1. Introduction
Immune tolerance is defined as a lack of response to an antigen, induced by previous exposure to said antigen; therefore, tolerance to self-antigens is a fundamental property of a “normal” immune system. Loss of tolerance to self-antigens leads to an inappropriate immune reaction, called autoimmunity [
1,
2].
Diseases caused by such reactions are called autoimmune diseases (AIDs) and are characterized by pathogenic inflammatory responses induced by T lymphocytes (TL) and B lymphocytes (BL), which can induce “autotoxic” effects in virtually any organ or system [
3,
4].
When there is multi-organ involvement, the AID is classified as non-organ-specific (as in systemic lupus erythematosus and rheumatoid arthritis, among others), and when it affects a specific organ, it is classified as an organ-specific AID (such as type 1 diabetes, pernicious anemia, and autoimmune thyroid diseases (AITD), among others) [
5,
6].
Although the molecular mechanisms that induce AIDs are complex, it is clear that some genetic, non-genetic, epigenetic, and environmental factors are the basis for explaining the pathogenesis of these diseases and predicting clinical and biochemical responses [
7,
8].
AITD is the most common AID globally, presenting two classic phenotypes: hypothyroidism (subclinical or primary) in the context of Hashimoto’s thyroiditis (HT), or hyperthyroidism (subclinical or primary) in the context of Graves–Basedow disease (GBD) [
9,
10].
However, there are other thyroid conditions within the AITD group, such as postpartum thyroiditis, thyroiditis associated with autoimmune polyglandular syndromes, and drug-induced thyroiditis (for example, amiodarone), among others [
11,
12].
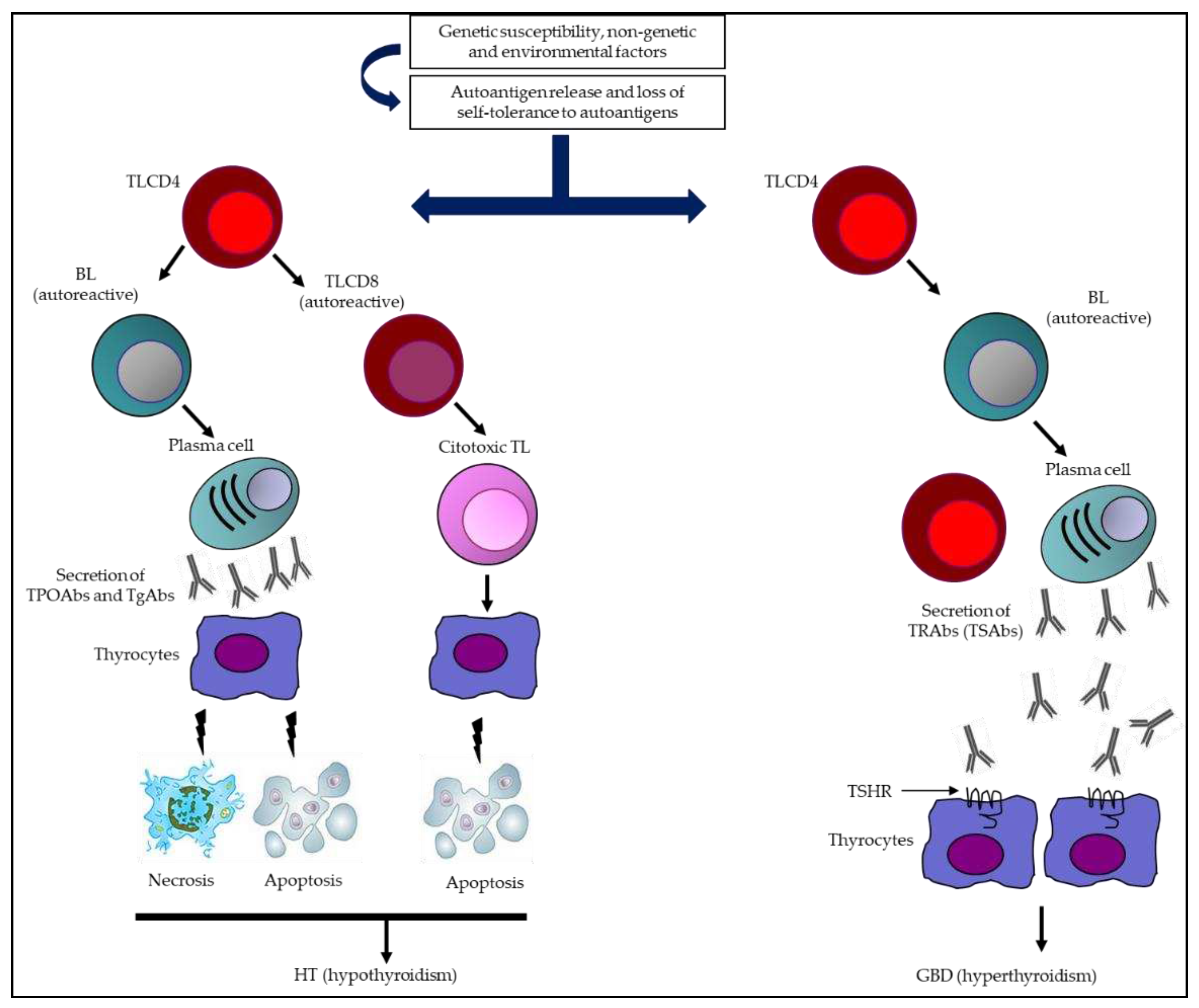
AITD is characterized by lymphocytic infiltration of the thyroid gland. For instance, in HT, the consequent inflammation induces follicular cell destruction, necrosis, and apoptosis, with subsequent fibrosis (and potentially hypothyroidism), with a humoral antibodies (Abs)-mediated response, directed against one or several thyroid antigens. These can include thyroid peroxidase (TPO) and thyroglobulin (Tg), among others [
13,
14].
In GBD on the other hand, a humoral response predominates, with the presence of Abs that stimulate the thyrotropin (TSH) receptor (TSHR). It can be accompanied by goiter, hyperthyroidism, ophthalmopathy, and dermopathy (
Figure 1) [
15,
16].
In this review, the three Abs directed against the major thyroid antigens (TPO, Tg, and TSHR) and against other minor thyroid antigens (pendrin (PDN), sodium iodide symporter (NIS), and megalin (Meg)) are described, as well as the prevalence of the positivity of said antibodies in individuals with AITD and their usefulness in clinical practice.
2. Methods (Search Strategy)
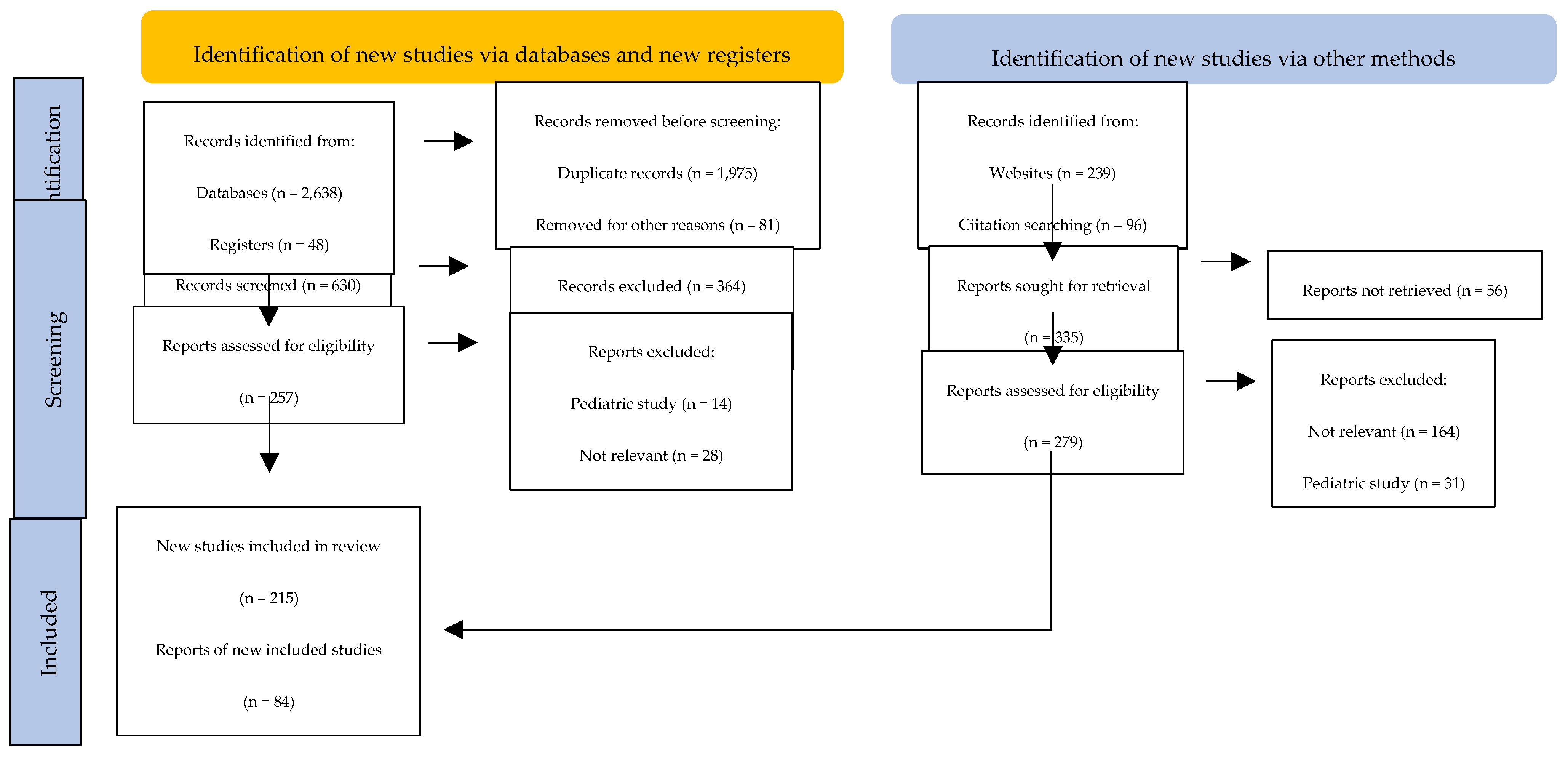
We performed a detailed search in the following databases: PubMed, PubMed Central, Scopus, EMBASE, BIOSIS, UpToDate, and Web of Science. Articles were selected according to the following keywords: autoimmune thyroiditis, Hashimoto’s thyroiditis, Graves–Basedow disease, thyroid antibodies, thyroid antigens, and autoimmune thyroid disease. Only English-written articles were included (
Figure 2).
3. Major Thyroid Antigens
3.1. Tg
The gene encoding Tg synthesis is a single copy gene (270 kb in length) located on chromosome 8q24.2–8q24.3, which contains an 8.5 kb coding sequence divided into 48 exons [
17].
Tg is a glycosylated protein synthesized exclusively in the thyroid and is the largest (660 kDa) and most abundant autoantigen of the thyroid. It is essential for the synthesis of both thyroid hormones (TH)-T4 and T3-, since the synthesis of these hormones depends on the conformation, iodination, and post-translational modification of Tg [
18].
TH synthesis (from Tg) occurs in the thyroid via iodination and coupling of pairs of tyrosines, and it is completed by Tg proteolysis. Additionally, follicular Tg is capable of suppressing the thyroid feedback phenomenon, since it can inhibit the expression of TTF-1, TTF-2, and PAX-8, decreasing the expression of the genes that code for the synthesis of TPO, NIS, TSHR, and Tg [
18,
19].
This suggests that Tg is not only the substrate for the synthesis of TH, but that it is also a regulator of thyroid function [
18,
19].
3.2. TPO
The gene encoding TPO synthesis is a single copy gene (2pter—p12) that codes for a protein of 933 amino acids, spanning the cell membrane, with a large extracellular domain (with 848 amino acids and five potential glycosylation sites) facing the follicular lumen and a cytoplasmic tail with a length of 61 amino acids [
20,
21].
The extracellular domain consists of three regions, which denote a high degree of sequence similarity, with other domains of specific three-dimensional structure, such as the myeloperoxidase-like domain, the complement control protein-like domain, and the epidermal growth factor-like domain. TPO expression is under the control of transcription factors such as TTF-1, TTF-2, and PAX-8 [
22,
23].
TPO has two active sites, which facilitate the iodination of tyrosine residues in Tg, together with the dual oxidase enzyme and hydrogen peroxide, which subsequently allows intrachain coupling of two iodotyrosine residues for TH synthesis. In addition, TPO catalyzes two reactions within the thyroid: the oxidation of iodine and the coupling of iodinated tyrosines in the process of TH synthesis [
23,
24].
Therefore, TPO plays a key role in the biosynthesis of TH and is a fundamental component of normal thyroid function.
3.3. TSHR
TSHR is encoded by a gene located at chromosome 14q31 and belongs to the family of G protein-coupled receptors. It has a large extracellular domain (containing an N-terminal domain, a leucine-rich repeat domain (and a hinge region or cleavage domain), seven transmembrane passageways, and a small intracellular domain [
25,
26]).
TSHR couples to four G protein subfamilies, including Gs (inducing adenylyl cyclase activity and cAMP production), phospholipase C (which activates Gq/G11), G13 (and in turn is capable of inducing p44/42 mitogen-activated protein kinase), and Gi (which inhibits adenylyl cyclase activity) [
27,
28].
Mature TSHR contains two subunits (A and B). The A subunit is made up of a large extracellular domain mainly determined by multiple leucine-rich repeats, and an N-terminal tail, where specific amino acids fold to form a complex TSH-binding pocket. The B subunit contains a short portion that is anchored to the membrane and to the intracellular portion of the receptor [
29,
30].
TSHR expression occurs mainly on the basolateral membrane of thyrocytes, and its activation stimulates iodine uptake, TH synthesis and secretion, and thyrocyte proliferation [
30,
31].
However, TSHR expression has also been demonstrated in other tissues, such as the hypothalamus, cerebellum, amygdala, cortex, cingulate gyrus, frontal, occipital and temporal lobes, periorbital tissue, epidermis, hair follicles, kidneys, ovaries, testicles, adrenals, bone marrow hematopoietic cells, thymocytes, and antigen presenting cells (APCs), among others [
31,
32].
In the thyroid, TSHR activation and G-protein binding induce a series of complex intracellular events that, consequently, stimulate the growth of thyroid cells and the production of TH [
33].
4. Minor Thyroid Antigens
4.1. NIS
The gene that codes for the synthesis of NIS is located on chromosome 19p12–13.2 and encodes a glycoprotein of 643 amino acids, with a molecular mass of about 70–90 kDa [
34].
NIS is an intrinsic membrane protein belonging to the superfamily of sodium/solute symporters and to the human transporter family (SLC5), containing 13 transmembrane domains, an extracellular amino terminus, and an intracellular carboxy terminus [
34,
35].
In thyrocytes, NIS is located at the basal cell level and is a mediator of active iodine transport to thyroid follicular cells involved in the first step of TH synthesis [
36].
Iodine transport, mediated by NIS, is a vector process stimulated by TSH; NIS is also expressed in other tissues, such as the salivary glands, ductal cells, placenta, testicular cells, stomach, and mammary glands (during lactation). However, our understanding of the physiological role of the symporter in these tissues is not yet conclusive [
36,
37].
This symporter is capable of co-transporting two sodium ions with one iodide ion, and the resulting sodium gradient across the membrane acts as a driving force for iodide uptake. Furthermore, TSH is considered to be the factor that regulates NIS expression; in fact, TSH can stimulate iodide uptake by increasing NIS transcription (through cAMP) [
37,
38].
Additionally, TSH can also mediate this phenomenon through post-transcriptional mechanisms. Therefore, NIS catalyzes the accumulation of iodide into thyroid cells, an essential step in the TH synthesis [
39].
4.2. PDN
The gene encoding PDN synthesis is the same gene responsible for Pendred syndrome, which is an autosomal recessive disease that manifests with goiter and congenital sensorineural deafness. PDN is encoded by the SLC26A
4 gene, which is located on chromosome 7q21-31 and contains 21 exons with an open reading frame of 2343 bp [
40,
41].
PDN (SLC26A4) is a glycoprotein composed of 780 amino acids. It contains three putative extracellular asparagine-glycosylation sites and is considered an apical membrane-bound iodide transporter that acts as a multifunctional exchanger of multiple monovalent anions (for example, iodide, chloride, and bicarbonate), and is highly expressed both in the thyroid and in extrathyroid tissues (inner ear and kidneys). In the thyroid, PDN is expressed in the apical membrane of thyrocytes and participates in the transport of iodide to the colloid, indicating its importance in the TH synthesis [
42,
43,
44].
4.3. Meg
Meg is a giant 600 kDa cell surface protein. Its gene covers 235,000 base pairs on the human chromosome 2q24-q31, and consists of 79 exons. It belongs to the endocytic low-density lipoprotein receptor family, which is expressed on the apical surface of thyrocytes; this expression is mediated by TSH. Meg has a high binding affinity for Tg and allows (at least in part) its uptake by thyrocytes; once Tg is internalized by Meg, lysosomal metabolism is avoided, and it is able to reach the basolateral membrane of the thyrocytes (by trancytosis), from where it is released into the blood [
45,
46,
47].
During this trancytosis process, a portion of Meg remains complexed with Tg (and enters circulation), so it could be considered an autoantigen that eventually leads to an Abs-mediated response. However, despite the fact that about half of patients with AITD may present MegAbs, its role in the pathogenesis, and usefulness in the diagnosis, management, and follow-up, of AITD is unknown [
48,
49].
The molecular characteristics and the prevalence of positivity of the different thyroid Abs are summarized in
Table 1.
5. Major Thyroid Abs
5.1. TgAbs
The ability of Tg to induce an immune response depends, at least in part, on its content of both T4 and T3. The concentration of TH within Tg is capable of changing its conformation, stimulating the formation of masked and unmasked epitopes. Consequently, the binding capacity of the Abs can be affected by the content of T4 and T3 in Tg [
50].
TgAbs identified in individuals without AITD generally recognize highly conserved epitopes (located in the T4 and T3-containing regions of Tg), whereas in individuals with AITD, TgAbs are less restricted. Additionally, TgAbs mainly recognize “conformational” and, to a lesser extent, “linear” epitopes, suggesting that the immunogenic potential of Tg increases to the extent that its fragments have a greater capacity to generate conformational epitopes [
51,
52].
In general, TgAbs are polyclonal (of the IgG class), with different contributions according to the four subclasses (IgG4 > IgG3 > IgG2 > IgG1), although low levels of IgA, kappa, and lambda light chains have also been described [
53].
Likewise, this humoral response is highly restricted to two immunodominant regions of Tg (143, 144, 147, and 150–154). In fact, TgAbs are responsive towards restricted epitopes located mainly in the central region and at the C-terminus of Tg (144, 153, and 155–159) [
54].
TgAbs formation can be generated by the massive release of Tg after tissue destruction (as a consequence of thyroiditis, or by direct tissue trauma, or by manipulation of the thyroid), although the intrathyroid iodine content must also be taken into account. For example, excessive iodine consumption can change the conformation of Tg, making it much more antigenic [
55,
56].
5.2. TPOAbs
TPOAbs are considered the hallmark of AITD. The prevalence of positivity in individuals with AITD is higher than for TgAbs [
57].
TPOAbs are capable of recognizing discontinuous determinants in TPO, which have been named immunodominant region A (IDR-A) and B (IDR-B), and several contact residues constituting IDR-A and IDR-B have been identified: 225, 353–363, 377–386, 597–604, 611–618, 620, 624, 627, 630, 646, 707, 713–720, and 766–775 [
58,
59].
TPOAbs can also react against conformational or linear epitopes, and the polyclonal Abs present both in healthy individuals and those with AITD are directed against the same epitopes, taking into account that TPOAbs from healthy individuals do not block TPO action, while those identified in AITD patients can fix complement, produce lysis of thyrocytes, and competitively inhibit enzymatic activity [
60].
Additionally, they can induce oxidative stress. However, despite the cytotoxic effect of TPOAbs on HT, their role in individuals with GBD has not been fully established. It has also been found that the spatial arrangement of the epitopes, together with the domain architecture and the positioning in the membrane, suggest that the interaction of TPO with TPOAbs may require radical changes in the tertiary structure of the antigen [
61,
62].
TPOAbs can be of any IgG class, although the estimated prevalence in descending order is: IgG1 (70%), IgG4 (66.1%), IgG2 (35.1%), and IgG3 (19.6%). Low levels of IgA type TPOAbs have also been described in some patients [
63].
The most frequently used laboratory methods in the evaluation of TgAbs and TPOAbs, in addition to the prevalence of positivity in patients with AITD and in healthy individuals, are summarized in
Table 2 and
Table 3.
5.3. TRAbs
Just as TPOAbs are considered the hallmark of HT, TRAbs are the hallmark of GBD. The prevalence of TRAbs in subjects with HT is 10–20%, and in GBD, it is 90–95%; hence, its detection is recommended in the differential diagnosis of patients with hyperthyroidism [
71].
The mechanism by which hyperthyroidism occurs in GBD is due to the presence of TRAbs, which simulate the effects of TSH on thyrocytes. TSHR is a receptor that belongs to the 7TM G protein-coupled receptor family and is expressed in thyroid follicular cells (and also in thymocytes and retroorbital tissue fibroblasts) [
72,
73].
From the functional and biological points of view, TRAbs can be classified in three ways: stimulators, blockers, and neutral; for GBD, the most frequent are the stimulators. Stimulator TRAbs bind to the N-terminus of the TSH extracellular domain, and consequently stimulate TH production (independently of the feedback phenomenon of the hypothalamic–pituitary–thyroid axis) [
74,
75].
TRAbs have a high receptor affinity; however, their absolute concentration is low. One explanation for this may that they are produced by a limited number of BLs and APCs. Moreover, in some individuals, the immune response may alternate and change from a state in which stimulatory TRAbs are initially (and predominantly) produced, to an opposite state in which the production of blocking or neutral TRAbs is increased, resulting in changes in the clinical and biochemical findings of the disease [
76,
77].
TRAbs are a combination of highly related IgGs, which have the ability to bind to specific epitopes of the TSHR. However, these Abs can vary and fluctuate within the same individual (and between individuals). Therefore, the presence of subtle changes in the affinity or specificity of TRAbs can cause radical changes in their ability to activate the TSHR [
77,
78].
According to the detection methods commonly used for the measurement of TRAbs, these can be divided into competitive immunoassays, bioassays, and enzyme-linked immunosorbent assay (ELISA) [
79].
The former can detect all types of TRAbs, determining their ability to compete with a labeled ligand (TSH or a monoclonal antibody against TSHR) and their ability to bind to TSHR. Furthermore, bioassays can measure the stimulatory or blocking effect of TRAbs by detecting cAMP production and the intracellular TSHR signal (via TSHR-expressing cells). Finally, the ELISA method is based on inhibition of human monoclonal TRAb (M22) binding [
71,
79,
80].
The measurement methods and precision of the most commonly used TRAbs in the diagnostic approach of GBD are described in
Table 4.
5.4. NISAbs and PDNAbs
The prevalence of positivity for NISAbs and PDNAbs in individuals with AITD and in the general population is highly variable. In general terms, the prevalence of NISAb positivity in healthy individuals is very low; however, its prevalence is increased in those with AITD (especially in GBD subjects) [
85].
Moreover, some studies have documented that the prevalence of positivity for NISAbs and PDNAbs is similar in patients with AITD (prevalence close to 10% for each Ab). Likewise, the prevalence of PDNAbs positivity is only slightly higher in individuals with AITD (relative to healthy controls, being also detectable in the latter), and the prevalence is higher in individuals with GBD (versus HT and participants without AITD) [
85,
86].
Several explanations for these findings can be given, such as the type of population studied (populations with low prevalence of the disease), studies with small sample sizes, and the type of technology used to measure Abs. For these reasons, the role of NISAbs and PDNAbs in the diagnosis, prediction, and response and relapse rate of AITD still needs to be clarified.
The measurement methods for NISAbs and PDNAbs most frequently used in the evaluation of individuals with AITD are summarized in
Table 5 and
Table 6. Likewise,
Table 7 summarizes the prevalence of positivity for NISAbs and PDNAbs in subjects with GBD, HT, and in participants without AITD in different studies.
5.5. MegAbs
As previously noted, Meg transports Tg through the thyroid epithelial cells, subsequently entering circulation in a Tg–Meg complex; thus, Meg is capable of eliciting an Abs-mediated immune response [
105].
In rodent models of Heymann’s nephritis, Meg has the ability to induce Abs production and secretion (Heymann’s nephritis is an experimental rat model for active and passive immune-mediated nephritis). However, Meg, which is the target antigen, is localized in podocytes in the rat model, but in humans, megalin is found in the proximal tubule and not in podocytes [
106,
107].
This experimental model in rodents allowed us to propose that MegAbs could be generated and manifest in individuals with AITD in the same way. Initially, studies measuring IgG binding to L2 cells (a rat yolk sac carcinoma cell line known to express Meg) found a prevalence of 50% in subjects with HT and a lower percentage in individuals with GBD (10.5%), while it was not present in healthy individuals [
108].
These results led to theories of a possible role of MegAbs in AITD being disconfirmed, although in fact there are very few studies evaluating the usefulness of MegAbs in different clinical and/or biochemical outcomes in individuals with AITD.
Some of the methods used for the detection of MegAbs are summarized in
Table 8.
6. Clinical Utility of Thyroid Abs in AITD
In AITD, the high prevalence of thyroid Abs (especially TRAbs, TPOAbs, and TgAbs) has made it possible to assess their usefulness in the initial approach and in the follow-up of these patients.
These Abs have been evaluated in aspects such as diagnosis (their mere presence determines the diagnosis of thyroid autoimmunity), differential diagnosis (for example, in cases where the symptoms and images do not allow differentiation between “Hashitoxicosis” and GBD), treatment (making it possible to predict, to a certain extent, which of the individuals affected with GBD may respond better to treatment with antithyroid drugs), risk of relapse (in people affected by GBD with strongly positive TRAbs, the risk of relapse or recurrence of the disease is higher), and prognosis (in individuals with any of the major antibody positives, there is an increased risk of developing hypothyroidism or hyperthyroidism over time, depending on the type of Ab or Abs present) [
111].
6.1. Clinical Utility of TgAbs
Routine measurement of TgAbs in iodine “sufficient” areas does not seem to be very useful as a screening method in the study of AITD; however, it may be useful in iodine “deficient” areas, particularly in individuals with nodular goiter [
112].
It has also been found that, in geographic regions where universal salt iodization programs (for consumption) have been developed, there has been a significant increase in the positivity of TgAbs after salt iodization, indicating that an eventual excess consumption of iodine (through salt) may also increase the immunogenicity of Tg, leading to a higher rate of thyroid autoimmunity. Therefore, it could be argued that, in areas where program of salt iodization have been implemented and where excess consumption has been documented, the measurement of TgAbs could play an important role in the population characterization of thyroid autoimmunity and the potential risk of developing thyroid functional disorders [
9,
63,
113].
6.2. Clinical Utility of TPOAbs
In patients with hypothyroidism (subclinical or primary), TPOAb positivity determines the diagnosis of HT, while in euthyroid individuals, TPOAb positivity is associated with a significant increase in the risk of developing hypothyroidism over time [
114,
115].
Likewise, TPOAbs have been associated with the development of ocular alterations in patients with GBD. However, the results have been contradictory, especially in children. These findings have not been corroborated on a large scale in adults [
14,
116].
Moreover, TPOAbs have been related to a higher rate of infertility, premature birth, and spontaneous abortions (independent of TSH or T4 levels). In fact, it is recommended to evaluate TPOAbs levels in the pre-pregnancy period and once a pregnancy is confirmed, since their presence (even with TSH values in the normal range of 2.5–4.0 mIU/L) could indicate the need for levothyroxine replacement [
117].
In addition, the presence of TPOAbs during early pregnancy may be associated (in children) with a lower intelligence quotient, although it seems that this is mediated by population iodine status, taking into account that TPOAbs can cross the placenta (based on the levels of TPOAbs in umbilical cord blood at the time of birth being similar to those of the mother in the last trimester of pregnancy). This finding does not seem to be related to changes in fetal thyroid function [
116,
117].
Furthermore, TPOAbs measurement during pregnancy may predict the risk of postpartum thyroiditis (with a higher predictive value when measured in the first trimester of pregnancy). There has also been increasing interest in the relationship between TPOAb positivity and the risk of hypothyroidism, destructive thyroiditis, and/or decreased thyroid volume in individuals receiving interferon-α, kinase inhibitor therapy, interleukin-2, amiodarone, and lithium [
118].
6.3. Clinical Utility of TRAbs
The clinical utility of TRAbs in the diagnosis and monitoring of AITD remains controversial, despite the fact that their presence defines the diagnosis of GBD, especially in those individuals with long-standing hyperthyroidism associated with extrathyroid manifestations (ophthalmopathy, myxedema) [
119].
Although in the diagnostic approach of hyperthyroidism (subclinical or primary) imaging methods such as ultrasound and/or thyroid scintigraphy can be used, the measurement of TRAbs can resolve the diagnosis in the majority of affected individuals, differentiating GBD from a “Hashitoxicosis” or other type of thyroiditis that debuts with hyperthyroidism, or also a fictitious thyrotoxicosis, and even toxic nodular goiter [
120].
On the other hand, TRAbs can be detected in practically all patients with thyroid ophthalmopathy. In fact, TRAbs levels correlate with the severity and clinical activity of the disease, and in addition, a high level of TRAbs in patients with early ophthalmopathy predicts a poor prognosis. TRAbs are also useful in those individuals with clinical features of thyroid ophthalmopathy, but with normal or discordant thyroid function (for example, with hypothyroidism) [
121,
122].
Likewise, elevated TRAbs prior to treatment with radioactive iodine (RAI) in individuals with GBD is associated with exacerbation of thyroid ophthalmopathy, suggesting that thyroidectomy could be considered (in the case of non-response to management with antithyroid drugs) in this type of patients, since in the long term, an increase in TRAbs levels has not been demonstrated in patients undergoing thyroidectomy [
123].
The usefulness of measuring TRAbs in individuals with pretibial myxedema must be demonstrated. It may be useful in patients with findings suggestive of this entity but with normal thyroid function [
124].
The detection of TRAbs can also help to make the differential diagnosis in individuals with amiodarone-induced thyroiditis (AmIT), since in this scenario, the presence of TRAbs increases the positive predictive value of a type 1 AmIT; however, a minority of patients can coexist with an associated inflammatory component (type 2 AmIT). Consequently, some patients could be managed with antithyroid drugs when this is not warranted [
125].
Additionally, it should also be taken into account that the absence of TRAbs does not rule out the diagnosis of AmIT; for this reason, the measurement of TRAbs, together with imaging studies (ultrasonography, scintigraphy) and evolution over time will allow confirmation of this diagnosis [
126].
Measurement of TRAbs may also be useful in predicting the course of GBD, since clinical remission of the disease is more likely to be achieved when a decrease in the level of TRAbs is documented (in individuals receiving treatment with antithyroid drugs (ATD)). In this sense, in patients with very high levels of TRAbs (together with prominent extraocular manifestations and large goiter), an “attenuated” and ineffective response can be predicted for the use of ATD [
116,
127].
Furthermore, after treatment with ATD, the recurrence rate of GBD is higher in those patients who initially had a decrease in TRAbs levels, but over time, their levels rose again, which suggests that TRAbs should be measured (in the long-term) in patients who have successfully completed ATD treatment [
128].
Moreover, the presence of TRAbs predicts the risk of fetal/neonatal hyperthyroidism in offspring of women with a history of AITD; in fact, 2–10% of pregnant women with very high levels of TRAbs have children with hyperthyroidism [
129].
It should also be taken into account that the risk of fetal/neonatal hyperthyroidism is significantly reduced after ATD treatment in the mother, but may be high in cases where RAI treatment was performed and TRAbs levels remained elevated. The measurement of TRAbs should be routine in these gestants (in the first and third trimester) [
129,
130].
Likewise, the measurement of TRAbs in pregnant women receiving ATD (in the third trimester) is also recommended. The presence of TRAbs in these situations requires an exhaustive evaluation of hyperthyroidism in the fetus (throughout gestation) and in the neonate. In the latter, evaluation of complete thyroid tests and TRAbs should be performed at birth from blood of the umbilical cord, and sequentially until seven days postpartum (the time by which the transplacental passage of ATDs has disappeared) [
130,
131].
Moreover, and as previously noted, there are several types of TRAbs (in addition to those that stimulate TSHR (TSAb) and are involved in the pathogenesis of GBD) [
13,
14].
TRAbs that have the ability to block TSHr (TBAb) could have utility in the evaluation of subjects with autoimmune thyroiditis; however, the routine measurement of TRAbs (containing both TSAb and TBAb) is not recommended, since they have a low prevalence in the global context of chronic autoimmune thyroiditis. Moreover, in those clinical settings where it is feasible to measure them, their presence does not change the treatment strategy [
132,
133,
134,
135].
However, there are some considerations or clinical situations where the detection of TBAb may be useful:
In individuals with HT and hypothyroidism, where there is adequate and stable control with very low doses of levothyroxine, since it is possible in this type of patient that a release of TBAb has occurred, causing a type of transient hypothyroidism, which could have a high recovery rate;
In a newborn born to a mother with HT, but with the presence of contradictory or bizarre clinical findings;
In patients with HT and clinical findings suggestive of thyroid ophthalmopathy;
When in individuals with long-standing hypothyroidism, under treatment with stable doses of levothyroxine, a change to the state of hyperthyroidism is noted;
When alternating periods of hyperthyroidism and hypothyroidism occur in the same patient.
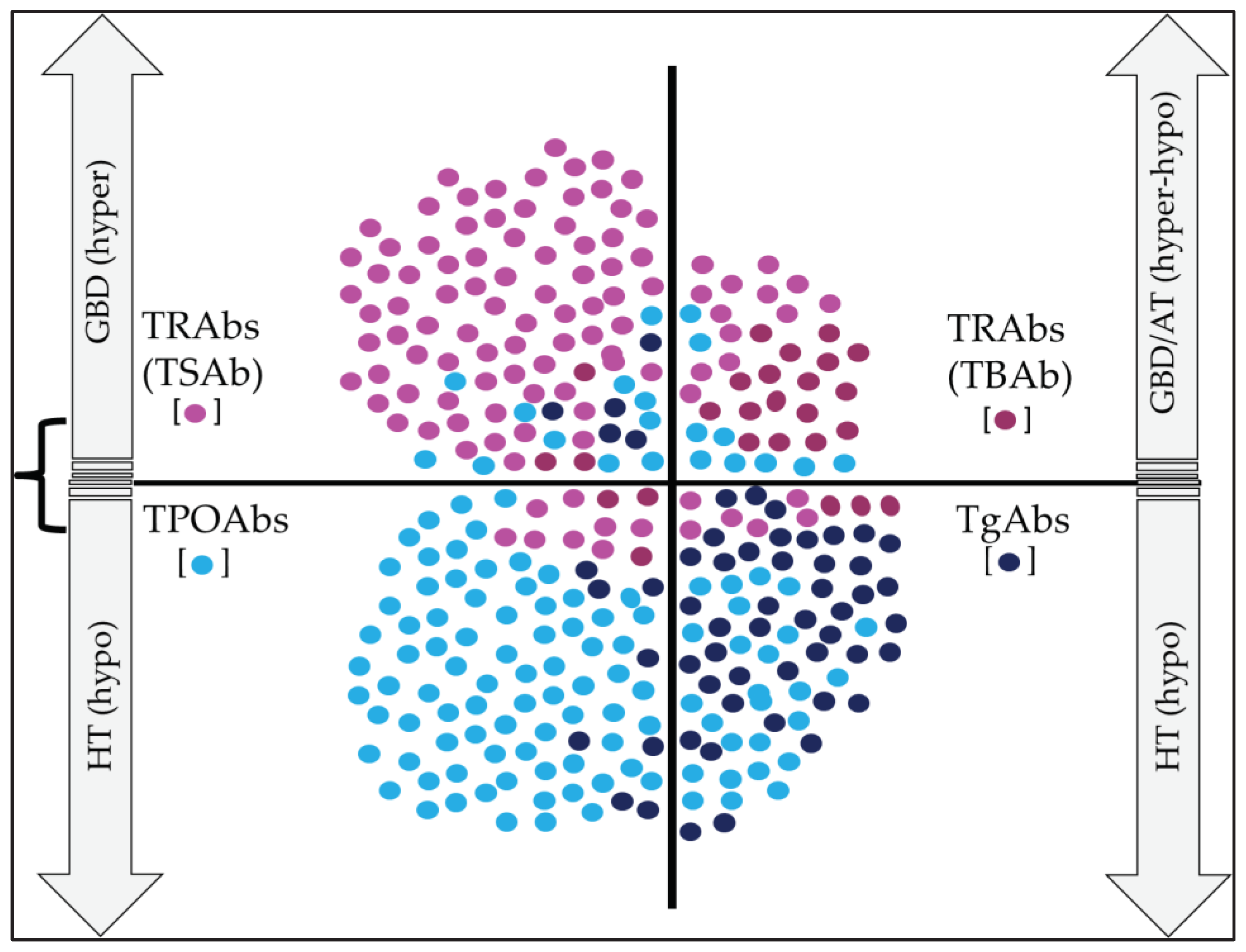
Finally, the usefulness of TRAbs that have a “neutral” effect on thyroid function has not been demonstrated; therefore, their role in individuals with AITD is unknown (
Figure 3) [
133,
134,
135].
6.4. Clinical Utility of PDNAbs, NISAbs, MegAbs, and Other Abs
The utility of these Abs in the diagnosis, treatment, and prognosis of AITD remains to be demonstrated; to date, none have been shown to have a superior diagnostic performance to that provided by TPOAbs, TgAbs, and TRAbs in individuals with HT and GBD [
46,
47,
85,
86,
102,
107].
Other Abs that have been described in patients with AITD are THAbs. THAbs are Abs that can bind to TH, and the presence of such Abs seems to be related to a massive “leakage” of Tg, exposing the immune system to several hormonogenic epitopes of the Tg and inducing a humoral (Ig-mediated) response. Four types of THAb have been described based on the presence of IgM or IgG (T4-IgM, T4-IgG, T3-IgM, and T3-IgG), with T4-IgG and T3-IgG being the most prevalent [
136,
137].
The occurrence of THAbs is variable; for example, in the general population, the prevalence is approximately 1%, but in individuals with AITD the prevalence is 20–23% and 32–46% (in HT and GBD, respectively) [
138,
139].
Additionally, THAbs can also be found in the serum of patients with other autoimmune diseases, such as Sjögren’s syndrome and rheumatoid arthritis, suggesting the presence of epitopes that can induce a “cross-reaction” in other tissues, such as connective tissue. Moreover, a high frequency of THAbs has also been demonstrated in patients with type 1 diabetes mellitus or with vitiligo, and in individuals with autoimmune polyglandular syndrome [
140,
141].
THAbs are not usually measured in clinical practice. In fact, their role is specifically related to the possibility of being able to interfere with peripheral levels of thyroid hormones (T4 and T3), inducing a false elevation (or decrease) of their values [
142].
Another antibody has also been described in individuals with AITD. This antibody is capable of binding to colloidal antigens other than Tg (second colloidal antigen); however, the properties and function of this antibody have not been determined [
143,
144].
7. Conclusions
There have been great advances in the development of the methods used in the detection of thyroid Abs in the last few decades. Measuring these Abs helps to establish the cause of thyroid function abnormalities, as their positivity (at least for major thyroid antibodies) defines the concept of thyroid autoimmunity. For instance, TPOAbs are the hallmark of HT, while TRAbs are the hallmark of GBD.
TRAbs help in the diagnostic confirmation of GBD, but also in differential diagnosis with other types of hyperthyroidism; additionally, they serve as a prognostic factor in thyroid ophthalmopathy, in the risk of relapse, and in the risk of fetal/neonatal hyperthyroidism, among other functions.
Other thyroid Abs (such as NISAbs, PDNAbs, and MegAbs) have not yet been shown to have a higher diagnostic performance than major thyroid antibodies; therefore, their usefulness in the study of AITD has yet to be demonstrated.
Author Contributions
Conceptualization, H V-U; writing—original draft preparation, H V-U, JP N, MV P-F, S D; writing—review and editing, H V-U, JP N, S D, M P-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received financial support from the Colombian Association of Endocrinology, Diabetes and Metabolism (Funding number 003, 15 june 2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Skevaki C, Wesemann DR. Antibody repertoire and autoimmunity. J Allergy Clin Immunol. 2023;151(4):898-900. [CrossRef]
- Huffaker MF, Sanda S, Chandran S, Chung SA, St Clair EW, Nepom GT, Smilek DE. Approaches to Establishing Tolerance in Immune Mediated Diseases. Front Immunol. 2021;12:744804. [CrossRef]
- Petersone L, Edner NM, Ovcinnikovs V, Heuts F, Ross EM, Ntavli E, Wang CJ, Walker LSK. T Cell/B Cell Collaboration and Autoimmunity: An Intimate Relationship. Front Immunol. 2018;9:1941. [CrossRef]
- Corneth OBJ, Neys SFH, Hendriks RW. Aberrant B Cell Signaling in Autoimmune Diseases. Cells. 2022;11(21):3391. [CrossRef]
- Samuels H, Malov M, Saha Detroja T, Ben Zaken K, Bloch N, Gal-Tanamy M, Avni O, Polis B, Samson AO. Autoimmune Disease Classification Based on PubMed Text Mining. J Clin Med. 2022;11(15):4345. [CrossRef]
- Hundt JE, Hoffmann MH, Amber KT, Ludwig RJ. Editorial: Autoimmune pre-disease. Front Immunol. 2023;14:1159396. [CrossRef]
- Chi X, Huang M, Tu H, Zhang B, Lin X, Xu H, Dong C, Hu X. Innate and adaptive immune abnormalities underlying autoimmune diseases: the genetic connections. Sci China Life Sci. 2023:1–36. [CrossRef]
- Mazzone R, Zwergel C, Artico M, Taurone S, Ralli M, Greco A, Mai A. The emerging role of epigenetics in human autoimmune disorders. Clin Epigenetics. 2019;11(1):34. [CrossRef]
- Bogusławska J, Godlewska M, Gajda E, Piekiełko-Witkowska A. Cellular and molecular basis of thyroid autoimmunity. Eur Thyroid J. 2022;11(1):e210024. [CrossRef]
- McLachlan SM, Rapoport B. Discoveries in Thyroid Autoimmunity in the Past Century. Thyroid. 2023;33(3):278-286. [CrossRef]
- Rahimova, RR. Autoimmune thyroiditis (review of literature). Klin Lab Diagn. 2022;67(5):286-291. [CrossRef]
- Stasiak M, Lewiński A. New aspects in the pathogenesis and management of subacute thyroiditis. Rev Endocr Metab Disord. 2021;22(4):1027-1039. [CrossRef]
- Vargas-Uricoechea, H. Molecular Mechanisms in Autoimmune Thyroid Disease. Cells. 2023;12(6):918. [CrossRef]
- Daramjav N, Takagi J, Iwayama H, Uchino K, Inukai D, Otake K, Ogawa T, Takami A. Autoimmune Thyroiditis Shifting from Hashimoto's Thyroiditis to Graves' Disease. Medicina (Kaunas). 2023;59(4):757. [CrossRef]
- Wémeau JL, Klein M, Sadoul JL, Briet C, Vélayoudom-Céphise FL. Graves' disease: Introduction, epidemiology, endogenous and environmental pathogenic factors. Ann Endocrinol (Paris). 2018;79(6):599-607. [CrossRef]
- Chang, Y. Graves' Disease is a Thyroid Autoimmune Disorder Identified by Excessive Thyroid Hormone Production. Thyroid Disorders Ther. 2023;12:294.
- Coscia F, Taler-Verčič A, Chang VT, Sinn L, O'Reilly FJ, Izoré T, Renko M, Berger I, Rappsilber J, Turk D, Löwe J. The structure of human thyroglobulin. Nature. 2020;578(7796):627-630. [CrossRef]
- Citterio CE, Rivolta CM, Targovnik HM. Structure and genetic variants of thyroglobulin: Pathophysiological implications. Mol Cell Endocrinol. 2021;528:111227. [CrossRef]
- Tosatto L, Coscia F. A glance at post-translational modifications of human thyroglobulin: potential impact on function and pathogenesis. Eur Thyroid J. 2022;11(3):e220046. [CrossRef]
- Godlewska M, Gawel D, Buckle AM, Banga JP. Thyroid Peroxidase Revisited - What's New? Horm Metab Res. 2019;51(12):765-769. [CrossRef]
- Ruf J, Carayon P. Structural and functional aspects of thyroid peroxidase. Arch Biochem Biophys. 2006;445(2):269-77. [CrossRef]
- Godlewska M, Banga PJ. Thyroid peroxidase as a dual active site enzyme: Focus on biosynthesis, hormonogenesis and thyroid disorders of autoimmunity and cancer. Biochimie. 2019;160:34-45. [CrossRef]
- Williams DE, Le SN, Godlewska M, Hoke DE, Buckle AM. Thyroid Peroxidase as an Autoantigen in Hashimoto's Disease: Structure, Function, and Antigenicity. Horm Metab Res. 2018;50(12):908-921. [CrossRef]
- Mondal S, Raja K, Schweizer U, Mugesh G. Chemistry and Biology in the Biosynthesis and Action of Thyroid Hormones. Angew Chem Int Ed Engl. 2016;55(27):7606-7630. [CrossRef]
- Chu YD, Yeh CT. The Molecular Function and Clinical Role of Thyroid Stimulating Hormone Receptor in Cancer Cells. Cells. 2020;9(7):1730. [CrossRef]
- Vieira IH, Rodrigues D, Paiva I. The Mysterious Universe of the TSH Receptor. Front Endocrinol (Lausanne). 2022;13:944715. [CrossRef]
- Marín-Sánchez A, Álvarez-Sierra D, González O, Lucas-Martin A, Sellés-Sánchez A, Rudilla F, Enrich E, Colobran R, Pujol-Borrell R. Regulation of TSHR Expression in the Thyroid and Thymus May Contribute to TSHR Tolerance Failure in Graves' Disease Patients via Two Distinct Mechanisms. Front Immunol. 2019;10:1695. [CrossRef]
- Kleinau G, Vassart G. TSH Receptor Mutations and Diseases. 2017 Jul 24. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. [PubMed]
- Gershengorn MC, Osman R. Molecular and cellular biology of thyrotropin-releasing hormone receptors. Physiol Rev. 1996;76(1):175-191. [CrossRef]
- Rapoport B, McLachlan SM. TSH Receptor Cleavage Into Subunits and Shedding of the A-Subunit; A Molecular and Clinical Perspective. Endocr Rev. 2016;37(2):114-34. [CrossRef]
- Helfinger L, Tate CG. Expression and Purification of the Human Thyroid-Stimulating Hormone Receptor. Methods Mol Biol. 2022;2507:313-325. [CrossRef]
- Führer, D. Constitutive TSH receptor activation as a hallmark of thyroid autonomy. Endocrine. 2020;68(2):274-278. [CrossRef]
- Korta P, Pocheć E. Glycosylation of thyroid-stimulating hormone receptor. Endokrynol Pol. 2019;70(1):86-100. [CrossRef]
- Darrouzet E, Lindenthal S, Marcellin D, Pellequer JL, Pourcher T. The sodium/iodide symporter: state of the art of its molecular characterization. Biochim Biophys Acta. 2014;1838(1 Pt B):244-253. [CrossRef]
- Ravera S, Reyna-Neyra A, Ferrandino G, Amzel LM, Carrasco N. The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications. Annu Rev Physiol. 2017;79:261-289.
- Riesco-Eizaguirre G, Santisteban P, De la Vieja A. The complex regulation of NIS expression and activity in thyroid and extrathyroidal tissues. Endocr Relat Cancer. 2021;28(10):T141-T165. [CrossRef]
- Thompson RJ, Fletcher A, Brookes K, Nieto H, Alshahrani MM, Mueller JW, Fine NHF, Hodson DJ, Boelaert K, Read ML, Smith VE, McCabe CJ. Dimerization of the Sodium/Iodide Symporter. Thyroid. 2019;29(10):1485-1498. [CrossRef]
- Portulano C, Paroder-Belenitsky M, Carrasco N. The Na+/I- symporter (NIS): mechanism and medical impact. Endocr Rev. 2014;35(1):106-149. [CrossRef]
- Ravera S, Nicola JP, Salazar-De Simone G, Sigworth FJ, Karakas E, Amzel LM, Bianchet MA, Carrasco N. Structural insights into the mechanism of the sodium/iodide symporter. Nature. 2022;612(7941):795-801.
- Rozenfeld J, Efrati E, Adler L, Tal O, Carrithers SL, Alper SL, Zelikovic I. Transcriptional regulation of the pendrin gene. Cell Physiol Biochem. 2011;28(3):385-396. [CrossRef]
- Dossena S, Nofziger C, Tamma G, Bernardinelli E, Vanoni S, Nowak C, Grabmayer E, Kössler S, Stephan S, Patsch W, Paulmichl M. Molecular and functional characterization of human pendrin and its allelic variants. Cell Physiol Biochem. 2011;28(3):451-466. [CrossRef]
- Dossena S, Bizhanova A, Nofziger C, Bernardinelli E, Ramsauer J, Kopp P, Paulmichl M. Identification of allelic variants of pendrin (SLC26A4) with loss and gain of function. Cell Physiol Biochem. 2011;28(3):467-476. [CrossRef]
- Bizhanova A, Kopp P. Controversies concerning the role of pendrin as an apical iodide transporter in thyroid follicular cells. Cell Physiol Biochem. 2011;28(3):485-490. [CrossRef]
- Twyffels L, Massart C, Golstein PE, Raspe E, Van Sande J, Dumont JE, Beauwens R, Kruys V. Pendrin: the thyrocyte apical membrane iodide transporter? Cell Physiol Biochem. 2011;28(3):491-496. [CrossRef]
- Zheng G, Marino' M, Zhao J, McCluskey RT. Megalin (gp330): a putative endocytic receptor for thyroglobulin (Tg). Endocrinology. 1998;139(3):1462-1465. [CrossRef]
- Marinò M, Pinchera A, McCluskey RT, Chiovato L. Megalin in thyroid physiology and pathology. Thyroid. 2001;11(1):47-56. [CrossRef]
- Marinò M, Zheng G, Chiovato L, Pinchera A, Brown D, Andrews D, McCluskey RT. Role of megalin (gp330) in transcytosis of thyroglobulin by thyroid cells. A novel function in the control of thyroid hormone release. J Biol Chem. 2000;275(10):7125-7137. [CrossRef]
- Goto S, Hosojima M, Kabasawa H, Saito A. The endocytosis receptor megalin: From bench to bedside. Int J Biochem Cell Biol. 2023;157:106393. [CrossRef]
- Lee J, Sul HJ, Kim KH, Chang JY, Shong M. Primary Cilia Mediate TSH-Regulated Thyroglobulin Endocytic Pathways. Front Endocrinol (Lausanne). 2021;12:700083. [CrossRef]
- Soh SB, Aw TC. Laboratory Testing in Thyroid Conditions - Pitfalls and Clinical Utility. Ann Lab Med. 2019;39(1):3-14. [CrossRef]
- Doggui, R. Immunoanalytical profile of thyroglobulin antibodies. Ann Biol Clin (Paris). 2018;76(6):695-704. [CrossRef]
- Dwivedi SN, Kalaria T, Buch H. Thyroid autoantibodies. J Clin Pathol. 2023;76(1):19-28. [CrossRef]
- Li Y, Zhao C, Zhao K, Yu N, Li Y, Yu Y, Zhang Y, Song Z, Huang Y, Lu G, Gao Y, Zhang J, Guo X. Glycosylation of Anti-Thyroglobulin IgG1 and IgG4 Subclasses in Thyroid Diseases. Eur Thyroid J. 2021;10(2):114-124. [CrossRef]
- McIntosh RS, Weetman AP. Molecular analysis of the antibody response to thyroglobulin and thyroid peroxidase. Thyroid. 1997;7(3):471-487. [CrossRef]
- Khan FA, Al-Jameil N, Khan MF, Al-Rashid M, Tabassum H. Thyroid dysfunction: an autoimmune aspect. Int J Clin Exp Med. 2015;8(5):6677-6681.
- Bílek R, Dvořáková M, Grimmichová T, Jiskra J. Iodine, thyroglobulin and thyroid gland. Physiol Res. 2020;69(Suppl 2):S225-S236. [CrossRef]
- McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42(2):252-265. [CrossRef]
- McLachlan SM, Rapoport B. Thyroid Autoantibodies Display both "Original Antigenic Sin" and Epitope Spreading. Front Immunol. 2017;8:1845. [CrossRef]
- Baker S, Miguel RN, Thomas D, Powell M, Furmaniak J, Smith BR. Cryo-electron microscopy structures of human thyroid peroxidase (TPO) in complex with TPO antibodies. J Mol Endocrinol. 2023;70(3):e220149. [CrossRef]
- Espenbetova M, Kuzmina N, Zubkov A, Akhmetova V, Zamanbekova Z, Krykpaeva A, Zhumanbayeva Z, Amrenova K, Smailova Z, Glushkova N. Epitopes specificity of antibodies to thyroid peroxidase in patients with Graves' disease, Hashimoto's thyroiditis and overlap-syndrome. J Clin Transl Endocrinol. 2022;27:100293. [CrossRef]
- Tian X, Li N, Su R, Dai C, Zhang R. Selenium Supplementation May Decrease Thyroid Peroxidase Antibody Titer via Reducing Oxidative Stress in Euthyroid Patients with Autoimmune Thyroiditis. Int J Endocrinol. 2020;2020:9210572. [CrossRef]
- Williams DE, Le SN, Hoke DE, Chandler PG, Gora M, Godlewska M, Banga JP, Buckle AM. Structural Studies of Thyroid Peroxidase Show the Monomer Interacting With Autoantibodies in Thyroid Autoimmune Disease. Endocrinology. 2020;161(2):bqaa016. [CrossRef]
- Fröhlich E, Wahl R. Thyroid Autoimmunity: Role of Anti-thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front Immunol. 2017;8:521. [CrossRef]
- Nishihara E, Amino N, Kudo T, Ito M, Fukata S, Nishikawa M, Nakamura H, Miyauchi A. Comparison of thyroglobulin and thyroid peroxidase antibodies measured by five different kits in autoimmune thyroid diseases. Endocr J. 2017;64(10):955-961. [CrossRef]
- Wolffenbuttel BHR, Wouters HJCM, Muller Kobold AC, Roozendaal C, Klauw MM. Comparison of four commercially available thyroid peroxidase autoantibody and two thyroglobulin autoantibody assays. Available in: https://assets.researchsquare.com/files/rs-1550125/v1/a9384112-5494-4d6c-9c27-3e8e464faebd.pdf?c=1655112553 (Accessed May 3, 2023).
- La'ulu SL, Slev PR, Roberts WL. Performance characteristics of 5 automated thyroglobulin autoantibody and thyroid peroxidase autoantibody assays. Clin Chim Acta. 2007;376(1-2):88-95. [CrossRef]
- Hu X, Chen Y, Shen Y, Tian R, Sheng Y, Que H. Global prevalence and epidemiological trends of Hashimoto's thyroiditis in adults: A systematic review and meta-analysis. Front Public Health. 2022;10:1020709. [CrossRef]
- Pedersen IB, Knudsen N, Jørgensen T, Perrild H, Ovesen L, Laurberg P. Thyroid peroxidase and thyroglobulin autoantibodies in a large survey of populations with mild and moderate iodine deficiency. Clin Endocrinol (Oxf). 2003;58(1):36-42. [CrossRef]
- Camargo RY, Tomimori EK, Neves SC, Knobel M, Medeiros-Neto G. Prevalence of chronic autoimmune thyroiditis in the urban area neighboring a petrochemical complex and a control area in Sao Paulo, Brazil. Clinics (Sao Paulo). 2006;61(4):307-312. [CrossRef]
- Tammaro A, Pigliacelli F, Fumarola A, Persechino S. Trends of thyroid function and autoimmunity to 5 years after the introduction of mandatory iodization in Italy. Eur Ann Allergy Clin Immunol. 2016;48(3):77-81.
- Ehlers M, Allelein S, Schott M. TSH-receptor autoantibodies: pathophysiology, assay methods, and clinical applications. Minerva Endocrinol. 2018;43(3):323-332. [CrossRef]
- Nicolì F, Lanzolla G, Mantuano M, Ionni I, Mazzi B, Leo M, Sframeli A, Posarelli C, Maglionico MN, Figus M, Nardi M, Marcocci C, Marinò M. Correlation between serum anti-TSH receptor autoantibodies (TRAbs) and the clinical feature of Graves' orbitopathy. J Endocrinol Invest. 2021;44(3):581-585. [CrossRef]
- Kahaly GJ, Diana T, Olivo PD. TSH Receptor Antibodies: Relevance & Utility. Endocr Pract. 2020;26(1):97-106. [CrossRef]
- McLachlan SM, Rapoport B. Thyrotropin-blocking autoantibodies and thyroid-stimulating autoantibodies: potential mechanisms involved in the pendulum swinging from hypothyroidism to hyperthyroidism or vice versa. Thyroid. 2013;23(1):14-24. [CrossRef]
- Da Silva Santos T, Oliveira JC, Freitas C, Couto de Carvalho A. Thyroid-Stimulatory Antibody as a Predictive Factor for Graves' Disease Relapse. Cureus. 2022;14(2):e22190. [CrossRef]
- Arshad I, Zahra T, Vargas-Jerez J. New-Onset Graves' Disease in the Background of Hashimoto's Thyroiditis: Spectrums of the Same Disease With Changing Autoantibodies. Cureus. 2022;14(8):e28296. [CrossRef]
- Kotwal A, Stan M. Thyrotropin Receptor Antibodies-An Overview. Ophthalmic Plast Reconstr Surg. 2018;34(4S Suppl 1):S20-S27. [CrossRef]
- Michalek K, Morshed SA, Latif R, Davies TF. TSH receptor autoantibodies. Autoimmun Rev. 2009;9(2):113-116. [CrossRef]
- Gupta AK, Kumar S. Utility of Antibodies in the Diagnoses of Thyroid Diseases: A Review Article. Cureus. 2022;14(11):e31233. [CrossRef]
- Autilio C, Morelli R, Locantore P, Pontecorvi A, Zuppi C, Carrozza C. Stimulating TSH receptor autoantibodies immunoassay: analytical evaluation and clinical performance in Graves' disease. Ann Clin Biochem. 2018;55(1):172-177. [CrossRef]
- López Ortega JM, Martínez PS, Acevedo-León D, Capell NE. Anti-TSH receptor antibodies (TRAb): Comparison of two third generation automated immunoassays broadly used in clinical laboratories and results interpretation. PLoS ONE.2022;17(7): e0270890.
- Struja T, Jutzi R, Imahorn N, Kaeslin M, Boesiger F, Kutz A, Mundwiler E, Huber A, Kraenzlin M, Mueller B, Meier C, Bernasconi L, Schuetz P. Comparison of Five TSH-Receptor Antibody Assays in Graves' disease: results from an observational pilot study. BMC Endocr Disord. 2019;19(1):38.
- Allelein S, Ehlers M, Goretzki S, Hermsen D, Feldkamp J, Haase M, Dringenberg T, Schmid C, Hautzel H, Schott M. Clinical Evaluation of the First Automated Assay for the Detection of Stimulating TSH Receptor Autoantibodies. Horm Metab Res. 2016;48(12):795-801.
- Liu T, Zhang X, Long L, Zhou L, Chen J, Li M, Gao Y, Zhou X, Han X, Ji L. Clinical evaluation of an automated TSI bridge immunoassay in the diagnosis of Graves' disease and its relationship to the degree of hyperthyroidism. BMC Endocr Disord. 2022;22(1):218.
- Brix TH, Hegedüs L, Weetman AP, Kemp HE. Pendrin and NIS antibodies are absent in healthy individuals and are rare in autoimmune thyroid disease: evidence from a Danish twin study. Clin Endocrinol (Oxf). 2014;81(3):440-444.
- Eleftheriadou AM, Mehl S, Renko K, Kasim RH, Schaefer JA, Minich WB, Schomburg L. Re-visiting autoimmunity to sodium-iodide symporter and pendrin in thyroid disease. Eur J Endocrinol. 2020;183(6):571-580. [CrossRef]
-
https://www.biossusa.com/products/bs-0448r (Accesed May 5, 2023).
-
https://www.mybiosource.com/polyclonal-nis-human-antibody/sodium-iodide-symporter/2026331 (Accesed May 5, 2023).
-
https://www.arp1.com/anti-nai-symporter-nis-polyclonal-antibody-03-16093.html (Accesed may 5, 2023).
-
https://www.lsbio.com/antibodies/ihc-plus-slc5a5-antibody-nis-antibody-if-immunofluorescence-ihc-wb-western-ls-b15569/697202?trid=247 (Accesed May 5, 2023).
-
https://www.biorbyt.com/nis-antibody-orb11131.html (Accesed May 5, 2023).
-
https://www.genetex.com/Product/Detail/NIS-antibody/GTX37599?utm_source=Biocompare&utm_medium=referral&utm_campaign=Biocompare_GeneTex (Accesed May 5, 2023). 5 May.
-
https://www.biocompare.com/9776-Antibodies/17754578-SLC26A4-Pendrin-Antibody/?pda=9776|17754578_0_1||1|Pendrin&dfp=true (Accesed May 8, 2023).
-
https://www.biocompare.com/9776-Antibodies/8077560-SLC26A4-antibody/?pda=9776|8077560_0_1||2|Pendrin&dfp=true (Accesed May 8, 2023).
-
https://www.biocompare.com/9776-Antibodies/4920922-SLC26A4-Pendrin-Mouse-anti-Human-Monoclonal-3D2-Antibody/?pda=9776|4920922_0_0||3|Pendrin (Accesed May 8, 2023).
-
https://www.biocompare.com/9776-Antibodies/12145581-Pendrin-Antibody-SLC26A4/?pda=9776|12145581_0_0||4|Pendrin (Accesed May 8, 2023).
-
https://www.biocompare.com/9776-Antibodies/14074098-Immunotag-8482-S26A4-Polyclonal-Antibody/?pda=9776|14074098_0_0||5|Pendrin (Accesed May 8, 2023).
- Seissler J, Wagner S, Schott M, Lettmann M, Feldkamp J, Scherbaum WA, Morgenthaler NG. Low frequency of autoantibodies to the human Na(+)/I(-) symporter in patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 2000;85:4630–4634.
- Morris JC, Bergert ER, Bryant WP. Binding of immunoglobulin G from patients with autoimmune thyroid disease to rat sodium-iodide symporter peptides: evidence for the iodide transporter as an autoantigen. Thyroid. 1997;7:527–534.
- Yoshida A, Hisatome I, Taniguchi S, Shirayoshi Y, Yamamoto Y, Miake J, Ohkura T, Akama T, Igawa O, Shigemasa C et al. Pendrin is a novel autoantigen recognized by patients with autoimmune thyroid diseases. J Clin Endocrinol Metab. 2009;94:442–448.
- Ajjan RA, Kemp EH, Waterman EA, Watson PF, Endo T, Onaya T, Weetman AP. Detection of binding and blocking autoantibodies to the human sodium-iodide symporter in patients with autoimmune thyroid disease. J Clin Endocrinol Metab.2000;85:2020–2027.
- Brix TH, Hegedus L, Weetman AP & Kemp HE. Pendrin and NIS antibodies are absent in healthy individuals and are rare in autoimmune thyroid disease: evidence from a Danish twin study. Clinical Endocrinology. 2014;81:440–444.
- Ajjan RA, Findlay C, Metcalfe RA, Watson PF, Crisp M, Ludgate M & Weetman AP. The modulation of the human sodium iodide symporter activity by Graves’ disease sera. J Clin Endocrinol Metab. 1998;83:1217–1221.
- Eleftheriadou AM, Mehl S, Renko K, Kasim RH, Schaefer JA, Minich WB, Schomburg L. Re- visiting autoimmunity to sodium-iodide symporter and pendrin in thyroid disease. Eur J Endocrinol. 2020;183(6):571-580.
- Lisi S, Pinchera A, McCluskey RT, Willnow TE, Refetoff S, Marcocci C, Vitti P, Menconi F, Grasso L, Luchetti F, Collins AB, Marino M. Preferential megalin-mediated transcytosis of low-hormonogenic thyroglobulin: a control mechanism for thyroid hormone release. Proc Natl Acad Sci U S A. 2003;100(25):14858-14863.
- Wang YM, Lee VWS, Wu H, Harris DCH, Alexander SI. Heymann nephritis in Lewis rats. Curr Protoc Immunol. 2015;109:15.29.1-15.29.6.
- Akiyama S, Imai E, Maruyama S. Immunology of membranous nephropathy. F1000Res. 2019;8:F1000 Faculty Rev-734.
- Marinò M, Chiovato L, Friedlander JA, Latrofa F, Pinchera A, McCluskey RT. Serum antibodies against megalin (GP330) in patients with autoimmune thyroiditis. J Clin Endocrinol Metab. 1999;84(7):2468-2474.
-
https://www.biocompare.com/Assay-Kit-Product-Search/?search=megalin (Accesed May 10, 2023).
-
https://www.mybiosource.com/human-elisa-kits/megalin/3804658 (Accesed May 10, 2023).
- Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. 2014;13(4-5):391-7. [CrossRef]
- Esfandiari NH, Papaleontiou M. Biochemical Testing in Thyroid Disorders. Endocrinol Metab Clin North Am. 2017;46(3):631-648. [CrossRef]
- Vargas-Uricoechea H, Agredo-Delgado V, Vargas-Sierra HD, Pinzón-Fernández MV. Prevalence of Functional Alterations and The Effects of Thyroid Autoimmunity on The Levels of TSH in an Urban Population of Colombia: A Population-Based Study. Endocr Metab Immune Disord Drug Targets. 2022. [CrossRef]
- Czarnocka, B. Thyroperoxidase, thyroglobulin, Na(+)/I(-) symporter, pendrin in thyroid autoimmunity. Front Biosci (Landmark Ed). 2011;16(2):783-802. [CrossRef]
- Hadj-Kacem H, Rebuffat S, Mnif-Féki M, Belguith-Maalej S, Ayadi H, Péraldi-Roux S. Autoimmune thyroid diseases: genetic susceptibility of thyroid-specific genes and thyroid autoantigens contributions. Int J Immunogenet. 2009;36(2):85-96. [CrossRef]
- Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, LiVosli VA, Niccoli-Sire P, John R, Ruf J, Smyth PP, Spencer CA, Stockigt JR; Guidelines Committee, National Academy of Clinical Biochemistry. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3-126. [CrossRef]
- Demers LM, Spencer CA. Laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Clin Endocrinol (Oxf). 2003;58(2):138-140. [CrossRef]
- Dhillon-Smith RK, Coomarasamy A. TPO antibody positivity and adverse pregnancy outcomes. Best Pract Res Clin Endocrinol Metab. 2020;34(4):101433. [CrossRef]
- Furmaniak J, Sanders J, Sanders P, Miller-Gallacher J, Ryder MM, Rees Smith B. Practical applications of studies on the TSH receptor and TSH receptor autoantibodies. Endocrine. 2020;68(2):261-264. [CrossRef]
- Kahaly GJ, Bartalena L, Hegedüs L, Leenhardt L, Poppe K, Pearce SH. 2018 European Thyroid Association Guideline for the Management of Graves' Hyperthyroidism. Eur Thyroid J. 2018;7(4):167-186. [CrossRef]
- Parameswaran R, de Jong MC, Kit JLW, Sek K, Nam TQ, Thang TV, Khue NT, Aye TT, Tun PM, Cole T, Miller JA, Villa M, Khiewvan B, Sirinvaravong S, Sin YL, Muhammad R, Jap TS, Agrawal A, Rajput R, Fernando R, Sumanatilleke M, Suastika K, Shong YK, Lang B, Bartalena L, Yang SP; Asian Graves Consortium Study. 2021 Asia-Pacific Graves' Disease Consortium Survey of Clinical Practice Patterns in the Management of Graves' Disease. Endocrine. 2023;79(1):135-142. [CrossRef]
- Hoang TD, Stocker DJ, Chou EL, Burch HB. 2022 Update on Clinical Management of Graves Disease and Thyroid Eye Disease. Endocrinol Metab Clin North Am. 2022;51(2):287-304. [CrossRef]
- Bartalena L, Tanda ML. Current concepts regarding Graves' orbitopathy. J Intern Med. 2022;292(5):692-716. [CrossRef]
- Fatourechi, V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6(5):295-309. [CrossRef]
- Trohman RG, Sharma PS, McAninch EA, Bianco AC. Amiodarone and thyroid physiology, pathophysiology, diagnosis and management. Trends Cardiovasc Med. 2019;29(5):285-295. [CrossRef]
- Medić F, Bakula M, Alfirević M, Bakula M, Mucić K, Marić N. Amiodarone and thyroid dysfunction. Acta Clin Croat. 2022;61(2):327-341. [CrossRef]
- Martinez Quintero B, Yazbeck C, Sweeney LB. Thyroiditis: Evaluation and Treatment. Am Fam Physician. 2021;104(6):609-617.
- John M, Jagesh R, Unnikrishnan H, Jalaja MMN, Oommen T, Gopinath D. Utility of TSH Receptor Antibodies in the Differential Diagnosis of Hyperthyroidism in Clinical Practice. Indian J Endocrinol Metab. 2022;26(1):32-37. [CrossRef]
- Mooij CF, Cheetham TD, Verburg FA, Eckstein A, Pearce SH, Léger J, van Trotsenburg ASP. 2022 European Thyroid Association Guideline for the management of pediatric Graves' disease. Eur Thyroid J. 2022;11(1):e210073. [CrossRef]
- Ashkar C, Sztal-Mazer S, Topliss DJ. How to manage Graves' disease in women of childbearing potential. Clin Endocrinol (Oxf). 2023;98(5):643-648. [CrossRef]
- Pyrżak B, Rumińska M, Witkowska-Sędek E, Kucharska A. Follow-Up of Thyroid Function in Children With Neonatal Hyperthyroidism. Front Endocrinol (Lausanne). 2022;13:877119. [CrossRef]
- Orgiazzi, J. Anti-TSH Receptor Antibodies in Clinical Practice. Endocrinol Metab Clin North Am. 2000;29(2):339–355. [CrossRef]
- McLachlan SM, Rapoport B. Thyrotropin-Blocking Autoantibodies and Thyroid-Stimulating Autoantibodies: Potential Mechanisms Involved in the Pendulum Swinging From Hypothyroidism to Hyperthyroidism or Vice Versa. Thyroid. 2013;23(1):14–24. [CrossRef]
- Diana T, Olivo PD, Kahaly GJ. Thyrotropin Receptor Blocking Antibodies. Horm Metab Res. 2018;50(12):853–862. [CrossRef]
- Napolitano G, Bucci I, Di Dalmazi G, Giuliani C. Non-Conventional Clinical Uses of TSH Receptor Antibodies: The Case of Chronic Autoimmune Thyroiditis. Front. Endocrinol.2021; 12:769084. [CrossRef]
- Benvenga S, Bartolone L, Squadrito S, Trimarchi F. Thyroid hormone autoantibodies elicited by diagnostic fine needle biopsy. J Clin Endocrinol Metab. 1997;82(12):4217-4223. [CrossRef]
- Benvenga S, Burek CL, Talor M, Rose NR, Trimarchi F. Heterogeneity of the thyroglobulin epitopes associated with circulating thyroid hormone autoantibodies in Hashimoto's thyroiditis and non-autoimmune thyroid diseases. J Endocrinol Invest. 2002;25(11):977-982. [CrossRef]
- Sakata S, Nakamura S, Miura K. Autoantibodies against thyroid hormones or iodothyronines. Ann Intern Med. 1985;103(4):579-589.
- Ni J, Long Y, Zhang L, Yang Q, Kou C, Li S, Li J, Zhang H. High prevalence of thyroid hormone autoantibody and low rate of thyroid hormone detection interference. J Clin Lab Anal. 2022;36(1):e24124. [CrossRef]
- Ruggeri RM, Galletti M, Mandolfino MG, Aragona P, Bartolone S, Giorgianni G, Alesci D, Trimarchi F, Benvenga S. Thyroid hormone autoantibodies in primary Sjögren syndrome and rheumatoid arthritis are more prevalent than in autoimmune thyroid disease, becoming progressively more frequent in these diseases. J Endocrinol Invest. 2002;25(5):447-454.
- Vita R, Santaguida MG, Virili C, Segni M, Galletti M, Mandolfino M, Di Bari F, Centanni M, Benvenga S. Serum Thyroid Hormone Antibodies Are Frequent in Patients with Polyglandular Autoimmune Syndrome Type 3, Particularly in Those Who Require Thyroxine Treatment. Front Endocrinol (Lausanne). 2017;8:212. [CrossRef]
- Ghazal K, Brabant S, Prie D, Piketty ML. Hormone Immunoassay Interference: A 2021 Update. Ann Lab Med. 2022;42(1):3-23. [CrossRef]
- Doniach D, Roitt IM. In: Gell PGH, Coombs RA, Lachmann PJ, eds. Clinical aspects of immunology. Oxford: Blackwell Science, 1975:1355.
- Ochi, Y. Possible participation of colloid antigen 2 and abhormone (IgG with hormone activity) for the etiology of Grave’s Disease. Med Hypotheses.2019;127:23-25. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).