1. Introduction
Antimicrobial resistance has become a global concern in recent years, with many bacterial species developing resistance to commonly used antibiotics.[
1] One of the most significant contributors to this problem is the emergence and spread of antibiotic-resistant strains of bacteria in healthcare settings. [
2]
Enterococcus faecalis has been identified as a major source of nosocomial infections, and its prevalence in these settings has raised concerns about the efficacy of current treatment options, so much so that vaccines are under development to combat it. [
3] Despite being among the main antibiotic-resistant microorganisms,
E. faecalis is still commonly treated with cell wall-active antibiotics. [
1,
2] However, the prevalence of strains resistant to multiple drugs is a significant challenge for healthcare professionals. To address this issue, drug sensitivity testing is recommended to ensure appropriate treatment strategies are implemented. In recent years, there has been growing interest in the use of medicinal plants as an alternative or complementary approach to treating bacterial infections.
Sutherlandia frutescens, a plant commonly found in South Africa, has been shown to possess antimicrobial properties. The use of plant extracts, such as
S. frutescens, in combination with existing antibiotics such as Penicillin G provides a promising approach for addressing this issue. [
3,
4] It is on that premise that the research was needed to determine the efficacy and safety of such combinations.
Enterococcus faecalis
Enterococcus faecalis is a Gram-positive, commensal bacterium commonly found in the human gastrointestinal tract, responsible for various hospital-acquired infections.[
4] This bacterium is particularly challenging to treat due to its ability to form biofilms, secrete cytolysin, and acquire antibiotic resistance genes, leading to the development of multidrug-resistant strains. Infections caused by
E. faecalis are often linked to surgeries in the gastrointestinal area or puncturing injuries, as well as the prolonged use of intravenous devices and catheters. [
5] Treatment options for
E. faecalis infections include ampicillin, linezolid, and daptomycin, but drug sensitivity testing is crucial to prevent the development of resistant strains. Vancomycin has minimal bactericidal action against
E. faecalis, and
Enterococcus faecium isolates are more resistant to penicillin than
E. faecalis as reported in isolated from Indian and Turkish studies. [
6,
7] In recent years, there has been growing interest in natural products as alternative treatments for
E. faecalis infections. Studies have reported the antimicrobial activity of various plant extracts, including those from medicinal herbs and spices, against
E. faecalis. However, more research is needed to determine their safety and efficacy as a treatment option.[
4] In addition, infection control measures are crucial in preventing the spread of multidrug-resistant strains of
E. faecalis in hospitals worldwide. [
6,
7] Overall
, E. faecalis is a significant contributor to nosocomial infections, [
4] and its resistance to multiple drugs highlights the need for continued research and development of new treatment options to combat this challenging pathogen.
Penicillin G
Penicillin G, a natural penicillin antibiotic, is widely employed for the treatment of diverse bacterial infections [
8]. Its mechanism of action centers on interfering with susceptible bacteria’s cell wall synthesis, culminating in their demise [
8]. Gram-positive bacteria, including
Streptococcus and
Staphylococcus species, are notably vulnerable to its effects. Penicillin G is typically administered via injection, and various formulations such as aqueous solutions and pro-caine or benzathine salts allow for sustained release of the antibiotic. However, the emergence of antibiotic-resistant strains has diminished its efficacy against certain bacterial species, underscoring the significance of judicious antibiotic stewardship [
8]. Penicillin G, or benzylpenicillin, is a potent antibiotic with a well-established mechanism of action within the realm of microbiology. As a beta-lactam antibiotic, it disrupts bacterial cell wall synthesis, leading to bactericidal activity. Upon administration, penicillin G binds specifically to penicillin-binding proteins (PBPs) situated on the bacterial cell wall. PBPs play a vital role in catalyzing the cross-linking of peptidoglycan chains, a critical component of the bacterial cell wall structure. By inhibiting the transpeptidase activity of PBPs, penicillin G disrupts the formation of cross-links between peptidoglycan chains, thereby compromising the integrity and strength of the bacterial cell wall. Consequently, the weakened cell wall becomes more susceptible to osmotic pressure changes, resulting in cellular lysis and eventual bacterial death. Additionally, penicillin G triggers the activation of autolytic enzymes within the bacterial cell wall, further degrading the peptidoglycan structure, exacerbating cell wall damage, and promoting bacterial lysis. Although penicillin G primarily targets Gram-positive bacteria due to their thicker peptidoglycan layer, certain bacterial strains have developed resistance mechanisms, such as the production of beta-lactamases that can inactivate penicillin G. To address this challenge, it is imperative to develop a comprehensive understanding of penicillin G’s mechanism of action. This knowledge aids in the formulation of effective strategies to combat bacterial infections and promotes the responsible use of antibiotics in clinical microbiology practice.
Combining medicinal plants with routine antibiotics
To briefly explain
Table 1: In a series of studies investigating the combination of plants with antibiotics against different organisms, several interesting findings have emerged. One study focused on
Jatropha elliptica, ciprofloxacin, and norfloxacin, revealing that their combination exhibited additive activity against
Staphylococcus aureus [
9]
. This suggests that the combined effect was equal to the sum of their individual activities, potentially providing a more comprehensive antimicrobial action. In another study, α-mangostin and oxacillin were combined to target
Staphylococcus saprophyticus, resulting in a synergistic activity [
10]
. The combination demonstrated a greater antimicrobial effect compared to either compound used alone, indicating a potential enhancement of their individual abilities. The combination of
Juglans regia,
Camellia sinensis, and oxacillin showed promising results against
Staphylococcus aureus, particularly methicillin-resistant Staphylococcus aureus (MRSA) [
11]
. The synergistic activity observed in this combination suggests a potentiated effect that surpasses the individual actions of each component, offering new possibilities in combating MRSA infections.
Zygophyllum album combined with penicillin demonstrated synergistic activity against
Staphylococcus aureus, including MRSA [
12]
. This synergistic effect suggests a collaborative interaction between the plant and the antibiotic, potentially enhancing the antimicrobial efficacy.
Moringa oleifera, when combined with various antibiotics such as Penicillin-G, chloramphenicol, erythromycin, gentamicin, and tetracycline, displayed synergistic activity against multiple bacteria, including
Klebsiella pneumoniae,
Proteus vulgaris,
Acinetobacter baylyi, and
Pseudomonas aeruginosa [
13]
. This combination exhibited an enhanced antimicrobial effect compared to each antibiotic used individually, presenting a promising approach in addressing these challenging bacterial infections. Similarly,
Salvia limbata in combination with ceftazidime and neomycin showed synergistic activity against a range of organisms, including
Staphylococcus aureus,
Pseudomonas aeruginosa,
Acinetobacter baumannii, and MRSA [
13]
. This combination displayed a potentiated antimicrobial effect, suggesting a valuable strategy for combating these troublesome infections. Lastly,
Salvia officinalis combined with ciprofloxacin, cefotaxime, and ticarcillin plus clavulanic acid exhibited synergistic activity against
Pseudomonas aeruginosa [
14]
. The combination demonstrated an enhanced antimicrobial effect compared to the individual antibiotics used alone, highlighting the potential benefits of combining plant extracts with conventional antibiotics. These findings collectively indicate that the combination of specific plants with antibiotics can lead to synergistic or additive antimicrobial effects against various organisms. These interactions have the potential to enhance the efficacy of antibiotic treatments and address the growing challenge of antibiotic resistance. Further research is necessary to elucidate the underlying mechanisms and optimize these combinations for potential clinical applications.
The use of medicinal plants alongside penicillins for bacterial infections has been a topic of interest for many researchers and healthcare practitioners. Medicinal plants have been used for centuries to treat various ailments and have been found to possess antimicrobial properties. [
5] Penicillins, on the other hand, are a class of antibiotics that are commonly used to treat bacterial infections.[
8] Studies have shown that combining penicillins, as well as other types of antibiotics with certain medicinal plants can enhance their antibacterial activity.
Evidence from literature indicate that the combining of antibiotics with medicinal plants and their extracts has been demonstrated to be effective against multi-drug resistant strains by; increasing the activity of the antibiotic against the bacteria; or enhancing its effectiveness. However, despite the promising scholarly works of the use of medicinal plants alongside antibiotics such as penicillin should be done with caution. It is important to note that some medicinal plants may interfere with the absorption, distribution, and metabolism of penicillins, leading to adverse effects. Furthermore, the quality and potency of medicinal plant extracts can vary widely, which can affect their efficacy. In conclusion, the use of medicinal plants alongside penicillins for bacterial infections is a promising area of research.
Sutherlandia frutescens [syn. Lessertia frutescens] (Fabaceae)
Sutherlandia frutescens [syn.
Lessertia frutescens] (Fabaceae) (commonly known as cancer bush) has a long history of use as a medicinal plant in Southern Africa. [
15] It has been used in various cultural groups traditional medicine systems (including
AmaZulu, AmaXhosa, BaSotho,
Khoisan, and Cape Dutch). [
3]
Medicinal uses
Fever, wounds, [
16] insomnia, immune booster, [
17] anti-depressant including stress and anxiety, [
18] prevent muscle wasting from cancer, Tuberculosis, HIVAIDS, [
19] an appetite stimulant in wasted patients, [
20] influenza, viral hepatitis, asthma and chronic bronchitis, oesophagitis, heartburn, dysentery, type 2 diabetes mellitus (T2DM), , [
21] mild to moderate hypertension, [
22] rheumatoid arthritis, [
23] heart failure, [
24] urinary tract infections,[
25] peptic ulcer, [
25] gastritis, [
25] reflux oesophagitis, [
26] hot flashes and irritability in menopause. [
27]
The objective of this study was to assess the antibacterial activity of a combination of Sutherlandia frutescens water-based extract and penicillin G against Enterococcus faecalis, with the aim of evaluating the potential synergistic effect and determining the efficacy of the combination as a treatment option.
2. Materials and Methods
Preparation of the Water–Based Extraction ofSutherlandia frutescens extract
Sutherlandia frutescens was prepared according to an adjusted method 3a of the German Homeopathic Pharmacopoeia.Sutherlandia frutescens was harvested early in the morning. Plant material was immediately minced, and one part was added to three parts of distilled water (1:3) and calculated accordingly.
The mixture was shaken for 5 minutes and left in a glass jar for 10 days at a temperature not exceeding 20˚C. Mixture was agitated once a day. At the end of the incubation period the mixture was pressed through 100 % cotton muslin cloth and filtered through a No 1 Whatman filter paper. Volume was made up by adding distilled water.
Preparation ofpenicillin G
Penicillin G was dissolved in the solvent (water) in the ratio 1:3 and then used for disc diffusion tests. It was then added to Mueller Hinton agar for MIC studies. Dilutions of the penicillin G were prepared in sterile distilled water by way of serial dilutions. The dilutions ranged from 1 in 2 up to 1 in 16. All MIC ranges were according to the National Committee for Clinical Laboratory Standard guidelines. The original concentration of penicillin G was penicillin G was 200mg/ml.
Preparation of the combination of Sutherlandia frutescens water-based extract and penicillin G
Sutherlandia frutescens water – based extract was combined with penicillin G suspension in the ratio 1:1 for synergy testing. This was done by diluting 50ml of the extract with 50ml penicillin G suspension and this was utilised for the disc diffusion test. It was then added to Mueller Hinton agar for MIC studies. Dilutions of the combination were prepared in sterile distilled water by way of serial dilutions. The dilutions ranged from 1 in 2 up to 1 in 16. All MIC ranges were according to the National Committee for Clinical Laboratory Standard guidelines. This was used immediately after preparation.
Antibiotic Assay (AA) Discs
Antibiotic Assay discs purchased from Davies Diagnostics (Batch number: 277653). Mueller Hinton agar, nutrient agar slopes and nutrient broth were prepared according to manufacturer’s instructions. Blood Agar Base (BA) was also prepared accordingly.
Microbial cultures and preparation of inoculums
The cultures of E. faecalis American Type Colony Collection (ATCC number: 29212) were maintained on nutrient agar slopes at 4˚C and sub-cultured on to blood agar plates for 24 hours before use. These are known ATCC strains obtainable from Davies Diagnostics (Pty) Ltd. A few colonies from the overnight cultures of E. faecalis were suspended in 5ml of nutrient broth in bijou bottles. This was swirled to allow even distribution of the culture. The suspension was then vortexed in a vortex mixer to enable adequate mixing. The suspension was made up to the equivalent of 0.5 McFarland turbidity standard.
Bacterial sensitivity testing (screening)
The methodology was in accordance with a modification of the Kirby-Bauer Antimicrobial Sensitivity Test Procedure. Inoculum containing 1x106 colony forming units (CFU) per millilitre (ml) was introduced on to the surface of Mueller Hinton Agar plates.
Inoculation of plates and Placement of Disc on the agar plate
Six Mueller Hinton agar plates were inoculated with E. faecalis using a sterile swab dipped into the well mixed overnight nutrient broth culture. The agar surface of each plate was inoculated in such a way that there was uniform growth. The plates were then divided into 3 quadrants and labelled as: water control and vancomycin control but the third quadrat was for the combination or penicillin G.
The antibiotic assay discs were soaked for 10 seconds in each solution, except for the vancomycin discs which were purchased ready for use from Davies Diagnostics. Each disc was then gently placed on the surface of the agar plates and incubated at 37oC for 24 hours. After incubation, the plates were examined for the inhibition of growth around the discs. A positive result was indicated by a clear zone of inhibition surrounding the discs.
Zone of inhibition was measured using a ruler and compared to the controls. Each extract testing was repeated six times to ensure consistency and to allow for statistical analysis. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined using agar dilution technique. The combination was serially diluted using nutrient broth and was then incorporated into the Mueller Hinton agar plates. The plates were then inoculated with 0.5 MacFarland standard of bacterial suspension and incubated overnight at 37oC. Growth was compared to the negative control. If growth was present, colonies were counted.
Statistical Procedures
The combination of water extract from the plant and penicillin G was tested six times with E. faecalis. In total, the experiment was repeated 12 times for the plant extract. For each replication, the zone of inhibition produced, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were recorded. The response of E. faecalis was coded: growth or no growth. The number of replicates was determined in consultation with the statistician. A Fisher’s exact test was used to compare the number of replications that responded with the organism in the extract. The analysis was repeated for the combination of plant extract and penicillin. Statistically significant values are indicated by the asterisk (*).
3. Results
Table 3.
Zones of inhibition for the combination of S.frutescens water based extract and penicillin G on S. faecalis.
Table 3.
Zones of inhibition for the combination of S.frutescens water based extract and penicillin G on S. faecalis.
| Extract/penicillin |
Bacteria inhibtion zone |
P-value |
| Penicillin G |
0 mm |
0.000* |
| S.frutescens + penicillin G |
35 mm |
0.000* |
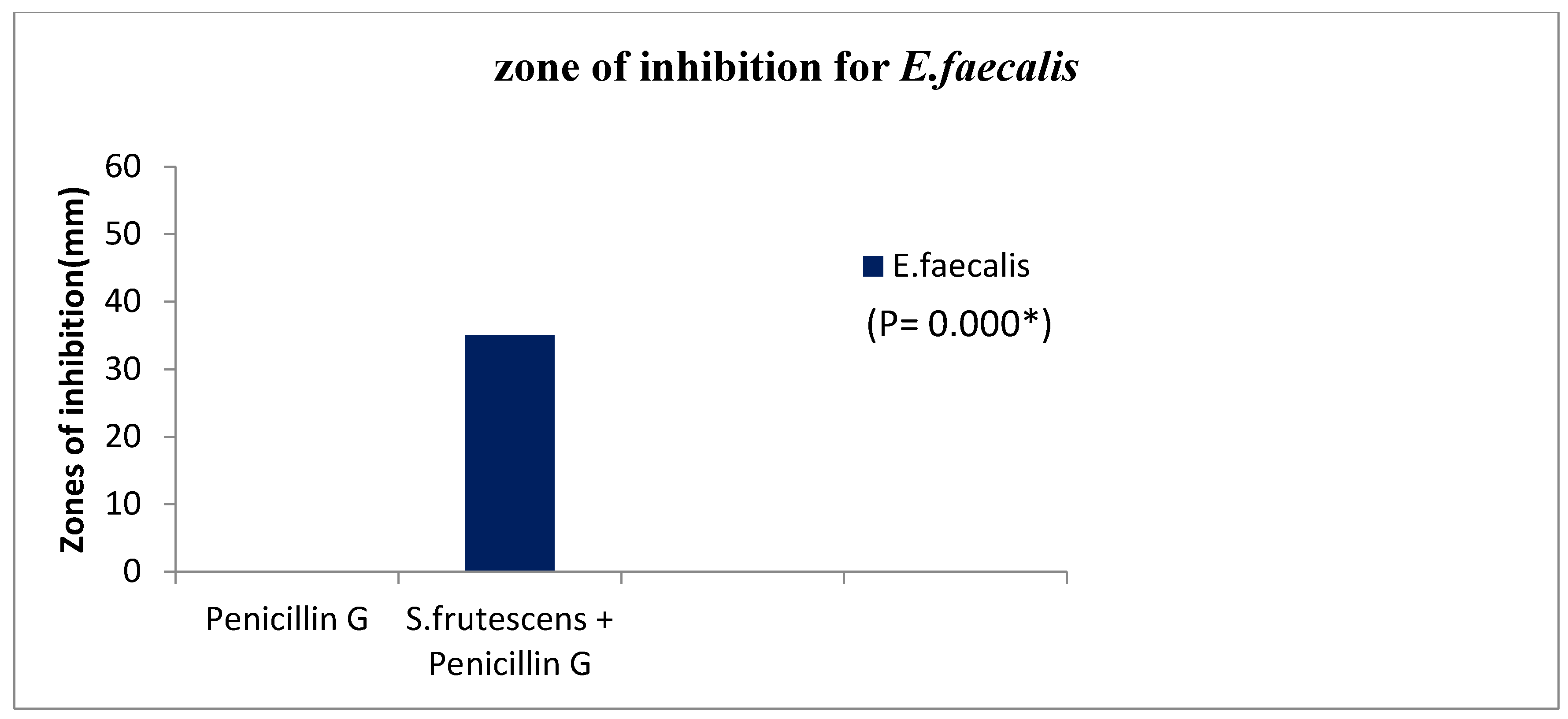
Figure 2.
Zones of inhibition for a combination of S. frutescens water-based extract and penicillin G compared to penicillin G alone on bacteria.
Figure 2.
Zones of inhibition for a combination of S. frutescens water-based extract and penicillin G compared to penicillin G alone on bacteria.
Table 4.
MIC results for the combination of S.frutescens water based extract and penicillin G against E.faecalis.
Table 4.
MIC results for the combination of S.frutescens water based extract and penicillin G against E.faecalis.
| Dilution |
E.faecalis % growth |
| 1 in 2 |
0% |
| 1 in 4 |
0% |
| 1 in 8 |
100% |
| 1 in 16 |
100% |
4. Discussion
The aim of the study was to assess the antibacterial activity of
S. frutescens water-based extract in combination with penicillin G against
E. faecalis. Since the recent years pathogenic microorganisms have mutated and become resistant to routine antibiotics and caused nosocomial infections. It was suggested that alternative natural products of plants could be of interest when considering the increase in the incidence of resistance to antibiotics. [
30] Some plants extracts, and phytochemicals are known to have antimicrobial properties, which could be significant in therapeutic treatment. In the last few years, various studies have been conducted in different countries demonstrating the efficacy of this type of treatment. [
31] Many plants have been evaluated not only for direct antimicrobial activity but also as resistance- modifying agents. [
31]
Zone of inhibition
The combination of S. frutescens water-based extract and penicillin G was tested for its inhibitory effect on S. faecalis bacteria. The inhibitory effect was measured by the size of the zone of inhibition, which is the area around the spot where the extract and penicillin G were applied where the bacteria did not grow. The table shows two treatments: penicillin G alone and a combination of S. frutescens water-based extract and penicillin G. The zone of inhibition for penicillin G alone was 0 mm, indicating that there was no inhibitory effect on S. faecalis bacteria.
In contrast, the combination of S. frutescens water-based extract and penicillin G resulted in a zone of inhibition of 35 mm, indicating a strong inhibitory effect. The P-values shown in the table represent the statistical significance of the differences between the two treatments. In this case, both P-values are (0.000), indicating that the difference between the two treatments is statistically significant. This result suggests that the combination of S. frutescens water-based extract and penicillin G has a strong inhibitory effect on S. faecalis bacteria, whereas penicillin G alone does not.
Zones of inhibition for a combination S. frutescens water-based extract and penicillin G
The second phase of investigating the inhibitory effect of a combination of S. frutescens water-based extract and penicillin G on bacterial growth compared to penicillin G alone. The zones of inhibition were measured to evaluate the effect on bacterial growth. The results showed that the combination of S. frutescens water-based extract and penicillin G produced a significantly larger zone of inhibition compared to penicillin G alone, indicating a stronger inhibitory effect on bacterial growth. These findings suggest that the combination of S. frutescens water-based extract and penicillin G may have a synergistic antimicrobial effect on bacteria, potentially enhancing the effectiveness of penicillin G.
Minimum Inhibitory Concentration (MIC)
The second phase of the investigation evaluated the inhibitory effect of a combination of S. frutescens water-based extract and penicillin G on the growth of E. faecalis bacteria. The minimum inhibitory concentration (MIC) of the combination was determined by measuring the visible growth of bacteria at different dilutions. The results showed that the combination completely inhibited bacterial growth at a dilution of 1 in 2 and 1 in 4 but was ineffective at inhibiting bacterial growth at a dilution of 1 in 8 and 1 in 16. These findings suggest that the combination of S. frutescens water-based extract and penicillin G has a potential antimicrobial effect against E. faecalis at low concentrations but may not be effective at higher dilutions.
Several studies have investigated the synergistic or additive effects of plant extracts and antibiotics on bacterial growth by measuring the agar zones of inhibition. These studies have focused on various plant extracts and antibiotics and have shown promising results in terms of enhancing the antibacterial activity of antibiotics. A previous study conducted to establish the effects of pomegranate rind (
Punica granatum Linn.) and guava leaf extract (
Psidium guajava Linn.) for inhibition of multidrug resistant
Escherichia coli. The combination of the plant extract and antibiotics resulted in larger zones of inhibition compared to the antibiotics alone, indicating an enhanced antibacterial effect. [
32]
The study involving
Coridothymus capitatus (L.)
Reichenb. fil. (Lamiaceae) alone and in combination, revealed a synergistic effect with itraconazole against
Staphylococcus aureus,
Staphylococcus epidermidis,
Listeria monocytogenes;
Bacillus subtilis and some clinical (methicillin resistant
S. aureus-MRSA),
Pseudomonas aeruginosa,
Candida krusei, and an additive effect with tetracycline against methicillin-resistant
Staphylococcus aureus strains. The suggested mechanism is that the extracts altered the membrane permeability of the microorganisms, and the mitochondrial function of yeasts. In sum, found that the combination of the plant extract and antibiotics produced larger zones of inhibition compared to the antibiotics alone, suggesting an additive and synergistic effect. [
33] A study carried out on Jordanian plants and established that the effectiveness of the antibiotics,
gentamycin and
chloramphenicol against
S. aureus were allegedly enhanced using plant materials. [
34] Similarly, a study stated that crude extracts of Indian medicinal plants revealed synergistic interaction with tetracycline and ciprofloxacin against extended spectrum β-lactamase (ESβL)-producing multidrug-resistant enteric microorganisms. [
35] A recent study investigating selected crude extracts as adjuvants to antibiotics to treat resistant bacterial infections. The study provided evidence that certain plant extracts can enhance the effectiveness of cefixime in treating infections caused by antibiotic-resistant strains of
S. aureus, K. pneumoniae, and
E. coli. This demonstrates the potential of plant-derived compounds in managing antimicrobial resistance. The study emphasizes the importance of phytochemicals in combating antibiotic-resistant infections. [
36]
The Penicillin G and Sutherlandia antimicrobial effect
In investigating the combined antimicrobial therapy of penicillin G and Sutherlandia frutescens, we can propose a potential synergistic mechanism of action based on their individual properties. Penicillin G, a beta-lactam antibiotic, exerts its effect by disrupting bacterial cell wall synthesis through binding to penicillin-binding proteins (PBPs) and inhibiting their transpeptidase activity. This disruption weakens the bacterial cell wall, leading to cell lysis and bacterial death, primarily targeting Gram-positive bacteria. Sutherlandia frutescens, on the other hand, displays broad-spectrum antimicrobial activity and immune modulation. This medicinal plant contains various phytochemicals such as flavonoids, triterpenes, alkaloids, and saponins, which contribute to its antimicrobial properties. These phytochemicals can disrupt microbial cell membranes, inhibit key enzymes, and stimulate immune responses against microbial infections. When penicillin G and Sutherlandia frutescens are used in combination, their potential mechanisms of action may synergize. Penicillin G’s disruption of the bacterial cell wall could render bacteria more susceptible to the antimicrobial components present in Sutherlandia frutescens. The phytochemicals within Sutherlandia frutescens, known for membrane disruption and enzyme inhibition, may further augment the antimicrobial effects. Additionally, Sutherlandia frutescens’ immune-modulating properties may enhance the body’s immune response, aiding in the clearance of microbial pathogens. However, it is important to note that the proposed synergistic mechanism of action is speculative, as no specific studies have explored the combined effects of penicillin G and Sutherlandia frutescens. Further research is necessary to elucidate the actual interactions and confirm the efficacy, safety, and compatibility of this combination therapy. Careful consideration should be given to ensure proper antibiotic stewardship and optimal clinical outcomes when considering such combinations.
Conclusion and recommendations
The present study aimed to assess the antibacterial activity of a combination of Sutherlandia frutescens water-based extract and penicillin G against Enterococcus faecalis. The results obtained shed light on the potential synergistic effect of the combination and provide valuable insights into its efficacy as a treatment option. In this section, we will comprehensively analyze and interpret the findings, discuss their implications in the context of existing literature, and address any limitations or potential sources of error in the study.
The results demonstrated that the combination of Sutherlandia frutescens extract and penicillin G exhibited a significantly stronger inhibitory effect on Enterococcus faecalis compared to penicillin G alone. The zones of inhibition observed in the combination group were notably larger, indicating enhanced antibacterial activity. Additionally, the minimum inhibitory concentration (MIC) of the combination was significantly lower than that of penicillin G alone, further supporting the synergistic effect.
These findings align with previous studies investigating the antibacterial properties of Sutherlandia frutescens extract. Several bioactive compounds present in the plant, such as canavanine, pinitol, and L-canavanine sulfate, have been reported to possess antimicrobial properties. These compounds can inhibit the growth of various bacterial strains, including those resistant to conventional antibiotics. The combination of Sutherlandia frutescens extract with penicillin G may have resulted in a synergistic interaction, enhancing the overall antibacterial activity against Enterococcus faecalis.
Furthermore, the observed synergistic effect may be attributed to the different mechanisms of action of Sutherlandia frutescens extract and penicillin G. Penicillin G is a β-lactam antibiotic that primarily targets the bacterial cell wall synthesis, while Sutherlandia frutescens extract may act on multiple targets within the bacterial cell, such as enzymes involved in nucleic acid synthesis or membrane integrity. This multi-target approach can disrupt bacterial homeostasis and potentiate the effects of penicillin G.
The findings of this study have important implications for the development of new treatment strategies against Enterococcus faecalis infections. Enterococcal infections are a growing concern in both community and healthcare settings, and the emergence of antibiotic-resistant strains poses a significant challenge (Klare et al., 2016). The combination of Sutherlandia frutescens extract and penicillin G could serve as a promising alternative or adjunct to conventional antibiotics, particularly in cases where resistance to penicillin G is encountered.
Despite the encouraging results, it is essential to acknowledge the limitations and potential sources of error in this study. Firstly, the in vitro nature of the study may not fully reflect the complexity of an in vivo environment, where additional factors such as host immune response and drug metabolism come into play. Therefore, further investigations involving animal models or clinical trials are necessary to validate the efficacy and safety of the combination.
Another limitation is the focus on a single bacterial strain, Enterococcus faecalis, which may not fully represent the spectrum of enterococcal infections. Future studies should include other clinically relevant enterococcal species to assess the broad-spectrum activity of the combination.
Furthermore, the study relied on the Sutherlandia frutescens water-based extract, and the specific concentrations and formulation used in this study may not be optimal. It would be valuable to explore different extraction methods, solvents, and concentrations to maximize the antibacterial activity and identify the active compounds responsible for the observed effects. In conclusion, the results of this study indicate that the combination of Sutherlandia frutescens water-based extract and penicillin G.
Author Contributions
Conceptualization, NWN. and N.W.N; methodology, N.W.N.; software, N.T; validation, N.W.N., and N.T.; formal analysis, N.W.N.; investigation, N.W.N; resources, N.W.N; data curation, N.W.N; writing—original draft preparation, N.W.N. and N.T.; writing—review and editing, N.T.; visualization, N.T.; supervision, N.W.N.; project administration, N.W.N.; funding acquisition, N.W.N. All authors have read and agreed to the published version of the manuscript.
Contribution: NWN: Conceptualization, Design, Literature review, Analysis, Writing; NT: Design, Literature review, Analysis and Interpretations
Financial Support: No support was received for this study
Statement: The authors state that the manuscript had been read and approved by all of them, and that the requirements for authorship as outlined earlier in the document had been met. They also confirmed that each author believed that the manuscript represented honest work.
Data Availability Statement
Data will be made available on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Conflicts of Interest
No potential conflict of interest was reported by the authors.
References
- Sengupta, M.; Sarkar, R.; Sarkar, S.; Sengupta, M.; Ghosh, S.; Banerjee, P. Vancomycin and Linezolid-Resistant Enterococcus Isolates from a Tertiary Care Center in India. Diagnostics 2023, 13, 945. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Browning, J.D.; Eichen, P.A.; Brownstein, K.J.; Folk, W.R.; Sun, G.Y.; Lubahn, D.B.; Rottinghaus, G.E.; Fritsche, K.L. Unveiling the anti-inflammatory activity of Sutherlandia frutescens using murine macrophages. Int. Immunopharmacol. 2015, 29, 254–262. [Google Scholar] [CrossRef]
- Hlongwane, M.M.; Mohammed, M.; Mokgalaka, N.S.; Dakora, F.D. The Potential of Rhizobacteria to Mitigate Abiotic Stress in Lessertia frutescens. Plants 2023, 12, 196. [Google Scholar] [CrossRef]
- Krawczyk B, Wityk P, Gałęcka M, Michalik M. The Many Faces of Enterococcus spp.—Commensal, Probiotic and Opportunistic Pathogen. Microorganisms [Internet]. 2021 Sep 7 [cited 2023 ];9(9):1900. 9 May 2076.
- Zonyane S, Fawole OA, La Grange C, Stander MA, Opara UL, Makunga NP. The Implication of Chemotypic Variation on the Antioxidant and Anti-Cancer Activities of Sutherlandia frutescens (L.) R.Br. (Fabaceae) from Different Geographic Locations. Antioxidants [Internet]. 2020 Feb 13 [cited 2023 ];9(2):152. 10 May 2076.
- Nsele NW, Moodley S. The Effect of Aqueous Extract of Sutherlandia frutescens (Unwele) and Benzathine Penicillin on Enterococcus faecalis. In: Ikeno DrT, editor. Challenges and Advances in Pharmaceutical Research Vol 10 [Internet]. B P International (a part of SCIENCEDOMAIN International); 2022 [cited 2023 ]. p. 92–102. 9 May 8800.
- Sweedan, E.G. The Antimicrobial Effects of Alcoholic Leaves Extract of Salvia Officinalis Against Multidrug Resistant Pseudomonas Aeruginosa. Iraqi J. Sci. 2021, 441–448. [Google Scholar] [CrossRef]
- Farooqui, A.; Khan, A.; Borghetto, I.; Kazmi, S.U.; Rubino, S.; Paglietti, B. Synergistic Antimicrobial Activity of Camellia sinensis and Juglans regia against Multidrug-Resistant Bacteria. PLOS ONE 2015, 10, e0118431–e0118431. [Google Scholar] [CrossRef]
- Al-Saiym RA, Al-Kamali HH, Al-Magboul AZ. Synergistic Antibacterial Interaction between Trachyspermum ammi, Senna alexandrina Mill and Vachellia nilotica spp. Nilotica Extract and Antibiotics. Pakistan J of Biological Sciences [Internet]. 2015 Mar 15 [cited 2023 ];18(3):115–21. 10 May.
- Tavakoli* F, Emami A, Ranjbar AM, Beyk M. Synergistic Activity of Three Iranian Medicinal Plants in Combination with Ceftazidime and Neomycin against Bacterial Strains Causing Nosocomial Infections. Res J Pharmacogn [Internet]. 2022 Jul [cited 2023 ];9(3). 10 May. [CrossRef]
- Phitaktim, S.; Chomnawang, M.; Sirichaiwetchakoon, K.; Dunkhunthod, B.; Hobbs, G.; Eumkeb, G. Synergism and the mechanism of action of the combination of α-mangostin isolated from Garcinia mangostana L. and oxacillin against an oxacillin-resistant Staphylococcus saprophyticus. BMC Microbiol. 2016, 16, 1–14. [Google Scholar] [CrossRef]
- Sergeant, C.; Africander, D.; Swart, P.; Swart, A. Sutherlandia frutescens modulates adrenal hormone biosynthesis, acts as a selective glucocorticoid receptor agonist (SEGRA) and displays anti-mineralocorticoid properties. J. Ethnopharmacol. 2017, 202, 290–301. [Google Scholar] [CrossRef]
- Aboyade, O.M.; Styger, G.; Gibson, D.; Hughes, G. Sutherlandia frutescens: The Meeting of Science and Traditional Knowledge. J. Altern. Complement. Med. 2014, 20, 71–76. [Google Scholar] [CrossRef]
- Abibu, M.A.; Takuwa, D.T.; Sichilongo, K. Quantification of eight water soluble vitamins in Sutherlandia frutescens species from Botswana using a validated reversed phase HPLC method. Sep. Sci. PLUS 2019, 2, 200–209. [Google Scholar] [CrossRef]
- Sharaf, M.H.; El-Sherbiny, G.M.; Moghannem, S.A.; Abdelmonem, M.; Elsehemy, I.A.; Metwaly, A.M.; Kalaba, M.H. New combination approaches to combat methicillin-resistant Staphylococcus aureus (MRSA). Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Dai, L.; Sahin, O.; Grover, M.; Zhang, Q. New and alternative strategies for the prevention, control, and treatment of antibiotic-resistant Campylobacter. Transl. Res. 2020, 223, 76–88. [Google Scholar] [CrossRef]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural Products as Platforms To Overcome Antibiotic Resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef]
- Marquez, B.; Neuville, L.; Moreau, N.J.; Genet, J.-P.; dos Santos, A.F.; de Andrade, M.C.C.; Sant’ana, A.E.G. Multidrug resistance reversal agent from Jatropha elliptica. Phytochemistry 2005, 66, 1804–1811. [Google Scholar] [CrossRef]
- E. Rajitha. Molecular Studies and Pharmacological Role of Sutherlandia Frutescens. 2022 Nov 18 [cited 2023 ]. 10 May 7336.
- Loggenberg SR, Twilley D, De Canha MN, Lall N. Medicinal plants used in South Africa as antibacterial agents for wound healing. In: Medicinal Plants as Anti-Infectives [Internet]. Elsevier; 2022 [cited 2023 ]. p. 139–82. 10 May.
- Mncwangi N, Viljoen A, Mulaudzi N, Fouche G. Lessertia frutescens. In: The South African Herbal Pharmacopoeia [Internet]. Elsevier; 2023 [cited 2023 ]. p. 321–44. 10 May.
- Hübsch, Z.; Van Zyl, R.; Cock, I.; Van Vuuren, S. Interactive antimicrobial and toxicity profiles of conventional antimicrobials with Southern African medicinal plants. South Afr. J. Bot. 2014, 93, 185–197. [Google Scholar] [CrossRef]
- Ilanko, P.; McDonnell, P.A.; van Vuuren, S.; Cock, I.E. Interactive antibacterial profile of Moringa oleifera Lam. extracts and conventional antibiotics against bacterial triggers of some autoimmune inflammatory diseases. South Afr. J. Bot. 2019, 124, 420–435. [Google Scholar] [CrossRef]
- Ahmad I, Aqil F. In vitro efficacy of bioactive extracts of 15 medicinal plants against ESβL-producing multidrug-resistant enteric bacteria. Microbiological Research [Internet]. 2007 Jul [cited 2023 ];162(3):264–75. 10 May.
- Brown, L.; Heyneke, O.; Brown, D.; van Wyk, J.; Hamman, J. Impact of traditional medicinal plant extracts on antiretroviral drug absorption. J. Ethnopharmacol. 2008, 119, 588–592. [Google Scholar] [CrossRef]
- Gao, W.; Howden, B.P.; Stinear, T.P. Evolution of virulence in Enterococcus faecium, a hospital-adapted opportunistic pathogen. Curr. Opin. Microbiol. 2018, 41, 76–82. [Google Scholar] [CrossRef]
- Marino, A.; Nostro, A.; Mandras, N.; Roana, J.; Ginestra, G.; Miceli, N.; Taviano, M.F.; Gelmini, F.; Beretta, G.; Tullio, V. Evaluation of antimicrobial activity of the hydrolate of Coridothymus capitatus (L.) Reichenb. fil. (Lamiaceae) alone and in combination with antimicrobial agents. BMC Complement. Med. Ther. 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ngcobo, M.; Gqaleni, N.; Cheluled, P.; Serumula, M.; Assounga, A. Effects of Sutherlandia frutescens Extracts on Normal T-Lymphocytes In Vitro. Afr. J. Tradit. Complement. Altern. Med. 2011, 9, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kridda Chukiatsiri, Kittiphong Tippaya, Ruttayaporn Ngasaman2. Effects of pomegranate rind (Punica granatum Linn.) and guava leaf extract (Psidium guajava Linn.) for inhibition of multidrug resistant Escherichia coli recovered from diarrhoeal piglets. Songklanakarin J Sci Technol [Internet]. [cited 2023 ]. Available online: https://www.thaiscience.info/Journals/Article/SONG/10993204.
- Pandey N, Cascella M. Beta Lactam Antibiotics. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2023 ]. Available online: http://www.ncbi.nlm.nih.gov/books/NBK545311/.
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Asgin, N.; Otlu, B. Antibiotic Resistance and Molecular Epidemiology of Vancomycin-Resistant Enterococci in a Tertiary Care Hospital in Turkey. Infect. Drug Resist. 2020, ume 13, 191–198. [Google Scholar] [CrossRef]
- Atta, S.; Waseem, D.; Fatima, H.; Naz, I.; Rasheed, F.; Kanwal, N. Antibacterial potential and synergistic interaction between natural polyphenolic extracts and synthetic antibiotic on clinical isolates. Saudi J. Biol. Sci. 2023, 30, 103576. [Google Scholar] [CrossRef]
- Buthelezi NMD, Gololo SS, Mugivhisa LL. An Assessment of Moringa (Moringa oleifera L.) Seed Extract on Crop Water Productivity and Physico-Biochemical Properties of Cancer Bush (Sutherlandia frutescens L.) under Deficit Irrigation. Horticulturae [Internet]. 2022 Oct 13 [cited 2023 ];8(10):938.
- van Wyk, B.-E.; Albrecht, C. A review of the taxonomy, ethnobotany, chemistry and pharmacology of Sutherlandia frutescens (Fabaceae). J. Ethnopharmacol. 2008, 119, 620–629. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Combination of antibiotics and medicinal plants in literature.
Table 1.
Combination of antibiotics and medicinal plants in literature.
| Plant & Antibiotic combination |
Organism |
Results |
Reference |
|
Jatropha elliptica, ciprofloxacin & norfloxacin |
Staphylococcus aureus |
Additive activity |
[9] |
| α-mangostin & oxacillin |
Staphylococcus Saprophyticus. |
Synergistic activity |
[10] |
|
Juglans regia, Camellia sinensis & oxacillin |
Staphylococcus aureus (MRSA) |
Synergistic activity |
[11] |
| Zygophyllum album & penicillin |
Staphylococcus aureus (MRSA) |
Synergistic activity |
[12] |
|
Moringa oleifera & Penicillin-G chloramphenicol, erythromycin , gentamicin, & tetracycline
|
Klebsiella pneumoniae, Proteus vulgaris, Acinetobacter baylyi & Pseudomonas aeruginosa). |
Synergistic activity |
[13] |
|
Salvia limbata with ceftazidime & neomycin |
Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter baumannii & methicillin-resistant Staphylococcus aureus (MRSA) |
Synergistic activity |
[13] |
|
Salvia officinalis, Ciprofloxacin, Cefotaxime, & Ticarcillin+ Clavulanic Acid |
Pseudomonas aeruginosa |
Synergistic activity |
[14] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).