Submitted:
13 June 2023
Posted:
14 June 2023
You are already at the latest version
Abstract

Keywords:
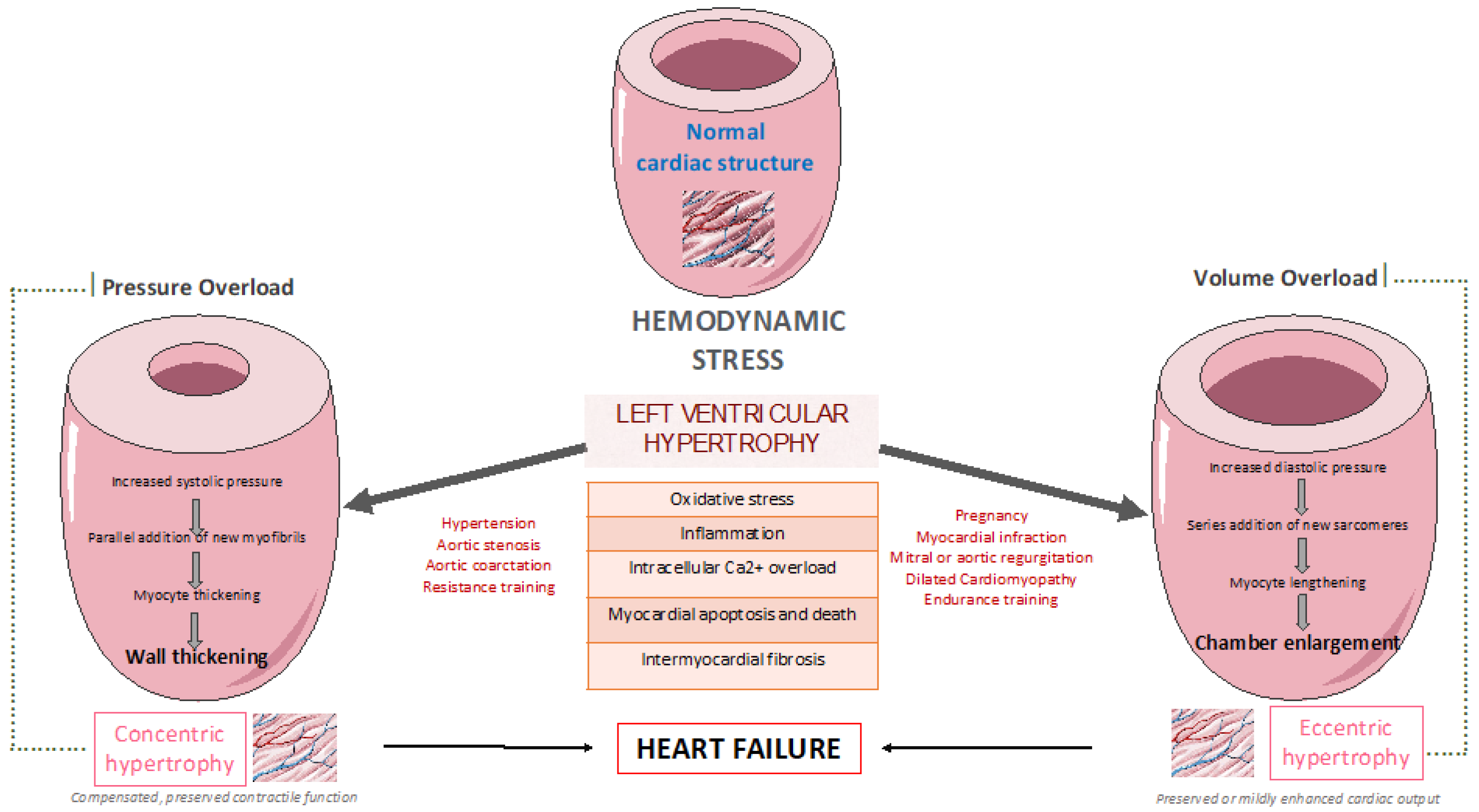
1. Introduction
2. LV remodelling pattern among patients with cardiometabolic risk factors
2.1. Athlete’s heart
2.2. Hypertensive heart disease
2.3. Diabetic cardiomyopathy
2.4. Obesity-related cardiomyopathy
3. The effect of exercise training on left ventricular remodelling among patients with cardiometabolic risk factors
3.1. In patients with hypertension
3.2. In patients with type 2 diabetes
3.3. In patients with type obesity
| Author | Type of study | Patient Characteristics | Main findings |
|---|---|---|---|
| Zanettini et al. [61] | Prospective cohort study | ∙14 sedentary patients with untreated diastolic BP (90-104 mmHg) ∙12-week supervised exercise program |
∙Exercise-mediated increase in aerobic fitness significantly reduced resting systolic and diastolic BP, mean systolic and diastolic 24-hour BP, as well as LV mass index. |
| Kokkinos et al. [62] | Randomized controlled trial | ∙46 male patients with severe hypertension ∙35-76 years of age ∙16 or 32-week exercise program plus antihypertensive medication or antihypertensive medication alone |
∙Diastolic BP decreased in the patients who exercised, whereas it increased slightly, in those who did not exercise. ∙Thickness of interventricular septum, LV mass, and mass index decreased significantly only in the patients who exercised. |
| Turner et al. [67] | Prospective cohort study | ∙11 patients with mild to moderate hypertension vs 7 sedentary hypertensive patients as controls ∙65.5± 1.2 vs 68.5±1 years of age ∙6.8±3.8- month exercise program |
∙Exercise training decreased systolic and diastolic BP, LV wall thickness and mass, as well as wall thickness-to-radius. ∙Only the reduction in resting systolic BP was correlated significantly with the regression of concentric remodeling. |
| Pitsavos et al. [66] | Randomized controlled trial | ∙40 patients with borderline to mild hypertension ∙53±7 years of age ∙16-week exercise aerobic program or standard care |
∙Systolic and diastolic BP, as well as heart rate were significantly lower in the exercise group compared to the control group. ∙LV mass index decreased significantly only in the exercise group. |
| Palatini et al. [72] | Prospective cohort study | ∙454 patients with stage 1 hypertension ∙33.1±8.4 years of age ∙median follow-up of 8.3 years |
∙Physically active groups were less likely to develop LVH than sedentary group. ∙BP declined in physically active patients and slightly increased in the sedentary peers. |
| Cassidy et al. [75]C | Randomized controlled trial | ∙28 patients with type 2 diabetes ∙61±9 vs 59±9 years of age ∙12-week HIIT or standard care |
∙HIIT improved LV wall mass and stroke volume. ∙Early diastolic filling rates increased, and peak torsion decreased in the treatment group. |
| Otten et al. [76] | Randomized controlled trial | ∙22 overweight and obese subjects with type 2 diabetes ∙61(58–66) vs 59(52–64) years of age ∙12-week PD-EX vs PD and standard care |
∙Significant decreases in LV mass to EDV ratio was observed in the PD-EX group. ∙LVEDV and stroke volume increased significantly only in the PD-EX group. |
| Gulsin et al. [77] | Randomized controlled trial | ∙87 patients with type 2 diabetes and 36 matched controls ∙50.5±6.5 vs 48.6±6.2 years of age ∙12-week supervised aerobic exercise training vs low-energy MRP diet vs routine care |
∙Supervised aerobic exercise training program improved diastolic function in the absence of any major effects on LV remodeling, perfusion, or aortic stiffening. ∙MRP resulted in weight loss, and improved blood pressure, glycemia, LV mass/volume, and aortic stiffness but not diastolic function. |
| Kamimura et al. [79] | Retrospective cohort study | ∙1,300 African Americans with preserved LVEF (>50%) ∙63 (57, 69) years of age ∙physical activity was calculated as 3*heavy activity hours + 2*moderate activity hours + slight activity hours/day |
∙Higher physical activity index was independently associated with lower LV mass. ∙Higher physical activity index was associated with lower LV mass index more in obese or hypertensive participants compared with non-obese or non-hypertensive participants. |
| Himeno et al. [80] | Prospective cohort study | ∙11 obese and hypertensive patients and 11 obese and normotensive patients ∙37±11 vs 35±7 years of age ∙12-week weight-reduction program consisted of mild exercise and mild hypocaloric intake |
∙Systolic, diastolic, and mean BP were significantly reduced only in the hypertensive group. ∙LV mas was significantly reduced both among hypertensive and normotensive obese patients. |
3.4. In patients with coronary artery disease
3.5. In patients with heart failure
4. Pathophysiological mechanisms of exercise-mediated favorable cardiovascular outcomes
5. Recommendations and new perspectives
6. Conclusions
Funding
Conflicts of Interest
References
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Lond Engl 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Gupta A, Fonarow GC. The Hospital Readmissions Reduction Program-learning from failure of a healthcare policy. Eur J Heart Fail 2018, 20, 1169–1174. [Google Scholar] [CrossRef]
- Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 2000, 102, 470–479. [Google Scholar] [CrossRef]
- Stein EJ, Fearon WF, Elmariah S, et al. Left Ventricular Hypertrophy and Biomarkers of Cardiac Damage and Stress in Aortic Stenosis. J Am Heart Assoc 2022, 11, e023466. [Google Scholar] [CrossRef]
- Rader F, Sachdev E, Arsanjani R, et al. Left ventricular hypertrophy in valvular aortic stenosis: mechanisms and clinical implications. Am J Med 2015, 128, 344–352. [Google Scholar] [CrossRef]
- Lovic D, Narayan P, Pittaras A, et al. Left ventricular hypertrophy in athletes and hypertensive patients. J Clin Hypertens 2017, 19, 413–417. [Google Scholar] [CrossRef]
- Gupta S, Berry JD, Ayers CR, et al. Association of Health Aging and Body Composition (ABC) Heart Failure score with cardiac structural and functional abnormalities in young individuals. Am Heart J 2010, 159, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Devereux RB, Casale PN, Hammond IW, et al. Echocardiographic detection of pressure-overload left ventricular hypertrophy: effect of criteria and patient population. J Clin Hypertens 1987, 3, 66–78. [Google Scholar]
- Polese A, De Cesare N, Montorsi P, et al. Upward shift of the lower range of coronary flow autoregulation in hypertensive patients with hypertrophy of the left ventricle. Circulation 1991, 83, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation 2013, 128, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J 2012, 33, 1058–1066. [Google Scholar] [CrossRef]
- Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990, 322, 1561–1566. [Google Scholar] [CrossRef]
- Dimitriadis K, Bletsa E, Lazarou E, et al. A Narrative Review on Exercise and Cardiovascular Events: “Primum Non Nocere”. Heart Mind 2022, 6, 127. [Google Scholar] [CrossRef]
- Rauch B, Davos CH, Doherty P, et al. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: A systematic review and meta-analysis of randomized and non-randomized studies – The Cardiac Rehabilitation Outcome Study (CROS). Eur J Prev Cardiol 2016, 23, 1914–1939. [Google Scholar] [CrossRef] [PubMed]
- Lewinter C, Doherty P, Gale CP, et al. Exercise-based cardiac rehabilitation in patients with heart failure: a meta-analysis of randomised controlled trials between 1999 and 2013. Eur J Prev Cardiol 2015, 22, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med 2004, 116, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Coates AM, Cheung CP, Currie KD, et al. Cardiac Remodeling in Elite Aquatic Sport Athletes. Clin J Sport Med 2022, 32, e485–e491. [Google Scholar] [CrossRef] [PubMed]
- Lazzeroni D, Rimoldi O, Camici PG. From Left Ventricular Hypertrophy to Dysfunction and Failure. Circ J Off J Jpn Circ Soc 2016, 80, 555–564. [Google Scholar]
- Albaeni A, Davis JW, Ahmad M. Echocardiographic evaluation of the Athlete’s heart. Echocardiography 2021, 38, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Merghani A, Maestrini V, Rosmini S, et al. Prevalence of Subclinical Coronary Artery Disease in Masters Endurance Athletes With a Low Atherosclerotic Risk Profile. Circulation 2017, 136, 126–137. [Google Scholar] [CrossRef]
- Pelliccia A, Maron BJ, Spataro A, et al. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med 1991, 324, 295–301. [Google Scholar] [CrossRef]
- Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef]
- Maron BJ, Pelliccia A. The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation 2006, 114, 1633–1644. [Google Scholar] [CrossRef]
- Maron, BJ. Structural features of the athlete heart as defined by echocardiography. J Am Coll Cardiol 1986, 7, 190–203. [Google Scholar] [CrossRef]
- Augustine DX, Howard L. Left Ventricular Hypertrophy in Athletes: Differentiating Physiology From Pathology. Curr Treat Options Cardiovasc Med 2018, 20, 96. [Google Scholar] [CrossRef]
- Go AS, Mozaffarian D, Roger VL, et al. Heart Disease and Stroke Statistics—2014 Update: A Report From the American Heart Association. Circulation; 129. Epub ahead of print 21 January 2014. [CrossRef]
- Lattanzi F, Di Bello V, Picano E, et al. Normal ultrasonic myocardial reflectivity in athletes with increased left ventricular mass. A tissue characterization study. Circulation 1992, 85, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia A, Maron BJ, De Luca R, et al. Remodeling of Left Ventricular Hypertrophy in Elite Athletes After Long-Term Deconditioning. Circulation 2002, 105, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Gosse P, Cremer A, Vircoulon M, et al. Prognostic value of the extent of left ventricular hypertrophy and its evolution in the hypertensive patient. J Hypertens 2012, 30, 2403–2409. [Google Scholar] [CrossRef]
- Drazner, MH. The Progression of Hypertensive Heart Disease. Circulation 2011, 123, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013, 34, 2159–2219. [Google Scholar] [CrossRef]
- Ohkuma T, Komorita Y, Peters SAE, et al. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia 2019, 62, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Gulsin GS, Swarbrick DJ, Hunt WH, et al. Relation of Aortic Stiffness to Left Ventricular Remodeling in Younger Adults With Type 2 Diabetes. Diabetes 2018, 67, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Matsue Y, Suzuki M, Nakamura R, et al. Prevalence and prognostic implications of pre-diabetic state in patients with heart failure. Circ J Off J Jpn Circ Soc 2011, 75, 2833–2839. [Google Scholar]
- Miki T, Yuda S, Kouzu H, et al. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev 2013, 18, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Marwick TH, Ritchie R, Shaw JE, et al. Implications of Underlying Mechanisms for the Recognition and Management of Diabetic Cardiomyopathy. J Am Coll Cardiol 2018, 71, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Boyer JK, Thanigaraj S, Schechtman KB, et al. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol 2004, 93, 870–875. [Google Scholar] [CrossRef]
- Zabalgoitia M, Ismaeil MF, Anderson L, et al. Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol 2001, 87, 320–323. [Google Scholar] [CrossRef]
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Dawson A, Morris AD, Struthers AD. The epidemiology of left ventricular hypertrophy in type 2 diabetes mellitus. Diabetologia 2005, 48, 1971–1979. [Google Scholar] [CrossRef]
- Huynh K, McMullen JR, Julius TL, et al. Cardiac-specific IGF-1 receptor transgenic expression protects against cardiac fibrosis and diastolic dysfunction in a mouse model of diabetic cardiomyopathy. Diabetes 2010, 59, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Bowden MA, Tesch GH, Julius TL, et al. Earlier onset of diabesity-Induced adverse cardiac remodeling in female compared to male mice. Obes Silver Spring Md 2015, 23, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Shimizu M, Umeda K, Sugihara N, et al. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol 1993, 46, 32–36. [Google Scholar] [CrossRef]
- Di Carli MF, Janisse J, Grunberger G, et al. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol 2003, 41, 1387–1393. [Google Scholar] [CrossRef]
- Huynh K, Bernardo BC, McMullen JR, et al. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther 2014, 142, 375–415. [Google Scholar] [CrossRef] [PubMed]
- Ritchie RH, Abel ED. Basic Mechanisms of Diabetic Heart Disease. Circ Res 2020, 126, 1501–1525. [Google Scholar] [CrossRef]
- Jastreboff AM, Kotz CM, Kahan S, et al. Obesity as a Disease: The Obesity Society 2018 Position Statement. Obes Silver Spring Md 2019, 27, 7–9. [Google Scholar] [CrossRef]
- Gordon-Larsen P, Heymsfield SB. Obesity as a Disease, Not a Behavior. Circulation 2018, 137, 1543–1545. [Google Scholar] [CrossRef]
- GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Katta N, Loethen T, Lavie CJ, et al. Obesity and Coronary Heart Disease: Epidemiology, Pathology, and Coronary Artery Imaging. Curr Probl Cardiol 2021, 46, 100655. [Google Scholar] [CrossRef] [PubMed]
- Koliaki C, Liatis S, Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Després J-P. Body fat distribution and risk of cardiovascular disease: an update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar]
- Bastien M, Poirier P, Lemieux I, et al. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 2014, 56, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Taqueti VR, Di Carli MF. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J Am Coll Cardiol 2018, 72, 2625–2641. [Google Scholar] [CrossRef]
- Bogers RP, Bemelmans WJE, Hoogenveen RT, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med 2007, 167, 1720–1728. [Google Scholar] [CrossRef]
- Csige I, Ujvárosy D, Szabó Z, et al. The Impact of Obesity on the Cardiovascular System. J Diabetes Res 2018, 2018, 3407306. [Google Scholar]
- Neeland IJ, Gupta S, Ayers CR, et al. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging 2013, 6, 800–807. [Google Scholar] [CrossRef]
- Pandey A, LaMonte M, Klein L, et al. Relationship Between Physical Activity, Body Mass Index, and Risk of Heart Failure. J Am Coll Cardiol 2017, 69, 1129–1142. [Google Scholar] [CrossRef]
- Zanettini R, Bettega D, Agostoni O, et al. Exercise Training in Mild Hypertension: Effects on Blood Pressure, Left Ventricular Mass and Coagulation Factor VII and Fibrinogen. Cardiology 1997, 88, 468–473. [Google Scholar] [CrossRef]
- Kokkinos PF, Narayan P, Colleran JA, et al. Effects of Regular Exercise on Blood Pressure and Left Ventricular Hypertrophy in African-American Men with Severe Hypertension. N Engl J Med 1995, 333, 1462–1467. [Google Scholar] [CrossRef]
- Korsager Larsen M, Matchkov VV. Hypertension and physical exercise: The role of oxidative stress. Med Kaunas Lith 2016, 52, 19–27. [Google Scholar]
- Vona M, Rossi A, Capodaglio P, et al. Impact of physical training and detraining on endothelium-dependent vasodilation in patients with recent acute myocardial infarction. Am Heart J 2004, 147, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Gielen S, Schuler G, Adams V. Cardiovascular Effects of Exercise Training: Molecular Mechanisms. Circulation 2010, 122, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Pitsavos C, Chrysohoou C, Koutroumbi M, et al. The impact of moderate aerobic physical training on left ventricular mass, exercise capacity and blood pressure response during treadmill testing in borderline and mildly hypertensive males. Hell J Cardiol HJC Hell Kardiologike Epitheorese 2011, 52, 6–14. [Google Scholar]
- Turner MJ, Spina RJ, Kohrt WM, et al. Effect of endurance exercise training on left ventricular size and remodeling in older adults with hypertension. J Gerontol A Biol Sci Med Sci 2000, 55, M245–M251. [Google Scholar] [CrossRef] [PubMed]
- Seals DR, Reiling MJ. Effect of regular exercise on 24-hour arterial pressure in older hypertensive humans. Hypertens Dallas Tex 1979 1991, 18, 583–592. [Google Scholar]
- Hagberg JM, Montain SJ, Martin WH, et al. Effect of exercise training in 60- to 69-year-old persons with essential hypertension. Am J Cardiol 1989, 64, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Lou M, Zong X-F, Wang L-L. Curative treatment of hypertension by physical exercise. Eur Rev Med Pharmacol Sci 2017, 21, 3320–3326. [Google Scholar]
- Kokkinos P, Pittaras A, Narayan P, et al. Exercise Capacity and Blood Pressure Associations With Left Ventricular Mass in Prehypertensive Individuals. Hypertension 2007, 49, 55–61. [Google Scholar] [CrossRef]
- Palatini P, Visentin P, Dorigatti F, et al. Regular physical activity prevents development of left ventricular hypertrophy in hypertension. Eur Heart J 2009, 30, 225–232. [Google Scholar]
- Gusso S, Pinto T, Baldi JC, et al. Exercise Training Improves but Does Not Normalize Left Ventricular Systolic and Diastolic Function in Adolescents With Type 1 Diabetes. Diabetes Care 2017, 40, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Verboven M, Van Ryckeghem L, Belkhouribchia J, et al. Effect of Exercise Intervention on Cardiac Function in Type 2 Diabetes Mellitus: A Systematic Review. Sports Med Auckl NZ 2019, 49, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Cassidy S, Thoma C, Hallsworth K, et al. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: a randomised controlled trial. Diabetologia 2016, 59, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Otten J, Andersson J, Ståhl J, et al. Exercise Training Adds Cardiometabolic Benefits of a Paleolithic Diet in Type 2 Diabetes Mellitus. J Am Heart Assoc 2019, 8, e010634. [Google Scholar] [CrossRef] [PubMed]
- Gulsin GS, Swarbrick DJ, Athithan L, et al. Effects of Low-Energy Diet or Exercise on Cardiovascular Function in Working-Age Adults With Type 2 Diabetes: A Prospective, Randomized, Open-Label, Blinded End Point Trial. Diabetes Care 2020, 43, 1300–1310. [Google Scholar] [CrossRef]
- Piché M-E, Poirier P, Marette A, et al. Benefits of 1-Year Lifestyle Modification Program on Exercise Capacity and Diastolic Function Among Coronary Artery Disease Men With and Without Type 2 Diabetes. Metab Syndr Relat Disord 2019, 17, 149–159. [Google Scholar] [CrossRef]
- Kamimura D, Loprinzi PD, Wang W, et al. Physical Activity Is Associated With Reduced Left Ventricular Mass in Obese and Hypertensive African Americans. Am J Hypertens 2017, 30, 617–623. [Google Scholar] [CrossRef]
- Himeno E, Nishino K, Nakashima Y, et al. Weight reduction regresses left ventricular mass regardless of blood pressure level in obese subjects. Am Heart J 1996, 131, 313–319. [Google Scholar] [CrossRef]
- Voulgari C, Pagoni S, Vinik A, et al. Exercise improves cardiac autonomic function in obesity and diabetes. Metabolism 2013, 62, 609–621. [Google Scholar] [CrossRef]
- Carbone S, Del Buono MG, Ozemek C, et al. Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog Cardiovasc Dis 2019, 62, 327–333. [Google Scholar] [CrossRef]
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990, 81, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 2000, 35, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med 2004, 116, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Koren MJ, Devereux RB. Mechanism, effects, and reversal of left ventricular hypertrophy in hypertension. Curr Opin Nephrol Hypertens 1993, 2, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Giallauria F, Cirillo P, Lucci R, et al. Left ventricular remodelling in patients with moderate systolic dysfunction after myocardial infarction: favourable effects of exercise training and predictive role of N -terminal pro-brain natriuretic peptide. Eur J Cardiovasc Prev Rehabil 2008, 15, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Giallauria F, Galizia G, Lucci R, et al. Favourable effects of exercise-based Cardiac Rehabilitation after acute myocardial infarction on left atrial remodeling. Int J Cardiol 2009, 136, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Haykowsky M, Scott J, Esch B, et al. A Meta-analysis of the effects of Exercise Training on Left Ventricular Remodeling Following Myocardial Infarction: Start early and go longer for greatest exercise benefits on remodeling. Trials 2011, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Giallauria F, Lorenzo AD, Pilerci F, et al. Reduction of N terminal-pro-brain (B-type) natriuretic peptide levels with exercise-based cardiac rehabilitation in patients with left ventricular dysfunction after myocardial infarction. Eur J Cardiovasc Prev Rehabil 2006, 13, 625–632. [Google Scholar] [CrossRef]
- Wen Y, Zhang X, Lan W, et al. Effects of Cardiac Rehabilitation on Cardiac Function and Quality of Life in Patients with Ischemic Nonobstructive Coronary Artery Disease and Diabetes Mellitus. BioMed Res Int 2022, 2022, 1–5. [Google Scholar]
- Zhang Y-M, Lu Y, Tang Y, et al. The effects of different initiation time of exercise training on left ventricular remodeling and cardiopulmonary rehabilitation in patients with left ventricular dysfunction after myocardial infarction. Disabil Rehabil 2016, 38, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Wisløff U, Støylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef] [PubMed]
- Pandey A, LaMonte M, Klein L, et al. Relationship Between Physical Activity, Body Mass Index, and Risk of Heart Failure. J Am Coll Cardiol 2017, 69, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Pandey A, Allen NB, Ayers C, et al. Fitness in Young Adulthood and Long-Term Cardiac Structure and Function. JACC Heart Fail 2017, 5, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Kitzman DW, Nicklas B, Kraus WE, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol 2014, 306, H1364–H1370. [Google Scholar] [CrossRef] [PubMed]
- Horwich TB, Fonarow GC, Clark AL. Obesity and the Obesity Paradox in Heart Failure. Prog Cardiovasc Dis 2018, 61, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Pandey A, Cornwell WK, Willis B, et al. Body Mass Index and Cardiorespiratory Fitness in Mid-Life and Risk of Heart Failure Hospitalization in Older Age. JACC Heart Fail 2017, 5, 367–374. [Google Scholar] [CrossRef]
- Pandey A, Patel M, Gao A, et al. Changes in mid-life fitness predicts heart failure risk at a later age independent of interval development of cardiac and noncardiac risk factors: The Cooper Center Longitudinal Study. Am Heart J 2015, 169, 290–297.e1. [Google Scholar] [CrossRef]
- Heran BS, Chen JM, Ebrahim S, et al. Exercise-based cardiac rehabilitation for coronary heart disease. In: The Cochrane Collaboration (ed) Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd, p. CD001800.pub2.
- Pandey A, Parashar A, Kumbhani DJ, et al. Exercise Training in Patients With Heart Failure and Preserved Ejection Fraction: Meta-Analysis of Randomized Control Trials. Circ Heart Fail 2015, 8, 33–40. [Google Scholar] [CrossRef]
- Davies EJ, Moxham T, Rees K, et al. Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. Eur J Heart Fail 2010, 12, 706–715. [Google Scholar] [CrossRef]
- Edwards JJ, O’Driscoll JM. Exercise Training in Heart failure with Preserved and Reduced Ejection Fraction: A Systematic Review and Meta-Analysis. Sports Med - Open 2022, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and Safety of Exercise Training in Patients With Chronic Heart Failure: HF-ACTION Randomized Controlled Trial. JAMA 2009, 301, 1439. [Google Scholar] [CrossRef] [PubMed]
- van Tol BAF, Huijsmans RJ, Kroon DW, et al. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: A meta-analysis. Eur J Heart Fail 2006, 8, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Sandri M, Kozarez I, Adams V, et al. Age-related effects of exercise training on diastolic function in heart failure with reduced ejection fraction: The Leipzig Exercise Intervention in Chronic Heart Failure and Aging (LEICA) Diastolic Dysfunction Study. Eur Heart J 2012, 33, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Haykowsky MJ, Timmons MP, Kruger C, et al. Meta-analysis of aerobic interval training on exercise capacity and systolic function in patients with heart failure and reduced ejection fractions. Am J Cardiol 2013, 111, 1466–1469. [Google Scholar] [CrossRef]
- Hegde SM, Claggett B, Shah AM, et al. Physical Activity and Prognosis in the TOPCAT Trial (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist). Circulation 2017, 136, 982–992. [Google Scholar] [CrossRef]
- Heizer J, Carbone S, Billingsley HE, et al. Left ventricular concentric remodeling and impaired cardiorespiratory fitness in patients with heart failure and preserved ejection fraction. Minerva Cardiol Angiol. Epub ahead of print September 2020. [CrossRef]
- Whelton SP, Chin A, Xin X, et al. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 2002, 136, 493–503. [Google Scholar] [CrossRef]
- Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol 1997, 59, 551–571. [Google Scholar] [CrossRef]
- Pearson MJ, Smart NA. Effect of exercise training on endothelial function in heart failure patients: A systematic review meta-analysis. Int J Cardiol 2017, 231, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Siasos G, Athanasiou D, Terzis G, et al. Acute effects of different types of aerobic exercise on endothelial function and arterial stiffness. Eur J Prev Cardiol 2016, 23, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Ehsani AA, Biello DR, Schultz J, et al. Improvement of left ventricular contractile function by exercise training in patients with coronary artery disease. Circulation 1986, 74, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Belardinelli R, Georgiou D, Ginzton L, et al. Effects of Moderate Exercise Training on Thallium Uptake and Contractile Response to Low-Dose Dobutamine of Dysfunctional Myocardium in Patients With Ischemic Cardiomyopathy. Circulation 1998, 97, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Brehm M, Picard F, Ebner P, et al. Effects of exercise training on mobilization and functional activity of blood-derived progenitor cells in patients with acute myocardial infarction. Eur J Med Res 2009, 14, 393. [Google Scholar] [CrossRef] [PubMed]
- Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J 2002, 16, 1335–1347. [Google Scholar] [CrossRef]
- Thomas DP, McCormick RJ, Zimmerman SD, et al. Aging- and training-induced alterations in collagen characteristics of rat left ventricle and papillary muscle. Am J Physiol 1992, 263, H778–H783. [Google Scholar]
- Melo SFS, Fernandes T, Baraúna VG, et al. Expression of MicroRNA-29 and Collagen in Cardiac Muscle after Swimming Training in Myocardial-Infarcted Rats. Cell Physiol Biochem 2014, 33, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Martinez DG, Nicolau JC, Lage RL, et al. Effects of Long-Term Exercise Training on Autonomic Control in Myocardial Infarction Patients. Hypertension 2011, 58, 1049–1056. [Google Scholar] [CrossRef]
- Malfatto G, Facchini M, Bragato R, et al. Short and long term effects of exercise training on the tonic autonomic modulation of heart rate variability after myocardial infarction. Eur Heart J 1996, 17, 532–538. [Google Scholar] [CrossRef]
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e563–e595. [Google Scholar]
- Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016, 37, 2315–2381. [Google Scholar]
- Pelliccia A, Sharma S, Gati S, et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2021, 42, 17–96. [Google Scholar] [CrossRef] [PubMed]
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Hansen D, Dendale P, Coninx K, et al. The European Association of Preventive Cardiology Exercise Prescription in Everyday Practice and Rehabilitative Training (EXPERT) tool: A digital training and decision support system for optimized exercise prescription in cardiovascular disease. Concept, definitions and construction methodology. Eur J Prev Cardiol 2017, 24, 1017–1031. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





