Submitted:
08 June 2023
Posted:
09 June 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
2.1. Methodology
3. Relevant sections
3.1. Definition and types of biostimulants
3.2. Advantages of natural biostimulants over conventional ones
3.2.1. Sustainability and Environmental Impact
3.2.2. Security
3.2.3. Broad spectrum of activity
3.2.4. Positive interactions
3.2.5. Regulatory compliance
3.3. Production Processes of NBs by SSF
3.3.1. Substrate Selection for SSF
3.3.2. Substrate Pretreatment
3.3.3. Inoculation of microorganisms
3.3.4. Control of SSF Conditions
4. Methods of NBs Production
4.1. Microorganisms used in NBs Production
4.2. Characteristics of SSF for NBs Production
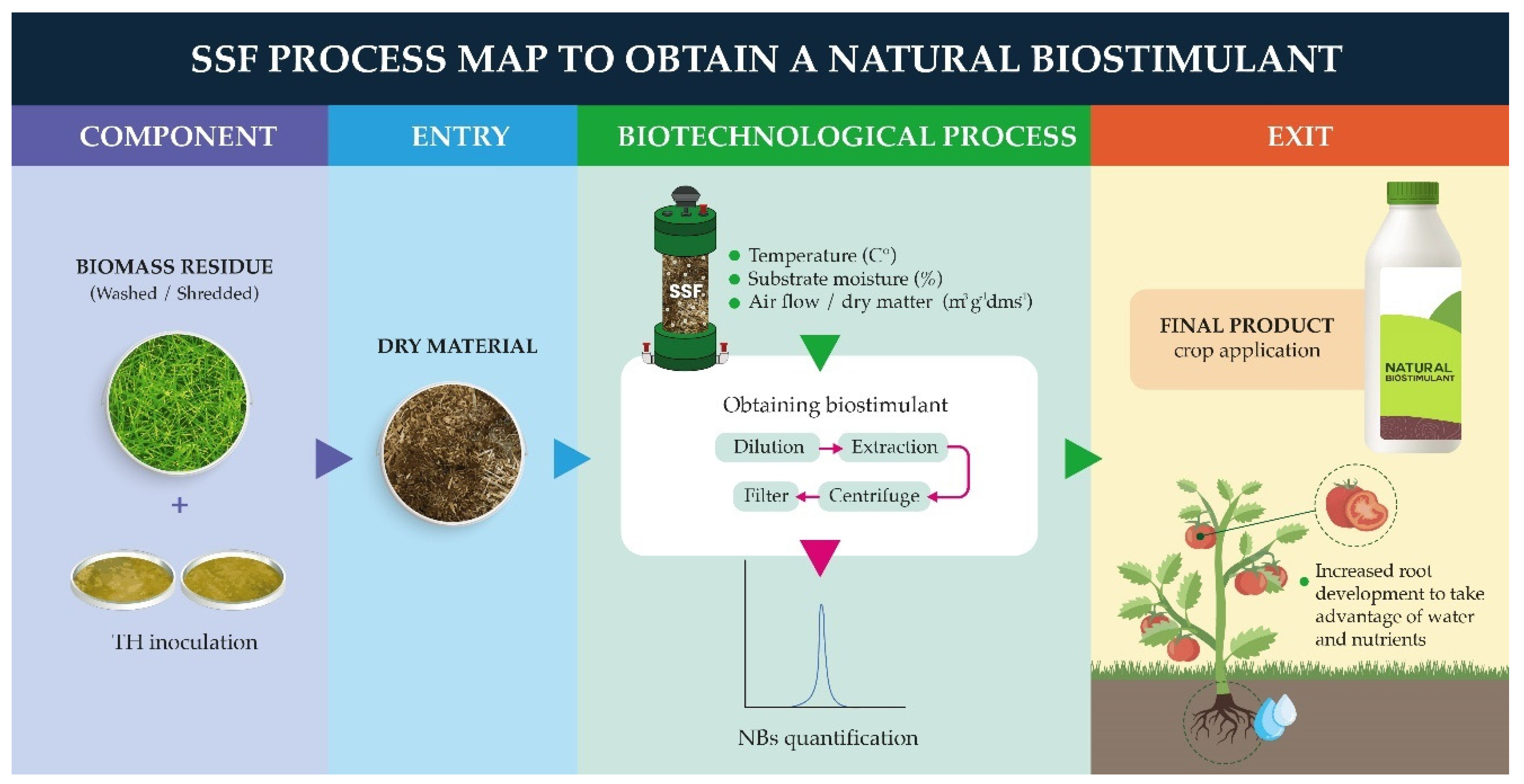
4.3. Effect of the NBs on crops
4.3.1. Improvement of plant growth and development
| Crop | NBs type | Effect | Scale | Ref |
|---|---|---|---|---|
| Arabidopsis thaliana | Low molecular weight peptides | Increase in plant biomass | laboratory | [99] |
| Sesame | GA3 | Improvement of plant architecture | laboratory | [100] |
| Rice | GA3 | Improvement of plant architecture | laboratory | [101] |
| Tomato Pepper seed Arabidopsis Orchid |
IAA | Promotion of seed germination and seedling emergence | Greenhouse laboratory |
[102,103,104] |
4.3.2. Increased resistance to adverse conditions
4.3.3. Effect of NBs on improving crop quality
| Crop | NBs type | Effect | Scale | Ref |
|---|---|---|---|---|
| Gerbera Tectona grandis Peas Yarrow |
Humic acid |
Increased nutrient concentration | Greenhouse | [114,115,116,117] |
| Tomato Apple |
Amino acids | Improved organoleptic quality | Greenhouse | [118,119,120] |
| Soy Petunia flowers lettuce |
Cytokinins | Delayed tissue senescence |
Greenhouse | [121,122,123] |
4.3.4. Optimization of nutrient use efficiency
4.3.5. Effect NBs on agricultural productivity
| Crop | NBs type | Effect of productivity on crops | Scale | Ref |
|---|---|---|---|---|
| Corn | Seaweed extract | Increase in grain yield, crop residue, and improvement in nutritional quality | field | [129,130,131] |
| Grapes | Seaweed extract | Increase in grape production, improvement in stress resistance, and higher polyphenol content. | Greenhouse | [132,133,134] |
| Tomato | Seaweed extract | Increased fruit yield and quality. | Greenhouse | [135,136,137] |
| Lettuce | Seaweed extract | Higher yield increase and increased shoot growth | Greenhouse | [138,139,140] |
| Strawberries | Seaweed extract | Improvement in fruit quality and flavour, higher yield | Greenhouse | [112,141] |
| Onion | Seaweed extract | Increased bulb diameter and weight | field | [142,143] |
| Potato | Seaweed extract | Increased tuber yield and quality. | Field | [144,145] |
| Corn | IAA | Stimulation of vegetative growth and increased grain production | Greenhouse | [146,147,148] |
| Lettuce | IAA | Increase in biomass | Greenhouse | [149] |
| Potato | IAA | Promotes tuber growth and improves yield | Greenhouse | [150,151,152] |
| Onion | IAA | Increases bulb size and enhances production | Greenhouse Laboratory |
[153,154,155] |
| Quinoa | IAA | Boosts grain yield and improves quality | Field | [156,157] |
| Wheat | IAA | Stimulates plant growth and increases yield | Field | [158,159] |
| Tomato | IAA | Improves rooting, increases fruit production, and enhances antioxidant content. | Greenhouse |
[160,161] |
| Soybean | IAA | Improves root development and increases production. | Greenhouse |
[162,163] |
| Rice | IAA | Promotes rooting and improves yield | Field | [164,165] |
| broad beans | IAA | Stimulates vegetative growth and increases production | Greenhouse |
[163,166] |
| Grapes | IAA | Enhances root formation and increases yield | Greenhouse |
[167,168,169] |
| Corn | Cytokinins | Stimulates cell division and increases yield | Greenhouse |
[170,171] |
| Rice | Cytokinins | Promotes grain growth and improves yield. | Greenhouse | [172,173] |
| Wheat | Cytokinins | Increases the number of grains per spike and improves production. | Field | [174,175,176] |
| Soybean | Cytokinins | Improves vegetative growth and increases production | Greenhouse | [177,178] |
| Tomato | Cytokinins | Stimulates flower formation and increases yield. | Greenhouse | [29,179] |
| Potato | Cytokinins | Promotes tuber development and improves yield | Field | [180,181] |
| Grapes | Cytokinins | Enhances cluster size and quality | Greenhouse | [182,183] |
| Strawberry | Cytokinins | Increases stolon formation and improves production. | Greenhouse | [184,185] |
| Strawberry | Cytokinins | Stimulates bud break and improves yield | Greenhouse | [186] |
| Citrus | Cytokinins | Increases fruit size and improves production | Greenhouse | [187,188] |
| Onion | Humic acids | Enhances bulb yield, improves quality, and disease resistance. | Greenhouse | [189,190] |
| Corn | Humic acids | Improves nutrient absorption and increases yield | Greenhouse | [29,191] |
| Wheat | Humic acids | Increases grain size and weight. | Greenhouse | [192,193] |
| Rice | Humic acids | Boosts the number of spikes and improves production | Greenhouse | [194,195] |
| Tomato | Humic acids | Enhances fruit quality and increases yield | Greenhouse | [196,197] |
| Beans | Humic acids | Improves vegetative growth and increases production. | Field | [198] |
| Onion | Humic acids | Increases bulb size and quality. | Greenhouse | [199,200] |
| Carrot | Humic acids | Promotes root development and improves production | Greenhouse | [201] |
| Lettuce | Humic acids | Stimulates leaf growth and increases yield. | Greenhouse | [202] |
4.4. Limitations and Challenges of NBs by SSF
4.4.1. Standardization issues in NBs production by SSF
4.4.2. Challenges in the Application of NBs from SSF in Sustainable Agriculture
4.4.3. Factors Limiting the Effectiveness of Natural Bio-Stimulants Produced by SSF in Different Crops
5. Conclusions and future research perspectives
5.1. Future research prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Natural biostimulants (NBs) |
| Solid state fermentation (SSF) |
| The European Biostimulants Industry Council (EBIC) |
| Humic substances (HS) |
| Hormone-containing products (HCP) |
| Amino acid-containing products (AACP) |
| Indole-3-Acetic Acid (IAA) |
| Abscisic acid (ABA) |
References
- 2.4.1 Agricultural Sustainability | Sustainable Development Goals | Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/sustainable-development-goals/indicators/241/en/ (accessed on 23 May 2023).
- Sumberg, J.; Giller, K.E. What Is ‘Conventional’ Agriculture? Global Food Security 2022, 32, 100617. [Google Scholar] [CrossRef]
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a Biostimulant on the Heat Tolerance Associated with Photosynthetic Capacity, Membrane Thermostability, and Polyphenol Production of Perennial Ryegrass. Crop Science 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Wong, W.S.; Tan, S.N.; Ge, L.; Chen, X.; Letham, D.S.; Yong, J.W.H. The Importance of Phytohormones and Microbes in Biostimulants: Mass Spectrometric Evidence and Their Positive Effects on Plant Growth. Acta Horticulturae 2016, 1148, 49–60. [Google Scholar] [CrossRef]
- Boivin, S.; Fonouni-Farde, C.; Frugier, F. How Auxin and Cytokinin Phytohormones Modulate Root Microbe Interactions. Frontiers in Plant Science 2016, 7, 1240. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Scientia Horticulturae 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Sauer, M.; Robert, S.; Kleine-Vehn, J. Auxin: Simply Complicated. Journal of Experimental Botany 2013, 64, 2565–2577. [Google Scholar] [CrossRef]
- Rady, M.M.; Desoky, E.-S.M.; Elrys, A.S.; Boghdady, M.S. Can Licorice Root Extract Be Used as an Effective Natural Biostimulant for Salt-Stressed Common Bean Plants? South African Journal of Botany 2019, 121, 294–305. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Zaman, M.; Pharis, R.P. Phytohormonal Basis for the Plant Growth Promoting Action of Naturally Occurring Biostimulators. Journal of the Science of Food and Agriculture 2014, 94, 1715–1722. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Szafrańska, K. Biostimulators: A New Trend towards Solving an Old Problem. Frontiers in Plant Science 2016, 7. [Google Scholar] [CrossRef]
- Hellequin, E.; Monard, C.; Chorin, M.; Le bris, N.; Daburon, V.; Klarzynski, O.; Binet, F. Responses of Active Soil Microorganisms Facing to a Soil Biostimulant Input Compared to Plant Legacy Effects. Sci Rep 2020, 10, 13727. [Google Scholar] [CrossRef]
- EBIC – The European Biostimulants Industry Council. Available online: https://biostimulants.eu/ (accessed on 8 March 2022).
- Biological Products Industry Alliance | Advancing Knowledge About Biopesticides & Biostimulants. Available online: https://www.bpia.org/ (accessed on 23 April 2023).
- Prado, D.Z. do; Okino-Delgado, C.H.; Zanutto-Elgui, M.R.; Silva, R.B.G. da; Pereira, M.S.; Jahn, L.; Ludwig-Müller, J.; Silva, M.R. da; Velini, E.D.; Fleuri, L.F. Screening of Aspergillus, Bacillus and Trichoderma Strains and Influence of Substrates on Auxin and Phytases Production through Solid-State Fermentation. Biocatalysis and Agricultural Biotechnology 2019, 19, 101165. [Google Scholar] [CrossRef]
- Ghoreishi, G.; Barrena, R.; Font, X. Using Green Waste as Substrate to Produce Biostimulant and Biopesticide Products through Solid-State Fermentation. Waste Management 2023, 159, 84–92. [Google Scholar] [CrossRef]
- Chen, H. Biotechnology Principles of Solid State Fermentation. Modern Solid State Fermentation 2013, 23–74. [Google Scholar] [CrossRef]
- Sanchez-Montesinos, B.; Dianez, F.; Moreno-Gavira, A.; Gea, F.J.; Santos, M. Role of Trichoderma Aggressivum f. Europaeumas Plant-Growth Promoter in Horticulture. AGRONOMY-BASEL 2020, 10. [Google Scholar] [CrossRef]
- Zanoni do Prado, D.; Oliveira, S.L.; Okino-Delgado, C.H.; Auer, S.; Ludwig-Müller, J.; Ribeiro da Silva, M.; Júnior da Costa Fernandes, C.; Carbonari, C.A.; Zambuzzi, W.F.; Fleuri, L.F. Aspergillus Flavipes as a Novel Biostimulant for Rooting-Enhancement of Eucalyptus. Journal of Cleaner Production 2019, 234, 681–689. [Google Scholar] [CrossRef]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Del Buono, D. The Opportunity of Valorizing Agricultural Waste, Through Its Conversion into Biostimulants, Biofertilizers, and Biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An Overview of Plant-Based Natural Biostimulants for Sustainable Horticulture with a Particular Focus on Moringa Leaf Extracts. Plant Science 2020, 295, 110194. [Google Scholar] [CrossRef]
- EBIC – El Consejo Europeo de La Industria de Bioestimulantes. Available online: https://biostimulants.eu/ (accessed on 14 June 2022).
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant and Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Cui, Y.; Liu, R.; Li, Y.; Chen, Q.; Gu, Y.; Zhao, K.; Xiang, Q.; Xu, K.; et al. An Indoleacetic Acid-Producing Ochrobactrum Sp. MGJ11 Counteracts Cadmium Effect on Soybean by Promoting Plant Growth. Journal of Applied Microbiology 2017, 122, 987–996. [Google Scholar] [CrossRef]
- Karnwal, A.; Dohroo, A. Effect of Maize Root Exudates on Indole-3-Acetic Acid Production by Rice Endophytic Bacteria under Influence of L-Tryptophan. F1000Research 2018, 7. [Google Scholar] [CrossRef]
- Do, T.C.V.; Tran, D.T.; Le, T.G.; Nguyen, Q.T. Characterization of Endogenous Auxins and Gibberellins Produced by Chlorella Sorokiniana TH01 under Phototrophic and Mixtrophic Cultivation Modes toward Applications in Microalgal Biorefinery and Crop Research. Journal of Chemistry 2020, 2020, e4910621. [Google Scholar] [CrossRef]
- Ma, D.; Liu, B.; Ge, L.; Weng, Y.; Cao, X.; Liu, F.; Mao, P.; Ma, X. Identification and Characterization of Regulatory Pathways Involved in Early Flowering in the New Leaves of Alfalfa (Medicago Sativa L.) by Transcriptome Analysis. BMC Plant Biology 2021, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Rusmin, D.; Basmal, J.; Kusumawati, R.; Darwati, I. Improving the Growth of Clove Seedlings by the Application of Seaweed Waste as Organic Fertilizers. IOP Conf. Ser.: Earth Environ. Sci. 2020, 418, 012029. [Google Scholar] [CrossRef]
- Jain, P.; Farooq, B.; Lamba, S.; Koul, B. Foliar Spray of Moringa Oleifera Lam. Leaf Extracts (MLE) Enhances the Stevioside, Zeatin and Mineral Contents in Stevia Rebaudiana Betoni. South African Journal of Botany 2020, 132, 249–257. [Google Scholar] [CrossRef]
- Basra, S.M.A.; Lovatt, C.J. Exogenous Applications of Moringa Leaf Extract and Cytokinins Improve Plant Growth, Yield, and Fruit Quality of Cherry Tomato. HortTechnology 2016, 26, 327–337. [Google Scholar] [CrossRef]
- Ravindran, B.; Wong, J.W.C.; Selvam, A.; Sekaran, G. Influence of Microbial Diversity and Plant Growth Hormones in Compost and Vermicompost from Fermented Tannery Waste. Bioresource Technology 2016, 217, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, B.; Contreras-Ramos, S.M.; Sekaran, G. Changes in Earthworm Gut Associated Enzymes and Microbial Diversity on the Treatment of Fermented Tannery Waste Using Epigeic Earthworm Eudrilus Eugeniae. Ecological Engineering 2015, 74, 394–401. [Google Scholar] [CrossRef]
- Ali, H.M.; Khan, H.Z.; Afzal, I. EXOGENOUS APPLICATION OF GROWTH PROMOTING SUBSTANCES IMPROVES GROWTH, YIELD AND QUALITY OF SPRING MAIZE (ZEA MAYS L.) HYBRIDS UNDER LATE SOWN CONDITIONS. Bulletin of Biological and Allied Sciences Research 2017, 2017, 9–9. [Google Scholar] [CrossRef]
- 戚海乐 Method for Producing Abscisic Acid by Solid State Fermentation of Fungi 2013.
- Vandenberghe, L.P.S.; Pandey, A.; Carvalho, J.C.; Letti, L.A.J.; Woiciechowski, A.L.; Karp, S.G.; Thomaz-Soccol, V.; Martínez-Burgos, W.J.; Penha, R.O.; Herrmann, L.W.; et al. Solid-State Fermentation Technology and Innovation for the Production of Agricultural and Animal Feed Bioproducts. Syst Microbiol and Biomanuf 2021, 1, 142–165. [Google Scholar] [CrossRef]
- Rodrigues, C.; Vandenberghe, L.P.D.S.; De Oliveira, J.; Soccol, C.R. New Perspectives of Gibberellic Acid Production: A Review. Critical Reviews in Biotechnology 2012, 32, 263–273. [Google Scholar] [CrossRef]
- Yang, S.; Xie, J.; Hu, N.; Liu, Y.; Zhang, J.; Ye, X.; Liu, Z. Bioconversion of Gibberellin Fermentation Residue into Feed Supplement and Organic Fertilizer Employing Housefly (Musca domestica L.) Assisted by Corynebacterium variabile. PLOS ONE 2015, 10, e0110809. [Google Scholar] [CrossRef] [PubMed]
- Camara, M.C.; Vandenberghe, L.P.S.; Rodrigues, C.; de Oliveira, J.; Faulds, C.; Bertrand, E.; Soccol, C.R. Current Advances in Gibberellic Acid (GA3) Production, Patented Technologies and Potential Applications. Planta 2018, 248, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Brückner, B.; Blechschmidt, D. The Gibberellin Fermentation. Critical Reviews in Biotechnology 1991, 11, 163–192. [Google Scholar] [CrossRef]
- Maria, C.; Machado, M.; Soccol, C.R. Gibberellic Acid Production. In Current Developments in Solid-state Fermentation; Pandey, A., Soccol, C.R., Larroche, C., Eds.; Springer: New York, NY, 2008; pp. 277–301. ISBN 978-0-387-75213-6. [Google Scholar]
- Saeed, S.; Mehmood, T.; Irfan, M. Statistical Optimization of Cultural Parameters for the Optimized Production of Alginic Acid Using Apple (Malus Domestica) Peels through Solid-State Fermentation. Biomass Conv. Bioref. 2023, 13, 1269–1277. [Google Scholar] [CrossRef]
- dos Santos Silva, M.C.; De Farias Silva, C.E.; dos Santos, L.M.; Medeiros, J.A.; Vieira, R.C.; de Souza Abud, A.K.; Almeida, R.M.R.G.; Tonholo, J. Alginate Lyase Produced by Filamentous Fungus Through Solid State Fermentation Using Sargassum from the Brazilian Coast. Waste Biomass Valor 2022, 13, 2947–2962. [Google Scholar] [CrossRef]
- Biostimulant Mode of Action.
- Rodríguez-Jasso, R.M.; Mussatto, S.I.; Sepúlveda, L.; Agrasar, A.T.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Fungal Fucoidanase Production by Solid-State Fermentation in a Rotating Drum Bioreactor Using Algal Biomass as Substrate. Food and Bioproducts Processing 2013, 91, 587–594. [Google Scholar] [CrossRef]
- Marine Biotechnology : An Overview of Leading Field.
- Patel, J.S.; Selvaraj, V.; More, P.; Bahmani, R.; Borza, T.; Prithiviraj, B. A Plant Biostimulant from Ascophyllum Nodosum Potentiates Plant Growth Promotion and Stress Protection Activity of Pseudomonas Protegens CHA0. Plants 2023, 12, 1208. [Google Scholar] [CrossRef]
- Zou, P.; Yang, X.; Yuan, Y.; Jing, C.; Cao, J.; Wang, Y.; Zhang, L.; Zhang, C.; Li, Y. Purification and Characterization of a Fucoidan from the Brown Algae Macrocystis Pyrifera and the Activity of Enhancing Salt-Stress Tolerance of Wheat Seedlings. International Journal of Biological Macromolecules 2021, 180, 547–558. [Google Scholar] [CrossRef]
- Solid-State Fermentation for Humic Acids Production by a Trichoderma Reesei Strain Using an Oil Palm Empty Fruit Bunch as the Substrate | SpringerLink. Available online: https://link.springer.com/article/10.1007/s12010-013-0668-2 (accessed on 27 May 2023).
- Zhang, Y.; Dou, S.; Hamza, B.; Ye, S.; Zhang, D. Mechanisms of Three Fungal Types on Humic-Like Substances Formation during Solid-State Fermentation of Corn Straw. International Journal of Agriculture and Biology 2020, 23, 970–976. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Zhang, Y.; Li, L.; Shi, X.; Liu, X.; Ren, X.; Dou, S. Transformation of Corn Stalk Residue to Humus-Like Substances during Solid-State Fermentation. Sustainability 2019, 11, 6771. [Google Scholar] [CrossRef]
- Bettoni, M.M.; Mogor, Á.F.; Pauletti, V.; Goicoechea, N. Growth and Metabolism of Onion Seedlings as Affected by the Application of Humic Substances, Mycorrhizal Inoculation and Elevated CO2. Scientia Horticulturae 2014, 180, 227–235. [Google Scholar] [CrossRef]
- Hölker, U.; Höfer, M. Solid Substrate Fermentation of Lignite by the Coal-Solubilizing Mould, Trichoderma Atroviride, in a New Type of Bioreactor. Biotechnology Letters 2002, 24, 1643–1645. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and Fulvic Acids as Biostimulants in Horticulture. Scientia Horticulturae 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Deswal, D.; Khasa, Y.P.; Kuhad, R.C. Optimization of Cellulase Production by a Brown Rot Fungus Fomitopsis Sp. RCK2010 under Solid State Fermentation. Bioresource Technology 2011, 102, 6065–6072. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, H.K.; Sharma, K.; Gupta, J.K.; Soni, S.K. Production of a Thermostable α-Amylase from Bacillus Sp. PS-7 by Solid State Fermentation and Its Synergistic Use in the Hydrolysis of Malt Starch for Alcohol Production. Process Biochemistry 2005, 40, 525–534. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The Effect of a Plant-Derived Biostimulant on Metabolic Profiling and Crop Performance of Lettuce Grown under Saline Conditions. Scientia Horticulturae 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Mohamad Asri, N.; Muhialdin, B.J.; Zarei, M.; Saari, N. Low Molecular Weight Peptides Generated from Palm Kernel Cake via Solid State Lacto-Fermentation Extend the Shelf Life of Bread. LWT 2020, 134, 110206. [Google Scholar] [CrossRef]
- Li, W.; Wang, T. Effect of Solid-State Fermentation with Bacillus Subtilis Lwo on the Proteolysis and the Antioxidative Properties of Chickpeas. International Journal of Food Microbiology 2021, 338, 108988. [Google Scholar] [CrossRef]
- Cai, C.; Hua, Y.; Liu, H.; Dai, X. A New Approach to Recycling Cephalosporin Fermentation Residue into Plant Biostimulants. Journal of Hazardous Materials 2021, 413, 125393. [Google Scholar] [CrossRef]
- Prabhu, G.N.; Bindu, P. Optimization of Process Parameters for Siderophore Production Under Solid State Fermentation Using Polystyrene Beads as Inert Support. Journal of Scientific & Industrial Research 2016, 75, 621–625. [Google Scholar]
- Le, H.; ZongHao, Y.; Can, C.; ChunYan, L.; Juan, L.; ZhongKe, S. Enhancing Iron Uptake and Alleviating Iron Toxicity in Wheat by Plant Growth-Promoting Bacteria: Theories and Practices. International Journal of Agriculture and Biology 2020, 23, 190–196. [Google Scholar]
- Marschner, H.; Römheld, V.; Kissel, M. Different Strategies in Higher Plants in Mobilization and Uptake of Iron. Journal of Plant Nutrition 1986, 9, 695–713. [Google Scholar] [CrossRef]
- Stanley-Raja, V.; Senthil-Nathan, S.; Chanthini, K.M.P.; Sivanesh, H.; Ramasubramanian, R.; Karthi, S.; Shyam-Sundar, N.; Vasantha-Srinivasan, P.; Kalaivani, K. Biological Activity of Chitosan Inducing Resistance Efficiency of Rice (Oryza Sativa L.) after Treatment with Fungal Based Chitosan. Scientific Reports 2021 11:1 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nwe, N.; Chandrkrachang, S.; Stevens, W.F.; Maw, T.; Tan, T.K.; Khor, E.; Wong, S.M. Production of Fungal Chitosan by Solid State and Submerged Fermentation. Carbohydrate Polymers 2002, 49, 235–237. [Google Scholar] [CrossRef]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent Advances in the Molecular Effects of Biostimulants in Plants: An Overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef]
- Mattedi, A.; Sabbi, E.; Farda, B.; Djebaili, R.; Mitra, D.; Ercole, C.; Cacchio, P.; Del Gallo, M.; Pellegrini, M. Solid-State Fermentation: Applications and Future Perspectives for Biostimulant and Biopesticides Production. Microorganisms 2023, 11, 1408. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in Horticulture. Scientia Horticulturae 2015, 196, 1–2. [Google Scholar] [CrossRef]
- Tarafdar, J.C. Chapter 15 - Biostimulants for Sustainable Crop Production. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, H.B., Vaishnav, A., Eds.; Elsevier, 2022; pp. 299–313. ISBN 978-0-323-85579-2. [Google Scholar]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Frontiers in Plant Science 2020, 11. [Google Scholar] [CrossRef]
- Lau, S.-E.; Teo, W.F.A.; Teoh, E.Y.; Tan, B.C. Microbiome Engineering and Plant Biostimulants for Sustainable Crop Improvement and Mitigation of Biotic and Abiotic Stresses. Discov Food 2022, 2, 9. [Google Scholar] [CrossRef]
- Ben Mrid, R.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary Metabolites as Biostimulant and Bioprotectant Agents: A Review. Science of The Total Environment 2021, 777, 146204. [Google Scholar] [CrossRef]
- Caccavo, V.; Forlano, P.; Mang, S.M.; Fanti, P.; Nuzzaci, M.; Battaglia, D.; Trotta, V. Effects of Trichoderma Harzianum Strain T22 on the Arthropod Community Associated with Tomato Plants and on the Crop Performance in an Experimental Field. Insects 2022, 13, 418. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Rasera, R.; Causin, R. Trichoderma Harzianum Seed Treatment Controls Fusarium Verticillioides Colonization and Fumonisin Contamination in Maize under Field Conditions. Crop Protection 2014, 65, 51–56. [Google Scholar] [CrossRef]
- Xu, L.; Geelen, D. Developing Biostimulants From Agro-Food and Industrial By-Products. Frontiers in Plant Science 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Francesca, S.; Arena, C.; Hay Mele, B.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The Use of a Plant-Based Biostimulant Improves Plant Performances and Fruit Quality in Tomato Plants Grown at Elevated Temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef]
- Nanda, S.; Kumar, G.; Hussain, S. Utilization of Seaweed-Based Biostimulants in Improving Plant and Soil Health: Current Updates and Future Prospective. Int. J. Environ. Sci. Technol. 2022, 19, 12839–12852. [Google Scholar] [CrossRef]
- da Silva, L.C.A.; Honorato, T.L.; Cavalcante, R.S.; Franco, T.T.; Rodrigues, S. Effect of PH and Temperature on Enzyme Activity of Chitosanase Produced Under Solid Stated Fermentation by Trichoderma Spp. Indian J Microbiol 2012, 52, 60–65. [Google Scholar] [CrossRef]
- Akram, N.A.; Saleem, M.H.; Shafiq, S.; Naz, H.; Farid-ul-Haq, M.; Ali, B.; Shafiq, F.; Iqbal, M.; Jaremko, M.; Qureshi, K.A. Phytoextracts as Crop Biostimulants and Natural Protective Agents—A Critical Review. Sustainability 2022, 14, 14498. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Lopez, A.; Martos, V.; Reyes, A.; Maksimovic, I.; Eichler-Löbermann, B.; Malusà, E. Unexploited Potential of Some Biotechnological Techniques for Biofertilizer Production and Formulation. Appl Microbiol Biotechnol 2015, 99, 4983–4996. [Google Scholar] [CrossRef]
- Chen, H. Modern Solid State Fermentation. Modern Solid State Fermentation 2013. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent Advances in Solid-State Fermentation. Biochemical Engineering Journal 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Szabo, O.E.; Csiszar, E.; Koczka, B.; Kiss, K. Ultrasonically Assisted Single Stage and Multiple Extraction of Enzymes Produced by Aspergillus Oryzae on a Lignocellulosic Substrate with Solid-State Fermentation. Biomass and Bioenergy 2015, 75, 161–169. [Google Scholar] [CrossRef]
- Yadav, J.S.; Tripathi, J.P. Optimization of Cultivation and Nutrition Conditions and Substrate Pretreatment for Solid-Substrate Fermentation of Wheat Straw ByCoriolus Versicolor. Folia Microbiol 1991, 36, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Kunamneni, A.; Permaul, K.; Singh, S. Amylase Production in Solid State Fermentation by the Thermophilic Fungus Thermomyces Lanuginosus. Journal of Bioscience and Bioengineering 2005, 100, 168–171. [Google Scholar] [CrossRef]
- Elibol, M.; Moreira, A.R. Optimizing Some Factors Affecting Alkaline Protease Production by a Marine Bacterium Teredinobacter Turnirae under Solid Substrate Fermentation. Process Biochemistry 2005, 40, 1951–1956. [Google Scholar] [CrossRef]
- do Prado, D.Z.; Oliveira, S.L.; Okino-Delgado, C.H.; Auer, S.; Ludwig-Mueller, J.; da Silva, M.R.; da Costa Fernandes, C.J.; Carbonari, C.A.; Zambuzzi, W.F.; Fleuri, L.F. Aspergillus Flavipes as a Novel Biostimulant for Rooting-Enhancement of Eucalyptus. JOURNAL OF CLEANER PRODUCTION 2019, 234, 681–689. [Google Scholar] [CrossRef]
- Alias, C.; Bulgari, D.; Gobbi, E. It Works! Organic-Waste-Assisted Trichoderma Spp. Solid-State Fermentation on Agricultural Digestate. Microorganisms 2022, 10, 164. [Google Scholar] [CrossRef]
- Werle, L.B. Obtenção de ácido giberélico por fermentação em estado sólido empregando resíduo de cervejaria e farelo de arroz bruto como substratos. Obtention of gibberellic acid by solid state fermentation employing brewery residue and crude rice brand as substrates 2017. [Google Scholar]
- Monrroy, M.; García, J.R. Gibberellic Acid Production from Corn Cob Residues via Fermentation with Aspergillus Niger. Journal of Chemistry 2022, 2022, e1112941. [Google Scholar] [CrossRef]
- de Oliveira, J.; Rodrigues, C.; Vandenberghe, L.P.S.; Câmara, M.C.; Libardi, N.; Soccol, C.R. Gibberellic Acid Production by Different Fermentation Systems Using Citric Pulp as Substrate/Support. BioMed Research International 2017, 2017, e5191046. [Google Scholar] [CrossRef]
- Jain, B.M.; Badve, M.P. A Novel Process for Synthesis of Soybean Protein Hydrolysates and Study of Its Effectiveness as a Biostimulant and Emulsifier. Chemical Engineering and Processing - Process Intensification 2022, 174, 108880. [Google Scholar] [CrossRef]
- Wei, X.; Sui, Z.; Guo, M.; Chen, S.; Zhang, Z.; Geng, J.; Xiao, J.; Huang, D. The Potential of Degrading Natural Chitinous Wastes to Oligosaccharides by Chitinolytic Enzymes from Two Talaromyces Sp. Isolated from Rotten Insects (Hermetia Illucens) under Solid State Fermentation. Braz J Microbiol 2023, 54, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Volpi, M.P.C.; Corzo, I.J.M.; Bastos, R.G.; Santana, M.H.A. Production of Humic Acids by Solid-State Fermentation of Trichoderma Reesei in Raw Oil Palm Empty Fruit Bunch Fibers. 3 Biotech 2019, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Ghanavati, H.; Ramezanipour, N.; Salehi Jouzani, G.; Kowsari, M.; Valijanian, E.; Nikrad, M.; Mostajeran, F.; Tahmasbi, M. Submerged Fermentation as a Suitable Solution to Produce Humic and Fulvic Acids from Sugarcane Bagasse. Scientia Iranica 2022, 29, 3554–3569. [Google Scholar] [CrossRef]
- Amadou, I.; Le, G.-W.; Shi, Y.-H.; Gbadamosi, O.S.; Kamara, M.T.; Jin, S. Optimized Lactobacillus Plantarum Lp6 Solid-State Fermentation and Proteolytic Hydrolysis Improve Some Nutritional Attributes of Soybean Protein Meal. Journal of Food Biochemistry 2011, 35, 1686–1694. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as Biostimulant: Exploiting the Multilevel Properties of a Plant Beneficial Fungus. Scientia Horticulturae 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Dikilitas, M.; Tuna, A.L. Alleviation of Salt Stress-Induced Adverse Effects on Maize Plants by Exogenous Application of Indoleacetic Acid (IAA) and Inorganic Nutrients - A Field Trial. Australian Journal of Crop Science 2013, 7, 249–254. [Google Scholar] [CrossRef]
- Richards, D.E.; King, K.E.; Ait-ali, T.; Harberd, N.P. HOW GIBBERELLIN REGULATES PLANT GROWTH AND DEVELOPMENT: A Molecular Genetic Analysis of Gibberellin Signaling. Annual Review of Plant Physiology and Plant Molecular Biology 2001, 52, 67–88. [Google Scholar] [CrossRef]
- Brian, P.W. Effects of Gibberellins on Plant Growth and Development. Biological Reviews 1959, 34, 37–77. [Google Scholar] [CrossRef]
- Soper, F.M.; Paungfoo-Lonhienne, C.; Brackin, R.; Rentsch, D.; Schmidt, S.; Robinson, N. Arabidopsis and Lobelia Anceps Access Small Peptides as a Nitrogen Source for Growth. Functional Plant Biol. 2011, 38, 788–796. [Google Scholar] [CrossRef]
- Sheng, C.; Song, S.; Zhou, W.; Dossou, S.S.K.; Zhou, R.; Zhang, Y.; Li, D.; You, J.; Wang, L. Integrating Transcriptome and Phytohormones Analysis Provided Insights into Plant Height Development in Sesame. Plant Physiology and Biochemistry 2023, 198, 107695. [Google Scholar] [CrossRef]
- Luo, L.; Xie, Y.; Yu, S.; Yang, J.; Chen, S.; Yuan, X.; Guo, T.; Wang, H.; Liu, Y.; Chen, C.; et al. The DnaJ Domain-Containing Heat-Shock Protein NAL11 Determines Plant Architecture by Mediating Gibberellin Homeostasis in Rice (Oryza Sativa). New Phytologist 2023, 237, 2163–2179. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Montesinos, B.; Diánez, F.; Moreno-Gavíra, A.; Gea, F.J.; Santos, M. Role of Trichoderma Aggressivum f. Europaeum as Plant-Growth Promoter in Horticulture. Agronomy 2020, Vol. 10, Page 1004 2020, 10, 1004. [Google Scholar] [CrossRef]
- Tsavkelova, E.A.; Cherdyntseva, T.A.; Klimova, S.Yu.; Shestakov, A.I.; Botina, S.G.; Netrusov, A.I. Orchid-Associated Bacteria Produce Indole-3-Acetic Acid, Promote Seed Germination, and Increase Their Microbial Yield in Response to Exogenous Auxin. Arch Microbiol 2007, 188, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D. Alleviation of Salt Stress by Plant Growth Regulators and IAA Producing Bacteria in Wheat. Acta Physiol Plant 2009, 31, 861–864. [Google Scholar] [CrossRef]
- Florido Bacallao, M.; Bao Fundora, L. Tolerancia a Estrés Por Déficit Hídrico En Tomate (Solanum Lycopersicum L.). Cultivos Tropicales 2014, 35, 70–88. [Google Scholar]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Luo, P.; Shen, Y.; Jin, S.; Huang, S.; Cheng, X.; Wang, Z.; Li, P.; Zhao, J.; Bao, M.; Ning, G. Overexpression of Rosa Rugosa Anthocyanidin Reductase Enhances Tobacco Tolerance to Abiotic Stress through Increased ROS Scavenging and Modulation of ABA Signaling. Plant Science 2016, 245, 35–49. [Google Scholar] [CrossRef]
- Yazdani, M.; Croen, M.G.; Fish, T.L.; Thannhauser, T.W.; Ahner, B.A. Overexpression of Native ORANGE (OR) and OR Mutant Protein in Chlamydomonas Reinhardtii Enhances Carotenoid and ABA Accumulation and Increases Resistance to Abiotic Stress. Metabolic Engineering 2021, 68, 94–105. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, M.; Hu, J.; Zhang, X.; Wang, K.; Ashraf, M. Modulation Role of Abscisic Acid (ABA) on Growth, Water Relations and Glycinebetaine Metabolism in Two Maize (Zea Mays L.) Cultivars under Drought Stress. International Journal of Molecular Sciences 2012, 13, 3189–3202. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patel, J.S. Seaweed Extract: Biostimulator of Plant Defense and Plant Productivity. Int. J. Environ. Sci. Technol. 2020, 17, 553–558. [Google Scholar] [CrossRef]
- Berthon, J.-Y.; Michel, T.; Wauquier, A.; Joly, P.; Gerbore, J.; Filaire, E. Seaweed and microalgae as major actors of blue biotechnology to achieve plant stimulation and pest and pathogen biocontrol – a review of the latest advances and future prospects. The Journal of Agricultural Science 2021, 159, 523–534. [Google Scholar] [CrossRef]
- Kapur, B.; Sarıdaş, M.A.; Çeliktopuz, E.; Kafkas, E.; Paydaş Kargı, S. Health and Taste Related Compounds in Strawberries under Various Irrigation Regimes and Bio-Stimulant Application. Food Chemistry 2018, 263, 67–73. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Corio-Costet, M.-F.; Stadnik, M.J.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.-T.; Dumas, B. An Ulva Armoricana Extract Protects Plants against Three Powdery Mildew Pathogens. Eur J Plant Pathol 2011, 131, 393–401. [Google Scholar] [CrossRef]
- Khaled, H.; Fawy, H.A. Effect of Different Levels of Humic Acids on the Nutrient Content, Plant Growth, and Soil Properties under Conditions of Salinity. Soil Water Res. 2011, 6, 21–29. [Google Scholar] [CrossRef]
- Fagbenro, J.A.; Agboola, A.A. Effect of Different Levels of Humic Acid on the Growth and Nutrient Uptake of Teak Seedlings. Journal of Plant Nutrition 1993, 16, 1465–1483. [Google Scholar] [CrossRef]
- Khan, R.U.; Khan, M.Z.; Khan, A.; Saba, S.; Hussain, F.; Jan, I.U. Effect of Humic Acid on Growth and Crop Nutrient Status of Wheat on Two Different Soils. Journal of Plant Nutrition 2018, 41, 453–460. [Google Scholar] [CrossRef]
- Bayat, H.; Shafie, F.; Aminifard, M.H.; Daghighi, S. Comparative Effects of Humic and Fulvic Acids as Biostimulants on Growth, Antioxidant Activity and Nutrient Content of Yarrow (Achillea Millefolium L.). Scientia Horticulturae 2021, 279, 109912. [Google Scholar] [CrossRef]
- Maach, M.; Boudouasar, K.; Akodad, M.; Skalli, A.; Moumen, A.; Baghour, M. Application of Biostimulants Improves Yield and Fruit Quality in Tomato. International Journal of Vegetable Science 2021, 27, 288–293. [Google Scholar] [CrossRef]
- Chanthini, K.M.-P.; Senthil-Nathan, S.; Pavithra, G.-S.; Asahel, A.-S.; Malarvizhi, P.; Murugan, P.; Deva--Andrews, A.; Sivanesh, H.; Stanley-Raja, V.; Ramasubramanian, R.; et al. The Macroalgal Biostimulant Improves the Functional Quality of Tomato Fruits Produced from Plants Grown under Salt Stress. Agriculture 2023, 13, 6. [Google Scholar] [CrossRef]
- Graziani, G.; Ritieni, A.; Cirillo, A.; Cice, D.; Di Vaio, C. Effects of Biostimulants on Annurca Fruit Quality and Potential Nutraceutical Compounds at Harvest and during Storage. Plants 2020, 9, 775. [Google Scholar] [CrossRef]
- Zwack, P.J.; Rashotte, A.M. Cytokinin Inhibition of Leaf Senescence. Plant Signaling & Behavior 2013, 8, e24737. [Google Scholar] [CrossRef]
- Chang, H.; Jones, M.L.; Banowetz, G.M.; Clark, D.G. Overproduction of Cytokinins in Petunia Flowers Transformed with PSAG12-IPT Delays Corolla Senescence and Decreases Sensitivity to Ethylene. Plant Physiology 2003, 132, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Balibrea Lara, M.E.; Gonzalez Garcia, M.-C.; Fatima, T.; Ehneß, R.; Lee, T.K.; Proels, R.; Tanner, W.; Roitsch, T. Extracellular Invertase Is an Essential Component of Cytokinin-Mediated Delay of Senescence[W]. The Plant Cell 2004, 16, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, F.; Fiori, G.; Noferini, M.; Sprocatti, M.; Costa, G. A Novel Type of Seaweed Extract as a Natural Alternative to the Use of Iron Chelates in Strawberry Production. Scientia Horticulturae 2010, 125, 263–269. [Google Scholar] [CrossRef]
- Kumari, R.; Kaur, I.; Bhatnagar, A.K. Enhancing Soil Health and Productivity of Lycopersicon Esculentum Mill. Using Sargassum Johnstonii Setchell & Gardner as a Soil Conditioner and Fertilizer. J Appl Phycol 2013, 25, 1225–1235. [Google Scholar] [CrossRef]
- Meng, C.; Gu, X.; Liang, H.; Wu, M.; Wu, Q.; Yang, L.; Li, Y.; Shen, P. Optimized Preparation and High-Efficient Application of Seaweed Fertilizer on Peanut. Journal of Agriculture and Food Research 2022, 7, 100275. [Google Scholar] [CrossRef]
- Matthews, S.; Ali, A.; Siddiqui, Y.; Supramaniam, C.V. Plant Bio-Stimulant: Prospective, Safe and Natural Resources. J Soil Sci Plant Nutr 2022, 22, 2570–2586. [Google Scholar] [CrossRef]
- Zeljković, S.; Parađiković, N.; Maksimović, I.; Teklić, T.; Tkalec Kojić, M. Growth and Nutrient Status of French Marigold (Tagetes Patula L.) under Biostimulant Application. New Zealand Journal of Crop and Horticultural Science 2022, 0, 1–11. [Google Scholar] [CrossRef]
- Basavaraja, P.K.; Yogendra, N.D.; Zodape, S.T.; Prakash, R.; Ghosh, A. Effect of Seaweed Sap as Foliar Spray on Growth and Yield of Hybrid Maize. Journal of Plant Nutrition 2018, 41, 1851–1861. [Google Scholar] [CrossRef]
- Pal, A.; Dwivedi, S.K.; Maurya, P.K.; Kanwar, P. Effect of Seaweed Saps on Growth, Yield, Nutrient Uptake and Economic Improvement of Maize (Sweet Corn). Journal of Applied and Natural Science 2015, 7, 970–975. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of Seaweed Extracts From Laminaria and Ascophyllum Nodosum Spp. as Biostimulants in Zea Mays L. Using a Combination of Chemical, Biochemical and Morphological Approaches. Frontiers in Plant Science 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; McClements, D.J.; Decker, E.A. Antioxidant Activity of a Proanthocyanidin-Rich Extract from Grape Seed in Whey Protein Isolate Stabilized Algae Oil-in-Water Emulsions. J Agric Food Chem 2004, 52, 5272–5276. [Google Scholar] [CrossRef] [PubMed]
- Efecto Del Extracto de Células de Alga Verde Como Aerosol Foliar Sobre El Crecimiento Vegetativo, El Rendimiento y La Calidad de Las Bayas de Vides Superiores. Available online: https://www.researchgate.net/publication/237566381_Effect_of_Green_Alga_Cells_Extract_as_Foliar_Spray_on_Vegetative_Growth_Yield_and_Berries_Quality_of_Superior_Grapevines (accessed on 3 June 2023).
- Arioli, T.; Mattner, S.W.; Hepworth, G.; McClintock, D.; McClinock, R. Effect of Seaweed Extract Application on Wine Grape Yield in Australia. J Appl Phycol 2021, 33, 1883–1891. [Google Scholar] [CrossRef]
- Jayaraj Jayaraman, J.J.; Ali, N. Use of Seaweed Extracts for Disease Management of Vegetable Crops. Sustainable crop disease management using natural products 2015, 160–183. [Google Scholar] [CrossRef]
- Murtic, S.; Oljaca, R.; Smajic Murtic, M.; Vranac, A.; Akagic, A.; Civic, H. Cherry Tomato Productivity as Influenced by Liquid Organic Fertilizer under Different Growth Conditions. Journal of Central European Agriculture 2018, 19, 503–516. [Google Scholar] [CrossRef]
- Demir, N.; Dural, B.; Yildirim, K. Effect of Seaweed Suspensions on Seed Germination of Tomato, Pepper and Aubergine; Asian Network for Scientific Information, Pakistan, 2006.
- Yusuf, R.; Kristiansen, P.; Warwick, N. Effect of Two Seaweed Products and Equivalent Mineral Treatments on Lettuce (Lactuca Sativa L.) Growth. Journal of Agronomy 2019, 18, 100–106. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; Colla, G.; Mori, M. Effect of Vegetal- and Seaweed Extract-Based Biostimulants on Agronomical and Leaf Quality Traits of Plastic Tunnel-Grown Baby Lettuce under Four Regimes of Nitrogen Fertilization. Agronomy 2019, 9, 571. [Google Scholar] [CrossRef]
- Nardelli, A.E.; Chiozzini, V.G.; Braga, E.S.; Chow, F. Integrated Multi-Trophic Farming System between the Green Seaweed Ulva Lactuca, Mussel, and Fish: A Production and Bioremediation Solution. J Appl Phycol 2019, 31, 847–856. [Google Scholar] [CrossRef]
- Righini, H.; Roberti, R.; Baraldi, E. Use of Algae in Strawberry Management. J Appl Phycol 2018, 30, 3551–3564. [Google Scholar] [CrossRef]
- Abbas, M.; Anwar, J.; Zafar-ul-Hye, M.; Iqbal Khan, R.; Saleem, M.; Rahi, A.A.; Danish, S.; Datta, R. Effect of Seaweed Extract on Productivity and Quality Attributes of Four Onion Cultivars. Horticulturae 2020, 6, 28. [Google Scholar] [CrossRef]
- Almaroai, Y.A.; Eissa, M.A. Role of Marine Algae Extracts in Water Stress Resistance of Onion Under Semiarid Conditions. J Soil Sci Plant Nutr 2020, 20, 1092–1101. [Google Scholar] [CrossRef]
- Prajapati, A.; Jain, S.; Chongtham, S.; Maheshwari, M.; Patel, C.; Patel, R.; Patel, C.; Singh, N.; Prajapati, A. Evaluation of Seaweed Extract on Growth and Yield of Potato. 2015.
- Dziugieł, T.; Wadas, W. Possibility of Increasing Early Crop Potato Yield with Foliar Application of Seaweed Extracts and Humic Acids. Journal of Central European Agriculture 2020, 21, 300–310. [Google Scholar] [CrossRef]
- Manpuhro, N.; Dawson, J. Influence of Indole Acetic Acid (IAA) and Boron on Growth and Yield of Maize (Zea Mays. L). International Journal of Plant & Soil Science 2023, 35, 33–41. [Google Scholar] [CrossRef]
- Hagaggi, N.Sh.A.; Mohamed, A.A.A. Enhancement of Zea Mays (L.) Growth Performance Using Indole Acetic Acid Producing Endophyte Mixta Theicola Isolated from Solenostemma Argel (Hayne). South African Journal of Botany 2020, 134, 64–71. [Google Scholar] [CrossRef]
- Marag, P.S.; Suman, A. Growth Stage and Tissue Specific Colonization of Endophytic Bacteria Having Plant Growth Promoting Traits in Hybrid and Composite Maize (Zea Mays L.). Microbiological Research 2018, 214, 101–113. [Google Scholar] [CrossRef]
- Visconti, D.; Fiorentino, N.; Cozzolino, E.; Woo, S.L.; Fagnano, M.; Rouphael, Y. Can Trichoderma-Based Biostimulants Optimize N Use Efficiency and Stimulate Growth of Leafy Vegetables in Greenhouse Intensive Cropping Systems? Agronomy 2020, 10, 121. [Google Scholar] [CrossRef]
- Kondhare, K.R.; Patil, A.B.; Giri, A.P. Auxin: An Emerging Regulator of Tuber and Storage Root Development. Plant Science 2021, 306, 110854. [Google Scholar] [CrossRef]
- Romanov, G.A.; Aksenova, N.P.; Konstantinova, T.N.; Golyanovskaya, S.A.; Kossmann, J.; Willmitzer, L. Effect of Indole-3-Acetic Acid and Kinetin on Tuberisation Parameters of Different Cultivars and Transgenic Lines of Potato in Vitro. Plant Growth Regulation 2000, 32, 245–251. [Google Scholar] [CrossRef]
- Ekin, Z. Integrated Use of Humic Acid and Plant Growth Promoting Rhizobacteria to Ensure Higher Potato Productivity in Sustainable Agriculture. Sustainability 2019, 11, 3417. [Google Scholar] [CrossRef]
- Hye, M.; Haque, M.; Karim, M. Influence of Growth Regulators and Their Time of Application on Yield of Onion. Pakistan Journal of Biological Sciences 2002, 5. [Google Scholar] [CrossRef]
- Bista, D.; Sapkota, D.; Paudel, H.; Adhikari, G. Effect of Foliar Application of Growth Regulators on Growth and Yield of Onion (Allium Cepa). International Journal of Horticultural Science and Technology 2022, 9, 247–254. [Google Scholar] [CrossRef]
- Gupta, S.; Stirk, W.A.; Plačková, L.; Kulkarni, M.G.; Doležal, K.; Van Staden, J. Interactive Effects of Plant Growth-Promoting Rhizobacteria and a Seaweed Extract on the Growth and Physiology of Allium Cepa L. (Onion). Journal of Plant Physiology 2021, 262, 153437. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant Growth Enhancement Using Rhizospheric Halotolerant Phosphate Solubilizing Bacterium Bacillus Licheniformis QA1 and Enterobacter Asburiae QF11 Isolated from Chenopodium Quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef]
- Azarakhsh, M.R.; Bagherieh-Najjar, M.B.; Sadeghipour, H.R.; Raeisi, S. Improved Grain Yield by Phytohormones-Driven Suppression of Pod Abscission and Revitalization of Source-Sink Relationships in Soybean. Int. J. Plant Prod. 2022, 16, 467–481. [Google Scholar] [CrossRef]
- Hanaa, H.; Safaa, A. Foliar Application Foliar Application of IAA at Different Growth Stages and Their Influenced on Growth and Productivity of Bread Wheat (Triticum Aestivum l.). J. Phys.: Conf. Ser. 2019, 1294, 092029. [Google Scholar] [CrossRef]
- Çakmakçı, R.; Erat, M.; Erdoğan, Ü.; Dönmez, M.F. The Influence of Plant Growth–Promoting Rhizobacteria on Growth and Enzyme Activities in Wheat and Spinach Plants. Journal of Plant Nutrition and Soil Science 2007, 170, 288–295. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-Inoculation of Glomus Intraradices and Trichoderma Atroviride Acts as a Biostimulant to Promote Growth, Yield and Nutrient Uptake of Vegetable Crops. Journal of the Science of Food and Agriculture 2015, 95, 1706–1715. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma Virens, a Plant Beneficial Fungus, Enhances Biomass Production and Promotes Lateral Root Growth through an Auxin-Dependent Mechanism in Arabidopsis. Plant Physiology 2009, 149, 1579–1592. [Google Scholar] [CrossRef]
- Tandon, S.; Dubey, A. Effects of Biozyme (Ascophyllum Nodosum) Biostimulant on Growth and Development of Soybean [Glycine Max (L.) Merill]. Communications in Soil Science and Plant Analysis 2015, 46, 845–858. [Google Scholar] [CrossRef]
- Marathe, R.; Phatake, Y.; Shaikh, A.; Shinde, B.; Gajbhiye, M. Effect of IAA Produced by Pseudomonas Aeruginosa 6a (Bc4) on Seed Germination and Plant Growth of Glycin Max. Journal of Experimental Biology and Agricultural Sciences 2017, 5, 351–358. [Google Scholar] [CrossRef]
- Susilowati, D.N.; Riyanti, E.I.; Setyowati, M.; Mulya, K. Indole-3-Acetic Acid Producing Bacteria and Its Application on the Growth of Rice. AIP Conference Proceedings 2018, 2002, 020016. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Zhao, Y.; Li, Y.; Zhang, G.; Peng, Z.; Zhang, J. Enhancing Auxin Accumulation in Maize Root Tips Improves Root Growth and Dwarfs Plant Height. Plant Biotechnology Journal 2018, 16, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Husen, A.; Iqbal, M.; Aref, I.M. Plant Growth and Foliar Characteristics of Faba Bean (Vicia Faba L.) as Affected by Indole-Acetic Acid under Water-Sufficient and Water-Deficient Conditions. Journal of Environmental Biology 2017, 38, 179–186. [Google Scholar] [CrossRef]
- Hamidon, A.; Shah, R.M.; Razali, R.M.; Lob, S. EFFECT OF DIFFERENT TYPES AND CONCENTRATION OF ROOTING HORMONES ON Momordica Cochinensis (GAC FRUIT) ROOT VINE CUTTINGS. Malaysian Applied Biology 2020, 49, 127–132. [Google Scholar] [CrossRef]
- Sabir, A. Improvement of Grafting Efficiency in Hard Grafting Grape Berlandieri Hybrid Rootstocks by Plant Growth-Promoting Rhizobacteria (PGPR). Scientia Horticulturae 2013, 164, 24–29. [Google Scholar] [CrossRef]
- Shahzad, K.; Siddiqi, E.H.; Ahmad, S.; Zeb, U.; Muhammad, I.; Khan, H.; Zhao, G.-F.; Li, Z.-H. Exogenous Application of Indole-3-Acetic Acid to Ameliorate Salt Induced Harmful Effects on Four Eggplants (Solanum Melongena L.) Varieties. Scientia Horticulturae 2022, 292, 110662. [Google Scholar] [CrossRef]
- Lur, H.-S.; Setter, T.L. Endorsperm Development of Maize Defective Kernel ( Dek ) Mutants. Auxin and Cytokinin Levels. Annals of Botany 1993, 72, 1–6. [Google Scholar] [CrossRef]
- Rady, M.M.; Talaat, N.B.; Abdelhamid, M.T.; Shawky, B.T.; Desoky, E.-S.M. Maize (Zea Mays L.) Grains Extract Mitigates the Deleterious Effects of Salt Stress on Common Bean (Phaseolus Vulgaris L.) Growth and Physiology. The Journal of Horticultural Science and Biotechnology 2019, 94, 777–789. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, D.; Zhang, G.; Gao, S.; Liu, L.; Xu, F.; Che, R.; Wang, Y.; Tong, H.; Chu, C. Big Grain3, Encoding a Purine Permease, Regulates Grain Size via Modulating Cytokinin Transport in Rice. Journal of Integrative Plant Biology 2019, 61, 581–597. [Google Scholar] [CrossRef]
- Yin, W.; Xiao, Y.; Niu, M.; Meng, W.; Li, L.; Zhang, X.; Liu, D.; Zhang, G.; Qian, Y.; Sun, Z.; et al. ARGONAUTE2 Enhances Grain Length and Salt Tolerance by Activating BIG GRAIN3 to Modulate Cytokinin Distribution in Rice[OPEN]. The Plant Cell 2020, 32, 2292–2306. [Google Scholar] [CrossRef]
- Yang, D.; Li, Y.; Shi, Y.; Cui, Z.; Luo, Y.; Zheng, M.; Chen, J.; Li, Y.; Yin, Y.; Wang, Z. Exogenous Cytokinins Increase Grain Yield of Winter Wheat Cultivars by Improving Stay-Green Characteristics under Heat Stress. PLOS ONE 2016, 11, e0155437. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, M.S.; Raza, M.A.S.; Saleem, M.F.; Erinle, K.O.; Iqbal, R.; Ahmad, S. Effect of Rhizobacteria and Cytokinins Application on Wheat Growth and Yield under Normal vs Drought Conditions. Communications in Soil Science and Plant Analysis 2019, 50, 2521–2533. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, Y.; Liu, W. High Nitrogen Application Rate and Planting Density Reduce Wheat Grain Yield by Reducing Filling Rate of Inferior Grain in Middle Spikelets. The Crop Journal 2021, 9, 412–426. [Google Scholar] [CrossRef]
- Nagel, L.; Brewster, R.; Riedell, W.E.; Reese, R.N. Cytokinin Regulation of Flower and Pod Set in Soybeans (Glycine Max(L.) Merr.). Annals of Botany 2001, 88, 27–31. [Google Scholar] [CrossRef]
- Kron, A.P.; Souza, G.M.; Ribeiro, R.V. Water Deficiency at Different Developmental Stages of Glycine Max Can Improve Drought Tolerance. Bragantia 2008, 67, 43–49. [Google Scholar] [CrossRef]
- Mady, M.A. EFFECT OF FOLIAR APPLICATION WITH SALICYLIC ACID AND VITAMIN E ON GROWTH AND PRODUCTIVITY OF TOMATO (Lycopersicon Esculentum, Mill.) PLANT. Journal of Plant Production 2009, 34, 6715–6726. [Google Scholar] [CrossRef]
- Caldiz, D.O. Seed Potato (Solanum Tuberosum L.) Yield and Tuber Number Increase after Foliar Applications of Cytokinins and Gibberellic Acid under Field and Glasshouse Conditions. Plant Growth Regul 1996, 20, 185–188. [Google Scholar] [CrossRef]
- Pavlista, A.D. Growth Regulators Increased Yield of Atlantic Potato. Am. J. Pot Res 2011, 88, 479–484. [Google Scholar] [CrossRef]
- Carvajal-Millán, E.; Carvallo, T.; Orozco, J.A.; Martínez, M.A.; Tapia, I.; Guerrero, V.M.; Rascón-Chu, A.; Llamas, J.; Gardea, A.A. Polyphenol Oxidase Activity, Color Changes, and Dehydration in Table Grape Rachis during Development and Storage As Affected by N-(2-Chloro-4-Pyridyl)-N-Phenylurea. J. Agric. Food Chem. 2001, 49, 946–951. [Google Scholar] [CrossRef]
- Peppi, M.C.; Fidelibus, M.W. Effects of Forchlorfenuron and Abscisic Acid on the Quality of ‘Flame Seedless’ Grapes. HortScience 2008, 43, 173–176. [Google Scholar] [CrossRef]
- Qiu, Y.; Guan, S.C.; Wen, C.; Li, P.; Gao, Z.; Chen, X. Auxin and Cytokinin Coordinate the Dormancy and Outgrowth of Axillary Bud in Strawberry Runner. BMC Plant Biol 2019, 19, 528. [Google Scholar] [CrossRef]
- Dale, A.; Elfving, D.C.; Chandler, C.K. Benzyladenine and Gibberellic Acid Increase Runner Production in Dayneutral Strawberries. HortScience 1996, 31, 1190–1194. [Google Scholar] [CrossRef]
- Costa, G.; Corelli-Grappadelli, L.; Bucchi, F. STUDIES ON APPLE FRUIT ABSCISSION AND GROWTH AS AFFECTED BY CYTOKININS. Acta Hortic. 2001, 243–252. [Google Scholar] [CrossRef]
- Kumari, S.; Bakshi, P.; Sharma, A.; Wali, V.; Jasrotia, A.; Kour, S. Use of Plant Growth Regulators for Improving Fruit Production in Sub Tropical Crops. International Journal of Current Microbiology and Applied Sciences 2018, 7, 659–668. [Google Scholar] [CrossRef]
- Ferrer, C.; Martiz, J.; Saa, S.; Cautín, R. Increase in Final Fruit Size of Tangor (Citrus Reticulata×C. Sinensis) Cv W. Murcott by Application of Benzyladenine to Flowers. Scientia Horticulturae 2017, 223, 38–43. [Google Scholar] [CrossRef]
- Yousif, K.H. Application Method of Potassium Humate on Growth And Yield of Green Onion (Allium Cepa L.). Science Journal of University of Zakho 2014, 2, 323–328. [Google Scholar] [CrossRef]
- Amiri Forotaghe, Z.; Souri, M.K.; Ghanbari Jahromi, M.; Mohammadi Torkashvand, A. Influence of Humic Acid Application on Onion Growth Characteristics under Water Deficit Conditions. Journal of Plant Nutrition 2022, 45, 1030–1040. [Google Scholar] [CrossRef]
- Kaya, C.; Akram, N.A.; Ashraf, M.; Sonmez, O. Exogenous Application of Humic Acid Mitigates Salinity Stress in Maize (Zea Mays L.) Plants by Improving Some Key Physico-Biochemical Attributes. Cereal Research Communications 2018, 46, 67–78. [Google Scholar] [CrossRef]
- Izhar Shafi, M.; Adnan, M.; Fahad, S.; Wahid, F.; Khan, A.; Yue, Z.; Danish, S.; Zafar-ul-Hye, M.; Brtnicky, M.; Datta, R. Application of Single Superphosphate with Humic Acid Improves the Growth, Yield and Phosphorus Uptake of Wheat (Triticum Aestivum L.) in Calcareous Soil. Agronomy 2020, 10, 1224. [Google Scholar] [CrossRef]
- Lamlom, S.F.; Irshad, A.; Mosa, W.F.A. The Biological and Biochemical Composition of Wheat (Triticum Aestivum) as Affected by the Bio and Organic Fertilizers. BMC Plant Biol 2023, 23, 111. [Google Scholar] [CrossRef]
- Mindari, W.; Sasongko, P.E.; Kusuma, Z.; Syekhfani; Aini, N. Efficiency of Various Sources and Doses of Humic Acid on Physical and Chemical Properties of Saline Soil and Growth and Yield of Rice. AIP Conference Proceedings 2018, 2019, 030001. [Google Scholar] [CrossRef]
- Khedr, R.A.; Sorour, S.Gh.R.; Aboukhadrah, S.H.; El Shafey, N.M.; Abd Elsalam, H.E.; El-Sharnouby, M.E.; El-Tahan, A.M. Alleviation of Salinity Stress Effects on Agro-Physiological Traits of Wheat by Auxin, Glycine Betaine, and Soil Additives. Saudi Journal of Biological Sciences 2022, 29, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.Y.; Yoo, K.S.; Suh, S.G. Effect of Foliar Application of Fulvic Acid on Plant Growth and Fruit Quality of Tomato (Lycopersicon Esculentum L.). Hortic. Environ. Biotechnol. 2014, 55, 455–461. [Google Scholar] [CrossRef]
- Yildirim, E. Foliar and Soil Fertilization of Humic Acid Affect Productivity and Quality of Tomato. Acta Agriculturae Scandinavica, Section B — Soil & Plant Science 2007, 57, 182–186. [Google Scholar] [CrossRef]
- Hemida, K.A.; Eloufey, A.Z.A.; Seif El-Yazal, M.A.; Rady, M.M. Integrated Effect of Potassium Humate and α-Tocopherol Applications on Soil Characteristics and Performance of Phaseolus Vulgaris Plants Grown on a Saline Soil. Archives of Agronomy and Soil Science 2017, 63, 1556–1571. [Google Scholar] [CrossRef]
- Kandil, A.A.; Sharief, A.E.; Fathalla, F.H. ONION YIELD AS AFFECTED BY FOLIAR APPLICATION WITH AMINO AND HUMIC ACIDS UNDER NITROGEN FERTILIZER LEVELS. Crop Production 2013, 2, 62–72. [Google Scholar]
- Sruthi, B.; E, S. Influence of organic manures on yield, quality and economics of aggregatum onion (Allium cepa. L. var. aggregatum). J Pharmacogn Phytochem 2019, 8, 1768–1770. [Google Scholar]
- Omar, M.; Ramadan, A. Response of Carrot (Daucus Carota L.) to Foliar Application of Potassium Fertilizers and Some Soil Amendments under Clay Soil Conditions. Journal of Soil Sciences and Agricultural Engineering 2018, 9, 197–202. [Google Scholar] [CrossRef]
- Raheem, S.M.; Al-Jaf, H.I.; Tofiq, G.K. Influence of Foliar and Soil Application of Humic Acid on Growth and Yield of Lettuce. 2018.
- Srivastava, N.; Srivastava, M.; Ramteke, P.W.; Mishra, P.K. Chapter 23 - Solid-State Fermentation Strategy for Microbial Metabolites Production: An Overview. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: Amsterdam, 2019; pp. 345–354. ISBN 978-0-444-63504-4. [Google Scholar]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae Biostimulants: A Critical Look at Microalgal Biostimulants for Sustainable Agricultural Practices. Biotechnology Advances 2021, 49, 107754. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant Properties of Seaweed Extracts in Plants: Implications towards Sustainable Crop Production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Malik, A.; Mor, V.S.; Tokas, J.; Punia, H.; Malik, S.; Malik, K.; Sangwan, S.; Tomar, S.; Singh, P.; Singh, N.; et al. Biostimulant-Treated Seedlings under Sustainable Agriculture: A Global Perspective Facing Climate Change. Agronomy 2021, 11, 14. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef] [PubMed]
| Natural Products | Type NBs | Molecules presents | Action mode | Biostimulant effect | Produced by SSF? | Ref. |
|---|---|---|---|---|---|---|
| Hormone containing products (HCP ) | Auxins | 3-indoleacetic acid (IAA) | Promotes cell elongation | Stimulates cell elongation and rooting | Produced by SSF | [23,24] |
| Indole propionic acid (AIP) | Promotes vegetative growth and cell division | Stimulation of growth, flowering and rooting in plants | Not produced by SSF | [25,26] | ||
| Cytokinins | Zeatin | Stimulates cell division and vegetative growth | Promotion of growth and development of plants | Not produced by SSF | [27,28,29] | |
| Kinetin | Stimulates cell division and vegetative growth | Improves the quality of the crops, increasing the size and weight of the fruits | Produced by SSF and vermicompost | [30,31,32] | ||
| Abscisic acid (ABA) | ABA | Regulation of stress responses and plant development | Improves stress tolerance and fruit ripening | Produced by SSF | [33,34] | |
| Gibberellins | Gibberellin A3 (GA3) | Stimulation of growth and vigor in plants | Induction to germination, flowering | Produced by SSF | [35,36,37] | |
| Gibberellin A4 (GA4) | Promotion of plant growth and development | Stimulates germination, development of lateral shoots, flowering | Produced by SSF | [38,39] | ||
| Seaweed Extract (AM) | Alginic acids | Improves nutrient absorption and stimulates enzyme activity | Increased growth, resistance to abiotic stress | Produced by SSF |
[40,41,42] | |
| AM | Fucoidan | Improves the defense mechanisms of plants | Resistance to abiotic stress | Produced by SSF |
[43,44,45,46] | |
| Oligosaccharides | Stimulation of physiological responses in plants | Improves immune response and growth | Produced by SSF |
[[64–67] | ||
|
humic substances |
Humic and Fulvic Acids (AHF) |
Humic acids | Improved soil structure and nutrient availability | Stimulates root growth and nutrient absorption | Produced by SSF | [47,48,49,50] |
| humic acids | Stimulation of plant growth and development | Improves nutrient uptake and stress resistance. | Produced by SSF | [51,52] | ||
| Amino acid-containing products Peptides (AACP) | Amino acids | L-proline | Regulation of plant stress and development | Enhances stress tolerance and resistance | Produced by SSF | [53,54,55] |
| Peptides | Low molecular weight peptides | Stimulation of plant growth and development | Improvement of plant nutrition and growth | Produced by SSF | [56,57,58] | |
| Other NBs | Siderophores | Siderophores | binds to Fe and is solubilized | Improve absorption and mobilization of Fe | Produced by SSF | [59,60,61] |
| Chitosan Fungal | Chitosan Fungal | promote plant growth, cell division, increase enzyme activity and improve nutrient transport | presented biostimulant activity in seed germination | Produced by SSF | [62,63] |
| NBs | Substrate | Microorganism | Pretreatment | Optimal conditions SSF | Effect NBs and Crop |
Ref | |||
|---|---|---|---|---|---|---|---|---|---|
| Trituration | pH | sterilization | % moisture | Temperature C° | |||||
| IIA | pruning waste + Grass |
Trichoderma harzianum | 1 cm | 6.8 |
2 times |
74 |
25 |
[15] | |
| IIA | Yuca bagasse Soy bran Wheat bran Sorghum dried distiller's grains Corn dried distiller´s grains |
Aspergillus flavipes Aspergillus ustus Bacillus subtilis Bacillus megaterium Bacillus amyloliquefaciens Trichoderma atroviride Trichoderma koningii Trichoderma harzianum |
0,5, 1,0 y > 1,0 mm |
50 |
room temperature |
clon IPB2 Eucalyptus grandis x Eucalyptus urophylla increasing rooting |
[14,85] |
||
| Kinetin | cow dung + leaf litter |
Selenomonas ruminantium | 2 - 5 mm | 6.9 | 70-75 | 25 ± 3 | [30] | ||
| ABA | millet rice |
Botrytis cinerea | millet and rice | 1 time | 26.5 - 25.5 | [33] | |||
| GA3 | rice bran |
Gibberella fujikuroi | 50° C | 65,95% | 28 ± 2 | [87] | |||
| GA3 | Corn Cob Residues | Aspergillus niger | 5.1 | 24% | [88] | ||||
| GA3 | Citric Pulp |
Fusarium moniliforme LPB03 + Gibberella fujikuroi |
5.5 - 5.8 | 75 | 29 | [89] | |||
| Alginic acids | Apple peels | Azotobacter vinelandii , NRRL-14641 | 0.1 mm |
7 |
60 °C |
70 | 37.5 | [40] | |
| Alginic acids | Sargassum macroalgae |
Cunninghamella echinulate Aspergillus niger Penicillium oxalicum |
7 – 8.5 |
1 time 121 °C |
65-75 | 28-30 | [41] | ||
| Fucoida | seaweed Fucus vesiculosus |
Aspergillus niger Mucor sp |
80 | 30 | [43] | ||||
| Oligosaccharides | oybean meal | - | room temperature | effect on germination | [90] | ||||
| chitin oligosaccharides | powder of molting of mealworms |
Talaromyces allahabadensis Hi-4 Talaromyces funiculosus |
|
6 | 40 | [91] | |||
| Humic Acid | Oil Palm Empty Fruit Bunch | Trichoderma reesei | 6 | 64-72 | 30 | [47,92] | |||
| Fulvic Acid | sugarcane bagasse | Trichoderma Sp. | 70 | 20 | [93] | ||||
| L-proline | wheat straw ice straw wheat bran corn cob corn stover |
Fomitopsis sp. | small pieces |
5.5 |
25 - 30 | [53] | |||
|
Low molecular weight peptides |
chickpeas | Bacillus subtilis | [57] | ||||||
| Siderophores | soybean protein meal | Lactobacillus plantarum | 37 | [94] | |||||
| Chitosan Fungal | sweet potato | Gongronella butleri USDB 0201 | 28 | [63] | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





