Submitted:
08 June 2023
Posted:
08 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
Is there an antidiabetics’ misusing issue?
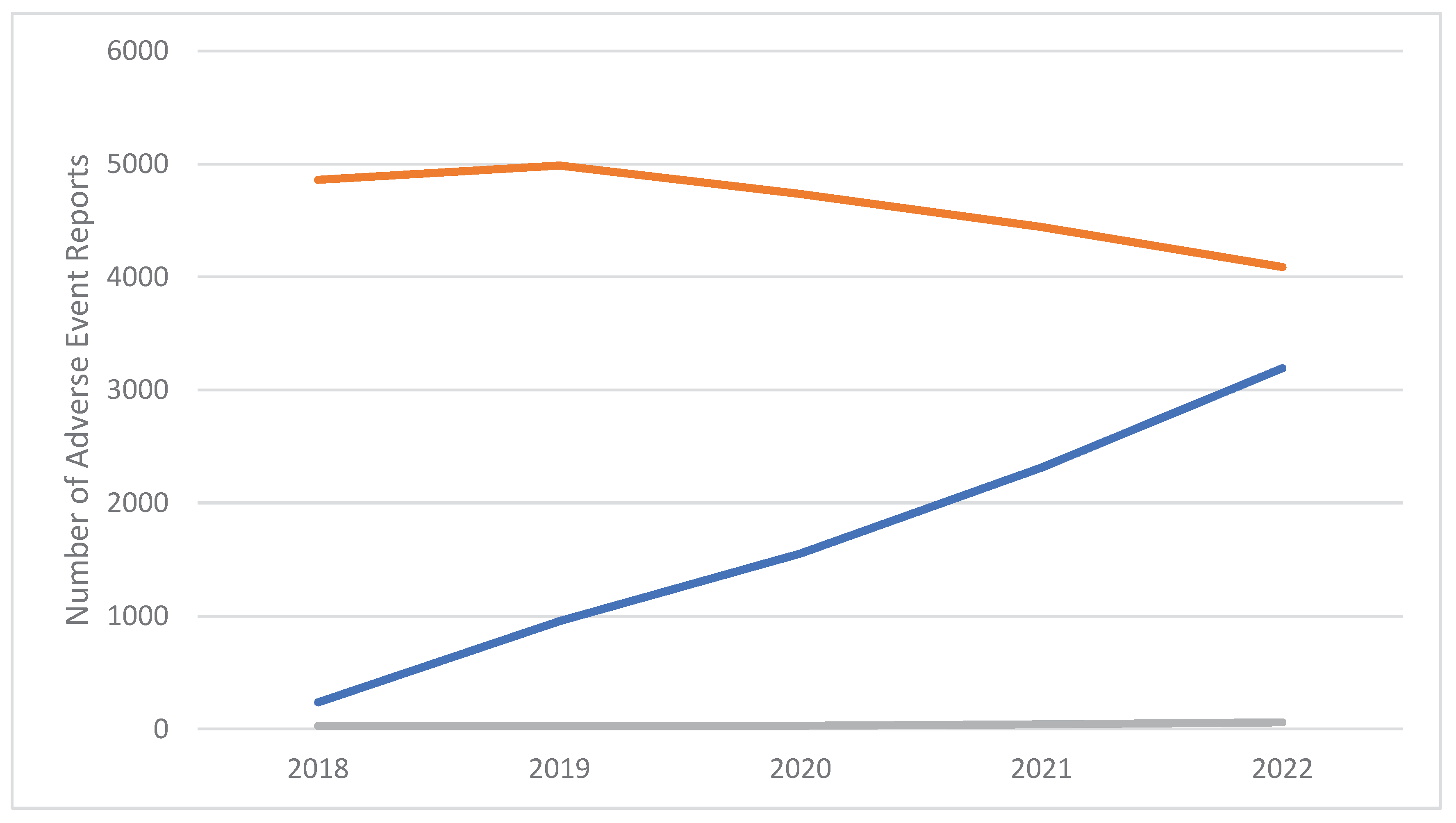
2. Results
Pharmacovigilance signals
3. Discussion
3.1. Semaglutide and GLP-1RA as image- and performance-enhancing drugs (IPED)
3.2. Semaglutide and GLP-1RAs as molecules acting on the reward system?
3.3. The potential use of GLP-1 Ras in neurology
3.4. Limitations
4. Materials and Methods
4.1. Data source
4.2. Data analysis
5. Conclusions
Data Availability Statement:
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gettman, L. New Drug: Tirzepatide (Mounjaro™). Sr. Care Pharm. 2023, 38, 50–62. [Google Scholar] [CrossRef]
- Rendell, M.S. Obesity and diabetes: the final frontier. Expert Rev. Endocrinol. Metab. 2023, 18, 81–94. [Google Scholar] [CrossRef]
- Novograd, J.; Mullally, J.A.; Frishman, W.H. Tirzepatide for Weight Loss: Can Medical Therapy “Outweigh” Bariatric Surgery? Cardiol. Rev. 2023, 31, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Slahor, L. CME: Metformin – Dos und Don’ts]. Praxis 2021, 110, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Dokmak, G.; Bader, M.; Karaman, R. A Comprehensive Review on Weight Loss Associated with Anti-Diabetic Medications. Life 2023, 13, 1012. [Google Scholar] [CrossRef]
- Azuri, J.; Hammerman, A.; Aboalhasan, E.; Sluckis, B.; Arbel, R. Tirzepatide versus semaglutide for weight loss in patients with type 2 diabetes mellitus: A value for money analysis. Diabetes, Obes. Metab. 2022, 25, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.H.; Deanfield, J.E.; Jacob, S. Prioritizing obesity treatment: expanding the role of cardiologists to improve cardiovascular health and outcomes. Cardiovasc. Endocrinol. Metab. 2023, 12, e0279. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Raj, R.; Elshimy, G.; Zapata, I.; Kannan, L.; Majety, P.; Edem, D.; Correa, R. Adverse Events Related to Tirzepatide. J. Endocr. Soc. 2023, 7, bvad016. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Dual GIP/GLP-1 receptor agonists: New advances for treating type-2 diabetes. Ann. d'Endocrinologie 2023, 84, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Neuville, M.F.; Paquot, N.; Scheen, A.J. [A new era for glucagon-like peptide-1 receptor agonists]. . 2023, 78, 40–45. [Google Scholar]
- Bhusal, A. Advent of tirzepatide: boon for diabetic and obese? Ann. Med. Surg. 2023, 85, 71–72. [Google Scholar] [CrossRef]
- Sinha, R.; Papamargaritis, D.; Sargeant, J.A.; Davies, M.J. Efficacy and Safety of Tirzepatide in Type 2 Diabetes and Obesity Management. J. Obes. Metab. Syndr. 2023, 32, 25–45. [Google Scholar] [CrossRef]
- Ebell, M.H. Tirzepatide Helps Adults With Obesity Without Diabetes Lose 15% to 21% of Their Body Weight Over 72 Weeks. 2023, 107, 99–99.
- Alkhezi, O.S.; Alahmed, A.A.; Alfayez, O.M.; Alzuman, O.A.; Almutairi, A.R.; Almohammed, O.A. Comparative effectiveness of glucagon-like peptide-1 receptor agonists for the management of obesity in adults without diabetes: A network meta-analysis of randomized clinical trials. Obes. Rev. 2022, 24, e13543. [Google Scholar] [CrossRef]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of obesity: an update on the available medications and drugs under investigation. EClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef] [PubMed]
- BBC News. Weight loss drug emaglutide approved for NHS use. https://www.bbc.com/news/health-64874243. Accessed , 2023. 08 March.
- Li, A.; Su, X.; Hu, S.; Wang, Y. Efficacy and safety of oral semaglutide in type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res. Clin. Pr. 2023, 198, 110605. [Google Scholar] [CrossRef]
- Rodríguez, J.E.; Campbell, K.M. Past, Present, and Future of Pharmacologic Therapy in Obesity. Prim. Care: Clin. Off. Pr. 2016, 43, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.; Munro, J. Drug treatment and obesity. Pharmacol. Ther. 1982, 18, 351–373. [Google Scholar] [CrossRef] [PubMed]
- Makówka, A.; Zawiasa, A.; Nowicki, M. Prescription-medication sharing among family members: an unrecognized cause of a serious drug adverse event in a patient with impaired renal function. Clin. Nephrol. 2015, 83, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-B.; Shao, X.-T.; Liu, S.-Y.; Tan, D.-Q.; Wang, Z.; Wang, D.-G. Assessment of metformin, nicotine, caffeine, and methamphetamine use during Chinese public holidays. Chemosphere 2020, 258, 127354. [Google Scholar] [CrossRef]
- Burtscher, M. Metformin for high-altitude performance? Clin. Exp. Pharmacol. Physiol. 2017, 44, 903–903. [Google Scholar] [CrossRef] [PubMed]
- Geer, B.; Gibson, D.; Grayeb, D.; Benabe, J.; Victory, S.; Mehler, S.; Mehler, P. Metformin abuse: A novel and dangerous purging behavior in anorexia nervosa. Int. J. Eat. Disord. 2019, 52, 319–321. [Google Scholar] [CrossRef] [PubMed]
- The Independent. Jameela Jamil calls out ‘extreme’ weight loss at Oscars amid ozempic controversy. https://www.independent.co.uk/life-style/ozempic-weight-loss-jameela-jamil-oscars-b2300525.html. Accessed on , 2023. 14 March.
- Le Monde, 2023. https://www.lemonde.fr/en/health/article/2023/03/02/ozempic-french-authorities-issue-alert-for-anti-diabetic-drug-misused-for-weight-loss_6017913_14.html#:~:text=While%20misuse%20of%20Ozempic%20appears,them%20of%20this%20essential%20treatment.%22. Accessed on , 2023. 08 April.
- Alvarez-Mon, M.A.; Llavero-Valero, M.; del Barco, A.A.; Zaragozá, C.; A Ortega, M.; Lahera, G.; Quintero, J.; Alvarez-Mon, M. Areas of Interest and Attitudes Toward Antiobesity Drugs: Thematic and Quantitative Analysis Using Twitter. J. Med Internet Res. 2021, 23, e24336. [Google Scholar] [CrossRef] [PubMed]
- The Guardian, 2023. https://www.theguardian.com/australia-news/2023/jan/06/tga-investigates-influencers-after-diabetes-drug-ozempic-promoted-as-weight-loss-treatment. Accessed on , 2023. 08 April.
- Valdesolo, F. What You Need to Know About Ozempic: The Diabetes Drug Fuelling Hollywood’s Harmful Weight-Loss Obsession; 10 February 2023. https: //www.vogue.co.uk/beauty/article/what-is-ozempic. Accessed on April 08, 2023. [Google Scholar]
- Orsolini, L.; Francesconi, G.; Papanti, D.; Giorgetti, A.; Schifano, F. Profiling online recreational/prescription drugs' customers and overview of drug vending virtual marketplaces. Hum. Psychopharmacol. Clin. Exp. 2015, 30, 302–318. [Google Scholar] [CrossRef] [PubMed]
- Zaprutko, T.; Kopciuch, D.; Paczkowska, A.; Sprawka, J.; Cynar, J.; Pogodzińska, M.; Niewczas, K.; Stolecka, A.; Sygit, M.; Michalak, M.; et al. Facebook as a source of access to medicines. PLOS ONE 2022, 17, e0275272. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, S.; Vickers-Smith, R.; Guirguis, A.; Corkery, J.M.; Martinotti, G.; Harris, D.R.; Schifano, F. Pharmacovigilance Signals of the Opioid Epidemic over 10 Years: Data Mining Methods in the Analysis of Pharmacovigilance Datasets Collecting Adverse Drug Reactions (ADRs) Reported to EudraVigilance (EV) and the FDA Adverse Event Reporting System (FAERS). Pharmaceuticals 2022, 15, 675. [Google Scholar] [CrossRef] [PubMed]
- Schifano, N.; Capogrosso, P.; Boeri, L.; Fallara, G.; Cakir, O.O.; Castiglione, F.; Alnajjar, H.M.; Muneer, A.; Deho’, F.; Schifano, F.; et al. Medications mostly associated with priapism events: assessment of the 2015–2020 Food and Drug Administration (FDA) pharmacovigilance database entries. Int. J. Impot. Res. 2022, 36, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Dahlén, A.D.; Dashi, G.; Maslov, I.; Attwood, M.M.; Jonsson, J.; Trukhan, V.; Schiöth, H.B. Trends in Antidiabetic Drug Discovery: FDA Approved Drugs, New Drugs in Clinical Trials and Global Sales. Front. Pharmacol. 2022, 12, 807548. [Google Scholar] [CrossRef]
- Engler, C.; Leo, M.; Pfeifer, B.; Juchum, M.; Chen-Koenig, D.; Poelzl, K.; Schoenherr, H.; Vill, D.; Oberdanner, J.; Eisendle, E.; et al. Long-term trends in the prescription of antidiabetic drugs: real-world evidence from the Diabetes Registry Tyrol 2012–2018. BMJ Open Diabetes Res. Care 2020, 8, e001279. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Mol. Metab. 2020, 46, 101102. [Google Scholar] [CrossRef]
- Yamamoto-Honda, R.; Takahashi, Y.; Mori, Y.; Yamashita, S.; Yoshida, Y.; Kawazu, S.; Iwamoto, Y.; Kajio, H.; Yanai, H.; Mishima, S.; et al. Changes in Antidiabetic Drug Prescription and Glycemic Control Trends in Elderly Patients with Type 2 Diabetes Mellitus from 2005-2013: An Analysis of the National Center Diabetes Database (NCDD-03). Intern. Med. 2018, 57, 1229–1240. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Wang, L.; Chen, C.; Chen, L. Association between different GLP-1 receptor agonists and gastrointestinal adverse reactions: A real-world disproportionality study based on FDA adverse event reporting system database. Front. Endocrinol. 2022, 13, 1043789. [Google Scholar] [CrossRef] [PubMed]
- Sarayani, A.; Hampp, C.; Brown, J.D.; Donahoo, W.T.; Winterstein, A.G. Topiramate Utilization After Phentermine/Topiramate Approval for Obesity Management: Risk Minimization in the Era of Drug Repurposing. Drug Saf. 2022, 45, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Verma, S.; Vaidya, S.; Kalia, K.; Tiwari, V. Recent updates on GLP-1 agonists: Current advancements & challenges. Biomed. Pharmacother. 2018, 108, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.M.; Van Raalte, D.H. Safety of Semaglutide. Front. Endocrinol. 2021, 12, 786732. [Google Scholar] [CrossRef]
- Berkovic, M.C.; Strollo, F. Semaglutide-eye-catching results. World J. Diabetes 2023, 14, 424–434. [Google Scholar] [CrossRef]
- EMCDDA, 2020. Health and social responses to problems associated with the use of performance- and image-enhancing drugs A background paper for the updated European Responses Guide. https://www.emcdda.europa.eu/system/files/media/attachments/documents/14197/ERG2021_BackgroundPaper_FINAL.pdf. Accessed on , 2023. 06 May.
- Bruening, A.B.; Perez, M.; Ohrt, T.K. Exploring weight control as motivation for illicit stimulant use. Eat. Behav. 2018, 30, 72–75. [Google Scholar] [CrossRef]
- Milano, G.; Chiappini, S.; Mattioli, F.; Martelli, A.; Schifano, F. β-2 Agonists as Misusing Drugs? Assessment of both Clenbuterol- and Salbutamol-related European Medicines Agency Pharmacovigilance Database Reports. Basic Clin. Pharmacol. Toxicol. 2018, 123, 182–187. [Google Scholar] [CrossRef]
- Dakanalis, A.; Colmegna, F.; Zanetti, M.A.; Di Giacomo, E.; Riva, G.; Clerici, M. Evaluation of the DSM-5 Severity Specifier for Bulimia Nervosa in Treatment-Seeking Youth. Child Psychiatry Hum. Dev. 2017, 49, 137–145. [Google Scholar] [CrossRef]
- Potts, A.J.; Bowman, N.J.; Seger, D.L.; Thomas, S.H.L. Toxicoepidemiology and predictors of death in 2,4-dinitrophenol (DNP) toxicity. Clin. Toxicol. 2020, 59, 515–520. [Google Scholar] [CrossRef]
- Corazza, O.; Bersani, F.S.; Brunoro, R.; Valeriani, G.; Martinotti, G.; Schifano, F. The diffusion of Performance and Image-Enhancing Drugs (PIEDs) on the Internet: The Abuse of the Cognitive Enhancer Piracetam. Subst. Use Misuse 2014, 49, 1849–1856. [Google Scholar] [CrossRef]
- Hendricks, E.J. Off-label drugs for weight management. Diabetes, Metab. Syndr. Obesity: Targets Ther. 2017; 10. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; In, S.; Choi, H.; Chung, H.; Chung, K.H. Detection of phentermine in hair samples from drug suspects. Forensic Sci. Int. 2011, 207, e5–e7. [Google Scholar] [CrossRef]
- Targher, G.; Mantovani, A.; Byrne, C.D. Mechanisms and possible hepatoprotective effects of glucagon-like peptide-1 receptor agonists and other incretin receptor agonists in non-alcoholic fatty liver disease. Lancet Gastroenterol. Hepatol. 2023, 8, 179–191. [Google Scholar] [CrossRef]
- Reiner, D.J.; Leon, R.M.; E McGrath, L.; Koch-Laskowski, K.; Hahn, J.D.; E Kanoski, S.; Mietlicki-Baase, E.G.; Hayes, M.R. Glucagon-Like Peptide-1 Receptor Signaling in the Lateral Dorsal Tegmental Nucleus Regulates Energy Balance. Neuropsychopharmacology 2017, 43, 627–637. [Google Scholar] [CrossRef]
- DI Chiara, G.; Tanda, G.; Bassareo, V.; Pontieri, F.; Acquas, E.; Fenu, S.; Cadoni, C.; Carboni, E. Drug Addiction as a Disorder of Associative Learning: Role of Nucleus Accumbens Shell/Extended Amygdala Dopamine. Ann. New York Acad. Sci. 1999, 877, 461–485. [Google Scholar] [CrossRef]
- Dickson, S.L.; Shirazi, R.H.; Hansson, C.; Bergquist, F.; Nissbrandt, H.; Skibicka, K.P. The Glucagon-Like Peptide 1 (GLP-1) Analogue, Exendin-4, Decreases the Rewarding Value of Food: A New Role for Mesolimbic GLP-1 Receptors. J. Neurosci. 2012, 32, 4812–4820. [Google Scholar] [CrossRef] [PubMed]
- Eren-Yazicioglu, C.Y.; Yigit, A.; Dogruoz, R.E.; Yapici-Eser, H. Can GLP-1 Be a Target for Reward System Related Disorders? A Qualitative Synthesis and Systematic Review Analysis of Studies on Palatable Food, Drugs of Abuse, and Alcohol. Front. Behav. Neurosci. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Listos, J.; Listos, P.; Baranowska-Bosiacka, I.; Karpiuk, A.; Filarowska, J.; Łupina, M.; Słowik, T.; Zawiślak, S.; Kotlińska, J. Linagliptin, a Selective Dipeptidyl Peptidase-4 Inhibitor, Reduces Physical and Behavioral Effects of Morphine Withdrawal. Molecules 2022, 27, 2478. [Google Scholar] [CrossRef]
- Jerlhag, E. The therapeutic potential of glucagon-like peptide-1 for persons with addictions based on findings from preclinical and clinical studies. Front. Pharmacol. 2023, 14, 1063033. [Google Scholar] [CrossRef]
- New York Times, 2023. https://www.nytimes.com/2023/02/03/well/live/ozempic-wegovy-weight-loss.html. Accessed on , 2023. 06 May.
- Wilding, J.P.H.; Batterham, R.L.; Davies, M.; Van Gaal, L.F.; Kandler, K.; Konakli, K.; Lingvay, I.; McGowan, B.M.; Oral, T.K.; Rosenstock, J.; et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes, Obes. Metab. 2022, 24, 1553–1564. [Google Scholar] [CrossRef]
- van Bloemendaal, L.; Ijzerman, R.G.; Kulve, J.S.T.; Barkhof, F.; Konrad, R.J.; Drent, M.L.; Veltman, D.J.; Diamant, M. GLP-1 Receptor Activation Modulates Appetite- and Reward-Related Brain Areas in Humans. Diabetes 2014, 63, 4186–4196. [Google Scholar] [CrossRef] [PubMed]
- Urbanik, L.A.; Acharya, N.K.; Grigson, P.S. Acute treatment with the glucagon-like peptide-1 receptor agonist, liraglutide, reduces cue- and drug-induced fentanyl seeking in rats. Brain Res. Bull. 2022, 189, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Douton, J.E.; Acharya, N.K.; Stoltzfus, B.; Sun, D.; Grigson, P.S.; Nyland, J.E. Acute glucagon-like peptide-1 receptor agonist liraglutide prevents cue-, stress-, and drug-induced heroin-seeking in rats. Behav. Pharmacol. 2022, 33, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Colvin, K.J.; Killen, H.S.; Kanter, M.J.; Halperin, M.C.; Engel, L.; Dickinson, M.B.; Fimmel, A.I.; Holland, J.G.; Currie, P.J. Differential effects of intra-ventral tegmental area ghrelin and glucagon-like peptide-1 on the stimulatory action of D-amphetamine and cocaine-induced ethanol intake in male Sprague Dawley rats. Behav. Brain Res. 2021, 421, 113726. [Google Scholar] [CrossRef] [PubMed]
- Douton, J.E.; Augusto, C.; Stoltzfus, B.; Carkaci-Salli, N.; Vrana, K.E.; Grigson, P.S. Glucagon-like peptide-1 receptor agonist, exendin-4, reduces reinstatement of heroin-seeking behavior in rats. Behav. Pharmacol. 2020, 32, 265–277. [Google Scholar] [CrossRef]
- Marty, V.N.; Farokhnia, M.; Munier, J.J.; Mulpuri, Y.; Leggio, L.; Spigelman, I. Long-Acting Glucagon-Like Peptide-1 Receptor Agonists Suppress Voluntary Alcohol Intake in Male Wistar Rats. Front. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Yammine, L.; E Green, C.; Kosten, T.R.; de Dios, C.; Suchting, R.; Lane, S.D.; Verrico, C.D.; Schmitz, J.M. Exenatide Adjunct to Nicotine Patch Facilitates Smoking Cessation and May Reduce Post-Cessation Weight Gain: A Pilot Randomized Controlled Trial. Nicotine Tob. Res. 2021, 23, 1682–1690. [Google Scholar] [CrossRef]
- Klausen, M.K.; Jensen, M.E.; Møller, M.; Le Dous, N.; Jensen, A.-M. .; Zeeman, V.A.; Johannsen, C.-F.; Lee, A.M.; Thomsen, G.K.; Macoveanu, J.; et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. J. Clin. Investig. 2022, 7. [Google Scholar] [CrossRef]
- Harkavyi, A.; Abuirmeileh, A.; Lever, R.; E Kingsbury, A.; Biggs, C.S.; Whitton, P.S. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson's disease. J. Neuroinflammation 2008, 5, 19–19. [Google Scholar] [CrossRef]
- Hölscher, C. Protective properties of GLP-1 and associated peptide hormones in neurodegenerative disorders. Br. J. Pharmacol. 2021, 179, 695–714. [Google Scholar] [CrossRef]
- Athauda, D.; Maclagan, K.; Skene, S.S.; Bajwa-Joseph, M.; Letchford, D.; Chowdhury, K.; Hibbert, S.; Budnik, N.; Zampedri, L.; Dickson, J.; et al. Exenatide once weekly versus placebo in Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1664–1675. [Google Scholar] [CrossRef]
- Athauda, D.; Foltynie, T. Protective effects of the GLP-1 mimetic exendin-4 in Parkinson's disease. Neuropharmacology 2018, 136, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Bomba, M.; Granzotto, A.; Castelli, V.; Massetti, N.; Silvestri, E.; Canzoniero, L.M.; Cimini, A.; Sensi, S.L. Exenatide exerts cognitive effects by modulating the BDNF-TrkB neurotrophic axis in adult mice. Neurobiol. Aging 2018, 64, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Food & Drug Administration (FDA, 2021). FDA Adverse Event Reporting System (FAERS) Public Dashboard. U.S. Food & Drug Administration. 2021. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-eventreporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard. Accessed on , 2023. 08 April.
- Schifano, F. Coming Off Prescribed Psychotropic Medications: Insights from Their Use as Recreational Drugs. Psychother. Psychosom. 2020, 89, 274–282. [Google Scholar] [CrossRef] [PubMed]
- ICH. ‘MedDRA ® TERM SELECTION : POINTS TO CONSIDER. ICH-Endorsed Guide for MedDRA Users’. London Release 4.21. 21. https://alt.meddra.org/files_acrobat/000571_termselptc_r4_21_mar2021.pdf. Accessed on April 08, 2023. 20 March.
- Ahmed I, Poncet A. PhViD: An R Package for PharmacoVigilance Signal Detection. R Package Version 1.0.8., , 2022. https://cran.r-project.org/web/packages/PhViD/PhViD.pdf. Accessed on April 08, 2023. 12 October.
- Poluzzi, E.; Raschi, E.; Piccinni, C.; De Ponti, F. Data Mining Techniques in Pharmacovigilance: Analysis of the Publicly Accessible FDA Adverse Event Reporting System (AERS). In Data Mining Applications in Engineering and Medicine; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Subeesh, V.; Maheswari, E.; Saraswathy, G.R.; Swaroop, A.M.; Minnikanti, S.S. A Comparative Study of Data Mining Algorithms used for Signal Detection in FDA AERS Database. J. Young- Pharm. 2018, 10, 444–449. [Google Scholar] [CrossRef]
- Ahmed I, Thiessard F, Miremont-Salam G, et al. Early Detection of Pharmacovigilance Signals with Automated Methods Based on False Discovery Rates: A Comparative Study. Drug Saf. 2012, 35, 495–506.
- Suling, M.; Pigeot, I. Signal Detection and Monitoring Based on Longitudinal Healthcare Data. Pharmaceutics 2012, 4, 607–640. [Google Scholar] [CrossRef]
- Websites apparently offering novel diabetes medicines for sale; no prescription was required (search carried out on 9 March 2023 and on 6 May 2023).
- https://www.myjuniper.co.uk/weight-loss-medication?dki=Weight+Loss+Drug+Programme&utm_source=google&utm_medium=cpc&utm_campaign=search_jpruk&utm_content=weight_loss_drug&utm_ad=637429004630&utm_term=weight%20loss%20drug&matchtype=b&device=c&geoloc=9046033&placement=&network=g&campaign_id=17048534585&adset_id=145545873764&ad_id=637429004630&gclid=CjwKCAiAjPyfBhBMEiwAB2CCIgwHphPNS9t80ZR9OHh2uaJD0S9CqRy9HQB32cS_lXlVYkoHgsebpBoClyMQAvD_BwE.
- https://www.peptidesciences.com/tirzepatide-5mg.
- https://www.dailychemist.com/product/semaglutide-tablets-rybelsus/?landingPage&gclid=CjwKCAiAjPyfBhBMEiwAB2CCIt99gQcAEROnisBlxK4z81PbDS4r-vsayS73n-8x74Z6TkEufr-CVhoCt0AQAvD_BwE.
- https://www.simplymedsonline.co.uk/weight-loss.html?gclid=CjwKCAiAjPyfBhBMEiwAB2CCIvuolAv1u8G1R03kjnoUqJfuukMJ5rVhRzcHTdggLbjHsm92wuaf3RoCwbMQAvD_BwE.
- www.semaspace.com.
- https://www.echemi.com/produce/pr2211081437-semaglutide-1006-white-loose-powder-human-api-arshine.html.
- https://www.echemi.com/produce/pr2211241658-semaglutide-998-powder-weight-loss-beiyina.html.
| SEMAGLUTIDE | PHENTERMINE-TOPIRAMATE | OTHER GLP-1-RA* | |||
|---|---|---|---|---|---|
| Preferred Term | # AER | Preferred Term | # AER | Preferred Term | # AER |
| Nausea | 1,047 | Dizziness | 15 | Nausea | 1,843 |
| Vomiting | 921 | Nephrolithiasis | 14 | Blood glucose increased | 1,604 |
| Diarrhoea | 699 | Headache | 11 | Vomiting | 1,586 |
| Pancreatitis | 492 | Weight increased | 10 | Pancreatitis | 1,459 |
| Off label use | 483 | Angle closure glaucoma | 9 | Diarrhoea | 1,426 |
| Weight decreased | 465 | Vision blurred | 9 | Acute kidney injury | 1,112 |
| Blood glucose increased | 424 | Suicidal ideation | 8 | Weight decreased | 1,082 |
| Decreased appetite | 387 | Chronic kidney disease | 7 | Fatigue | 794 |
| Fatigue | 357 | Hypoesthesia | 7 | Decreased appetite | 711 |
| Dehydration | 352 | Paraesthesia | 6 | Chronic kidney disease | 689 |
| Semaglutide | Phentermine-topiramate | Other GLP-1-RA* | |||
|---|---|---|---|---|---|
| Outcome | # AER | Outcome | # AER | Outcome | # AER |
| Other outcomes | 5418 | Other outcomes | 154 | Other outcomes | 14206 |
| Hospitalized | 3479 | Hospitalized | 46 | Hospitalized | 10287 |
| Life threatening | 306 | Disabled | 14 | Died | 1705 |
| Disabled | 299 | Life threatening | 3 | Life threatening | 1103 |
| Died | 273 | Died | 1 | Disabled | 671 |
| Required intervention | 67 | Required intervention | 1 | Required intervention | 76 |
| SEMAGLUTIDE VS. OTHER GLP-1-RA | SEMAGLUTIDE VS. PHENTERMINE-TOPIRAMATE | |||||||
|---|---|---|---|---|---|---|---|---|
| PT (MedDRA) | PRR | ROR | IC025 | EB05 | PRR | ROR | IC025 | EB05 |
| Accidental overdose | 0.59 (0.60) | 0.59 (0.60) | -1.62 (0.34) | 0.50 (0.41) | Inf (<0.01) | Inf (<0.01) | -1.41 (0.50) | 0.99 (0.52) |
| Drug abuse | 4.05 (<0.01) | 4.05 (<0.01) | -0.63 (0.16) | 0.80 (0.12) | Inf (<0.01) | Inf (<0.01) | -1.74 (0.52) | 0.99 (0.53) |
| Drug levelincreased | 0.85 (0.46) | 0.85 (0.46) | -1.12 (0.27) | 0.62 (0.29) | Inf (<0.01) | Inf (<0.01) | -1.21 (0.49) | 0.99 (0.52) |
| Drug withdrawal syndrome | 4.05 (<0.01) | 4.05 (<0.01) | -0.63 (0.16) | 0.80 (0.12) | Inf (<0.01) | Inf (<0.01) | -1.74 (0.52) | 0.99 (0.53) |
| Incorrect route of product administration | 0.55 (0.61) | 0.55 (0.61) | -1.65 (0.34) | 0.48 (0.42) | Inf (<0.01) | Inf (<0.01) | -1.34 (0.50) | 0.99 (0.52) |
| Intentional product misuse | 0.42 (0.64) | 0.42 (0.64) | -1.68 (0.35) | 0.40 (0.45) | 0.32 (<0.01) | 0.32 (<0.01) | -1.01 (0.48) | 0.99 (0.53) |
| Intentional product use issue | 1.80 (<0.01) | 1.80 (<0.01) | 0.08 (<0.01) | 1.11 (<0.01) | Inf (<0.01) | Inf (<0.01) | -0.54 (0.41) | 0.99 (0.50) |
| Overdose | 0.92 (0.46) | 0.92 (0.46) | -0.66 (0.17) | 0.72 (0.19) | Inf (<0.01) | Inf (<0.01) | -0.71 (0.44) | 0.99 (0.51) |
| Prescription drug used without a prescription | 3.60 (<0.01) | 3.60 (<0.01) | -0.42 (0.10) | 0.85 (0.08) | Inf (<0.01) | Inf (<0.01) | -1.50 (0.51) | 0.99 (0.53) |
| Substance use | Inf (0.70) | Inf (0.70) | -0.29 (0.06) | 0.91 (0.04) | Inf (0.04) | Inf (0.04) | -1.74 (0.53) | 0.99 (0.53) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





