Submitted:
19 June 2023
Posted:
19 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
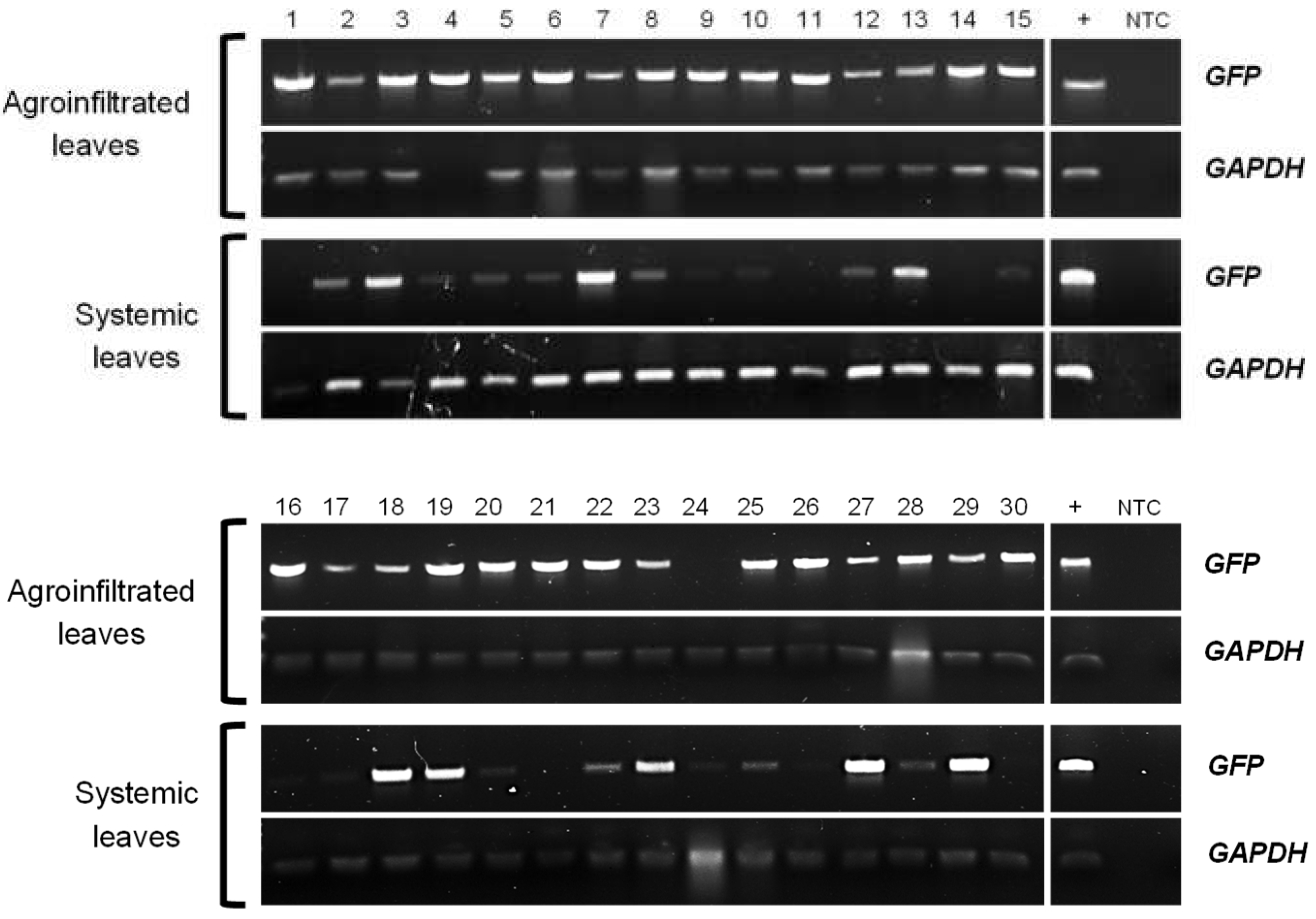
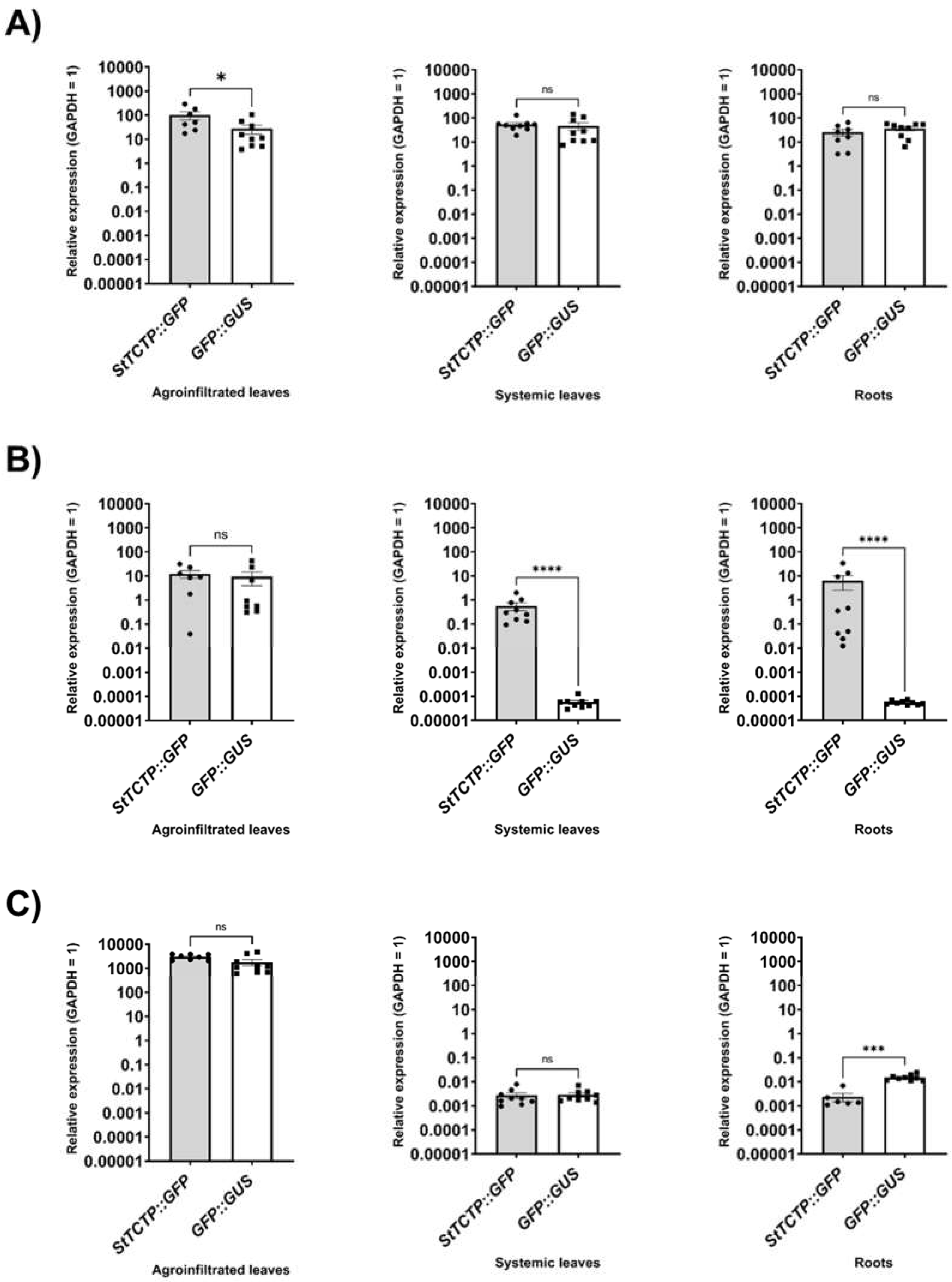
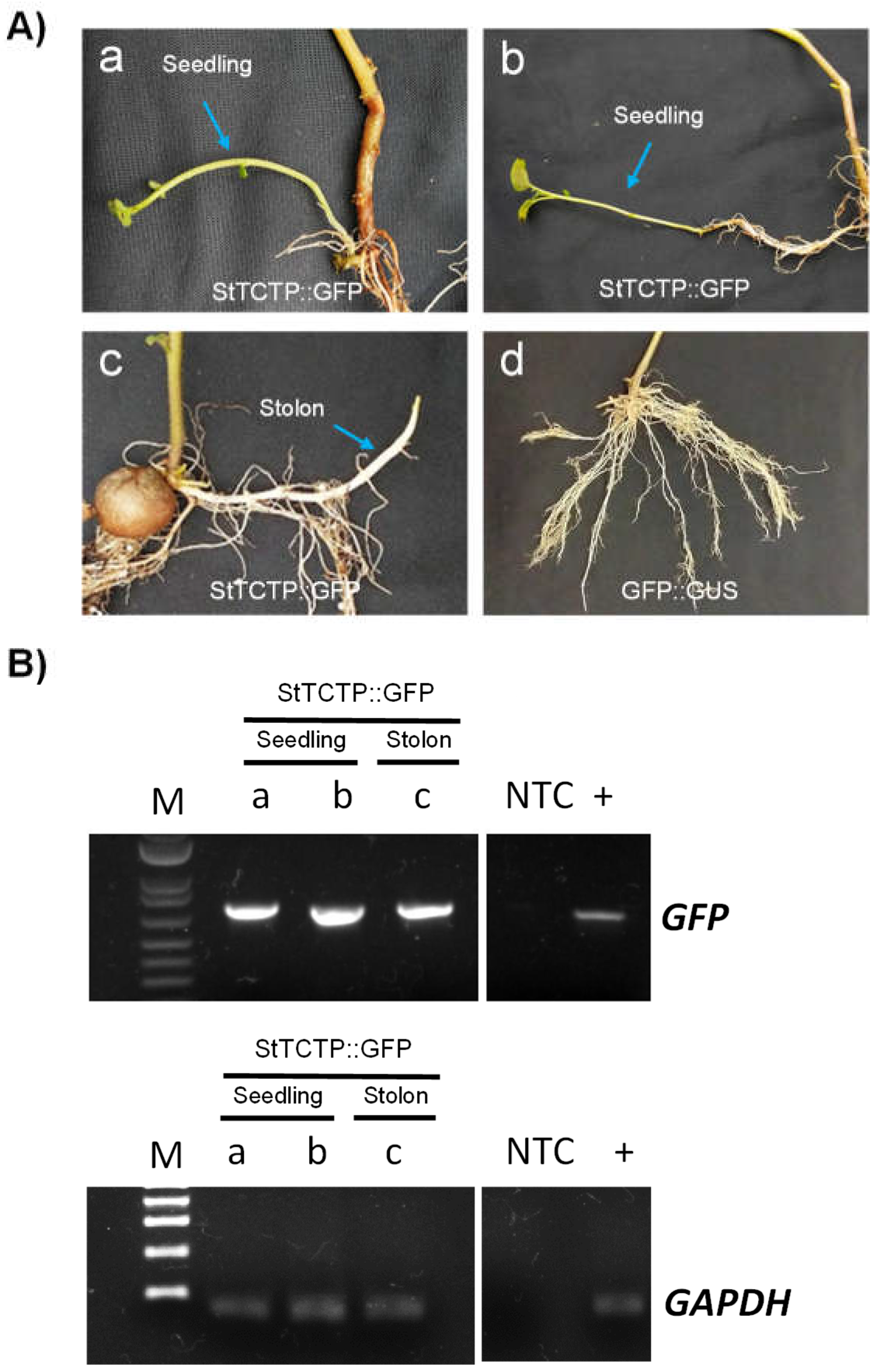
2.1. GFP-fused StTCTP transcript is transported long distance
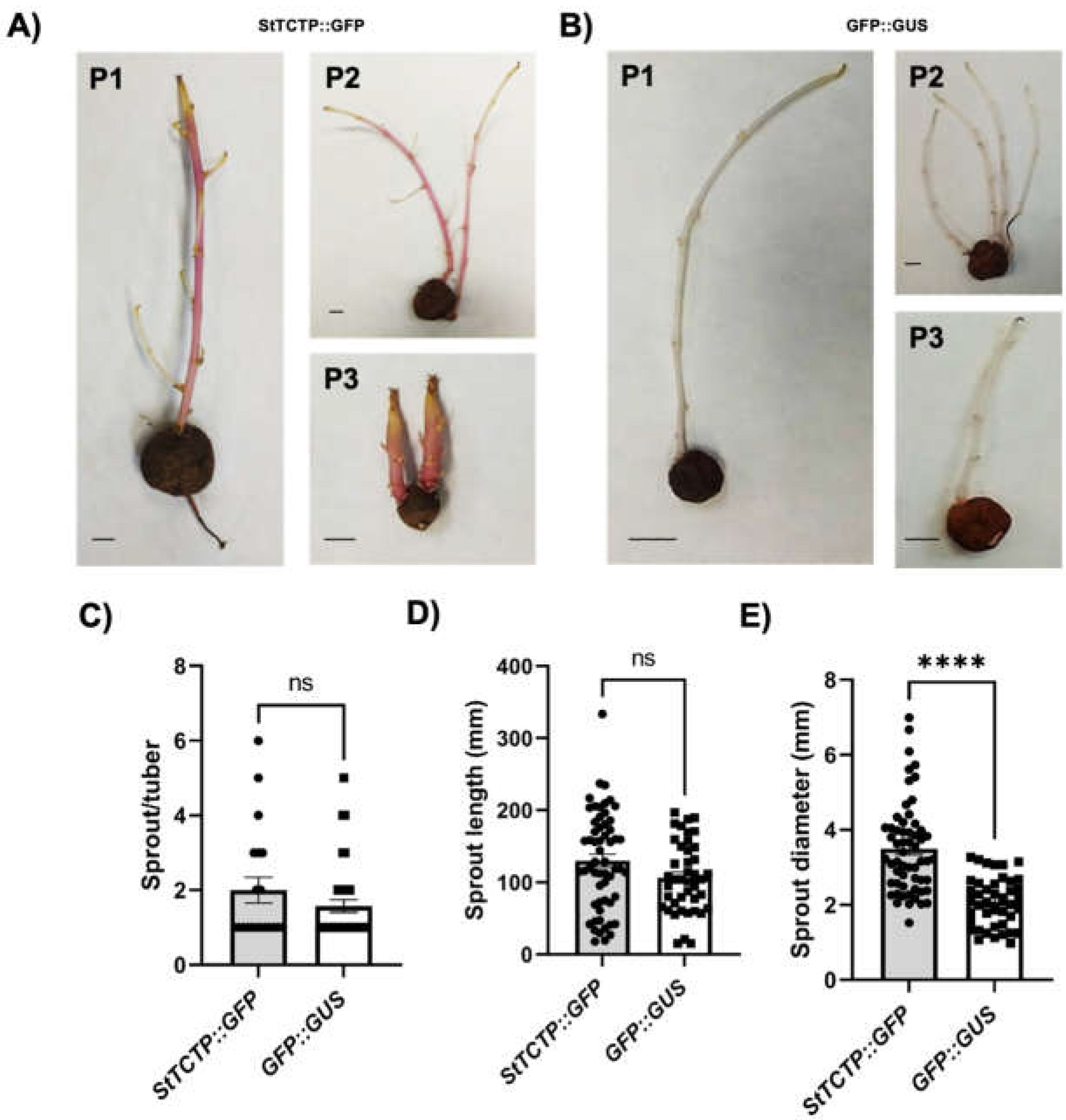
2.2. Phenotype of plants agroinfiltrated with StTCTP::GFP or GFP::GUS
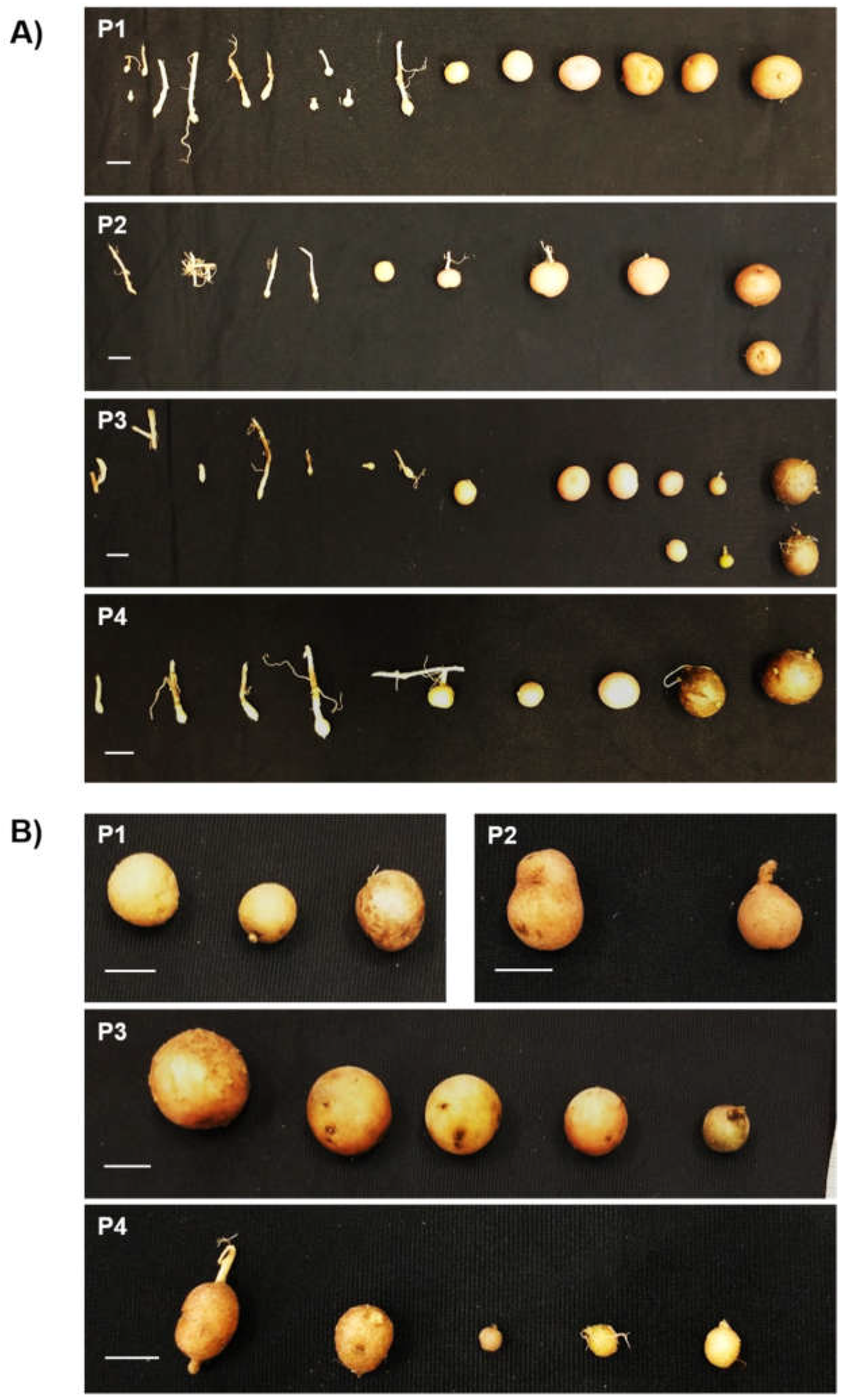
2.2.1. StTCTP induces an increase in stolon diameter and early stages of tuberization
2.2.2. StTCTP promotes increase in shoot diameter
2.3. Molecular docking simulation indicates interaction between StTCTP and PTB1/6
3. Discussion
4. Materials and Methods
4.1. Plant material
4.2. RNA extraction and vector construction
4.3. Potato transient transformation
4.4. Endpoint RT-PCR
4.5. Quantitative RT-PCR (RT-qPCR)
4.6. Shoot induction assay
4.7. Prediction of tertiary structures
4.7.1. Prediction of 3D structures of mRNA
4.7.2. Prediction of 3D structures of proteins
4.7.3. Protein-RNA interactions by molecular docking simulation
4.8. Statistical analysis
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Bommer:, U.-A.; Thiele, B.-J. The Translationally Controlled Tumour Protein (TCTP). The International Journal of Biochemistry & Cell Biology 2004, 36, 379–385. [Google Scholar] [CrossRef]
- Zhang, D.; Li, F.; Weidner, D.; Mnjoyan, Z.H.; Fujise, K. Physical and Functional Interaction between Myeloid Cell Leukemia 1 Protein (MCL1) and Fortilin. Journal of Biological Chemistry 2002, 277, 37430–37438. [Google Scholar] [CrossRef]
- Liu, H.; Peng, H.-W.; Cheng, Y.-S.; Yuan, H.S.; Yang-Yen, H.-F. Stabilization and Enhancement of the Antiapoptotic Activity of Mcl-1 by TCTP. Molecular and Cellular Biology 2005, 25, 3117–3126. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, F.; Xiong, Z.; Yan, Y.; Wang, X.; Nishino, M.; Mirkovic, D.; Nguyen, J.; Wang, H.; Yang, X.-F. An N-Terminal Region of Translationally Controlled Tumor Protein Is Required for Its Antiapoptotic Activity. Oncogene 2005, 24, 4778–4788. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Jarolim, S.; Heeren, G.; Palle, E.; Perju, S.; Klinger, H.; Bogengruber, E.; Madeo, F.; Braun, R.J.; Breitenbach-Koller, L.; et al. MMI1 (YKL056c, TMA19), the Yeast Orthologue of the Translationally Controlled Tumor Protein (TCTP) Has Apoptotic Functions and Interacts with Both Microtubules and Mitochondria. Biochimica et Biophysica Acta (BBA) - Bioenergetics 2006, 1757, 631–638. [Google Scholar] [CrossRef]
- Mak, C.H.; Poon, M.W.; Lun, H.M.; Kwok, P.Y.; Ko, R.C. Heat-Inducible Translationally Controlled Tumor Protein of Trichinella Pseudospiralis: Cloning and Regulation of Gene Expression. Parasitol Res 2007, 100, 1105–1111. [Google Scholar] [CrossRef]
- Betsch, L.; Boltz, V.; Brioudes, F.; Pontier, G.; Girard, V.; Savarin, J.; Wipperman, B.; Chambrier, P.; Tissot, N.; Benhamed, M.; et al. TCTP and CSN4 Control Cell Cycle Progression and Development by Regulating CULLIN1 Neddylation in Plants and Animals. PLoS Genet 2019, 15, e1007899. [Google Scholar] [CrossRef]
- Li, S.; Ge, F. Current Understanding of the TCTP Interactome. In TCTP/tpt1 - Remodeling Signaling from Stem Cell to Disease; Telerman, A., Amson, R., Eds.; Results and Problems in Cell Differentiation; Springer International Publishing: Cham, 2017; Vol. 64, pp. 127–136; ISBN 978-3-319-67590-9. [Google Scholar]
- Li, F.; Zhang, D.; Fujise, K. Characterization of Fortilin, a Novel Antiapoptotic Protein. Journal of Biological Chemistry 2001, 276, 47542–47549. [Google Scholar] [CrossRef]
- Acunzo, J.; Baylot, V.; So, A.; Rocchi, P. TCTP as Therapeutic Target in Cancers. Cancer Treatment Reviews 2014, 40, 760–769. [Google Scholar] [CrossRef]
- Thébault, S.; Agez, M.; Chi, X.; Stojko, J.; Cura, V.; Telerman, S.B.; Maillet, L.; Gautier, F.; Billas-Massobrio, I.; Birck, C.; et al. TCTP Contains a BH3-like Domain, Which Instead of Inhibiting, Activates Bcl-XL. Sci Rep 2016, 6, 19725. [Google Scholar] [CrossRef]
- Susini, L.; Besse, S.; Duflaut, D.; Lespagnol, A.; Beekman, C.; Fiucci, G.; Atkinson, A.R.; Busso, D.; Poussin, P.; Marine, J.-C.; et al. TCTP Protects from Apoptotic Cell Death by Antagonizing Bax Function. Cell Death Differ 2008, 15, 1211–1220. [Google Scholar] [CrossRef]
- Jung, J.; Kim, H.Y.; Maeng, J.; Kim, M.; Shin, D.H.; Lee, K. Interaction of Translationally Controlled Tumor Protein with Apaf-1 Is Involved in the Development of Chemoresistance in HeLa Cells. BMC Cancer 2014, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Rho, S.B.; Lee, J.H.; Park, M.S.; Byun, H.-J.; Kang, S.; Seo, S.-S.; Kim, J.-Y.; Park, S.-Y. Anti-Apoptotic Protein TCTP Controls the Stability of the Tumor Suppressor P53. FEBS Letters 2011, 585, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Amson, R.; Pece, S.; Lespagnol, A.; Vyas, R.; Mazzarol, G.; Tosoni, D.; Colaluca, I.; Viale, G.; Rodrigues-Ferreira, S.; Wynendaele, J.; et al. Reciprocal Repression between P53 and TCTP. Nat Med 2012, 18, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekar, M.; Dakshinamoorthy, G.; Ramaswamy, K. Translationally Controlled Tumor Protein Is a Novel Heat Shock Protein with Chaperone-like Activity. Biochemical and Biophysical Research Communications 2009, 386, 333–337. [Google Scholar] [CrossRef]
- Lucibello, M.; Gambacurta, A.; Zonfrillo, M.; Pierimarchi, P.; Serafino, A.; Rasi, G.; Rubartelli, A.; Garaci, E. TCTP Is a Critical Survival Factor That Protects Cancer Cells from Oxidative Stress-Induced Cell-Death. Experimental Cell Research 2011, 317, 2479–2489. [Google Scholar] [CrossRef]
- Graidist, P.; Yazawa, M.; Tonganunt, M.; Nakatomi, A.; Lin, C.C.-J.; Chang, J.-Y.; Phongdara, A.; Fujise, K. Fortilin Binds Ca2+ and Blocks Ca2+-Dependent Apoptosis in Vivo. Biochemical Journal 2007, 408, 181–191. [Google Scholar] [CrossRef]
- Gachet, Y.; Tournier, S.; Lee, M.; Lazaris-Karatzas, A.; Poulton, T.; Bommer, U.A. The Growth-Related, Translationally Controlled Protein P23 Has Properties of a Tubulin Binding Protein and Associates Transiently with Microtubules during the Cell Cycle. Journal of Cell Science 1999, 112, 1257–1271. [Google Scholar] [CrossRef]
- Tani, T.; Shimada, H.; Kato, Y.; Tsunoda, Y. Bovine Oocytes with the Potential to Reprogram Somatic Cell Nuclei Have a Unique 23-KDa Protein, Phosphorylated Transcriptionally Controlled Tumor Protein (TCTP). Cloning and Stem Cells 2007, 9, 267–280. [Google Scholar] [CrossRef]
- Jeon, H.-J.; You, S.Y.; Park, Y.S.; Chang, J.W.; Kim, J.-S.; Oh, J.S. TCTP Regulates Spindle Microtubule Dynamics by Stabilizing Polar Microtubules during Mouse Oocyte Meiosis. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2016, 1863, 630–637. [Google Scholar] [CrossRef]
- Chen, S.H.; Wu, P.-S.; Chou, C.-H.; Yan, Y.-T.; Liu, H.; Weng, S.-Y.; Yang-Yen, H.-F. A Knockout Mouse Approach Reveals That TCTP Functions as an Essential Factor for Cell Proliferation and Survival in a Tissue- or Cell Type–Specific Manner. MBoC 2007, 18, 2525–2532. [Google Scholar] [CrossRef] [PubMed]
- Koide, Y.; Kiyota, T.; Tonganunt, M.; Pinkaew, D.; Liu, Z.; Kato, Y.; Hutadilok-Towatana, N.; Phongdara, A.; Fujise, K. Embryonic Lethality of Fortilin-Null Mutant Mice by BMP-Pathway Overactivation. Biochimica et Biophysica Acta (BBA) - General Subjects 2009, 1790, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-C.; Chern, J.J.; Cai, Y.; Liu, M.; Choi, K.-W. Drosophila TCTP Is Essential for Growth and Proliferation through Regulation of DRheb GTPase. Nature 2007, 445, 785–788. [Google Scholar] [CrossRef]
- Hong, S.-T.; Choi, K.-W. TCTP Directly Regulates ATM Activity to Control Genome Stability and Organ Development in Drosophila Melanogaster. Nat Commun 2013, 4, 2986. [Google Scholar] [CrossRef] [PubMed]
- Brioudes, F.; Thierry, A.-M.; Chambrier, P.; Mollereau, B.; Bendahmane, M. Translationally Controlled Tumor Protein Is a Conserved Mitotic Growth Integrator in Animals and Plants. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 16384–16389. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; Blenis, J. Molecular Mechanisms of MTOR-Mediated Translational Control. Nat Rev Mol Cell Biol 2009, 10, 307–318. [Google Scholar] [CrossRef]
- Bommer, U.-A.; Iadevaia, V.; Chen, J.; Knoch, B.; Engel, M.; Proud, C.G. Growth-Factor Dependent Expression of the Translationally Controlled Tumour Protein TCTP Is Regulated through the PI3-K/Akt/MTORC1 Signalling Pathway. Cellular Signalling 2015, 27, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Han, Y.-J.; Hwang, O.-J.; Lee, S.-S.; Shin, A.-Y.; Kim, S.Y.; Kim, J.-I. Overexpression of Arabidopsis Translationally Controlled Tumor Protein Gene AtTCTP Enhances Drought Tolerance with Rapid ABA-Induced Stomatal Closure. Mol Cells 2012, 33, 617–626. [Google Scholar] [CrossRef]
- De Carvalho, M.; Acencio, M.L.; Laitz, A.V.N.; De Araújo, L.M.; De Lara Campos Arcuri, M.; Do Nascimento, L.C.; Maia, I.G. Impacts of the Overexpression of a Tomato Translationally Controlled Tumor Protein (TCTP) in Tobacco Revealed by Phenotypic and Transcriptomic Analysis. Plant Cell Rep 2017, 36, 887–900. [Google Scholar] [CrossRef]
- Berkowitz, O.; Jost, R.; Pollmann, S.; Masle, J. Characterization of TCTP, the Translationally Controlled Tumor Protein, from Arabidopsis Thaliana. The Plant Cell 2009, 20, 3430–3447. [Google Scholar] [CrossRef]
- Liao, F.; Wang, L.; Yang, L.-B.; Peng, X.; Sun, M. NtGNL1 Plays an Essential Role in Pollen Tube Tip Growth and Orientation Likely via Regulation of Post-Golgi Trafficking. PLoS ONE 2010, 5, e13401. [Google Scholar] [CrossRef]
- Zou, X.; Li, L.; Liao, F.; Chen, W. ITRAQ-Based Quantitative Proteomic Analysis Reveals NtGNL1-Dependent Regulatory Network Underlying Endosome Trafficking for Pollen Tube Polar Growth. Plant Physiology and Biochemistry 2021, 161, 200–209. [Google Scholar] [CrossRef]
- Corbesier, L.; Vincent, C.; Jang, S.; Fornara, F.; Fan, Q.; Searle, I.; Giakountis, A.; Farrona, S.; Gissot, L.; Turnbull, C.; et al. FT Protein Movement Contributes to Long-Distance Signaling in Floral Induction of Arabidopsis. Science 2007, 316, 1030–1033. [Google Scholar] [CrossRef]
- Pant, B.D.; Buhtz, A.; Kehr, J.; Scheible, W.-R. MicroRNA399 Is a Long-Distance Signal for the Regulation of Plant Phosphate Homeostasis. Plant J 2008, 53, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, Y.; Ham, B.-K.; Chen, J.; Yoshida, A.; Kochian, L.V.; Fei, Z.; Lucas, W.J. Vascular-Mediated Signalling Involved in Early Phosphate Stress Response in Plants. Nature Plants 2016, 2, 16033. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.-C.; Kragler, F.; Varkonyi-Gasic, E.; Haywood, V.; Archer-Evans, S.; Lee, Y.M.; Lough, T.J.; Lucas, W.J. A Systemic Small RNA Signaling System in Plants. Plant Cell 2004, 16, 1979–2000. [Google Scholar] [CrossRef] [PubMed]
- Champigny, M.J.; Isaacs, M.; Carella, P.; Faubert, J.; Fobert, P.R.; Cameron, R.K. Long Distance Movement of DIR1 and Investigation of the Role of DIR1-like during Systemic Acquired Resistance in Arabidopsis. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef]
- Martin, A.; Adam, H.; Díaz-Mendoza, M.; Żurczak, M.; González-Schain, N.D.; Suárez-López, P. Graft-Transmissible Induction of Potato Tuberization by the MicroRNA MiR172. Development 2009, 136, 2873–2881. [Google Scholar] [CrossRef]
- Navarro, C.; Abelenda, J.A.; Cruz-Oró, E.; Cuéllar, C.A.; Tamaki, S.; Silva, J.; Shimamoto, K.; Prat, S. Control of Flowering and Storage Organ Formation in Potato by FLOWERING LOCUS T. Nature 2011, 478, 119–122. [Google Scholar] [CrossRef]
- Peres, L.E.P.; Carvalho, R.F.; Zsögön, A.; Bermúdez-Zambrano, O.D.; Robles, W.G.R.; Tavares, S. Grafting of Tomato Mutants onto Potato Rootstocks: An Approach to Study Leaf-Derived Signaling on Tuberization. Plant Science 2005, 169, 680–688. [Google Scholar] [CrossRef]
- Rosin, F.M.; Hart, J.K.; Horner, H.T.; Davies, P.J.; Hannapel, D.J. Overexpression of a Knotted -Like Homeobox Gene of Potato Alters Vegetative Development by Decreasing Gibberellin Accumulation. Plant Physiology 2003, 132, 106–117. [Google Scholar] [CrossRef]
- Yu, Y.; Lashbrook, C.C.; Hannapel, D.J. Tissue Integrity and RNA Quality of Laser Microdissected Phloem of Potato. Planta 2007, 226, 797–803. [Google Scholar] [CrossRef]
- Campbell, B.A.; Hallengren, J.; Hannapel, D.J. Accumulation of BEL1-like Transcripts in Solanaceous Species. Planta 2008, 228, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Bhogale, S.; Kang, I.H.; Hannapel, D.J.; Banerjee, A.K. The MRNA of a Knotted1-like Transcription Factor of Potato Is Phloem Mobile. Plant Mol Biol 2012, 79, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.K.; Chatterjee, M.; Yu, Y.; Suh, S.-G.; Miller, W.A.; Hannapel, D.J. Dynamics of a Mobile RNA of Potato Involved in a Long-Distance Signaling Pathway. The Plant Cell 2007, 18, 3443–3457. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Moya, J.J.; Xoconostle-Cázares, B.; Toscano-Morales, R.; Ramírez-Ortega, F.; Luis Cabrera-Ponce, J.; Ruiz-Medrano, R. Characterization of the Pumpkin Translationally-Controlled Tumor Protein CmTCTP. Plant Signaling & Behavior 2013, 8, e26477. [Google Scholar] [CrossRef]
- Toscano-Morales, R.; Xoconostle-Cázares, B.; Martínez-Navarro, A.C.; Ruiz-Medrano, R. Long Distance Movement of an Arabidopsis Translationally Controlled Tumor Protein (AtTCTP2) MRNA and Protein in Tobacco. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Toscano-Morales, R.; Xoconostle-Cázares, B.; Cabrera-Ponce, J.L.; Hinojosa-Moya, J.; Ruiz-Salas, J.L.; Galván-Gordillo, S.V.; Guevara-González, R.G.; Ruiz-Medrano, R. AtTCTP2, an Arabidopsis Thaliana Homolog of Translationally Controlled Tumor Protein, Enhances in Vitro Plant Regeneration. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Thieme, C.J.; Rojas-Triana, M.; Stecyk, E.; Schudoma, C.; Zhang, W.; Yang, L.; Miñambres, M.; Walther, D.; Schulze, W.X.; Paz-Ares, J.; et al. Endogenous Arabidopsis Messenger RNAs Transported to Distant Tissues. Nature Plants 2015, 1, 15025. [Google Scholar] [CrossRef]
- Doering-Saad, C.; Newbury, H.; Couldridge, C.; Bale, J.; Pritchard, J. A Phloem-Enriched CDNA Library from Ricinus: Insights into Phloem Function. Journal of Experimental Botany 2006, 57, 3183–3193. [Google Scholar] [CrossRef]
- Giavalisco, P.; Kapitza, K.; Kolasa, A.; Buhtz, A.; Kehr, J. Towards the Proteome OfBrassica Napus Phloem Sap. Proteomics 2006, 6, 896–909. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Medina, C.; Atkins, C.A.; Mann, A.J.; Jordan, M.E.; Smith, P.M. Macromolecular Composition of Phloem Exudate from White Lupin (Lupinus AlbusL.). BMC Plant Biol 2011, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Toscano-Morales, R.; Xoconostle-Cázares, B.; Martínez-Navarro, A.C.; Ruiz-Medrano, R. AtTCTP2 MRNA and Protein Movement Correlates with Formation of Adventitious Roots in Tobacco. Plant Signaling & Behavior 2016, 11, e1071003. [Google Scholar] [CrossRef]
- Gutiérrez-Galeano, D.F.; Toscano-Morales, R.; Calderón-Pérez, B.; Xoconostle-Cázares, B.; Ruiz-Medrano, R. Structural Divergence of Plant TCTPs. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Weeda, S.M.; Mohan Kumar, G.N.; Richard Knowles, N. Developmentally Linked Changes in Proteases and Protease Inhibitors Suggest a Role for Potato Multicystatin in Regulating Protein Content of Potato Tubers. Planta 2009, 230, 73–84. [Google Scholar] [CrossRef]
- Cho, S.K.; Sharma, P.; Butler, N.M.; Kang, I.-H.; Shah, S.; Rao, A.G.; Hannapel, D.J. Polypyrimidine Tract-Binding Proteins of Potato Mediate Tuberization through an Interaction with StBEL5 RNA. EXBOTJ 2015, 66, 6835–6847. [Google Scholar] [CrossRef]
- Kondhare, K.R.; Kumar, A.; Hannapel, D.J.; Banerjee, A.K. Conservation of Polypyrimidine Tract Binding Proteins and Their Putative Target RNAs in Several Storage Root Crops. BMC Genomics 2018, 19, 124. [Google Scholar] [CrossRef]
- Reuveni, M. Sex and Regeneration. Biology 2021, 10, 937. [Google Scholar] [CrossRef]
- Yang, L.; Perrera, V.; Saplaoura, E.; Apelt, F.; Bahin, M.; Kramdi, A.; Olas, J.; Mueller-Roeber, B.; Sokolowska, E.; Zhang, W.; et al. M5C Methylation Guides Systemic Transport of Messenger RNA over Graft Junctions in Plants. Current Biology 2019, 29, 2465–2476. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Abiotic Stress-induced Anthocyanins in Plants: Their Role in Tolerance to Abiotic Stresses. Physiologia Plantarum 2021, 172, 1711–1723. [Google Scholar] [CrossRef]
- Kumlay, A.M.; Ercisli, S. Callus Induction, Shoot Proliferation and Root Regeneration of Potato ( Solanum Tuberosum L.) Stem Node and Leaf Explants under Long-Day Conditions. Biotechnology & Biotechnological Equipment 2015, 29, 1075–1084. [Google Scholar] [CrossRef]
- Logemann, J.; Schell, J.; Willmitzer, L. Improved Method for the Isolation of RNA from Plant Tissues. Analytical Biochemistry 1987, 163, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Muñoz, L.; Vargas-Hernández, B.; Hinojosa-Moya, J.; Ruiz-Medrano, R.; Xoconostle-Cázares, B. Plant Drought Tolerance Provided through Genome Editing of the Trehalase Gene. Plant Signaling & Behavior 2021, 16, 1877005. [Google Scholar] [CrossRef]
- Mba’u, Y.J.; Iriawati; Faizal, A. Transient Transformation of Potato Plant (Solanum Tuberosum L.) Granola Cultivar Using Syringe Agroinfiltration. Agrivita.J.Agr.Sci 2018, 40. [Google Scholar] [CrossRef]
- Ruiz-Salas, J.L.; Ruiz-Medrano, R.; Montes-Horcasitas, M.D.C.; Agreda-Laguna, K.A.; Hinojosa-Moya, J.; Xoconostle-Cázares, B. Vascular Expression of Trehalose Phosphate Synthase1 (TPS1) Induces Flowering in Arabidopsis. Plant Omics 2016, 9, 344–351. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hofacker, I.L. Vienna RNA Secondary Structure Server. Nucleic Acids Research 2003, 31, 3429–3431. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.; Xiao, Y. 3dDNA: A Computational Method of Building DNA 3D Structures. Molecules 2022, 27, 5936. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Xiao, Y. 3dRNA: 3D Structure Prediction from Linear to Circular RNAs. Journal of Molecular Biology 2022, 434, 167452. [Google Scholar] [CrossRef]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and Better Reference Data for Improved All-Atom Structure Validation: PROTEIN SCIENCE.ORG. Protein Science 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Wadley, L.M.; Keating, K.S.; Duarte, C.M.; Pyle, A.M. Evaluating and Learning from RNA Pseudotorsional Space: Quantitative Validation of a Reduced Representation for RNA Structure. Journal of Molecular Biology 2007, 372, 942–957. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Hassabis, D. Protein Structure Predictions to Atomic Accuracy with AlphaFold. Nat Methods 2022, 19, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Shuvo, M.H.; Gulfam, M.; Bhattacharya, D. DeepRefiner: High-Accuracy Protein Structure Refinement by Deep Network Calibration. Nucleic Acids Research 2021, 49, W147–W152. [Google Scholar] [CrossRef]
- Colovos, C.; Yeates, T.O. Verification of Protein Structures: Patterns of Nonbonded Atomic Interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A Program to Check the Stereochemical Quality of Protein Structures. J Appl Crystallogr 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Lüthy, R.; Bowie, J.U.; Eisenberg, D. Assessment of Protein Models with Three-Dimensional Profiles. Nature 1992, 356, 83–85. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, D.; Zhou, P.; Li, B.; Huang, S.-Y. HDOCK: A Web Server for Protein–Protein and Protein–DNA/RNA Docking Based on a Hybrid Strategy. Nucleic Acids Research 2017, 45, W365–W373. [Google Scholar] [CrossRef]
- Yan, Y.; Tao, H.; He, J.; Huang, S.-Y. The HDOCK Server for Integrated Protein–Protein Docking. Nat Protoc 2020, 15, 1829–1852. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera?A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Science 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




