1. Introduction
Surgical myocardial revascularization is a surgical procedure with a mortality of about 1% in elective cases. A condition for the controlled performance of this surgical procedure is the application of an extracorporeal blood flow device (ECB), which during the intervention ensures tissue oxygenation and thermoregulation, replacing cardiac action and pulmonary circulation. The greatest danger in its performance lies in ischemia-reperfusion injuries of the myocardium after revascularization with the development of hemorrhagic infarction with all its complications [
1]. Revascularization of the myocardium under conditions of extracorporeal circulation has also opened up problems related to the effects of the intervention itself on the immune system and inflammatory response [
2]. Due to its importance on the morbidity and mortality of patients, the appearance of a generalized, systemic inflammatory response (Engl. Systemic Inflammatory Response Syndrome, SIRS).
The strength of the inflammatory response depends on several factors: biomaterials used, oxygenator components, patient-related factors (age, sex, comorbidities) and surgical factors in terms of length of operation and surgical technique. In addition, the effects of anesthetic agents, techniques of perioperative myocardial protection and the use of pharmacological cardiosurgical supports can collectively modify the immune response and affect its characteristics [
3].
Basically, the onset of the inflammatory response is considered to be a consequence of blood exposure to artificial materials as well as sheer stress during the passage of blood through an extracorporeal circulation machine. Then there is a disturbance of coagulation, activation of leukocytes and the complement system, as well as the release of inflammatory and vasoactive substances into the bloodstream [
4].
Various authors have shown that cytokines have a crucial pathogenetic importance in the initiation and later maintenance of SIRS and sepsis, and their determination would also enable early diagnosis of these disorders. However, it is insufficiently known how the use of the extracorporeal blood flow system affects the level of cytokines of innate (IL-1β; IL-6 and TNF-α) and acquired immunity in terms of the polarization of the immune response in the direction of Th1 (IL-12p70 and IFN-γ), Th2 (IL-4), Th17 (IL-17) and regulatory T cells (IL-10) and how these changes are reflected in the postoperative recovery of patients. Today, special attention is paid to IL-6, which has been shown to increase the concentration of C-reactive protein (CRP). The soluble form of the CD14 molecule (presepsin) is also one of the more recently defined, early parameters of systemic inflammation. In the proposed study, by determining the level of the mentioned parameters in patients who undergo cardiac surgery with or without the use of extracorporeal blood flow systems as well as their postoperative monitoring, the systemic immune response would be looked at and parameters for the early identification of patients at risk of developing SIRS or sepsis would be determined. These findings would enable timely identification of high-risk patients in whom there is a possible need for additional therapeutic action.
The aim of this research was to determine whether the use of an extracorporeal blood flow system during myocardial surgical revascularization leads to an increased systemic inflammatory response of the body, which contributes to the development of postoperative complications. Analysis of the systemic cytokine response in these patients would indicate the influence of extracorporeal blood flow on the polarization of the immune response in the direction mediated by Th1 or Th2 cells.
2. Materials and Methods
This prospective study included 100 patients who underwent single, double and triple surgical revascularization of the myocardium, at the Clinic for Cardiac Surgery of CC Niš, in the period from January 15., 2020. until January 15., 2021. 22 female persons—22.0% and 78 male persons—78.0%) were included. The average age of the studied population was 64.63 ± 7.48 years (from 43 to 80 years).
After preoperative preparation, patients were operated according to standard cardiac surgical protocols. Patients were divided into 2 groups, group A (EKK group) in which a device for extracorporeal blood flow was used during the intervention (52 subjects) and group B (Off pump group), in which this system was not applied intraoperatively (48 patients).
Preoperatively, as well as 8 hours after the surgical intervention, the following parameters were determined for the subjects:
1. blood count (erythrocyte count—Rbc, hemoglobin—Hgb, hematocrit—Hct, leukocyte count—Wbc, platelet count—Plt) and CRP on the hematological analyzer Microsemi CRP LC-667G (Horiba Medical, France), from 4 mL of whole blood sampled in a tube with EDTA anticoagulant.
2. soluble CD14 molecule (sCD 14)—presepsin, by the immunofluorescence method, using a cartridge on the Pathfast device (Mitsubishi, Japan) from 4 mL of the patient’s whole blood sampled in a test tube with EDTA anticoagulant.
Presepsin values above 600 pg/mL were considered a state of presepsis, and this is also the cut-off value for distinguishing systemic inflammatory response syndrome (SIRS) from a septic state.
3. Levels of the following cytokines: TNFα, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12 p70 and IFN γ, by ELISA technique from serum obtained by centrifugation of a tube with 4 ml of sampled blood without anticoagulants.
The Magnetic Luminex® Performance assay was used to determine cytokines. Standard cytokine cocktail detectors, produced by Biotechne RnD systems, were used, and the reading was performed on a FlexMap3D-invitrogen flow analyzer, Thermo Fisher scientific, according to the manufacturer’s instructions.
Early postoperative complications in terms of SIRS, sepsis and septic shock were monitored. According to the American Society of Anesthesiologists, the diagnosis of SIRS was made if two or more parameters were present: temperature ≥ 38 °C or ≤36 °C, tachycardia ≥ 90/min, tachypnea ≥ 20/min or pCO2 ≤ 32 mmHg, leukocytes ≥ 12,000 or ≤4000/mm3. Sepsis was defined as SIRS with proven infection; septic shock as sepsis with hypotension and marked organ hypoperfusion.
Statistical data Processing
The data are presented in the form of arithmetic mean and standard deviation, minimum and maximum values, as well as in the form of absolute and relative numbers.
The normality of continuous variables was tested with the Kolomogor-Smirnov test. If the data distribution was normal, the preoperative and postoperative values were compared using the ANOVA test. If the data distribution is not normal, the Friedman test was used for this comparison. If the data distribution is normal, the comparison between the two groups was performed using the t test, if the data distribution is not normal, this comparison was performed using the Mann-Whitney test. The hypothesis was tested with a significance threshold of p < 0.05. Data analysis was performed in the IBM SPSS 26.0.0 software package.
3. Results
3.1. Type of Surgical Revascularisation and Basic Parameters
The prospective study included 100 patients who underwent single, double and triple surgical revascularization of the myocardium, at the Cardiac Surgery Clinic of the CC Niš, in the period from January 15., 2020. until January 15., 2021.
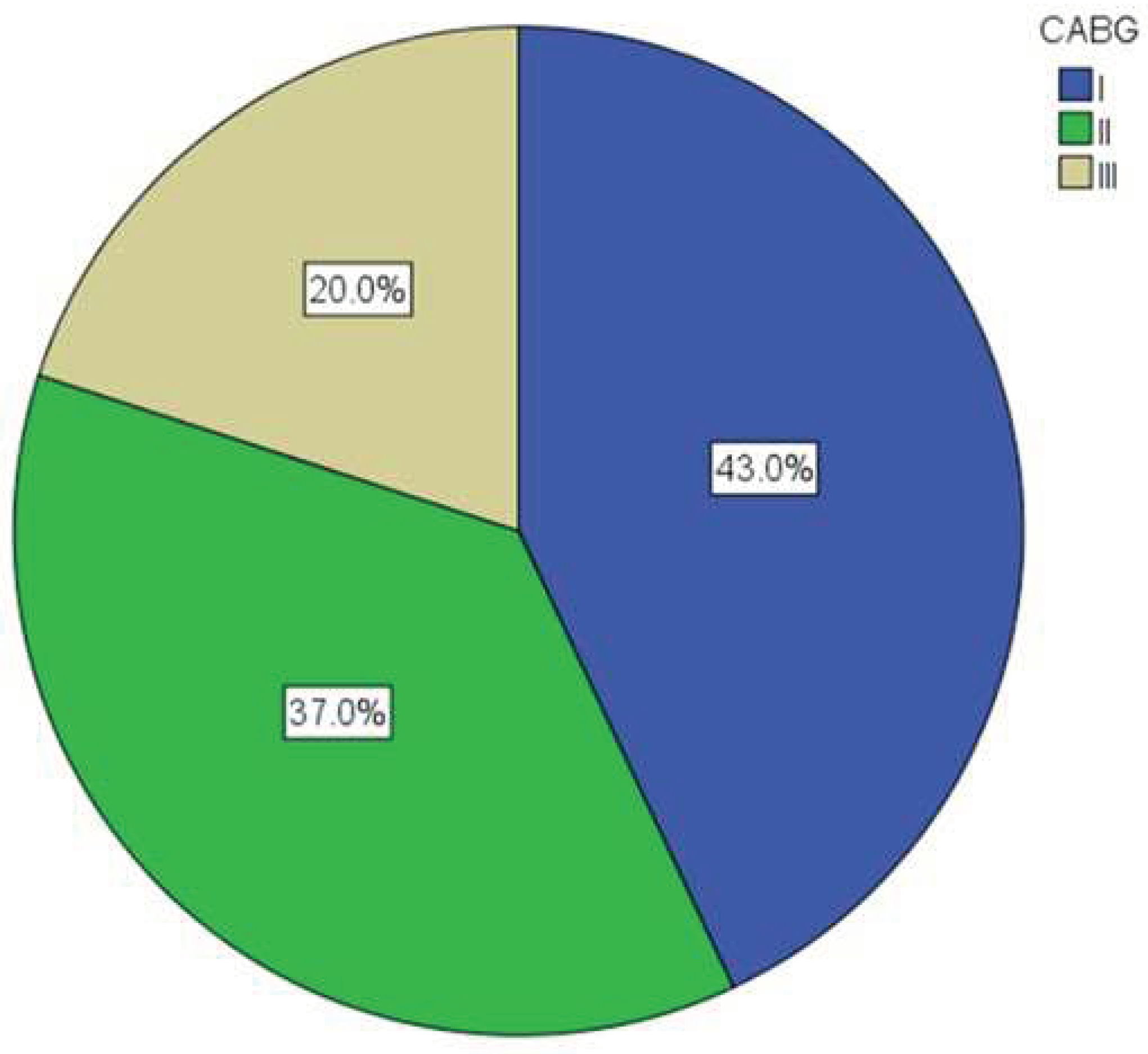
Figure 1 shows the representation of certain types of surgical revascularization—single, double or triple aorto-coronary bypass (CABG I, II or III). The largest number of patients (43.0%) underwent CABG I.
Patients were divided into two groups in relation to the use of devices for extracorporeal blood flow. This method was used in 51 patients (51.0%).
Table 1 shows the intergroup differences in the preoperative parameters of the patients.
The only difference found between the two groups was in the type of surgical intervention—a device for extracorporeal blood flow was used more often in patients underwent multiple aorto-coronary bypass (p < 0.001). All subjects had normal preoperative presepsin values (≤ 600 pg/mL).
Table 1.
Basic characteristics and preoperative parameters in patients with and without the use of an extracorporeal blood flow device (ECB).
Table 1.
Basic characteristics and preoperative parameters in patients with and without the use of an extracorporeal blood flow device (ECB).
| |
ECB (N = 51) |
off ECB (N = 49) |
t * ili χ2 ** ili Z *** (p) |
| Age |
63.90 ± 7.49 |
65.39 ± 7.48 |
0.992 (0.323) * |
| Gender (male) |
39 (76.5%) |
39 (79.6%) |
0.018 (0.811) ** |
| CABG |
I |
9 (17.6%) |
34 (69.4%) |
35.834 (0.000) **
5.916 (0.000) ***
|
| II |
22 (43.1%) |
15 (30.6%) |
| III |
20 (39.2%) |
0 (0.0%) |
| RBC (1 × 1012/L) |
4.45 ± 0.46 |
4.49 ± 0.49 |
0.376 (0.707) * |
| HGB (g/L) |
136.80 ± 11.68 |
138.80 ± 10.25 |
0.905 (0.368)* |
| HCT (%) |
38.78 ± 4.48 |
39.58 ± 3.80 |
0.965 (0.337) * |
| WBC (1 × 109/L) |
7.34 ± 1.51 |
6.95 ± 1.13 |
1.431 (0.156) * |
| PLT (1 × 109/L) |
241.72 ± 70.05 |
232.51 ± 56.70 |
0.721 (0.472) * |
| CRP (ng/mL) |
9.0 (4.0–14.0) |
10.0 (5.0–13.0) |
0.252 (0.801) *** |
| PSEP (pg/mL) |
246.0 (167.0–366.0) |
235.0 (166.0–337.0) |
0.538 (0.591) *** |
| PSEP (≥600 pg/mL) |
0 (0.0%) |
0 (0.0%) |
|
Eight hours after the surgical intervention, hematological parameters and the concentration of CRP and presepsin were measured again. Postoperatively, there was a decrease in the number of erythrocytes (p < 0.001), hemoglobin (p < 0.001), the number of platelets (p < 0.001), and an increase in the number of leukocytes (p < 0.001), an increase in the concentration of CRP (p < 0.001) and presepsin (p < 0.001). An identical change was found in the group of patients with ECB, with a statistical significance of p < 0.001 for all measured variables.
3.2. Influence of Extracorporeal Circuit on Basic Postoperative Parameters
Patients in whom the extracorporeal circulation method was applied had, postoperatively (
Table 2), a higher concentration of CRP (p < 0.001), as well as a concentration of presepsin (p < 0.05)—a greater number of patients had a concentration of presepsin above the limit of 600 pg/ mL (p < 0.01).
In patients in whom ECB was applied, postoperatively (
Table 3) there was a smaller absolute decrease in hemoglobin value (p < 0.05). In addition, a greater increase in the absolute concentrations of CRP (p < 0.001) and presepsin (p < 0.001) was found. However, analyzing the relative changes in CRP levels does not show a difference between patients on ECB compared to patients without (p = 0.137). On the other hand, a greater relative increase in the concentration of presepsin (p < 0.01) was found in patients on ECB in the postoperative period.
Four patients out of 51 (7.84%) in the ECB group had elevated presepsin values over 1000 pg/mL and high risk according to clinical criteria for the development of systemic bacterial infection and sepsis. No such patients were recorded in the off pump group. Also, in the group where the extracorporeal blood flow system was applied, 17.64% of patients (9/51) met the clinical criteria for SIRS with elevated presepsin values over 600 pg/mL, while in the other group of patients, 2 out of 49 (4.08%) met the clinical criteria and laboratory criteria for SIRS.
Table 3.
Absolute and relative differences between postoperative and preoperative parameters in patients with and without the use of extracorporeal blood flow devices.
Table 3.
Absolute and relative differences between postoperative and preoperative parameters in patients with and without the use of extracorporeal blood flow devices.
| |
ECB (N = 51) |
off ECB (N = 49) |
t * ili Z ** (p) |
| Absolute differencies |
|
|
|
| WBC (1 × 109/L) |
3.8 (0.1–6.2) |
2.2 (−0.2–5.2) |
1.393 (0.164) ** |
| CRP (ng/mL) |
100.0 (89.0–119.0) |
79.0 (68.0–98.0) |
4.072 (0.000) ** |
| PSEP (pg/mL) |
200.0 (104.0–377.0) |
109.0 (59.0–189.5) |
3.427 (0.001) ** |
| Relative changes |
|
|
|
| WBC (1 × 109/L) |
1.47 ± 0.45 |
1.37 ± 0.53 |
1.051 (0.296) * |
| CRP (ng/mL) |
11.6(9.1–23.8) |
9.9 (5.6–18.3) |
1.486 (0.137) ** |
| PSEP (pg/mL) |
1.9 (1.5–2.5) |
1.5 (1.2–2.0) |
2.748 (0.006) ** |
3.3. Cytokines Response
Except for TNFa, the levels of IL-1β, IL-2, IL-6 and IL-10 showed an increase after the intervention in all groups compared to the preoperative period (p < 0.01) (
Table 4). However, comparing the degree of cytokine increase in the postoperative period between the control (off pump) and the extracorporeal blood flow group did not show a statistical difference (IL-2; p = 0.881, IL-1; p = 0.709, IL-6; p = 0.911, IL-10, p = 0.179).
It should be emphasized that in both groups of patients the levels of IL-4, IL-12, p70 and IFNγ were determined both before revascularization and 8 hours after the intervention. It was observed that no changes were recorded.
4. Discussion
Cardiosurgical intervention is mutilating in itself, regardless of whether it is performed under ECB conditions or without a pump. Access to the heart through a medial sternotomy, using vibration testers and electrocautery, causes the release of tissue fragments into the circulation and the expression of damage-associated molecular patterns (DAMPs). These molecules act on multiple receptors from the PRR family and initiate an intracellular signaling pathway that ultimately leads to the activation of the transcription factor NF-κB and p38 with the subsequent initiation of an inflammatory response [
5].
An early increase in reactants of the acute phase can also occur due to ischemia—that is, due to reperfusion injury of the brain, heart, lungs, kidneys and liver, due to cross-clamping of the aorta. Gut perfusion may be reduced even when whole-body perfusion indices are normal due to the consequent release of vasopressin and catecholamines that reduce flow through the mesenteric arteries [
6]. The use of ECB potentiates the hypoperfusion effect on the mucosa of the gastrointestinal tract, with a consequent increase in intestinal permeability and facilitated penetration of bacterial endotoxins. In this way, additional conditions are created for the occurrence of SIRS and damage to other organs. After the use of ECB, 2–4% of patients undergo gastrointestinal surgery, due to bleeding, peritonitis or intestinal obstruction, with a mortality rate of up to 30% [
7].
It can be assumed that the early inflammatory response, present after cardiac surgery, is a consequence of the cumulative action of the above-mentioned factors on the immune system. Therefore, the findings of elevated values of presepsin, CRP and leukocytes after cardiac surgery are not surprising, with a proposal for their use as independent predictors of SIRS in cardiac surgery [
8]. In this sense, for example, presepsin values of 600–700 pg/mL have been defined as clinical significant for the development of infectious complications after surgery [
9]. In our study, the postoperative increase in the level of CRP, prespsin and leukocytes after revascularization, recorded in all investigated groups, fully correlates with the results of other researchers.
Proinflammatory cytokines play a key role in stimulating the inflammatory process, with the concentration of specific cytokines in plasma, such as IL-1β and IL-6, predicting the outcome in certain critical groups of patients. Tumor necrosis factor α (TNF-α) and IL-1β are elevated early after cardiac surgery, and IL-6 and IL-8 peak later. Although a direct cause-and-effect relationship has not been proven, the elevation of pro-inflammatory cytokines is strongly associated with an unfavorable outcome after cardiac surgery. Patients who develop SIRS show a significant elevation of cytokine concentrations compared to patients with an uncomplicated course after cardiac surgery [
10].
In our study, convincing findings of an increase in IL-1, IL-2 and IL-6 were obtained postoperatively in all groups, but the differences between the control -off pump group and patients with extracorporeal blood flow are not convincing. TNFα levels did not differ between groups.
Variable detection of certain cytokines after coronary artery bypass graft surgery (CABG) is not a rare finding in the literature. Conflicting results also refer to the time course of detection of maximal cytokine levels. There are numerous reasons for these differences: sampling time, assay sensitivity, circadian variation in cytokine release, short half-life of TNF-α and IL-1β, all of which may affect the measurement of desired parameters during CABG surgery [
11,
12].
In their research, Diegeler et al. indicated that cardiopulmonary bypass (CPB) causes systemic release of inflammatory cytokines. This study compares the humoral immune response in patients undergoing CABG with standard, minimally invasive and “off-pump” techniques [
13]. In support of the increased systemic inflammation, the significantly increased release of the breakdown products of complement activation C5a and C3d as well as IL-8 speak. This trend was most pronounced in patients who underwent EKK during the initial period and in the short period of time after perfusion. Also, TNF-α receptors p55 and p75 were increased and at the same time prolonged expression (up to 48 hours) in the CPB group compared to the off-pump group. Interestingly, in this study, IL-6 did not show a different release among the three surgical groups over the entire period. There was no significant difference in any parameter measured in relation to the type of operative approach.
An interesting finding in our study is the postoperative increase in IL-10 levels in all analyzed groups. The finding of increased production of IL-10 after cardiac surgery has been described in the literature and may represent a compensatory mechanism for suppressing excessive activity of the pro-inflammatory cytokines IL-6 and IL-8 [
14,
15,
16]. IL-10 is a strong inhibitor of the production of other cytokines such as TNF-α, IL-1β, which is why it is assumed that the clinical outcome after cardiopulmonary bypass depends on the balance between pro- and anti-inflammatory cytokines. It is believed that prior administration of steroids and use of aprotinin can significantly improve IL-10 production in hepatocytes in patients undergoing cardiopulmonary bypass [
17].
Finally, the authors of this research believe that the absence of changes in IL-4, IL-12p70 and IFNγ levels between groups and different time intervals may be due to the study design. It is likely that the blood sampling interval of 8 hours after reperfusion surgery is too short for the polarization, development of Th1 and Th2 lymphocytes and the secretion of detectable concentrations of their cytokines, specifically IL-12p70, IFN-γ and IL-4.
Contradictions in the findings of cytokine levels in our work and in the literature indicate that there are still no definitive answers and point to the need for new studies with a larger number of patients.
5. Conclusions
Based on the obtained results, the following conclusions were drawn:
1. After surgical revascularization of the myocardium, a greater relative increase in the concentration of presepsin, the number of leukocytes and CRP was found in the ECB group of patients compared to the control group, which proves the significant influence of the ECB system on the immune status of patients.
2. Postoperative increase in the levels of IL-1, IL-2, IL-6 and IL-10 was noted in all patients in all groups, but the level of increase between the ECB group and the control had no statistical significance.
3. After 8 hours of the intervention, there were no detectable levels of cytokines involved in the response mediated by Th2 cells (IL-4) nor cytokines secreted by Th1 cells (IFNγ and IL-12 p70), which is why the influence of extracorporeal blood flow on the polarization of the adaptive immune response must be evaluated by determining the cytokine level in later time intervals.
4. In the postoperative period, a higher incidence rate of SIRS was noted in patients in whom the ECB system was used, which also indicates its importance in the development of systemic inflammation and immune dysregulation.
Author Contributions
Conceptualization, Dragan Milic and Milan Lazarevic.; methodology, Isidora Milić.; software, Pavle Marjanovic.; validation, Vesna Marjanovic; formal analysis, Sasa Zivic; investigation, Zorica Lazarevic; resources, Mlađan Golubovic.; data curation, Velimir Peric; writing—original draft preparation, Aleksandar Kamenov and Vladimir Stojiljkovic.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Ethics Committee of Clinical Center Nis Serbia 5257/20 06.01.2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wijns, W.; Kolh, P.; Danchin, N.; et al. Guidelines on myocardialrevascularization: The Task Force on Myocardial Revascularization of the European Society ofCardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2010, 31, 2501–2555. [Google Scholar] [PubMed]
- Welsby, I.J.; Bennett-Guerrero, E.; Atwell, D.; et al. The association of complication type with mortality and prolonged stay after cardiac surgery with cardiopulmonary bypass. Anesth Analg 2002, 94, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Fitch, J.C.; Rollins, S.; Matis, L.; Alford, B.; Aranki, S.; Collard, C.D.; Dewar, M.; Elefteriades, J.; Hines, R.; Kopf, G.; Kraker, P.; Li, L.; O’Hara, R.; Rinder, C.; Rinder, H.; Shaw, R.; Smith, B.; Stahl, G.; Shernan, S.K. Pharmacology and biological efficacy of a recombinant, humanized, single-chain antibody C5 complement inhibitor in patients undergoing coronary artery bypass graft surgery with cardiopulmonary bypass. Circulation 1999, 100, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Day, J.R.; Taylor, K.M. The systemic inflammatory response syndrome and cardiopulmonary bypass. Int J Surg 2005, 3, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.Y.; Rainer, T.H.; Chan, L.Y.; et al. Time course of early and late changes in plasma DNA in trauma patients. Clin Chem 2003, 49, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Ohri, S.K.; Bowles, C.W.; Mathie, R.T.; et al. Effect of cardiopulmonary bypass perfusion protocols on gut tissue oxygenation and blood flow. Ann Thorac Surg 1997, 64, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Byhahn, C.; Strouhal, U.; Martens, S.; et al. Incidence of gastrointestinal complications in cardiopulmonary bypass patients. World J Surg 2001, 25, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Junichi, S.; Eiji, H.; Akio, M.; Tomoyuki, K.; Hidetomo, N.; Kazuyoshi, H. Pilot Study of Changes in Presepsin Concentrations Compared With Changes in Procalcitonin and C-Reactive Protein Concentrations After Cardiovascular Surgery. J Cardiothorac Vasc Anesth 2017, 31, 1262–1267. [Google Scholar]
- Popov, D.; Plyushch, M.; Ovseenko, S.; Abramyan, M.; Podshchekoldina, O.; Yaroustovsky, M. Prognostic value of sCD14-ST (presepsin) in cardiac surgery. Kardiochir Torakochirurgia Pol 2015, 12, 30–36. [Google Scholar] [PubMed]
- Sablotzki, A.; Mann, V.; Simm, A.; Czeslick, E. Changes in the cytokine network through escalating SIRS after heart surgery. Anasthesiol Intensivmed Notfallmed Schmerzther 2001, 36, 552–559. [Google Scholar] [CrossRef] [PubMed]
-
Clin Exp Immunol 1999, 118, 242–246. [CrossRef]
- Perioperative serum levels of tumour-necrosis-factor alpha (TNF-α), IL-1β, IL-6, IL-10 and soluble IL-2 receptor in patients undergoing cardiac surgery with cardiopulmonary bypass without and with correction for haemodilution. Roth-Isigkeit, A.; Borstel, T.V.; Seyfarth, M.; Schmucker, P..
- Diegeler, A.; Doll, N.; Rauch, T.; Haberer, D.; Walther, T.; Falk, V.; et al. Humoral immune response during coronary artery bypass grafting: a comparison of limited approach, “off-pump” technique, and conventional cardiopulmonary bypass. Circulation 2000, 102, III95–III100. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Miyamoto, H.; Suzuki, K.; Horikoshi, S.; Arai, T.; Kurosawa, H. Evidence of organ damage after cardiopulmonary bypass. The role of elastase and vasoactive mediators. J Thorac Cardiovasc Surg 1992, 104, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Kukielka, G.L.; Smith, C.W.; LaRosa, G.J.; et al. Interleukin8 gene induction in the myocardium after ischemia and reperfusion in vivo. J Clin Invest 1995, 95, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.C.; Liao, H.; Naka, Y.; et al. Ischemia-induced interleukin-8 release after human heart transplantation. A potential role for endothelial cells. Circulation 1995, 92, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; LeClerc, J.L.; Schmartz, D.; Barvais, L.; Huynh, C.H.; Devière, J.; DeSmet, J.M.; Vincent, J.L. Hepatic release of interleukin-10 during cardiopulmonary bypass in steroid-pretreated patients. Am Heart J 1997, 133, 335–339. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).