1. Introduction
Emerging and re-emerging tick-borne diseases (TBD) are reported to be on the rise globally [
1,
2] many of them are known to be of potential zoonotic origin [
3]. Due to the recent global warming phenomenon, the expanding potential of the geographical distribution of ticks has also increased [
4,
5]. The incursion of exotic tick fauna into the naïve areas determines the introduction and spreading of emerging and remerging tick-borne pathogens (TBP) which necessitates the routine surveillance, estimation and understanding of risk factors ([
6]. Even though the estimation of the tick-borne rickettsial disease burden is difficult due to misdiagnosis, its infection in Southeast Asia is estimated to be the second most reported next to malaria ([
7]. The rickettsial bacteria are mainly transmitted by the bite of infected ticks to rodents, dogs, and wild animals, as well as humans engaging in outdoor activities.
The genus
Rickettsia is genetically subdivided into four groups based on molecular analysis, such as the spotted fever group (SFG), the typhus group,
Rickettsia bellii group,
and Rickettsia canadensis group
[8]. SFG includes the causative agent of
R. rickettsii, R. conorii, R. africae, R. parkeri, R. honei, R. felis, R. conorii subsp.
raoultii,
R. conorii subsp.
indica and many others. Tick-borne rickettsioses are mainly caused by the SFG; its identification at the species level with immunological methods is difficult since ticks are a more competent and versatile vector [
9]. The studies on spotted fever group (SFG) and typhus group (TG) rickettsioses are limited when compared with Scrub typhus (ST) [
10]. However, worldwide, improved diagnostic methods have contributed to the reliable identification of tick-borne pathogens (TBP) in humans, domestic animals and vectors [
11].
In India, the distributions of 106 tick species have been documented and only a few of them have been incriminated as potential vectors of known TBP [
12]. Apart from its significant role in the transmission of viruses, rickettsia remains one of the most important tick-transmitted aetiology of causing rocky mountain spotted fever, rickettsialpox, other spotted fevers, epidemic typhus, and murine typhus in humans and animals in Southeast Asia (SEA) [
13,
14]. However, their true magnitude in terms of disease prevalence is yet to be determined [
13].
Rickettsial isolates from various tick faunas of
Amblyomma sp.,
Ceratophyllus fasciatus (rat flea),
C.orientis felis,
Haemaphysalis sp., and
Rhipicephalous sp. were recorded. In addition, molecular studies conducted Tamil Nadu, the north-eastern state of Nagaland and Punjab have also reported rickettsial infection in humans [
15,
16,
17]. Our recent study has recorded the dominant occurrence of
Haemaphysalis intermedia in certain parts of Tamil Nadu (unpublished data) and has already been known to transmit many public health important viruses, namely the Nairobi sheep disease virus in Sri Lanka, Bhanja and the Ganjam viruses in Odisha state, India. [
18,
19].
Though outbreaks due to rickettsial etiology have been well documented in many Indian states [
20],
the available data on the circulation of
rickettsia through serological and molecular evidence and their clinical relevance in causing illness in humans is very scanty, but these evidence confirm its widespread distribution in the country
[21,22,23]. The recent reports of encephalitis cases due the rickettsial infection spread by ectoparasites stress attention to molecular diagnosis SFG and TG because only >10% outbreaks of acute encephalitis syndrome (AES) aetiology were confirmed and the reaming is not confirmed with molecular methods [
10].
The data on the prevalence of rickettsial pathogens in ticks from the southern parts of Tamil Nadu, India is limited and no substantiated records are available from the Eastern Ghats. In addition, this area is known to have a favorable climate and rich biodiversity suitable for the breeding and spread of ticks and tick-borne diseases. Considering the emerging potential of tick-borne pathogens in India, the present study was conducted as a pilot laboratory-based screening of tick-borne rickettsial organisms in dominant tick species of
H. intermedia through PCR based on the multilocus sequence typing (MLST) [
24]. Since this method is robust, eliminates phylogenetic inconsistencies due to lateral gene transfer and is widely used as a complementary tool for the identification of rickettsial species [
25].
2. Materials and Methods
2.1. Study area
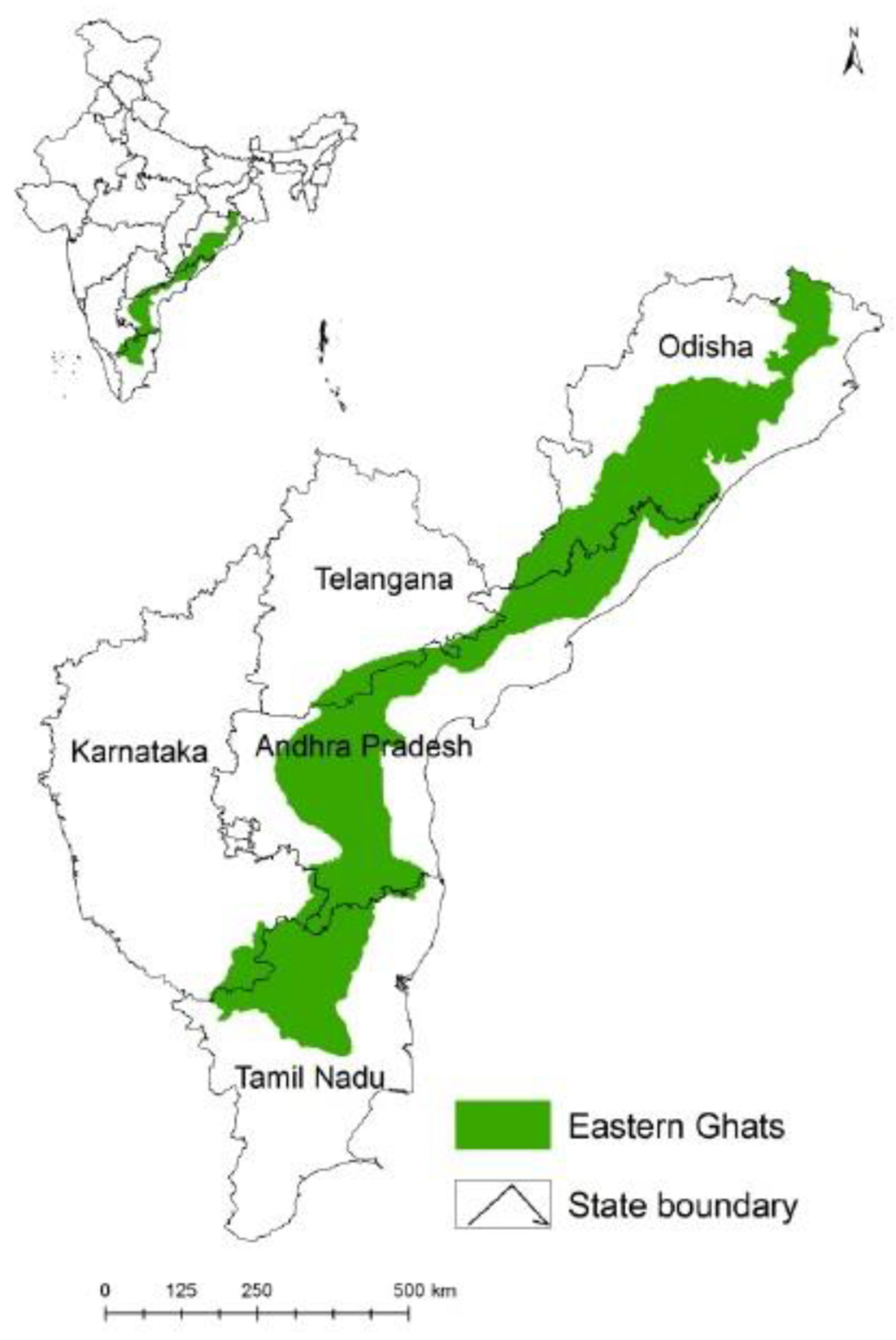
The Sirumalai foothill ranges cover approximately 60,000 acres and are located in the last mountain range of the Eastern Ghats, Tamil Nadu, South India, at longitude: 77.99, latitude: 10.19 and elevation: 1600 metres (
Figure 1). The villages situated in the Sirumalai foothills area geographically have a dense forest with a moderate climate (14˚C to 30˚C) throughout the year. The village population is mainly engaged in agricultural activities and cattle rearing. Livestock such as cows, sheep, goats, dogs and horses are domesticated in the villages. The prevailing climatic and ecological conditions are highly favorable for the breeding of ticks in this area.
2.2. Tick Collection and Identification
Tick specimens were collected from domestic animals manually, using forceps, during October 2020–December 2020. The individual specimens were immediately placed into a 2 ml vial containing 70% ethanol and transported to the laboratory for further processing. The samples were morphologically identified using standard keys [
26,
27] and pooled by sex and stage.
2.3. DNA Extraction
2.3.1. Rickettsia screening
Tick samples were removed from 70% ethanol (EtoH) washed twice in clean de-ionized water to remove the residue of EtOH and allowed to air dry before DNA extraction. Before the commencement of the homogenization, single female ticks, pools of males and other stages (5 ticks per pool) kept in 1.5 ml microtubes were incubated at 80°C in a dry bath for 2 hours to remove the moisture contents in the sample. All the microtube lids are kept open during the incubation to avoid the formation of moisture in the sample tubes. After complete drying, the whole body of samples in 1.5 ml microtubes was manually ground using the sterile stainless steel pellet pestle (Sigma-Aldrich, cat No. Z359963) without adding ATL buffer, until the tick was completely ground to powder. After manual grinding, 50 µl of the ATL buffer was added to each tube and homogenized with a motor tissue homogenizer (REMI-RQ-127A/D, Mumbai, India) at the optimum speed for 2 minutes using an individual pestle for each pool. Finally, the pestles were washed with 130 µl of ATL buffer (a total of 180 µl).
Since the H. intermedia females are bigger than the males and other stages, the adult female ticks were homogenized individually, and after centrifugation for 1 minute at 8000 rpm, the supernatant of each tube was collected and pooled into a single tube (five female ticks constitute a pool). On the other hand, males, larvae and nymphs were pooled as five specimens per pool for homogenization and after centrifugation, the supernatant was collected into fresh microtubes. All the supernatant was incubated at 56°C in a dry bath for 1 hour with 20 µl proteinase-K after a brief vortex. The samples were removed from a dry bath after one-hour of protease digestion and column-based DNA extraction protocol was followed as per the manufacturer’s instructions (QIAamp DNA mini kit (Qiagen, Hilden, Germany). The DNA was eluted with 50 µl of nuclease-free water (Qiagen, Cat. No. 129115) and stored at -80°C.
2.3.2. DNA Barcoding of H. intermedia
DNA was extracted from
H. intermedia male and female ticks after morphological identification with standard keys [
26,
27]. Each tick specimen was cut into two parts vertically, half of the specimen was used for DNA extraction and the remaining half was preserved in the VCRC tick museum as a voucher specimen for future reference (Plates. 5 & 6). The DNA extraction procedure for screening of rickettsia was also followed, except that the collection of the supernatant after centrifugation for 1 minute at 8000 rpm was skipped; instead, the entire homogenate was used for protease digestion and kit the manufacturer’s protocol for column-based DNA extraction was followed (QIAamp DNA mini kit,Qiagen, Hilden, Germany). The DNA was eluted with 30 µl of nuclease free water (Qiagen, Cat. No. 129115) and stored at -80°C.
2.4. PCR methods
2.4.1. Multilocus sequence typing (MLST)
The total DNA extracted from each pool was screened for rickettsial species by targeting the OmpB gene using the nested PCR method. The positive samples for OmpB were further subjected to multi-locus sequence typing (MLST) by covering the genes encoding 16s rRNA, OmpA, and gltA with gene specific primers. Different types of PCR methods, such as single-stage, semi-nested, and nested PCR, were carried out as per the standardized thermal cycler conditions for amplification of the four genes (
Table 1) using the primer sequences for the detection of rickettsial species [
28]. Both the first and second rounds of each PCR were carried out in a final volume of 25 µl. Two µl of template DNA was used for the first round of PCR, and one µl of the first PCR product was used as a template for the second round of nested PCR.
2.4.2. DNA barcoding
Mitochondrial cytochrome c oxidase subunit I gene (COI) fragment of 710 bp was PCR amplified with a touchdown PCR protocol using forward and reverse primers LCO1490: 5'-GGTCAACAAATCATAAAGATATTGG-3', HC02198: 5'-TAAACTTCAGGGT GACCAAAAAATCA-3' as described by Folmer et al., [
29]. Two µl of DNA was used as template and PCR was carried out with a total volume of 50 µl, using the Taq PCR core kit (Cat. No. 201223, Qiagen, Hilden, Germany).
2.5. DNA sequencing and analysis
2.5.1. Rickettsial DNA sequences
Ten fragments (16s rRNA- 2, OmpA-2, OmpB-3 and GltA-3) from three PCR positive pools were subjected to the Sanger DNA sequencing method. The forward and reverse DNA sequences were aligned and manually edited comparing with sequencing chromatogram. The partial DNA sequences thus obtained were analyzed in the BLASTn nucleotide database (
https://www.ncbi.nlm.nih.gov) to find out their pair-wise nucleotide similarity with the sequences available in the nucleotide database (NCBI). A phylogenetic tree was constructed using the sequences with a higher percentage of identity with the query sequence, applying the maximum likelihood (ML) method using the Kimura 2 parameter (K2P) distances with 1000 bootstrap replicates on the MEGA 7 software (
https://www.megasoftware.net) [
30]. The outlier group of DNA sequences for
R. raoultii and
R. felis were also included in the FASTA file in order to separate the phylogenetic clade, and one DNA sequence from a genetically more distant species was incorporated to serve as an outgroup in the phylogenetic tree (
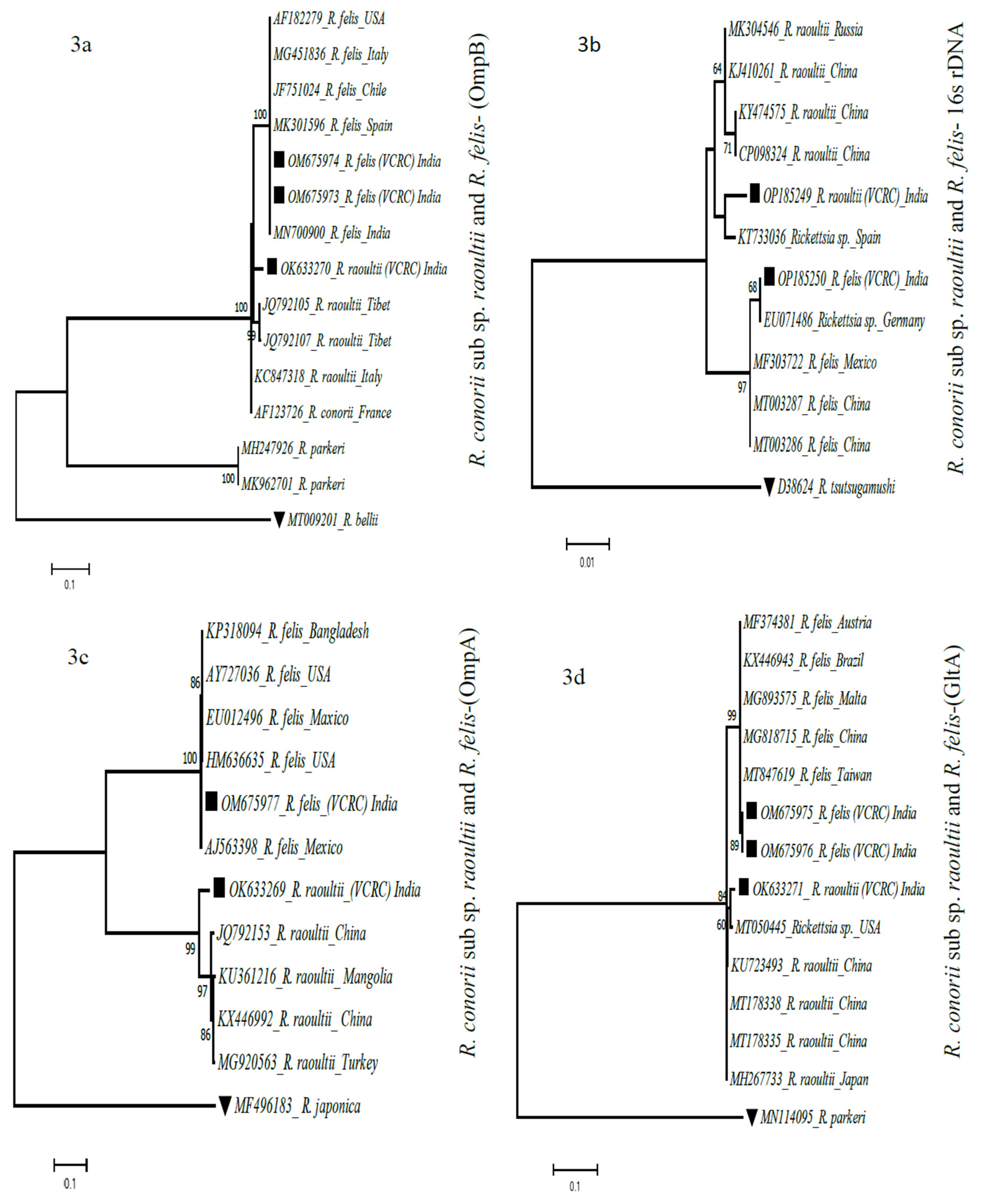
Table 3,
Figure 3).
2.5.2. H. intermedia COI sequences
DNA barcodes generated to complement the taxonomic identification of tick using the mitochondrial cytochrome c oxidase subunit I gene (COI) of
H. intermedia were aligned in MEGA 7 software [
30] and the final sequences were analyzed in the NCBI, BLASTn database for confirmation of the tick species.
3. Results
3.1. Detection of rickettsial pathogen
Tick-borne rickettsial pathogens remain an important clinical entity in causing serious illnesses in humans and animals, which are mostly unreported or underreported. In the present study, H. intermedia males, females, larvae and nymph life stages were screened for rickettsial organisms. During the survey, 330 H. intermedia were collected from cows, goats and dogs (Plate.1-4) subjected to rickettsial screening. Table, 2 shows the number of pools processed in each life stage, by sex, the number of genes sequenced, the number of positives, and the Rickettsia species detected from the respective pools of H. intermedia using MLST.
The outer membrane protein B (OmpB) was PCR amplified from a pool of females (R. felis), and two pools of males (R. felis and R. raoultii). The sequence similarities of OmpB with the previous DNA sequences were 97.39% and 100% for R. raoultii and R. felis, respectively. In addition, one pool positive for OmpB in the nymph stage (437 bp) detected showed 94% query coverage and 99.76% DNA sequence identity with R. massiliae strain 80 (GenBank No. KJ663753) isolated from Ixodid ticks R. sanguineus infesting humans in Italy. Since the other three loci did not yield PCR amplification, the OmpB nymph stage positive sequence was not submitted in the GenBank database.
In the case of 16s RNA gene, single-step PCR was carried out to amplify 426 bp of the rickettsia genome. Among the three positive pools obtained, the rRNA gene sequence could be amplified only in two pools. The multiple sequence analysis in the BLASTn database showed that each belongs to R. felis and R. conorii supsp. raoultii. The nucleotide identity of the query sequence with Rickettsia sp. and R. felis was found to be 99.54%. Another pool had 99.32% and 99.08% sequence identity with Rickettsia sp. and R. raoultii, respectively.
Rickettsia outer membrane protein-A was amplified in the female and male pools and the BLASTn analysis of OmpA DNA sequences showed 100% and 93.38% and sequence identity with R. raoultii and R. felis, respectively. The citrate synthase protein coding gene (gltA) was amplified from R. felis in female and male pools and from R. raoultii in a male pool. The BLASTn analysis showed 99.41% and 98.57% sequence identity, respectively. DNA sequences (OM675975, OM675976 and OK633271) of both pathogens generated in this study have shown a close similarity with rickettsial sequences submitted from China and USA (GenBank Acc. Nos. MG818715 and MT050445).
Table 2.
Details of the number of positive pool, gene amplified, and detection of Rickettsia species by PCR assay.
Table 2.
Details of the number of positive pool, gene amplified, and detection of Rickettsia species by PCR assay.
| Stage/ Sex |
No. of pool |
No. of ticks |
No
+ve |
Host +ve |
Gene sequenced |
Rickettsia spp. |
| rRNA |
OmpA |
OmpB |
gltA |
| Larva |
6 |
30 |
0 |
0 |
-ve |
-ve |
-ve |
-ve |
- |
| Nymph |
14 |
70 |
0 |
0 |
-ve |
-ve |
Rickettsia spp. |
-ve |
Rickettsia spp. |
| Female |
8 |
40 |
1 |
Cow |
× |
Rf |
Rf |
Rf |
R. felis |
| Male |
38 |
190 |
2 |
Goat |
Rf, Rr |
Rr * |
Rr, Rf |
Rr, Rf |
R. felis,
R. raoultii |
Overall, the nucleotide identity of the rickettsial species was confirmed based on the values of the BLASTn DNA sequence analysis, such as the e-value, the higher the percentage of query coverage and sequence identity. The cut-off value (%) as proposed by Fournier et al., was considered for rickettsial species identification. The details of the rickettsial DNA sequence generated from the present study and the output values of BLASTn analysis are given in
Table 3.
3.2. DNA Barcoding of Haemaphysalis intermedia
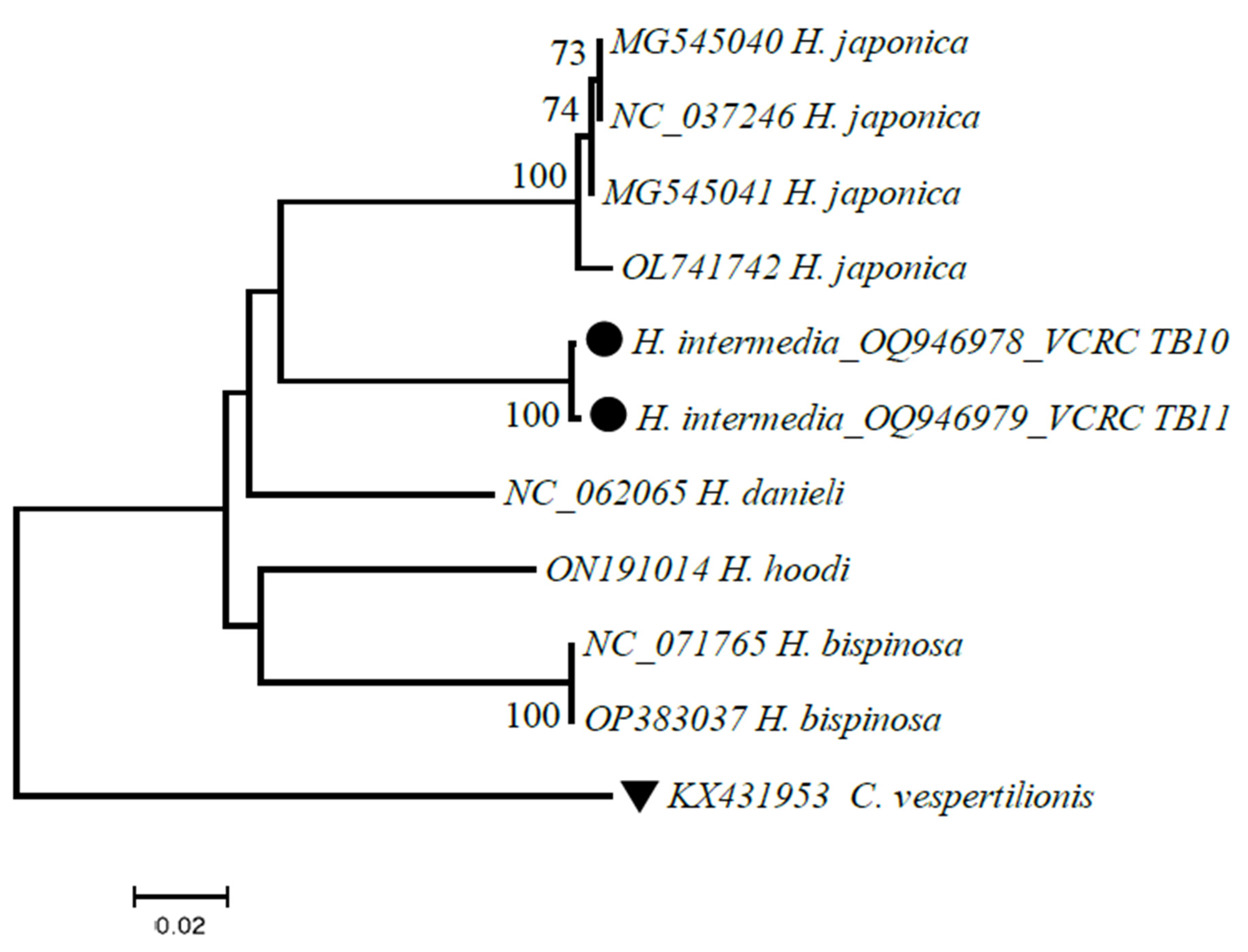
To complement the taxonomical identification of H. intermedia, it was subjected to DNA barcoding and 710 bp of the mitochondrial COI gene was amplified using template DNA extracted from a half portion of a male and female tick specimen collected from the study area. DNA sequences generated (700 bp, 699 bp) were subjected to BLASTn analysis and the result showed close similarity with the species viz., H. japonica (MK863383), H. bispinosa (MW078971), H. danieli (NC_062065) and H. hoodi (ON191014) with 85% to 90% query coverage. Therefore, the COI sequences generated in this study were submitted to GenBank as H. intermedia for the first time (Accession Nos. OQ946978 and OQ946979).
Figure 2.
Phylogenetic analysis of partial COI gene sequences of H. intermedia using the neighbor-joining K2P model with 1000 bootstrap replication in MEGA 7. Sequences of the closest neighbors from the BLASTn analysis were incorporated in the phylogenetic analysis. Legends: ● sequences generated in this study; ▼ out-group.
Figure 2.
Phylogenetic analysis of partial COI gene sequences of H. intermedia using the neighbor-joining K2P model with 1000 bootstrap replication in MEGA 7. Sequences of the closest neighbors from the BLASTn analysis were incorporated in the phylogenetic analysis. Legends: ● sequences generated in this study; ▼ out-group.
Table 3.
Details of rickettsial species detected from H. intermedia tick, locus amplified, GenBank accession number, percentage of identity with study sequence, and cut-off value.
Table 3.
Details of rickettsial species detected from H. intermedia tick, locus amplified, GenBank accession number, percentage of identity with study sequence, and cut-off value.
| Gene |
Taxon |
Isolation
Source |
GenBank Acc. No. |
Nucleotide identity (%) |
Closest match in GenBank |
Cut-off
value (%) |
| R. conorii subsp. raoultii |
| 16s rDNA |
R. conorii subsp. raoultii
|
H. intermedia |
OP185249 |
Present study |
Present study |
rrs> 98.1%
(97.7-98.1) with at least one Rickettsia species |
|
Rickettsia sp. |
Sheep |
KT733036 |
99.32 |
Spain |
| R. conorii subsp. raoultii |
D. reticulatus |
MK304546 |
99.08 |
Russia |
| H. asiaticum |
KJ410261 |
99.08 |
China |
| Homo sapiens |
KY474575 |
98.86 |
China |
| D. silvarum |
CP098324 |
98.86 |
China |
| rOmpA |
R. conorii subsp. raoultii
|
H. intermedia |
OK633269 |
Present study |
Present study |
Either possess ompA or nucleotide homologies with gltA ≥92.7% & ompB ≥85.2% |
| Rickettsial sp. |
D. marginatus |
MG920563 |
93.38% |
Turkey |
|
R. conorii subsp. raoultii
|
D. nuttalli |
KU361216 |
93.38% |
Mongolia |
| I. ovatus |
KX446992 |
93.38% |
China |
| D. niveus |
JQ792153 |
93.37% |
Tibet |
| rOmpB |
R. raoultii |
H. intermedia |
OK633270 |
Present study |
Present study |
≥85.8% |
| Rickettsia conorii |
R. sanguineus |
AF123726 |
97.39% |
France |
| R. conorii raoultii |
D.niveus |
JQ792105
|
96.1% |
Tibet |
| D. everestianus |
JQ792107 |
96.1% |
Tibet |
| Human Blood |
KC847318 |
97.09% |
Italy |
| gltA |
R. conorii subsp. raoultii
|
H. intermedia |
OK633271 |
Present study |
Present study |
≥92.7% |
| Rickettsial sp. |
Dog blood |
MT050445 |
98.57% |
USA |
|
R. conorii subsp. raoultii
|
D. marginatus |
KU723493 |
98.29% |
China |
| D. nuttalli |
MT178338 |
98% |
China |
| Human blood |
MH267733 |
98% |
Japan |
| Rickettsia felis |
| 16s rDNA |
R. felis |
H. intermedia |
OP185250 |
Present study |
Present study |
rrs> 98.1% (97.7-98.1) with at least one Rickettsia species |
|
Rickettsia species |
ESTEC HYDRA facility |
EU071486 |
99.54% |
Germany |
| R. felis |
Cultured |
CP000053 |
99.54% |
USA |
|
Rickettsia endosymbiont |
L. bostrychophila |
DQ407743 |
99.31% |
China |
| R. felis |
C. felis |
MF303722 |
99.08 |
Mexico |
| rOmpA |
R. felis |
H. intermedia |
OM675977 |
Present study |
Present study |
Either possess ompA or pairwise nucleotide homologies with gltA ≥92.7% & ompB ≥85.2% |
| C. felis |
AJ563398 |
100% |
Mexico |
| C. felis |
AY727036 |
99.6% |
USA |
| Infested fleas |
EU012496 |
99.6% |
Mexico |
| L. bostrychophila |
HM636635 |
99.6% |
USA |
| Human Blood |
KP318094 |
99.4% |
Bangladesh |
| rOmpB |
R. felis |
H. intermedia |
OM675973 |
Present study |
Present study |
≥85.8% |
| H. intermedia |
OM675974 |
Present study |
Present study |
| Ixodes ricinus |
MK301596 |
100% |
Spain |
| Dog Blood |
MG451836 |
99.72% |
Italy |
| |
AF182279 |
99.72% |
USA |
| R. sanguineus |
JF751024 |
99.15% |
Chile |
| GltA |
R. felis |
H. intermedia |
OM675975 |
Present study |
Present study |
92.7% |
| H. intermedia |
OM675976 |
Present study |
Present study |
| Booklice |
MG818715 |
99.41% |
China |
| Cat flea |
MG893575 |
Malta |
| C. felis |
MF374381 |
Austria |
| Xenopsylla cheopis |
KX446943 |
Brazil |
| Ixodes granulatus |
MT847619 |
Taiwan |
4. Discussion
Rickettsial diseases are considered important public health problems globally and ticks play a major role in transmitting them to humans and animals [
1,
2]. Despite their true burden among the global community is not known, the recent reports on the escalating case incidences of tick-borne infections stress an in-depth understanding of the occurrence of the pathogen and strengthening of surveillance on TBP around the globe [
31,
32]. In India, tick-transmitted rickettsial infections are very common, but their true health impact is either unreported or underreported [
23,
35]. In Tamil Nadu, the occurrence of different tick species has been well documented [
36] and their impact in causing diseases needs to be determined. The present study reports the circulation of
R. felis and
R. raoultii among tick species that are prevalent in Sirumalai foothill villages in Tamil Nadu. Though the pathogens detected were not associated with any clinical diseases in India, they were found to be etiological agents of the diseases, namely scalp eschar and neck lymphadenopathy after a tick bite (SENLAT) [
37]. Hence, it is noteworthy to determine its clinical relevance to local animals and humans in causing diseases and their impact on the local community.
In order to generate adequate molecular evidence of the detected rickettsial sequences, multi-locus sequencing typing was carried out to fulfill the recommended essential criteria for determining the species within the genus
Rickettsia [
24,
38]. A set of four genes, namely 16s rRNA, OmpA, OmpB and gltA sequences, confirm its identity as
R. raoultii and
R. felis in
H. intermedia ticks in the study area. At the same time, the problems of non-amplification of these four genes from all the positive pools obtained were noted. Though the loci
OmpB and gltA were successfully amplified in all three positive pools, (OmpB:
R. felis OM675973 -OM675974 and
R. raoultii OK633270; gltA:
R. felis OM675975, OM675976 and OK633271
R. raoultii), the genes OmpA (OK633269-
R. raoultii, OM675977-
R. felis) and 16s rRNA (OP185249-
R. raoultii and OP185250-
R. felis) were amplified in two pools. In a pool of males, the occurrence of
R. felis was confirmed based on three loci, viz., 16S
rRNA, OmpB, and
gltA gene sequences. The reason behind the failure of amplification of the
OmpA fragment may be due to the truncated nucleotides, ranging from 36 bp to 4.5 kb, as reported by Ellison et al., [
39].
All four loci of
R. conorii subsp.
raoultii were amplified in one of the male positive pool. The rRNA
, OmpA
, OmpB
, and gltA gene sequences
of R. conorii subsp.
raoultii were found to be clustered with isolates from Russia (MK3044546), Tibet (JQ792153), and the United States (MT050445) [
Figure 3]. Similarly, in the phylogenetic analysis,
R. felis partial gene sequences of rRNA, OmpA, OmpB and gltA were found clustered with rickettsial strains originating from Germany and Mexico (EU071486, MF303722), Mexico (AJ563398), Spain (MK301596) and Taiwan (MT847619), respectively, which denotes the widespread distribution of rickettsia around the globe. The OmpB positive in a pool of nymph stage, confirm that the tick carries rickettsial bacteria in all developmental stage and the transstadial transmission of rickettsia by ticks.
Though the available evidence documents the widespread distribution of rickettsia in various places in India, with our limited knowledge, this is the first report on the molecular evidence with MLST-based identification of R. felis and R. conorii subsp. raoultii in the H. intermedia ticks from south India. To complement the taxonomical identification of the tick species, partial DNA sequences of the cytochrome oxidase subunit I (COI) gene were generated. Upon comparison with BLASTn database sequences, they showed close identity to tick species, viz., H. japonica (MK863383), H. bispinosa (MW078971), H. danieli (NC_062065) and H. hoodi (ON191014 with 85% to 90% query coverage. Eventually, the vouched specimens of H. intermedia were maintained at the institute for future examination. The identity of the species was also reexamined with taxonomists unanimously, and the COI sequence generated in this study has been deposited for the first time as H. intermedia in the GenBank database (OQ946978 and OQ946979).
The report on
R. raoultii’s isolation from
Dermacentor reticulatus,
D. marginatus,
D. silvarum and
D. nuttalli ticks [
40] and
R. felis from Ctenocephalides felis
(Cat flea),
L. bostrychophila (book louse),
R. sanguineus (the brown dog tick),
Xenopsylla cheopis (rat flea) and
Ixodes granulatus (tick) [
41] have already been documented in the literature. At the same time, the
mere circulation of rickettsial pathogens in ticks in this area cannot be correlated with the transmission risk to humans without adequate laboratory evidence. However, their existence in these areas could be considered a risk factor in the future, since, in India, six episodes of rickettsial outbreaks have already been reported [
20] including the involvement of the first human case of
R. felis from the north-eastern state of Nagaland, India [
16].
5. Conclusions
In conclusion, the present study has shown laboratory evidence for the circulation of R. conorii subsp. raoultii and R. felis in H. intermedia ticks in the Sirumalai area of Tamil Nadu, India. The MLST is very useful and complementary in identifying the rickettsial agent. Application of this tool will reveal the actual rickettsial species in circulation in the environment. Further studies are highly warranted to determine the extent of the circulation of these agents and their impact on the community. It is worth understanding their role in causing clinical diseases in animals and humans and the potential risks of acquiring the diseases for future prevention and control measures.
Author Contributions
Conceptualization, Krishnamoorthy Nallan and Paramasivan Rajaiah; Methodology, Krishnamoorthy Nallan, Veerapathiran Ayyavu, Elango Ayyanar, Balaji Thirupathi, Bhavna Gupta and Panneer Devaraju; Formal analysis, Krishnamoorthy Nallan and Bhavna Gupta; Resources, Veerapathiran Ayyavu, Elango Ayyanar, Balaji Thirupathi and Paramasivan Rajaiah; Writing – original draft, Krishnamoorthy Nallan; Writing – review & editing, Elango Ayyanar, Balaji Thirupathi and Panneer Devaraju; Supervision, Ashwani Kumar and Paramasivan Rajaiah; Project administration, Ashwani Kumar and Paramasivan Rajaiah; Funding acquisition, Krishnamoorthy Nallan and Ashwani Kumar.
Funding
ICMR-VCRC Intra-mural funding, IM 2106.
Institutional Review Board Statement
This study has been approved by the institutional Research Integrity Unit (RIU) of the Centre.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Dr. N. Pradeep Kumar, Senior Consultant, Vector Control Research Centre Field Unit, Kottayam for the valuable comments and suggestions. The laboratory staff and Ms. A. Rajeshwari Research Scholar at Vector Control Research Centre, Field Unit, Madurai are acknowledged for their support during the processing of samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mysterud, A.; Jore, S.; Østerås, O.; Vijiugrein, H. Emergence of tick-borne diseases at northern latitudes in Europe: A comparative approach. Sci. Rep. 2017, 16316. [CrossRef]
- Rochlin, I.; Toledo, A. Emerging tick-borne pathogens of public health importance: A mini-review. J. Med. Microbial. 2020, 69(6), 781–791. [CrossRef]
- Springer, A.; Glass, A.; Topp, A.K.; Strube, C Zoonotic tick-borne pathogens in temperate and cold regions of Europe—A review on the prevalence in domestic animals. Fron. Vet. Sci. 2020,7, 604910. [CrossRef]
- Ogden, NH.; Mechai, S.; Margos, G. Changing geographic ranges of ticks and tick-borne pathogens: Drivers, mechanisms and consequences for pathogen diversity. Fron. Cell. Infec. Microbial. 2013, 3, 46. [CrossRef]
- Molaei, G.; Little, E.A.H.; Williams, S.C.; Stafford, K.C. Bracing for the Worst - Range Expansion of the Lone Star Tick in the Northeastern United States. N. Engl. J. Med. 2019, 381(23), 2189-2192. PMID: 31800982. [CrossRef]
- Eisen, Rebecca J., and Christopher D. Paddock. "Tick and tickborne pathogen surveillance as a public health tool in the United States." Journal of medical entomology 58, no. 4 (2021): 1490-1502. [CrossRef]
- Low VL, Tan TK, Khoo JJ, Lim FS, AbuBakar S. An overview of rickettsiae in Southeast Asia: Vector-animal-human interface. Acta Trop. 2020 Feb;202:105282. Epub 2019 Nov 25. PMID: 31778642. [CrossRef]
- Tariq, M., Seo, JW., Kim, D.Y. et al. First report of the molecular detection of human pathogen Rickettsia raoultii in ticks from the Republic of Korea. Parasites Vectors 14, 191 (2021). [CrossRef]
- de la Fuente J, Antunes S, Bonnet S, Cabezas-Cruz A, Domingos AG and others. Tick-Pathogen Interactions and Vector Competence: Identification of Molecular Drivers for Tick-Borne Diseases. Front Cell Infect Microbiol. 2017 Apr 7;7:114. PMID: 28439499; PMCID: PMC5383669. [CrossRef]
- Krishnamoorthi, S.; Goel, S.; Kaur, J.; Bisht, K.; Biswal, M. A Review of Rickettsial Diseases Other Than Scrub Typhus in India. Trop. Med. Infect. Dis. 2023. [CrossRef]
- Raoult D. A new rickettsial disease in the United States. Clin Infect Dis. 2004 Mar 15;38(6):812-3. Epub 2004 Mar 1. PMID: 14999623. [CrossRef]
- Ghosh, S.; Nagar, G. Problem of ticks and tick-borne diseases in India with special emphasis on progress in tick control research: A review. J. Vec. Born. Dis. 2014, 51(4), 259.
- Available online: https://www.rickettsia.net/infocat.aspx?pageID=104&contentID=1040102 (accessed on 15 June 2022).
- Chaisiri, K.; Tanganuchitcharnchai, A.; Kritiyakan, A.; Thinphovong, C.; Tanita, M.; Morand, S.; Blacksell, S.D. Risk factors analysis for neglected human rickettsiosis in rural communities in Nan province, Thailand: A community-based observational study along a landscape gradient. PLoS Negl. Trop. Dis. 2022, 16(3), e0010256. [CrossRef]
- Prakash JAJ, SohanLal T, Rosemol V, Verghese VP, Pulimood SA, Reller M; et al. Molecular detection and analysis of spotted fever group Rickettsia in patients with fever and rash at a tertiary care centre in Tamil Nadu, India. Path glob hlth. 2012; 106(1): 40-45.
- Khan, S.; Bora, T.; Richards, A. Human Rickettsia felis infection in India. J. Vec. Born. Dis. 2020, 57(2), 187-187. [CrossRef]
- Biswal M, Krishnamoorthi S, Bisht K, Sehgal A, Kaur J, Sharma N, Rickettsial Diseases: Not Uncommon Causes of Acute Febrile Illness in India. Trop Med and Infec Dis. 2020; 5(2):59. [CrossRef]
- Negi, T.; Kandari, L.S.; Arunachalam, K. Update on prevalence and distribution pattern of tick-borne diseases among humans in India: A review. Parasitol. Res. 2021, 120(5),1523-1539. [CrossRef]
- Perera, L.P.; Peiris, J.S.; Weilgama, D.J. Nairobi sheep disease virus isolated from Haemaphysalis intermedia ticks collected in Sri Lanka. Ann. Trop. Med. Parasitol. 1996, 90(1), 91-93. PMID: 8729633. [CrossRef]
- Sharma, A.; Mishra, B. Rickettsial disease existence in India: Resurgence in outbreaks with the advent of 20th century. Ind. J. Hlth. Sci. Biomed. Res. 2020, 13(1), 5. [CrossRef]
- Mathai, E.; Lloyd, G.; Cherian. T.; Abraham, O.C.; Cherian, A.M. Serological evidence for the continued presence of human rickettsiosis in southern India. Ann. Trop. Med. Parasitol. 2001, 95(4), 395-398. [CrossRef]
- Kamarasu, K.; Malathi, M.; Rajagopal, V.; Subramani, K.; Jagadeeshramasamy, D.; Mathai, E. Serological evidence for wide distribution of spotted fevers & typhus fever in Tamil Nadu. Ind. J. Med. Res. 2007, 126(2), 128-30. PMID: 17932437.
- Kalal, B.S.; Puranik, P.; Nagaraj, S.; Rego, S.; Shet, A. Scrub typhus and spotted fever among hospitalised children in South India: Clinical profile and serological epidemiology. Ind. J. Med. Microbiol. 2016, 34(3), 293–298. [CrossRef]
- Maiden MCJ Bygraves JA Feil E .et al. (1998) Multilocus sequence typing: A portable approach to the identification of clones within populations of pathogenic microorganisms .P Natl Acad Sci USA 95 :3140 –3145,. [CrossRef]
- Leclerque A, Kleespies RG (2012) A Rickettsiella Bacterium from the Hard Tick, Ixodes woodi: Molecular Taxonomy Combining Multilocus Sequence Typing (MLST) with Significance Testing. PLoS ONE 7(5): e38062. [CrossRef]
- Geevargheese, G.; Mishra, A.C. Haemaphysalis ticks of India. Elsevier, London, UK, 2011, 253.
- Sharif, M.A. Revision of the Indian Ixodidae with special reference to the collection in the Indian Museum. Records of the Zoological Survey of India. 1928, 30(3), 217-344. [CrossRef]
- Santibáñez, S.; Portillo, A.; Santibáñez, P.; Palomar, A.M.; Oteo, J.A. Usefulness of Rickettsial PCR assays for the molecular diagnosis of human rickettsiosis. Enferm. Infec. Microbiol. Clin. 2013, 31(5), 283-8. [CrossRef]
- Folmer, O.; Black, M., Hoeh, W., Lutz, R. and Vrijenhoek, R., 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates 3, 294–299.
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016 Jul; 33(7):1870-4. Epub 2016 Mar 22. PMID: 27004904; PMCID: PMC8210823. [CrossRef]
- Clow, K.M.; Leighton, P.A.; Pearl, D.L.; Jardine, C.M. A framework for adaptive surveillance of emerging tick-borne zoonosis. One Hlth. 2019, 100083. PMID: 30809583; PMCID: PMC6376153. [CrossRef]
- Johnson, N.; Phipps, L.P.; Hansford, K.M.; Folly, A.J.; Fooks, A.R.; Medlock, J.M.; Mansfield, K.L. One Health Approach to Tick and Tick-Borne Disease Surveillance in the United Kingdom. Int. J. Environ. Res. Pub. Hlth. 2022, 19(10), 5833. PMID: 35627370; PMCID: PMC9142090. [CrossRef]
- Salje, J.; Weitzel, T.; Newton, P.N.; Varghese, G.M.; Day, N. Rickettsial infections: A blind spot in our view of neglected tropical diseases. PLoS Neg. Trop. Dis. 2021, 15(5), p.e0009353. [CrossRef]
- Stewart, A.; Armstrong, M.; Graves, S.; Hajkowicz, K. Clinical manifestations and outcomes of Rickettsia australis infection: A 15-year retrospective study of hospitalized patients. Trop. Med. Infec. Dis. 2017, 2(2), 19. [CrossRef]
- https://www.rickettsia.net/infocat.aspx?pageID=104&contentID=1040502 (accessed on 30-03-2023).
- Krishnamoorthi, R.; Govindarajan, R.; Elango, A.; Rajamannar, V.; Philip Samuel, P. Species composition of hard ticks (Acari: Ixodidae) on domestic animals and their public health importance in Tamil Nadu, South India. Acar. Stu. 2021, 3(1), 16-21. [CrossRef]
- Li, H.; Zhang, P.H.; Huang, Y.; Du, J.; Cui, N.; Yang, Z.D.; Tang, F.; Fu, F.X.; Li, X.M.; Cui, X.M.; Fan, Y.D. Isolation and identification of Rickettsia raoultii in human cases: A surveillance study in 3 medical centers in China. Clin. Infec. Dis. 2018, 66(7), 1109-1115. [CrossRef]
- Fournier, P.E.; Dumler, J.S.; Greub, G.; Zhang, J.; Wu, Y,; Raoult, D. Gene sequence-based criteria for identification of new Rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J. Clin, Microbiol. 2003, 41(12), 5456-5465. [CrossRef]
- Ellison, D.W.; Clark, T.R.; Sturdevant, D.E.; Virtaneva, K.; Porcella, S.F.; Hackstadt, T. Genomic comparison of virulent Rickettsia rickettsii Sheila Smith and avirulent Rickettsia rickettsii Iowa. Infect Immun. 2008, 76(2), 542-50. Epub 2007 Nov 19. PMID: 18025092; PMCID: PMC2223442. [CrossRef]
- Olivieri, E.; Wijnveld, M.; Bonga, M.; Manfredi, M.T.; Veronesi, F.; Jongejan, F. Transmission Rickettsia raoultii and Rickettsia massiliae DNA Dermacentor reticulatus and Rhipicephalus sanguineus (s.l.) ticks during artificial feeding. Parasi. Vec. 2018, 11, 494. [CrossRef]
- Brown, L.D.; Macaluso, K.R. Rickettsia felis, an Emerging Flea-Borne Rickettsiosis. Curr. Trop. Med. Rep. 2016, 3, 27–39. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).