Submitted:
14 June 2023
Posted:
15 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Therapeutic drug candidates for the treatment of NAFLD
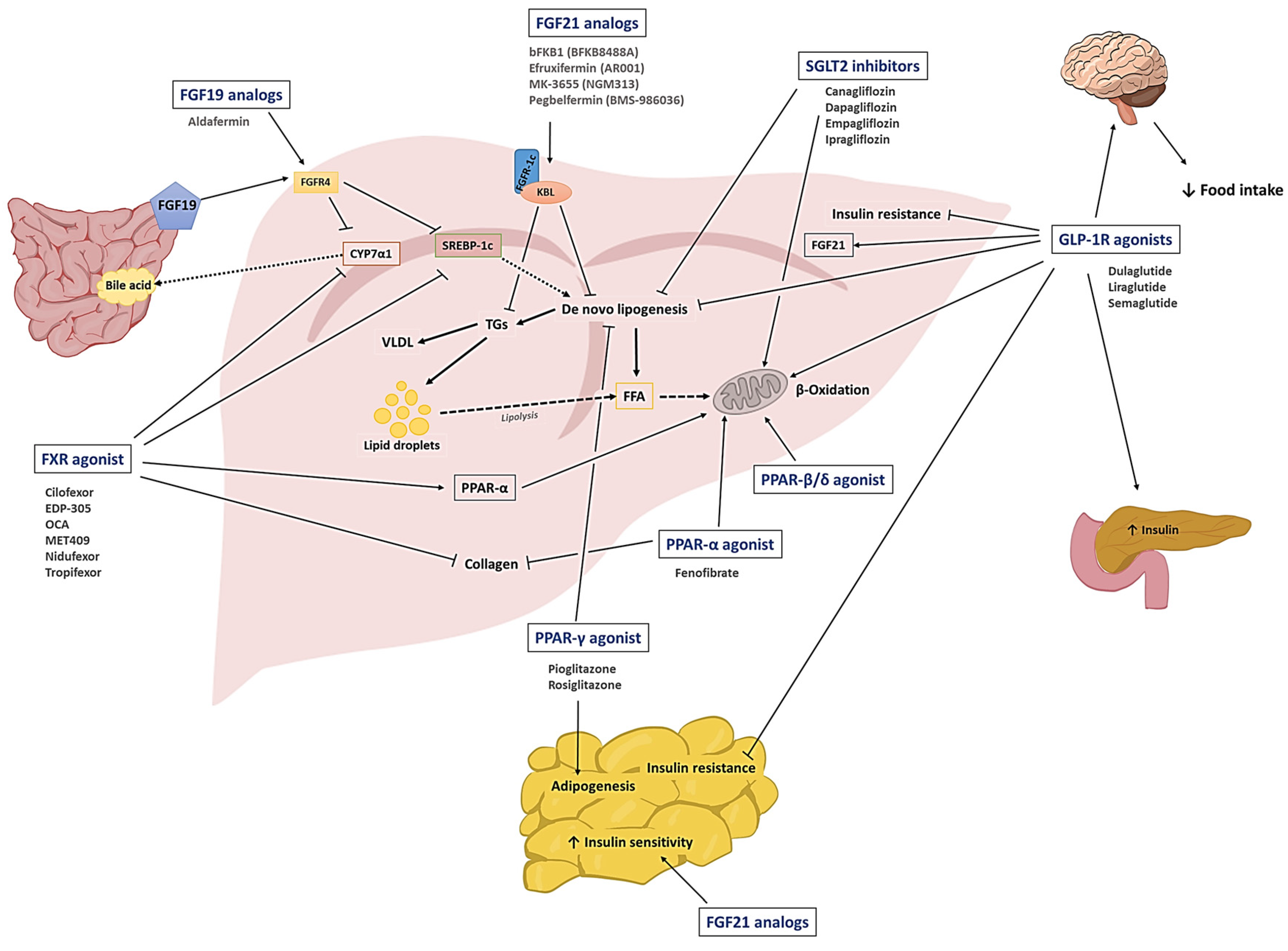
2.1. SGLT2 inhibitors
2.2. GLP-1R agonists
2.3. PPAR agonists
2.4. FGFs
2.5. FXR agonists
3. Strategies to enhance the efficacy of NAFLD drug candidates
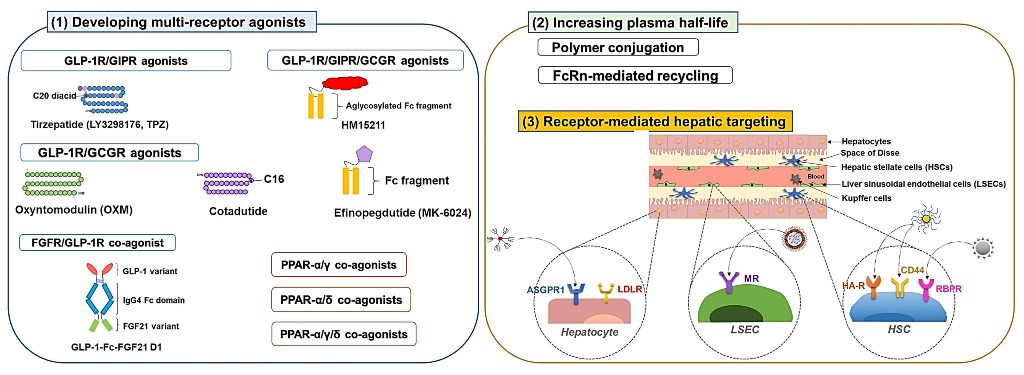
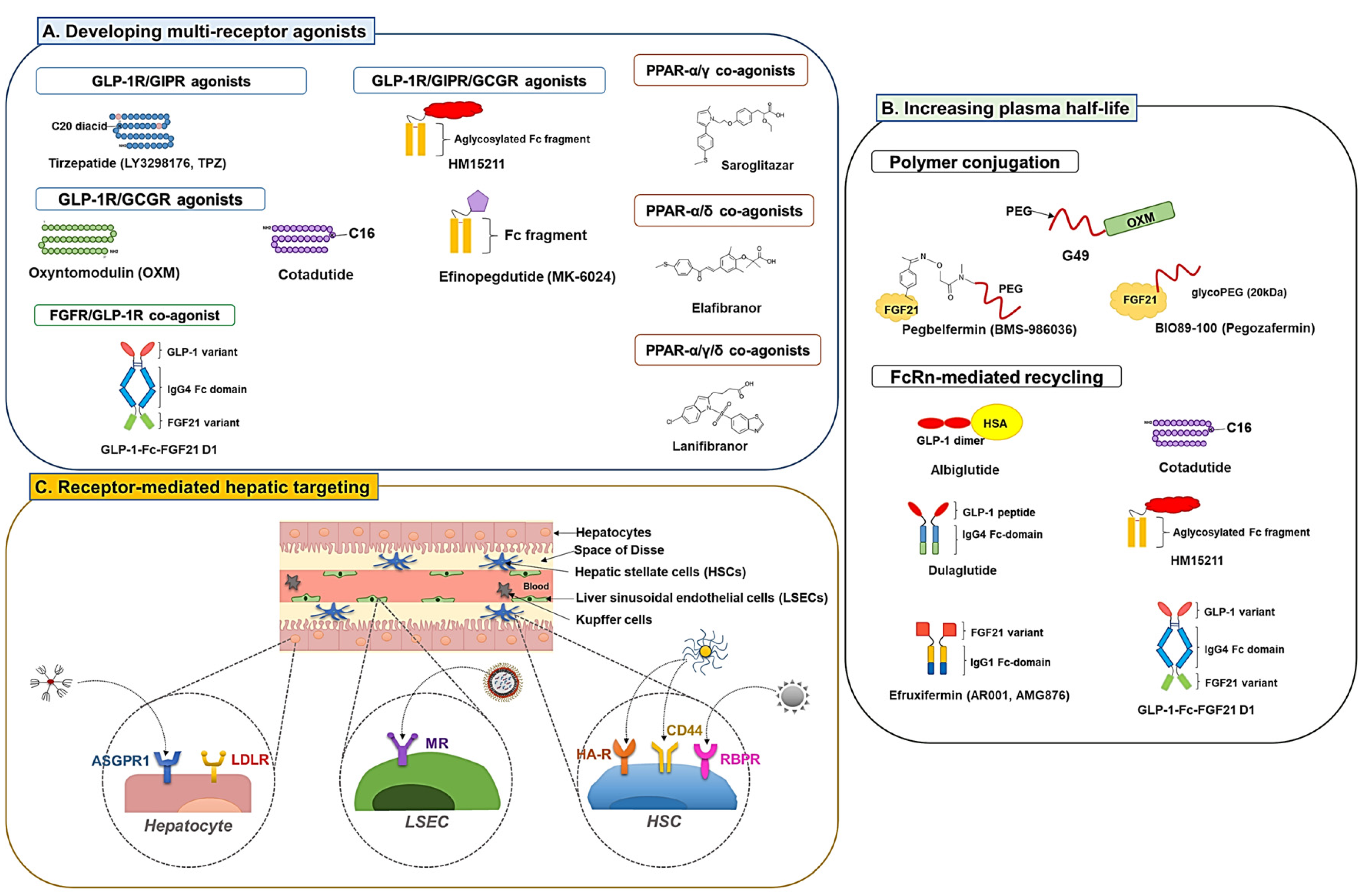
3.1. Developing multiple receptor agonists
3.1.1. GLP-1R co-agonists
3.1.2. PPAR dual and pan agonists
3.1.3. FGFR dual agonists
3.2. Development of long-acting derivatives
3.2.1. Polymer conjugation
3.2.2. Exploiting neonatal Fc receptor (FcRn)-mediated recycling
3.3. Receptor-mediated hepatic targeting
4. Conclusion and future perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clark, J.M.; Brancati, F.L.; Diehl, A.M. Nonalcoholic fatty liver disease. Gastroenterology 2002, 122, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nature reviews Gastroenterology & hepatology 2018, 15, 11–20. [Google Scholar]
- Afolabi, B.I.; Ibitoye, B.O.; Ikem, R.T.; Omisore, A.D.; Idowu, B.M.; Soyoye, D.O. The relationship between glycaemic control and non-alcoholic fatty liver disease in Nigerian type 2 diabetic patients. Journal of the National Medical Association 2018, 110, 256–264. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Zeng, J.; Cao, X.; He, Y. Association of NAFLD with diabetes and the impact of BMI changes: a 5-year cohort study based on 18,507 elderly. The Journal of Clinical Endocrinology & Metabolism 2017, 102, 1309–1316. [Google Scholar]
- Younossi, Z.M. Non-alcoholic fatty liver disease–a global public health perspective. Journal of hepatology 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-h.; Cho, Y.; Lee, B.-W.; Park, C.-Y.; Lee, D.H.; Cha, B.-S.; Rhee, E.-J. Nonalcoholic fatty liver disease in diabetes. Part I: epidemiology and diagnosis. Diabetes & metabolism journal 2019, 43, 31–45. [Google Scholar]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nature medicine 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Treeprasertsuk, S.; Björnsson, E.; Enders, F.; Suwanwalaikorn, S.; Lindor, K.D. NAFLD fibrosis score: a prognostic predictor for mortality and liver complications among NAFLD patients. World journal of gastroenterology: WJG 2013, 19, 1219. [Google Scholar] [CrossRef]
- Eren, F.; Kaya, E.; Yilmaz, Y. Accuracy of Fibrosis-4 index and non-alcoholic fatty liver disease fibrosis scores in metabolic (dysfunction) associated fatty liver disease according to body mass index: failure in the prediction of advanced fibrosis in lean and morbidly obese individuals. European journal of gastroenterology & hepatology 2022, 34, 98–103. [Google Scholar]
- Sanyal, A.J.; Harrison, S.A.; Ratziu, V.; Abdelmalek, M.F.; Diehl, A.M.; Caldwell, S.; Shiffman, M.L.; Aguilar Schall, R.; Jia, C.; McColgan, B. The natural history of advanced fibrosis due to nonalcoholic steatohepatitis: data from the simtuzumab trials. Hepatology 2019, 70, 1913–1927. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Duan, Q.; Wu, R.; Harris, E.N.; Su, Q. Pathophysiological communication between hepatocytes and non-parenchymal cells in liver injury from NAFLD to liver fibrosis. Advanced Drug Delivery Reviews 2021, 176, 113869. [Google Scholar] [CrossRef]
- Hammoutene, A.; Rautou, P.-E. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. Journal of hepatology 2019, 70, 1278–1291. [Google Scholar] [CrossRef]
- Lang, A.; Schoonhoven, R.; Tuvia, S.; Brenner, D.A.; Rippe, R.A. Nuclear factor κB in proliferation, activation, and apoptosis in rat hepatic stellate cells. Journal of hepatology 2000, 33, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Efsen, E.; Romanelli, R.G.; Caligiuri, A.; Pastacaldi, S.; Batignani, G.; Bonacchi, A.; Caporale, R.; Laffi, G.; Pinzani, M. Ligands of peroxisome proliferator-activated receptor γ modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology 2000, 119, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. Journal of Biological Chemistry 2000, 275, 2247–2250. [Google Scholar] [CrossRef]
- Pierantonelli, I.; Rychlicki, C.; Agostinelli, L.; Giordano, D.M.; Gaggini, M.; Fraumene, C.; Saponaro, C.; Manghina, V.; Sartini, L.; Mingarelli, E. Lack of NLRP3-inflammasome leads to gut-liver axis derangement, gut dysbiosis and a worsened phenotype in a mouse model of NAFLD. Scientific reports 2017, 7, 12200. [Google Scholar] [CrossRef]
- Tilg, H.; Effenberger, M. From NAFLD to MAFLD: when pathophysiology succeeds. Nature reviews Gastroenterology & hepatology 2020, 17, 387–388. [Google Scholar]
- Finotti, M.; Romano, M.; Auricchio, P.; Scopelliti, M.; Brizzolari, M.; Grossi, U.; Piccino, M.; Benvenuti, S.; Morana, G.; Cillo, U. Target therapies for NASH/NAFLD: from the molecular aspect to the pharmacological and surgical alternatives. Journal of Personalized Medicine 2021, 11, 499. [Google Scholar] [CrossRef]
- Sumida, Y.; Naito, Y.; Tanaka, S.; Sakai, K.; Inada, Y.; Taketani, H.; Kanemasa, K.; Yasui, K.; Itoh, Y.; Okanoue, T. Long-term (>= 2 yr) efficacy of vitamin E for non-alcoholic steatohepatitis. Hepatogastroenterology 2013, 60, 1445–1450. [Google Scholar]
- Sasaki, A.; Nitta, H.; Otsuka, K.; Umemura, A.; Baba, S.; Obuchi, T.; Wakabayashi, G. Bariatric surgery and non-alcoholic Fatty liver disease: current and potential future treatments. Frontiers in endocrinology 2014, 5, 164. [Google Scholar] [CrossRef]
- Saeed, N.; Glass, L.; Sharma, P.; Shannon, C.; Sonnenday, C.J.; Tincopa, M.A. Incidence and risks for nonalcoholic fatty liver disease and steatohepatitis post-liver transplant: systematic review and meta-analysis. Transplantation 2019, 103, e345–e354. [Google Scholar] [CrossRef]
- Cardoso, A.C.; de Figueiredo-Mendes, C.A.; Villela-Nogueira, C.; A. Current management of NAFLD/NASH. Liver International 2021, 41, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Androutsakos, T.; Nasiri-Ansari, N.; Bakasis, A.-D.; Kyrou, I.; Efstathopoulos, E.; Randeva, H.S.; Kassi, E. SGLT-2 inhibitors in NAFLD: expanding their role beyond diabetes and cardioprotection. International journal of molecular sciences 2022, 23, 3107. [Google Scholar] [CrossRef] [PubMed]
- Jojima, T.; Tomotsune, T.; Iijima, T.; Akimoto, K.; Suzuki, K.; Aso, Y. Empagliflozin (an SGLT2 inhibitor), alone or in combination with linagliptin (a DPP-4 inhibitor), prevents steatohepatitis in a novel mouse model of non-alcoholic steatohepatitis and diabetes. Diabetology & metabolic syndrome 2016, 8, 1–11. [Google Scholar]
- Wallenius, K.; Kroon, T.; Hagstedt, T.; Löfgren, L.; Sörhede-Winzell, M.; Boucher, J.; Lindén, D.; Oakes, N.D. The SGLT2 inhibitor dapagliflozin promotes systemic FFA mobilization, enhances hepatic β-oxidation, and induces ketosis. Journal of lipid research 2022, 63. [Google Scholar] [CrossRef]
- Komiya, C.; Tsuchiya, K.; Shiba, K.; Miyachi, Y.; Furuke, S.; Shimazu, N.; Yamaguchi, S.; Kanno, K.; Ogawa, Y. Ipragliflozin improves hepatic steatosis in obese mice and liver dysfunction in type 2 diabetic patients irrespective of body weight reduction. PloS one 2016, 11, e0151511. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kessoku, T.; Kawanaka, M.; Nonaka, M.; Hyogo, H.; Fujii, H.; Nakajima, T.; Imajo, K.; Tanaka, K.; Kubotsu, Y. Ipragliflozin improves the hepatic outcomes of patients with diabetes with NAFLD. Hepatology communications 2022, 6, 120–132. [Google Scholar] [CrossRef]
- Han, E.; Lee, Y.-h.; Lee, B.-W.; Kang, E.S.; Cha, B.-S. Ipragliflozin additively ameliorates non-alcoholic fatty liver disease in patients with type 2 diabetes controlled with metformin and pioglitazone: a 24-week randomized controlled trial. Journal of clinical medicine 2020, 9, 259. [Google Scholar] [CrossRef]
- He, K.; Li, J.; Xi, W.; Ge, J.; Sun, J.; Jing, Z. Dapagliflozin for nonalcoholic fatty liver disease: a systematic review and meta-analysis. Diabetes research and clinical practice 2022, 109791. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.W.; Lundkvist, P.; Jansson, P.-A.; Johansson, L.; Kvarnström, M.; Moris, L.; Miliotis, T.; Forsberg, G.-B.; Risérus, U.; Lind, L. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia 2018, 61, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Murotani, K.; Saito, M.; Tamasawa, A.; Osonoi, Y.; Yoneda, M.; Osonoi, T. Effect of luseogliflozin on hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease: a prospective, single-arm trial (LEAD trial). Hepatology Research 2019, 49, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Farooqui, K.J.; Singh, M.K.; Wasir, J.S.; Bansal, B.; Kaur, P.; Jevalikar, G.; Gill, H.K. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial). Diabetes care 2018, 41, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Itani, T.; Ishihara, T. Efficacy of canagliflozin against nonalcoholic fatty liver disease: a prospective cohort study. Obesity Science & Practice 2018, 4, 477–482. [Google Scholar]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like peptide 1 in health and disease. Nature Reviews Endocrinology 2018, 14, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Liang, Y.; Luo, Y.; Li, Z.; Wen, Y.; Shen, J.; Li, R.; Zheng, H.; Gu, H.F.; Xia, N. Liraglutide ameliorates nonalcoholic fatty liver disease in diabetic mice via the IRS2/PI3K/Akt signaling pathway. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 2019, 12, 1013. [Google Scholar] [CrossRef]
- Ao, N.; Ma, Z.; Yang, J.; Jin, S.; Zhang, K.; Luo, E.; Du, J. Liraglutide ameliorates lipotoxicity-induced inflammation through the mTORC1 signalling pathway. Peptides 2020, 133, 170375. [Google Scholar] [CrossRef]
- Ali, E.S.; Girard, D.; Petrovsky, N. Impaired Ca2+ signaling due to hepatic steatosis mediates hepatic insulin resistance in Alström syndrome mice that is reversed by GLP-1 analog treatment. American Journal of Physiology-Cell Physiology 2021, 321, C187–C198. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.-n.; Ye, C.-y.; Feng, W.-b.; Zhou, Q.-t.; Yang, D.-h.; Wang, M.-w. GLP-1 mimetics as a potential therapy for nonalcoholic steatohepatitis. Acta Pharmacologica Sinica 2022, 43, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Duparc, T.; Briand, F.; Trenteseaux, C.; Merian, J.; Combes, G.; Najib, S.; Sulpice, T.; Martinez, L.O. Liraglutide improves hepatic steatosis and metabolic dysfunctions in a 3-week dietary mouse model of nonalcoholic steatohepatitis. American Journal of Physiology-Gastrointestinal and Liver Physiology 2019, 317, G508–G517. [Google Scholar] [CrossRef] [PubMed]
- Somm, E.; Montandon, S.A.; Loizides-Mangold, U.; Gaïa, N.; Lazarevic, V.; De Vito, C.; Perroud, E.; Bochaton-Piallat, M.-L.; Dibner, C.; Schrenzel, J. The GLP-1R agonist liraglutide limits hepatic lipotoxicity and inflammatory response in mice fed a methionine-choline deficient diet. Translational Research 2021, 227, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Pontes-da-Silva, R.M.; de Souza Marinho, T.; de Macedo Cardoso, L.E.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Obese mice weight loss role on nonalcoholic fatty liver disease and endoplasmic reticulum stress treated by a GLP-1 receptor agonist. International Journal of Obesity 2022, 46, 21–29. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Hull, D.; Guo, K.; Barton, D.; Hazlehurst, J.M.; Gathercole, L.L.; Nasiri, M.; Yu, J.; Gough, S.C.; Newsome, P.N. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. Journal of hepatology 2016, 64, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. The Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Jia, Y.; Li, Z.; Wang, F.; Ren, L.; Chen, S. Effects of liraglutide on nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Therapy 2021, 12, 1735–1749. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.; Francque, S.; Harrison, S.; Ratziu, V.; Van Gaal, L.; Calanna, S.; Hansen, M.; Linder, M.; Sanyal, A. Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity. Alimentary pharmacology & therapeutics 2019, 50, 193–203. [Google Scholar]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Lonardo, A.; Targher, G. Glucagon-like peptide-1 receptor agonists for treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: an updated meta-analysis of randomized controlled trials. Metabolites 2021, 11, 73. [Google Scholar] [CrossRef]
- Rubino, D.M.; Greenway, F.L.; Khalid, U.; O’Neil, P.M.; Rosenstock, J.; Sørrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T.; Arauz-Pacheco, C. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. Jama 2022, 327, 138–150. [Google Scholar] [CrossRef]
- Choudhary, N.S.; Kumar, N.; Duseja, A. Peroxisome proliferator-activated receptors and their agonists in nonalcoholic fatty liver disease. Journal of Clinical and Experimental Hepatology 2019, 9, 731–739. [Google Scholar] [CrossRef]
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nature Reviews Endocrinology 2017, 13, 36–49. [Google Scholar] [CrossRef]
- Grygiel-Górniak, B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications-a review. Nutrition journal 2014, 13, 1–10. [Google Scholar] [CrossRef]
- Lian, J.; Fu, J. Pioglitazone for NAFLD patients with prediabetes or type 2 diabetes mellitus: a meta-analysis. Frontiers in endocrinology 2021, 12, 615409. [Google Scholar] [CrossRef]
- Powers, C.; McLeskey, S.; Wellstein, A. Fibroblast growth factors, their receptors and signaling. Endocrine-related cancer 2000, 7, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A. FGF-21 as a novel metabolic regulator. The Journal of clinical investigation 2005, 115, 1627–1635. [Google Scholar] [CrossRef]
- Wang, W.-F.; Li, S.-M.; Ren, G.-P.; Zheng, W.; Lu, Y.-J.; Yu, Y.-H.; Xu, W.-J.; Li, T.-H.; Zhou, L.-H.; Liu, Y. Recombinant murine fibroblast growth factor 21 ameliorates obesity-related inflammation in monosodium glutamate-induced obesity rats. Endocrine 2015, 49, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Keinicke, H.; Sun, G.; Mentzel, C.M.J.; Fredholm, M.; John, L.M.; Andersen, B.; Raun, K.; Kjaergaard, M. FGF21 regulates hepatic metabolic pathways to improve steatosis and inflammation. Endocrine connections 2020, 9, 755–768. [Google Scholar] [CrossRef]
- Kolumam, G.; Chen, M.Z.; Tong, R.; Zavala-Solorio, J.; Kates, L.; van Bruggen, N.; Ross, J.; Wyatt, S.K.; Gandham, V.D.; Carano, R.A. Sustained brown fat stimulation and insulin sensitization by a humanized bispecific antibody agonist for fibroblast growth factor receptor 1/βKlotho complex. EBioMedicine 2015, 2, 730–743. [Google Scholar] [CrossRef]
- Wong, C.; Dash, A.; Fredrickson, J.; Lewin-Koh, N.; Chen, S.; Yoshida, K.; Liu, Y.; Gutierrez, J.; Kunder, R. Fibroblast growth factor receptor 1/Klothoβ agonist BFKB8488A improves lipids and liver health markers in patients with diabetes or NAFLD: A phase 1b randomized trial. Hepatology 2022. [Google Scholar] [CrossRef] [PubMed]
- Depaoli, A.; Phung, V.; Bashir, M.R.; Morrow, L.; Beysen, C.; Yan, A.; Ling, L.; Baxter, B.; Luskey, K.L.; Olefsky, J.M. 140-LB: NGM313, a novel activator of b-Klotho/FGFR1c, improves insulin resistance and reduces hepatic fat in obese, nondiabetic subjects. Diabetes 2019, 68. [Google Scholar] [CrossRef]
- Kurosu, H.; Choi, M.; Ogawa, Y.; Dickson, A.S.; Goetz, R.; Eliseenkova, A.V.; Mohammadi, M.; Rosenblatt, K.P.; Kliewer, S.A.; Kuro-o, M. Tissue-specific expression of βKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. Journal of Biological Chemistry 2007, 282, 26687–26695. [Google Scholar] [CrossRef]
- Wunsch, E.; Milkiewicz, M.; Wasik, U.; Trottier, J.; Kempińska-Podhorodecka, A.; Elias, E.; Barbier, O.; Milkiewicz, P. Expression of hepatic fibroblast growth factor 19 is enhanced in primary biliary cirrhosis and correlates with severity of the disease. Scientific reports 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Janus, D.; Dolezal-Oltarzewska, K.; Kalicka-Kasperczyk, A.; Poplawska, K.; Drozdz, D.; Sztefko, K.; Starzyk, J.B. A decrease in fasting FGF19 levels is associated with the development of non-alcoholic fatty liver disease in obese adolescents. Journal of Pediatric Endocrinology and Metabolism 2012, 25, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Gadaleta, R.M.; Scialpi, N.; Peres, C.; Cariello, M.; Ko, B.; Luo, J.; Porru, E.; Roda, A.; Sabbà, C.; Moschetta, A. Suppression of hepatic bile acid synthesis by a non-tumorigenic FGF19 analogue protects mice from fibrosis and hepatocarcinogenesis. Scientific Reports 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Rossi, S.J.; Paredes, A.H.; Trotter, J.F.; Bashir, M.R.; Guy, C.D.; Banerjee, R.; Jaros, M.J.; Owers, S.; Baxter, B.A. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology 2020, 71, 1198–1212. [Google Scholar] [CrossRef]
- Rinella, M.E.; Trotter, J.F.; Abdelmalek, M.F.; Paredes, A.H.; Connelly, M.A.; Jaros, M.J.; Ling, L.; Rossi, S.J.; DePaoli, A.M.; Harrison, S.A. Rosuvastatin improves the FGF19 analogue NGM282-associated lipid changes in patients with non-alcoholic steatohepatitis. Journal of Hepatology 2019, 70, 735–744. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, F.; Guo, G.L. Tissue-specific function of farnesoid X receptor in liver and intestine. Pharmacological research 2011, 63, 259–265. [Google Scholar] [CrossRef]
- Bjursell, M.; Wedin, M.; Admyre, T.; Hermansson, M.; Böttcher, G.; Göransson, M.; Lindén, D.; Bamberg, K.; Oscarsson, J.; Bohlooly-Y, M. Ageing Fxr deficient mice develop increased energy expenditure, improved glucose control and liver damage resembling NASH. PloS one 2013, 8, e64721. [Google Scholar] [CrossRef]
- Cariou, B.; Staels, B. FXR: a promising target for the metabolic syndrome? Trends in pharmacological sciences 2007, 28, 236–243. [Google Scholar] [CrossRef]
- Zhou, S.; You, H.; Qiu, S.; Yu, D.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. A new perspective on NAFLD: Focusing on the crosstalk between peroxisome proliferator-activated receptor alpha (PPARα) and farnesoid X receptor (FXR). Biomedicine & Pharmacotherapy 2022, 154, 113577. [Google Scholar]
- Miyazaki, T.; Honda, A.; Ikegami, T.; Iida, T.; Matsuzaki, Y. Human-specific dual regulations of FXR-activation for reduction of fatty liver using in vitro cell culture model. Journal of Clinical Biochemistry and Nutrition 2019, 64, 112–123. [Google Scholar] [CrossRef]
- Fiorucci, S.; Mencarelli, A.; Palladino, G.; Cipriani, S. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends in pharmacological sciences 2009, 30, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Alemi, F.; Kwon, E.; Poole, D.P.; Lieu, T.; Lyo, V.; Cattaruzza, F.; Cevikbas, F.; Steinhoff, M.; Nassini, R.; Materazzi, S. The TGR5 receptor mediates bile acid–induced itch and analgesia. The Journal of clinical investigation 2013, 123, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Pellicciari, R.; Fiorucci, S.; Camaioni, E.; Clerici, C.; Costantino, G.; Maloney, P.R.; Morelli, A.; Parks, D.J.; Willson, T.M. 6α-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. Journal of medicinal chemistry 2002, 45, 3569–3572. [Google Scholar] [PubMed]
- Zhang, Y.; Jackson, J.P.; St. Claire III, R.L.; Freeman, K.; Brouwer, K.R.; Edwards, J.E. Obeticholic acid, a selective farnesoid X receptor agonist, regulates bile acid homeostasis in sandwich-cultured human hepatocytes. Pharmacology Research & Perspectives 2017, 5, e00329. [Google Scholar]
- Goto, T.; Itoh, M.; Suganami, T.; Kanai, S.; Shirakawa, I.; Sakai, T.; Asakawa, M.; Yoneyama, T.; Kai, T.; Ogawa, Y. Obeticholic acid protects against hepatocyte death and liver fibrosis in a murine model of nonalcoholic steatohepatitis. Scientific reports 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, X.; Chung, T.-Y.; Ye, W.; Hodge, L.; Zhang, L.; Chng, K.; Xiao, Y.-F.; Wang, Y.J. Carbon tetrachloride (CCl4) accelerated development of non-alcoholic fatty liver disease (NAFLD)/steatohepatitis (NASH) in MS-NASH mice fed western diet supplemented with fructose (WDF). BMC gastroenterology 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Rizzo, G.; Passeri, D.; De Franco, F.; Ciaccioli, G.; Donadio, L.; Rizzo, G.; Orlandi, S.; Sadeghpour, B.; Wang, X.X.; Jiang, T. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Molecular pharmacology 2010, 78, 617–630. [Google Scholar] [CrossRef]
- Roth, J.D.; Feigh, M.; Veidal, S.S.; Fensholdt, L.K.; Rigbolt, K.T.; Hansen, H.H.; Chen, L.C.; Petitjean, M.; Friley, W.; Vrang, N. INT-767 improves histopathological features in a diet-induced ob/ob mouse model of biopsy-confirmed non-alcoholic steatohepatitis. World Journal of Gastroenterology 2018, 24, 195. [Google Scholar] [CrossRef]
- Comeglio, P.; Cellai, I.; Mello, T.; Filippi, S.; Maneschi, E.; Corcetto, F.; Corno, C.; Sarchielli, E.; Morelli, A.; Rapizzi, E. INT-767 prevents NASH and promotes visceral fat brown adipogenesis and mitochondrial function. Journal of Endocrinology 2018, 238, 107–127. [Google Scholar] [CrossRef]
- Chau, M.; Li, Y.; Roqueta-Rivera, M.; Garlick, K.; Shen, R.; Wang, G.; Or, Y.S.; Jiang, L.-J. Characterization of EDP-305, a highly potent and selective farnesoid X receptor agonist, for the treatment of non-alcoholic steatohepatitis. Int J Gastroenterol 2019, 3, 4–16. [Google Scholar] [CrossRef]
- Schwabl, P.; Hambruch, E.; Budas, G.R.; Supper, P.; Burnet, M.; Liles, J.T.; Birkel, M.; Brusilovskaya, K.; Königshofer, P.; Peck-Radosavljevic, M. The non-steroidal FXR agonist cilofexor improves portal hypertension and reduces hepatic fibrosis in a rat NASH model. Biomedicines 2021, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Chianelli, D.; Rucker, P.V.; Roland, J.; Tully, D.C.; Nelson, J.; Liu, X.; Bursulaya, B.; Hernandez, E.D.; Wu, J.; Prashad, M. Nidufexor (LMB763), a novel FXR modulator for the treatment of nonalcoholic steatohepatitis. Journal of medicinal chemistry 2020, 63, 3868–3880. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, J.-H.; Yi, J.; Han, O.; Kim, Y.; Baek, E.; Jung, S.Y.; Kwon, S.C.; Trautmann, M.E.; Hompesch, M. The ultra-long acting LAPS GLP/GCG dual agonist, HM12525A, demonstrated safety and prolonged pharmacokinetics in healthy volunteers: a phase 1 first-in-human study. Diabetologia 2015, 58. [Google Scholar]
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L. A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. New England Journal of Medicine 2021, 385, 1547–1558. [Google Scholar] [PubMed]
- Gawrieh, S.; Noureddin, M.; Loo, N.; Mohseni, R.; Awasty, V.; Cusi, K.S.; Kowdley, K.; Lai, M.; Schiff, E.; Parmar, D. a PPAR-α/γ Agonist, for Treatment of Nonalcoholic Fatty Liver Disease: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology 2021, 74, 1809–1824. [Google Scholar] [PubMed]
- Gabe, M.B.N.; van der Velden, W.J.; Smit, F.X.; Gasbjerg, L.S.; Rosenkilde, M.M. Molecular interactions of full-length and truncated GIP peptides with the GIP receptor–A comprehensive review. Peptides 2020, 125, 170224. [Google Scholar] [CrossRef]
- Bastin, M.; Andreelli, F. Dual GIP–GLP1-receptor agonists in the treatment of type 2 diabetes: a short review on emerging data and therapeutic potential. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 2019, 1973–1985. [Google Scholar] [CrossRef]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the two incretin hormones: similarities and differences. Journal of diabetes investigation 2010, 1, 8–23. [Google Scholar] [CrossRef]
- Yamada, Y.; Seino, Y. Physiology of GIP-a lesson from GIP receptor knockout mice. Hormone and metabolic research 2004, 36, 771–774. [Google Scholar]
- Nauck, M.A.; Meier, J.J. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. The lancet Diabetes & endocrinology 2016, 4, 525–536. [Google Scholar]
- Hartman, M.L.; Sanyal, A.J.; Loomba, R.; Wilson, J.M.; Nikooienejad, A.; Bray, R.; Karanikas, C.A.; Duffin, K.L.; Robins, D.A.; Haupt, A. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care 2020, 43, 1352–1355. [Google Scholar] [PubMed]
- Zhao, F.; Zhou, Q.; Cong, Z.; Hang, K.; Zou, X.; Zhang, C.; Chen, Y.; Dai, A.; Liang, A.; Ming, Q. Structural insights into multiplexed pharmacological actions of tirzepatide and peptide 20 at the GIP, GLP-1 or glucagon receptors. Nature Communications 2022, 13, 1057. [Google Scholar]
- Unger, R.H.; Orci, L. Glucagon and the A cell: physiology and pathophysiology. New England Journal of Medicine 1981, 304, 1575–1580. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Pan, Y.-H.; Huang, Y.-M.; Zhao, H.-L. Neuroendocrine hormone amylin in diabetes. World journal of diabetes 2016, 7, 189. [Google Scholar] [CrossRef]
- Habegger, K.M.; Heppner, K.M.; Geary, N.; Bartness, T.J.; DiMarchi, R.; Tschöp, M.H. The metabolic actions of glucagon revisited. Nature Reviews Endocrinology 2010, 6, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Galsgaard, K.D. The vicious circle of hepatic glucagon resistance in non-alcoholic fatty liver disease. Journal of Clinical Medicine 2020, 9, 4049. [Google Scholar] [PubMed]
- Cohen, M.A.; Ellis, S.M.; Le Roux, C.W.; Batterham, R.L.; Park, A.; Patterson, M.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Oxyntomodulin suppresses appetite and reduces food intake in humans. The Journal of Clinical Endocrinology & Metabolism 2003, 88, 4696–4701. [Google Scholar]
- Ma, T.; Huo, S.; Xu, B.; Li, F.; Wang, P.; Liu, Y.; Lei, H. A novel long-acting oxyntomodulin analogue eliminates diabetes and obesity in mice. European journal of medicinal chemistry 2020, 203, 112496. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. Enteroglucagon. Annual Review of Physiology 1997, 59, 257–271. [Google Scholar] [CrossRef]
- Pocai, A.; Carrington, P.E.; Adams, J.R.; Wright, M.; Eiermann, G.; Zhu, L.; Du, X.; Petrov, A.; Lassman, M.E.; Jiang, G. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes 2009, 58, 2258–2266. [Google Scholar] [CrossRef]
- Henderson, S.; Konkar, A.; Hornigold, D.; Trevaskis, J.; Jackson, R.; Fritsch Fredin, M.; Jansson-Löfmark, R.; Naylor, J.; Rossi, A.; Bednarek, M. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes, Obesity and Metabolism 2016, 18, 1176–1190. [Google Scholar] [CrossRef]
- Boland, M.L.; Laker, R.C.; Mather, K.; Nawrocki, A.; Oldham, S.; Boland, B.B.; Lewis, H.; Conway, J.; Naylor, J.; Guionaud, S. Resolution of NASH and hepatic fibrosis by the GLP-1R and GCGR dual-agonist cotadutide via modulating mitochondrial function and lipogenesis. Nature metabolism 2020, 2, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, J.S.; Park, E.; Kim, D.J.; Kim, Y.H.; Choi, I.Y. Therapeutic effect of a novel long-acting GLP-1/GIP/Glucagon triple agonist (HM15211) in NASH and fibrosis animal models. In.EASD annual meeting; 2018.
- Choi, I.Y.; Lee, J.S.; Kim, J.K.; Park, Y.J.; Jung, S.Y.; Kim, Y.H.; Kwon, S.C. Potent body weight loss and efficacy in a NASH animal model by a novel long-acting GLP-1/Glucagon/GIP triple-agonist (HM15211). American Diabetes Association’s 77th Scientific Session. 2017. [Google Scholar]
- Choi, J.; Jo, H.; Kim, J.K.; Lee, S.D.; Lee, S.H.; Choi, I.Y. 996-P: Therapeutic Effect of a Novel Long-Acting GLP-1/GIP/Glucagon Triple Agonist (HM15211) in a Dyslipidemia Animal Model. Diabetes 2019, 68. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, J.S.; Kwon, H.; Lee, J.; Kim, D.; Bae, S.; Lee, S.H.; Choi, I.Y. 1803-P: Antifibrotic Effect of a Novel Long-Acting GLP-1/GIP/Glucagon Triple Agonist (HM15211) in BDL-Induced Liver Fibrosis Mice. Diabetes 2020, 69. [Google Scholar] [CrossRef]
- Zarei, M.; Aguilar-Recarte, D.; Palomer, X.; Vázquez-Carrera, M. Revealing the role of peroxisome proliferator-activated receptor β/δ in nonalcoholic fatty liver disease. Metabolism 2021, 114, 154342. [Google Scholar] [CrossRef] [PubMed]
- Fajas, L.; Auboeuf, D.; Raspé, E.; Schoonjans, K.; Lefebvre, A.-M.; Saladin, R.; Najib, J.; Laville, M.; Fruchart, J.-C.; Deeb, S. The organization, promoter analysis, and expression of the human PPARγ gene. Journal of Biological Chemistry 1997, 272, 18779–18789. [Google Scholar] [CrossRef]
- Kalliora, C.; Drosatos, K. The glitazars paradox: Cardiotoxicity of the metabolically beneficial dual PPARα and PPARγ activation. Journal of cardiovascular pharmacology 2020, 76, 514. [Google Scholar] [CrossRef]
- Agrawal, R. The first approved agent in the Glitazar’s class: saroglitazar. Current drug targets 2014, 15, 151–155. [Google Scholar] [CrossRef]
- Munigoti, S.P.; Harinarayan, C. Role of Glitazars in atherogenic dyslipidemia and diabetes: Two birds with one stone? Indian Journal of Endocrinology and Metabolism 2014, 18, 283. [Google Scholar] [CrossRef]
- Kumar, D.P.; Caffrey, R.; Marioneaux, J.; Santhekadur, P.K.; Bhat, M.; Alonso, C.; Koduru, S.V.; Philip, B.; Jain, M.R.; Giri, S.R. The PPAR α/γ agonist saroglitazar improves insulin resistance and steatohepatitis in a diet induced animal model of nonalcoholic fatty liver disease. Scientific Reports 2020, 10, 9330. [Google Scholar] [CrossRef]
- Staels, B.; Rubenstrunk, A.; Noel, B.; Rigou, G.; Delataille, P.; Millatt, L.J.; Baron, M.; Lucas, A.; Tailleux, A.; Hum, D.W. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2013, 58, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Okanoue, T.; Nakajima, A.; NAFLD, J.S.G.o. Phase 3 drug pipelines in the treatment of non-alcoholic steatohepatitis. Hepatology Research 2019, 49, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Lefere, S.; Puengel, T.; Hundertmark, J.; Penners, C.; Frank, A.K.; Guillot, A.; De Muynck, K.; Heymann, F.; Adarbes, V.; Defrêne, E. Differential effects of selective-and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages☆. Journal of hepatology 2020, 73, 757–770. [Google Scholar] [CrossRef]
- Liu, J.; Yang, K.; Yang, J.; Xiao, W.; Le, Y.; Yu, F.; Gu, L.; Lang, S.; Tian, Q.; Jin, T. Liver-derived fibroblast growth factor 21 mediates effects of glucagon-like peptide-1 in attenuating hepatic glucose output. EBioMedicine 2019, 41, 73–84. [Google Scholar]
- Hong, H.; Kim, J.; Choi, H.; Kim, D.; Kim, T.; Tølbøl, K.; Feigh, M.; Illemann, M.; Rigbolt, K.; Vrang, N. YH25724, a novel long-acting GLP-1/FGF21 dual agonist lowers both non-alcoholic fatty liver disease activity score and fibrosis stage in a diet-induced obese mouse model of biopsy-confirmed non-alcoholic steatohepatitis. Journal of Hepatology 2017, 1, S16–S17. [Google Scholar] [CrossRef]
- Pan, Q.; Lin, S.; Li, Y.; Liu, L.; Li, X.; Gao, X.; Yan, J.; Gu, B.; Chen, X.; Li, W. A novel GLP-1 and FGF21 dual agonist has therapeutic potential for diabetes and non-alcoholic steatohepatitis. EBioMedicine 2021, 63, 103202. [Google Scholar] [CrossRef] [PubMed]
- Zaman, R.; Islam, R.A.; Ibnat, N.; Othman, I.; Zaini, A.; Lee, C.Y.; Chowdhury, E.H. Current strategies in extending half-lives of therapeutic proteins. Journal of Controlled Release 2019, 301, 176–189. [Google Scholar] [CrossRef]
- Schjoldager, B.; Baldissera, F.; Mortensen, P.; Holst, J.; Christiansen, J. Oxyntomodulin: a potential hormone from the distal gut. Pharmacokinetics and effects on gastric acid and insulin secretion in man. European journal of clinical investigation 1988, 18, 499–503. [Google Scholar] [CrossRef]
- Ye, X.; Qi, J.; Sun, G.; Ren, G.; Zhu, S.; Wu, Y.; Yu, D.; Zhao, J.; Liu, M.; Li, D. Enhancement of the pharmacological efficacy of FGF-21 by genetic modification and PEGylation. Current pharmaceutical biotechnology 2013, 14, 1287–1298. [Google Scholar] [CrossRef]
- Cui, A.; Li, J.; Ji, S.; Ma, F.; Wang, G.; Xue, Y.; Liu, Z.; Gao, J.; Han, J.; Tai, P. The effects of B1344, a novel fibroblast growth factor 21 analog, on nonalcoholic steatohepatitis in nonhuman primates. Diabetes 2020, 69, 1611–1623. [Google Scholar]
- Xu, J.; Bussiere, J.; Yie, J.; Sickmier, A.; An, P.; Belouski, E.; Stanislaus, S.; Walker, K.W. Polyethylene glycol modified FGF21 engineered to maximize potency and minimize vacuole formation. Bioconjugate chemistry 2013, 24, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Pinkstaff, J.; Li, Z.; Skidmore, L.; Li, N.; Myler, H.; Dallas-Yang, Q.; Putnam, A.-M.; Yao, J.; Bussell, S. FGF21 analogs of sustained action enabled by orthogonal biosynthesis demonstrate enhanced antidiabetic pharmacology in rodents. Diabetes 2012, 61, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Verzijl, C.R.; Van De Peppel, I.P.; Struik, D.; Jonker, J.W. Pegbelfermin (BMS-986036): an investigational PEGylated fibroblast growth factor 21 analogue for the treatment of nonalcoholic steatohepatitis. Expert opinion on investigational drugs 2020, 29, 125–133. [Google Scholar] [CrossRef]
- Sanyal, A.; Charles, E.D.; Neuschwander-Tetri, B.A.; Loomba, R.; Harrison, S.A.; Abdelmalek, M.F.; Lawitz, E.J.; Halegoua-DeMarzio, D.; Kundu, S.; Noviello, S. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. The Lancet 2018, 392, 2705–2717. [Google Scholar] [CrossRef]
- Charles, E.D.; Neuschwander-Tetri, B.A.; Pablo Frias, J.; Kundu, S.; Luo, Y.; Tirucherai, G.S.; Christian, R. Pegbelfermin (BMS-986036), PEGylated FGF21, in patients with obesity and type 2 diabetes: results from a randomized phase 2 study. Obesity 2019, 27, 41–49. [Google Scholar] [CrossRef]
- Abdelmalek, M.F.; Charles, E.D.; Sanyal, A.J.; Harrison, S.A.; Neuschwander-Tetri, B.A.; Goodman, Z.; Ehman, R.A.; Karsdal, M.; Nakajima, A.; Du, S. The FALCON program: Two phase 2b randomized, double-blind, placebo-controlled studies to assess the efficacy and safety of pegbelfermin in the treatment of patients with nonalcoholic steatohepatitis and bridging fibrosis or compensated cirrhosis. Contemporary Clinical Trials 2021, 104, 106335. [Google Scholar] [CrossRef] [PubMed]
- Pierce, A.; Rosenstock, M.; Margalit, M.; Mansbach, H. BIO89-100, a novel glycoPEGylated FGF21 Analog, Demonstrates Triglyceride Reduction and Broad Metabolic Effects in Spontaneously Diabetic Obese Cynomolgus Monkeys. Journal of Clinical Lipidology 2020, 14, 584–585. [Google Scholar] [CrossRef]
- Rosenstock, M.; Ayalon, M.; Mansbach, H.; Liu, Y.; Margalit, M. LBP-29-BIO89-100, a novel PEG-FGF21 analogue, is efficacious following weekly and every 2-week subcutaneous dosing in spontaneous diabetic cynomolgus monkeys. Journal of Hepatology 2019, 70, e155. [Google Scholar] [CrossRef]
- Loomba, R.; Lawitz, E.J.; Frias, J.P.; Ortiz-Lasanta, G.; Johansson, L.; Franey, B.B.; Morrow, L.; Rosenstock, M.; Hartsfield, C.L.; Chen, C.-Y. Safety, pharmacokinetics, and pharmacodynamics of pegozafermin in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 1b/2a multiple-ascending-dose study. The Lancet Gastroenterology & Hepatology 2023, 8, 120–132. [Google Scholar]
- Saxena, R.; Nanjan, M.J. Elastin-like polypeptides and their applications in anticancer drug delivery systems: a review. Drug delivery 2015, 22, 156–167. [Google Scholar] [CrossRef]
- Gilroy, C.; Capozzi, M.; Varanko, A.; Tong, J.; D'alessio, D.; Campbell, J.; Chilkoti, A. Sustained release of a GLP-1 and FGF21 dual agonist from an injectable depot protects mice from obesity and hyperglycemia. Science advances 2020, 6, eaaz9890. [Google Scholar] [CrossRef] [PubMed]
- Roopenian, D.C.; Akilesh, S. FcRn: the neonatal Fc receptor comes of age. Nature reviews immunology 2007, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Kontermann, R.E. Strategies for extended serum half-life of protein therapeutics. Current opinion in biotechnology 2011, 22, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Fanali, G.; Di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: from bench to bedside. Molecular aspects of medicine 2012, 33, 209–290. [Google Scholar]
- Chaudhury, C.; Brooks, C.L.; Carter, D.C.; Robinson, J.M.; Anderson, C.L. Albumin binding to FcRn: Distinct from the FcRn− IgG interaction. Biochemistry 2006, 45, 4983–4990. [Google Scholar]
- Bhattacharya, A.A.; Grüne, T.; Curry, S. Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. Journal of molecular biology 2000, 303, 721–732. [Google Scholar] [CrossRef]
- Brønden, A.; Knop, F.K.; Christensen, M.B. Clinical pharmacokinetics and pharmacodynamics of albiglutide. Clinical pharmacokinetics 2017, 56, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.B.; Nielsen, P.F.; Huusfeldt, P.O.; Johansen, N.L.; Madsen, K.; Pedersen, F.Z.; Thøgersen, H.; Wilken, M.; Agersø, H. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. Journal of medicinal chemistry 2000, 43, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Bloch, P.; Schaffer, L.; Pettersson, I.; Spetzler, J.; Kofoed, J.; Madsen, K.; Knudsen, L.B.; McGuire, J.; Steensgaard, D.B. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. Journal of medicinal chemistry 2015, 58, 7370–7380. [Google Scholar] [CrossRef]
- Elbrønd, B.; Jakobsen, G.; Larsen, S.; Agersø, H.; Jensen, L.B.; Rolan, P.; Sturis, J.; Hatorp, V.; Zdravkovic, M. Pharmacokinetics, pharmacodynamics, safety, and tolerability of a single-dose of NN2211, a long-acting glucagon-like peptide 1 derivative, in healthy male subjects. Diabetes care 2002, 25, 1398–1404. [Google Scholar] [CrossRef]
- Hui, H.; Farilla, L.; Merkel, P.; Perfetti, R. The short half-life of glucagon-like peptide-1 in plasma does not reflect its long-lasting beneficial effects. European journal of endocrinology 2002, 146, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Ly, N.; Li, J.; Arends, R.H. Population Pharmacokinetics of Cotadutide in Subjects with Type 2 Diabetes. Clinical Pharmacokinetics 2022, 61, 833–845. [Google Scholar] [CrossRef]
- Monnet, C.; Jorieux, S.; Urbain, R.; Fournier, N.; Bouayadi, K.; De Romeuf, C.; Behrens, C.K.; Fontayne, A.; Mondon, P. Selection of IgG variants with increased FcRn binding using random and directed mutagenesis: impact on effector functions. Frontiers in Immunology 2015, 6, 39. [Google Scholar] [CrossRef]
- Vaughn, D.E.; Bjorkman, P.J. Structural basis of pH-dependent antibody binding by the neonatal Fc receptor. Structure 1998, 6, 63–73. [Google Scholar] [CrossRef]
- Naver, S.V.; Jimenez-Solem, E.; Christensen, M.; Andersen, J.T.; Knop, F.K. Dulaglutide: a novel once-weekly glucagon-like peptide-1 receptor agonist. Clinical investigation 2014, 4, 729–743. [Google Scholar] [CrossRef]
- Choi, J.D.; Baek, S.; Kim, Y.; Eun, K.; Kwon, S.C.; Morrow, L.; Hompesch, M.; Kang, J. 982-P: A Double-Blinded, Placebo Controlled, Single Ascending Dose Study for Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics after Subcutaneous Administration of Novel Long-Acting GLP-1/GIP/Glucagon Triple Agonist (HM15211) in Healthy Obese Subjects. Diabetes 2019, 68. [Google Scholar]
- Stanislaus, S.; Hecht, R.; Yie, J.; Hager, T.; Hall, M.; Spahr, C.; Wang, W.; Weiszmann, J.; Li, Y.; Deng, L. A novel Fc-FGF21 with improved resistance to proteolysis, increased affinity toward β-klotho, and enhanced efficacy in mice and cynomolgus monkeys. Endocrinology 2017, 158, 1314–1327. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, A.; Abuqayyas, L.; Denney, W.S.; Tillman, E.J.; Rolph, T. AKR-001, an Fc-FGF21 analog, showed sustained pharmacodynamic effects on insulin sensitivity and lipid metabolism in type 2 diabetes patients. Cell Reports Medicine 2020, 1, 100057. [Google Scholar] [CrossRef]
- Harrison, S.A.; Ruane, P.J.; Freilich, B.L.; Neff, G.; Patil, R.; Behling, C.A.; Hu, C.; Fong, E.; de Temple, B.; Tillman, E.J. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial. Nature medicine 2021, 27, 1262–1271. [Google Scholar] [CrossRef]
- Boey, A.; Ho, H.K. All roads lead to the liver: metal nanoparticles and their implications for liver health. Small 2020, 16, 2000153. [Google Scholar] [CrossRef]
- Sadauskas, E.; Wallin, H.; Stoltenberg, M.; Vogel, U.; Doering, P.; Larsen, A.; Danscher, G. Kupffer cells are central in the removal of nanoparticles from the organism. Particle and fibre toxicology 2007, 4, 1–7. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Molecular pharmaceutics 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Xin, X.; Ma, J.; Tan, C.; Osna, N.; Mahato, R.I. Therapeutic targets, novel drugs, and delivery systems for diabetes associated NAFLD and liver fibrosis. Advanced Drug Delivery Reviews 2021, 176, 113888. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-K.; Utsumi, T.; Seo, Y.-E.; Deng, Y.; Satoh, A.; Saltzman, W.M.; Iwakiri, Y. Cellular distribution of injected PLGA-nanoparticles in the liver. Nanomedicine: Nanotechnology, Biology and Medicine 2016, 12, 1365–1374. [Google Scholar] [CrossRef]
- Monestier, M.; Charbonnier, P.; Gateau, C.; Cuillel, M.; Robert, F.; Lebrun, C.; Mintz, E.; Renaudet, O.; Delangle, P. ASGPR-Mediated Uptake of Multivalent Glycoconjugates for Drug Delivery in Hepatocytes. ChemBioChem 2016, 17, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Petrov, R.A.; Maklakova, S.Y.; Ivanenkov, Y.A.; Petrov, S.A.; Sergeeva, O.V.; Yamansarov, E.Y.; Saltykova, I.V.; Kireev, I.I.; Alieva, I.B.; Deyneka, E.V. Synthesis and biological evaluation of novel mono-and bivalent ASGP-R-targeted drug-conjugates. Bioorganic & medicinal chemistry letters 2018, 28, 382–387. [Google Scholar]
- Bon, C.; Hofer, T.; Bousquet-Mélou, A.; Davies, M.R.; Krippendorff, B.-F. Capacity limits of asialoglycoprotein receptor-mediated liver targeting. In.MAbs: Taylor & Francis. 2017; 1360–1369. [Google Scholar]
- Sharma, R.; Porterfield, J.E.; An, H.-T.; Jimenez, A.S.; Lee, S.; Kannan, S.; Sharma, A.; Kannan, R.M. Rationally designed galactose dendrimer for hepatocyte-specific targeting and intracellular drug delivery for the treatment of liver disorders. Biomacromolecules 2021, 22, 3574–3589. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.; Laurent, T.C.; Pertoft, H.; Baxter, E. Plasma clearance, tissue distribution and metabolism of hyaluronic acid injected intravenously in the rabbit. Biochemical Journal 1981, 200, 415–424. [Google Scholar] [CrossRef]
- Fraser, J.R.E.; Appelgren, L.-E.; Laurent, T.C. Tissue uptake of circulating hyaluronic acid: a whole body autoradiographic study. Cell and tissue research 1983, 233, 285–293. [Google Scholar] [CrossRef]
- Yang, J.-A.; Kong, W.H.; Sung, D.K.; Kim, H.; Kim, T.H.; Lee, K.C.; Hahn, S.K. Hyaluronic acid–tumor necrosis factor-related apoptosis-inducing ligand conjugate for targeted treatment of liver fibrosis. Acta biomaterialia 2015, 12, 174–182. [Google Scholar] [CrossRef]
- Li, W.; Zhou, C.; Fu, Y.; Chen, T.; Liu, X.; Zhang, Z.; Gong, T. Targeted delivery of hyaluronic acid nanomicelles to hepatic stellate cells in hepatic fibrosis rats. Acta Pharmaceutica Sinica B 2020, 10, 693–710. [Google Scholar] [CrossRef]
- Newcomer, M.E.; Ong, D.E. Plasma retinol binding protein: structure and function of the prototypic lipocalin. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology 2000, 1482, 57–64. [Google Scholar] [CrossRef]
- Uchio, K.; Tuchweber, B.; Manabe, N.; Gabbiani, G.; Rosenbaum, J.; Desmouliere, A. Cellular retinol-binding protein-1 expression and modulation during in vivo and in vitro myofibroblastic differentiation of rat hepatic stellate cells and portal fibroblasts. Laboratory Investigation 2002, 82, 619–628. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jeong, W.I. Retinoic acids and hepatic stellate cells in liver disease. Journal of gastroenterology and hepatology 2012, 27, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.-B.; Fan, Q.-Q.; Xing, L.; Cui, P.-F.; He, Y.-J.; Zhu, J.-C.; Wang, L.; Pang, T.; Oh, Y.-K.; Zhang, C. Vitamin A-decorated biocompatible micelles for chemogene therapy of liver fibrosis. Journal of Controlled Release 2018, 283, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Tammam, S.N.; El Safy, S.; Abdel-Halim, M.; Asimakopoulou, A.; Weiskirchen, R.; Mansour, S. Prevention of hepatic stellate cell activation using JQ1-and atorvastatin-loaded chitosan nanoparticles as a promising approach in therapy of liver fibrosis. European Journal of Pharmaceutics and Biopharmaceutics 2019, 134, 96–106. [Google Scholar] [CrossRef]
- Magnusson, S.; Berg, T. Extremely rapid endocytosis mediated by the mannose receptor of sinusoidal endothelial rat liver cells. Biochemical Journal 1989, 257, 651–656. [Google Scholar] [CrossRef]
- Schlesinger, P.H.; Doebber, T.W.; Mandell, B.F.; White, R.; DeSchryver, C.; Rodman, J.; Miller, M.; Stahl, P. Plasma clearance of glycoproteins with terminal mannose and N-acetylglucosamine by liver non-parenchymal cells. Studies with β-glucuronidase, N-acetyl-β-d-glucosaminidase, ribonuclease B and agalacto-orosomucoid. Biochemical Journal 1978, 176, 103–109. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, M.; Hur, S.; Cho, Y.; Park, J.; Jung, H.; Seo, Y.; Woo, H.; Nam, K.; Lee, K. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Science Advances 2021, 7, eabf4398. [Google Scholar] [CrossRef]
- Go, G.-w.; Mani, A. Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. The Yale journal of biology and medicine 2012, 85, 19. [Google Scholar]
- Wang, Z.; Duan, X.; Lv, Y.; Zhao, Y. Low density lipoprotein receptor (LDLR)-targeted lipid nanoparticles for the delivery of sorafenib and Dihydroartemisinin in liver cancers. Life sciences 2019, 239, 117013. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Querbes, W.; De, S.; Qin, J.; Frank-Kamenetsky, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Cantley, W.L.; Dorkin, J.R. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Molecular Therapy 2010, 18, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
| No. | Molecules | Development stage | Clinical trial identification | Year of completion |
|---|---|---|---|---|
| 1 | Canagliflozin | N/A | NCT05422092 | 2023 |
| N/A | NCT05513729 | 2024 | ||
| 2 | Dapagliflozin | Phase 3 | NCT03723252 | 2022 |
| NCT05308160 | 2024 | |||
| Phase 4 | NCT05140694 | 2025 | ||
| 3 | Empagliflozin | Phase 2 | NCT03867487 | 2024 |
| NCT05140694 | 2025 | |||
| Phase 4 |
NCT03646292 | 2021 | ||
| NCT04642261 | 2022 | |||
| NCT04976283 | 2023 | |||
| NCT05140694 | 2025 | |||
| 4 | Ipragliflozin | Phase 4 | NCT02875821 | 2017 |
| No. | Molecules | Development stage | Clinical trial identification | Year of completion |
|---|---|---|---|---|
| 1 | Dulaglutide | N/A | NCT03590626 | 2020 |
| Phase 4 | NCT03648554 | 2024 | ||
| 2 | Liraglutide | Phase 4 | NCT02147925 | 2017 |
| NCT03068065 | 2017 | |||
| 3 | Semaglutide | Phase 1 | NCT03357380 | 2020 |
| Phase 2 | NCT03987074 | 2020 | ||
| NCT03987451 | 2021 | |||
| NCT02970942 | 2021 | |||
| NCT03884075 | 2023 | |||
| NCT04216589 | 2023 | |||
| NCT04971785 | 2024 | |||
| NCT05016882 | 2024 | |||
| Phase 3 | NCT04822181 | 2028 | ||
| Phase 4 | NCT04639414 | 2023 | ||
| NCT05195944 | 2024 |
| No. | Molecules | Target | Development stage | Clinical trial identification | Year of completion |
|---|---|---|---|---|---|
| 1 | Fenofibrate | PPAR-α | Phase 2 | NCT02354976 | 2016 |
| NCT02781584 | 2022 | ||||
| 2 | Pioglitazone | PPAR-γ | Phase 4 | NCT00227110 | 2006 |
| NCT00994682 | 2014 | ||||
| NCT02875821 | 2017 | ||||
| NCT03796975 | 2019 | ||||
| NCT04976283 | 2023 | ||||
| 3 | Rosiglitazone | PPAR-γ | Phase 2 | NCT02285205 | 2015 |
| Phase 4 | NCT01406704 | 2013 |
| No. | Molecules | Structure | Development stage | Clinical trial identification | Year of completion |
|---|---|---|---|---|---|
| 1 | Aldafermin (NGM282) | FGF19 analog | Phase 2 | NCT02443116 | 2020 |
| NCT03912532 | 2021 | ||||
| NCT04210245 | 2023 | ||||
| 2 | bFKB1 (BFKB8488A) | FGFR1c/β-klotho bispecific antibody | Phase 1 | NCT03060538 | 2019 |
| Phase 2 | NCT04171765 | 2023 | |||
| 3 | Efruxifermin (AR001) | Fc-FGF21 analog | Phase 2 | NCT03976401 | 2022 |
| NCT05039450 | 2024 | ||||
| NCT04767529 | 2024 | ||||
| 4 | MK-3655 (NGM313) | FGFR1c/β-klotho bispecific antibody | Phase 2 | NCT04583423 | 2024 |
| 5 | Pegbelfermin (BMS-986036) | PEG conjugated FGF21 analog | Phase 2 | NCT03486899 | 2021 |
| NCT03486912 | 2021 |
| No. | Molecules | Development stage | Clinical trial identification | Year of completion |
|---|---|---|---|---|
| 1 | Cilofexor (GS-9674) Combination with selonsertib and firsocostat | Phase 2 | NCT03449446 | 2019 |
| Cilofexor + selonsertib and firsocostat | NCT02781584 | 2020 | ||
| Cilofexor + semaglutide and firsocostat | NCT03987074 | 2020 | ||
| Cilofexor + semaglutide and firsocostat | NCT04971785 | 2024 | ||
| 2 | EDP-305 | Phase 2 | NCT03421431 | 2019 |
| NCT04378010 | 2022 | |||
| 3 | OCA, 6-α-ethyl-chenodeoxycholic acid (6-ECDCA, INT-747) | Phase 3 | NCT03439254 | 2022 |
| NCT02548351 | 2025 | |||
| 4 | MET409 | Phase 2 | NCT04702490 | 2022 |
| 5 | Nidufexor (LMB763) | Phase 2 | NCT02913105 | 2018 |
| 6 | Tropifexor (LJN452) + cenicriviroc | Phase 2 | NCT03517540 | 2020 |
| 7 | Tropifexor +licogliflozin | Phase 2 | NCT04065841 | 2024 |
| No. | Molecules | Target | Development stage | Clinical trial identification | Year of completion |
|---|---|---|---|---|---|
| 1 | Cotadutide (MEDI0382) | GLP-1R /GCGR |
Phase 1 | NCT03625778 | 2019 |
| NCT04208620 | 2020 | ||||
| NCT04091373 | 2020 | ||||
| Phase 2 | NCT03235050 | 2019 | |||
| NCT04019561 | 2021 | ||||
| NCT03555994 | 2021 | ||||
| 2 | CT-388 | GLP-1R /GIPR | Phase 1 | NCT04838405 | 2022 |
| 3 | CT-868 | GLP-1R /GIPR | Phase 2 | NCT05110846 | 2022 |
| Phase 1 | NCT04973111 | 2022 | |||
| 4 | DD01 | GLP-1R /GCGR |
Phase 1 | NCT04812262 | 2022 |
| 5 | Efinopegdutide (MK-6024) | GLP-1R/ GCGR | Phase 2 | NCT04944992 | 2022 |
| 6 | HM15211 | GLP-1R /GIPR/ GCGR |
Phase 1 | NCT03744182 | 2021 |
| Phase 2 | NCT04505436 | 2024 | |||
| 7 | Tirzepatide (LY3298176, TPZ) | GLP-1R/ GIPR |
N/A | NCT05078255 | 2023 |
| Phase 1 | NCT03951753 | 2021 | |||
| NCT04407234 | 2021 | ||||
| NCT04050553 | 2022 | ||||
| Phase 2 | NCT03131687 | 2018 | |||
| NCT03311724 | 2021 | ||||
| NCT04166773 | 2023 | ||||
| Phase 3 | NCT03954834 | 2020 | |||
| NCT03861039 | 2021 | ||||
| NCT03861052 | 2021 | ||||
| NCT05024032 | 2022 | ||||
| NCT04660643 | 2023 | ||||
| NCT04657016 | 2023 | ||||
| NCT04657003 | 2023 | ||||
| NCT04184622 | 2024 |
| No. | Molecules | Target | Development stage | Clinical trial identification | Year of completion |
|---|---|---|---|---|---|
| 1 | Elafibranor | PPAR α/δ agonist | Phase 2 |
NCT03883607 | 2020 |
| NCT03953456 | 2020 | ||||
| Phase 3 | NCT02704403 | 2020 | |||
| 2 | Lanifibranor | PPAR-α/γ/δ agonist | Phase 2 | NCT03008070 | 2020 |
| NCT03459079 | 2022 | ||||
| NCT05232071 | 2022 | ||||
| Phase 3 | NCT04849728 | 2028 | |||
| 3 | Saroglitazar | PPAR-α/γ agonist | Phase 2 | NCT03061721 | 2020 |
| NCT03863574 | 2020 | ||||
| NCT03617263 | 2023 | ||||
| NCT05011305 | 2023 | ||||
| NCT03639623 | 2022 | ||||
| NCT05211284 | 2025 | ||||
| Phase 3 | NCT02265276 | 2015 | |||
| NCT04193982 | 2021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





