Submitted:
15 June 2023
Posted:
16 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
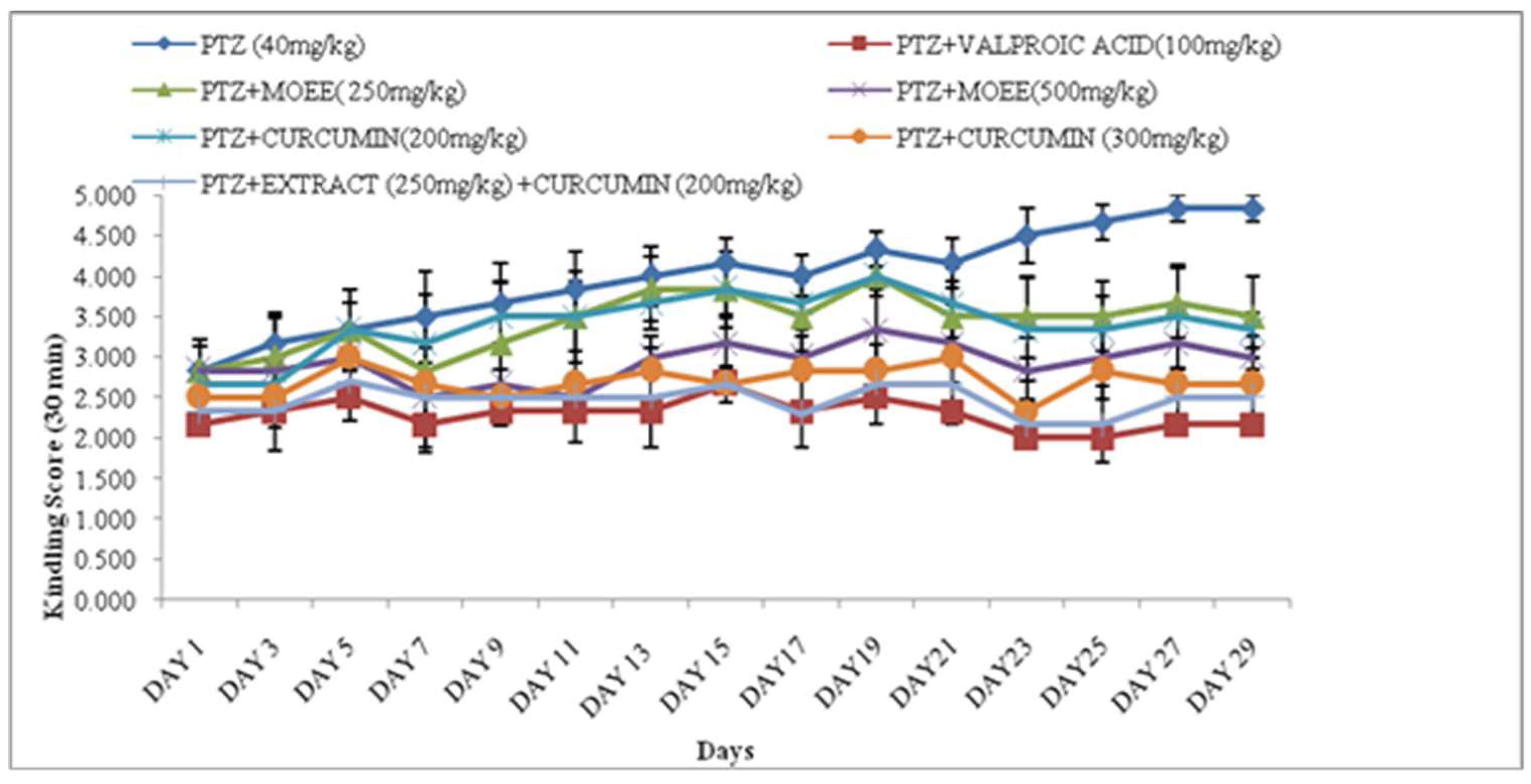
2.1. Effect of M. oleifera & curcumin on the seizure severity score in Pentyletetrazole treated rats
2.2. Neurobehavioral Observations
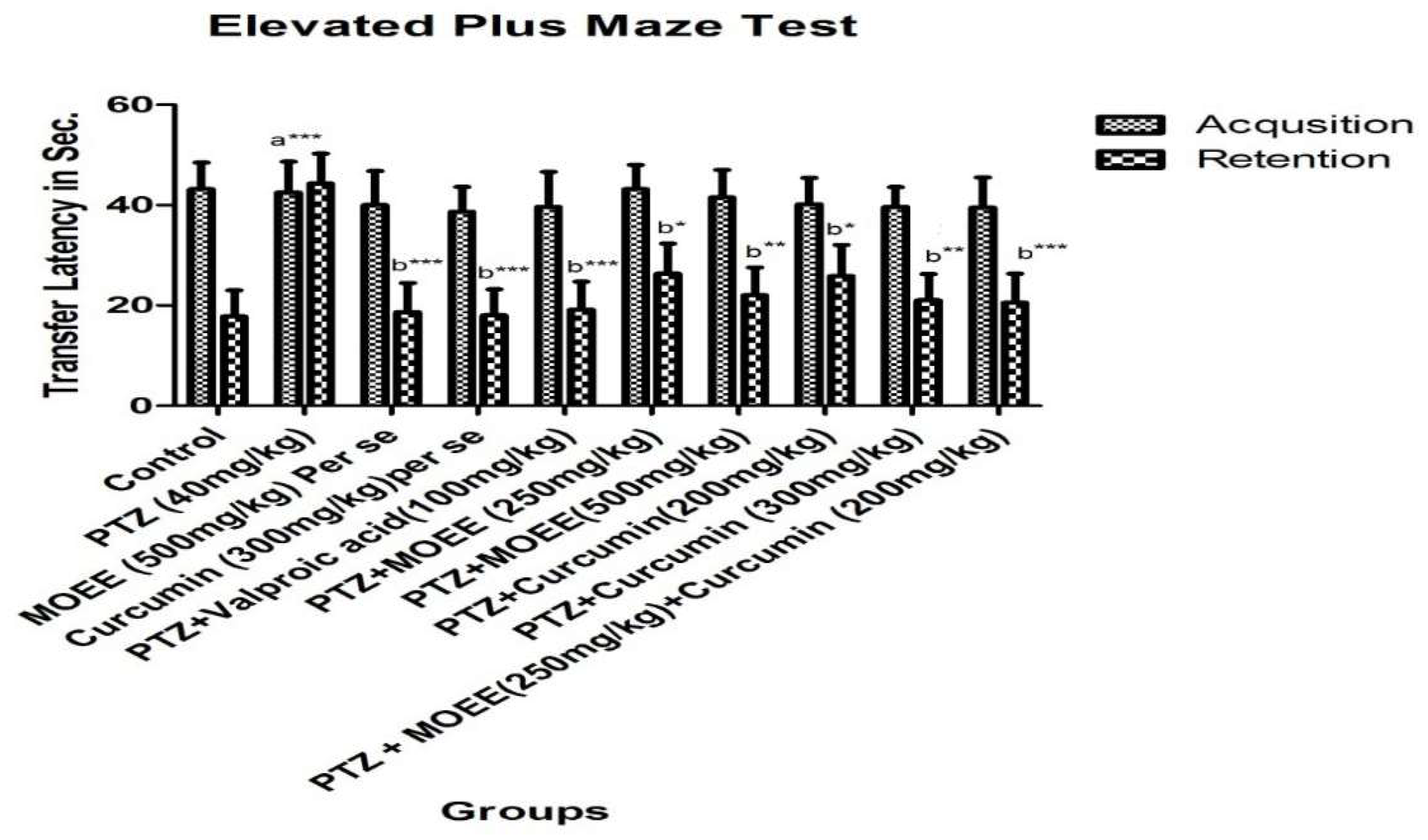
2.2.1. Effects of MOEE & curcumin on elevated plus maze test
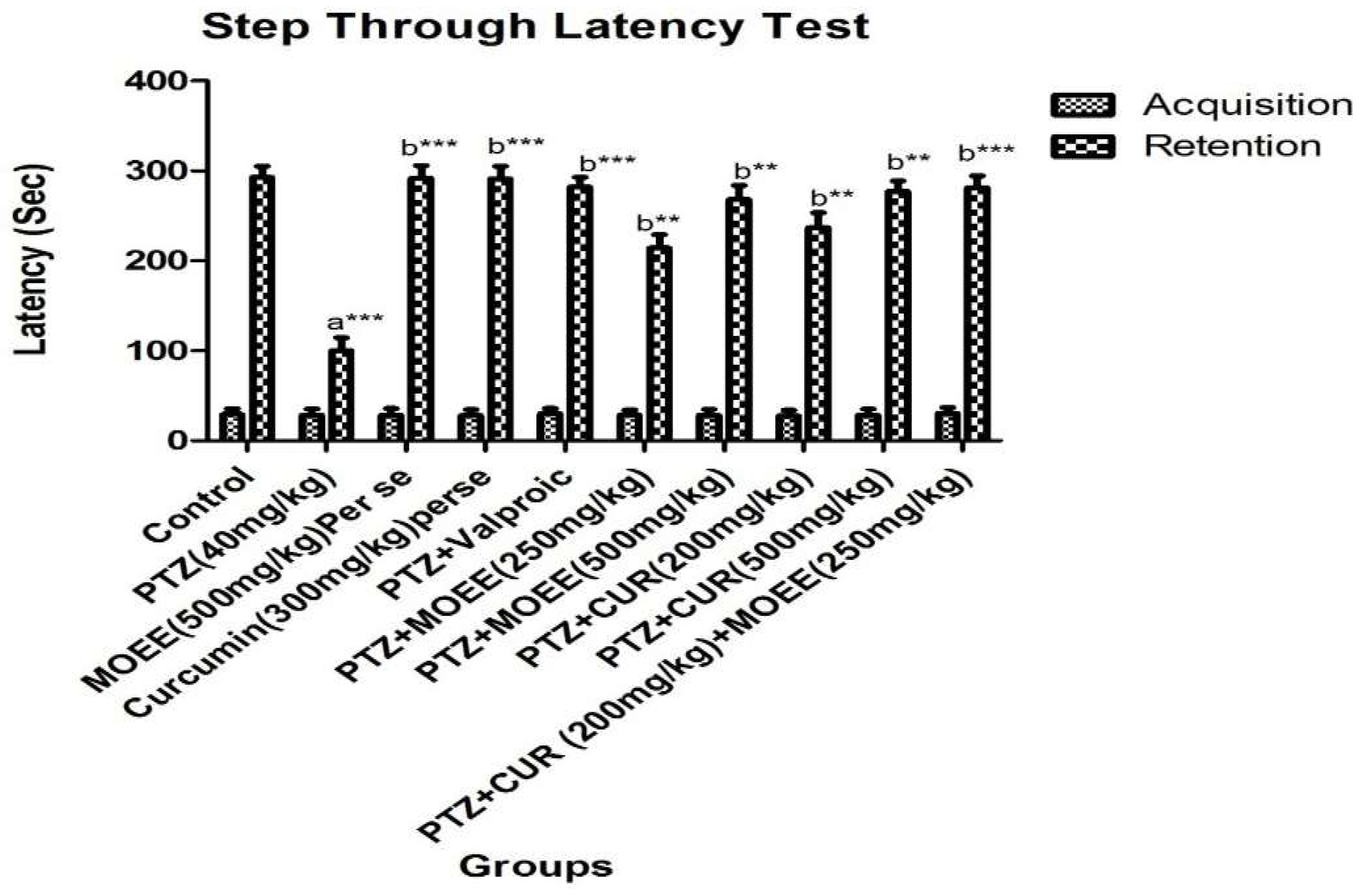
2.2.2. Effects of MOEE and curcumin on step through latency test
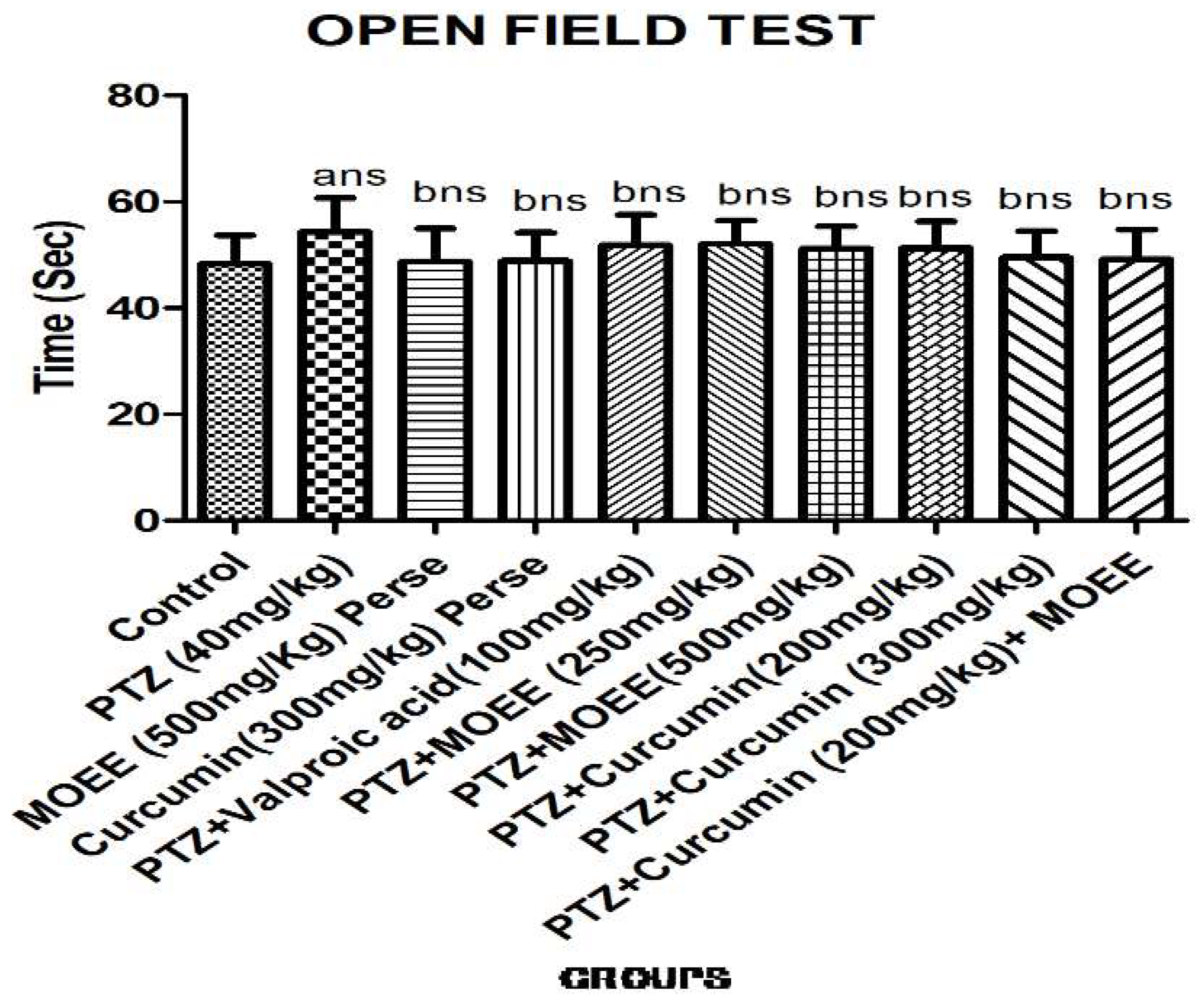
2.2.3. Effect of MOEE & curcumin in open field apparatus test
2.3. In-vivo antioxidant activity
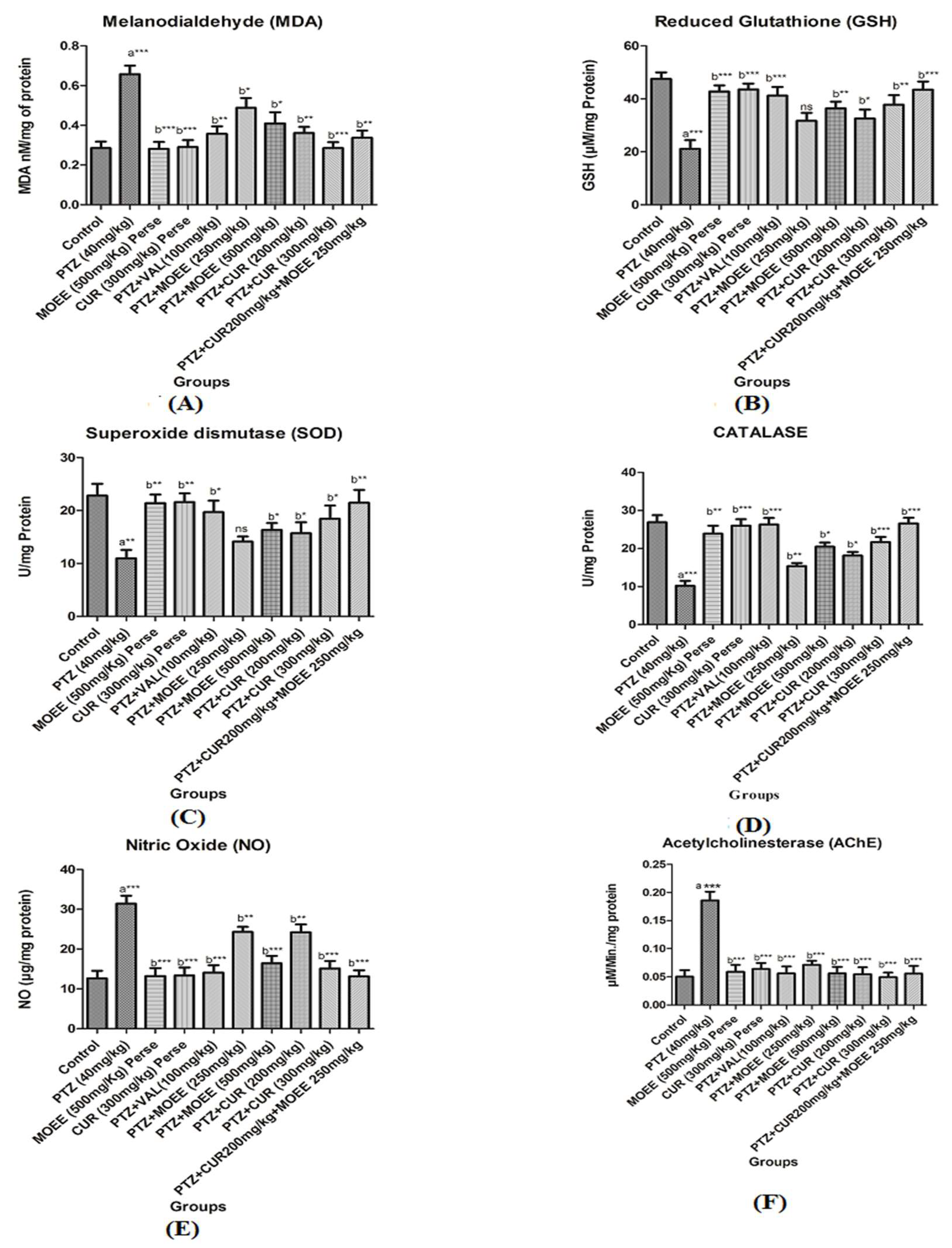
2.3.1. Effects of MOEE & curcumin on brain MDA level
2.3.2. Effects of MOEE & curcumin on brain of glutathione level
2.3.3. Effect of MOEE & curcumin on brain of SOD levels
2.3.4. Effect of MOEE & curcumin on brain catalase levels
2.3.5. Effects of MOEE & curcumin on brain of NO levels
2.3.6. Effects of MOEE & curcumin in brain of AChE levels
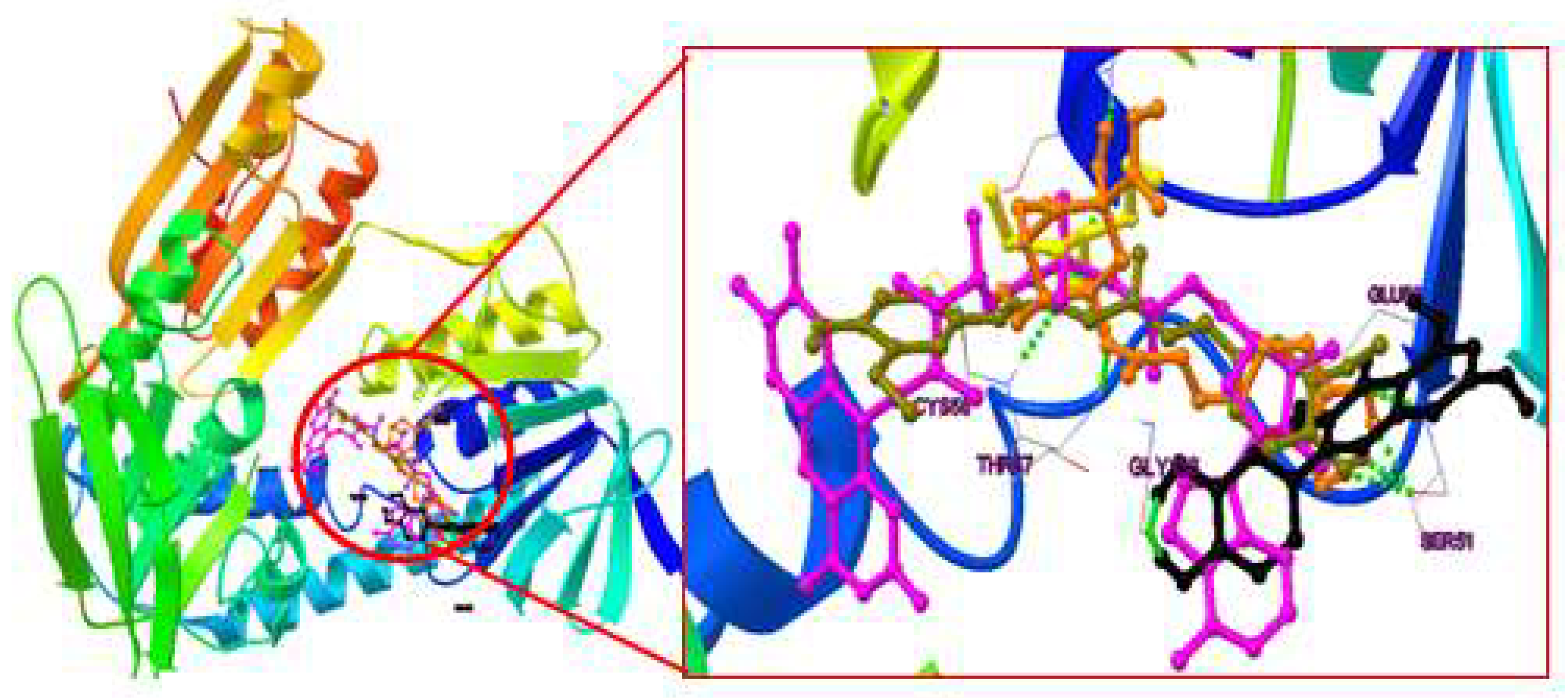
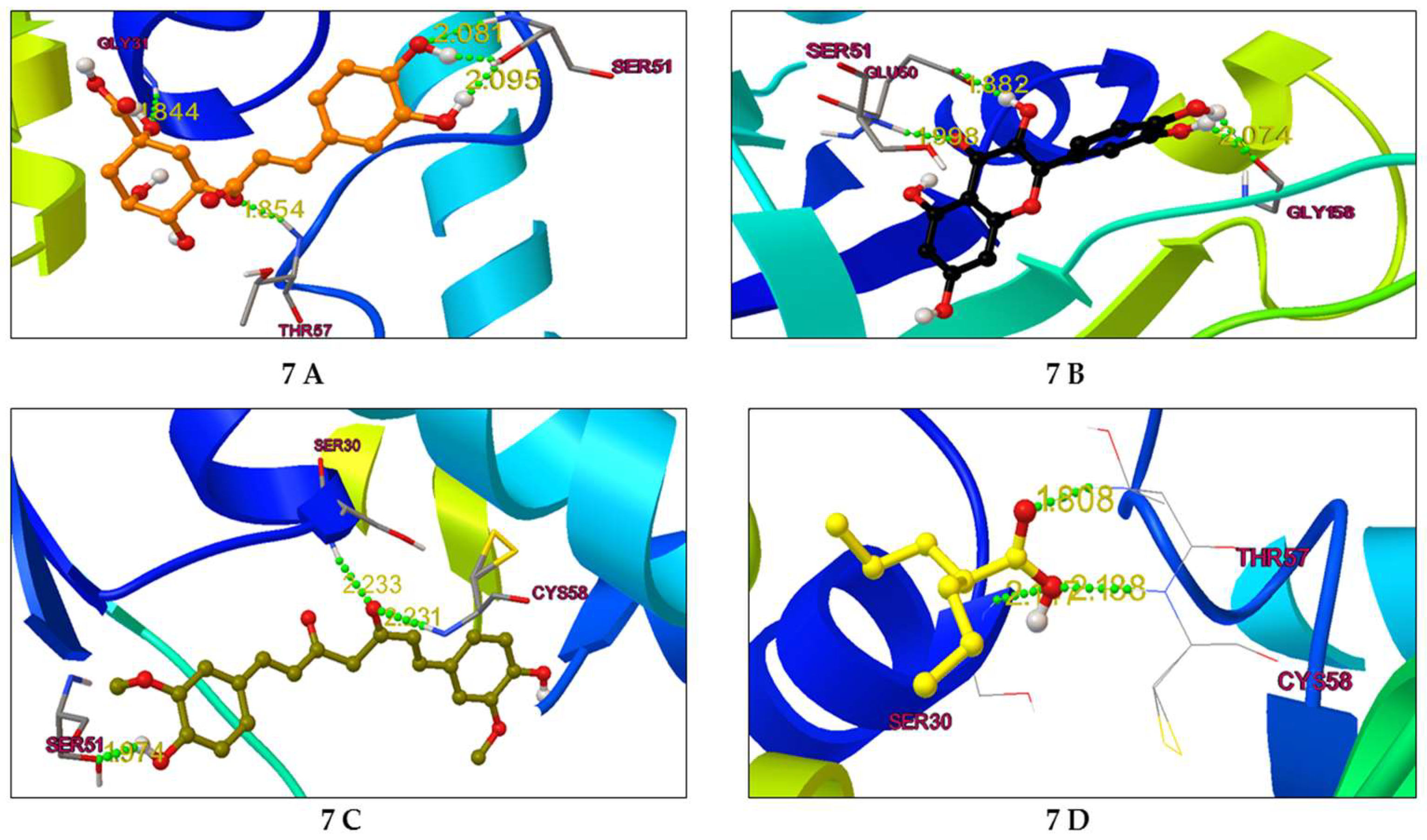
2.4. Binding mode analysis of curcumin, quercitin, chlorogenic acid & valproic acid
2.4.1. Post-Docking Analysis
2.4.2. Binding mode analysis
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Preparation of M. oleifera leaves extract
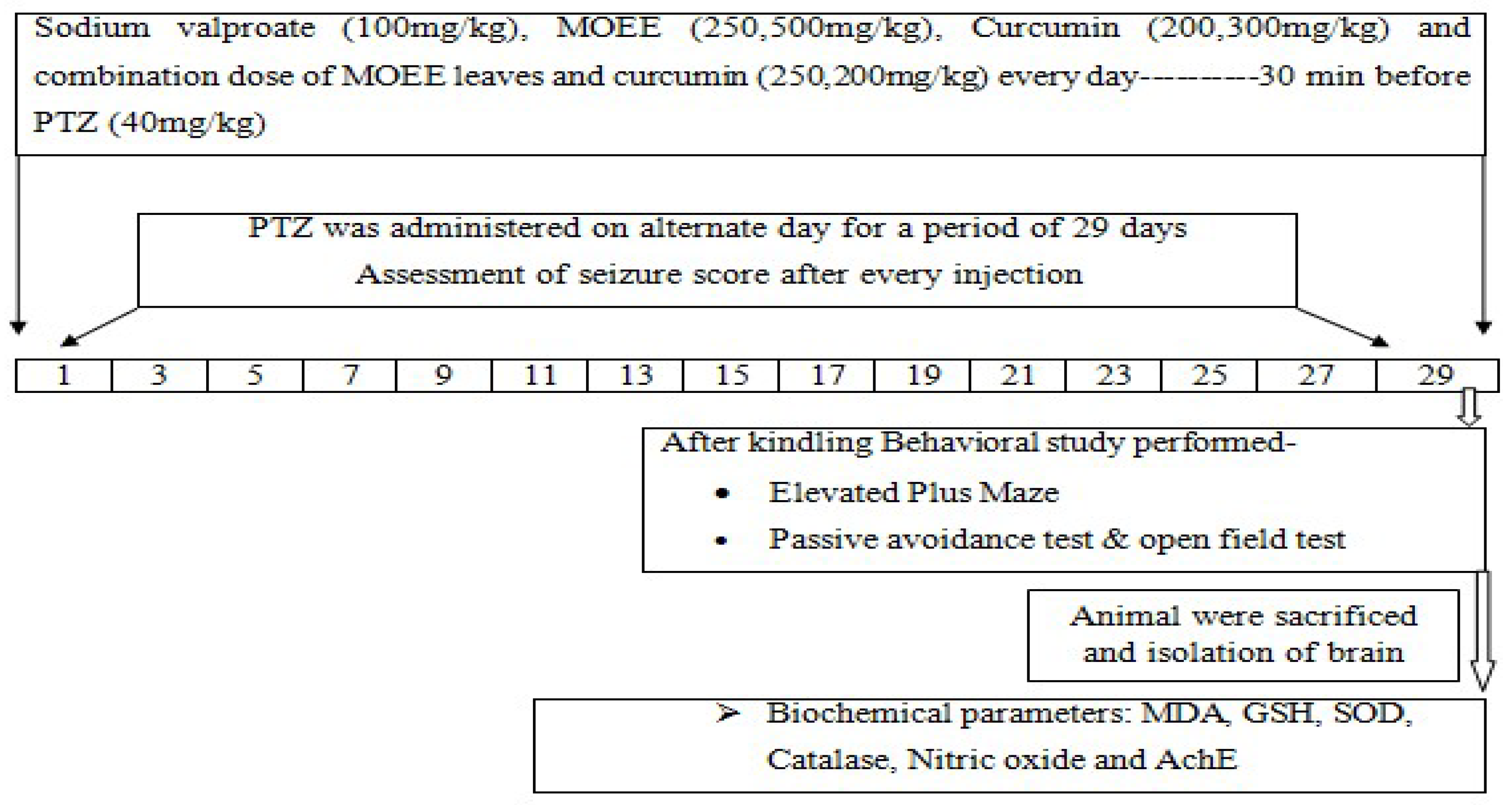
4.3. Inducing of kindled seizures & experiment design
4.3.1. Neurobehavioral Assessment
4.3.2. Elevated plus maze test
4.3.3. Step-through passive avoidance Test
4.3.4. Open field test (OFT)
4.4. Measurement of oxidative stress
4.4.1. Lipid peroxidation (MDA)
4.4.2. Reduced glutathione estimation
4.4.3. Superoxide dismutase estimation
4.4.4. Catalase estimation
4.4.5. Nitrite Estimation
4.4.6. Acetyl cholinesterase activity
4.5. In Silco docking analysis of curcumin, quercitin & chlorogenic acid in compare with valproic acid as anti-epileptic drug
4.6. Statistical analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Declaration of competing interest
References
- Huang, W.; Manglik, A.; Venkatakrishnan, A.J.; Laeremans, T.; Feinberg, E.N.; Sanborn, A.L.; Kato, H.E.; Livingston, K.E.; Thorsen, T.S.; Kling, R.C.; Granier, S.; Gmeiner, P.; Husbands, S.M.; Traynor, J.R.; Weis, W.I.; Steyaert, J.; Dror, R.O.; Kobilka, B.K. Structural insights into µ-opioid receptor activation. Nat. 2015, 524, 315–321. [Google Scholar] [CrossRef]
- Aboutabl, M.E. Antiepileptic drugs: progress and development. Egypt. Pharma J. 2018, 17, 129. [Google Scholar]
- Abraham, S.; Shaju, M. Innovations in epilepsy management–an overview. J Pharm & Pharma Sci. 2013, 16, 564–576. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods in enzymology 1984, 105, 121–126. [Google Scholar] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. The Int. J. Bio. & cell boil. 2009, 41, 40–59. [Google Scholar]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, C.C.T.; Almeida, A.B.; Araújo, P.V.P.; Abreu, R.N.D.C.D.; Chaves, E.M.C.; Vale, O.C.D.; Macêdo, D.S.; Woods, D.J.; Fonteles, M.M.D.F.; Vasconcelos, S.M.M. Oxidative stress and epilepsy: literature review. Oxi. Med. Cell. Long. 2012. [Google Scholar] [CrossRef]
- Aju, B.Y.; Rajalakshmi, R.; Mini, S. Protective role of M. oleifera leaf extract on cardiac antioxidant status and lipid peroxidation in streptozotocin induced diabetic rat. Heliyon. 2019, 5, e02935. [Google Scholar] [CrossRef]
- Alachkar, A.; Ojha, S.K.; Sadeq, A.; Adem, A.; Frank, A.; Stark, H.; Sadek, B. Experimental models for the discovery of novel anticonvulsant drugs: focus on pentylenetetrazole-induced seizures and associated memory deficits. Current Pharmaceu. Des. 2020, 26, 1693–1711. [Google Scholar] [CrossRef]
- Alhakmani, F.; Kumar, S.; Khan, S.A. Estimation of total phenolic content, in–vitro antioxidant and anti–inflammatory activity of flowers of Moringa oleifera. Asian Pac. J. Trop. Biomed. 2013, 3, 623–627. [Google Scholar] [CrossRef]
- Angelova, P.R. Sources and triggers of oxidative damage in neurodegeneration. Free Rad. Biol. Med. 2021, 173, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Awodele, O.; Oreagba, I.A.; Odoma, S.; da Silva, J.A.T.; Osunkalu, V.O. Toxicological evaluation of the aqueous leaf extract of M. oleifera Lam (Moringaceae). J. Ethnopharmacol. 2012, 139, 330–336. [Google Scholar] [CrossRef]
- Bakre, A.G.; Aderibigbe, A.O.; Ademowo, O.G. Studies on neuropharmacological profile of ethanol extract of M. oleiferaleaves in mice. J. Ethnopharmacol. 2013, 149, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, B.B.; Yilmaz, S.; Hacihasanoglu Cakmak, N.; Yanardag, R. The effects of edaravone, a free-radical scavenger in lung injury induced by valproic acid demonstrated via different biochemical parameters. J. Biochem. Mole. Toxicol. 2021, 35, e22847. [Google Scholar] [CrossRef]
- Berkholz, D.S.; Faber, H.R.; Savvides, S.N.; Karplus, P.A. Catalytic cycle of human glutathione reductase near 1 Å resolution. J. Mol. Biol. 2008, 382, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Kumar, A. Neuroprotective effect of lycopene against PTZ-induced kindling seizures in mice: possible behavioural, biochemical and mitochondrial dysfunction. Phytoth. Res. 2016, 30, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Bruce, A.J.; Baudry, M. Oxygen free radicals in rat limbic structures after kainite-induced seizures. Free Rad. Biol.Med. 1995, 18, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Chanioti, S.; Katsouli, M.; Tzia, C. Novel processes for the extraction of phenolic compounds from olive pomace and their protection by encapsulation. Mol. 2021, 26, 1781. [Google Scholar] [CrossRef] [PubMed]
- Corda, M.G.; Giorgi, O.; Longoni, B.; Orlandi, M.; Biggio, G. Decrease in the function of the γ-aminobutyric acid-coupled chloride channel produced by the repeated administration of pentylenetetrazol to rats. J. Neurochem. 1990, 55, 1216–1221. [Google Scholar] [CrossRef]
- Davoudi, M.; Shojaei, A.; Palizvan, M.R.; Javan, M.; Mirnajafi-Zadeh, J. Comparison between standard protocol and a novel window protocol for induction of pentylenetetrazol kindled seizures in the rat. Epilepsy Res. 2013, 106, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Eid, T.; Williamson, A.; Lee, T.S.W.; Petroff, O.A.; De Lanerolle, N.C. Glutamate and astrocytes—key players in human mesial temporal lobe epilepsy? Epilepsia. 2008, 49, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochemis and Biophy. 82(1),70-77. Fantoukh, O.I.; Albadry, M.A.; Parveen, A.; Hawwal, M.F.; Majrashi, T.; Ali, Z.; Khan, S.I.; Chittiboyina, A.G.; Khan, I.A.; 2019. Isolation, synthesis, and drug interaction potential of secondary metabolites derived from the leaves of miracle tree (Moringa oleifera) against CYP3A4 and CYP2D6 isozyme. Phytomed 1959, 60, 153010.
- Farrell, J.S.; Wolff, M.D.; Teskey, G.C. Neurodegeneration and pathology in epilepsy: clinical and basic perspectives. Neurodegen. Diseases: Pathol, Mechn, and Potent. Thera. Targ. 2017, 317–334. [Google Scholar]
- Fisher, R.S.; Boas, W.V.E.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J., Jr. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005, 46, 470–472. [Google Scholar] [CrossRef]
- Geronzi, U.; Lotti, F.; Grosso, S. Oxidative stress in epilepsy. Exp. Rev. Neurothera. 2018, 18, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, S.; Subedi, L.; Acharya, N.; Gaire, B.P. Moringa oleifera: A tree of life as a promising medicinal plant for neurodegenerative diseases. J. Agricul. Food Chem. 2021, 69, 14358–14371. [Google Scholar] [CrossRef] [PubMed]
- Gorun, V.; Proinov, I.; Băltescu, V.; Balaban, G.; Bârzu, O. Modified Ellman procedure for assay of cholinesterases in crude enzymatic preparations. Anal. Biochem. 1978, 86, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Güller, P.; Karaman, M.; Güller, U.; Aksoy, M.; Küfrevioğlu, Ö.İ. A study on the effects of inhibition mechanism of curcumin, quercetin, and resveratrol on human glutathione reductase through in vitro and in silico approaches. J. Biomol. Struc. Dyn. 2021, 39, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminforma. 2012, 4, 1–17. [Google Scholar] [CrossRef]
- Holmes, A.; Rodgers, R.J. Prior exposure to the elevated plus-maze sensitizes mice to the acute behavioral effects of fluoxetine and phenelzine. European J. Pharmacol. 2003, 459, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shan, Y.; Du, Q.; Ding, Y.; Shen, C.; Wang, S.; Ding, M.; Xu, Y. Gender and socioeconomic disparities in global burden of epilepsy: an analysis of time trends from 1990 to 2017. Frontiers in Neurol. 2021, 12, 643450. [Google Scholar] [CrossRef] [PubMed]
- Huey, R.; Morris, G.M.; Olson, A.J.; Goodsell, D.S. A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Young, K.N.; Moniruzzaman, M.; Beyene, A.M.; Do, K.; Kalaiselvi, S.; Min, T. Curcumin and its modified formulations on inflammatory bowel disease (IBD): The story so far and future outlook. Pharmaceut. 2021, 13, 484. [Google Scholar] [CrossRef]
- Kono, Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophy. 1978, 186, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Khanum, F. Neuroprotective potential of phytochemicals. Pharmacg. Rev. 2012, 6, 81. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, A.; Kumar, B. Ethnobotanical Investigation of Medicinal Plants used by Rural Communities of District Chatra, Jharkhand, India. IOSR J. Biotech. Biochem. 2019, 5, 34–49. [Google Scholar]
- Landmark, C.J.; Johannessen, S.I. Pharmacological management of epilepsy: recent advances and future prospects. Drugs. 2008, 68, 1925–1939. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacg. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Löscher, W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilep Res. 2002, 50, 105–123. [Google Scholar] [CrossRef]
- Löscher, W.; Klitgaard, H.; Twyman, R.E.; Schmidt, D. New avenues for anti-epileptic drug discovery and development. Nat. Rev. Drug. Disc. 2013, 12, 757–776. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Ehmann, W.D.; Butler, S.M.; Markesbery, W.R. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer's disease. Neurol. 1995, 45, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Freitas, M.; Xavier, J.A.; Moura, F.A.; Goulart, M.O.; Ribeiro, D.; Fernandes, E. The scavenging effect of curcumin, piperine and their combination against physiological relevant reactive pro-oxidant species using in vitro non-cellular and cellular models. Chem. Pap. 2021, 75, 5269–5277. [Google Scholar] [CrossRef]
- Mathern, G.W.; Bertram, E.H., III. Recurrent limbic seizures do not cause hippocampal neuronal loss: a prolonged laboratory study. Neurobiol. Disea. 2021, 148, 105183. [Google Scholar] [CrossRef] [PubMed]
- Mehla, J.; Reeta, K.H.; Gupta, P.; Gupta, Y.K. Protective effect of curcumin against seizures and cognitive impairment in a pentylenetetrazole-kindled epileptic rat model. Life Sci. 2010, 87, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.R.; Dasgupta, C.; Ullal, G.R. A neural network model for kindling of focal epilepsy: basic mechanism. Biol. Cybern. 1993, 68, 335–340. [Google Scholar] [CrossRef]
- Moavero, R.; Santarone, M.E.; Galasso, C.; Curatolo, P. Cognitive and behavioral effects of new antiepileptic drugs in pediatric epilepsy. Brain and Devel. 2017, 39, 464–469. [Google Scholar] [CrossRef]
- Morimoto, K.; Fahnestock, M.; Racine, R.J. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog. Neurobiol. 2004, 73, 1–60. [Google Scholar] [CrossRef]
- Mousa, A.A.; El-Gansh, H.A.I.; Eldaim, M.A.A.; Mohamed, M.A.E.G.; Morsi, A.H.; El Sabagh, H.S. Protective effect of M. oleiferaleaves ethanolic extract against thioacetamide-induced hepatotoxicity in rats via modulation of cellular antioxidant, apoptotic and inflammatory markers. Environ. Sci. Poll. Res. 2019, 26, 32488–32504. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochemi. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Padayachee, B.; Baijnath, H. An updated comprehensive review of the medicinal, phytochemical and pharmacological properties of Moringa oleifera. South African J. Bot. 2020, 129, 304–316. [Google Scholar] [CrossRef]
- Park, K.M.; Kim, S.E.; Lee, B.I. Antiepileptic drug therapy in patients with drug-resistant epilepsy. J. Epilepsy Res. 2019, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Puttachary, S.; Sharma, S.; Stark, S.; Thippeswamy, T. Seizure-induced oxidative stress in temporal lobe epilepsy. BioMed. Res. Inter. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephal. Clin. Neurophy. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Rauramaa, T.; Pikkarainen, M.; Englund, E.; Ince, P.G.; Jellinger, K.; Paetau, A.; Alafuzoff, I. Consensus recommendations on pathologic changes in the hippocampus: a postmortem multicenter inter-rater study. J. Neuropathol & Exp. Neurol. 2013, 72, 452–461. [Google Scholar]
- Reeta, K.H.; Mehla, J.; Gupta, Y.K. Curcumin is protective against phenytoin-induced cognitive impairment and oxidative stress in rats. Brain Res. 2009, 1301, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.A.; Williams, D.I. Effects of repeated testing on rats' locomotor activity in the open-field. Animal Beh. 1973, 21, 109–111. [Google Scholar] [CrossRef]
- Sachett, A.; Gallas-Lopes, M.; Benvenutti, R.; Marcon, M.; Aguiar, G.P.S.; Herrmann, A.P.; Oliveira, J.V.; Siebel, A.M.; Piato, A. Curcumin micronization by supercritical fluid: In vitro and in vivo biological relevance. Ind. Crops Prod. 2022, 177, 114501. [Google Scholar] [CrossRef]
- Sandeep, I.S.; Das, S.; Nasim, N.; Mishra, A.; Acharya, L.; Joshi, R.K.; Nayak, S.; Mohanty, S. Differential expression of CURS gene during various growth stages, climatic condition and soil nutrients in turmeric (Curcuma longa): Towards site specific cultivation for high curcumin yield. Plant Physiol. Biochem. 2017, 118, 348–355. [Google Scholar] [CrossRef]
- Sarangi, S.C.; Joshi, D.; Kumar, R.; Kaleekal, T.; Gupta, Y.K. Pharmacokinetic and pharmacodynamic interaction of hydroalcoholic extract of Ocimum sanctum with valproate. Epil. & Behav. 2017, 75, 203–209. [Google Scholar]
- Schachter, S.C. Translating Nature to Nurture: Back to the Future for “New” Epilepsy Therapies: Epil. Curr. 2015, 15, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Taskiran, A.S.; Tastemur, Y. The role of nitric oxide in anticonvulsant effects of lycopene supplementation on pentylenetetrazole-induced epileptic seizures in rats. Exp. Brain Res. 2021, 239, 591–599. [Google Scholar] [CrossRef]
- Tham, C.L.; Liew, C.Y.; Lam, K.W.; Mohamad, A.S.; Kim, M.K.; Cheah, Y.K.; Zakaria, Z.A.; Sulaiman, M.R.; Lajis, N.H.; Israf, D.A. A synthetic curcuminoid derivative inhibits nitric oxide and proinflammatory cytokine synthesis. Euro. J. Pharma. 2010, 628, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ge, T.; Pan, Z.; Leng, Y.; Lv, J.; Li, B. The effects of herbal medicine on epilepsy. Oncotarget. 2017, 18, 48385–48397. [Google Scholar] [CrossRef]
- Pearl, P.L.; Drillings, I.M.; Conry, J.A. Herbs in epilepsy: evidence for efficacy, toxicity, and interactions. Semin Pediatr Neurol. 2011, 18, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Hsieh, C.L. Chinese Herbal Medicine for Treating Epilepsy. Front Neurosci. 2021, 2, 682821. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kabra, A.; Rao, M.M.; Prajapati, P.K. Herbal and Holistic Solutions for Neurodegenerative and Depressive Disorders: Leads from Ayurveda. Curr Pharm Des. 2018, 24, 2597–2608. [Google Scholar] [CrossRef]
- Shalini, V.T.; Neelakanta, S.J.; Sriranjini, J.S. Neuroprotection with Bacopa monnieri-A review of experimental evidence. Mol Biol Rep. 2021, 48, 2653–2668. [Google Scholar] [CrossRef]
- Khan, A.U.; Akram, M.; Daniyal, M.; Akhter, N.; Riaz, M.; Akhtar, N.; Shariati, M.A.; Anjum, F.; Khan, S.G.; Parveen, A.; Ahmad, S. Awareness and current knowledge of epilepsy. Metab Brain Dis. 2020, 35, 45–63. [Google Scholar] [CrossRef] [PubMed]
| Molecule | KI value | Docking Score (Kcl/mol) | Hydrogen Bond Interaction |
|---|---|---|---|
| Curcumin | 0.264 μM | -8.97 | SER 51, GLY29, CYS 58 |
| Quercetin | 2.33 μM | -7.68 | GLU 50, SER 51, GLY 158 |
| Chlorogenic acid | 3.29 μM | -7.48 | GLY 31, THR 57, SER51 |
| Valproic Acid | 190.91uM | -5.07 | SER 30, THR 57, CYS 58 |
| Groups (n = 10) | Treatments |
|---|---|
| I | Normal control with vehicle |
| II | PTZ (40mg/kg, i.p.) –PTZ was administered on alternate day for a period of 29 days |
| III | PTZ treated (40mg/kg, i.p.) + MOEE (250mg/kg, p.o.) - for a period of 29 days and 30 min. before PTZ treatment. |
| IV | PTZ treated (40mg/kg, i.p.) + MOEE (500mg/kg, p.o.) - for a period of 29 days and 30 min. before PTZ treatment. |
| V | PTZ treated (40mg/kg, i.p.) + Curcumin (200mg/kg, p.o.) - for a period of 29 days and 30 min. before PTZ treatment. |
| VI | PTZ treated (40mg/kg, i.p.) + Curcumin (300mg/kg, p.o.) - for a period of 29 days and 30 min. before PTZ treatment |
| VII | PTZ treated (40mg/kg, i.p.) + curcumin (200mg/kg, p.o.) + MOEE (250mg/kg, p.o) –for a period of 29 days and 30 min. before PTZ treatment. |
| VIII | PTZ treated (40mg/kg, i.p.) + valproic acid (100 mg/kg, i.p.) - for a period of 29 days and 30 min. before PTZ treatment. |
| XI | MOEE (500mg/kg, p.o.) per se - for a period of 29 days |
| X | Curcumin (300mg/kg, p.o.) per se- given daily by oral route for a period of 29 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





