Submitted:
19 June 2023
Posted:
20 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Clinical Evidence and Controversies
2.1. Justification and rationale
2.2. Summary of clinical evidence
|
Study (Ref) |
N | Imaging/ Nº MET |
Type of lesion | MDT/ Design |
Median FU |
Endpoints | Outcome |
|---|---|---|---|---|---|---|---|
| Harrows (11) SABR-COMET Phase II RCT |
16/99* | Conv. 1-5 |
Palliative SOC (PSOC) vs SABR + PSOC |
5.7 yrs | P: OS S: PFS Toxicity, QoL, |
8-yr OS: HR 0.50 8-yr PFS: HR. 0.45 |
|
| Ost (13) STOMP Phase II RCT |
62 | PET-Cho (1-3) |
Nodal 55% M1a 16% 1-2 met 78% |
Surveillance vs MDT: SBRT (81%) or S) |
3 yrs | P: ADTF | 13 vs 21 mo (HR 0.60; p=0.11) |
| Phillips (14) ORIOLE Phase II RCT |
4 | Conv PSMA-PET* (1-3) |
Nodal alone 58% mean nº lesions 1.6 |
Surveillance vs SBRT | 19 mo | 6 months PFS Median PFS |
81% vs 39% (p=0.005) HR 0.30; p=0.002) |
| Siva (17) POPSTAR Phase I |
33 | CT, BS, F-PET 1-3 |
Nodal 39% 1 lesion: 67% |
SBRT (1x20 Gy) (ADT 33%) |
24 mo | Local-PFS | 2-yr L-PFS 93% 2-yr DFS 39% 2-yr ADTF 48% |
| Glicksman (16) PSMA MRgRT Phase II |
74 |
PSMA PET-CT/MR 2 lesions |
Nodal 34/37 N1 <=3 in 31/37 M1a: 4 |
SBRT (87%) or Surgery No ADT |
41 mo | P: PSA response S: PSA-PFS and ADTF |
51% Median 21 months Median 45 months |
| Hölscher (18) OLI-P Phase II |
63 | PSMA-PET/MR 1 lesion |
1 lesion: 71% Nodal alone 68% |
SBRT 77% CRT 50 Gy 23% No ADT |
37 mo | P: Treatment-related toxicity S: PSAFS Time to ADT |
No grade ≥2 tox Median 13.2 mo Median 20. 6 mo |
| Conde Moreno (43) SBRT-SG05 Phase II |
67 |
PET-Cho/MR 1-5 |
Nodal 57% Non-spinal bone 36% Spinal bone 6% |
SBRT and ADT | 41 mo | DPFS | Median DPFS 54,2 mo No grade ≥3 tox |
2.3. New molecular imaging. Clinical meaning on treatment strategies
2.4. Tailoring treatment approach
3. Combined RT and Systemic Therapy
4. Volume of Treatment and RT Scheme. N1 vs M1A? Role of Elective Pelvic RT
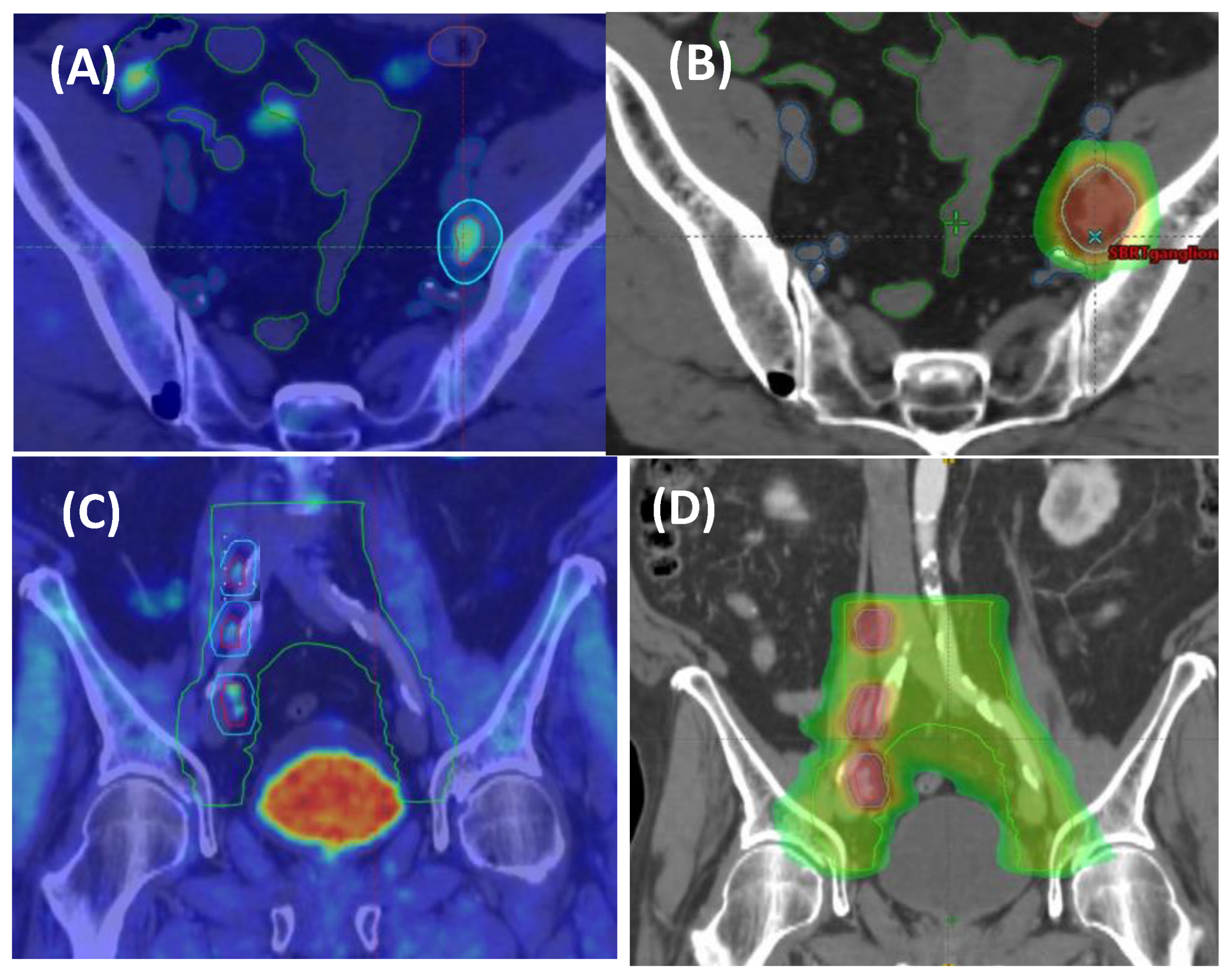
5. Trials Ongoing and Future Directions
| Trial (Type) | n | Patient characteristics | Design | Primary outcome | Status /Estimated completion date |
|---|---|---|---|---|---|
| Diagnosis with PET/TC w/wo WB MRI | |||||
| NCT03304418RROPE (phase II) | 20 | Metachronous oligometastatic (≤3) HSPC (PSMA-PET) | Radium-223+ SBRT (16-32.4 Gy/1-6fx) | Time to ADT | Active, not recruiting/Aug 2023 |
| NCT03902951 (phase II) | 28 | Metachronous oligometastatic (≤5) M1a,b prostate cancer patients (exclusive of pelvic nodal N1 metastases) | ADT + Abiraterone + Apalutamide + SBRT (1-5fx) | % of patients achieving PSA <0.05 ng/mL | Recruiting/Jan 2025 |
| NCT04557059 PRIMORDIUM (phase II) | 412 | Metachronous oligometastatic pelvic nodes HSPC (PSMA-PET |
Arm A: pbRT + Pelvic RT +/- SBRT (ns) + ADT Arm B: pbRT + Pelvic RT +/- SBRT (ns) + ADT + Apalutamide |
PSMA-PET DPFS | Recruiting/Jan 2028 |
| NCT03569241 PEACE V-STORM (phase II) | 178 | Metachronous nodal oligometastatic HSPC |
Arm A: ADT + SBRT (ns) Arm B: ADT + WPRT + SBRT (ns) |
DPFS | Active, not recruiting/April 2025 |
| NCT04641078 DART (phase II) | 128 | Metachronous oligometastatic pelvic nodes (≤5) HSPC (PSMA-PET) |
Arm A: SBRT (ns) + Darolutamide Arm B: SBRT (ns ) |
DPFS | Recruiting/Feb 2026 |
| NCT03795207 POSTCARD (phase II) | 96 | Metachronous oligometastatic Pelvic nodes (≤5) HSPC | Arm A: SBRT (3fx) + Durvalumab Arm B: SBRT (3fx) | 2 years PFS | Active, not recruiting /Nov 2024 |
| NCT04748042 FAALCON (phase II) | 29 | Metachronous oligometastatic (≤5) HSPC (PSMA-PET) | Arm A: RT (ns) + Abiraterone + ADT + Olaparib | % without treatment failure at 24 months | Recruiting/May 2025 |
| NCT05146973 ProstACT TARGET/SATURN (phase II) | 50 | Metachronous biochemical & oligometastatic HSPC (PSMA-PET) | Arm A: 177Lu-DOTA-TLX951 + RT (ns) | BRFS | Recruiting/June 2025 |
| NCT05404139 DIRECT (phase II) | 66 | Metachronous oligometastatic (≤10) HSPC (PSMA-PET) |
Arm A: ADT + SBRT (ns) Arm B: Enzalutamide + ADT + SBRT (ns) |
PFS | Not yet recruiting/March 2026 |
| NCT04031378 (phase II) | 100 | Synchronous/metachronous oligometastatic (≤3) prostate cancer |
Arm A: SBRT (24 G/1fx) Arm B: SBRT (24Gy/1fx) + systemic therapy |
BRFS | Unknown |
| NCT04599686 (not applicable) | 100 | Metachronous oligometastatic (≤3) M1a,b prostate cancer patients (PSMA-PET) | ADT vs. SBRT (30-50 Gy/3-5 fx) | 1-year ADT-free survival | Recruiting/Oct 2025 |
| NCT05352178 SPARKLE (phase III) | 873 | Metachronous oligometastatic pelvic nodes (≤5) HSPC (PSMA-PET) |
Arm A: MDT Arm B: ADT +ADT Arm C: MDT + ADT+ Enzalutamide |
Poly-metastatic-free survival | Recruiting/Apr 2032 |
| NCT03630666 OLIGOPELVIS2 (phase III) | 256 | Metachronous oligometastatic pelvic nodes (≤5) HSPC (PSMA-PET) | Arm A: Intermittent ADT Arm B: Intermittent ADT + IMRT (ns) | PFS | Recruiting/Jun 2026 |
| NCT04423211 INDICATE (phase III) | 804 | Metachronous biochemical & oligometastatic HSPC |
Arm A: (PET negative extra-pelvic metastases): ADT + Pelvic RT (ns) Arm B: (PET negative extra-pelvic metastases): ADT + Pelvic RT (ns) + Apalutamide Arm C: (PET positive extra-pelvic metastases): ADT + Pelvic RT (ns) + Apalutamide Arm D: (PET positive extra-pelvic metastases): ADT + Pelvic RT (ns) + Apalutamide + SBRT |
PFS | Recruiting/Dec 2032 |
| NCT04983095 METRO (phase III) | 114 | Synchronous/metachronous oligometastatic (≤3) HSPC (PSMA-PET) |
Arm A: pRT (ns) + ADT Arm B: pRT (ns) + ADT + SBRT |
PFS | Recruiting/Dec 2029 |
| NCT04115007 PRESTO (phase II) | 350 | Synchronous/metachronous oligometastatic (≤5 at least 1 bone/lung mtx) HSPC |
Arm A: pRT (60Gy/20fx) + ADT +/- ST + (SBRT 30Gy/3fx) Arm B: pRT (60Gy/20fx) + ADT +/- ST |
Castration resistant prostate cancer-free survival | Recruiting/Jun 2028 |
| NCT03940235 RADIOSA (phase II) | 150 | Metachronous oligometastatic (≤3) M1a,b prostate cancer patients | SBRT vs. SBRT + ADT | PFS | Recruiting/Apr 2024 |
| START-MET (phase III) | 266 | Synchronous/metachronous oligometastatic (≤3) with PSMA-PET (≤5) HSPC |
Arm A: pRT (ns) + ADT + SGADT + SBRT (ns) Arm B: pRT (ns) + ADT + SGADT |
rPFS | Recruiting /Januay 2027 |
| NCT03361735 RA 223 + SBRT (phase II) | 24 | Synchronous/metachronous oligometastatic (≤4, at least 1 bone mtx) HSPC | Arm A: ADT + Radium-223 + SBRT (3-5fx) | Time treatment failure & response rate | Recruiting/Dec 2023 |
| NCT02759783 CORE (phase II/III) | 245 | Metachronous oligometastatic (≤3): Prostate, Breast, NSCLC | SOC vs. SOC + SBRT (ns) | PFS | Active, not recruiting/Oct 2024 |
| NCT03043807 (phase II) | 26 | Metachronous oligometastatic (≤5) HSPC | Docetaxel + ADT+ RTp+ SBRT (ns) | Efficacy as 3y-BRFS | Active, not recruiting/Feb 2024 |
| NCT04037358 RAVENS (phase II) | 64 | Metachronous oligometastatic (≤3) HSPC (at least 1 bone mtx) |
Arm A: SBRT (1-5fx Arm B: Radium-223 + SBRT (1-5fx) |
PFS | Active, not recruiting /Dec 2025 |
| Diagnosis with conventional image or PET | |||||
| NCT03784755 PLATON (phase III) | 410 | Synchronous /metachronous oligometastatic (≤5) HSPC |
Arm A: SOC + pRT (ns) Arm B: SOC + pRT(ns) + SBRT (ns) |
PFS | Recruiting/Dec 2025 |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Routman, D.M.; Chera, B.S.; Gupta, G.P. Circulating Tumor DNA Biomarkers for Early Detection of Oligometastasis. Cancer J. 2020, 26, 116–123. [Google Scholar] [CrossRef]
- N. Mottet, P. N. Mottet, P. Cornford, R.C.N. van den Bergh, E. Briers, D. Eberli, G. De Meerleer, M. De Santis, S. Gillessen, J. Grummet, A.M. Henry et al. EAU-EANM-ESTRO- ESUR-ISUP-SIOG guidelines on prostate cancer update. Eur Urol 2022;79:243–62. http://uroweb.org/guideline/prostate-cancer/. Available online: http://uroweb.org/guideline/prostate-cancer/.
- Michael, J. Morris, Steven P. Rowe, Michael A. Gorin, Lawrence Saperstein, Frederic Pouliot, David Josephson, Jeffrey Y.C. Wong, Austin R. Pantel, Steve Y. Cho, Kenneth L. Gage et al. Diagnostic performance of 18F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: results from the CONDOR phase III, multicenter study. Clin Cancer Res 2021;27:3674–82. [CrossRef]
- Kenneth, J. Pienta, Michael A. Gorin, Steven P. Rowe, Peter R. Carroll, Frédéric Pouliot, Stephan Probst, Lawrence Saperstein, Mark A. Preston, Ajjai S. Alva, Akash Patnaik, et al. A phase 2/3 prospective multicenter study of the diagnostic accuracy of prostate specific membrane antigen PET/CT with 18F-DCFPyL in prostate cancer patients (OSPREY). J Urol 2021;206:52–61. [CrossRef]
- Gillessen, S.; Bossi, A.; Davis, I.D.; de Bono, J.; Fizazi, K.; James, N.D.; Mottet, N.; Shore, N.; Small, E.; Smith, M.; et al. Management of Patients with Advanced Prostate Cancer. Part I: Intermediate-/High-risk and Locally Advanced Disease, Biochemical Relapse, and Side Effects of Hormonal Treatment: Report of the Advanced Prostate Cancer Consensus Conference 2022. Eur. Urol. 2023, 83, 267–293. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Poon, I.; Erler, D.; Badellino, S.; Biswas, T.; Dagan, R.; Foote, M.; Louie, A.V.; Ricardi, U.; et al. Late metastatic presentation is associated with improved survival and delayed wide-spread progression after ablative stereotactic body radiotherapy for oligometastasis. Cancer Med. 2021, 10, 6189–6198. [Google Scholar] [CrossRef]
- Jeremie Calais, Johannes Czernin, Minsong Cao, Amar U Kishan, John V Hegde, Narek Shaverdian, Kiri Sandler, Fang-I Chu, Chris R King, Michael L Steinberg, et al. 68Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med 2018;59:230–7. [CrossRef]
- De Bruycker, A.; De Bleser, E.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Visschere, P.; De Man, K.; Delrue, L.; Lambert, B.; Ost, P. Nodal Oligorecurrent Prostate Cancer: Anatomic Pattern of Possible Treatment Failure in Relation to Elective Surgical and Radiotherapy Treatment Templates. Eur. Urol. 2019, 75, 826–833. [Google Scholar] [CrossRef]
- Soldatov, A.; von Klot, C.A.; Walacides, D.; Derlin, T.; Bengel, F.; Ross, T.L.; Wester, H.-J.; Derlin, K.; Kuczyk, M.A.; Christiansen, H.; et al. Patterns of Progression After 68Ga-PSMA-Ligand PET/CT-Guided Radiation Therapy for Recurrent Prostate Cancer. Int. J. Radiat. Oncol. 2019, 103, 95–104. [Google Scholar] [CrossRef]
- Ost, P.; Jereczek-Fossa, B.A.; Van As, N.; Zilli, T.; Muacevic, A.; Olivier, K.; Henderson, D.; Casamassima, F.; Orecchia, R.; Surgo, A.; et al. Progression-free Survival Following Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Treatment-naive Recurrence: A Multi-institutional Analysis. Eur. Urol. 2016, 69, 9–12. [Google Scholar] [CrossRef]
- Harrow, S.; Palma, D.A.; Olson, R.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Radiation for the Comprehensive Treatment of Oligometastases (SABR-COMET): Extended Long-Term Outcomes. Int. J. Radiat. Oncol. 2022, 114, 611–616. [Google Scholar] [CrossRef]
- Huynh, M.A.; Tang, C.; Siva, S.; Berlin, A.; Hannan, R.; Warner, A.; Koontz, B.; De Meeleer, G.; Palma, D.; Ost, P.; et al. Review of Prospective Trials Assessing the Role of Stereotactic Body Radiation Therapy for Metastasis-directed Treatment in Oligometastatic Genitourinary Cancers. Eur. Urol. Oncol. 2023, 6, 28–38. [Google Scholar] [CrossRef]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef]
- Ryan Phillips, William, Matthew Deek, Noura Radwan, Su Jin Lim, Emmanuel S. Antonarakis, Steven P. Rowe; Ashley E. Ross, Michael A. Gorin, et al: Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: The ORIOLE phase 2 randomized clinical trial. JAMA Oncol 6:650-659, 2020. [Google Scholar] [CrossRef]
- Deek, M.P.; Van der Eecken, K.; Sutera, P.; Deek, R.A.; Fonteyne, V.; Mendes, A.A.; Decaestecker, K.; Kiess, A.P.; Lumen, N.; Phillips, R.; et al. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials J Clin Oncol 2022: 40:3377-3382. https://doi. org/10.1200/JCO.22. 00644. [CrossRef]
- Glicksman, R.M.; Ramotar, M.; Metser, U.; Chung, P.W.; Liu, Z.; Vines, D.; Finelli, A.; Hamilton, R.; Fleshner, N.E.; Perlis, N.; et al. Extended Results and Independent Validation of a Phase 2 Trial of Metastasis-Directed Therapy for Molecularly Defined Oligometastatic Prostate Cancer. Int. J. Radiat. Oncol. 2022, 114, 693–704. [Google Scholar] [CrossRef]
- Siva, S.; Bressel, M.; Murphy, D.G.; Shaw, M.; Chander, S.; Violet, J.; Tai, K.H.; Udovicich, C.; Lim, A.; Selbie, L.; et al. Stereotactic Abative Body Radiotherapy (SABR) for Oligometastatic Prostate Cancer: A Prospective Clinical Trial. Eur. Urol. 2018, 74, 455–462. [Google Scholar] [CrossRef]
- Hölscher, T.; Baumann, M.; Kotzerke, J.; Zöphel, K.; Paulsen, F.; Müller, A.-C.; Zips, D.; Koi, L.; Thomas, C.; Löck, S.; et al. Toxicity and Efficacy of Local Ablative, Image-guided Radiotherapy in Gallium-68 Prostate-specific Membrane Antigen Targeted Positron Emission Tomography–staged, Castration-sensitive Oligometastatic Prostate Cancer: The OLI-P Phase 2 Clinical Trial. Eur. Urol. Oncol. 2021, 5, 44–51. [Google Scholar] [CrossRef]
- Marvaso, G.; Volpe, S.; Pepa, M.; Augugliaro, M.; Corrao, G.; Biffi, A.; Zaffaroni, M.; Bergamaschi, L.; La Fauci, F.M.; Mistretta, F.A.; et al. Oligorecurrent Prostate Cancer and Stereotactic Body Radiotherapy: Where Are We Now? A Systematic Review and Meta-analysis of Prospective Studies. Eur. Urol. Open Sci. 2021, 27, 19–28. [Google Scholar] [CrossRef]
- Kneebone, A.; Hruby, G.; Ainsworth, H.; Byrne, K.; Brown, C.; Guo, L.; Guminski, A.; Eade, T. Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Detected via Prostate-specific Membrane Antigen Positron Emission Tomography. Eur. Urol. Oncol. 2018, 1, 531–537. [Google Scholar] [CrossRef]
- Bowden, P.; See, A.W.; Frydenberg, M.; Haxhimolla, H.; Costello, A.J.; Moon, D.; Ruljancich, P.; Grummet, J.; Crosthwaite, A.; Pranavan, G.; et al. Fractionated stereotactic body radiotherapy for up to five prostate cancer oligometastases: Interim outcomes of a prospective clinical trial. Int. J. Cancer 2019, 146, 161–168. [Google Scholar] [CrossRef]
- Muacevic, A.; Kufeld, M.; Rist, C.; Wowra, B.; Stief, C.; Staehler, M. Safety and feasibility of image-guided robotic radiosurgery for patients with limited bone metastases of prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 455–460. [Google Scholar] [CrossRef]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef]
- Sutera, P.; Song, Y.; Van der Eecken, K.; Shetty, A.C.; English, K.; Hodges, T.; Chang, J.; Fonteyne, V.; Rana, Z.; Ren, L.; et al. Clinical and Genomic Differences Between Advanced Molecular Imaging-detected and Conventional Imaging-detected Metachronous Oligometastatic Castration-sensitive Prostate Cancer. Eur. Urol. 2023. [Google Scholar] [CrossRef]
- Gillessen, S.; Bossi, A.; Davis, I.D.; de Bono, J.; Fizazi, K.; James, N.D.; Mottet, N.; Shore, N.; Small, E.; Smith, M.; et al. Management of patients with advanced prostate cancer—metastatic and/or castration-resistant prostate cancer: Report of the Advanced Prostate Cancer Consensus Conference (APCCC) 2022. Eur. J. Cancer 2023, 185, 178–215. [Google Scholar] [CrossRef]
- LBA02-09 EMBARK: A Phase 3 Randomized Study of Enzalutamide or Placebo Plus Leuprolide Acetate and Enzalutamide Monotherapy in High-risk Biochemically Recurrent Prostate Cancer. J. Urol. 2023, 210, 224–226. [CrossRef]
- Deek, M.P.; Van der Eecken, K.; Phillips, R.; Parikh, N.R.; Velho, P.I.; Lotan, T.L.; Kishan, A.U.; Maurer, T.; Boutros, P.C.; Hovens, C.; et al. The Mutational Landscape of Metastatic Castration-sensitive Prostate Cancer: The Spectrum Theory Revisited. Eur. Urol. 2021, 80, 632–640. [Google Scholar] [CrossRef]
- Sutera, P.; Deek, M.P.; Van der Eecken, K.; Shetty, A.C.; Chang, J.H.; Hodges, T.; Song, Y.; Verbeke, S.; Van Dorpe, J.; Fonteyne, V.; et al. WNT Pathway Mutations in Metachronous Oligometastatic Castration-Sensitive Prostate Cancer. Int. J. Radiat. Oncol. 2023, 115, 1095–1101. [Google Scholar] [CrossRef]
- Soloway, M.S.; Sharifi, R.; Wajsman, Z.; McLeod, D.; Wood, D.P.; Puras-Baez, A. Randomized prospective study comparing radical prostatectomy alone versus radical prostatectomy preceded by androgen blockade in clinical stage B2 (T2bNxM0) prostate cancer. The Lupron Depot Neoadjuvant Prostate Cancer Study Group. J. Urol. 1995, 154. [Google Scholar] [CrossRef]
- Bolla, M.; Maingon, P.; Carrie, C.; Villa, S.; Kitsios, P.; Poortmans, P.M.; Sundar, S.; van der Steen-Banasik, E.M.; Armstrong, J.; Bosset, J.-F.; et al. Short Androgen Suppression and Radiation Dose Escalation for Intermediate- and High-Risk Localized Prostate Cancer: Results of EORTC Trial 22991. J. Clin. Oncol. 2016, 34, 1748–1756. [Google Scholar] [CrossRef]
- Hanks, G.E.; Pajak, T.F.; Porter, A.; Grignon, D.; Brereton, H.; Venkatesan, V.; Horwitz, E.M.; Lawton, C.; Rosenthal, S.A.; Sandler, H.M.; et al. Phase III Trial of Long-Term Adjuvant Androgen Deprivation After Neoadjuvant Hormonal Cytoreduction and Radiotherapy in Locally Advanced Carcinoma of the Prostate: The Radiation Therapy Oncology Group Protocol 92–02. J. Clin. Oncol. 2003, 21, 3972–3978. [Google Scholar] [CrossRef]
- Jones, C.U.; Hunt, D.; McGowan, D.G.; Amin, M.B.; Chetner, M.P.; Bruner, D.W.; Leibenhaut, M.H.; Husain, S.M.; Rotman, M.; Souhami, L.; et al. Radiotherapy and Short-Term Androgen Deprivation for Localized Prostate Cancer. New Engl. J. Med. 2011, 365, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Zapatero, A.; Guerrero, A.; Maldonado, X.; Alvarez, A.; Segundo, C.G.S.; Rodríguez, M.A.C.; Macias, V.; Olive, A.P.; Casas, F.; Boladeras, A.; et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol. 2015, 16, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; Collette, L.; Blank, L.; Warde, P.; Dubois, J.B.; Mirimanoff, R.-O.; Storme, G.; Bernier, J.; Kuten, A.; Sternberg, C.; et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 2002, 360, 103–108. [Google Scholar] [CrossRef]
- Zietman, A.L.; Prince, E.A.; Nakfoor, B.M.; Park, J.J. Androgen deprivation and radiation therapy: Sequencing studies using the shionogi in vivo tumor system. Int. J. Radiat. Oncol. 1997, 38, 1067–1070. [Google Scholar] [CrossRef]
- Granfors, T.; Damber, J.-E.; Bergh, A.; Landström, M.; Löfroth, P.-O.; Widmark, A. Combined castration and fractionated radiotherapy in an experimental prostatic adenocarcinoma. Int. J. Radiat. Oncol. 1997, 39, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Joseph, I.B.; Nelson, J.B.; Denmeade, S.R.; Isaacs, J.T. Androgens regulate vascular endothelial growth factor content in normal and malignant prostatic tissue. Clin. Cancer Res. 1997, 3. [Google Scholar]
- Milosevic, M.; Chung, P.; Parker, C.; Bristow, R.; Toi, A.; Panzarella, T.; Warde, P.; Catton, C.; Menard, C.; Bayley, A.; et al. Androgen Withdrawal in Patients Reduces Prostate Cancer Hypoxia: Implications for Disease Progression and Radiation Response. Cancer Res 2007, 67, 6022–6025. [Google Scholar] [CrossRef]
- Goodwin, J.F.; Schiewer, M.J.; Dean, J.L.; Schrecengost, R.S.; de Leeuw, R.; Han, S.; Ma, T.; Den, R.B.; Dicker, A.P.; Feng, F.Y.; et al. A Hormone–DNA Repair Circuit Governs the Response to Genotoxic Insult. Cancer Discov. 2013, 3, 1254–1271. [Google Scholar] [CrossRef]
- Kissick, H. T.; Sanda, M. G.; Dunn, L. K.; Pellegrini, K. L.; On, S. T.; Noel, J. K.; Arredouani, M. S. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc. Natl. Acad. Sci. USA Am. 2014, 111, 9887–9892. [Google Scholar] [CrossRef]
- Roden, A.C.; Moser, M.T.; Tri, S.D.; Mercader, M.; Kuntz, S.M.; Dong, H.; Hurwitz, A.A.; McKean, D.J.; Celis, E.; Leibovich, B.C.; et al. Augmentation of T Cell Levels and Responses Induced by Androgen Deprivation. J. Immunol. 2004, 173, 6098–6108. [Google Scholar] [CrossRef]
- Drake, C.G. Prostate cancer as a model for tumour immunotherapy. Nat. Rev. Immunol. 2010, 10, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Glicksman, R.M.; Metser, U.; Vines, D.; Valliant, J.; Liu, Z.; Chung, P.W.; Bristow, R.G.; Finelli, A.; Hamilton, R.; Fleshner, N.E.; et al. Curative-intent Metastasis-directed Therapies for Molecularly-defined Oligorecurrent Prostate Cancer: A Prospective Phase II Trial Testing the Oligometastasis Hypothesis. Eur. Urol. 2021, 80, 374–382. [Google Scholar] [CrossRef]
- Ahmed, M.; Li, L.-C. Adaptation and clonal selection models of castration-resistant prostate cancer: Current perspective. Int. J. Urol. 2013, 20, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Conde-Moreno, A.; Lopez, F.; Hervas, A.; Morillo, V.; Mendez, A.; Puertas, M.; Albarrán, J.; De Iturriaga, A.; Rico, M.; de la Torre, M.V.; et al. Phase II Trial of SBRT and Androgen Deprivation for Oligometastases in Prostate Cancer. Int. J. Radiat. Oncol. 2021, 111, S59. [Google Scholar] [CrossRef]
- Shipley, W.U.; Seiferheld, W.; Lukka, H.R.; Major, P.P.; Heney, N.M.; Grignon, D.J.; Sartor, O.; Patel, M.P.; Bahary, J.-P.; Zietman, A.L.; et al. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. New Engl. J. Med. 2017, 376, 417–428. [Google Scholar] [CrossRef]
- Carrie, C.; Magné, N.; Burban-Provost, P.; Sargos, P.; Latorzeff, I.; Lagrange, J.-L.; Supiot, S.; Belkacemi, Y.; Peiffert, D.; Allouache, N.; et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): a 112-month follow-up of a phase 3, randomised trial. Lancet Oncol. 2019, 20, 1740–1749. [Google Scholar] [CrossRef]
- Pollack, A.; Karrison, T.G.; Balogh, A.G.; Gomella, L.G.; A Low, D.; Bruner, D.W.; Wefel, J.S.; Martin, A.-G.; Michalski, J.M.; Angyalfi, S.J.; et al. The addition of androgen deprivation therapy and pelvic lymph node treatment to prostate bed salvage radiotherapy (NRG Oncology/RTOG 0534 SPPORT): an international, multicentre, randomised phase 3 trial. Lancet 2022, 399, 1886–1901. [Google Scholar] [CrossRef]
- C. Parker, N. C. Parker, N. Clarke, A. Cook, C. Catton, W.R. Cross, H. Kynaston, J. Logue, P.M. Petersen, P. Neville, R. Persad0,et al. LBA9 Duration of androgen deprivation therapy (ADT) with post-operative radiotherapy (RT) for prostate cancer: first results of the RADICALS-HD trial (ISRCTN40814031). Ann Oncol. 2022;33 (suppl 7):S1427. [CrossRef]
- Tang, C.; Sherry, A.D.; Haymaker, C.; Bathala, T.; Liu, S.; Fellman, B.; Cohen, L.; Aparicio, A.; Zurita, A.J.; Reuben, A.; et al. Addition of Metastasis-Directed Therapy to Intermittent Hormone Therapy for Oligometastatic Prostate Cancer. JAMA Oncol. 2023, 9, 825. [Google Scholar] [CrossRef]
- Deek MP, Taparra K, Dao D, et al: Patterns of recurrence and modes of progression after metastasis-directed therapy in oligometastatic castra- tion-sensitive prostate cancer. Int J Radiat Oncol Biol Phys 2020; 109:387- 395. [CrossRef]
- Decaestecker K, De Meerleer G, Lambert B, et al: Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol 2014; 9:135. [CrossRef]
- Devos G, Berghen C, Van Eecke H, et al: Oncological outcomes of metastasis-directed therapy in oligorecurrent prostate cancer patients following radical prostatectomy. Cancers (Basel) 2020; 12:2271. [CrossRef]
- De Bleser, E.; Jereczek-Fossa, B.A.; Pasquier, D.; Zilli, T.; Van As, N.; Siva, S.; Fodor, A.; Dirix, P.; Gomez-Iturriaga, A.; Trippa, F.; et al. Metastasis-directed Therapy in Treating Nodal Oligorecurrent Prostate Cancer: A Multi-institutional Analysis Comparing the Outcome and Toxicity of Stereotactic Body Radiotherapy and Elective Nodal Radiotherapy. Eur. Urol. 2019, 76, 732–739. [Google Scholar] [CrossRef]
- Supiot S, Vaugier L, Pasquier D, Buthaud X, Magné N, Peiffert D, Sargos P, Crehange G, Pommier P, Loos G,et al. OLIGOPELVIS GETUG P07, a Multicenter Phase II Trial of Combined High-dose Salvage Radiotherapy and Hormone Therapy in Oligorecurrent Pelvic Node Relapses in Prostate Cancer. Eur Urol. 2021;80(4):405-414. [CrossRef]
- De Bruycker, A.; Spiessens, A.; Dirix, P.; Koutsouvelis, N.; Semac, I.; Liefhooghe, N.; Gomez-Iturriaga, A.; Everaerts, W.; Otte, F.; Papachristofilou, A.; et al. PEACE V – Salvage Treatment of OligoRecurrent nodal prostate cancer Metastases (STORM): a study protocol for a randomized controlled phase II trial. BMC Cancer 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Zilli T, Siva S, Heikkilä R, Dirix P, Liefhooghe N, Otte FX, Gomez-Iturriaga A, Everaerts W, Shelan M, Conde-Moreno A. 2022. Available online: https://www.estro.org/Congresses/ESTRO-2022/581/oligometastaticdisease/5367/peacev-stormrandomizedphaseiitrialforoligorecurren.
- Chopade, P.; Maitre, P.; David, S.; Panigrahi, G.; Singh, P.; Phurailatpam, R.; Murthy, V. Common Iliac Node-Positive Prostate Cancer Treated With Curative Radiation Therapy: N1 or M1a? Int. J. Radiat. Oncol. 2022, 114, 711–717. [Google Scholar] [CrossRef]
- Rich, B.J.; Montoya, C.; Jin, W.H.; Spieler, B.O.; Mahal, B.A.; Delgadillo, R.; Bilusic, M.; Abramowitz, M.C.; Pollack, A.; Pra, A.D. Para-Aortic Radiation Therapy for Oligorecurrent Prostate Cancer. Int. J. Radiat. Oncol. 2022, 114, 718–724. [Google Scholar] [CrossRef]
- Zilli, T.; Achard, V.; Pra, A.D.; Schmidt-Hegemann, N.; Jereczek-Fossa, B.A.; Lancia, A.; Ingrosso, G.; Alongi, F.; Aluwini, S.; Arcangeli, S.; et al. Recommendations for radiation therapy in oligometastatic prostate cancer: An ESTRO-ACROP Delphi consensus. Radiother. Oncol. 2022, 176, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Swami, U.; Velho, P.I.; Nussenzveig, R.; Chipman, J.; Santos, V.S.; Erickson, S.; Dharmaraj, D.; Alva, A.S.; Vaishampayan, U.N.; Esther, J.; et al. Association of SPOP Mutations with Outcomes in Men with De Novo Metastatic Castration-sensitive Prostate Cancer. Eur. Urol. 2020, 78, 652–656. [Google Scholar] [CrossRef]
- Zilli, T.; Dirix, P.; Heikkilä, R.; Liefhooghe, N.; Siva, S.; Gomez-Iturriaga, A.; Everaerts, W.; Otte, F.; Shelan, M.; Mercier, C.; et al. The Multicenter, Randomized, Phase 2 PEACE V-STORM Trial: Defining the Best Salvage Treatment for Oligorecurrent Nodal Prostate Cancer Metastases. Eur. Urol. Focus 2021, 7, 241–244. [Google Scholar] [CrossRef]
- Kucharczyk, M.J.; So, J.; Gravis, G.; Sweeney, C.; Saad, F.; Niazi, T. A combined biological and clinical rationale for evaluating metastasis directed therapy in the management of oligometastatic prostate cancer. Radiother. Oncol. 2020, 152, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Deek, M.; Sutera, P.; Jing, Y.; Pryor, D.; Day, H.; Huynh, M.A.; Koontz, B.; Armstrong, A.; Debruycker, E.; Dirix, P.; et al. Multi-institutional analysis of metastasis directed therapy with or without androgen deprivation therapy in oligometastatic castration sensitive prostate cancer. J. Urol. 2023, 209. [Google Scholar] [CrossRef]
- Arcos, M.B.L.; López-Campos, F.; Valcarcel, M.L.; Rubio, M.G.; de Manzanos, I.V.F.; Duque-Santana, V.; Aparicio, M.G.; Martin, J.Z.; Kishan, A.U.; Achard, V.; et al. Oligometastatic Hormone-Sensitive Prostate Cancer. Why Radiotherapy? Clin. Genitourin. Cancer 2022, 21, e93–e103. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





