1. Introduction—A New Paradigm of Perioperative Cancer Care
The last decade has brought about a revolution in cancer therapies that harness our immune system to target cancer-specific antigens. These novel agents increasingly play a role in neo-adjuvant therapy and have greatly improved quality of life for patients with advanced cancer. More than ever, it is necessary for the perioperative provider to be up to date in the physiology underlying these therapies, as well as their unique toxicity profiles.
Early generation chemotherapeutics relied on nonspecific destruction of cell-division, with toxicities mostly related to en-mass destruction of rapidly dividing cells. Newer immunotherapies have a more benign acute safety profile, but nonetheless can exert chronic toxicities that may be more insidious and require a higher index of suspicion for detection. The predominant cause of toxicities results from unwanted upregulated immune system activity, or molecular mimicry leading to destruction of “bystander” tissue.
To provide an example: Ipilumumab and nivolumab are two immune-checkpoint inhibitors (ICIs) that have recently been shown to improve survival when included as part of neoadjuvant therapy for squamous cell esophageal cancer [
1]. However, the two drugs in combination can lead to a non-negligible incidence of ICI pneumonitis and ICI hypophysitis. It is thus becoming more important for thoracic surgeons and anesthesiologists who care for these patients to be aware of the potential risks of adrenal insufficiency and reduced pulmonary capacity. A thorough therapeutic history and close collaboration with oncologists are necessary to provide safe care.
The purpose of this review is to give the perioperative provider physiologic insight into new targeted immunotherapies, with a focus on their implications for safe perioperative care.
2. Immune Checkpoint Inhibitors
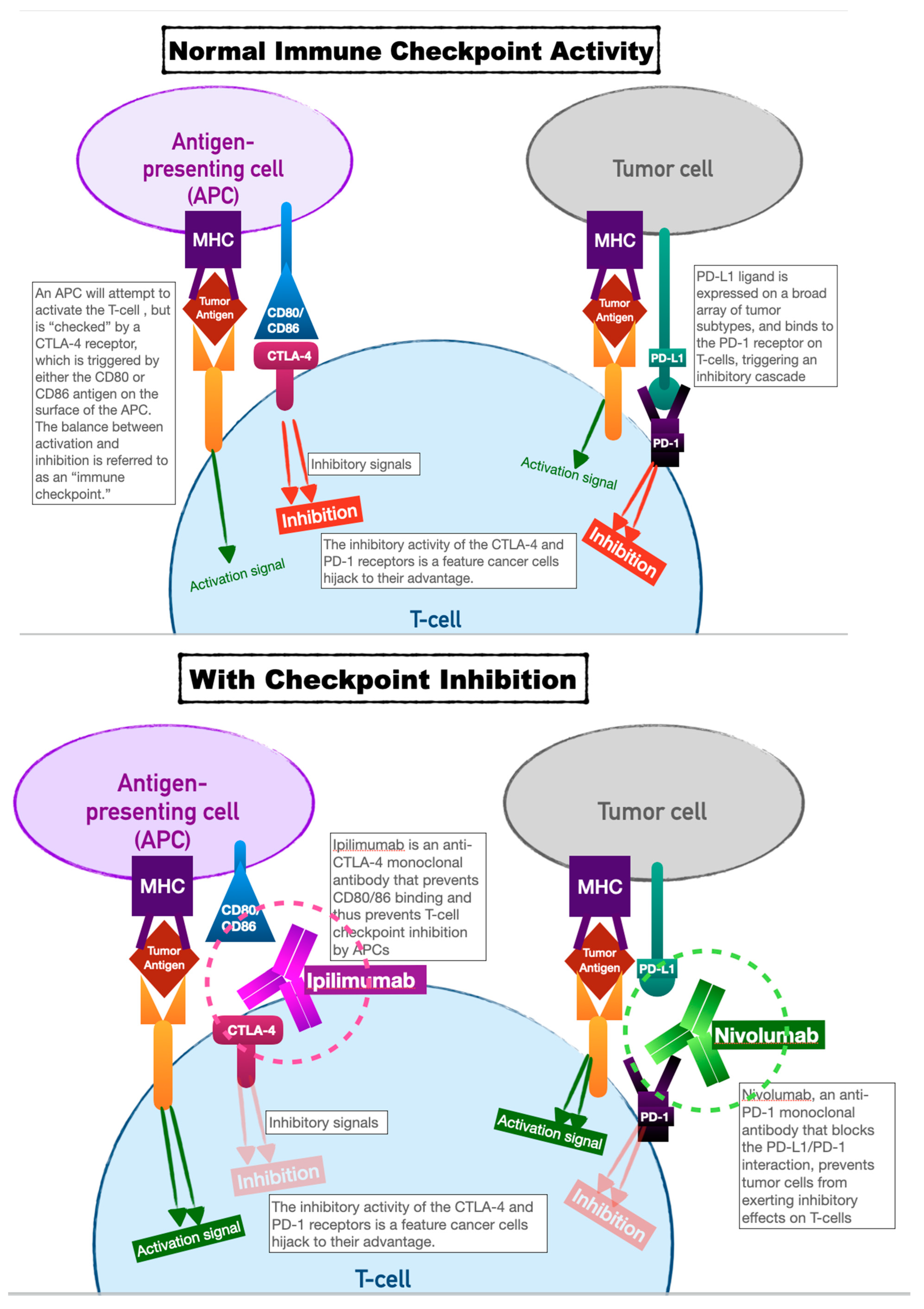
Immune checkpoint inhibitors have earned their keep as tremendously successful adjuncts to all manner of chemotherapeutic regimens. Our innate immune system relies on T cell activation and proliferation to destroy foreign cells. T cell activation requires both antigen-receptor coupling as well as co-stimulation by other immune effectors cells. This two-fold process underpins the versatility of our immune system but is also the process by which cancer cells can evade detection. T cell activation can be inhibited by two pathways: the cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) pathway, and the programmed cell death protein (PD-LA1) pathway. This interplay of T cell activation and inhibition is referred to as an “immune checkpoint.”
Cancer cells have evolved to thwart T cell activation by taking advantage of the inhibition role of this immune checkpoint, since CTLA-4 and PD-LA1 antigens are commonly found on the surfaces of tumor cells. Activation of these ligands by tumor cells allows them to evade detection.
Immune checkpoint inhibitors (ICIs), as a therapeutic target, prevent tumor cell evasion by acting as monoclonal antibodies against the CTLA-4 and PD-LA1 ligands. In preventing their ability to inactivate T cells, ICIs ensure that tumor cells are vulnerable to destruction (see
Figure 1).
This has proven to be a very effective therapeutic target, with successes in the treatment of melanoma, breast cancer, lymphoma, and prostate cancer.
Checkpoint inhibition technology has continued to advance, as new agents are developed targeting additional T cell pathways, and as existing ones are used on new tumor subtypes.
The first immune checkpoint inhibitors approved for human use were Ipilimumab and Nivolumab (2011 and 2014, respectively). Ipilimumab acts as a monoclonal antibody against CTLA-4; Nivolumab against PD-1. Ipilimumab was initially shown to dramatically improve survival in patients with metastatic melanoma [
2]. The subsequent arrival of Nivolumab showed promise of the two used as combination therapy, both for metastatic melanoma and several other cancers - renal cell carcinoma, unresectable non-small-cell lung cancer (NSCL), prostate cancer, and esophageal cancer [
3,
4].
Immune checkpoint inhibitors have shown promise in treating tumors that have been historically resistant to other chemo or immunotherapeutic agents, but there are highly specific indications for the use of each. Up to 45% of patients with cancer are candidates for ICI therapy [
5], so the perioperative footprint of these patients continues to rise. Their efficacy is determined by the rate of target PD-L1 or CTLA-4 receptor expression on the tumor cells, the mutational burden of tumors, and the relative inefficacy of classic chemotherapy agents for the tumor in question.
ICI therapy in the perioperative period is an area of ongoing study. Thus far, a few studies at major cancer centers have shown no association perioperative morbidity of any type [
6], and in fact, most show greatly improved results when added as neo-adjuvant therapy [
7,
8].
2.1. Specific Toxicities and Perioperative Implications
Toxicity from ICIs occurs via multiple mechanisms. The up-regulation of T-cell activity in general can lead to lymphocyte infiltration in normal parenchyma and cause nonspecific tissue damage. ICIs can lead to a loss of immunologic self-tolerance, wherein more T and B cells will be activated regardless of the presence of tumor cells. Molecular mimicry can occur, wherein ICI targets may appear on non-tumor cells, which are inadvertently triggered for destruction. Direct drug toxicity is less common, owing to their relatively inert antibody structure [
9].
2.1.1. Gastrointestinal
The increase in T-cell activation by ICIs is most clinically significant in sites that have a high number of T-cells: notably the lining of the GI tract, the airways, and skin. As such, the most prevalent condition is immune-checkpoint inhibitor induced enterocolitis, which can occur in up to 20% of patients and onset is typically within 2-3 months of therapy initiation. Mild cases of ICI enterocolitis (a few loose stools per day) are treated with low doses of oral corticosteroids. Endoscopy is typically obtained for patients with an increase in stool of over 4-6 more events over their baseline, to establish diagnosis and exclude other infectious or malabsorptive causes. These patients with moderate disease will be started on steroids and considered for biologic agents including infliximab. Repeat endoscopy is indicated after 2-3 months to gauge endoscopic response. For patients with mild symptoms, temporary cessation or low dose corticosteroids might suffice. For moderate to severe symptoms, ICI therapy is usually stopped permanently [
10]. Surgical intervention is extremely rare, and reserved for cases of severe, refractory colitis with megacolon or perforation [
11].
Perioperative implications of this condition track with disease severity. Patients on ICI should be screened for gastritis or other upper GI conditions that might affect anesthetic management - i.e.: rapid sequence induction. Patients with malabsorptive diarrhea warrant a comprehensive metabolic panel and screening for electrolyte analysis.
ICI hepatitis is a less common entity, but critical not to miss. It has an incidence of 5-10% [
12], usually occurring within 2-3 months of therapy. It will typically manifest as an occult increase in liver function testing (LFTs). The pattern of liver dysfunction typically follows a hepatocellular pattern (AST and ALT elevation) and less commonly a cholestatic pattern (ALK elevation and hyperbilirubinemia). The risk increases with dual-ICI therapy [
13]. CT imaging should be performed to rule out liver metastases, and abdominal ultrasound should be obtained to rule out biliary obstruction in patients with hyperbilirubinemia. Workup should include hepatitis viral panels, history of alcohol use, and presence of concomitant hepatotoxins.
ICI hepatitis is graded on a scale: Grades 1 and 2 are defined by AST and ALT levels of 2.5 to 5 times normal. These cases are typically treated with corticosteroids, which can be tapered over a 4–6-week period once LFTs return to normal. Very mild cases in patients with known liver metastases may warrant observation alone. Grade 3 and 4 ICI hepatitis are defined by a 5 to 20-fold elevation of LFTs and the presence of any clinical signs of liver dysfunction (asterixis, encephalopathy, ascites). In addition to drug cessation and a course of IV corticosteroids (typically methylprednisolone 1 mg/kg/day), these patients should undergo a liver biopsy to confirm the diagnosis or evaluate for other causes of liver dysfunction. For patients with steroid-refractory liver dysfunction, mycophenolate mofetil and tacrolimus can be considered, in coordination with a hepatologist experienced in treating the condition [
14]. Once treated and resolved, ICI therapy is only resumed for patients who achieved improvement from grade 2 toxicity or less.
To mitigate perioperative risk, patients requiring surgery on ICI therapy should be screened with recent LFTs. LFT elevation should prompt additional workup should including hepatitis viral panels, history of alcohol use, CT scan of the liver, and evaluation for concomitant hepatotoxic agents. Perioperative studies on patients with known ICI hepatitis are limited. It is recommended to postpone elective surgery on patients with hepatitis of any cause. Patients with ICI hepatitis should be treated as patients with liver dysfunction of any other cause. The Child-Pugh classification of liver dysfunction has been validated as a classification system of patients with cirrhosis to distinguish surgical mortality, with an increased score assigned to patients with signs of decompensated hepatic failure such as coagulopathy, ascites, and encephalopathy [
15].
A less common effect of ICI therapy is pancreatic dysfunction. Approximately 4% of patients on ICIs will have an asymptomatically elevated lipase. The incidence of patients with symptomatic pancreatitis is <1% [
16,
17]. Symptoms of ICI pancreatitis are similar to those of all-cause pancreatitis, including epigastric pain, fever, and nausea. A CT scan should be obtained to rule out other causes of pancreatitis and biliary obstruction. Patients with asymptomatic lipase elevation are not recommended to receive any specific treatment other than monitoring. Symptomatic pancreatitis should be treated with usual care: fluid resuscitation, NPO status, and analgesia. It is notable that studies are extremely limited for ICI pancreatitis, including the lack of a standardized grading system. Most centers use steroids and ICI cessation for patients with severe symptoms requiring hospitalization [
18,
19]. As with usual pancreatitis, antibiotics are not routinely recommended in the absence of abscess or presence of another infectious source [
20]. There is no established role for surgery in ICI pancreatitis, which in general is a last resort intervention for complicated pancreatitis of all causes.
New-onset type 1 diabetes is a rare result of acute autoimmune pancreatic exocrine dysfunction, with an incidence of <1% [
21]. The most common presentation of this adverse effect is new-onset DKA. Destruction of islet cells renders patients permanently insulin-dependent in most cases, an outcome that is not treatable with steroids [
22].
2.1.2. Pulmonary
Pulmonary toxicity from ICIs is referred to as ICI pneumonitis. The overall incidence is approximately 5% [
23,
24]. Cough and dyspnea are the typical initial symptoms, but the clinical syndrome falls on a wide spectrum of respiratory disease: acute, chronic, waxing/waning, permanently disabling.
The acute form of ICI pulmonary toxicity follows a pattern of acute hypersensitivity pneumonitis and can progress to full-blown acute respiratory distress syndrome (ARDS). Chronic ICI pneumonitis occurs within 3 months to one year after initiation of therapy [
25]. In both conditions, alternative causes of respiratory symptoms should be ruled out with a history of exposures, toxins, smoking history, and CT scans with contrast should be obtained. Radiologic patterns of chronic pulmonary toxicity typically reveal ground glass opacities and peripheral multifocal consolidations, although there is no pathognomonic finding for ICI pneumonitis. Bronchoscopy is only recommended for cases that do not respond to initial treatment, or for whom alternative diagnoses are being ruled out - i.e.: organizing infection, progression of malignancy, etc. Bronchoscopy specimens, if obtained, will show alveolar lymphocytic infiltration. Pathology specimens in ICI pneumonitis show a pattern of bronchiolitis obliterans and organizing pneumonia, with a lymphocytic predominance [
26]. Smoking and prior radiation therapy increase the likelihood of ICI pneumonitis [
27].
ICI pneumonitis is graded on a scale of grade 1 to 5 [
28]. Patients with no symptoms and only mild radiographic findings are considered grade 1, and simply warrant observation with continuation of treatment. Patients with grade 2-5 (symptoms ranging from dyspnea/cough to fulminant respiratory failure) should be treated with glucocorticoids and cessation of treatment. Typical doses of prednisone range from 0.5-1mg/kg daily, and the typical course is 2-4 weeks. Addition of anti-IL-6 agents has shown some promising results in a subset of patients who are refractory to steroids. The prognosis of ICI pneumonitis varies, as the pattern of disease is heterogenous. Patients with grade 1-3 disease do well with early recognition and steroid treatment. Patients with grade 4 or above, or who have recurrent or treatment-refractory disease, have a significantly higher burden of inpatient care, oxygen dependence, and overall mortality. It is worth noting that radiographic findings can persist long after symptom resolution [
29].
Perioperative management of patients with ICI pneumonitis varies depending on the context in which it arises. For patients on ICI therapy as neo-adjuvant to surgery, such as in non-small cell lung cancer [
30], the benefits of therapy have been shown to outweigh the risks. However, it remains essential to screen patients for pneumonitis symptomatology, which will inform operative risk. A history of dyspnea or cough in any patient on ICIs should warrant a chest x ray, and their surgical risk should be stratified based on standard-of-care for patients with all-cause pulmonary disease. Recommendations for preoperative lung function testing are continually evolving, with recommendations including spirometry for all patients undergoing lung resection, and for patients with a history of severe obstructive pulmonary disease. Patients with forced expiratory volume in one second (FEV1) and diffusion capacity of the lungs for carbon monoxide (DLCO) that are both >80% of predicted are considered fit for surgery, while those with decreased scores may benefit from further workup. Stair climbing, ambulatory stress tests (such as walking 400m on level ground with dyspnea and pulse oximetry monitoring) have been evaluated, and there is increasing evidence supporting the use of cardio-pulmonary exercise testing for further risk stratification [
31]. While further studies on outcomes specific to ICI pneumonitis are lacking, it is prudent to follow these existing guidelines.
2.1.3. Cardiac
ICI myocarditis is rare, with an incidence of less than 0.1% [
32]. While low, the mortality is significantly higher than other ICI toxicities, and in cancer centers with a high relative volume of immunotherapy patients, its prevalence is not to be ignored. Toxicity mechanisms are unclear, but likely result from lymphocytic infiltration of myocardial tissue in the setting of upregulated T-cell activity [
33,
34]. Pembrolizumab is the most common causative agent; the incidence is higher when combined with nivolumab. The average time to onset of cardiac symptoms is 1-2 months.
Cardiac toxicity can present as a conduction system disorder (ranging from prolonged PR interval to complete heart block), pericarditis, myocarditis, or cardiomyopathy with reduced systolic function. A useful triage of myocarditis in the presence of cancer therapeutics was developed by Circulation in 2019 [
35], grouping patients into three categories: definite myocarditis, probable myocarditis, and possible myocarditis. A positive cardiac MRI plus one of either 1) an elevation in biomarkers, 2) an ECG pattern showing diffuse ST elevations, or 3) the clinical syndrome of myocarditis are required for the diagnosis.
Mild cases of ICI myocarditis are treated with oral prednisone. More severe cases may require intravenous methylprednisolone in high doses, which mimics the treatment strategy of viral myocarditis. A taper regimen will generally last 4-6 weeks. Treatment with intravenous immunoglobulins, mycophenolate, infliximab, and plasmapheresis have been considered for severe cases, and remain under investigation [
36].
In addition to steroids, it is recommended to follow the standard American College of Cardiology/American Heart Association guidelines for the treatment of heart failure: beta-blockade (weighed against the risk of exacerbating a concurrent conduction system abnormality), ACE-inhibition, lifestyle modifications, and avoidance of volume overload.
Perioperative considerations for ICI myocarditis include a thorough cardiac history and a recent EKG to evaluate for conduction system abnormalities, particularly with a history of pembrolizumab exposure. There have been several case reports of complete heart block noted on a baseline preoperative EKG among patients on checkpoint inhibitors planned for cancer resection surgery [
37]. Patients with dyspnea on exertion, chest pain, or unexplained hemodynamic compromise should be evaluated with an EKG, cardiac biomarkers, and an echocardiogram. Suspicions of myocarditis should prompt evaluation with a cardiologist, and consideration for a cardiac MRI.
2.1.4. Endocrine
Pituitary dysfunction is a well-established category of ICI toxicity. The incidence of hypophysitis is quite significant (10-15%), with the highest incidence found in patients on dual-ICI therapy with ipilimumab and nivolumab. The most common biochemical disturbances are ACTH deficiency, hypogonadism, and hypothyroidism. For patients not detected biochemically, common presenting symptoms are headache and fatigue, but anorexia, nausea, dizziness, and visual changes have also been described. Pituitary dysfunction typically present 2-3 months after therapy initiation, but onset can be as late as one year into therapy. In contrast to other adverse ICI effects, hypopituitarism is often permanent.
Patients on ICI therapy have quarterly thyroid function studies as part of maintenance therapy. Clinical signs of hypothyroidism include headache and fatigue, and laboratory findings include low-normal free T4 and inappropriately low or normal TSH.
A substantial portion of patients on ICI therapy have reduced ACTH and cortisol. Retrospective studies of ICI therapy cohorts have reported a wide range of incidences of low hormone values, ranging from one quarter to one half of patients. Most are associated with CTLA-4 inhibitors, but PD-1 inhibitors will also cause isolated ACTH deficiency [
38,
39].
Low serum cortisol resulting from central adrenocortical suppression can result in hypoaldosteronism with hyponatremia and hyperkalemia. It should be noted that hyponatremia is an extremely common adverse event in patients on any chemotherapeutics, including as a result of cancer related SIADH [
40].
Hypogonadism on ICI therapy has an incidence of 10-15% and presents with decreased libido, hot flashes, or infertility. Laboratory studies will reveal low testosterone and inappropriately normal luteinizing hormone (LH) [
41].
MRI findings in patients with ICI hypophysitis demonstrate pituitary enlargement in slightly more than half of cases, however MRI findings do not necessarily correlate with clinical symptom burden [
42,
43], and patients with significantly reduced ACTH or cortisol may have normal MRI findings. Pathology specimens for patients with this condition indicate lymphocytic infiltration and increased CTLA-4 antigen expression on pituitary endocrine cells (i.e.: molecular mimicry) [
44] as culprit pathology.
Hyperthyroidism has a lower incidence (3-8% [
45]) and can result both from a centrally mediated increase in TRH from hypophysitis, or a transient, local thyroiditis from molecular mimicry. Patients with locally mediated ICI thyroiditis will most commonly present with laboratory abnormalities (low TSH, elevated T3, T4), or present with symptoms of hyperthyroidism. Thyroiditis, after it subsides, will most often progress to hypothyroidism.
The perioperative provider should have a high index of suspicion for adrenal insufficiency. There are no studies thus far on the incidence of intraoperative hemodynamic instability related to ICI therapy, but anecdotal evidence exists for patients on ICI therapy to require higher doses of intraoperative vasopressors. Patients on ICI therapy should have recent thyroid-function testing and should be screened for headache and fatigue. If positive, it should be worth considering an underlying central adrenal insufficiency, and to treat empirically should hemodynamic instability occur. Hydration status should be optimized, and electrolytes should be checked pre and postoperatively for all patients on ICI therapy. Adrenally insufficient patients are more likely to decompensate in the setting of systemic infection, and benefit from early aggressive resuscitative strategy, as they have limited ability to compensate for physiologic stress.
| Immune Checkpoint Inhibitor Toxicity Category |
Manifestations |
Treatment |
Perioperative Considerations |
| Central Hypothyroidism |
Fatigue, headache, low T4, low/normal TSH |
Thyroid hormone replacement |
Thyroid function tests within 3 months for all patients on ICI therapy |
| Hypogonadism |
Hot flashes, decreased libido, decreased FSH and LH |
Hormone replacement therapy |
Screening for other signs of pituitary dysfunction |
| Pulmonary |
Hypersensitivity pneumonitis, pulmonary fibrosis, organizing pneumonia, ARDS |
Corticosteroids, supportive care. |
Screening for respiratory symptoms, baseline CXR, pulmonary risk-stratification with baseline spirometry and cardio-pulmonary exercise testing. |
| Enterocolitis |
Diarrhea, gastritis, hepatitis (manifests as occult rise in AST/ALT over first few months of therapy) |
Low dose corticosteroids |
Screening for reflux, gastritis symptoms. Recent LFTs if therapy was started within the last few months. |
| Pancreatic dysfunction |
New-onset type 1 diabetes, ICI pancreatitis |
Corticosteroids, resuscitative fluids; insulin therapy |
Amylase and lipase levels for patients reporting abdominal pain, ruling out other causes of abdominal pain or pancreatitis; screening with preoperative blood glucose |
| Cardiac |
Myocarditis, pericarditis, conduction system disorders. |
Corticosteroids, standard AHA/ACC heart failure guidelines |
Screening for symptoms of heart failure, and baseline EKGs for patients on ICI therapy, cardiac biomarkers and cardiac MRI for patients with symptoms of myocarditis |
3. Chimeric Antigen-receptor T Cells (CAR-T)
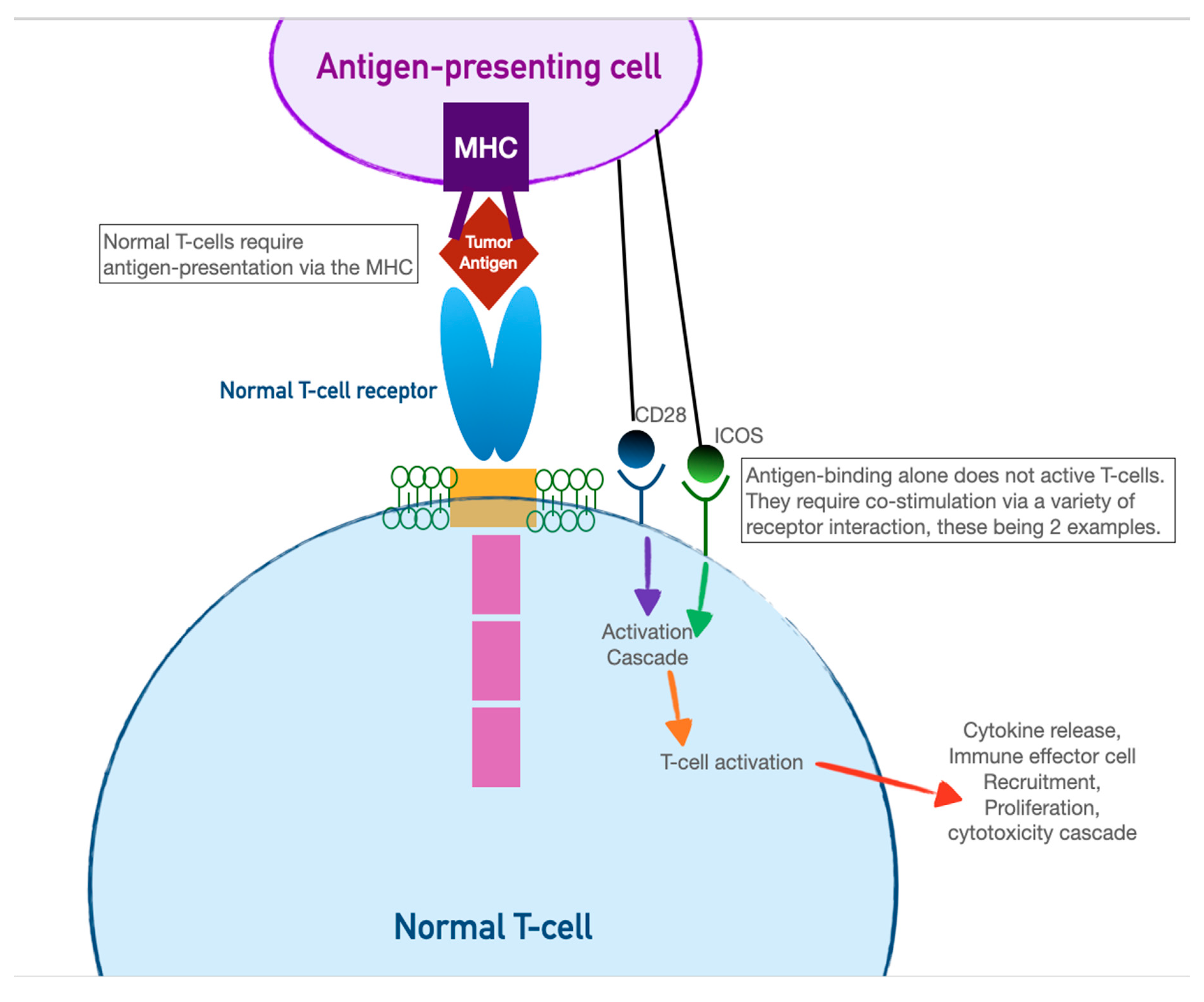
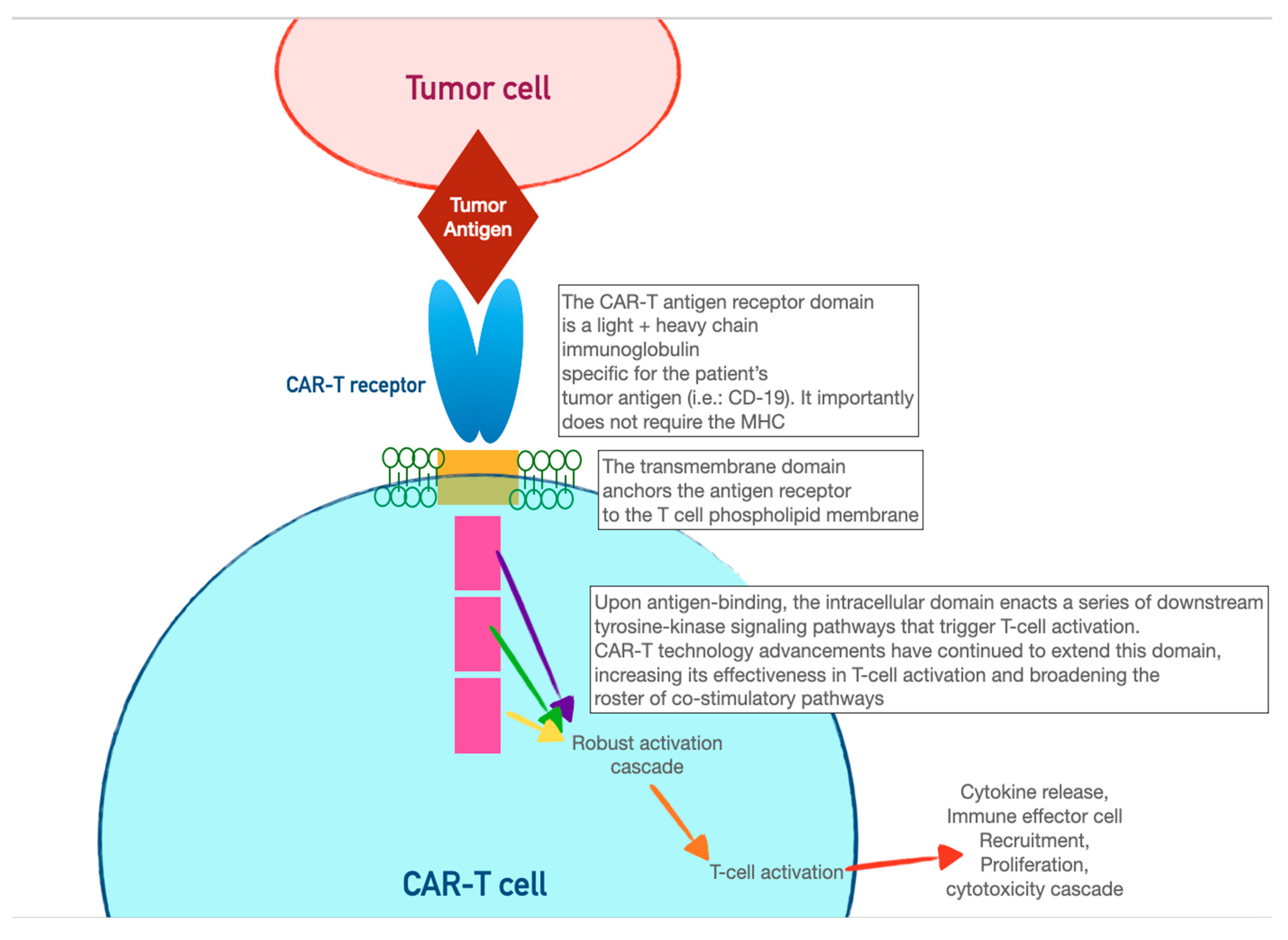
CAR-T cells harness the adaptive immunity offered by our T-lymphocytes to target tumor-specific antigens. As the first foray into cellular therapeutics, they represent one of the most consequential discoveries in cancer therapy in the last two decades, owing to their relative ease of administration and robust efficacy against highly fatal hematologic malignancies. As discussed in the previous section, the immune system relies on T-cell activation via 1) antigen presentation via an MHC complex and 2) costimulation via other immune effector cells. Cancer cells have historically evaded this process of T-cell activation by thwarting antigen recognition. Enter CAR-T cells: genetically modified host T-cells that force this process to occur without the ability of tumor-cell evasion.
First, T-cells are extracted from the patient (or an allogeneic donor) and modified via a retroviral vector to express a new antigen receptor and co-stimulatory domains (see
Figure 2). The term “chimeric” refers to the fact that this engineered receptor both
activates and
co-stimulates the T-cell [
46]. The patient is then leukoreduced, to increase the relative proportion of CAR-T cells, and CAR-T cells are infused into the patient. The CAR-T cells will recognize antigens, activate, and recruit immune effector cells to destroy tumor cells.
The first CAR-T cells used clinically targeted the CD19 receptor, making them effective for the treatment of ALL or diffuse large B-cell lymphoma [
47]. Trials have consistently shown a 50-90% remission rate for relapsed or refractory disease, which continues to improve with refinement of technique [
48]. CAR-T for solid tumors is a growing area of research, where re-modeled CAR-T cells can be introduced intra-tumor or intra-pleural [
49].
Once infused, CAR-T cell activity can be controlled via embedded biochemical switches. An “on” switch, or conversely, a “suicide gene” can induce CAR-T cell activation or apoptosis. These external controls allow CAR-T technology to be more pliable in the face of toxicities or other adverse events.
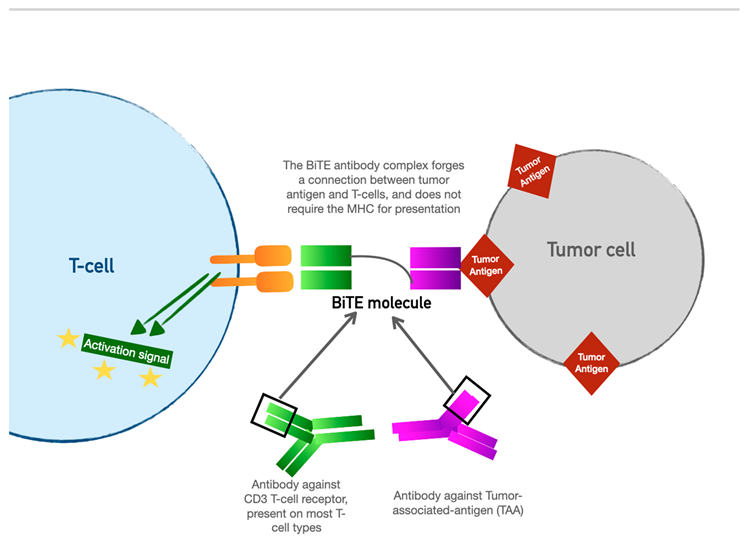
4. Bispecific T-cell Engager (BiTE) Therapy
BiTE therapy is one of the newest innovations in targeted immunomodulatory chemotherapy. BiTE molecules are an antibody with two domains (hence “bispecific”): one which recognizes tumor-specific antigens, and another which recognizes the universal CD-3 receptor on T cells. The binding sites are essentially two monoclonal antibodies bound together. In essence, the BiTE “forces” a recognition and activation of the T cell, so the tumor cell does not have a chance to present its own antigen and possibly release inhibitory signal receptors. Specific tumor antigen targets of BiTE molecules include CD19 (broadly expressed on B cell malignancies), B cell maturation antigen (expressed highly on malignant cells involved in multiple myeloma), CD33 (expressed in acute myeloid leukemia, myelodysplastic syndrome, and chronic myeloid leukemia). Under development are BiTE targets that include solid tumor antigens such as prostate-specific member antigen and delta-like protein 3 which is highly prevalent in small-cell lung cancer.
The most widely used BiTE agent at this time is Blinatumomab, approved for use in acute lymphoblastic leukemia (ALL). Trial response rates in children and adults with ALL have shown a resounding 90% complete remission rate, with a 6-month event-free survival of 67%, and a 78% overall survival rate [
50]. It is now approved for use around the globe. Solitumab is a BiTE molecule with a binding domain for epithelial cell adhesion molecule (EpCAM), which is being investigated for therapy against gastrointestinal, lung, and other solid tumors [
51].
These molecules are administered as continuous IV infusion over several days, owing to their 2–4-hour half-life. Not unsurprisingly, owing to their similar mechanisms, they have an adverse event profile that parallels that of CAR-T cells. In the original study for Blinatumomab, adverse events most common were neutropenia, infection, elevated LFTs, neurotoxicity, and CRS [
52]. CRS, neurotoxicity, and acute anaphylaxis are the most significant for the acute care and perioperative provider. There is perhaps a lower propensity than CAR-T for high-grade neurotoxicity [
53]. Treatment for these adverse events follows the same protocol - corticosteroids, IL-6 inhibition, and supportive care.
5. Other Cellular Therapies
Several novel cellular therapies are under investigation: natural killer (NK) CAR-T cells, engineered T cell receptors, and tumor-infiltrated lymphocytes. The latter involves harvesting lymphocytes that have already successfully infiltrated tumor cells (isolated via biopsy or surgical resection), and proliferating and re-infusing them. The increasing armamentarium of cellular therapy agents warrants an understanding of their mechanisms and toxicities so the perioperative provider can provide safe and informed care.
6. Toxicity of Cellular Therapy and Perioperative Considerations
CAR-T recipients are monitored as inpatients for acute toxicity. Immediate post-infusion reactions include anaphylaxis and anaphylactoid reactions including transient vasodilation. The most clinically significant toxicities are sub-acute and include cytokine release syndrome (CRS) and neurotoxicity. Chief considerations for these conditions are summarized in Table 1.
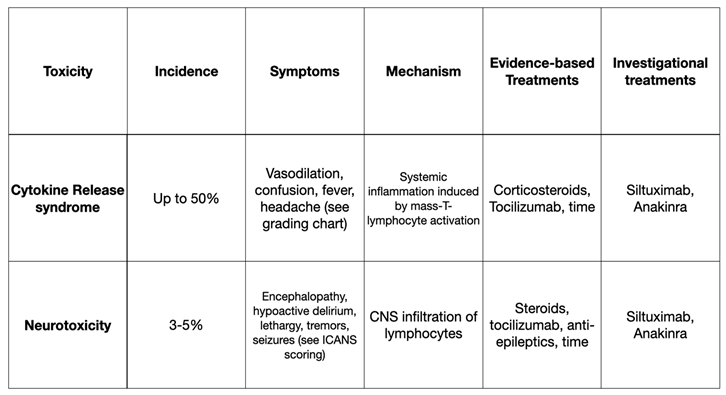
6.1. Cytokine Release Syndrome (CRS)
Around half of patients who have received CAR-T cells will develop some form of CRS [
54]. This systemic inflammatory response results from en-masse activation of lymphocytes, and CAR-T induced cytokine release. Interleukin-6 (IL-6) is highly associated with CRS severity and can be useful when attempting to differentiate between CAR-T related CRS and CRS related to sepsis. CRS can occur within hours, but the peak onset-time is 4-7 days post-infusion.
CRS is grading occurs on a scale of 1-5. Grade 1 is defined as mild systemic symptoms such as low-grade fever, headache, and myalgias. Grade 2 is defined by the requirement of oxygen or pressor support. Grade 3 is multi-organ dysfunction requiring high-dose or multiple pressors. Grade 4 requires the presence of mechanical ventilation and advanced multi-organ dysfunction. Treatment for CRS is mostly supportive, and it is notable that with just time alone, patients will make a significant recovery.
Patients with CRS in the perioperative period require close collaboration with the oncology services. Nearly all patients with CRS will receive glucocorticoids to blunt the overactive immune response. Grade 3 and 4 CRS should be treated with tocilizumab. Originally approved as a disease modifying agent for rheumatoid arthritis, tocilizumab is a monoclonal antibody which prevents IL-6 from binding to its receptor [
55]. It is administered on a three-times daily dosing schedule and is commonly combined with corticosteroids. Siltuximab is an anti-IL 6 monoclonal antibody that has shown safety and equivalence to Tocilizumab [
56]. Siltuximab should be noted as a strong CYP-450 inducer. Anakinra is an IL-1 antagonist which has been explored as an off-label agent to mitigate CRS and CAR-T neurotoxicity [
57,
58]. It can also be used to treat hemophagocytic lymphohistiocytosis (HLH), a severe complication of CAR-T therapy in which lymphocyte over-activation leads to destruction of all cell lines, liver dysfunction, coagulopathy, fever, and rash [
59].
It is important to note that none of the CRS-targeted therapies discussed here will significantly impact the efficacy of CAR-T cell therapy.
6.2. Neurotoxicity
3-5% of patients who receive CAR-T cells will develop neurotoxicity [
60]. Commonly referred to as immune effector cell-associated neurotoxicity syndrome (ICANS), the etiology of this condition is multifactorial and not yet fully understood. A likely early contributor is the breakdown of cerebral endothelial integrity by increased circulating cytokines, like the pathophysiology underlying septic encephalopathy [
61,
62]. The typical timeframe for onset of ICANS is 5 days post-infusion, but can be delayed by up to 2 weeks. Neurologic symptoms that are specific to early ICANS are outlined in Tables 2 and 3, and include word-finding difficulty, tremors, hypoactive delirium, lethargy, and impaired handwriting. Moderate ICANS can progress to hyperactive delirium, myoclonus, obtundation, and ataxia. Severe ICANS can result in neurologic catastrophe including seizures, coma, and death. The median duration of this syndrome is 4 days. Mortality is related to the degree of their resultant critical illness - ARDS, malnutrition, degree of shock, etc.
Neuroimaging and video-electroencephalography (vEEG) obtained from ICANS patients will demonstrate nonspecific patterns of cerebral edema, and generalized slowing, respectively. Lumbar puncture will reveal elevated lymphocytes in CSF, as well as the presence of CAR-T cells.
Time and supportive care are the mainstay of treatment. ICANS is less responsive to the typical IL-6-targeted therapies as compared to CRS. Corticosteroids are beneficial, but come with significant iatrogenic harm - immunosuppression, gastrointestinal bleeding, and increased delirium. There is some evidence showing seizure prophylaxis with levetiracetam to be useful for anyone with grade 2 or higher ICANS.
CRS and ICANS mortality are more closely linked to the degree of organ dysfunction and iatrogenic exposure (prolonged ICU stay, mechanical ventilation, pressor use, prolonged steroid use and immunosuppression, etc.) than the degree of the toxicity per se. With supportive care, a relatively high proportion of patients experience complete recovery [
63,
64]. The overall mortality of CAR-T cells remains low, at 5.4% [
65].
6.3. Perioperative Considerations
The increasing efficacy and safety profile of cellular therapy has placed them at the forefront of cancer therapeutics at a rapidly expanding array of academic and non-academic centers. At this time, around 150 centers across the country offer CAR-T therapy, and the technology has spread across the globe to Canada, China, Australia, Singapore, Israel, and Europe. Evidence for specific perioperative treatment is lacking, as are studies on outcomes for emergent surgery done in the immediate post-CAR-T period.
It is crucial to involve the critical care team for patients with high-grade CRS or who are at risk for developing high-grade CRS, to enable high-quality supportive care for respiratory and hemodynamic compromise.
Perioperative care of the CAR-T cell patient has several important implications. Centers that offer CAR-T therapy are always equipped with services that are familiar with post-infusion monitoring, and downstream complications such as CRS and neurotoxicity. It is essential to involve a multi-disciplinary team of oncologists, anesthesiologists, and critical care physicians in perioperative decision making. For patients with uneventful CAR-T therapy who have had no signs of CRS, neurotoxicity, or infectious complications: usual care should be pursued for urgent or emergent surgery. Elective surgery should ideally be deferred until hematologic dyscrasias have normalized, typically by 3-4 weeks. A discussion with the oncology team about intraoperative steroid dosing should be considered. For patients within a week of CAR-T infusion who need urgent surgery, there should be a low threshold for pre-emptive postoperative ICU admission so that acute changes in hemodynamics and neurologic status will not be missed. Coagulation profiles should be obtained prior to neuraxial anesthesia, and if no thrombocytopenia or elevated INR is present, there is no contraindication to regional anesthesia.
The physiologic milieu of CRS will render patients sensitive to the vasodilatory effects of anesthetics. They should be treated as patients with severe sepsis, with slow dosing of induction agents along with supplemental vasopressor support. Widespread endothelial dysfunction will lead to increased capillary leak, which portends to intravascular depletion and increased third spacing. Fevers and prolonged NPO status (in the setting of CRS or neurotoxicity) will exacerbate this. Insensible losses will be higher, so fluid-sparing strategies should be used with caution. As with any patient experiencing compromised end-organ perfusion, attention should be paid to not further worsen tissue oxygenation. Hypoxemia and hypercarbia should be avoided. Sedation without a protected airway should be administered cautiously in the presence of metabolic acidosis, and there should be a low threshold to provide an endotracheal airway. The presence of severe acute kidney injury (AKI) is uncommon in CRS, and most patients recover to their baseline renal function [
66]. In such cases, dosing of paralytic and analgesics should be done with attention to GFR.
Neurotoxic patients should be treated as at-risk for having elevations in intracranial pressure. Caution should be taken to avoid exacerbations of ICP (hypercarbia, hypoxemia, trendelenburg position), normo-glycemia, normothermia, normal sodium homeostasis. Anticonvulsants should be continued. The ICANS scoring system should be used pre and immediately postoperative, so post-procedure neurologic changes can be evaluated [
67].
7. Conclusion
Targeted cancer therapy is undergoing a seismic shift from one that exerts an imprecise attack on cell division to one that targets specific tumor antigens, engaging our innate immune system to assist in the process. With these more precise and efficient therapies comes an increasingly complex toxicity profile. Toxicities can occur chronically, and masquerade beneath other neoplastic and paraneoplastic conditions. These agents increasingly play a role in multimodal cancer care, which is significantly prolonging life expectancy and quality of life for patients with cancer. As the depth and breadth of cancer procedures increases, and as the footprint of these therapies increases, the perioperative provider plays a more central role in managing cancer therapies and drug toxicities. The above review serves to update the perioperative clinician on guidelines for screening, diagnosing, and treating toxicities associated with novel cellular therapies and immune-checkpoint therapies.
References
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.-H.; Adenis, A.; et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. New Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723, Erratum in: N. Engl. J. Med. 2010, 363, 1290. [Google Scholar] [CrossRef] [PubMed]
- Opdivo- nivolumab injection". DailyMed. 17 December 2019. Retrieved 11 March 2020.
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.; Gill, J.; Prasad, V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for Immune Checkpoint Inhibitor Drugs. JAMA Netw. Open 2020, 3, e200423. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.W.; Kasi, P.M.; Stauffer, J.A.; Thiel, D.D.; Colibaseanu, D.T.; Mody, K.; Joseph, R.W.; Bagaria, S.P. The Feasibility and Safety of Surgery in Patients Receiving Immune Checkpoint Inhibitors: A Retrospective Study. Front. Oncol. 2017, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M.; et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chen, Y.; Wright, G.P.; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.-G.; et al. Neoadjuvant–Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. New Engl. J. Med. 2023, 388, 813–823. [Google Scholar] [CrossRef]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2022, 184, 5309–5337, Erratum in: Cell 2022, 185, 576. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2022, 39, 4073–4126, Erratum in: J. Clin. Oncol. 2022, 40, 315. [Google Scholar] [CrossRef]
- Tunio, N.A.; Desai, A.; Dalal, S.; Kurin, M.; Waghray, N. S218 Prevalence and Outcomes of Immune Checkpoint Inhibitor (ICI) Associated Colitis: A Population-Based Cohort Study. Am. J. Gastroenterol. 2022, 117, e157. [Google Scholar] [CrossRef]
- Weber, J.S.; Kähler, K.C.; Hauschild, A. Management of Immune-Related Adverse Events and Kinetics of Response With Ipilimumab. J. Clin. Oncol. 2012, 30, 2691–2697. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2018, 377, 1345–1356, Erratum in: N. Engl. J. Med. 2018, 379, 2185. [Google Scholar] [CrossRef] [PubMed]
- Omori, G.; Takada, K.; Murase, K.; Hayasaka, N.; Nakamura, H.; Iyama, S.; Ohnuma, H.; Miyanishi, K.; Fukuta, F.; Tanaka, T.; et al. Successful mycophenolate mofetil treatment of a patient with severe steroid-refractory hepatitis evoked by nivolumab plus ipilimumab treatment for relapsed bladder cancer. Clin. Case Rep. 2020, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, N.; Fricker, Z.; Hubbard, R.A.; Ioannou, G.N.; Lewis, J.D.; Taddei, T.H.; Rothstein, K.D.; Serper, M.; Goldberg, D.S.; Kaplan, D.E. Risk Prediction Models for Post-Operative Mortality in Patients With Cirrhosis. Hepatology 2020, 73, 204–218. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Bajaj, D.; Sankaramangalam, K.; Yoo, J.W.; Joshi, N.S.; Gettinger, S.; Price, C.; Farrell, J.J. Incidence of pancreatitis with the use of immune checkpoint inhibitors (ICI) in advanced cancers: A systematic review and meta-analysis. Pancreatology 2019, 19, 587–594. [Google Scholar] [CrossRef]
- George, J.; Yoo, J.W.; Joshi, N.; Farrell, J.J. Mo1236 - Incidence of Acute Pancreatitis with the use of Immune Checkpoint Inhibitors (ICI) in Solid Tumors: A Systematic Review and Meta-Analysis. Gastroenterology 2018, 154. [Google Scholar] [CrossRef]
- Ikeuchi, K.; Okuma, Y.; Tabata, T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: A case report. Lung Cancer 2016, 99, 148–150. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Management of immunotherapy-related toxicities. V1.2020. 2020.
- Waller, A.; Long, B.; Koyfman, A.; Gottlieb, M. Acute Pancreatitis: Updates for Emergency Clinicians. J. Emerg. Med. 2018, 55, 769–779. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef]
- Clotman, K.; Janssens, K.; Specenier, P.; Weets, I.; Block, C.E.M.D. Programmed Cell Death-1 Inhibitor–Induced Type 1 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2018, 103, 3144–3154. [Google Scholar] [CrossRef]
- Banavasi, H.; Kim, S.; Alkassis, S.; Daoud, A.; Laktineh, A.; Nagasaka, M.; Sukari, A.; Soubani, A.O. Immune checkpoint inhibitor-induced pneumonitis: Incidence, clinical characteristics, and outcomes. Hematol. Stem Cell Ther. 2021, 16, 144–150. [Google Scholar] [CrossRef]
- Poulose, V. Incidence of Immune Checkpoint Inhibitor-Related Pneumonitis in Lung Cancer. Chest 2022, 161, e196–e197. [Google Scholar] [CrossRef]
- Naidoo, J.; Wang, X.; Woo, K.M.; Iyriboz, T.; Halpenny, D.; Cunningham, J.; Chaft, J.E.; Segal, N.H.; Callahan, M.K.; Lesokhin, A.M.; et al. Pneumonitis in Patients Treated With Anti–Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2017, 35, 709–717. [Google Scholar] [CrossRef]
- Naidoo, J.; Cottrell, T.R.; Lipson, E.J.; Forde, P.M.; Illei, P.B.; Yarmus, L.B.; Voong, K.R.; Feller-Kopman, D.; Lee, H.; Riemer, J.; et al. Chronic immune checkpoint inhibitor pneumonitis. J. Immunother. Cancer 2020, 8, e000840. [Google Scholar] [CrossRef]
- Barrón, F.; Sánchez, R.; Arroyo-Hernández, M.; Blanco, C.; Zatarain-Barrón, Z.L.; Catalán, R.; Ramos-Ramírez, M.; Cardona, A.F.; Flores-Estrada, D.; Arrieta, O. Risk of Developing Checkpoint Immune Pneumonitis and Its Effect on Overall Survival in Non-small Cell Lung Cancer Patients Previously Treated With Radiotherapy. Front. Oncol. 2020, 10, 570233. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2022, 39, 4073–4126. [Google Scholar] [CrossRef]
- Tiu, B.C.; Zubiri, L.; Iheke, J.; Pahalyants, V.; Theodosakis, N.; Ugwu-Dike, P.; Seo, J.; Tang, K.; E Sise, M.; Sullivan, R.; et al. Real-world incidence and impact of pneumonitis in patients with lung cancer treated with immune checkpoint inhibitors: a multi-institutional cohort study. J. Immunother. Cancer 2022, 10, e004670. [Google Scholar] [CrossRef]
- Qiu, B.; Cai, K.; Chen, C.; Chen, J.; Chen, K.-N.; Chen, Q.-X.; Cheng, C.; Dai, T.-Y.; Fan, J.; Fan, Z.; et al. Expert consensus on perioperative immunotherapy for local advanced non-small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 3713–3736. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Charloux, A.; Bolliger, C.T.; Rocco, G.; Sculier, J.-P.; Varela, G.; Licker, M.; Ferguson, M.K.; Faivre-Finn, C.; Huber, R.M.; et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur. Respir. J. 2009, 34, 17–41, Erratum in: Eur. Respir. J. 2009, 34, 782. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018, 19, e447–e458. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. New Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Bonaca Marc P, Olenchock Benjamin A, Salem J-E, Wiviott Stephen D, Ederhy S, Cohen A, Stewart Garrick C, Choueiri Toni K, Di Carli M, Allenbach Y, Kumbhani Dharam J, Heinzerling L, Amiri-Kordestani L, Lyon Alexander R, Thavendiranathan P, Padera R, Lichtman A, Liu Peter P, Johnson Douglas B, Moslehi J. Myocarditis in the setting of cancer therapeutics. Circulation. 2019; 140:80–91.
- Norwood, T.G.; Westbrook, B.C.; Johnson, D.B.; Litovsky, S.H.; Terry, N.L.; McKee, S.B.; Gertler, A.S.; Moslehi, J.J.; Conry, R.M. Smoldering myocarditis following immune checkpoint blockade. J. Immunother. Cancer 2017, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Vartanov, A.; Kalotra, A.; Varughese, J.; Gautam, S.; Kandel, S.; Hosmer, W. Immunotherapy-associated complete heart block in a patient with NSCLC: A case report and literature review. Respir. Med. Case Rep. 2021, 33, 101390. [Google Scholar] [CrossRef]
- Chang, L.-S.; Barroso-Sousa, R.; Tolaney, S.M.; Hodi, F.S.; Kaiser, U.B.; Min, L. Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints. Endocr. Rev. 2018, 40, 17–65. [Google Scholar] [CrossRef]
- Cui, K.; Wang, Z.; Zhang, Q.; Zhang, X. Immune checkpoint inhibitors and adrenal insufficiency: a large-sample case series study. Ann. Transl. Med. 2022, 10, 251. [Google Scholar] [CrossRef]
- Pelletier, K.; Škrtić, M.; Kitchlu, A. Cancer therapy-induced hyponatremia: A case-illustrated review. J. Onco-Nephrology 2021, 5, 70–78. [Google Scholar] [CrossRef]
- Özdemir, B.C. Immune checkpoint inhibitor-related hypogonadism and infertility: a neglected issue in immuno-oncology. J. Immunother. Cancer 2021, 9, e002220. [Google Scholar] [CrossRef]
- Jessel, S.; Weiss, S.A.; Austin, M.; Mahajan, A.; Etts, K.; Zhang, L.; Aizenbud, L.; Perdigoto, A.L.; Hurwitz, M.; Sznol, M.; et al. Immune Checkpoint Inhibitor-Induced Hypophysitis and Patterns of Loss of Pituitary Function. Front. Oncol. 2022, 12. [Google Scholar] [CrossRef]
- Caturegli, P.; Newschaffer, C.; Olivi, A.; Pomper, M.G.; Burger, P.C.; Rose, N.R. Autoimmune Hypophysitis. Endocr. Rev. 2005, 26, 599–614. [Google Scholar] [CrossRef]
- Caturegli, P.; Di Dalmazi, G.; Lombardi, M.; Grosso, F.; Larman, H.B.; Larman, T.; Taverna, G.; Cosottini, M.; Lupi, I. Hypophysitis Secondary to Cytotoxic T-Lymphocyte–Associated Protein 4 Blockade: Insights into Pathogenesis from an Autopsy Series. Am. J. Pathol. 2016, 186, 3225–3235. [Google Scholar] [CrossRef] [PubMed]
- El Sabbagh, R.; Azar, N.S.; A Eid, A.; Azar, S.T. Thyroid Dysfunctions Due to Immune Checkpoint Inhibitors: A Review. Int. J. Gen. Med. 2020, ume 13, 1003–1009. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, P.; Huang, H. Engineering better chimeric antigen receptor T cells. Exp. Hematol. Oncol. 2020, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kochenderfer, J.N.; Wilson, W.H.; Janik, J.E.; Dudley, M.E.; Stetler-Stevenson, M.; Feldman, S.A.; Maric, I.; Raffeld, M.; Nathan, D.-A.N.; Lanier, B.J.; et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010, 116, 4099–4102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, X.-A.; Yang, J.; Zhang, G.; Li, J.; Song, L.; Su, Y.; Shi, Y.; Zhang, M.; He, J.; et al. Efficacy and safety of anti-CD19 CAR T-cell therapy in 110 patients with B-cell acute lymphoblastic leukemia with high-risk features. Blood Adv. 2020, 4, 2325–2338. [Google Scholar] [CrossRef]
- Kyte, J.A. Strategies for Improving the Efficacy of CAR T Cells in Solid Cancers. Cancers 2022, 14, 571. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef]
- Helwick C. "Novel BiTE antibody mediates contact between T cells and cancer cells". Oncology NEWS International. 2008, 17 (6). 20 June.
- Dombret, H.; Topp, M.S.; Schuh, A.C.; Wei, A.H.; Durrant, S.; Bacon, C.L.; Tran, Q.; Zimmerman, Z.; Kantarjian, H. Blinatumomab versus chemotherapy in first salvage or in later salvage for B-cell precursor acute lymphoblastic leukemia. Leuk. Lymphoma 2019, 60, 2214–2222. [Google Scholar] [CrossRef]
- Diefenbach, C.; Assouline, S.; Bosch, F.; Cheah, C.Y.; Kim, W.S.; Matasar, M.J.; Panizo, C.; Yoon, D.H.; Bender, B.; Hernandez, G.; et al. An Individualized Risk Mitigation Approach for Safety: Experience from the Mosunetuzumab (CD20/CD3 Bispecific Antibody) Development Program in Relation to Neurotoxicity Risk. Blood 2019, 134, 4728. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Si, S.; Teachey, D.T. Spotlight on Tocilizumab in the Treatment of CAR-T-Cell-Induced Cytokine Release Syndrome: Clinical Evidence to Date. Ther. Clin. Risk Manag. 2020, 16, 705–714. [Google Scholar] [CrossRef]
- Patel, S.; Cenin, D.; Corrigan, D.; Hamilton, B.K.; Kalaycio, M.; Sobecks, R.M.; Anwer, F.; Khouri, J.; Dean, R.M.; Winter, A.; et al. Siltuximab for First-Line Treatment of Cytokine Release Syndrome: A Response to the National Shortage of Tocilizumab. Blood 2022, 140, 5073–5074. [Google Scholar] [CrossRef]
- Wehrli, M.; Gallagher, K.; Chen, Y.-B.; Leick, M.B.; McAfee, S.L.; El-Jawahri, A.R.; DeFilipp, Z.; Horick, N.; O'Donnell, P.; Spitzer, T.; et al. Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS). J. Immunother. Cancer 2022, 10, e003847. [Google Scholar] [CrossRef] [PubMed]
- Wehrli, M.; Gallagher, K.; Chen, Y.-B.; Leick, M.B.; McAfee, S.L.; El-Jawahri, A.R.; DeFilipp, Z.; Horick, N.; O'Donnell, P.; Spitzer, T.; et al. Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS). J. Immunother. Cancer 2022, 10, e003847. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; McClain, K.; Allen, C.E.; Parikh, S.A.; Otrock, Z.; Rojas-Hernandez, C.; Blechacz, B.; Wang, S.; Minkov, M.; Jordan, M.B.; et al. A consensus review on malignancy-associated hemophagocytic lymphohistiocytosis in adults. Cancer 2017, 123, 3229–3240. [Google Scholar] [CrossRef]
- Gust, J.; Hay, K.A.; Hanafi, L.-A.; Li, D.; Myerson, D.; Gonzalez-Cuyar, L.F.; Yeung, C.; Liles, W.C.; Wurfel, M.; Lopez, J.A.; et al. Endothelial Activation and Blood–Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017, 7, 1404–1419. [Google Scholar] [CrossRef]
- Ziaja, M. Septic Encephalopathy. Curr. Neurol. Neurosci. Rep. 2013, 13, 1–7. [Google Scholar] [CrossRef]
- Gust, J.; Hay, K.A.; Hanafi, L.-A.; Li, D.; Myerson, D.; Gonzalez-Cuyar, L.F.; Yeung, C.; Liles, W.C.; Wurfel, M.; Lopez, J.A.; et al. Endothelial Activation and Blood–Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017, 7, 1404–1419. [Google Scholar] [CrossRef]
- Cohen, A.D.; Parekh, S.; Santomasso, B.D.; Pérez-Larraya, J.G.; van de Donk, N.W.C.J.; Arnulf, B.; Mateos, M.-V.; Lendvai, N.; Jackson, C.C.; De Braganca, K.C.; et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. 2022, 12, 1–9. [Google Scholar] [CrossRef]
- Shah, N.N.; Highfill, S.L.; Shalabi, H.; Yates, B.; Jin, J.; Wolters, P.L.; Ombrello, A.; Steinberg, S.M.; Martin, S.; Delbrook, C.; et al. CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial. J. Clin. Oncol. 2020, 38, 1938–1950. [Google Scholar] [CrossRef]
- Cai, C.; Tang, D.; Han, Y.; Shen, E.; Ahmed, O.A.; Guo, C.; Shen, H.; Zeng, S. A comprehensive analysis of the fatal toxic effects associated with CD19 CAR-T cell therapy. Aging 2020, 12, 18741–18753. [Google Scholar] [CrossRef] [PubMed]
- Gutgarts, V.; Jain, T.; Zheng, J.; Maloy, M.A.; Ruiz, J.D.; Pennisi, M.; Jaimes, E.A.; Perales, M.-A.; Sathick, J. Acute Kidney Injury after CAR-T Cell Therapy: Low Incidence and Rapid Recovery. Biol. Blood Marrow Transplant. 2020, 26, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).