Submitted:
12 June 2023
Posted:
13 June 2023
You are already at the latest version
Abstract
Keywords:
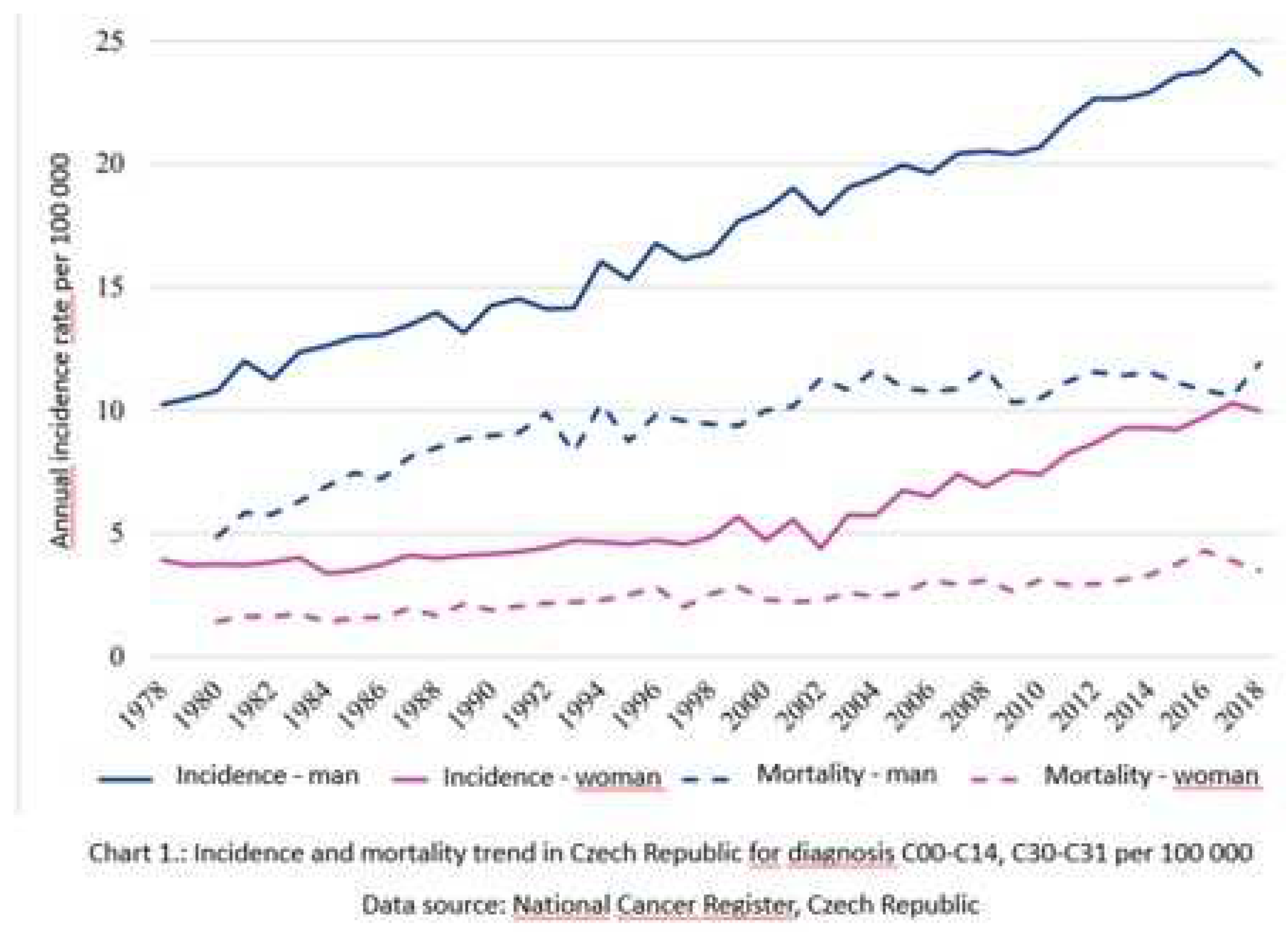
1. Introduction
2. Materials and Methods
3. Results
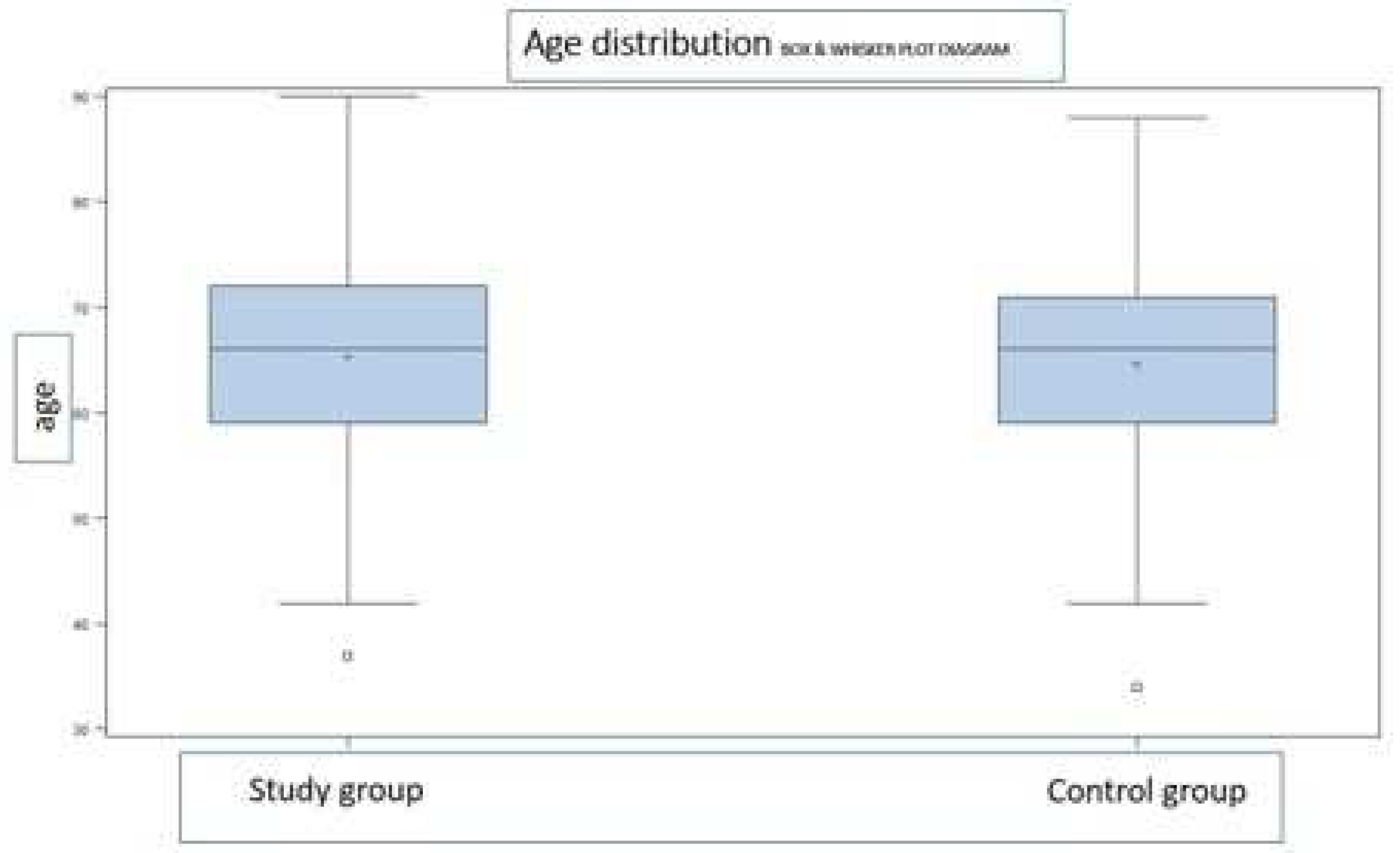
3.1. Groups characteristics
3.2. Treatment outcomes comparison
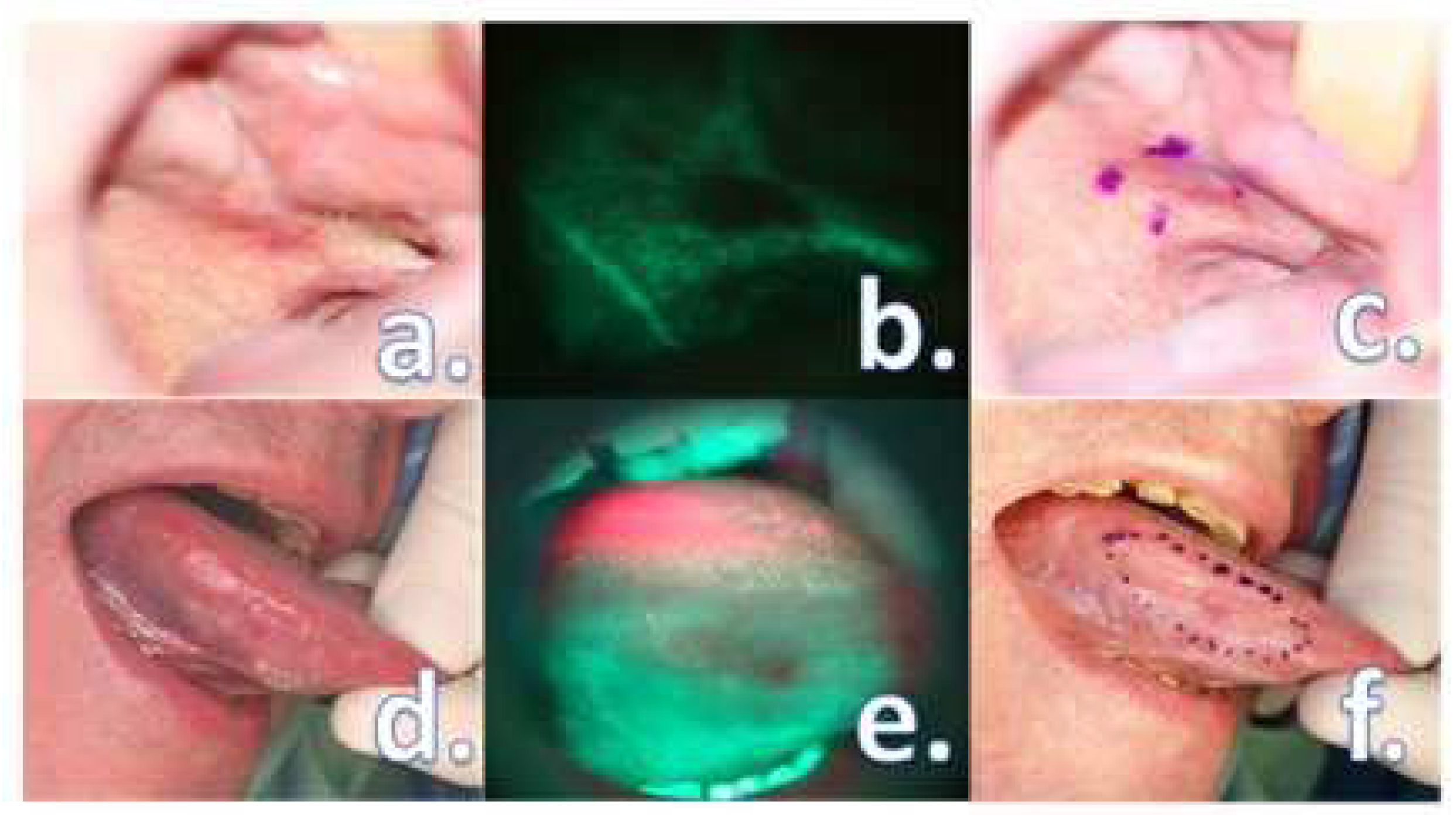
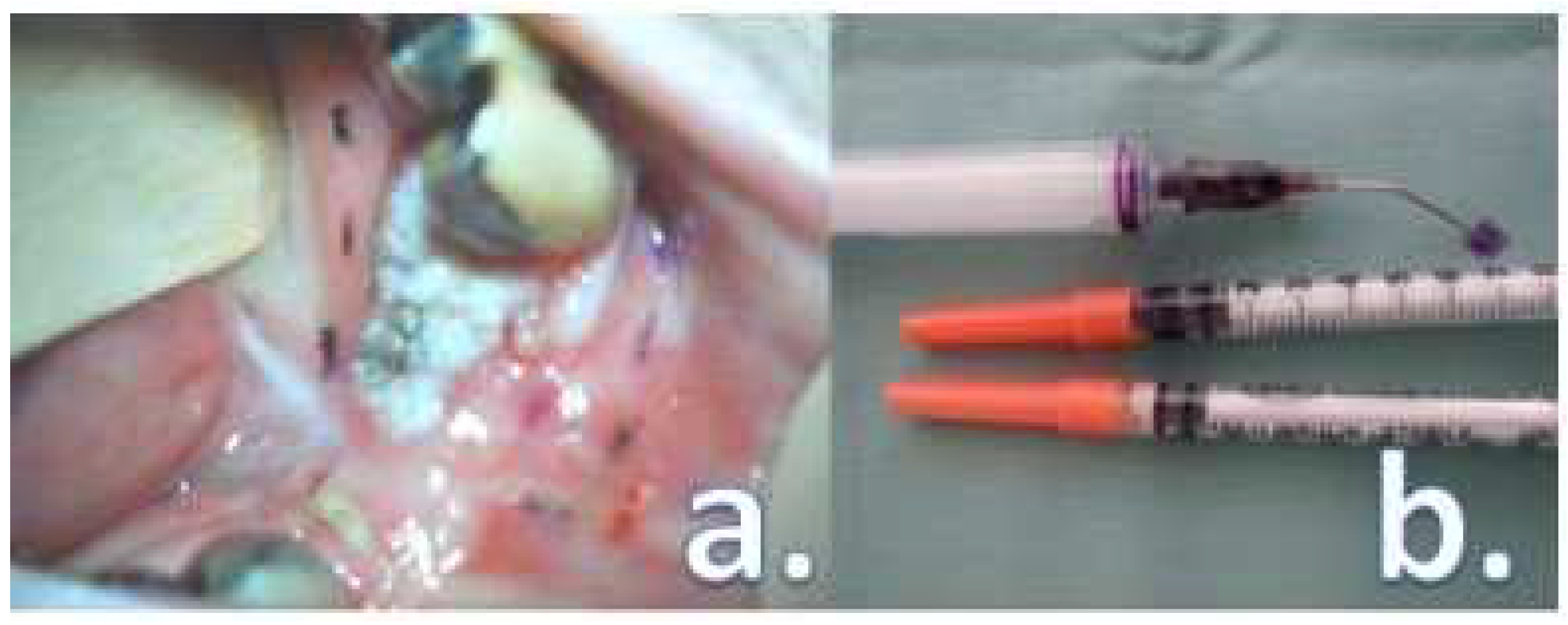
- The loss of physiologic fluorescence resulted in a resection enlargement of 4.68 mm in average (1 – 12 mm) compared to the polychromatic light and the tactile tumor borders assessment.
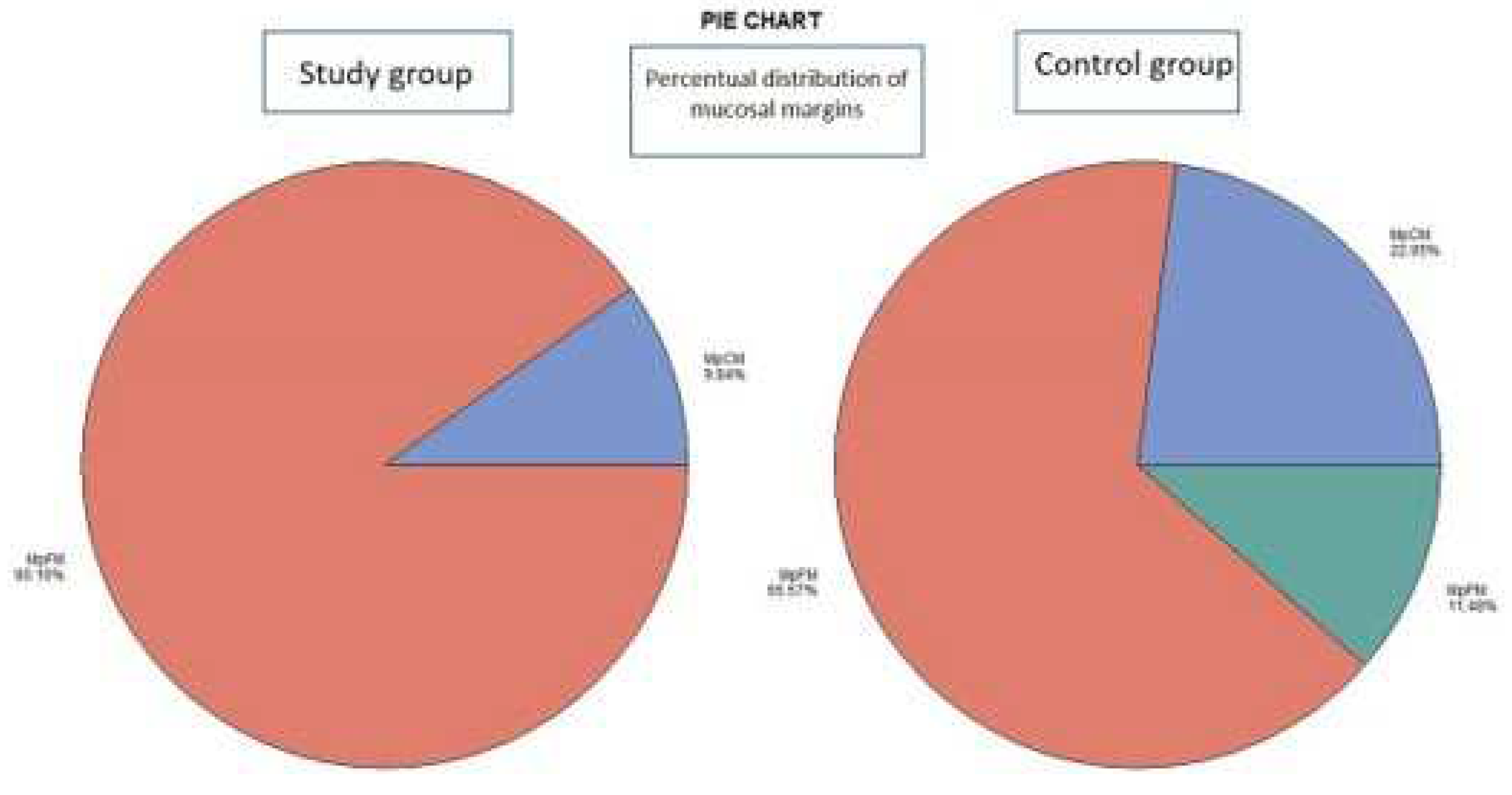
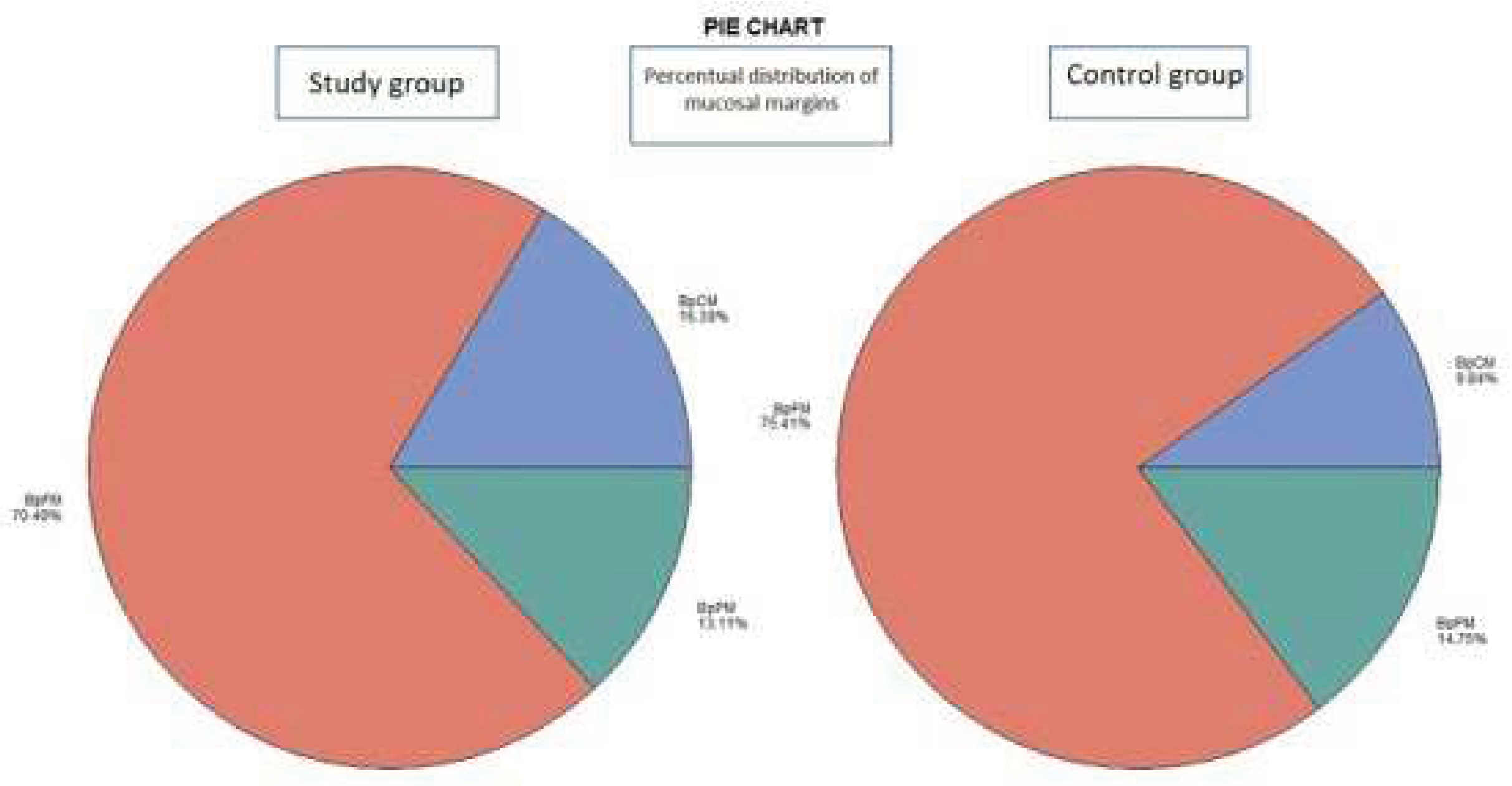
- The histological examination revealed no case of pPM (n = 0; 0 %), six cases of pCM (n = 6; 9.84 %) and fifty-five specimens were pFM (n = 55; 90.16 %) in the mucosal margins in the study group. The situation in the control group was as follows: pPM (n = 7; 11.48 %), pCM (n = 14; 22.95 %), pFM (n = 40; 65.57 %) (Chart 3). Although the autofluorescence technique has no influence on the deep surgical margins, we present the deep margin situation for comprehensiveness – in the study group we encountered pPM in eight cases (n = 8; 13.11 %), pCM in ten cases (n = 10; 16.39 %) and pFM in forty-three cases (n = 43; 70.49 %), in the control group situation is as follows: pPM (9; 14.75 %), pCM (6; 9.84 %), pFM (46; 75.41 %) (Chart 4).
3.3. Observed effect of autofluorescence assistance
- As expected, our study proved a statistically significant difference between the two groups in the mucosal layer only (see table 5). There was no significant difference in deep margins. Despite of the larger resection in the study group, there was no significant increase in postoperative morbidity with regard to either swallowing or speech. No patient experienced major complications in the postoperative period and no patient was discharged with a feeding tube or tracheostomy. The overall treatment outcome in terms of surgical tumor eradication was significantly better in the study group compared to the control group.
| Margin status | Frequency – amount / percentil | Total | |
| Study group | Control group | ||
| MpFM | 55 / 90.16 | 40 / 65.57 | 54 |
| MpCM | 6 / 9.84 | 14 / 22.95 | 56 |
| MpPM | 0 / 0.00 | 7 / 11.48 | 12 |
| Total | 61 | 61 | 122 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cariati, P.; Pampin Ozan, D.; Gonzalez Corcóles, C.; Tursun, R.; Peña Barreño, M.; Ferrari, S.; Arroyo Rodriguez, S. Clinical Behavior of T1–T2 Squamous Cell Carcinoma of the Oral Cavity. J. Cranio-Maxillofacial Surg. 2020, 48, 1152–1157. [Google Scholar] [CrossRef]
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European Cancer Burden in 2020: Incidence and Mortality Estimates for 40 Countries and 25 Major Cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef]
- Bschorer, M.; Schneider, D.; Goppold, K.; Sperling, J.; Schön, G.; Bschorer, R. Quality of Life and Survival Rate after Primary Surgical Treatment of Oral Squamous Cell Carcinoma: A Retrospective Study with 18 Years of Follow-Up. J. Cranio-Maxillofacial Surg. 2022, 50, 170–177. [Google Scholar] [CrossRef]
- Low, T.H.H.; Gao, K.; Gupta, R.; Clifford, A.; Elliott, M.; Ch’ng, S.; Milross, C.; Clark, J.R. Factors Predicting Poor Outcomes in T1N0 Oral Squamous Cell Carcinoma: Indicators for Treatment Intensification. ANZ J. Surg. 2016, 86, 366–371. [Google Scholar] [CrossRef]
- Loeffelbein, D.J.; Eiber, M.; Mayr, P.; Souvatzoglou, M.; Mücke, T.; von Bomhard, A.; Kesting, M.R.; Wolff, K.-D. Loco-Regional Recurrence after Surgical Treatment of Oral Squamous Cell Carcinoma: Proposals for Follow-up Imaging Based on Literature, National Guidelines and Institutional Experience. J. Cranio-Maxillo-Facial Surg. 2017, 43, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Ravasz, L.A.; Slootweg, P.J.; Hordijk, G.J.; Smit, F.; van der Tweel, I. The Status of the Resection Margin as a Prognostic Factor in the Treatment of Head and Neck Carcinoma. J. Cranio-Maxillo-Facial Surg. 2017, 19, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Ermer, M.A.; Kirsch, K.; Bittermann, G.; Fretwurst, T.; Vach, K.; Metzger, M.C. Recurrence Rate and Shift in Histopathological Differentiation of Oral Squamous Cell Carcinoma – A Long-Term Retrospective Study over a Period of 13.5 Years. J. Cranio-Maxillofacial Surg. 2015, 43, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- van der Waal, I. Are We Able to Reduce the Mortality and Morbidity of Oral Cancer; Some Considerations. Med. Oral Patol. Oral Cir. Bucal 2013, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Wang, C.P.; Ko, J.Y.; Yang, T.L.; Lou, P.J. The Impact of Pathologic Close Margin on the Survival of Patients with Early Stage Oral Squamous Cell Carcinoma. Oral Oncol. 2012.
- Wong, L.S.; McMahon, J.; Devine, J.; McLellan, D.; Thompson, E.; Farrow, A.; Moos, K.; Ayoub, A. Influence of Close Resection Margins on Local Recurrence and Disease-Specific Survival in Oral and Oropharyngeal Carcinoma. Br. J. Oral Maxillofac. Surg. 2012, 50, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Hinni, M.L.; Ferlito, A.; Brandwein-Gensler, M.S.; Takes, R.P.; Silver, C.E.; Westra, W.H.; Seethala, R.R.; Rodrigo, J.P.; Corry, J.; Bradford, C.R.; et al. Surgical Margins in Head and Neck Cancer: A Contemporary Review. Head Neck 2013, 35, 1362–1370. [Google Scholar] [CrossRef]
- Krishnan, G.; van den Berg, N.S.; Nishio, N.; Kapoor, S.; Pei, J.; Freeman, L.; Lee, Y.J.; Zhou, Q.; van Keulen, S.; Farkurnejad, S.; et al. Fluorescent Molecular Imaging Can Improve Intraoperative Sentinel Margin Detection in Oral Squamous Cell Carcinoma. J. Nucl. Med. 2022, 63, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J.; de Wit, J.G.; Voskuil, F.J.; Witjes, M.J.H. Improving Oral Cavity Cancer Diagnosis and Treatment with Fluorescence Molecular Imaging. Oral Dis. 2021, 27, 21–26. [Google Scholar] [CrossRef]
- Wang, R.; Naidu, A.; Wang, Y. Oral Cancer Discrimination and Novel Oral Epithelial Dysplasia Stratification Using FTIR Imaging and Machine Learning. Diagnostics 2021, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, S.; Teraphongphom, N.; Tweedle, M.F.; Basilion, J.P.; Rosenthal, E.L. Optical Surgical Navigation for Precision in Tumor Resections. Mol. Imaging Biol. 2017, 19, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Grochau, K.J.; Safi, A.F.; Drebber, U.; Grandoch, A.; Zöller, J.E.; Kreppel, M. Podoplanin Expression in Oral Leukoplakia─a Prospective Study. J. Cranio-Maxillofacial Surg. 2019, 47, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Deng, H.; Hu, S.; Xia, C.; Chen, Y.; Wang, J.; Wang, Y. Real-Time Surveillance of Surgical Margins via ICG-Based near-Infrared Fluorescence Imaging in Patients with OSCC. World J. Surg. Oncol. 2020, 18, 96. [Google Scholar] [CrossRef]
- Tian, Y.; Tang, C.; Shi, G.; Wang, G.; Du, Y.; Tian, J.; Zhang, H. Novel Fluorescent GLUT1 Inhibitor for Precision Detection and Fluorescence Image-Guided Surgery in Oral Squamous Cell Carcinoma. Int. J. cancer 2022, 151, 450–462. [Google Scholar] [CrossRef]
- Rocchetti, F.; Tenore, G.; Montori, A.; Cassoni, A.; Cantisani, V.; Di Segni, M.; Di Gioia, C.R.T.; Carletti, R.; Valentini, V.; Polimeni, A.; et al. Preoperative Evaluation of Tumor Depth of Invasion in Oral Squamous Cell Carcinoma with Intraoral Ultrasonography: A Retrospective Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 130–138. [Google Scholar] [CrossRef]
- Klein Nulent, T.J.W.; Noorlag, R.; Van Cann, E.M.; Pameijer, F.A.; Willems, S.M.; Yesuratnam, A.; Rosenberg, A.J.W.P.; de Bree, R.; van Es, R.J.J. Intraoral Ultrasonography to Measure Tumor Thickness of Oral Cancer: A Systematic Review and Meta-Analysis. Oral Oncol. 2018, 77, 29–36. [Google Scholar] [CrossRef]
- Caprioli, S.; Casaleggio, A.; Tagliafico, A.S.; Conforti, C.; Borda, F.; Fiannacca, M.; Filauro, M.; Iandelli, A.; Marchi, F.; Parrinello, G.; et al. High-Frequency Intraoral Ultrasound for Preoperative Assessment of Depth of Invasion for Early Tongue Squamous Cell Carcinoma: Radiological-Pathological Correlations. Int. J. Environ. Res. Public Health 2022, 19. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Su, Y.F.; Cheng, H.C.; Juan, Y.C.; Chiu, Y.W.; Wu, C.H.; Chen, P.Y.; Lee, Y.H.; Chen, Y.L.; Chen, Y.T.; et al. Improving the Diagnostic Performance by Adding Methylation Marker to Conventional Visual Examination in Identifying Oral Cancer. Diagnostics 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Fati, S.M.; Senan, E.M.; Javed, Y. Early Diagnosis of Oral Squamous Cell Carcinoma Based on Histopathological Images Using Deep and Hybrid Learning Approaches. Diagnostics 2022, 12. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kwon, T.G.; Kim, J.W.; Lee, S.T.; Hong, S.H.; Choi, S.Y. Evaluation of Depth of Invasion and Tumor Thickness as a Prognostic Factor for Early-Stage Oral Squamous Cell Carcinoma: A Retrospective Study. Diagnostics 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Tarsitano, A.; Ricotta, F.; Baldino, G.; Badiali, G.; Pizzigallo, A.; Ramieri, V.; Cascone, P.; Marchetti, C. Navigation-Guided Resection of Maxillary Tumours: The Accuracy of Computer-Assisted Surgery in Terms of Control of Resection Margins – A Feasibility Study. J. Cranio-Maxillofacial Surg. 2017. [Google Scholar] [CrossRef]
- Giovannacci, I.; Magnoni, C.; Vescovi, P.; Painelli, A.; Tarentini, E.; Meleti, M. Which Are the Main Fluorophores in Skin and Oral Mucosa? A Review with Emphasis on Clinical Applications of Tissue Autofluorescence. Arch. Oral Biol. 2019, 105, 89–98. [Google Scholar] [CrossRef]
- Laronde, D.M.; Williams, P.M.; Hislop, T.G.; Poh, C.; Ng, S.; Bajdik, C.; Zhang, L.; Macaulay, C.; Rosin, M.P. Influence of Fluorescence on Screening Decisions for Oral Mucosal Lesions in Community Dental Practices. J. Oral Pathol. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Burian, E.; Schulz, C.; Probst, F.; Palla, B.; Tröltzsch, M.; Maglitto, F.; Califano, L.; Ehrenfeld, M.; Otto, S. Fluorescence Based Characterization of Early Oral Squamous Cell Carcinoma Using the Visually Enhanced Light Scope Technique. J. Cranio-Maxillo-Facial Surg. 2017. [Google Scholar] [CrossRef]
- Scheer, M.; Neugebauer, J.; Lingohr, T.; Zöller, J.E. O. 317 Evaluation of Undiagnosed Mucosa Lesions with the VELscope System. J. Cranio-Maxillo-Facial Surg. 2017, 36, S80. [Google Scholar] [CrossRef]
- Sambandham, T.; Masthan, K.M.K.; Kumar, M.S.; Jha, A. The Application of Vizilite in Oral Cancer. J. Clin. Diagn. Res. 2013, 7, 185–186. [Google Scholar] [CrossRef]
- Low, T.H.; Gao, K.; Elliott, M.; Clark, J.R. Tumor Classification for Early Oral Cancer: Re-Evaluate the Current TNM Classification. Head Neck 2015, 37, 223–228. [Google Scholar] [CrossRef]
- Tasche, K.K.; Buchakjian, M.R.; Pagedar, N.A.; Sperry, S.M. Definition of “Close Margin” in Oral Cancer Surgery and Association of Margin Distance With Local Recurrence Rate Supplemental Content. JAMA Otolaryngol Head Neck Surg 2017, 143, 1166–1172. [Google Scholar] [CrossRef]
- Park, H. Surgical Margins for the Extirpation of Oral Cancer. J. Korean Assoc. Oral Maxillofac. Surg. 2016, 42, 325–326. [Google Scholar] [CrossRef]
- Brinkman, D.; Callanan, D.; O’Shea, R.; Jawad, H.; Feeley, L.; Sheahan, P. Impact of 3 Mm Margin on Risk of Recurrence and Survival in Oral Cancer. Oral Oncol. 2020, 110, 104883. [Google Scholar] [CrossRef]
- Fenton, M.; Foote, R.L.; Galloway, T.; Gillison, M.L.; Haddad, R.I.; Hicks, W.L.; Hitchcock, Y.J.; Jimeno, A.; Leizman, D.; Pinto, H.A.; et al. Continue NCCN Guidelines Panel Disclosures NCCN Gratefully Acknowledges the Following Subcommittee Member for Her Contributions on the Development of the Principles of Imaging (IMG-A) NCCN Guidelines Version 2. 2020. [Google Scholar]
- El-Fol, H.A.; Noman, S.A.; Beheiri, M.G.; Khalil, A.M.; Kamel, M.M. Significance of Post-Resection Tissue Shrinkage on Surgical Margins of Oral Squamous Cell Carcinoma. J. Craniomaxillofac. Surg. 2015, 43, 475–482. [Google Scholar] [CrossRef]
- Mistry, R.; Qureshi, S.; Kumaran, C. Post-Resection Mucosal Margin Shrinkage in Oral Cancer: Quantification and Significance. J. Surg. Oncol. 2005, 91, 131–133. [Google Scholar] [CrossRef]
- International Statistical Classification of Diseases and Related Health Problems. Tenth Revision - PubMed Available online:. Available online: https://pubmed.ncbi.nlm.nih.gov/3376487/ (accessed on 15 February 2023).
- Koerdt, S.; Röckl, J.; Rommel, N.; Mücke, T.; Wolff, K.-D.; Kesting, M.R. Lymph Node Management in the Treatment of Oral Cancer: Analysis of a Standardized Approach. J. Cranio-Maxillo-Facial Surg. 2017, 44, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Wolff, K.-D.; Rau, A.; Ferencz, J.; Langer, T.; Kesting, M.; Nieberler, M.; Wesselmann, S. Effect of an Evidence-Based Guideline on the Treatment of Maxillofacial Cancer: A Prospective Analysis. J. Cranio-Maxillo-Facial Surg. 2017, 45, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Low, T.H.H.; Lindsay, A.; Clark, J.; Chai, F.; Lewis, R. Reconstruction of Maxillary Defect with Musculo-Adipose Rectus Free Flap. Microsurgery 2017, 37, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.F.P.; Brand, L.M.; de Figueiredo, J.A.P.; Steier, L.; Lamers, M.L. Use of Autofluorescence and Fluorescent Probes as a Potential Diagnostic Tool for Oral Cancer: A Systematic Review. Photodiagnosis Photodyn. Ther. 2021, 33. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.F.; Wang, C.X.; Cao, Z.Y.; Han, W.; Guo, S.S.; Wang, Y.Z.; Meng, Y.; Hou, C.X.; Zhu, Q.H.; Tang, Y.T.; et al. Evaluation of Autofluorescence Visualization System in the Delineation of Oral Squamous Cell Carcinoma Surgical Margins. Photodiagnosis Photodyn. Ther. 2021, 36. [Google Scholar] [CrossRef] [PubMed]
- Durham, J.S.; Brasher, P.; Anderson, D.W.; Yoo, J.; Hart, R.; Dort, J.C.; Seikaly, H.; Kerr, P.; Rosin, M.P.; Poh, C.F. Effect of Fluorescence Visualization-Guided Surgery on Local Recurrence of Oral Squamous Cell Carcinoma: A Randomized Clinical Trial. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Poh, C.F.; Anderson, D.W.; Scott Durham, J.; Chen, J.; Berean, K.W.; MacAulay, C.E.; Rosin, M.P. Fluorescence Visualization-Guided Surgery for Early-Stage Oral Cancer. JAMA Otolaryngol. - Head Neck Surg. 2016, 142, 209–216. [Google Scholar] [CrossRef]
- Woolgar, J.A.; Rogers, S.; West, C.R.; Errington, R.D.; Brown, J.S.; Vaughan, E.D. Survival and Patterns of Recurrence in 200 Oral Cancer Patients Treated by Radical Surgery and Neck Dissection. Oral Oncol. 1999, 35, 257–265. [Google Scholar] [CrossRef]
- Jerjes, W.; Upile, T.; Petrie, A.; Riskalla, A.; Hamdoon, Z.; Vourvachis, M.; Karavidas, K.; Jay, A.; Sandison, A.; Thomas, G.J.; et al. Clinicopathological Parameters, Recurrence, Locoregional and Distant Metastasis in 115 T1-T2 Oral Squamous Cell Carcinoma Patients. Head Neck Oncol. 2010, 2, 9. [Google Scholar] [CrossRef]
- Popovic-Monevska, D.; Naumovski, S.; Popovski, V.; Benedetti, A.; Bozovic, S.; Iliev, A. O. 490 Loco Regional Recurrence of OSCC. J. Cranio-Maxillofacial Surg. 2008, 36, S123. [Google Scholar] [CrossRef]
- Alaeddini, M.; Etemad-Moghadam, S. Comparison of the Histologic Risk Assessment Model between Lower Lip and Oral Squamous Cell Carcinoma. J. Stomatol. Oral Maxillofac. Surg. 2017. [Google Scholar] [CrossRef]
- de Vicente, J.C.; Rúa-Gonzálvez, L.; Barroso, J.M.; Fernández del Valle-Fernández, Á.; de Villalaín, L.; Peña, I.; Cobo, J.L. Functional Results of Swallowing and Aspiration after Oral Cancer Treatment and Microvascular Free Flap Reconstruction: A Retrospective Observational Assessment. J. Craniomaxillofac. Surg. 2021, 49, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.D.; Oliver, D.A.; Varvares, M.A. Surgical Margin Determination in Head and Neck Oncology: Current Clinical Practice. The Results of an International American Head and Neck Society Member Survey. Head Neck 2005, 27, 952–958. [Google Scholar] [CrossRef]
- Woolgar, J.A.; Triantafyllou, A. A Histopathological Appraisal of Surgical Margins in Oral and Oropharyngeal Cancer Resection Specimens. Oral Oncol. 2005, 41, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Binahmed, A.; Nason, R.W.; Abdoh, A.A. The Clinical Significance of the Positive Surgical Margin in Oral Cancer. Oral Oncol. 2007, 43, 780–784. [Google Scholar] [CrossRef]
- Chiou, W.-Y.; Lin, H.-Y.; Hsu, F.-C.; Lee, M.-S.; Ho, H.-C.; Su, Y.-C.; Lee, C.-C.; Hsieh, C.-H.; Wang, Y.-C.; Hung, S.-K. Buccal Mucosa Carcinoma: Surgical Margin Less than 3 Mm, Not 5 Mm, Predicts Locoregional Recurrence. Radiat. Oncol. 2010, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Rougier, G.; Meningaud, J.P.; Ganry, L.; Hermeziu, O.; Bosc, R.; Sidahmed-Mezi, M.; Hersant, B. Oncological and Aesthetic Outcome Following Surgical Management of Orbito-Palpebral Skin Cancers: A Retrospective Study of 132 Patients. J. Cranio-Maxillofacial Surg. 2019, 47, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Kumakura, I.; Okui, T.; Karino, M.; Aoi, N.; Okuma, S.; Takeda, M.; Hayashida, K.; Sakamoto, T.; Kanno, T. Development of a Subjective Symptom Rating Scale for Postoperative Oral Dysfunction in Patients with Oral Cancer: Reliability and Validity of the Postoperative Oral Dysfunction Scale-10. Diagnostics 2021, Vol. 11, Page 2061 2021, 11, 2061. [Google Scholar] [CrossRef] [PubMed]
- Bungum, A.; Jensen, J.S.; Jakobsen, K.K.; Christensen, A.; Grønhøj, C.; von Buchwald, C. Impact of Surgical Resection Margins Less than 5 Mm in Oral Cavity Squamous Cell Carcinoma: A Systematic Review. Acta Otolaryngol. 2020, 140, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Yahalom, R.; Dobriyan, A.; Vered, M.; Talmi, Y.P.; Teicher, S.; Bedrin, L. A Prospective Study of Surgical Margin Status in Oral Squamous Cell Carcinoma: A Preliminary Report. J. Surg. Oncol. 2008, 98, 572–578. [Google Scholar] [CrossRef]
- Smithers, F.A.E.; Haymerle, G.; Palme, C.E.; Low, T.H.; Froggatt, C.; Gupta, R.; Clark, J.R. A Prospective Study of Intraoperative Assessment of Mucosal Squamous Cell Carcinoma Margins in the Head and Neck. Head Neck 2021, 43, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Gerber, S.M. The Impact of Frozen Sections on Surgical Margins in Squamous Cell Carcinoma of the Oral Cavity and Lips: A Retrospective Analysis 1998-2008. 2011. [CrossRef]
- Dedivitis, R.A.; Pfuetzenreiter, E.G.; Guimarães, A. V Contact Endoscopy of the Larynx as an Auxiliary Method to the Surgical Margins in Frontolateral Laryngectomy. Acta Otorhinolaryngol. Ital. 2009, 29, 16–20. [Google Scholar] [PubMed]
- Cikojević, D.; Glunčić, I.; Pešutić-Pisac, V. Comparison of Contact Endoscopy and Frozen Section Histopathology in the Intra-Operative Diagnosis of Laryngeal Pathology. J. Laryngol. Otol. 2008, 122, 836–839. [Google Scholar] [CrossRef]
- Esmaeili, N.; Boese, A.; Davaris, N.; Arens, C.; Navab, N.; Friebe, M.; Illanes, A. Cyclist Effort Features: A Novel Technique for Image Texture Characterization Applied to Larynx Cancer Classification in Contact Endoscopy—Narrow Band Imaging. Diagnostics 2021, 11. [Google Scholar] [CrossRef]
- Vu, A.N.; Farah, C.S. Efficacy of Narrow Band Imaging for Detection and Surveillance of Potentially Malignant and Malignant Lesions in the Oral Cavity and Oropharynx: A Systematic Review. Oral Oncol. 2014, 50, 413–420. [Google Scholar] [CrossRef]
- Tirelli, G.; Piovesana, M.; Gatto, A.; Tofanelli, M.; Biasotto, M.; Boscolo Nata, F. Narrow Band Imaging in the Intra-Operative Definition of Resection Margins in Oral Cavity and Oropharyngeal Cancer. Oral Oncol. 2015. [Google Scholar] [CrossRef]
- Piazza, C.; Cocco, D.; Del Bon, F.; Mangili, S.; Nicolai, P.; Peretti, G. Narrow Band Imaging and High Definition Television in the Endoscopic Evaluation of Upper Aero-Digestive Tract Cancer. Acta Otorhinolaryngol. Ital. 2011, 31, 70–75. [Google Scholar]
- Dobashi, A.; Ono, S.; Furuhashi, H.; Futakuchi, T.; Tamai, N.; Yamauchi, T.; Suka, M.; Sumiyama, K. Texture and Color Enhancement Imaging Increases Color Changes and Improves Visibility for Squamous Cell Carcinoma Suspicious Lesions in the Pharynx and Esophagus. Diagnostics 2021, Vol. 11, Page 1971 2021, 11, 1971. [Google Scholar] [CrossRef]
- Hatta, W.; Koike, T.; Ogata, Y.; Kondo, Y.; Ara, N.; Uno, K.; Asano, N.; Imatani, A.; Masamune, A. Comparison of Magnifying Endoscopy with Blue Light Imaging and Narrow Band Imaging for Determining the Invasion Depth of Superficial Esophageal Squamous Cell Carcinoma by the Japanese Esophageal Society’s Intrapapillary Capillary Loop Classification. Diagnostics 2021, Vol. 11, Page 1941 2021, 11, 1941. [Google Scholar] [CrossRef]
- Rezniczek, G.A.; Ertan, S.; Rehman, S.; Tempfer, C.B. Sequential Application of Lugol’s Iodine Test after Acetic Acid for Detecting Cervical Dysplasia: A Prospective Cohort Study. Diagnostics 2021, Vol. 11, Page 1598 2021, 11, 1598. [Google Scholar] [CrossRef]
- McMahon, J.; Devine, J.C.; McCaul, J.A.; McLellan, D.R.; Farrow, A. Use of Lugol’s Iodine in the Resection of Oral and Oropharyngeal Squamous Cell Carcinoma. Br. J. Oral Maxillofac. Surg. 2010, 48, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Umeda, M.; Shigeta, T.; Takahashi, H.; Minamikawa, T.; Komatsubara, H.; Oguni, A.; Shibuya, Y.; Komori, T. Clinical Evaluation of Lugol’s Iodine Staining in the Treatment of Stage I-II Squamous Cell Carcinoma of the Tongue. Int. J. Oral Maxillofac. Surg. 2011, 40, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Taniguchi, M.; Tsujie, H.; Hosokawa, M.; Fujita, M.; Sasaki, S. Clinical Impact of Iodine Staining for Diagnosis of Carcinoma in Situ in the Floor of Mouth, and Decision of Adequate Surgical Margin. Auris Nasus Larynx 2012, 39, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, G.; Shankar, A. Toluidine Blue: A Review of Its Chemistry and Clinical Utility. J. Oral Maxillofac. Pathol. 2012, 16, 251. [Google Scholar] [CrossRef] [PubMed]
- Poswillo, D. Evaluation, Surveillance and Treatment of Panoral Leukoplakia. J. Maxillofac. Surg. 1975, 3, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.S.; Donoghue, M.; Tauro, D.P.; Yadav, A.; Agarwal, S. Intraoperative Imprint Evaluation of Surgical Margins in Oral Squamous Cell Carcinoma. Acta Cytol. 2013, 57, 75–83. [Google Scholar] [CrossRef]
- Hamdoon, Z.; Jerjes, W.; McKenzie, G.; Jay, A.; Hopper, C. Assessment of Tumour Resection Margins Using Optical Coherence Tomography. Head Neck Oncol. 2010, 2, O7. [Google Scholar] [CrossRef]
- Zeng, S.; Huang, Y.; Huang, W.; Pathak, J.L.; He, Y.; Gao, W.; Huang, J.; Zhang, Y.; Zhang, J.; Dong, H. Real-Time Monitoring and Quantitative Evaluation of Resin In-Filtrant Repairing Enamel White Spot Lesions Based on Optical Coherence Tomography. Diagnostics 2021, Vol. 11, Page 2046 2021, 11, 2046. [Google Scholar] [CrossRef]
- Brennan, J.A.; Mao, L.; Hruban, R.H.; Boyle, J.O.; Eby, Y.J.; Koch, W.M.; Goodman, S.N.; Sidransky, D. Molecular Assessment of Histopathological Staging in Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 1995, 332, 429–435. [Google Scholar] [CrossRef]
- Braakhuis, B.J.M.; Bloemena, E.; Leemans, C.R.; Brakenhoff, R.H. Molecular Analysis of Surgical Margins in Head and Neck Cancer: More than a Marginal Issue. Oral Oncol. 2010, 46, 485–491. [Google Scholar] [CrossRef]
- Reis, P.P.; Waldron, L.; Perez-Ordonez, B.; Pintilie, M.; Galloni, N.N.; Xuan, Y.; Cervigne, N.K.; Warner, G.C.; Makitie, A.A.; Simpson, C.; et al. A Gene Signature in Histologically Normal Surgical Margins Is Predictive of Oral Carcinoma Recurrence. BMC Cancer 2011, 11, 437. [Google Scholar] [CrossRef]
- Sun, L.F.; Wang, C.X.; Cao, Z.Y.; Han, W.; Guo, S.S.; Wang, Y.Z.; Meng, Y.; Hou, C.X.; Zhu, Q.H.; Tang, Y.T.; et al. Evaluation of Autofluorescence Visualization System in the Delineation of Oral Squamous Cell Carcinoma Surgical Margins. Photodiagnosis Photodyn. Ther. 2021, 36. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Suzuki, T.; Saitou, H.; Ogane, S.; Hashimoto, K.; Takano, N.; Nomura, T. Usefulness of Fluorescence Visualization-Guided Surgery for Early-Stage Tongue Squamous Cell Carcinoma Compared to Iodine Vital Staining. Int. J. Clin. Oncol. 2020, 25, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Hom, M.E.; Rosenthal, E.L.; Varvares, M. The Future of Fluorescent-Guided Surgery. JAMA Otolaryngol. - Head Neck Surg. 2021, 147, 920. [Google Scholar] [CrossRef] [PubMed]
| Gender | Frequency – amount / percentil | Total | |
| Study group | Control group | ||
| Male | 40 / 65.57 | 36 / 59.02 | 76 |
| Female | 21 / 34.43 | 25 / 40.98 | 46 |
| Total | 61 | 61 | 122 |
| Diagnosis | Frequency – amount / percentil | Total | |
| Study group | Control group | ||
| C02 | 19 / 31.15 | 21 / 34.43 | 40 |
| C03 | 18 / 29.51 | 14 / 22.95 | 32 |
| C04 | 18 / 29.51 | 18 / 29.51 | 36 |
| C05 | 2 / 3.28 | 1 / 1.64 | 3 |
| C06 | 3 / 4.92 | 7 / 11.48 | 10 |
| C09 | 1 / 1.64 | 0 / 0.00 | 1 |
| Total | 61 | 61 | 122 |
| Grade | Frequency – amount / percentil | Total | |
| Study group | Control group | ||
| G1 | 26 / 42.62 | 28 / 45.90 | 54 |
| G2 | 31 / 50.82 | 25 / 40.98 | 56 |
| G3 | 4 / 6.56 | 8 / 13.11 | 12 |
| Total | 61 | 61 | 122 |
| Stage | Frequency – amount / percentil | Total | |
| Study group | Control group | ||
| I | 16 / 26.23 | 8 / 13.11 | 24 |
| II | 15 / 24.59 | 14 / 22.95 | 29 |
| III | 6 / 9.84 | 13 / 21.31 | 19 |
| Iva | 22 / 36.07 | 23 / 37.70 | 45 |
| IVb | 1 / 1.64 | 3 / 4.92 | 4 |
| IVc | 1 / 1.64 | 0 / 0.00 | 1 |
| Total | 61 | 61 | 122 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





