuela. Fitopatol. Venez. 2002, 15, 30–32. [Google Scholar]
- Punithalingam, E. Plant diseases attributed to Botryodiplodia theobromae Pat. Commonw. Mycol. Inst., Ferry Lane, Kew, Surrey, UK, 1980; 123 pp.
- Urtiaga, R. Índice de enfermedades en plantas de Venezuela y Cuba. Impresos Nuevo Siglo. S.R.L., Barquisimeto, Venezuela, 1986; pp. 324.
- Iturriaga, T.; Minter, D.W. Fungi of Venezuela. 2006. Available online: http://www.cybertruffle.org.uk/venefung/eng/.
- Hernandez de Parra, J.B.; Ortega, R.; Blanco, G. Diagnóstico de enfermedades en frutales en el estado Yaracuy, Venezuela entre los años 2001-2011. Agronomia Trop. 2012, 62, 111–122. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0002-192X2012000100009.
- Pacheco, C.; Suleima, L.; Manzanilla, E. Diversidad de hongos en cinco cultivares de mango (mangifera indica l.) del banco de germoplasma del inia-ceniap, Maracay. Bioagro 2016, 28, 201–208. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S1316-33612016000300007.
- Slippers, B.; Crous, P.W.; Denman, S.; Coutinho, T.A.; Wingfield, B.D. , Wingfield, M.J. Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 2004, 96, 83–101. [Google Scholar] [CrossRef]
- Slippers, B.; Fourie, G.; Crous, P.W.; Coutinho, T.A.; Wingfield, B.D.; Carnegie, A.J.; Wingfield, M.J. Multiple gene sequences delimit Botryosphaeria australis sp. nov. from B. lutea. Mycologia 2004, 96, 1030–1041. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J.L. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: a guide to methods and applications: 315-322; Innis, M.A., Gelfand, D.H., Sninsky, J.J., et al., Eds.; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous Ascomycetes. Mycologia 1999, 91, 553. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Farris, J.S.; Kallersjo, M.; Kluge, A.G.; Bult, C. , Testing significance of incongruence. Cladistics 1995, 10, 315–319. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4.0. Sinauer Associates, Sunderland, Massachusetts. 2003. Available online: https://paup.phylosolutions.com/.
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Baysian phylogenetic interference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Mohali, S.; Slippers, B.; Wingfield, M.J. Identification of Botryosphaeria species from Eucalyptus, Acacia, and Pinus in Venezuela. Fungal Divers. 2007, 25, 103–125. Available online: https://www.fungaldiversity.org/fdp/sfdp/25-7.pdf.
- Mohali, S.R.; Woodward, S.; Klopfenstein, N.B.; Kim, M.-S.; Stewart, J.E. Mycobiota associated with anthracnose and dieback symptoms on Theobroma cacao L. in Merida state, Venezuela. Summa Phytopathol. 2023, 1, 00–00. [Google Scholar]
- Mehl, J.W.M.; Slippers, B.; Roux, J.; Wingfield, M.J. Botryosphaeriaceae associated with Pterocarpus angolensis (kiaat) in South Africa. Mycologia 2011, 103, 534–553. [Google Scholar] [CrossRef]
- Liu, J.K.; Phookamsak, R.; Doilom, M.; Wikee, S.; Li, Y.M.; Ariyawansha, H.; Boonmee, S.; Chomnunti, P.; Dai, D.Q.; Bhat, J.D.; Romero, A.I.; Zhuang, W.Y.; Monkai, J.; Jones, E.B.G.; Chukeatirote, E.; Ko Ko, T.W.; Zhao, Y.C.; Wang, Y.; Hyde, K.D. Towards a natural classification of Botryosphaeriales. Fungal Divers. 2012, 57, 149–210. Available online: https://link.springer.com/article/10.1007/s13225-012-0207-4. [CrossRef]
- Li, G.Q.; Liu, F.F.; Li, J.Q.; Liu, Q.L.; Chen, S.F. Botryosphaeriaceae from Eucalyptus plantations and adjacent plants in China. Persoonia 2018, 40, 63–95. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.E.; Fonseca, W.L.; Viana, F.M.P.; Ootani, M.A.; Araújo, F.S.A.; Brasil, S.O.S.; Mesquita, A.L.M.; Lima, C.S. First Report of Cophinforma atrovirens Causing Stem Rot and Dieback of Cashew Plants in Brazil. Plant Dis. 2019, 103, 1772. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Castro-Medina, F.; Mohali, S.R.; Gubler, W.D. Botryosphaeriaceae species associated with cankers and dieback symptoms of Acacia mangium and Pinus caribaea var. hondurensis in Venezuela. Plant Dis. 2016, 100, 2455–2464. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Maddau, L.; Franceschini, A.; Alves, A.; Phillips, A.J.L. Botryosphaeriaceae species associated with lentisk dieback in Italy and description of Diplodia insularis sp. nov. Mycosphere 2016, 7, 962–977. Available online: https://www.mycosphere.org/pdf/Mycosphere_7_7_10-1.pdf. [CrossRef]
- Zhang, W.; Groenewald, J.Z.; Lombard. L.; Schumacher, R.K.; Phillips, A.J.L.; Crous, P.W. Evaluating species in Botryosphaeriales. Persoonia 2021, 46, 63–115. [Google Scholar] [CrossRef]
- Slippers, B.; Fourie, G.; Crous, P.W.; Coutinho, T.A.; Wingfield, B.D.; Carnegie, A.; Wingfield, M.J. Speciation and distribution of Botryosphaeria spp. on native and introduced Eucalyptus trees in Australia and South Africa. Stud. Mycol 2004, 50, 343–358. Available online: https://www.studiesinmycology.org/index.php/issue/52-studies-in-mycology-no-50.
- de Wet, J.; Wingfield, M.J.; Coutinho, T.A.; Wingfield, B.D. Characterization of Sphaeropsis sapinea isolates from South Africa, Mexico, and Indonesia. Plant Dis. 2000, 84, 151–156. [Google Scholar] [CrossRef]
- Hlaiem, S.; Boutiti, M.Z.; Jamaa, M.L.B. First report of shoot blight caused by Diplodia scrobiculata on Pinus halepensis in Tunisia. J. Plant Pathol. 2019, 101, 1237. [Google Scholar] [CrossRef]
- Jami, F.; Wingfield, M.J.; Gryzenhout, M.; Slippers, B. Diversity of tree-infecting Botryosphaeriales on native and non-native trees in South Africa and Namibia. Australasian Plant Pathol. 2017, 46, 529–545. [Google Scholar] [CrossRef]
- Manzanos, T.; Aragones, A.; Iturritxa, E. Diplodia scrobiculata: a latent pathogen of Pinus radiata reported in northern Spain. Phytopathol. Mediterr. 2017, 56, 274–277. Available online: https://www.jstor.org/stable/44809345.
- Burgess, T.I.; Gordon, T.R.; Wingfield, M.J.; Wingfield, B.D. Geographic isolation of Diplodia scrobiculata and its association with native Pinus radiata. Mycol. Res. 2004, 108, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.A.; Rooney-Latham, S.; Brown, A.A.; McCormick, M.; Baston, D. Pathogenicity of three Botryosphaeriaceae fungi, Diplodia scrobiculata, Diplodia mutila, and Dothiorella californica, isolated from coast redwood (Sequoia sempervirens) in California. Forest Pathol. 2022, 52, 1–11. [Google Scholar] [CrossRef]
- Netto, M.S.B.; Assunção, I.P.; Lima, G.S.A.; Marques, M.W.; Lima, W.G.; Monteiro, J.H.A.; Balbino, V. de Q.; Michereff, S.J.; Phillips, A.J.L.; Câmara, M.P.S. Species of Lasiodiplodia associated with papaya stem-end rot in Brazil. Fungal Divers. 2014, 67, 127–141. [Google Scholar] [CrossRef]
- Doilom, M.; Shuttleworth, L.A.; Roux, J.; Chukeatirote, E.; Hyde, K.D. Botryosphaeriaceae associated with Tectona grandis (teak) in northern Thailand. Phytotaxa 2015, 233, 1–26. [Google Scholar] [CrossRef]
- Rodriguez-Galvez, E.; Guerrero, P.; Barradas, C.; Crous, P.W.; Alves, A. Phylogeny and pathogenicity of Lasiodiplodia species associated with dieback of mango in Peru. Fungal Biol. 2017, 121, 452–465. [Google Scholar] [CrossRef]
- Cruywagen, E.M.; Slippers, B.; Roux, J.; Wingfield, M.J. Phylogenetic species recognition and hybridisation in Lasiodiplodia: A case study on species from baobabs. Fungal Biol. 2017, 121, 420–436. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, S.; Zhao, L.; Sun, X.; He, W.; Zhang, Y.; Dai, Y.C. Lasiodiplodia spp. associated with Aquilaria crassna in Laos. Mycol. Prog. 2019, 18, 683–701. [Google Scholar] [CrossRef]
- Bautista-Cruz, M.A.; Almaguer-Vargas, G.; Leyva-Mir, S.G.; Colinas-Leon, M.T.; Correia, K.C.; Camacho-Tapia, M.; Robles-Yerena, L.; Michereff, S.J.; Tovar-Pedraza, J.M. Phylogeny, distribution, and pathogenicity of Lasiodiplodia species associated with cankers and dieback symptoms of Persian lime in Mexico. Plant Dis. 2019, 103, 1156–1165. [Google Scholar] [CrossRef]
- Tan, Y.P.; Shivas, R.G.; Marney, T.S.; Edwards, J.; Dearnaley, J.; Jami, F.; Burgess, T.I. Australian cultures of Botryosphaeriaceae held in Queensland and Victoria plant pathology herbaria revisited. Australasian Plant Pathol. 2019, 48, 25–34. [Google Scholar] [CrossRef]
- Kee, Y.J.; Zakaria, L.; Mohd, M.H. Lasiodiplodia species associated with Sansevieria trifasciata leaf blight in Malaysia. J. Gen. Plant Pathol. 2019, 85, 66–71. [Google Scholar] [CrossRef]
- Serrato-Diaz, L.M.; Aviles-Noriega, A.; Soto-Bauzo, A.; Rivera-Vargas, L.I.; Goenaga, R.; Bayman, P. Botryosphaeriaceae fungi as causal agents of dieback and corky bark in Rambutan and Longan. Plant Dis. 2020, 104, 105–115. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Retrieved , 2023. Available online: https://nt.ars-grin.gov/fungaldatabases/ (accessed on 13 June 2023).
- Yildiz, A.; Benlioglu, K.; Benlioglu, H. First report of strawberry dieback caused by Lasiodiplodia theobromae. Plant Dis. 2014, 98, 1579. [Google Scholar] [CrossRef] [PubMed]
- Burgess, T.I.; Barber, P.A.; Mohali, S.; Pegg, G.; de Beer, W.; Wingfield, M.J. Three new Lasiodiplodia spp. from the tropics, recognized based on DNA comparisons and morphology. Mycologia 2006, 98, 423–435. [Google Scholar] [CrossRef] [PubMed]
- van Niekerk, J.M.; Bester, W.; Halleen, F.; Crous, P.W.; Fourie, P.H. First Report of Lasiodiplodia crassispora as a Pathogen of Grapevine Trunks in South Africa. Plant Dis. 2010, 94, 1063. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.A.; Wingfield, M.J.; Slippers, B.; Altier, N.A.; Blanchette, R.A. Endophytic and canker-associated Botryosphaeriaceae occurring on non-native Eucalyptus and native Myrtaceae trees in Uruguay. Fungal Divers. 2010, 41, 53–69. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Peduto, F.; Gubler, W.D. First report of grapevine cankers caused by Lasiodiplodia crassispora and Neofusicoccum mediterraneum in California. Plant Dis. 2010, 94, 785. [Google Scholar] [CrossRef]
- Sakalidis, M.L.; Hardy, G.E.S.J.; Burgess, T.I. Endophytes as potential pathogens of the baobab species Adansonia gregorii: a focus on the Botryosphaeriaceae. Fungal Ecol. 2011, 4, 1–14. [Google Scholar] [CrossRef]
- Marques, M.W.; Lima, N.B.; Morais, M.A. Jr.; Barbosa, M.A.G.; Souza, B.O.; Michereff, S.J.; Phillips, A.J.L.; Câmara, M.P.S. Species of Lasiodiplodia associated with mango in Brazil. Fungal Divers. 2013, 61, 181–193. [Google Scholar] [CrossRef]
- Correia, K.C.; Silva, M.A.; de Morais, M.A. Jr.; Armengold, J.; Phillips, A.J.L.; Camara, M.P.S.; Michereff, S.J. Phylogeny, distribution, and pathogenicity of Lasiodiplodia species associated with dieback of table grape in the main Brazilian exporting region. Plant Pathol. 2015, 65, 92–103. [Google Scholar] [CrossRef]
- Machado, A.R.; Custodio, F.A.; Cabral, P.G.C.; Capucho, A.S.; Pereira, O.L. Botryosphaeriaceae species causing dieback on Annonaceae in Brazil. Plant Pathol. 2019, 68, 1394–1406. [Google Scholar] [CrossRef]
- Rangel-Montoya, E.A.; Paolinelli, M.; Rolshausen, P.E.; Valenzuela-Solano, C.; Hernandez-Martinez, R. Characterization of Lasiodiplodia species associated with grapevines in Mexico. Phytopathol. Mediterr. 2021, 60, 237–251. [Google Scholar] [CrossRef]
- Slippers, B. , Roux, J.; Wingfield, M.J.; van der Walt, F.J.J.; Jami, F.; Mehl, J.W.M.; Marais, G.J. Confronting the constraints of morphological taxonomy in the Botryosphaeriales. Persoonia 2014, 33, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Castro-Medina, F.; Mohali, S.R.; Úrbez-Torres, J.R.; Gubler, W.D. First report of Lasiodiplodia pseudotheobromae causing trunk cankers in Acacia mangium in Venezuela. Plant Dis. 2014, 98, 686. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Crous, P.W.; Correia, A.; Phillips, A.J.L. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 2008, 28, 1–13. Available online: http://www.fungaldiversity.org/fdp/sfdp/28-1.pdf.
- Zhao, J.P.; Lu, Q.; Liang, J.; Decock, C.; Zhang, X.Y. Lasiodiplodia pseudotheobromae, a new record of pathogenic fungus from some subtropical and tropical trees in southern China. Cryptogam. Mycol. 2010, 31, 431–439. Available online: https://sciencepress.mnhn.fr/en/periodiques/mycologie/31/4/lasiodiplodia-pseudotheobromae-new-record-pathogenic-fungus-some-subtropical-and-tropical-trees-southern-china.
- Netto, M.S.B.; Lima, W.G.; Correia, K.C.; Silva, C.F.B.; Thon, M.; Martins, R.B.; Câmara, M.P.S. Analysis of phylogeny, distribution, and pathogenicity of Botryosphaeriaceae species associated with gummosis of Anacardium in Brazil, with a new species of Lasiodiplodia. Fungal Biol. 2017, 121, 437–451. [Google Scholar] [CrossRef]
- Trakunyingcharoen, T.; Lombard, L.; Groenewald, J.Z.; Cheewangkoon, R.; To-anun, C.; Crous, P.W. Caulicolous Botryosphaeriales from Thailand. Persoonia 2015, 34, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z.; Shafique, M.S.; Anwaar, H.A.; Sarfraz, S.; Tufail, M.R.; Fayyaz, A.; Muntaha, S.; Haque, K.; Ghuffar, S.; Amrao, L. First report of Lasiodiplodia pseudotheobromae causing trunk cankers in Citrus reticulata in Pakistan. Plant Dis. 2020, 104, 2522. [Google Scholar] [CrossRef]
- Abdollahzadeh, J.; Javadi, A.; Goltapeh, E.M.; Zare, R.; Phillips, A.J.L. Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia 2010, 25, 1–10. [Google Scholar] [CrossRef]
- Ismail, A.M.; Cirvilleri, G.; Polizzi, G.; Crous, P.W.; Groenewald, J.Z.; Lombard, L. Lasiodiplodia species associated with dieback disease of mango (Mangifera indica) in Egypt. Australasian Plant Pathol. 2012, 41, 649–660. [Google Scholar] [CrossRef]
- Kwon, J.H.; Choi, O.; Kang, B.; Lee, Y.; Park, J.; Kang, D.W.; Han, I.; Kim, J. Identification of Lasiodiplodia pseudotheobromae causing mango dieback in Korea. Can. J. Plant Pathol. 2017, 39, 241–245. [Google Scholar] [CrossRef]
- Lopez-Moral, A.; del Carmen Raya, M.; Ruiz-Blancas, C.; Medialdea, I.; Lovera, M.; Arquero, O.; Trapero, A.; Agusti-Brisach, C. Aetiology of branch dieback, panicle and shoot blight of pistachio associated with fungal trunk pathogens in southern Spain. Plant Pathol. 2020, 69, 1237–1269. [Google Scholar] [CrossRef]
- Endes, A.; Kayim, M.; Eskalen, A. First report of Lasiodiplodia theobromae, L. pseudotheobromae, and Diplodia seriata causing bot canker and gummosis of nectarines in Turkey. Plant Dis. 2016, 100, 2321. [Google Scholar] [CrossRef]
- Mehl, J.W.M.; Slippers, B.; Roux, J.; Wingfield, M.J. Botryosphaeriaceae associated with die-back of Schizolobium parahyba trees in South Africa and Ecuador. For. Pathol. 2014, 44, 396–408. [Google Scholar] [CrossRef]
- Rezgui, A.; Vallance, J.; Ghnaya-Chakroun, A.B.; Bruez, E.; Dridi, M.; Demasse, R.D.; Rey, P.; Sadfi-Zouaoui, N. Study of Lasiodiplodia pseudotheobromae, Neofusicoccum parvum and Schizophyllum commune, three pathogenic fungi associated with the grapevine trunk diseases in the north of Tunisia. Eur. J. Plant Pathol. 2018, 152, 127–142. [Google Scholar] [CrossRef]
- Summerbell, R.C.; Krajden, S.; Levine, R.; Fuksa, M. Subcutaneous phaeohyphomycosis caused by Lasiodiplodia theobromae and successfully treated surgically. Med. Mycol. 2004, 42, 543–547. [Google Scholar] [CrossRef]
- Mohali, S.; Burgess, T.I.; Wingfield, M.J. Diversity and host association of the tropical tree endophyte Lasiodiplodia theobromae revealed using simple sequence repeat markers. Forest Pathol. 2005, 35, 385–396. [Google Scholar] [CrossRef]
- Mohali, S.; Slippers, B.; Wingfield, M.J. Two new Fusicoccum species from Acacia and Eucalyptus in Venezuela, recognized based on morphology and DNA sequences data. Mycol. Res. 2006, 110, 405–413. [Google Scholar] [CrossRef]
- Mohali, S.R.; Castro-Medina, F.; Úrbez-Torres, J.R.; Gubler, W.D. First report of Lasiodiplodia theobromae and L. venezuelensis associated with blue stain on Ficus insipida wood from the Natural Forest of Venezuela. For. Pathol. 2017, 47, 1–5. [Google Scholar] [CrossRef]
- Li, G.; Slippers, B.; Wingfield, M.J.; Chen, S. Variation in Botryosphaeriaceae from Eucalyptus plantations in Yun Nan Province in southwestern China across a climatic gradient. IMA Fungus 2020, 11, 22. [Google Scholar] [CrossRef]
- Farr, D.F.; Elliot, M.; Rossman, A.Y.; Edmonds, R.L. Fusicoccum arbuti sp. nov. causing cankers on Pacific madrone in western North America with notes on Fusicoccum dimidiatum, the correct name for Scytalidium dimidiatum and Nattrassia mangiferae. Mycologia 2005, 97, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.G.; Briceño, E.X.; Chávez, E.R.; Úrbez-Torres, J.R.; Latorre, B.A. Neofusicoccum spp. associated with stem canker and dieback of blueberry in Chile. Plant Dis. 2009, 93, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Mohali, S.; Slippers, B.; Wingfield, M.J. Pathogenicity of seven species of the Botryosphaeriaceae on Eucalyptus clones in Venezuela. Australasian Plant Pathol. 2009, 38, 135–140. [Google Scholar] [CrossRef]
- Marques, M.W.; Lima, N.B.; Michereff, S.J.; Câmara, M.P.S.; Souza, C.R.B. First report of mango dieback caused by Pseudofusicoccum stromaticum in Brazil. Plant Dis. 2012, 96, 144. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.W.; Lima, N.B.; de Morais, N.A.; Michereff, S.J.; Phillips, A.J.L.; Câmara, M.P.S. Botryosphaeria, Neofusicoccum, Neoscytalidium and Pseudofusicoccum species associated with mango in Brazil. Fungal divers 2013, 61, 195–208. [Google Scholar] [CrossRef]
- Silveira, G.F.; Melo, M.P.; Teixeira, J.W.M.; Viana, D.C.; Silva, J.D.C.; Beserra, J.E.A. First report of Lasiodiplodia theobromae and Pseudofusicoccum stromaticum causing dieback in Syzygium malaccense tree in Brazil. For. Pathol. 2017, 48. [Google Scholar] [CrossRef]
- Coutinho, I.B. L.; Cardoso, J.E.; Lima, C.S.; Lima, J.S.; Goncalves, F.J.T.; Silva, A.M.S.; Freire, F.C.O. An emended description of Neofusicoccum brasiliense and characterization of Neoscytalidium and Pseudofusicoccum species associated with tropical fruit plants in northeastern Brazil. Phytotaxa 2018, 358, 251–264. [Google Scholar] [CrossRef]
- Sobreira, A.C.M.; Pinto, F.C.L.; Florencio, K.G.D.; Wilke, D.V.; Staats, C.C.; Streit, R.A.S.; de O Freire, F.C.; Pessoa, O.D.L.; Trindade-Silva, A.E.; Canuto, K.M. Endophytic fungus Pseudofusicoccum stromaticum produces cyclopeptides and plantrelated bioactive rotenoids. R. Soc. Chem. 2018, 8, 35575–35586. [Google Scholar] [CrossRef]
- Sessa, L.; Abreo, E.; Lupo, S. Pseudofusicoccum sp. causing shoot canker in peach in Uruguay. Australasian Plant Dis. Notes 2021, 16, 5. [Google Scholar] [CrossRef]
- Sharma, R.; Kulkarni, G.; Shouche, Y.S. Pseudofusicoccum adansoniae isolated as an endophyte from Jatropha podagrica: new record for India. Mycotaxon 2013, 123, 39–45. [Google Scholar] [CrossRef]
- Prasher, I.B.; Dhanda, R.K. First record of Pseudofusicoccum adonsoniae Pavlic, T.I. Burgess and M.J. Wingf. from Ficus krishnae (as endophyte) and new record for North India. J. New Biol. Rep. 2017, 6, 112–116. [Google Scholar]
- Jami, F.; Marincowitz, S.; Slippers, B.; Wingfield, M.J. New Botryosphaeriales on native red milkwood (Mimusops caffra). Australasian Plant Pathol. 2018, 47, 475–484. [Google Scholar] [CrossRef]
- Jayasiri, S.C.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Jeewon, R.; Phillips, A.J.L.; Bhat, D.J.; Wanasinghe, D.N.; Liu, J.K.; Lu, Y.Z.; Kang, J.C.; Xu, J.; Karunarathna, S.C. Diversity, morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere 2019, 10, 1–186. [Google Scholar] [CrossRef]
- Pavlic, D.; Wingfield, M.J.; Barber, P.; Slippers, B.; Hardy, G.E.S.T.J.; Burgess, T.I. Seven new species of the Botryosphaeriaceae from baobab and other native trees in Western Australia. Mycologia 2008, 100, 851–866. [Google Scholar] [CrossRef]
- Zink, P.; Fengel, D. Studies on the colouring matter of blue-stain fungi. Part 2. Electron microscopic observations of the hyphae walls. Holzforschung 1989, 43, 371–374. [Google Scholar] [CrossRef]
- Mohali, S. Estudio histológico de madera de pino caribe con manchado azul causado por Botryodiplodia theobromae. Fitopatol. Venez. 1993, 6, 14–17. [Google Scholar]
- Bauch, J. Development and characteristics of discolored wood. IAWA Bull ns, 1984, 5, 91–98. [Google Scholar] [CrossRef]
- Kreber, B.; Byrne, A. Discolorations of hem-fir wood: a review of the mechanisms. Forest Prod. J. 1994, 44, 35–42. [Google Scholar]
- Tattar, T.A. Diseases of shade trees; Academic Press: New York, 1978; p. 361. [Google Scholar] [CrossRef]
- de Wet, J.; Burgess, T.; Slippers, B.; Preisig, O.; Wingfield, B.D.; Wingfield, M.J. Multiple gene genealogies and microsatellite markers reflect relationships between morphotypes of Sphaeropsis sapinea and distinguish a new species of Diplodia. Mycol. Res. 2003, 107, 557–566. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Alves, A.; Pennycook, S.R.; Johnston, P.R.; Ramaley, A.; Akulov, A.; Crous, P.W. Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 2008, 21, 29–55. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.J.L.; Alves, A.; Correia, A.; Luque, J. Two new species of Botryosphaeria with brown, 1-septate ascospores and Dothiorella anamorphs. Mycologia 2005, 97, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Cedeño, L.; Mohali, S.; Palacios-Pru, E. Ultrastructure of Lasiodiplodia theobromae causal agent of Caribbean Pine blue stain in Venezuela. Interciencia 1996, 21, 264–271. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
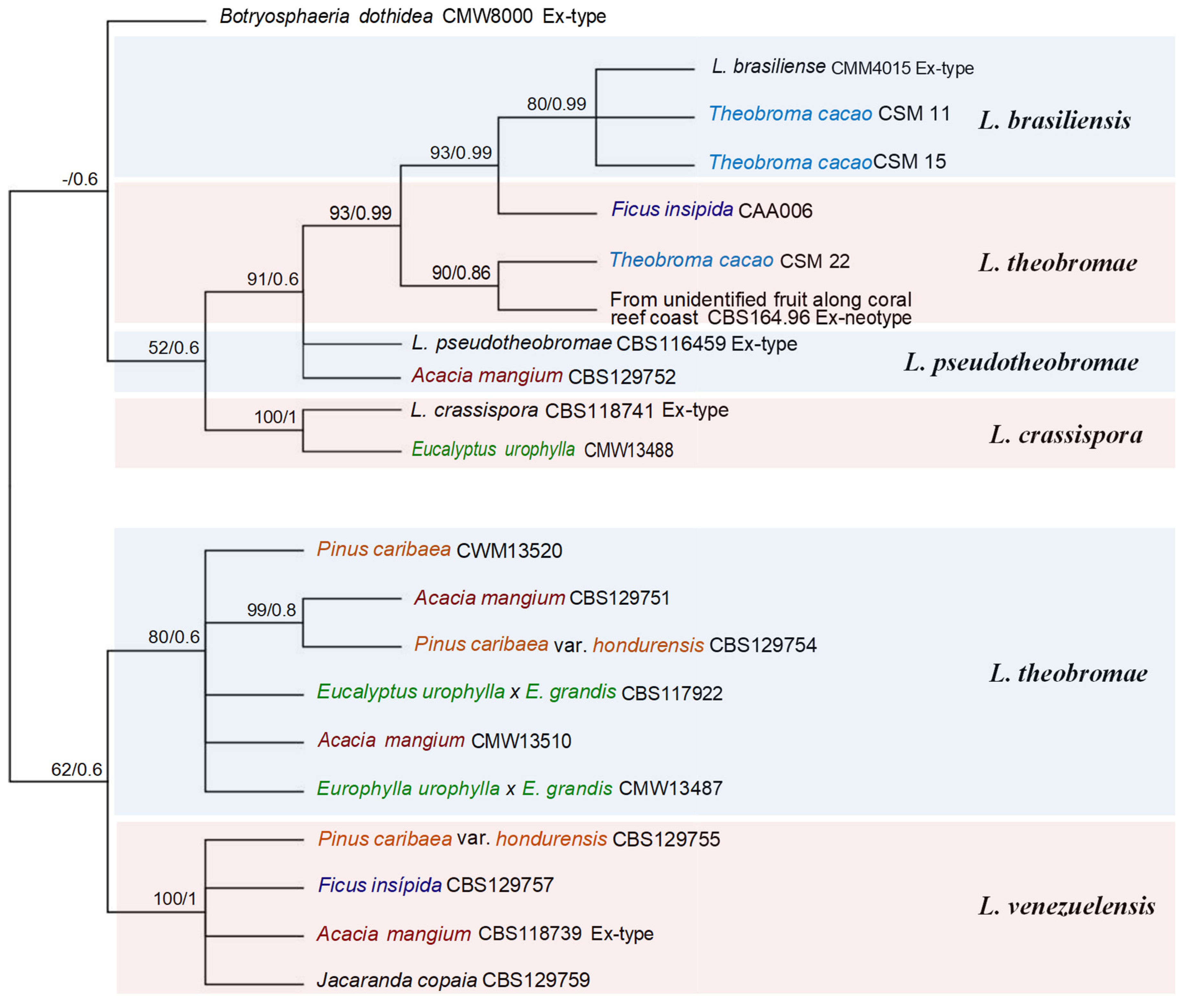
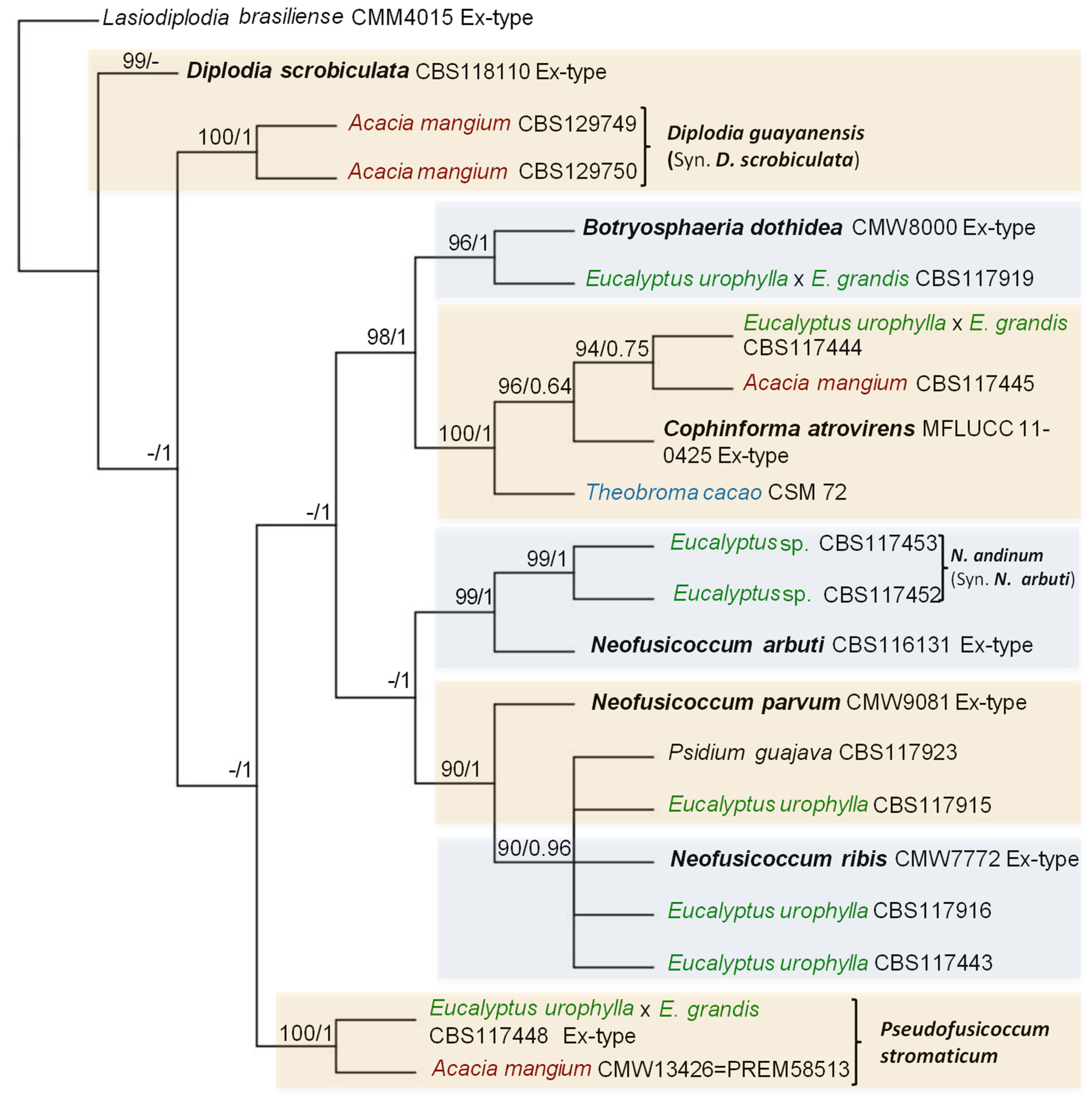


| Species | Accession number | Host | Locality | GenBank accession number | References | |||
|---|---|---|---|---|---|---|---|---|
| ITS | ITS | BTUB | ||||||
| Botryosphaeria dothidea | CMW8000 Ex-type | Prunus sp. | Switzerland | AY236949 | AY236898 | AY236927 | [43] | |
| B. dothidea | CMW13390= CBS117919 |
Eucalyptus urophylla x E.grandis |
CR and WCR | EF118044 | - | - | [53] | |
| Cophinforma atrovirens | CMW13416= CBS117444 |
E.urophylla x E.grandis |
CR and WCR | EF118050 | GU134938 | - | [53] | |
| C. atrovirens | CMW13425= CBS117445 | Acacia mangium | CR and WCR | EF118046 | GU134939 | - | [53] | |
| C. atrovirens | CSM 72 | Theobroma cacao | AR | MF436087 | MF436099 | MF436111 | [54] | |
| C. atrovirens | MFLUCC 11-0425 Ex-type |
Eucalyptus sp | Thailand | JX646800 | JX646865 | JX646848 | [56] | |
| Diplodia scrobiculata(syn. D. guayanensis) | CBS129749 | Acacia mangium | NER | JX545106 | JX545126 | JX545146 | [59] | |
| D. scrobiculata(syn. D. guayanensis) | CBS129750 | Acacia mangium | NER | JX545108 | JX545128 | JX545148 | [59] | |
| D. scrobiculata | CMW189 = CBS 118110 Ex-type | Pinus banksiana | United States | KF766160 | KF766399 | AY624258 | [60,127] | |
| Lasiodiplodia brasiliense | CMM4015 Ex-type | Mangifera indica | Brazil | JX464063 | JX464049 | - | [69] | |
| L. brasiliensis | CSM 11 | Theobroma cacao | AR | MF436018 | MF436006 | MF435998 | [54] | |
| L. brasiliensis | CSM 15 | Theobroma cacao | AR | MF436019 | MF436007 | MF435997 | [54] | |
| L. crassispora | WAC 12533= CBS118741 Ex-type | Santalum album | Australia | DQ103550 | EU673303 | KU887506 | [72,80] | |
| L. crassispora | CMW 13488 | Eucalyptus urophylla | CR and WCR | DQ103552 | DQ103559 | KU887507 | [72,80] | |
| L. pseudotheobromae | CBS 129752 | Acacia mangium | NER | JX545091 | JX545111 | JX545131 | [90] | |
| L. pseudotheobromae | CBS116459 Ex-type | Gmelina arborea | Costa Rica | KF766193 | EF622057 | EU673111 | [25,128] | |
| Lasiodiplodia theobromae | CBS 164.96 Ex-neotype | From unidentified fruit along coral reef coast | Papua New Guinea, Madang | AY640255 | AY640258 | KU887532 | [72,129] | |
| L. theobromae | CSM 22 | Theobroma cacao | AR | MF436023 | MF436011 | MF436005 | [54] | |
| L. theobromae | CBS129751 | Acacia mangium | NER | JX545096 | JX545116 | JX545136 | [59] | |
| L. theobromae | CMW13487 |
Europhylla urophylla x E. grandis |
CR and WCR | EF118053 | - | - | [53] | |
| L. theobromae | CBS129754 | Pinus caribaea var. hondurensis | NER | JX545099 | JX545119 | JX545139 | [59] | |
| L. theobromae | CMW13489= CBS117922 |
Eucalyptus urophylla x E. grandis | CR and WCR | DQ103525 | - | - | [53] | |
| L. theobromae | CMW13510 | Acacia mangium | CR and WCR | DQ103526 | - | - | [43] | |
| L. theobromae | CMW13520 | Pinus caribaea | CR and WCR | DQ103527 | - | - | [43] | |
| L. theobromae | CAA006 | Ficus insipida | GR | DQ458891 | DQ458876 | DQ458859 | [106] | |
| L. venezuelensis | CBS129755 |
Pinus caribaea var. hondurensis |
NER | JX545104 | JX545124 | JX545144 | [59] | |
| L. venezuelensis | CBS129757 | Ficus insípida | GR | JX545102 | JX545122 | - | [106] | |
| L. venezuelensis | WAC12539= CBS118739 Ex-type |
Acacia mangium | CR and WCR | DQ103547 | DQ103568 | KU887533 | [72,80] | |
| L. venezuelensis | CBS 129759 | Jacaranda copaia | GR | JX545101 | JX545121 | JX545141 | F. Castro-Medina/ S.R. Mohali-unpublished |
|
| Neofusicoccum arbuti | CBS 116131=AR 4014 Ex-type | Arbutus menziesii | USA | AY819720 | KF531792 | KF531793 | [8,108] | |
| Neofusicoccum arbuti(syn. N. andinum) | CMW13455= CBS117453 |
Eucalyptus sp. | AR | AY693976 | AY693977 | KX464923 | [26,105] | |
| Neofusicoccum arbuti(syn. N. andinum) | CMW13446= CBS117452 |
Eucalyptus sp. | AR | DQ306263 | DQ306264 | KX464922 | [26,105] | |
| N. parvum | CMW9081 Ex-type | Eucalyptus grandis | South Africa | AY236943 | AY236888 | [43] | ||
| N. parvum | CMW13350= CBS117923 | Psidium guajava | ZR | EF118036 | - | - | [53] | |
| N. parvum | CMW13355= CBS117915 | Eucalyptus urophylla | CR and WCR | EF118035 | - | - | [53] | |
| N. ribis | CMW7772 Ex-type | Ribes sp. | New York, United States | AY236935 | AY236877 | - | [43] | |
| N. ribis | CMW13360= CBS117916 | Eucalyptus urophylla | CR and WCR | EF118037 | - | - | [53] | |
| N. ribis | CMW13410= CBS117443 | Eucalyptus urophylla | CR and WCR | EF118038 | - | - | [53] | |
| Pseudofusicoccum stromaticum | CMW13434= CBS117448 Ex-type |
Eucalyptus urophylla x E. grandis |
CR and WCR | AY693974 | AY693975 | EU673094 | [105,128] | |
| P. stromaticum | CMW13426= PREM 58513 |
Acacia mangium | CR and WCR | EF118041 | - | - | [53] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





