1. Introduction
The superior gluteal nerve (SGN) is a branch of the sacral plexus that arises from the dorsal divisions of the fourth and fifth lumbar and first sacral ventral rami, and is the only nervous structure to emerge at the gluteal region through the greater sciatic foramen superiorly to the piriformis muscle, in conjunction with the superior gluteal artery and vein [
1,
2,
3,
4,
5,
6,
7,
8]. The SGN divides into superior and inferior branches: the superior branch innervates the gluteus medius and occasionally the gluteus minimus muscles, the inferior branch innervates the glutei medius and minimus, and ends in the tensor fasciae latae muscle [
1,
2,
3,
4,
5,
6,
7,
8]. The gluteus medius and minimus, acting from its proximal attachment, abduct the thigh, and their anterior and posterior fibers rotate it medially and laterally, respectively. Acting from the femur, they play a critical role in maintaining the upright position of the trunk when the foot of the opposite side is raised from the ground during gait [
8]. The actions of these muscles explain why lesions of the SGN may originate gait abnormalities, such as Trendelenburg gait [
8,
9]. The tensor fasciae latae is a hip flexor and abductor. Its role in medial rotation, from the anatomical position, is minimal. This muscle helps to maintain upright posture while minimizing energy expenditure on muscle activity. In standing, it acts from below to steady the pelvis on the head of the femur and, through the iliotibial tract, helps to maintain the extended knee in a locked position. When standing on one limb, the tensor fasciae latae aids the gluteus medius in stabilizing the pelvis over the femur in the coronal plane [
8].
The majority of proximal superior gluteal nerve (SGN) injuries are iatrogenic and occur during surgery, with such lesions described during surgical approaches of the hip, acetabulum, pelvis and sacroiliac joints [
10,
11,
12,
13]. These lesions can occur by traction of the adjacent structures in surgery, by compression of the nerve or its vascular supply by improper retractor placement, or even by direct neural transection, laceration or thermic injury with the use of electrocautery or cement. In hip arthroplasty, the general incidence of nerve injury is about 1-4% [
13]. Female sex and revision surgery are proven risk factors for iatrogenic surgical nerve injury [
13]. Furthermore, during percutaneous fixation of the sacroiliac joint, a direct injury to the superior gluteal neurovascular bundle was described in up to 18% of the cases, related with screw positioning [
11]. The most inferior branch of the SGN is most commonly injured during lateral and anterolateral approaches to the hip, corresponding to the injured nerve in 80% of the cases [
13,
14]. Regarding non-iatrogenic causes, SGN lesions are also described in acetabular fractures extending to the upper part of the greater sciatic notch (e.g., fractures of the posterior column) or fractures involving both columns, piriformis syndrome, pelvic fractures and rarely with extrinsic compression from inflammatory or neoplastic masses [
7,
15]. Lesions of the SGN seem to occur more often than expected and there are very few studies regarding the injury of the SGN overall and the existing ones usually refer to the injury of its most inferior branch, as it reaches the tensor fasciae latae muscle [
15].
To the best of our knowledge, there are no studies regarding the detailed position of the superior gluteal nerve or its branches as it exits the greater sciatic foramen and its injury in that location. The goal of this study was to describe the relation of the SGN or its branches with the greater sciatic notch, and measure the distances between these nervous structures at this location and selected bony references. Furthermore, we intended to evaluate if there was a correlation between the abovementioned parameters and the size of the pelvis. With this knowledge we hope to contribute to the safety of surgical approaches of the region and eventually describe palpable anatomical references useful in open and percutaneous surgery around the hip and pelvis.
2. Materials and Methods
The cadavers used in this study derived from body donation with informed consent, written and signed by the donator himself (Portuguese Decree-law nº 274/99). As so, this anatomic study did not require investigational review board or ethics committee approval. Cadavers were received and embalmed at the Unit of Anatomy, Department of Biomedicine, Faculty of Medicine, University of Porto. Twenty hemipelvises from ten cadavers were selected from all formalin-embalmed full body adult cadavers dissected for this study. The remaining dissected cadavers were excluded based on the following criteria: surgical scars, evidence of previous trauma or surgery involving the hip joint and pelvis and/or altered normal anatomy by dissection procedures. The cadavers included in this study were all caucasian (6 males, 4 females). The age of the specimens ranged from 58 to 86 years, with a median age of 78 years. The age of the female specimens ranged from 58 to 86 years, with a median age of 64 years. The age of the male specimens ranged from 75 to 85 years, with a median age of 80 years.
The cadavers were routinely dissected in our Unit, and cadavers in which the trunk wall, abdominopelvic cavity and lower limb were preserved were considered for inclusion in the present study. As routine in our Unit, appropriate dissection techniques were performed by using proper dissection tools in order to achieve the teaching and research objectives of the human cadaveric dissection [
16,
17,
18,
19,
20]. The specimens were carefully dissected in order not to disturb the normal anatomy of each region [
8,
20]. Regarding specifically the gluteal region (
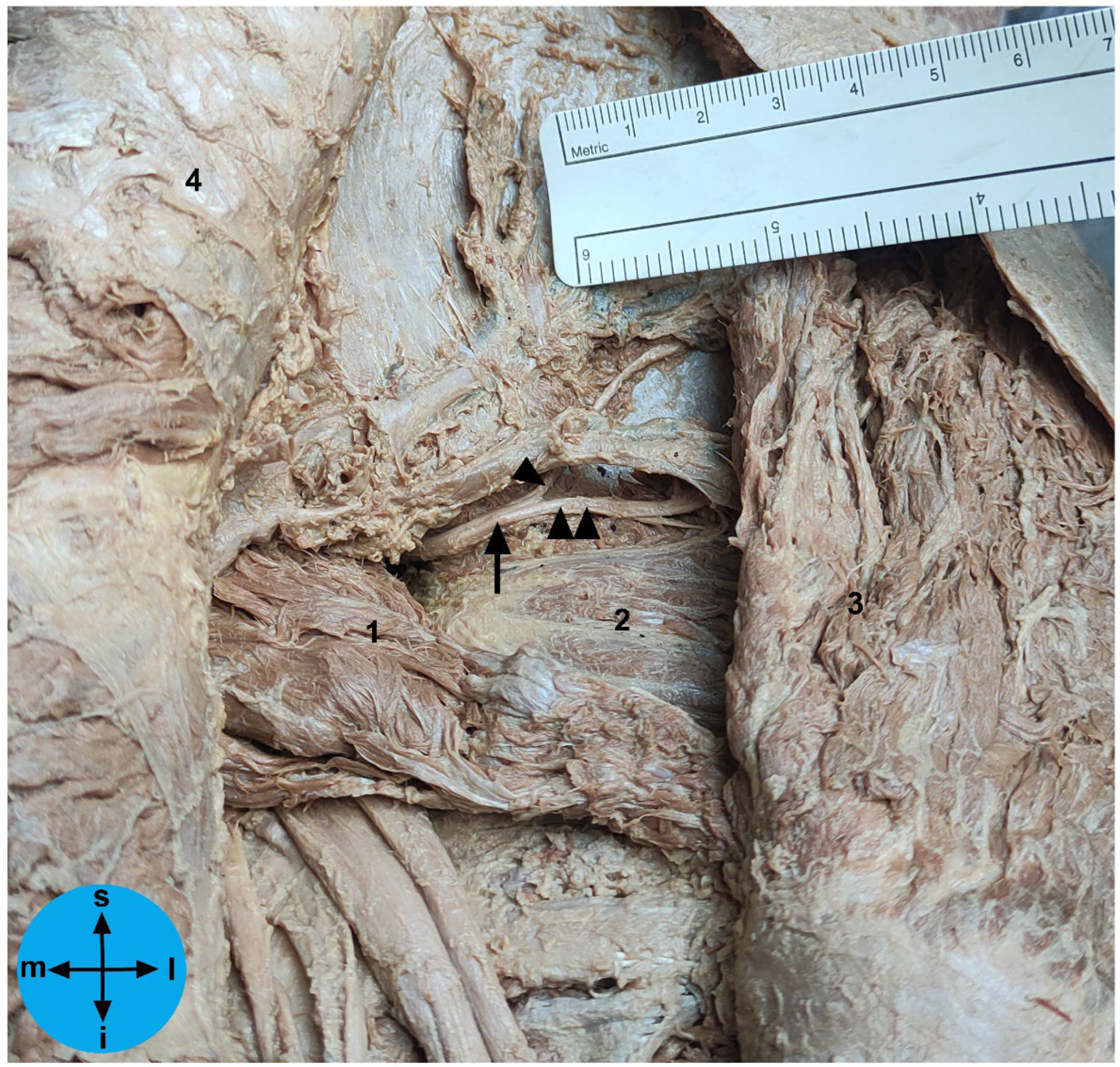
Figure 1), all dissection steps were based on those described previously in detail [
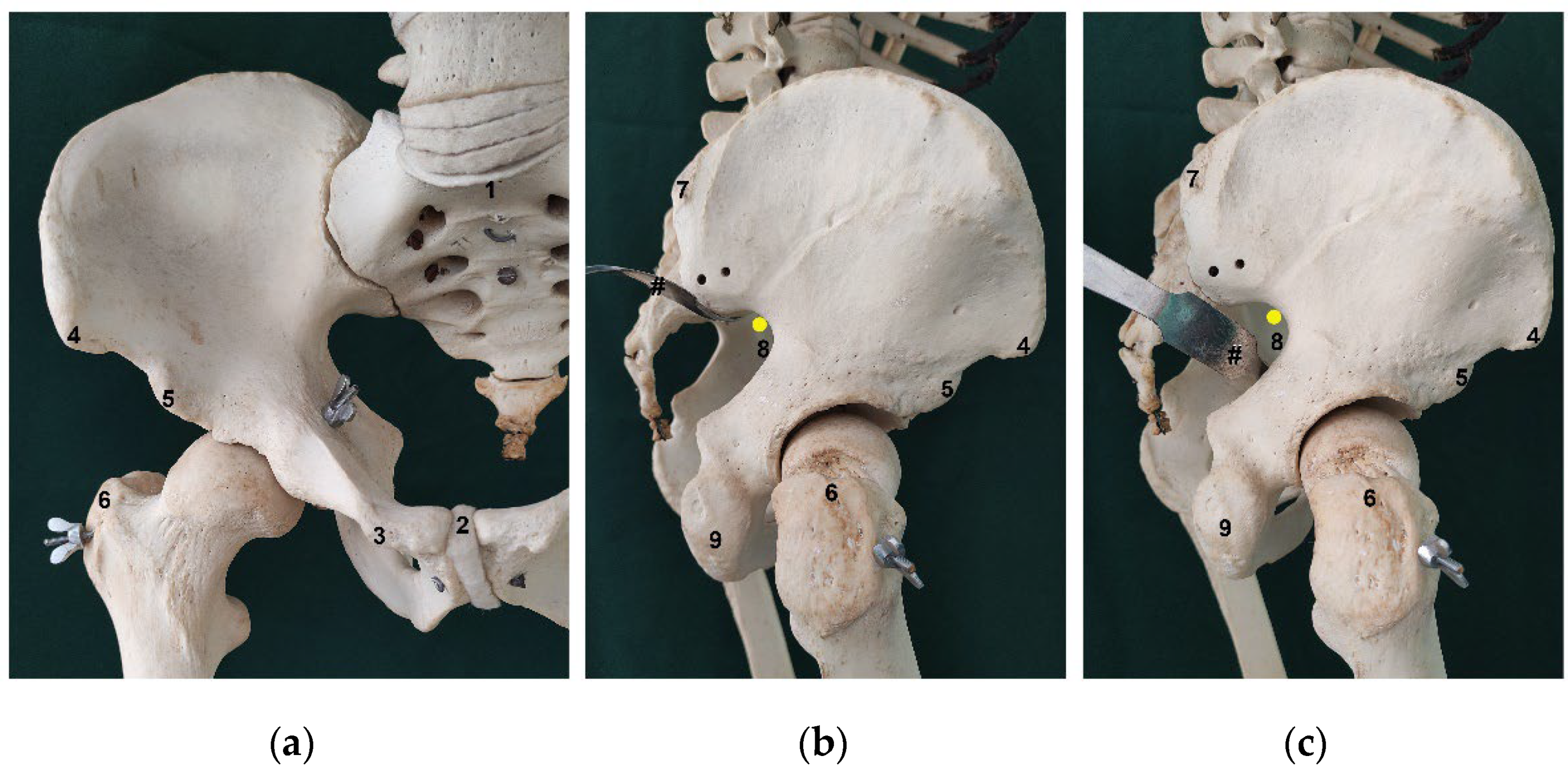
20]. After dissection, the bony landmarks (
Figure 2) were carefully identified and marked with a needle. A standardized measurement technique was developed, using a digital caliper and a standard surgical ruler, and all measurements were recorded by at least 2 different observers, over a period of 3 months and are expressed in centimeters (cm). Measurements were taken with the cadavers in the anatomical position, using four positions, i.e., supine, prone, and right and left lateral decubitus. Gender and laterality were also recorded.
Several distances were evaluated to describe the relation between the SGN or its branches, the greater sciatic notch and several chosen palpable bony landmarks, as well as to elucidate the possible differences in these distances with specimen pelvis size. To obtain an estimate of the size of the pelvis we did several measurements, including: the 1) distance between the anterior superior and posterior superior iliac spines (ASIS-PSIS), the 2) distance between both anterior superior iliac spines (DASIS), the 3) distance between the anterior superior iliac spine and the pubic tubercle (ASIS-PT), the 4) distance between both pubic tubercles (DPT), the 5) distance between the midpoint of the sacral promontory and the upper border of the pubic symphysis (SP-PS), the 6) distance between both posterior superior iliac spines (DPSIS) and the 7) distance between the anterior inferior and posterior superior iliac spines (AIIS-PSIS). To elucidate the association between the pelvis and the other anatomical references, that could possibly be used to infer the exact location of the SGN and/or its branches at its exit from the pelvis, we also evaluated the distance between the PSIS and the ipsilateral 1) apex of the greater trochanter (GT) and 2) inferior part of the ischial tuberosity (IT).
To take the measurements directly related to the SGN, firstly the superior gluteal neurovascular bundle was identified above the piriformis muscle and the SGN or its branches were carefully isolated at the greater sciatic foramen without moving it from its proper position (
Figure 1). Then, we recorded the distances from the exit point of the SGN or its branches at the greater sciatic notch to the: 1) apex of the GT, 2) PSIS, 3) ASIS and 4) the inferior part of the IT. We also determined the exact exit point of the SGN or its branches in the greater sciatic notch (
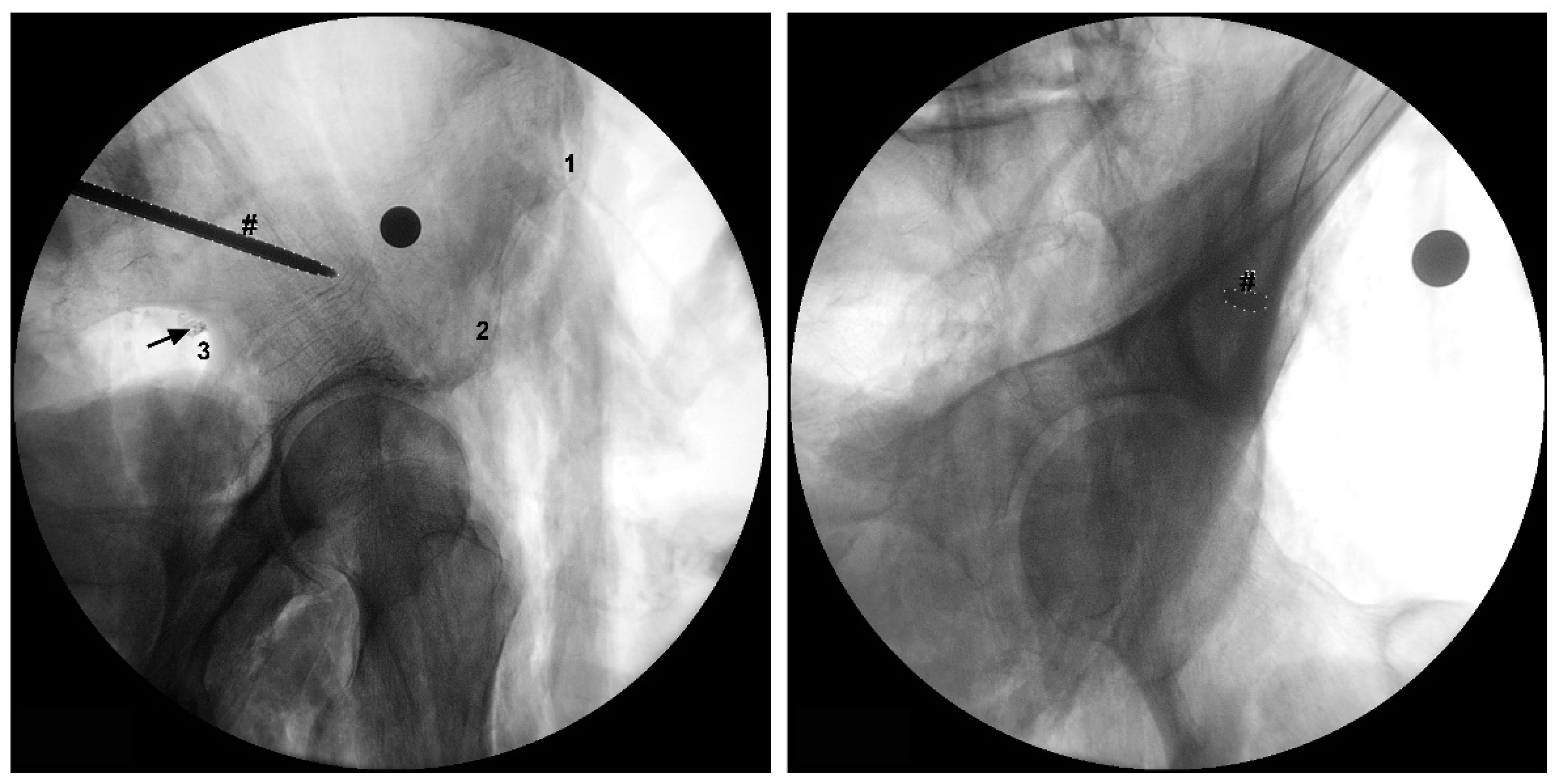
Figure 1). The emerging site of these nervous structures at the greater sciatic notch was recorded according to the following protocol: a) the distance values were considered positive if the nervous structures emerged superior to the greater sciatic notch apex; b) the distance values were considered negative if the nervous structures emerged inferior to the greater sciatic notch apex. We also determined the linear distance of each nervous structure to the bone at the greater sciatic notch. In one of the studied cadavers, we simulated the placement of an LC2 screw and we marked the SGN with a contrast medium at the point where it emerges from the pelvic cavity (
Figure 3).
With regard to the statistical analysis, considering the skewness of the distributions of the pelvic parameters, descriptive statistics of the sample were performed using median, interquartile range, minimum and maximum values. The Mann-Whitney U test was used to assess possible differences regarding sex and sidedness. The association between the different pelvic measurements and the location of the SGN emergence from the greater sciatic notch was evaluated through the Spearman’s correlation coefficient. The significance level was set at α=0.05 and statistical analysis was performed using SPSS software (version 26, SPSS Inc, Chicago, IL.).
3. Results
As mentioned before, the sample is composed of 20 hemipelvises from 10 cadaveric specimens.
Regarding the evaluated pelvic parameters representing the antero-posterior pelvic dimensions, the median distance between the anterior superior and posterior superior iliac spines (ASIS-PSIS) was 15.9 cm (min 13.5; max 17.7). Also, the distance between the sacral promontory and the upper border of the pubic symphysis (SP-PS) had a median value of 11.6 cm (min 10.5; max 12.2). Furthermore, the distance between the anterior inferior and posterior superior iliac spines (AIIS-PSIS) showed a median value of 15.7 cm (min 13.1; max 18.7) (
Table 1).
With respect to the transverse diameter of the pelvis, the measurements showed a median DPSIS of 9.2 cm (min 8.6; max 11.9). Furthermore, the DASIS had a median value of 22.6 cm (min 19.1; max 24.6). Also, the DPT had a median value of 5.2 cm (min 4.5; max 5.6). Representing the vertical diameter of the pelvis, the ASIS-PT distance showed a median value of 12.3 cm (min 10.2; max 21.4) (
Table 1). In our sample, there were no statistically significant differences in the measured pelvic parameters regarding side or gender, even though the anteroposterior diameter of the pelvic inlet (measured between the midpoints of the sacral promontory and upper border of the pubic symphysis) was close to being significantly different between sexes (median value of 11.75 cm in females and 11.15 cm in male cadavers; p=0.067).
In regard to the chosen anatomical references that could possibly be used to infer the exact location of the SGN or its branches at its exit from the pelvis, the distances between the PSIS and the ipsilateral 1) GT and the 2) IT showed a median of 14.4 cm (min 11.1; max 16.3; interquartile range (IQR) 2.9) and 16.9 cm (min 13.0; max 18.6; IQR 2.2).
Regarding the SGN, in all specimens included in this study, we found the SGN exiting the pelvic cavity through the greater sciatic foramen above the piriformis muscle. At the greater sciatic notch, the SGN emerges as a single branch in 15 hemipelvises (75%). In 5 hemipelvises (25%) the SGN emerges already divided in its two branches. In regard to the subgroup of hemipelvises in which the SGN emerges as a single branch, in the vast majority of cases (10 of the 15 hemipelvises) the nerve emerged in direct contact with the bone at the apex of the greater sciatic notch (median distance of 0.0 cm). In 4 hemipelvises the SNG emerged superiorly to the greater sciatic notch apex, distancing between 0.1 to 0.5 cm. In one hemipelvis the SGN emerged inferiorly to the greater sciatic notch apex. The median distance from the exit of the SGN at the greater sciatic notch were 7.6 cm to the PSIS (min 7.2; max 8.4), 10.9 cm to the ASIS (min 9.9; max 11.8), 7,5 cm to the apex of the GT (min 5.5; max 9.4) and 10.8 cm to the inferior part of the IT (min 8.8; max 12.1) (
Table 2).
Concerning the 5 hemipelvises in which the SGN emerged already dived in its superior and inferior branches, each of its branches were characterized separately. The superior branch emerged in all cases superior to the greater sciatic notch apex, with a median distance of 0.3 cm (min 0.1; max 1.0). The superior branch of the SGN was located at a median distance of 6.6 cm from the PSIS (min 6.2; max 7.0), 11,0 cm from the ASIS (min 9.5; max 11.3), 8.5 cm from the GT (min 6.7; max 9.0) and 10.7 cm from the IT (min 7.9; max 11.4). The inferior branch of the SGN emerged in all cases at the apex (1 hemipelvis) or inferior to the greater sciatic notch apex (4 hemipelvises), with a median distance of 0.2 cm from that reference (min -0.5; max 0.0). This branch was in closer relation with the GT (median distance of 8.1 cm; min 6.4; max 8.7) and the IT (median distance of 10.5 cm; min 7.7; max 11.1), but more distant from the PSIS (median distance 7.4 cm; min 6.6; max 7.8) and the ASIS (median distance of 10.5 cm; min 9.2; max 10.9) (
Table 2). There were no statistically significant differences according to side or gender regarding the distance between the SGN and the chosen pelvic bony anatomical references, although the distance to the apex of the GT showed a tendency to be inferior in females (median distance of 7.9 vs 6.5 cm, p=0.069).
Regarding the relation between the size of the pelvis and the distance from the SGN to the chosen bony structures, our data showed that pelvises with greater antero-posterior diameters (greater ASIS to PSIS distance) were associated with smaller distances from the SGN to the apex of the greater sciatic notch, either for the nerves that emerged as a common trunk or already divided in its branches. This association showed a modest negative correlation of -0.48 (SGN and superior branch) or -0.45 (SGN and inferior branch). There was also a significant association between greater pelvic diameter (ASIS-PSIS distance) and greater distance from the SGN to the inferior part of the IT, including hemipelvises in which the SGN emerged as a common trunk, as well as for both branches of the SGN that emerged already divided (p=0.025 and p=0.022 respectively). This association showed a modest positive correlation, with a correlation coefficient around 0.50 for both SGN branches (
Table 3).
Concerning the relationship between the SGN emerging site and the remaining pelvic parameters, most of the chosen parameters did not show a significant association with the distance from the SGN to the IT, GT or greater sciatic notch, including the 1) DASIS, 2) ASIS-PT distance, 3) DPT, 4) SP-PS distance, 5) DPSIS and 6) the AIIS-PSIS distance.
On the other hand, we found statistically significant associations between the distance from the PSIS to the GT and the distance from the SGN to the IT and to the GT (p<0.001), showing strong positive correlations with correlation coefficients ≥0.75. Furthermore, the distance between the PSIS and the IT also showed a significant association with the emerging SGN site and its distance to the IT, including the specimens with SGN emerging as a common trunk and both the superior and inferior branches (p<0.001). This relation showed a strong positive correlation (correlation coefficient of 0.78 and 0.77 respectively). The distance between the PSIS and the IT also showed a statistically significant association with the distance from the GT to the SGN emerging site, including the SGN trunk and the superior (p=0.018) or the inferior (p=0.012) branches, with a weak to moderate correlation (correlation coefficient of 0.52 and 0.55 respectively) (
Table 3).
4. Discussion
The main goal of this study was to define the precise exiting point of the SGN or its branches at the greater sciatic foramen, regarding various bone structures. Some of the anatomic references were chosen due to their easy accessibility to superficial palpation by the physician, and clinical importance in regard to the surgical anatomy of the region.
A sample of 20 hemipelvises were used to evaluate the pelvic morphology, the relation of the SGN with the greater sciatic notch, the distance from the SGN or its branches at the point they leave the pelvic cavity and the chosen bony anatomical landmarks. After the anatomical and morphological evaluation, our sample data was evaluated looking for associations between these parameters and differences according to laterality and gender.
Regarding laterality, no differences were found in any of the parameters evaluated and this can help exclude any role of a functional dominant limb. Also, there were no significant differences according to gender. However, the absence of statistically significant differences may be due to the small number of specimens. Indeed, the sexual differences in the pelvis are well known and widely described, and are unavoidably linked to function [
8]. For instance, in our sample, the antero-posterior diameter (true conjugate) of the pelvic inlet (superior pelvic aperture), measured between the midpoints of the sacral promontory and upper border of the pubic symphysis, had a median value of 11.75 cm in female and 11.15 cm in male cadavers. These results showing a trend to a greater antero-posterior diameter of the pelvic inlet in female are in line with those stated in classical textbooks of Anatomy where it is described that on average this diameter is 11.2 cm in the adult female and 10.0 cm in the adult male [
8].
In 75% of the hemipelvises the SGN emerged as a common trunk trough the greater sciatic foramen, and in 25% of cases emerged already divided. Concerning the 15 cases in which the SGN emerged as a single branch, 10 of them had the nerve in direct contact with the bone at the apex of the greater sciatic notch. In our sample, this is the location of greater risk of possible iatrogenic lesion to the SGN. According to our sample data, the most secure location for surgical exploration and for a surgical retractor placement, seems to be inferiorly to the apex of the greater sciatic notch when we approach the hip or near the posterior inferior iliac spine (PIIS) when doing a posterior approach to sacroiliac joint or to fixation of crescent iliac fracture.
In our sample, only 25% of hemipelvises had a SGN emerging already divided in its superior and inferior branches at the greater sciatic notch. However, in hemipelvises in which the SGN emerges already divided, care should be taken not to injure the inferior branch, which emerged in every case at or inferior to the greater sciatic notch apex, and in closer relation with the GT (median distance of 8.1 cm) and the IT (median distance of 10.5 cm) than the superior branch. There were no significant differences according to laterality. Furthermore, although no significant differences were recorded according to gender, the distance between the SGN and the apex of the GT showed a trend towards being smaller in females (p=0.069), possibly translating the general inferior height of females comparing to males. This can contribute to the established fact that female patients have greater risk for iatrogenic nerve injury in hip surgery [
13]. This difference is probably due to the smaller body height, originating smaller distances from bony landmarks to nerve structures and consequently higher risk of injury. The lower soft tissue mass present in females is also suggested to be an important factor in contributing for the higher risk [
21]. Although no significant differences were found between gender, the authors feel it’s important to remain attentive, especially in females, when surgically approaching the trochanteric area, namely when choosing the position of Hohmann retractors that can possibly cause entrapment of the SGN distally to the greater sciatic notch. Picado et al [
22] evaluated 40 patients subjected to total hip arthroplasty using the direct lateral approach for nerve injury using electromyography 4 weeks post-operatively. Injury to the SGN was found in 17 patients, and although most of these were transient, injury to the SGN can result in significant morbidity such as Trendelenburg gait [
22]. In our study, the SGN showed great proximity to the GT, with a median distance of 7.5 cm to its apex. Several studies evaluated the distance between the apex of the GT and the inferior branch of the SGN, in order to describe a safe zone for the lateral/anterolateral approach to the hip [
22,
23,
24,
25]. Although results vary, it was possible to define a safe zone, limiting the incision between 3-7 cm from the apex of the GT cranially. It is proposed that if this limit is exceeded, the neurovascular bundle is at risk of lesion [
22,
23,
24,
25]. Ray et al [
23] studied the branching pattern and length of the SGN, from its exit in the greater sciatic notch to the point where it pierces the glutei (medius and minimus) and the tensor fasciae latae muscles [
23]. The mean distance from the apex of the GT to its emergence was 7.26 ± 1.65 cm. These findings are in close range to ours, since in our study the median distance from the apex of the GT to the SGN emergence was 7.5 cm.
The SGN is also in close proximity to the greater sciatic notch which is frequently in contact with retractors used in hip arthroplasty for acetabulum exposure or when approaching the dorsal portion of the iliac bone for fixation of the crescent iliac fragment or sacroiliac joint (
Figure 3b,c). In our study, we found that the SGN emerged, in most cases, in direct contact with the bone at the apex of the greater sciatic notch. This places the SGN in the antero-superior quadrant of the greater sciatic notch, and so in close contact with the acetabular posterior wall/column. It’s the authors recommendation to exercise caution when placing Hohmann retractors in the postero-superior and superior region of the acetabular wall, due to the increased risk of entrapment of the SGN between the retractor and the bone at the greater sciatic notch. The same recommendation is mandatory for the posterior sacroiliac approach when we do an open reduction of the iliac fragment or sacroiliac joint and we need to stay in the safe zone in the proximity of PIIS or the first 7.6 cm of the superior border of greater sciatic notch. This relation between the SGN and the pelvic structures is particularly relevant in smaller patients. In our sample, cadavers with smaller pelvic diameters (smaller AIIS to PSIS distance) showed a course of the SGN significantly closer to the greater sciatic notch. As it should be expected, patients with smaller distances from the PSIS to the GT or IT, also had the SGN in closer relation with the GT and IT, and potentially at higher risk of iatrogenic injury.
There are also reports of injury to the SGN during percutaneous iliosacral screw insertion. Collinge et al [
11] performed a study in which the 58 sacroiliac screws were placed in the first sacral bodies, and observed lesion of the superior branch of the SGN and superior gluteal vessels in 10 of the 58 (18%) [
11]. In our study, besides the GT, the SGN was also in close proximity to the PSIS, distancing 7.6 cm from it. The PSIS is an important surgical reference, due to its superficial location and usefulness in the localization of PIIS, making it a useful guide for percutaneous fixation of crescent ilium fractures (LC2 Screw). Therefore, we recommend caution when placing these screws, as a screw directed at the AIIS with a too inferior orientation can induce fracture of the upper limit of the greater sciatic notch and concomitant lesion of the SGN or its branches taking into account the close relation between these nervous structures and the greater sciatic notch (
Figure 3).
Regarding the IT, this is a safe starting point to aim retrograde posterior column screws during pelvic percutaneous fixation, distancing in median 10.8 cm from the SGN. However, care should be taken not to direct the screw too posteriorly when trying to avoid the hip joint, due to the risk of entering the greater sciatic notch and injuring the contained structures.
Although our study showed important surgical and clinical anatomy findings, which we hope can influence surgical practice, it also presents some limitations that are generally observed in studies that are performed in cadavers. Nevertheless, we made everything possible to minimize, at least in part, these limitations. The main one is related to the changes in volume and trophicity of muscle mass with death and fixation techniques. We tried to minimize this limitation by using exclusively bony references. Furthermore, we measured some reference parameters and the obtained results were similar to data previously reported in the literature, a point that unequivocally provides robustness to our results. Furthermore, due to availability and cost, the number of cadaveric specimens is relatively reduced, possibly contributing to the fact that some of our results could not achieve statistical significance. Finally, another limitation of our study is the absence of clinical data related to the studied cadavers which prevented the establishment of any correlation between anatomical and clinical aspects.
5. Conclusions
It is of paramount importance to recognize that when SGN exits the pelvis trough the greater sciatic foramen as a common trunk, it does so, in most cases, in close contact with the bone at the apex of the greater sciatic notch or superior to it. The authors consider that although there is a safe distance to perform surgery around the hip or posterior sacroiliac approach without isolating the nerve, care should be taken when placing retractors around the greater sciatic notch. Besides that, in the surgical approach during hip arthroplasty, the SGN showed greater proximity to the GT, and the surgeon should be aware of this relation, especially in smaller patients in which this distance was significantly inferior. Regarding percutaneous fixation, these techniques seem to be relatively safe; however, depending on the entry point one should pay close attention to the screw trajectory to avoid any potential iatrogenic lesion of the SGN.
Author Contributions
Conceptualization, A.R.P., M.J.L., M.D.M. and P.A.P; Methodology, A.R.P., M.J.L., H.A., M.D.M. and P.A.P; Formal Analysis, M.J.L., H.A.; Investigation, A.R.P., J.L., M.R.S., P.V., J.N-M., M.C.B. and P.A.P; Resources, M.D.M. and P.A.P.; Data Curation, M.J.L. and H.A.; Writing – Original Draft Preparation, A.R.P., M.J.L. and J.L.; Writing – Review & Editing, A.R.P., M.J.L., H.A., M.D.M. and P.A.P; Supervision, A.R.P., M.D.M. and P.A.P. All authors read and approved the final version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to the fact that the used cadavers derived from body donation with informed consent, written and signed by the donator himself (Portuguese Decree-law nº 274/99).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.
Acknowledgments
The authors wish to thank those who donated their bodies to medical teaching and research, allowing an increase in the overall knowledge that can improve patient care. Thus, the donors, as well as their families, deserve our highest gratitude. The authors also wish to thank the Unit of Anatomy Technicians Filipe Silva and Hélder Bastos for their technical assistance, as well as the Radiological Technician Paula Cardoso for their technical assistance in the radiological procedures performed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Akita K, Sakamoto H, Sato T. Origin, course and distribution of the superior gluteal nerve. Acta Anat. (Basel). 1994, 149, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Bos JC, Stoeckart R, Klooswijk AI, van Linge B, Bahadoer R. The surgical anatomy of the superior gluteal nerve and anatomical radiologic bases of the direct lateral approach to the hip. Surg. Radiol. Anat. 1994, 16, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Ebraheim NA, Olexa TA, Xu R, Georgiadis G, Yeasting RA. The quantitative anatomy of the superior gluteal artery and its location. Am. J. Orthop. (Belle Mead NJ). 1998, 27, 427–431. [Google Scholar]
- Jacobs LG, Buxton RA. The course of the superior gluteal nerve in the lateral approach to the hip. J. Bone Joint Surg. Am. 1989, 71, 1239–1243. [Google Scholar] [CrossRef]
- Miguel-Pérez M, Ortiz-Sagristà JC, López I, Pérez-Bellmunt A, Llusá M, Alex L, Combalia A. How to avoid injuries of the superior gluteal nerve. Hip Int. 2010, 20 (suppl 7), S26–S31. [Google Scholar] [CrossRef] [PubMed]
- Stecco C, Macchi V, Baggio L, Porzionato A, Berizzi A, Aldegheri R, De Caro R. Anatomical and CT angiographic study of superior gluteal neurovascular pedicle: implications for hip surgery. Surg. Radiol. Anat. 2013, 35, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Collinge C, Ziran N, Coons D. Relationship Between the Superior Gluteal Vessels and Nerve at the Greater Sciatic Notch. Orthopedics. 2015, 38, e929–e933. [Google Scholar]
- Standring, S. Gray’s Anatomy - The Anatomical Basis of Clinical Practice, 42nd ed., Elsevier, 2021.
- Putzer, D, Haselbacher, M, Hörmann, R, Thaler M, Nogler M. The distance of the gluteal nerve in relation to anatomical landmarks: an anatomic study. Arch. Orthop. Trauma Surg. 2018, 138, 419–425. [Google Scholar] [CrossRef]
- Yang, IH. Neurovascular Injury in Hip Arthroplasty. Hip Pelvis. 2014, 26, 74–78. [Google Scholar] [CrossRef]
- Collinge C, Coons D, Aschenbrenner J. Risks to the superior gluteal neurovascular bundle during percutaneous iliosacral screw insertion: an anatomical cadaver study. J. Orthop. Trauma. 2005, 19, 96–101. [Google Scholar] [CrossRef]
- Grob K, Manestar M, Ackland T, Filgueira L, Kuster MS. Potential Risk to the Superior Gluteal Nerve During the Anterior Approach to the Hip Joint: An Anatomical Study. J. Bone Joint Surg. Am. 2015, 97, 1426–1431. [Google Scholar] [CrossRef]
- Hasija R, Kelly JJ, Shah NV, Newman JM, Chan JJ, Robinson J, Maheshwari AV. Nerve injuries associated with total hip arthroplasty. J. Clin. Orthop. Trauma. 2018, 9, 81–86. [Google Scholar] [CrossRef]
- Abitbol JJ, Gendron D, Laurin CA, Beaulieu MA. Gluteal nerve damage following total hip arthroplasty. A prospective analysis. J. Arthroplasty. 1990, 5, 319–322.
- Gänsslen A, Müller M, Nerlich M, Lindahl J. Acetabular Fractures: Diagnosis, Indications, Treatment Strategies, 1st ed., Thieme, 2017.
- Fernandes J, Pinho A, Pereira P, Madeira M, Raposo F, Sousa A, Lobo JM. Anterolateral ligament of the knee - Cadaver study in a Caucasian population. Rev. Esp. Cir. Ortop. Traumatol. 2023, 67, T134–T138. [Google Scholar]
- Leite MJ, Pinho AR, Silva MR, Lixa JC, Madeira MD, Pereira PG. Deep gluteal space anatomy and its relationship with deep gluteal pain syndromes. Hip Int. 2022, 32, 510–515. [Google Scholar] [CrossRef]
- Relvas-Silva M, Pinho AR, Lopes JG, Lixa J, Leite MJ, Sousa AN, Veludo V, Madeira D, Pereira P. Anatomy of the superficial peroneal nerve: Can we predict nerve location and minimize iatrogenic lesion? Morphologie. 2021, 105, 204–209. [Google Scholar] [CrossRef]
- Pinho AR, Pereira PA, Leite MJ, Santos CC, Vaz RP, Dulce Madeira M. The Surgical Vascular Anatomy of the Lower Lumbar Arteries and Its Implications in Minimally Invasive Spine Surgery: A Cadaveric Study. Int. J. Spine Surg. 2022, 16, 631–637. [Google Scholar] [CrossRef]
- Loukas M, Benninger B, Tubbs RS. Gray's Clinical Photographic Dissector of the Human Body, 2nd ed., Elsevier, 2018.
- Wang TI, Chen HY, Tsai CH, Hsu HC, Lin TL. Distances between bony landmarks and adjacent nerves: anatomical factors that may influence retractor placement in total hip replacement surgery. J. Orthop. Surg. Res. 2016; 11, 31.
- Picado CH, Garcia FL, Marques W Jr. Damage to the superior gluteal nerve after direct lateral approach to the hip. Clin. Orthop. Relat. Res. 2007, 455, 209–211. [Google Scholar] [CrossRef]
- Ray B, D'Souza AS, Saxena A, Nayak D, Sushma RK, Shetty P, Pugazhendi B. Morphology of the superior gluteal nerve: a study in adult human cadavers. Bratisl. Lek. Listy. 2013, 114, 409–412. [Google Scholar]
- Eksioglu F, Uslu M, Gudemez E, Atik OS, Tekdemir I. Reliability of the safe area for the superior gluteal nerve. Clin. Orthop. Relat. Res. 2003, 412, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Khan T, Knowles D. Damage to the superior gluteal nerve during the direct lateral approach to the hip: a cadaveric study. J. Arthroplasty. 2007, 22, 1198–1200. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).