1. Introduction
Acinetobacter baumannii (A. baumannii) is an obligate aerobic, nonfermenting, gram-negative nonmotile bacterium that was discovered by Dutch microbiologist Martinus Willem Beigerinck in 1911 (1). The ability of the microorganism to survive in various environmental conditions and to last longer on wet or dry surfaces has turned it into an endemic, health care-associated pathogen and a common cause of infection outbreaks (2). People who have a weakened immune system, chronic lung disease, or diabetes are at a greater risk of being more susceptible to A. baumannii-caused infections, especially patients with a prolonged hospital stay, those with open wounds, or anyone with urinary or other catheters (3).
A. baumannii is an important nosocomial pathogen that possesses not only intrinsic resistance to many classes of antibiotics, but also is capable to rapidly develop antimicrobial resistance during treatment. This pathogen has become resistant to almost all antimicrobial agents currently available including aminoglycosides, quinolones, and broad-spectrum β-lactams antibiotics. Nosocomial infections caused by A. baumannii are difficult to treat due to multidrug resistance (MDR), which severely limits the options for therapeutic treatment (4). When A. baumannii is responsible for antibiotic resistance to at least one antimicrobial agent from 3 or more classes of antimicrobials (for example penicillins, aminoglycosides, cephalosporins, fluoroquinolones, or tetracyclines), it is considered to be multidrug resistant (4). Antibiotic resistance in A. baumannii occurs due to the enzymatic degradation of antibiotics, mutations/modification of target sites, reduced expression of porins, and overexpression of multidrug efflux pumps. Resistance to carbapenems is often mediated by β-lactamases including extended-spectrum β-lactamases (ESBLs) and AmpC (5, 6).
ESBLs conferring resistance to broad-spectrum cephalosporins and carbapenemases conferring resistance to carbapenems are the greatest concern. An ESBL and an AmpC β-lactamase in a single isolate confer resistance to carbapenems that are usually the drug of choice in the treatment of A. baumannii infection (7). Due to resistance to multiple antibiotics, A. baumannii is associated with high mortality. Therefore, clinical trials are searching for new drugs and their combinations for the treatment of hospital-acquired A. baumannii infection. However, clinical data evaluating potentially effective treatment methods are currently insufficient, and randomized clinical trials have not shown that single antimicrobial agent or combination therapy is more efficient. Therefore, it is very important to maintain the effectiveness of already available antibiotics and to pay considerable attention to the prevention of the spread of A. baumannii infection in healthcare facilities (8, 9).
According to the European Antimicrobial Resistance Surveillance Network (EARS-Net) report, the resistance rate of Acinetobacter spp. to carbapenems in Lithuania in 2021 was 96.1% (10). The aim of this study was to evaluate changes in antibiotic resistance of A. baumannii strains comparing two different study periods, to determine the production of β-lactamases and their combinations, and to evaluate their association with antimicrobial resistance.
2. Materials and Methods
2.1. Bacterial strains
A total of 223 A. baumannii strains were isolated from different clinical specimens of patients treated in the Hospital of Lithuanian University of Health Sciences: 130 A. baumannii strains in 2016–2017 and 103, in 2021–2022.
Isolates were defined as MDR if they were resistant to at least one antimicrobial agent from three or more classes of antimicrobials: penicillins (ampicillin/sulbactam, piperacillin/tazobactam) or cephalosporins (ceftazidime, cefepime), fluoroquinolones (ciprofloxacin), and aminoglycosides (amikacin, tobramycin, and gentamicin). Specimens were obtained from wounds, biopsy, bronchial secretions, blood, sputum, pus, abdominal fluid, implant, central venous catheters, pleural fluid, urine, and cerebrospinal fluid of hospitalized patients. The identification of A. baumannii strains was made using a MALDI-TOF MS mass spectrometer (Brucker Daltonics Gmbh, Germany).
2.2. Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed by a disk diffusion method. All the strains were tested for sensitivity to the following antibiotics: ceftazidime, cefepime, gentamicin, amikacin, ciprofloxacin, ampicillin/sulbactam, piperacillin/tazobactam, imipenem, meropenem, doxycycline, tigecycline, tetracycline, and sulfamethoxazole/trimethoprim according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommendations (11). The results were interpreted according to the EUCAST breakpoints.
2.3. Phenotypic methods for the determination of ESBL type
A total of 233 A. baumannii strains resistant to carbapenems (imipenem and/or meropenem) were tested using a four combination disk test (CDT) to determine the production of ESBL and AmpC (Abtek Biologicals, UK, in 2016 and 2017; Liofilchem®, UK, in 2021 and 2022). Bacterial suspensions (0.5 McFarland) of A. baumannii strains were made and cultured on Mueller-Hinton (MH) agar using different swabs. With all aseptic precautions, the following antibiotic disks were placed on inoculated MH agar plates: 30-μg cefotaxime (CTX 30), 30-μg cefotaxime plus 10-μg clavulanic acid (CTL 40), 30-μg cefotaxime plus 200-μg cloxacillin (CTC 230), 30-μg cefotaxime plus 200-μg cloxacillin plus 10-μg clavulanic acid (CTLC 240). The plates were incubated at 37°C for 18–24 h in an ambient-air incubator. An increase of ≥ 5 mm in the zone diameter with the CTL disk as compared with the CTX disk alone indicates ESBL production. An increase of ≥ 5 mm in the zone diameter with the CTC 230 disk as compared to the CTX 30 disk or an increase of ≥ 5 mm in the zone diameter with the CTLC 240 disk as compared to the CTL 40 disk or the diameter of the CTLC 240 disk measured < 5 mm as compared to the CTC 230 disk indicated that the isolate was an AmpC producer.
A. baumannii strains were also considered as ESBL and AmpC producers if the diameter of the CTL 40 disk was smaller than ≤ 5 mm as compared to the CTX 30 disk, but the diameter of the CTLC 240 disk was greater than ≥ 5 mm as compared to the CTL 40 disk and the diameter of the CTLC 240 disk was greater than ≥ 5 mm as compared to CTC 230 disk. If the diameters of the disks did not differ from each other by 2 mm, the strain being tested was neither an ESBL nor an AmpC producer.
All A. baumannii strains resistant to carbapenems (imipenem and/or meropenem) were tested using a combination meropenem disk test. The advantage of the test is that it discriminates between carbapenem-susceptible, KPC-producing, metallo β-lactamase (MBL)-producing, and double carbapenemase-producing bacteria. Bacterial suspensions (0.5 McFarland) of A. baumannii were made and cultured on Mueller-Hinton agar using different swabs, and then four meropenem disks were placed. EDTA (0.1 M, 10 μL) was added on the second disk; 20 μL of phenylboronic acid (20 g/L), on the third disk; and 20 μl of phenylboronic acid (20 g/L) plus 10 μL EDTA (0.1 M), on the fourth disk. The plates were incubated at 37°C for 18–24 h in an ambient-air incubator. Interpretation of the results of the combination meropenem disk test was based on the comparison among the inhibition zones of four meropenem disks. If no carbapenemase was present, the diameter of the disk with added inhibitors was similar in size (≥ 5 mm) compared to the diameter of the meropenem disk alone. If an isolate was as a KPC producer, there was an increase of ≥ 5 mm in the diameters of the disks supplemented with phenylboronic acid as compared to the disks without phenylboronic acid. MBL production was confirmed by an increase of ≥ 5 mm in the diameters of the disks that were supplemented with EDTA. In case of a KPC plus MBL producer, the fourth disk had the largest zone diameter of all. EDTA- and phenylboronic acid-supplemented disks were ≥ 5 mm larger in diameter than the meropenem disk alone.
Kaunas Regional Biomedical Research Ethics Committee approved this study (No. BE10-0016).
2.4. Statistical analysis
The chi-square (χ2) criterion was used for the comparison of categorical data and the Student’s t test, continuous data. The results were considered statistically significant at p < 0.05. Statistical package SPSS 27.0 for Windows was used for the data analysis.
3. Results
A total of 130 A. baumannii strains were collected in the Hospital of Lithuanian University of Health Sciences from 2016 to 2017 and 103 strains, from 2021 to 2022. In 2016–2017, 44 (33.8%) A. baumannii isolates were collected from women and 86 (66.2%) from men, with a mean age being 62.9 (SD 17.5) years. In 2021–2022, 29 (28.2%) A. baumannii isolates were collected from women and 74 (71.8%) from men, with a mean age of 62.7 (SD 14.4) years.
In 2016–2017 and 2021–2022, the highest number of A. baumannii strains were isolated from patients hospitalized in intensive care units (67.7%, n = 88, and 59.2%, n = 61, respectively), followed by surgical wards (22.3%, n = 29, and 29.1%, n = 30, respectively), and medical wards (10.0%, n = 13, and 11.7%, n = 12, respectively). A. baumannii strains were most common isolated from bronchial secretions (62.9%, n = 88, and 37.1%, n = 52, respectively), followed by urinary specimens (50.0%, n = 12, and 50.0%, n = 12, respectively), ulcers and abdominal fluids (55.6%, n = 5, and 44.4%, n = 4, respectively), and blood (31.3%, n = 5, and 68.8%, n = 11, respectively).
Examination of all A. baumannii strains and determination of their sensitivity to antibiotics showed that all A. baumannii strains were resistant to ciprofloxacin, and more than 80% were resistant to carbapenems, piperacillin/tazobactam, gentamicin, and tobramycin.
We compared the resistance rates of
A. baumannii strains to antibiotics in 2016–2017 and 2021–2022. Resistance of
A. baumannii strains to doxycycline from 2016–2017 to 2021–2022 increased the most, i.e., almost 3-fold (
p < 0.001), while the resistance to tetracycline and tigecycline, more than 2-fold (
p < 0.001). An increase in resistance
A. baumannii strains to ampicillin/sulbactam was smallest (
p = 0.004). Contrary, resistance to trimethoprim/sulfamethoxazole showed almost a 30%-point decrease comparing these two periods (
p < 0.001). Resistance rates to all tested antibiotics are shown in
Table 1.
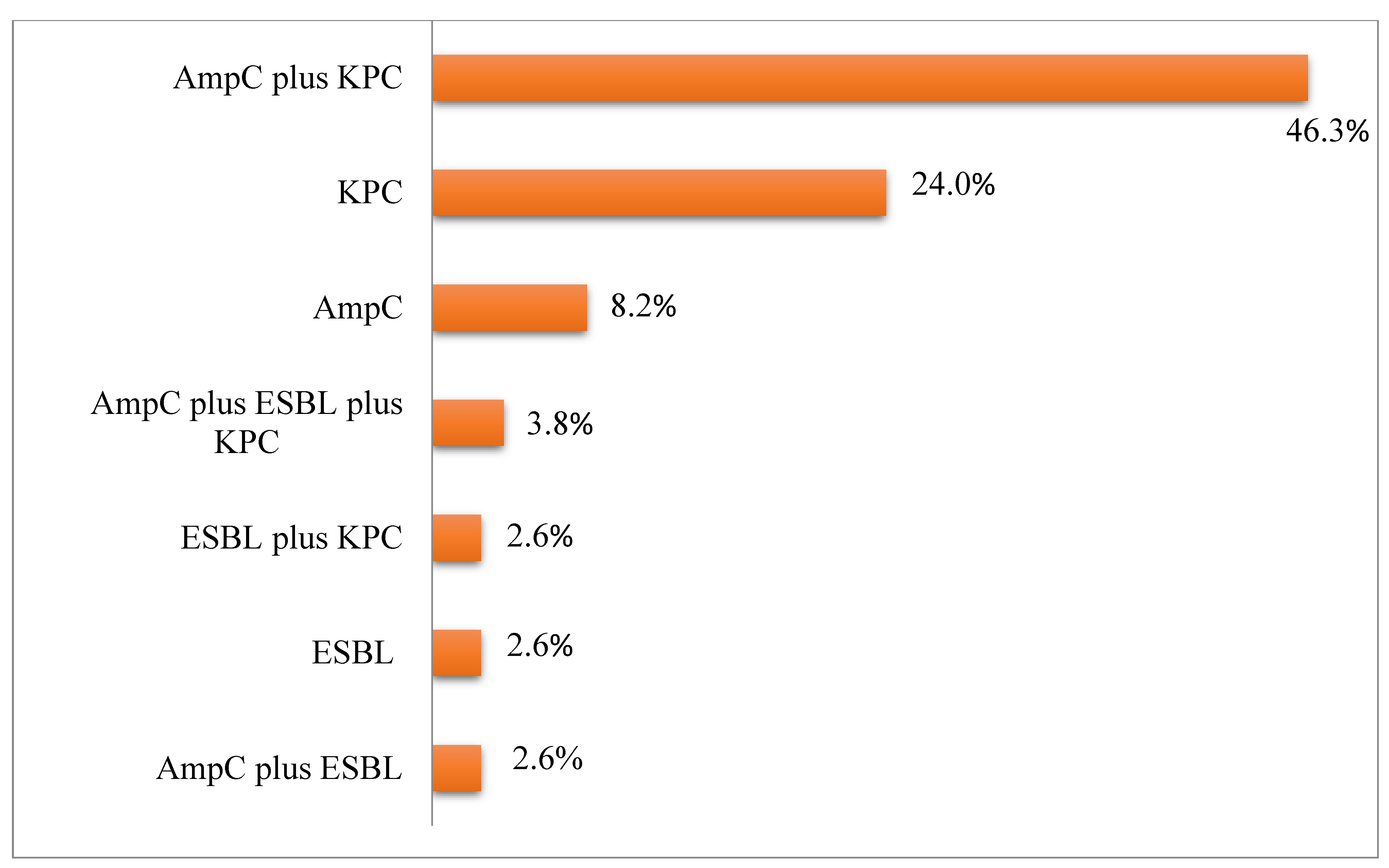
The following
β-lactamases or their combinations were found in
A. baumannii strains: 8.2% (n = 19) of strains had AmpC; 24% (n = 56), KPC; 2.6% (n = 6), ESBL; 46.3% (n = 108), a combination of AmpC and KPC; 2.6% (n = 6), a combination of AmpC and ESBL as well as ESBL and KPC; and 3.8% (n = 9), a combination of AmpC, KPC, and ESBL (
Figure 1).
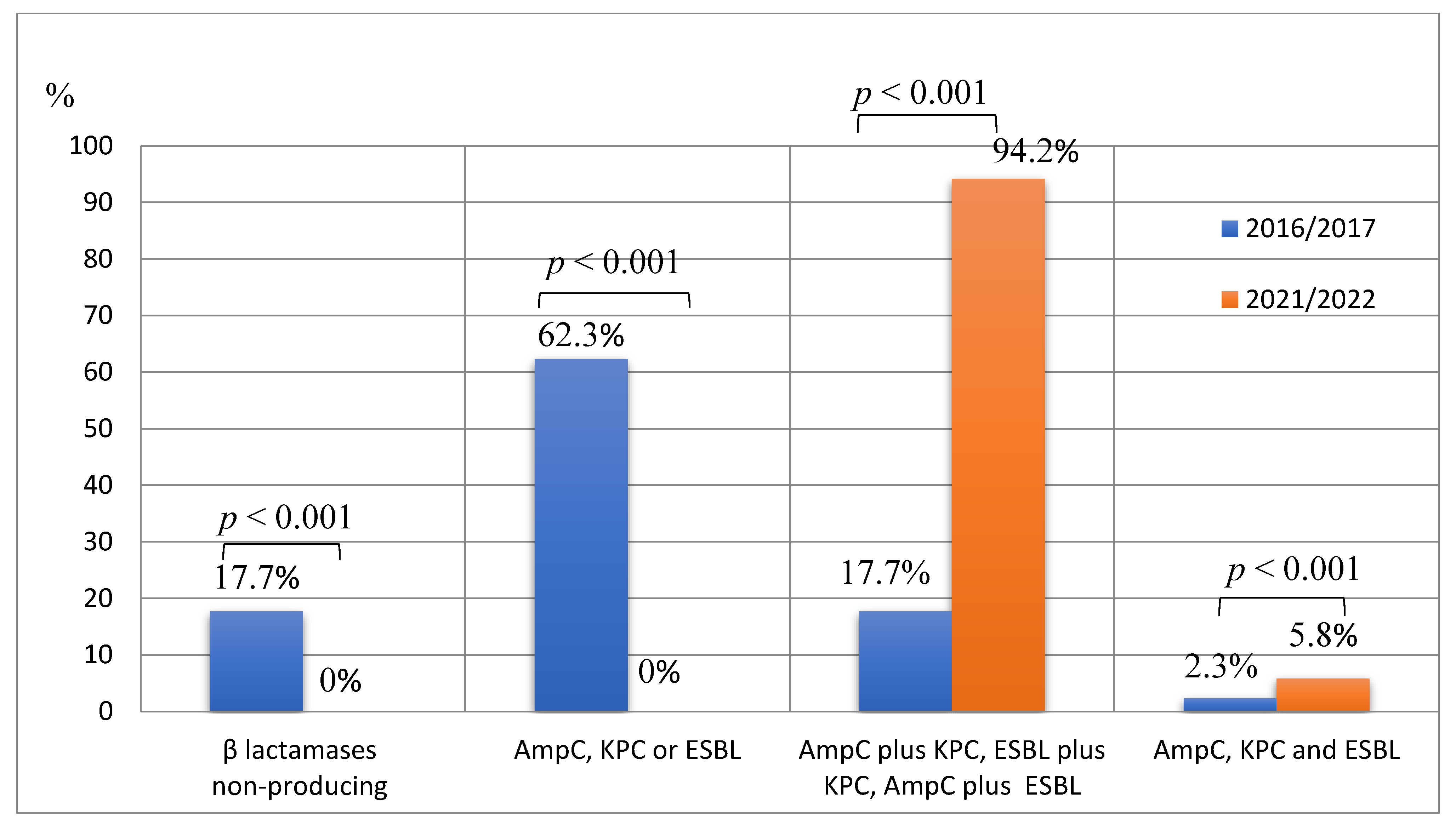
Of all A. baumannii strains tested, 9.9% (n = 23) did not produced any β-lactamase, 34.8% (n = 81) produced only one type of β-lactamase, i.e., AmpC or KPC or ESBL; 51.5% (n = 120) produced two different types of β-lactamases, i.e., AmpC plus KPC, AmpC plus ESBL, or ESBL plus KPC; and 3.8% (n = 9) produced all three types of β-lactamases. In 2016–2017, 17.7% (n = 23) of A. baumannii strains did not produce any β-lactamase, while in 2021–2022, there were no such A. baumannii strains (p < 0.001). In 2016–2017, 62.3% of all tested strains produced only one type of β-lactamase, while in 2021–2022, no such strains were detected (p < 0.001). There was a more than 5-fold increase in the percentage of A. baumannii strains producing two different types of β-lactamases when comparing 2016–2017 with 2021–2022 (17.7%, n = 23, vs. 94.2%, n = 97, p < 0.001). Only 2.3% (n = 3) of A. baumannii isolates produced all three types β-lactamases in 2016–2017, while in 2021–2022, this percentage accounted for 5.8% (n = 6) (p < 0.001) (Figure 2).
3.1. Assessment of antibiotic resistance based on β-lactamase type
AmpC-producing
A. baumannii strains were more frequently resistant to tigecycline, tetracycline, and doxycycline than non-producing AmpC strains (
p < 0.001 each). However, AmpC-producing strains were less frequently resistant to gentamicin (
p < 0.001), tobramycin (
p = 0.031), amikacin (
p < 0.001), and trimethoprim/sulfamethoxazole (
p < 0.001) as compared with AmpC-non-producing strains. Data are presented in
Table 2.
Significant differences in the resistance rates between ESBL-producing and ESBL-non-producing
A. baumannii strains were noted only for two groups of antimicrobial drugs – ampicillin/sulbactam (
p = 0.014) and tigecycline (
p = 0.021) – with the resistance rates being greater in ESBL-non-producing strains (
Table 3).
Table 4 shows the resistance rates of KPC-producing and KPC-non-producing
A. baumannii strains to all antibiotics investigated. The percentages of KPC-producing strains resistant to piperacillin/tazobactam (
p = 0.012), carbapenems (
p = 0.040), tigecycline (
p < 0.001), tetracycline (
p < 0.001), and doxycycline (
p < 0.001) were significantly greater than those of non-producing KPC. The resistance rate of KPC-producing strains to trimethoprim/sulfamethoxazole was significantly lower than that of KPC-non-producing strains (
p = 0.038).
A. baumannii strains producing one and two types of β-lactamase were more often resistant to ampicillin/sulbactam compared to all three types of β-lactamase producing strains (87.7% and 85.0% vs. 55.6%, p = 0.031). A. baumannii strains producing one type of β-lactamase were more often resistant to trimethoprim/sulfamethoxazole as compared with strains producing two or three types of β-lactamases (97.5% vs. 70.0% and 77.8%, p < 0.001). A. baumannii strains producing two or three types of β-lactamases were more often resistant to tigecycline as compared with strains producing one type of β-lactamase (87.5% and 77.8% vs. 67.9%, p < 0.001), tetracycline (94.2% and 88.4% vs. 64.2%, p < 0.001), and doxycycline (88.3% and 88.9% vs. 25.9%, p < 0.001) as compared with strains producing one type of β-lactamase. Antibiotic resistance rates of A. baumannii strains producing different types and numbers of β-lactamases are presented in Table 5.
4. Discussion
The number of cases of bacterial nosocomial infections resistant to antimicrobial agents has increased worldwide over the last decade. A. baumannii is considered as one of the most important causes of antimicrobial resistance in health care facilities. A. baumannii strains cause outbreaks around the world due to their remarkable ability to adapt to the changes in the environment and to acquire different resistance mechanisms against multiple antimicrobials (3). This study aimed to determine the types of β-lactamase and their combinations produced by MDR A. baumannii strains. It was also aimed to determine associations between antibiotic resistance and produced types of β-lactamase and to evaluate the changes in antibiotic resistance during the periods of 2016–2017 and 2020–2021.
According to the 2017 European Antimicrobial Resistance Surveillance Network (EARS-Net) report, proportions of carbapenem-resistant Acinetobacter spp. collected from invasive infections were particularly high in many southern and eastern European countries where resistance proportions often exceeded 50% (such as 95% in Greece, 79% in Italy, and 53% in Hungary). The EARS-Net analysis also showed that on average > 70% of carbapenem-resistant Acinetobacter spp. were also resistant to ciprofloxacin or gentamicin in Southern and Eastern Europe (12). In our study, all A. baumannii strains were resistant to ciprofloxacin, and resistance rates of A. baumannii strains to carbapenems, piperacillin/tazobactam, gentamicin, and tobramycin were high (more than 80%), supporting the findings of the EARS-Net report.
In our study, only 11.6% (n = 27) of 233 A. baumannii isolates were ESBL producers. The study by Kaur et al. reported (13) that of the 116 A. baumannii isolates, ESBL production was documented in 32 isolates (27.5%). Another study showed that ESBL production was observed in 55.8% (n = 24) of the 38 A. baumannii isolates (14). In both studies, the ESBL production by A. baumannii strains was greater compared with our study.
In the present study, 60.9% of the Acinetobacter isolates were AmpC producers as determined by the CDT method. Other authors also reported a similar percentage (64.63%) of AmpC producers among 82 Acinetobacter spp. isolates using the same method (15). With regard to KPC producers among A. baumannii isolates, we found that this percentage was 76.8%. Abouelfetouh et al. (16) investigated carbapenemase production in 74 carbapenem-resistant A. baumannii isolates by various phenotypic methods and determined 79.7% (n = 59) of the isolates being carbapenemase producers by the CDT method.
We showed that KPC-producing A. baumannii strains were more frequently resistant to carbapenems, piperacillin/tazobactam, and tetracyclines. This supports the statements by other authors that KPC plays an important role in resistance to carbapenems (imipenem, meropenem) and piperacillin/tazobactam among the mechanisms causing drug resistance. KPC-producing isolates are generally resistant to fluoroquinolones, aminoglycosides, and trimethoprim/sulfamethoxazole (17, 18). MDR A. baumannii strains remain susceptible to only few antibiotics such as minocycline/tigecycline and polymyxins (19). Monotherapy or combination therapy with next-generation tetracycline class antibiotics (e.g., tigecycline) is often employed as a last-resort measure to treat MDR and XDR A. baumannii infections (20); however, our study suggests that antibiotics of the tetracycline class are losing their effectiveness against infections caused by A. baumannii.
Compared with other studies, we found that A. baumannii strains producing two different types of β-lactamases – KPC and AmpC – were detected more frequently (46.3%), while the studies by Hans et al. (21) as well as by Das and Basak (22) reported these percentages being 10% and 23.3%, respectively. In our study, all three types of β-lactamases – AmpC, KPC, and ESBL – were produced by 3.8% of isolates. According to the results of other study, 1.78% of the isolates produced all three types of β-lactamases (23).
Comparison of two different periods revealed that the production of different types of β-lactamases was documented more frequently in 2020–2021 than 2016–2017. This is a serious warning as β-lactamases pose a significant threat to the effectiveness of antibiotics currently available for medical use. Other literature resources have also showed that β-lactamases are important in the emergence of antimicrobial-resistant strains and have reported a large increase in resistance among A. baumannii strains in healthcare settings (24, 25). Thus, due to such a high prevalence of resistance, antibiotics must be used judiciously by clinicians, and appropriate infection control measures need to be implemented to control the spread of infections in hospitals
5. Conclusions
The frequency of isolation of A. baumannii strains producing two or three β-lactamases types and the resistance rates to ampicillin/sulbactam, tigecycline, tetracycline, and doxycycline increased in 2020–2021 as compared with 2016–2017. The production of two or three types of β-lactamases by A. baumannii strains was associated with higher resistance rates to tetracyclines.
Author Contributions
Conceptualization, A.V. and A.D.; Data curation, K.Č. and A.D.; Formal analysis, K.Č.; Methodology, K.Č., A.D. and A.V.; Care, A.V.; Visualization, A.D.; Writing – original draft, K.Č.; Writing - revision and editing, K.Č., A.D. and A.V.
Informed Consent Statement
Patient informed consent was waived as this study analyzed bacterial strains and their resistance to antibiotics.
Acknowledgments
No funding was received.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Camp C, Tatum OL. A Review of Acinetobacter baumannii as a Highly Successful Pathogen in Times of War. Laboratory Medicine. 2010;41(11):649-57.
- Lynch JP, 3rd, Zhanel GG, Clark NM. Infections Due to Acinetobacter baumannii in the ICU: Treatment Options. Semin Respir Crit Care Med. 2017;38(3):311-25.
- Kirtikliene T, Mierauskaitė A, Razmienė I, Kuisiene N. Multidrug-Resistant Acinetobacter baumannii Genetic Characterization and Spread in Lithuania in 2014, 2016, and 2018. Life (Basel). 2021;11(2).
- Namiganda V, Mina Y, Meklat A, Touati D, Bouras N, Barakate M, et al. Antibiotic Resistance Pattern of Acinetobacter baumannii Strains Isolated from Different Clinical Specimens and Their Sensibility Against Bioactive Molecules Produced by Actinobacteria. Arabian Journal for Science and Engineering. 2019;44(7):6267-75. [CrossRef]
- Kim YJ, Kim SI, Kim YR, Hong KW, Wie SH, Park YJ, et al. Carbapenem-resistant Acinetobacter baumannii: diversity of resistant mechanisms and risk factors for infection. Epidemiology & Infection. 2012;140(1):137-45. [CrossRef]
- Lin MF, Lan CY. Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World J Clin Cases. 2014;2(12):787-814.
- Erfani Y. Detection of bla NDM -1, bla VIM, and bla IMP genes in multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa from clinical isolates in Tehran hospitals. International Journal of Advanced Biotechnology and Research. 2017;8:500-6.
- Kumar S, Anwer R, Azzi A. Virulence Potential and Treatment Options of Multidrug-Resistant (MDR) Acinetobacter baumannii. Microorganisms. 2021;9(10). [CrossRef]
- Bartal C, Rolston KVI, Nesher L. Carbapenem-resistant Acinetobacter baumannii: Colonization, Infection and Current Treatment Options. Infectious Diseases and Therapy. 2022;11(2):683-94. [CrossRef]
- ECDC Surveillance Atlas of Infectious Diseases (https://atlas.ecdc.europa.eu/). https://www.ecdc.europa.eu/sites/default/files/documents/Antimicrobial%20resistance%20surveillance%20in%20Europe%202023%20-%202021%20data.pdf.
- Giske CG, Turnidge J, Cantón R, Kahlmeter G. Update from the European Committee on Antimicrobial Susceptibility Testing (EUCAST). J Clin Microbiol. 2022;60(3):e0027621. [CrossRef]
- Ayobami O, Willrich N, Suwono B, Eckmanns T, Markwart R. The epidemiology of carbapenem-non-susceptible Acinetobacter species in Europe: analysis of EARS-Net data from 2013 to 2017. Antimicrob Resist Infect Control. 2020;9(1):89. [CrossRef]
- Kaur A, Singh S. Prevalence of Extended Spectrum Betalactamase (ESBL) and Metallobetalactamase (MBL) Producing Pseudomonas aeruginosa and Acinetobacter baumannii Isolated from Various Clinical Samples. Journal of pathogens. 2018, 2018, 6845985. [CrossRef]
- Solomon FB, Wadilo F, Tufa EG, Mitiku M. Extended spectrum and metalo beta-lactamase producing airborne Pseudomonas aeruginosa and Acinetobacter baumanii in restricted settings of a referral hospital: a neglected condition. Antimicrob Resist Infect Control. 2017;6:106. [CrossRef]
- Singla P, Sikka R, Deeep A, Gagneja D, Chaudhary U. Co-production of ESBL and AmpC β-Lactamases in Clinical Isolates of A. baumannii and A. lwoffii in a Tertiary Care Hospital From Northern India. Journal of clinical and diagnostic research: JCDR. 2014;8(4):Dc16-9.
- Abouelfetouh A, Torky AS, Aboulmagd E. Phenotypic and genotypic characterization of carbapenem-resistant Acinetobacter baumannii isolates from Egypt. Antimicrobial Resistance & Infection Control. 2019;8(1):185. [CrossRef]
- Kyriakidis I, Vasileiou E, Pana ZD, Tragiannidis A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens. 2021;10(3):373. [CrossRef]
- Raible KM, Sen B, Law N, Bias TE, Emery CL, Ehrlich GD, et al. Molecular characterization of β-lactamase genes in clinical isolates of carbapenem-resistant Acinetobacter baumannii. Ann Clin Microbiol Antimicrob. 2017;16(1):75. [CrossRef]
- Qureshi ZA, Hittle LE, O'Hara JA, Rivera JI, Syed A, Shields RK, et al. Colistin-Resistant Acinetobacter baumannii: Beyond Carbapenem Resistance. Clinical Infectious Diseases. 2015;60(9):1295-303.
- De Oliveira DMP, Forde BM, Phan MD, Steiner B, Zhang B, Zuegg J, et al. Rescuing Tetracycline Class Antibiotics for the Treatment of Multidrug-Resistant Acinetobacter baumannii Pulmonary Infection. mBio. 2022;13(1):e0351721. [CrossRef]
- Hans R, Bisht D, Agarwal R, Irfan M. Phenotypic detection of MBL, Ampc beta-lactamase and carbapenemases in multi drug resistant isolates of Acinetobacter baumannii. International Journal of Medical Research & Health Sciences. 2015;4:311.
- Das S, Basak S. Acinetobacter Baumannii Complex and Its Beta-Lactamase Production: Are We Moving Towards Pre Antibiotic Era? 2018; 8(3):60-69. [CrossRef]
- Neupane M, K.C S, Thakur SK, Panta OP, Joshi DR, Khanal S. Beta-Lactamases Production in Multi-drug Resistant Acinetobacter species Isolated from Different Clinical Specimens. Tribhuvan University Journal of Microbiology. 2019;6(0):44-50.
- Batra P, Khurana S, Govindaswamy A, Aravinda A, Bajpai V, Ayyanar M, et al. Antibiotic resistance profile and co-production of extended spectrum beta lactamases and AmpC in Acinetobacter spp. in a level 1 trauma center from India. Journal of laboratory physicians. 2019;11(2):128-32. [CrossRef]
- Davoudi-Monfared E, Khalili H. The threat of carbapenem-resistant gram-negative bacteria in a Middle East region. Infect Drug Resist. 2018;11:1831-80. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).