Submitted:
02 June 2023
Posted:
06 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
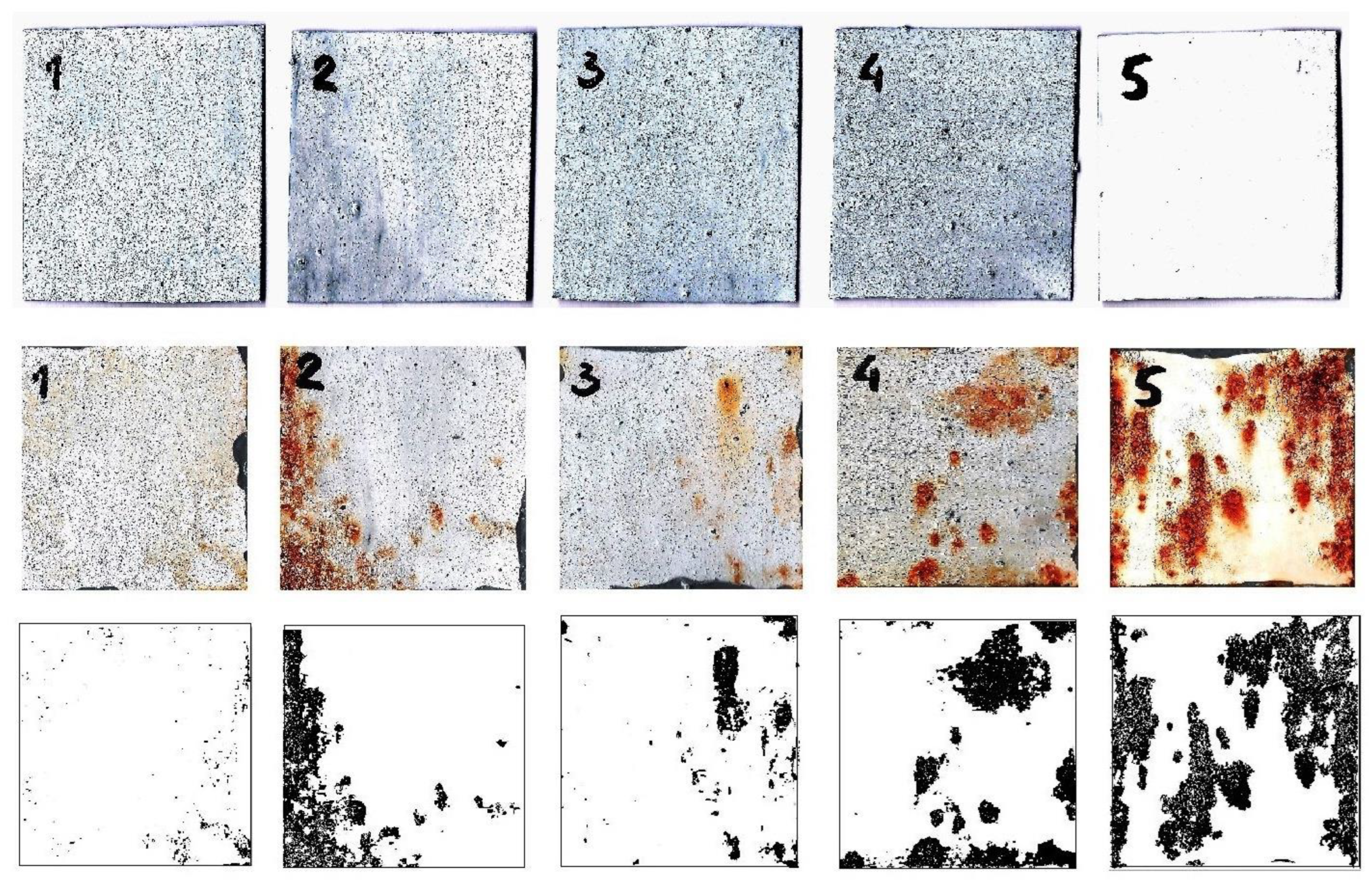
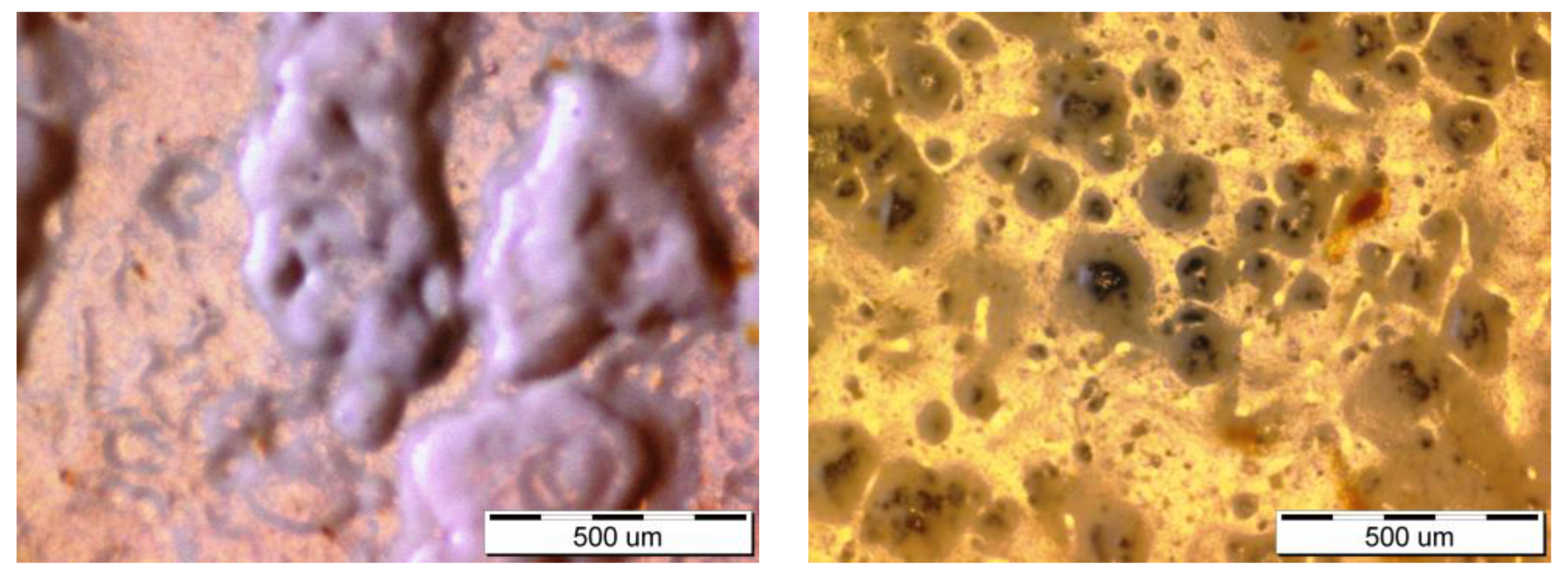
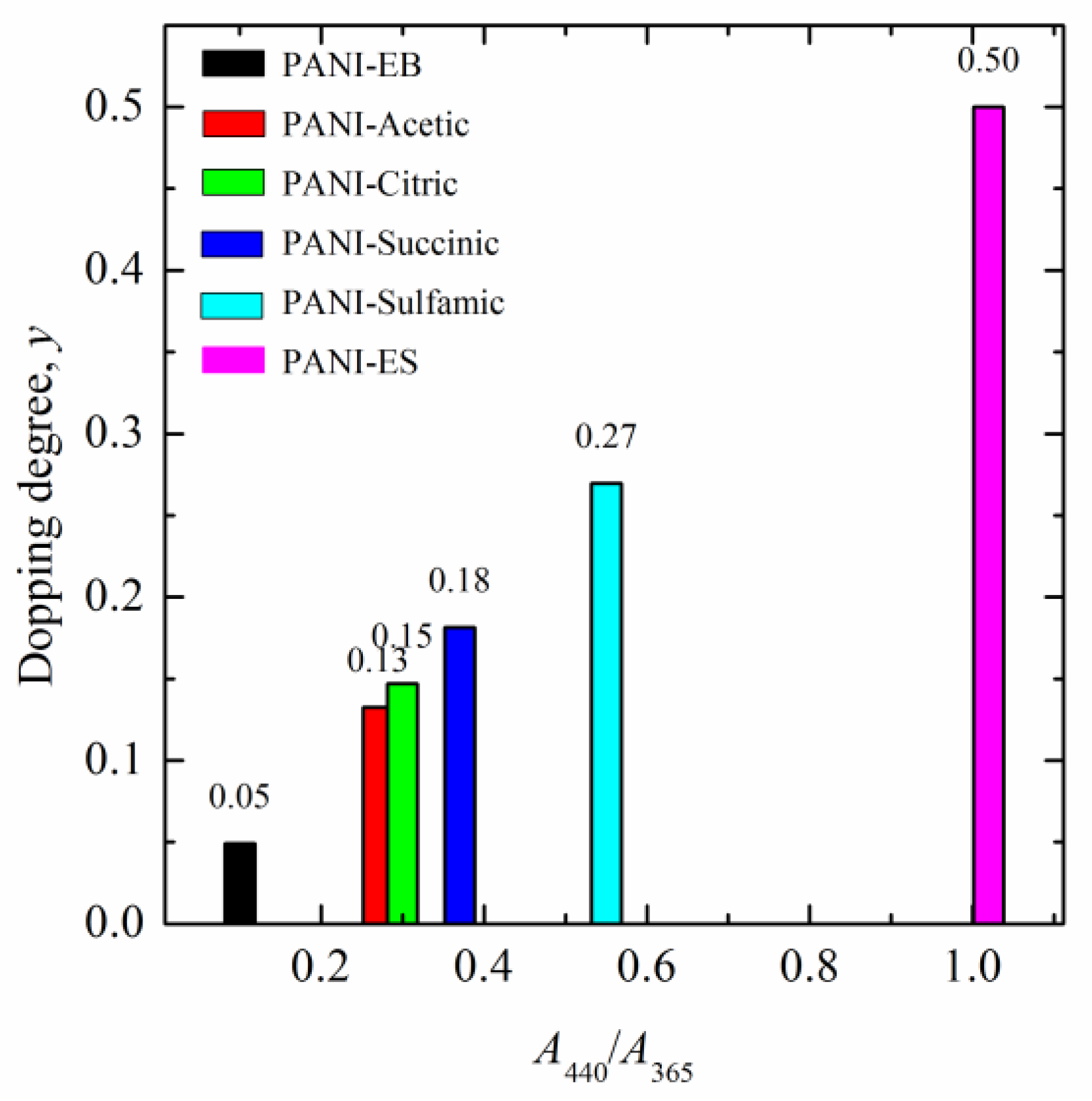
3.1. Characterization of the Samples
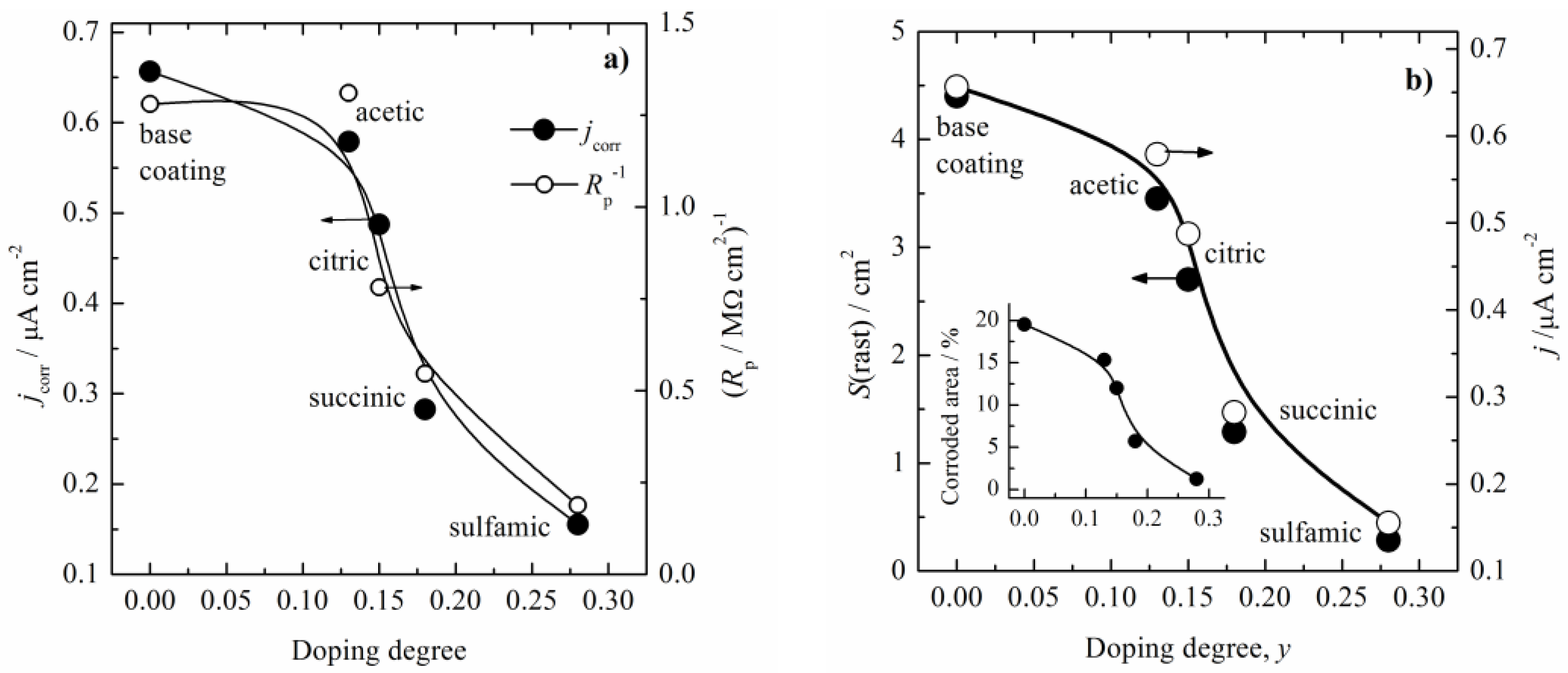
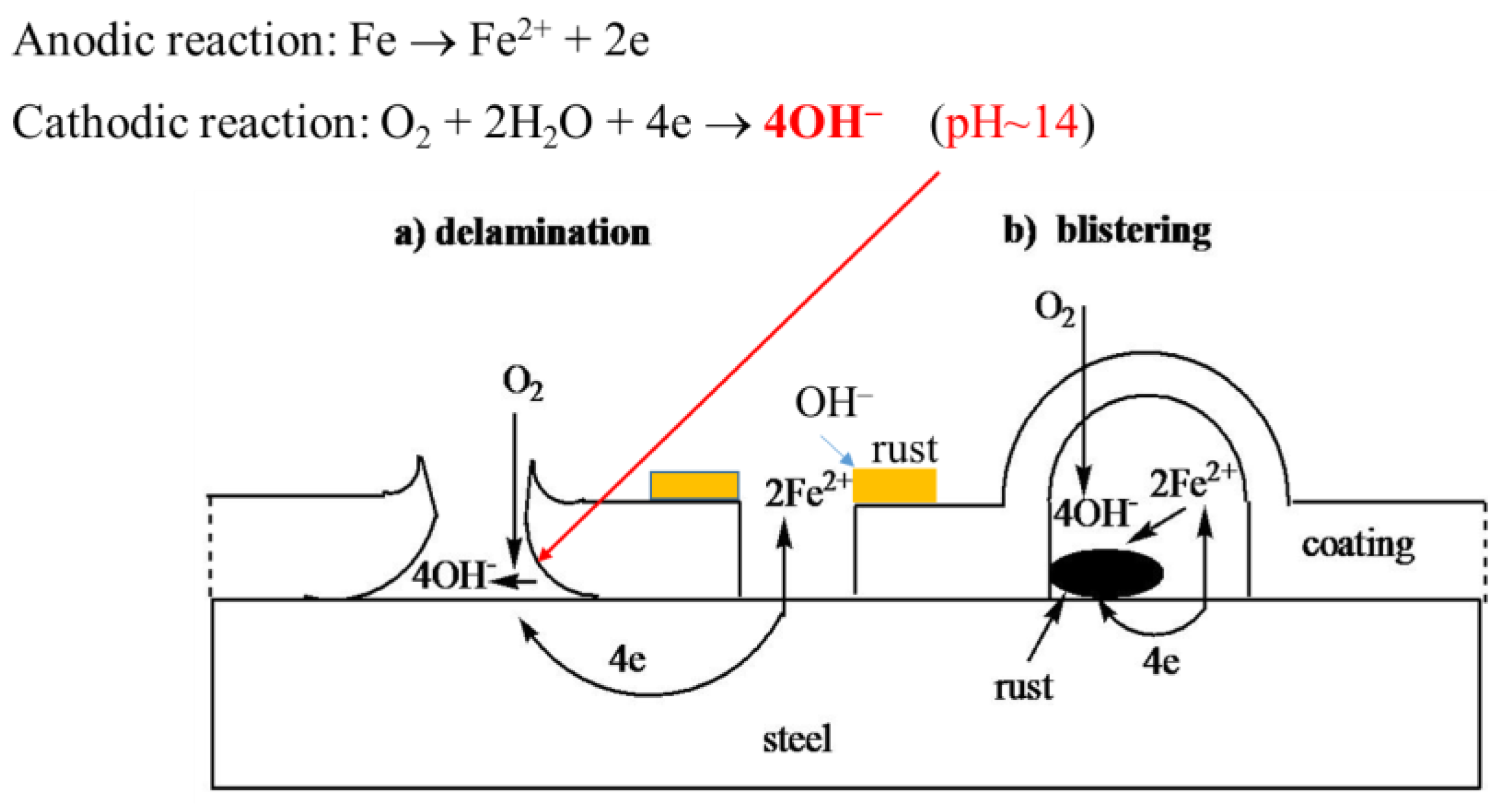
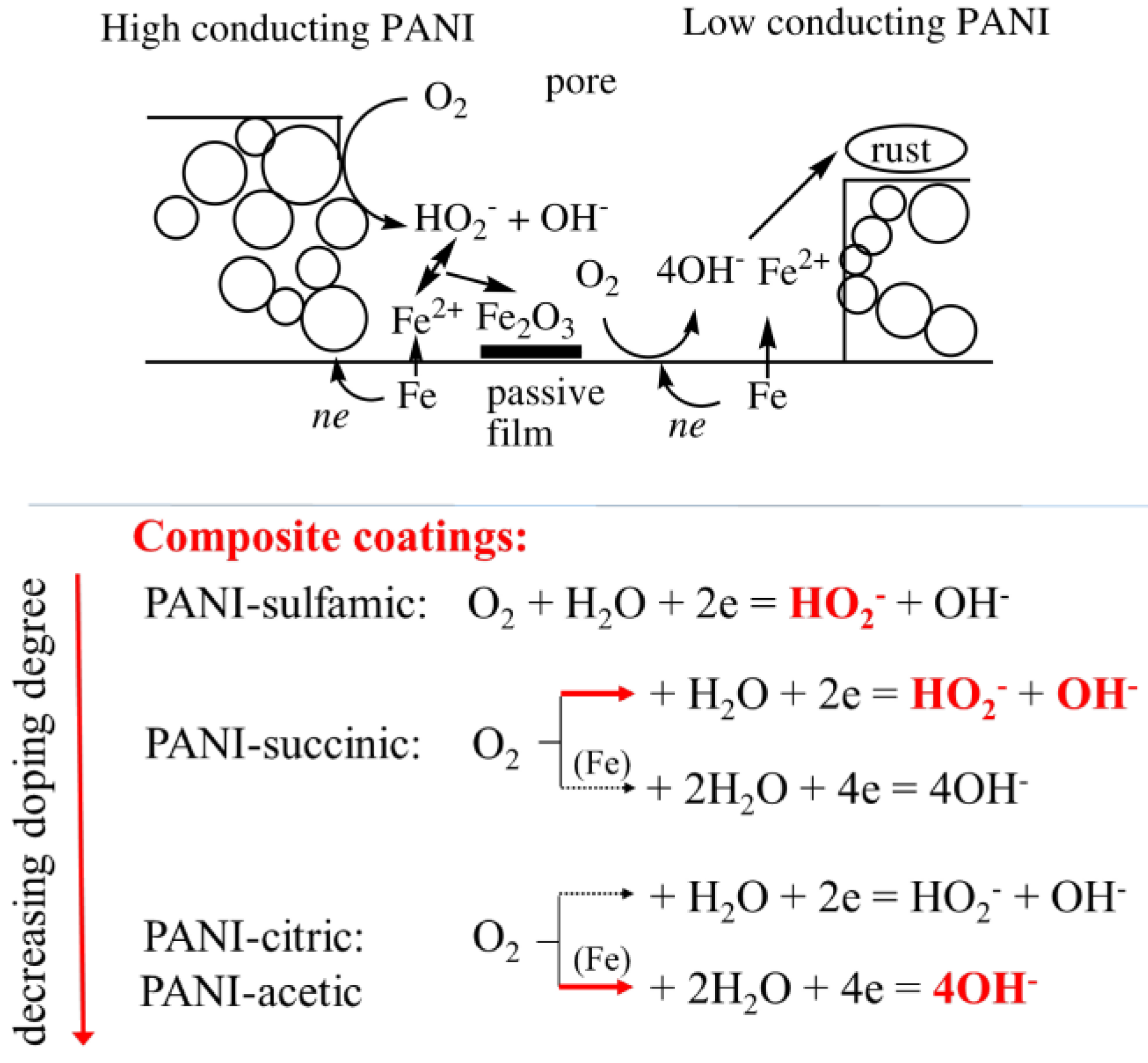
3.2. Corrosion Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Touileb, K.; Djoudjou, R.; Hedhibi, A.C.; Ouis, A.; Benselama, A.; Ibrahim, A.; Abdo, H.S.; Samad, U.A. Comparative microstructural, mechanical and corrosion study between dissimilar ATIG and conventional TIG weldments of 316L stainless steel and mild steel. Metals 2022, 12, 635. [CrossRef]
- Ruan, X.; Yang, L.; Wang, Y.; Dong, Y.; Xu, D.; Zhang, M. Biofilm-Induced Corrosion Inhibition of Q235 Carbon Steel by Tenacibaculum mesophilum D-6 and Bacillus sp. Y-6. Metals 2023, 13, 649. [CrossRef]
- Lyon, S.B.; Bingham, R.;. Mills, D.J. Advances in corrosion protection by organic coatings: What we know and what we would like to know. Prog. Org. Coat. 2017, 102, 2. [CrossRef]
- Rangel-Olivares, F.R.; Arce-Estrada, E.M.; Cabrera-Sierra, R. Synthesis and characterization of polyaniline-based polymer nanocomposites as anti-corrosion coatings. Coatings 2021, 11, 653. [CrossRef]
- Ćirić-Marjanović, G. Recent advances in polyaniline research: Polymerization mechanisms, structural aspects, properties and applications. Synth. Met. 2013, 177, 1. [CrossRef]
- Ćirić-Marjanović, G. Recent advances in polyaniline composites with metals, metalloids and nonmetals. Synth. Met. 2013, 170, 31. [CrossRef]
- Fangjian Gao, Jie Mu, Zhenxiao Bi, Shun Wang, Zili Li, Recent advances of polyaniline composites in anticorrosive coatings: A review, Prog. Org. Coat. 2021, 151, 106071. [CrossRef]
- Hu, C.; Li, T.; Yin, H.; Hu, L.; Tang, J.; Ren, K. Preparation and corrosion protection of three different acids doped polyaniline/epoxy resin composite coatings on carbon steel. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 126069. [CrossRef]
- Zhang, Y.J.; Shao, Y.W.; Liu, X.L.; Shi, C.; Wang, Y.Q.; Meng, G.Z.; Zeng, X.G.; Yang, Y. A study on corrosion protection of different polyaniline coatings for mild steel. Prog. Org. Coat. 2017, 111, 240.
- Liu, S.; Liu, L.; Meng, F.; Li, Y.; Wang, F. Protective performance of polyaniline-sulfosalicylic acid/epoxy coating for 5083 aluminum. Materials 2018, 11, 292. [CrossRef]
- Zhang, Y.; Shao, Y.; Zhang, T.; Meng, G.; Wang, F. The effect of epoxy coating containing emeraldine base and hydrofluoric acid doped polyaniline on the corrosion protection of AZ91D magnesium alloy. Corros. Sci. 2011, 53, 3747–3755. [CrossRef]
- Baloch, A.; Kannan, M.B. Electropolymerisation of aniline on AZ91 magnesium alloy: The effect of coating electrolyte corrosiveness. Metals 2017, 7, 533. [CrossRef]
- Ma, Y.; Fan, B.; Liu, H.; Fan, G.; Hao, H.; Yang, B. Enhanced corrosion inhibition of aniline derivatives electropolymerized coatings on copper: Preparation, characterization and mechanism modeling. Appl. Surf. Sci. 2020, 514, 146086. [CrossRef]
- Xu, H.; Zhang, Y. A review on conducting polymers and nanopolymer composite coatings for steel corrosion protection. Coatings 2019, 9, 807. [CrossRef]
- Armelin, E.; Alemán, C.; Iribarren, J.I. Anti-corrosion performances of epoxy coatings modified with polyaniline: A comparison between the emeraldine base and salt forms. Prog. Org. Coat. 2009, 65, 88. http://dx.doi.org/10.1016%2Fj.porgcoat.2008.10.001.
- Zhang, Y.; Shao, Y.; Liu, X.; Shi, C.; Wang, Y.; Meng, G.; Zeng, X.; Yang, Y. A study on corrosion protection of different polyaniline coatings for mild steel. Prog. Org. Coat. 2017, 111, 240. [CrossRef]
- Kohl, M.; Kalendová, A. Effect of polyaniline salts on the mechanical and corrosion properties of organic protective coatings. Prog. Org. Coat. 2015, 86, 96. [CrossRef]
- Liu, T.; Wei, J.; Ma, L.; Liu, S.; Zhang, D.; Zhao, H. Effect of polyaniline-based plate on the anticorrosion performance of epoxy coating. Prog. Org. Coat. 2021, 151, 106109, . [CrossRef]
- Kumar, A. Role of conducting polymers in corrosion protection, N.a. J. Adv. Res. Rev. 2023, 17(02), 045. [CrossRef]
- Salem, A.J.; Grgur, B.N. The influence of the polyaniline initial oxidation states on the corrosion of steel with composite coatings. Prog. Org. Coat. 2018, 119, 138. [CrossRef]
- Stejskal, J.; Gilbert, R.G. Polyaniline: Preparation of a conducting polymer (IUPAC Technical Report). Pure Appl. Chem. 2002, 74(5) 857. [CrossRef]
- Stejskal, J.; Prokeš, J.; Trchová, M. Reprotonation of polyaniline: A route to various conducting polymer materials, React. Funct. Polym. 2008, 68, 1355. [CrossRef]
- ASTM, Designation: E 394 – 00, Standard test method for iron in trace quantities using the 1,10-phenanthroline method, 2000.
- https://imagej.nih.gov/ij/docs/guide/user-guide.pdf.
- Jin, E.; Liu, N.; Lu, X. ; Zhang, W. Novel micro/nanostructures of polyaniline in the presence of different amino acids via a self-assembly process, Chem. Lett., 2007, 36, 1288. [CrossRef]
- Albuquerque de, J.E.;. Mattoso, L.H.C.; Faria, R.M.; Masters, J.G.; MacDiarmid, A.G. Study of the interconversion of polyaniline oxidation states by optical absorption spectroscopy. Synth. Met. 2004, 146, 1. [CrossRef]
- Sk, M.M.; Yue, C.Y. Synthesis of polyaniline nanotubes using the self-assembly behavior of vitamin C: a mechanistic study and application in electrochemical supercapacitors, J. Mater. Chem. A 2014, 2, 2830. [CrossRef]
- Han, Y-G.; Kusunose, T.; Sekino T. One-step reverse micelle polymerization of organic dispersible polyaniline nanoparticles, Synth. Met. 2009, 159, 123. [CrossRef]
- Wang, X.; Li, Y.; Zhao, Y.; Liu, J.; Tang, S.; Feng W. Synthesis of PANI nanostructures with various morphologies from fibers to micromats to disks doped with salicylic acid. Synth. Met. 2010, 160, 2008. [CrossRef]
- Prevost, V.; Petit, A.; Pla, F. Studies on chemical oxidative copolymerization of aniline and o-alkoxysulfonated anilines: I. Synthesis and characterization of novel self-doped polyanilines. Synth. Met. 1999, 104, 79. [CrossRef]
- Xia, H.; Wang Q. Synthesis and characterization of conductive polyaniline nanoparticles through ultrasonic assisted inverse microemulsion polymerization, J. Nanopart. Res. 2001, 3, 401. http://dx.doi.org/10.1023/A:1012564814745.
- Bertuoli, P.T.; Baldissera, A.F.; Zattera, A.J.; Ferreira, C.A.; Alemán, C.; Armelin, E. Polyaniline coated core-shell polyacrylates: Control of film formation and coating application for corrosion protection. Prog. Org. Coat. 2019, 128, 40. [CrossRef]
- Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, properties, and applications of polyaniline and polyaniline thin films—A Review. Polymers 2021, 13, 2003. [CrossRef]
- Rabl, H.; Wielend, D.; Tekoglu, S.; Seelajaroen, H.; Neugebauer, H.; Heitzmann, N.; Apaydin, DH.;, Scharber, MC.; Sariciftci, NS. Are polyaniline and polypyrrole electrocatalysts for oxygen (O2) reduction to hydrogen peroxide (H2O2)? ACS Appl Energy Mater. 2020, 3(11),:10611. [CrossRef]
- Grgur, B.N. Metal | polypyrrole battery with the air regenerated positive electrode. J. Power Sources, 2014, 272, 1053. [CrossRef]
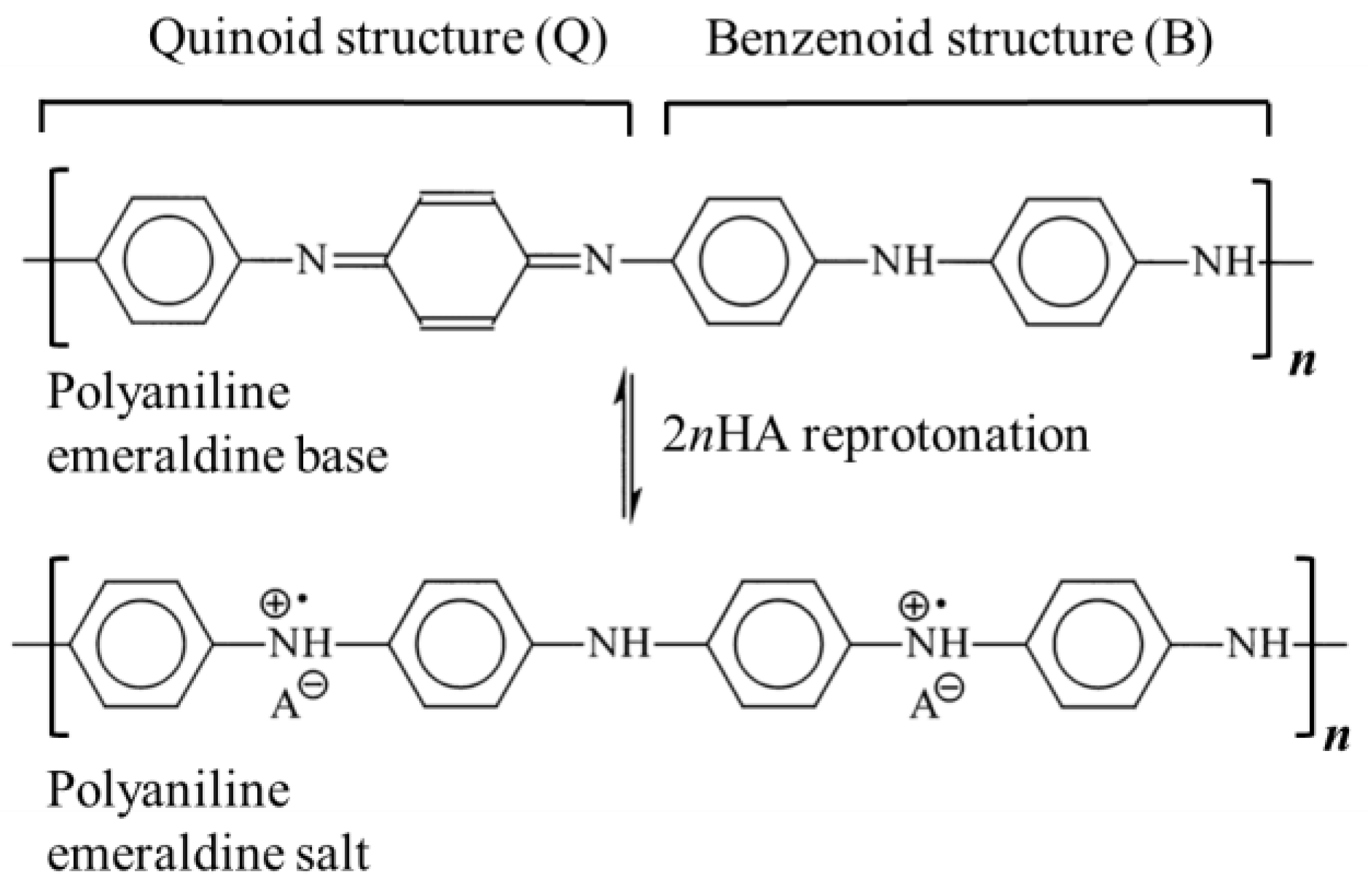
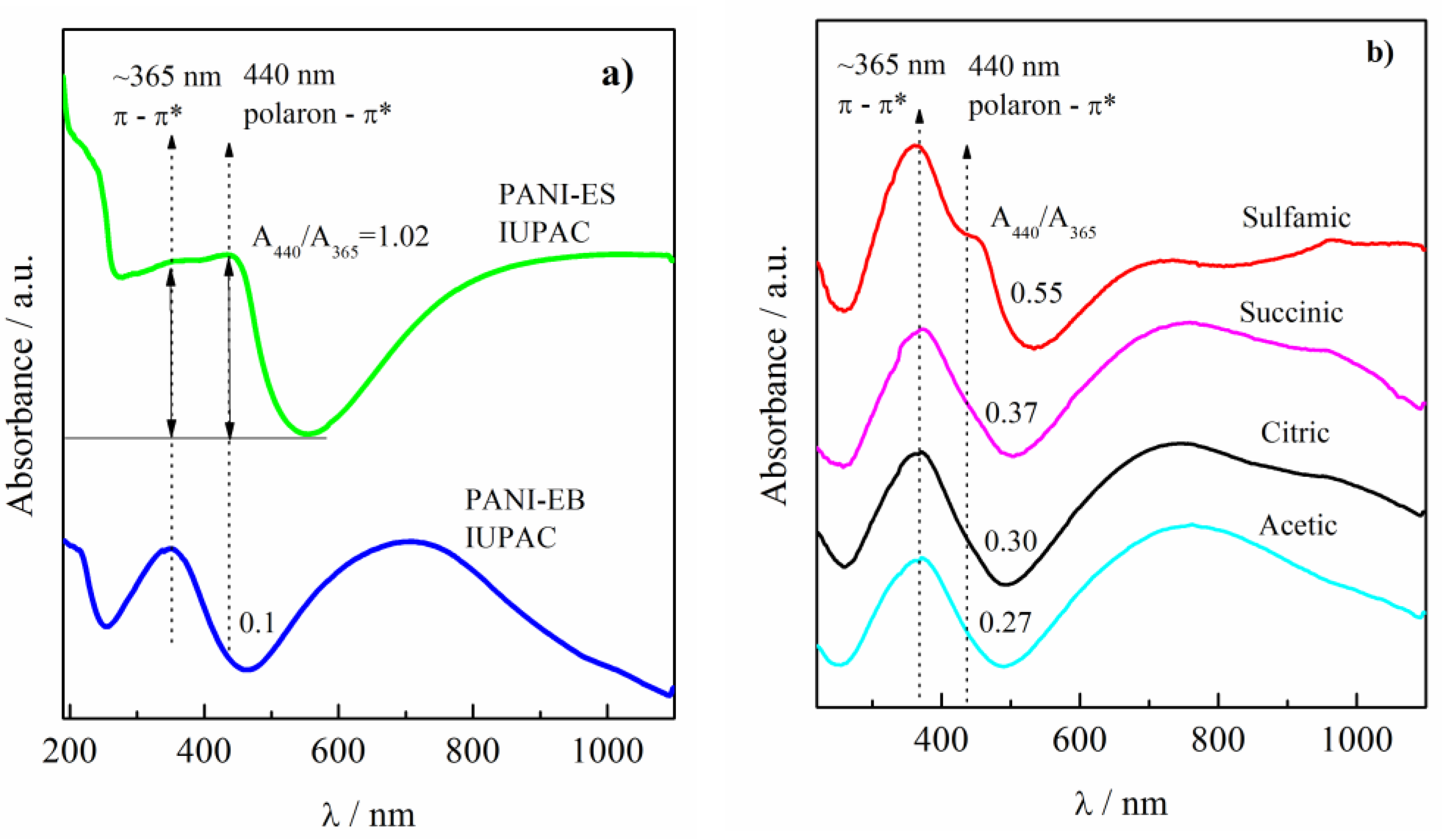
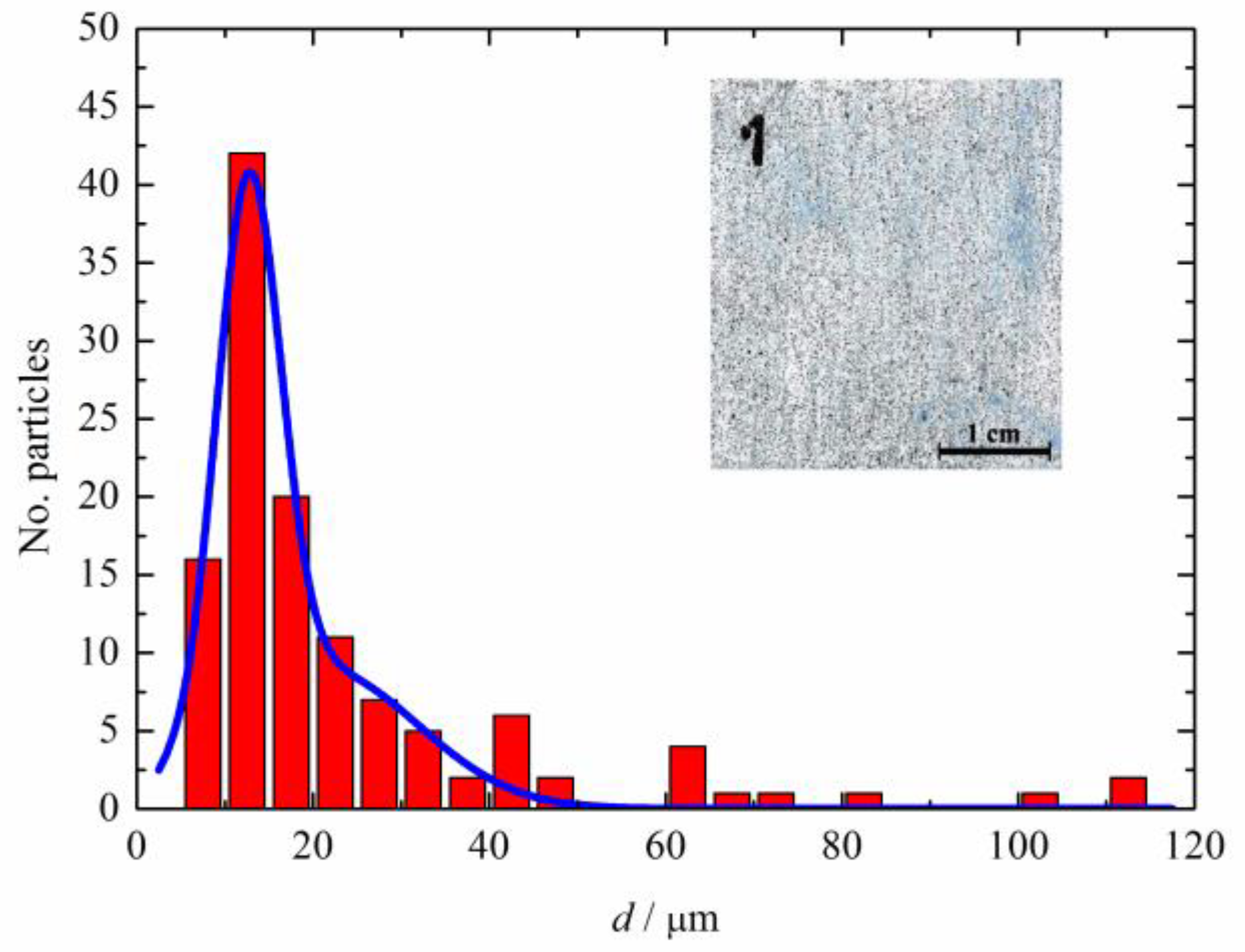
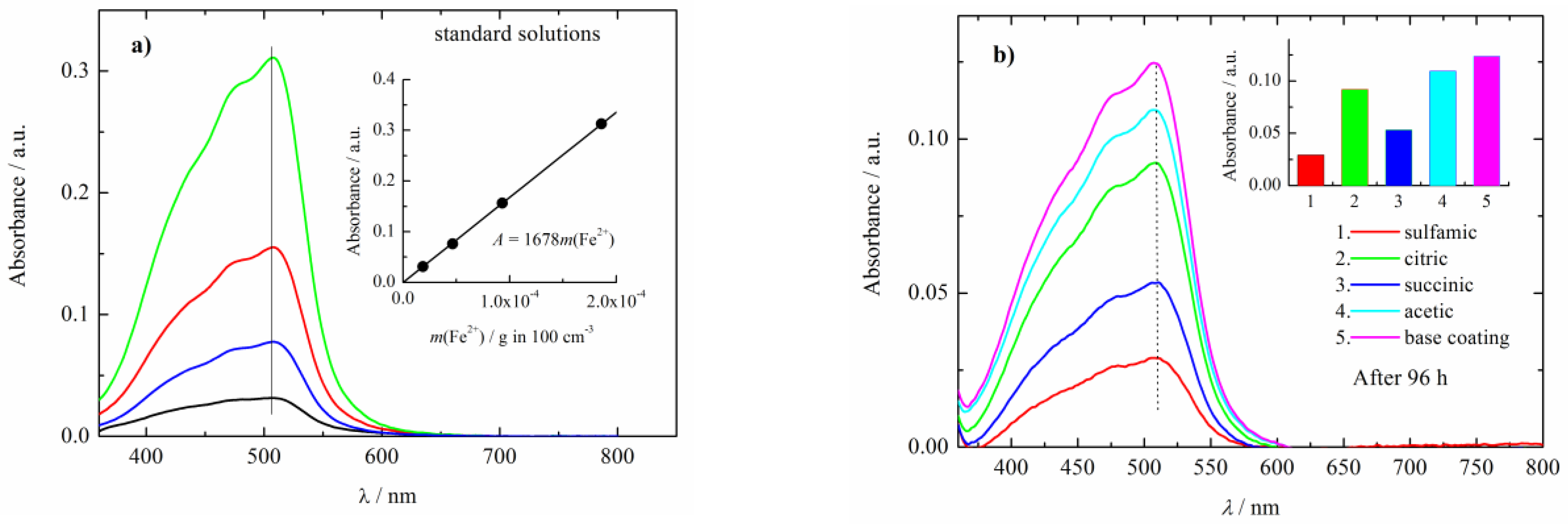
| Acid | Structure |
Mw g mol-1 |
pKa |
cAC mol dm-3 |
pH | σ / S cm-1 [22] |
|---|---|---|---|---|---|---|
|
Acetic |
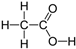 |
60.1 | 4.76 | 1 | 2.6 | 7.1×10-9 |
|
Succinic |
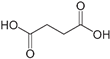 |
118.1 | pKa1= 4.2pKa2= 5.6 | 0.87 (sat) | 2.5 | 5.9×10-6 |
|
Citric |
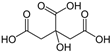 |
192.1 | pKa1= 3.13pKa2= 4.76 | 1 | 1.51 | 4.4×10-3 |
|
Sulfamic |
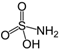 |
97.1 | 1.0 | 1 | 0.6 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





