Submitted:
05 June 2023
Posted:
05 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
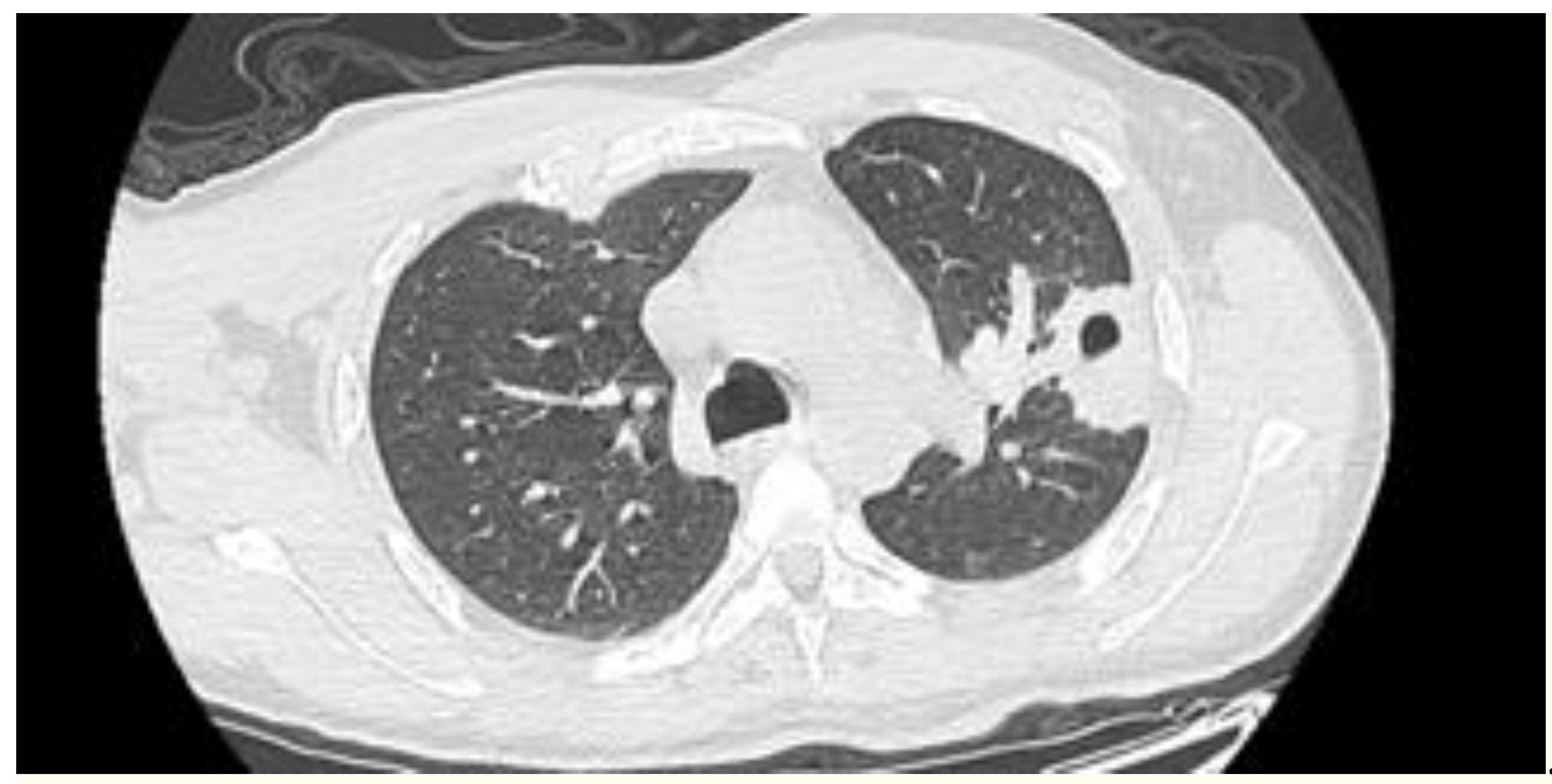
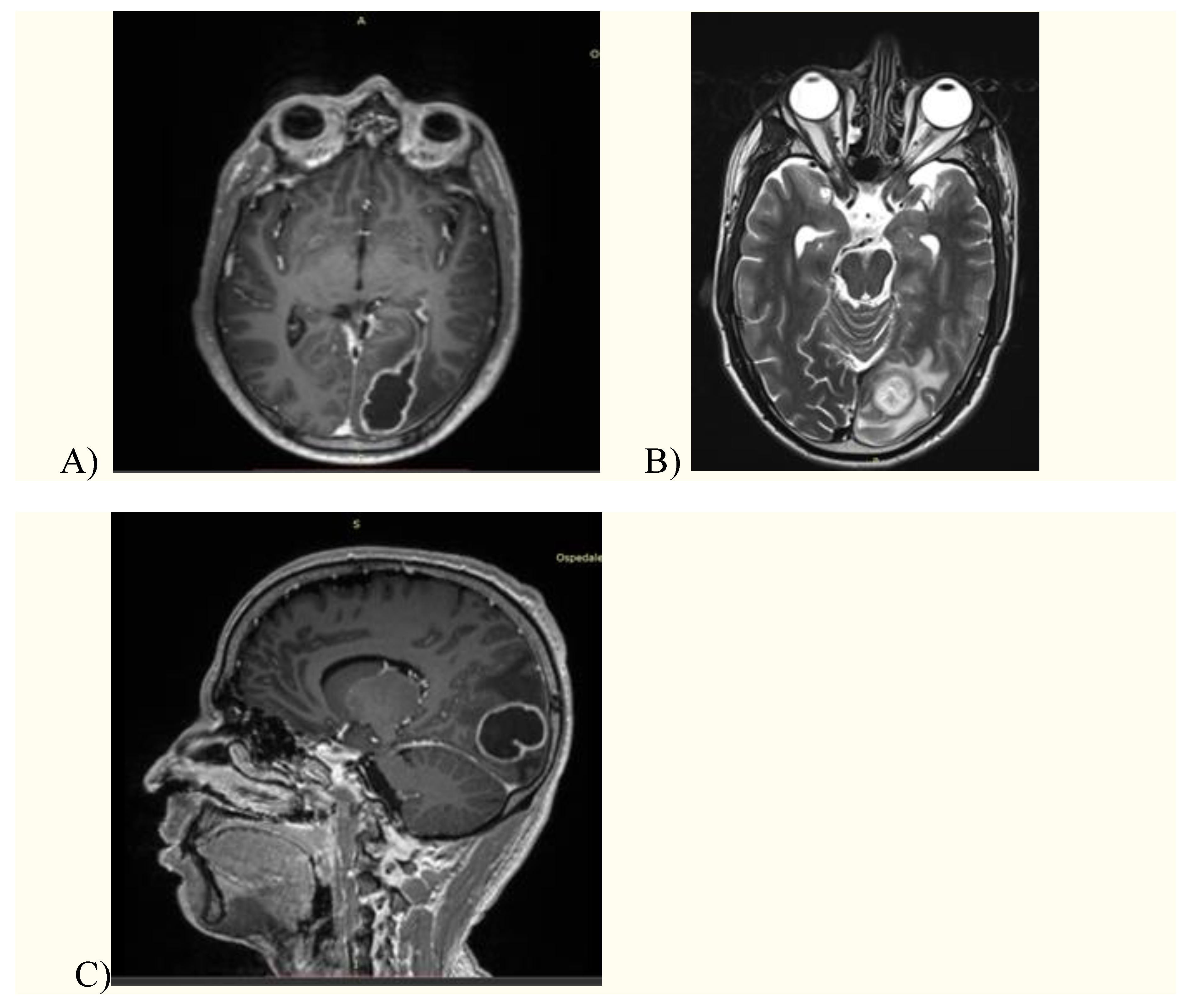
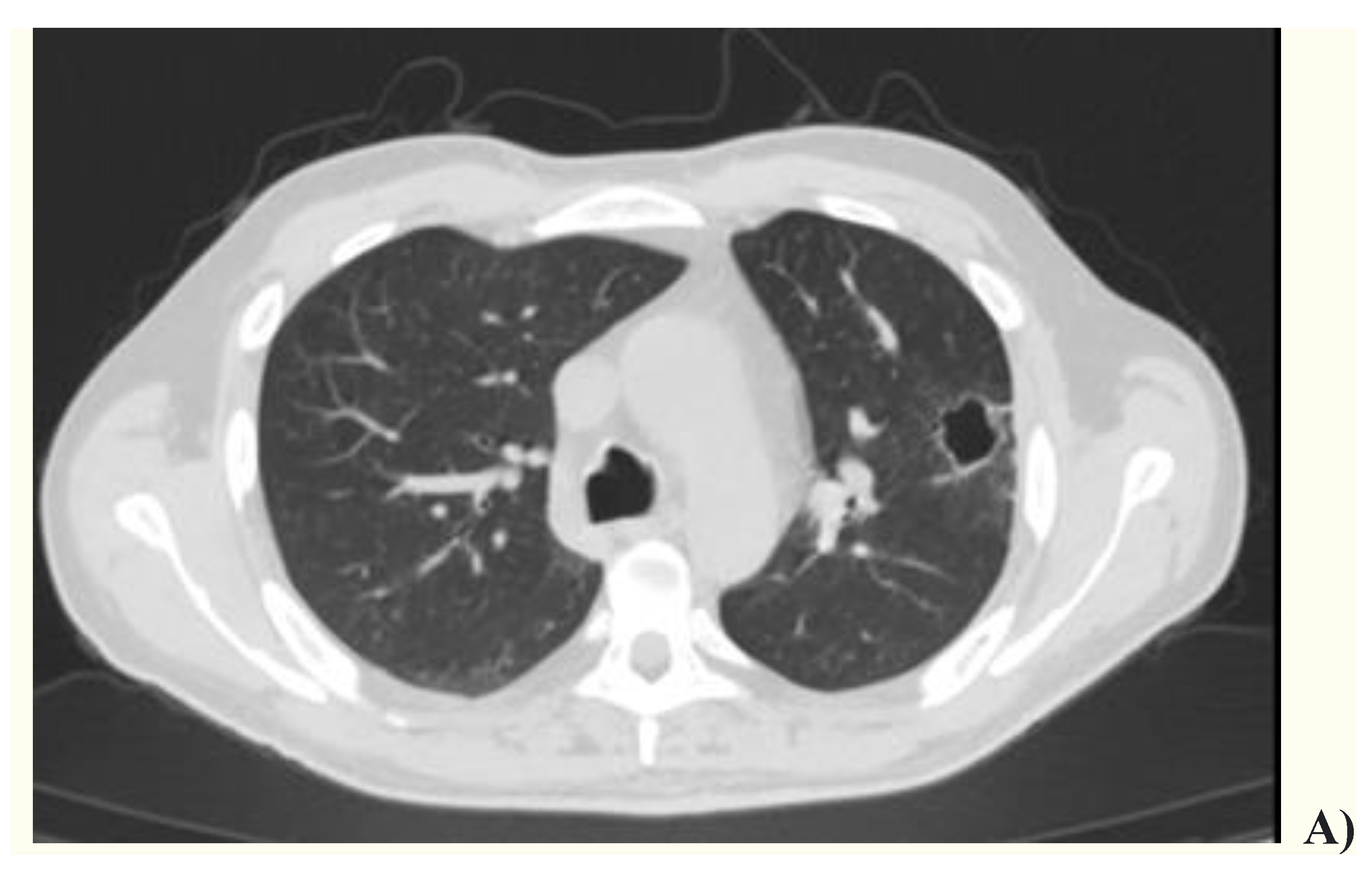
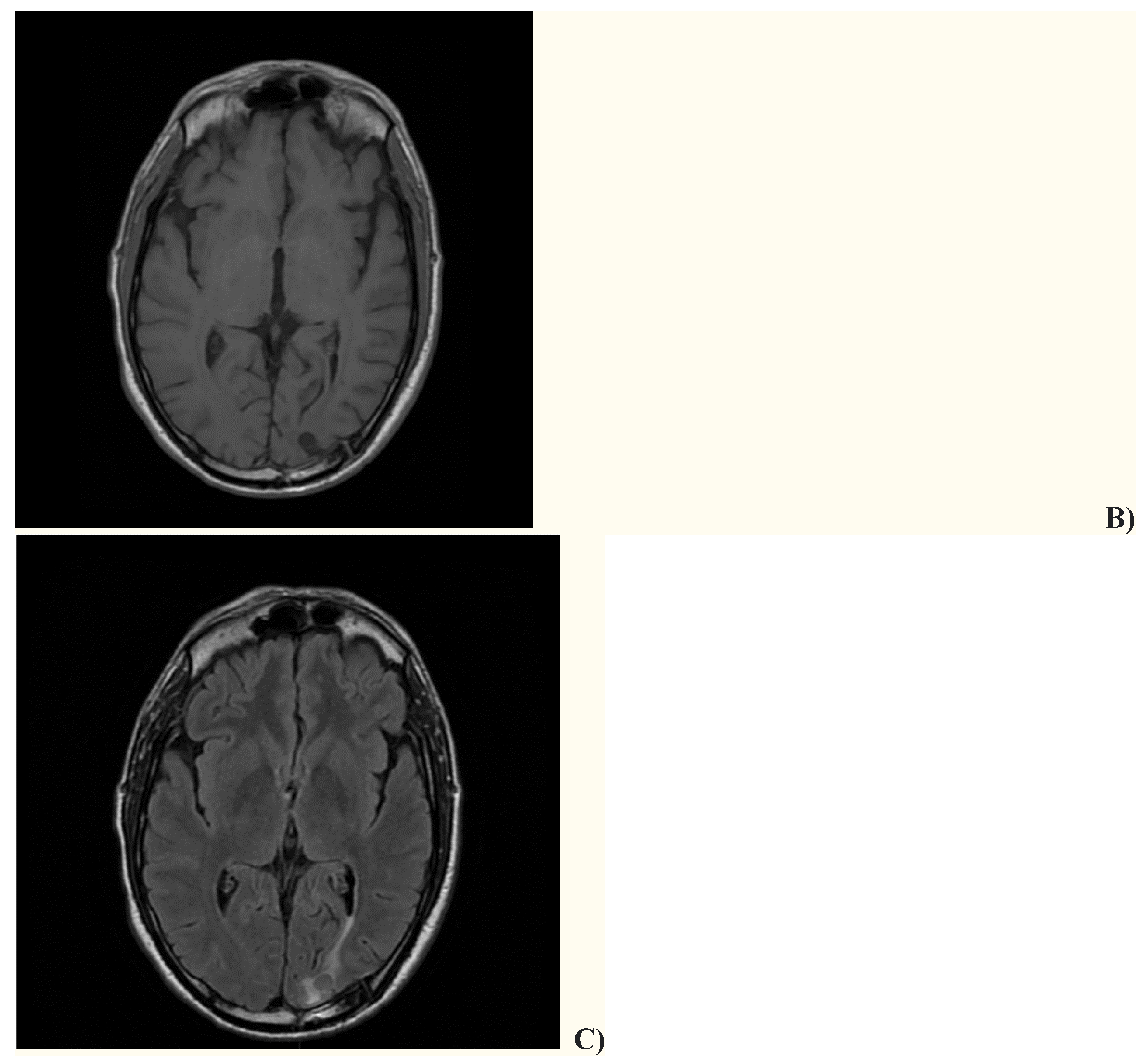
2. Case presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shinzato, T.; Saito, A. The Streptococcus milleri group as a cause of pulmonary infections. Clin. Infect. Dis. 1995, 21 Suppl 3, S238-43. [CrossRef]
- Noguchi, S.; Yatera, K.; Kawanami, T.; Yamasaki, K.; Naito, K.; Akata, K.; Shimabukuro, I.; Ishimoto, H.; Yoshii, C.; Mukae, H. The clinical features of respiratory infections caused by the Streptococcus anginosus group. BMC Pulm. Med. 2015, 15, 133. [CrossRef]
- Bueno, C.O.P.; Trillos, S.J.G.; Rosales, D.J.C.; García, E.A.B. Lung abscess due to Streptococcus intermedius associated with SARS CoV-2 infection in pregnancy: Unusual presentation and complication of a commensal bacteria during pregnancy. Clin. Case Rep. 2023, 11, e6763. [CrossRef]
- Erne, B.V.; Exner, C.; Frauenfelder, T.; Schmid, S.; Weder, W. A curious case of convulsion. Lancet 2010, 375, 2050. [CrossRef]
- Jerng, J.S.; Hsueh, P.R.; Teng, L.J.; Lee, L.N.; Yang, P.C.; Luh, K.T. Empyema thoracis and lung abscess caused by viridans streptococci. Am. J. Respir. Crit. Care Med. 1997, 156, 1508-14. [CrossRef]
- Chandy, B.; Todd, J.; Stucker, F.J.; Nathan, C.O. Pott's puffy tumor and epidural abscess arising from dental sepsis: a case report. Laryngoscope 2001, 111, 1732-4. [CrossRef]
- May, A.M.; Riede, F.N.; Riede, U.N. Acute subepicardial infarction associated with severe septic shock--insight in myocardial perfusion. Pathol Res Pract. 2010, 206, 401-4. [CrossRef]
- van Laren, M.; van Walree, N.C.; Kluytmans, J.A. Multiple lung abscesses secondary to a uterine empyema caused by an intrauterine device. Infection 2011, 39, 385-7. [CrossRef]
- de Kruif, M.D.; van Gorp, E.C.; Bel, E.H.; Gerlag, D.M.; Kunst, P.W. Streptococcal lung abscesses from a dental focus following tocilizumab: a case report. Clin. Exp. Rheumatol. 2012, 30, 951-3.
- Trabue, C.; Pearman, R.; Doering, T. Pyogenic brain and lung abscesses due to Streptococcus intermedius. J. Gen. Intern. Med. 2014, 29, 407. [CrossRef]
- Maeda, S.; Sado, T.; Sakurada, A.; Okada, Y.; Kondo, T. Successful closure of an open-window thoracostomy wound by negative-pressure wound therapy: report of a case. Surg. Today 2012, 42, 295-8. [CrossRef]
- Armendariz-Guezala, M.; Undabeitia-Huertas, J.; Samprón-Lebed, N.; Michan-Mendez, M.; Ruiz-Diaz, I.; Úrculo-Bareño, E. Absceso cerebral actinomicótico en paciente inmunocompetente [Actinomycotic brain abscess in immunocompetent patient]. Cir. Cir. 2017, 85 Suppl 1, 103-107.
- Sakurai, M.; Nagasawa, H.; Takeuchi, I.; Yanagawa, Y. A Case of an 80-Year-Old Man with Empyema and Psoas Abscess. Case Rep. Emerg. Med. 2020, 24, 2020:8895785. [CrossRef]
- Carrena, O.; Oluoha, O.; Wahba, A.; Eshun, D.; Endsley, M.; Okafor, H. Complicated Infective Endocarditis Limited to a Chiari Network. Case Rep. Cardiol. 2018, 26, 2018:3837825. [CrossRef]
- Fujihara, T.; Itoh, N.; Yamamoto, S.; Kurai, H. Lateral thoracic artery aneurysm with lung abscess and empyema caused by Streptococcus intermedius. J. Gen. Fam. Med. 2021, 22, 296-297. [CrossRef]
- Tasleem, A.; Mahmood, A.; Sharma, R. Streptococcus intermedius Pleuropulmonary Disease: A Not So Commonly Seen Radiological Picture. Cureus 2021, 13, 17385. [CrossRef]
- Manasrah, N.; Nanja Reddy, S.; Al Sbihi, A.; Hafeez, W. Streptococcus intermedius: unusual presentation and complication of lung abscess. BMJ Case Rep. 2021, 14, e245675. [CrossRef]
- Nakagawa, Y.; Otake, S.; Oue, T.; Ryu, H.; Kasai, M. Case of infant invasive Streptococcus intermedius infection suggesting the need for anaerobic cultures. J. Infect. Chemother. 2022, 28, 437-439. [CrossRef]
- Christensen, P.J.; Kutty, K.; Adlam, R.T.; Taft, T.A.; Kampschroer, B.H. Septic pulmonary embolism due to periodontal disease. Chest 1993, 104, 1927-9. [CrossRef]
- Patail, H.; Patail, H.; Ahmad, S. A Man in His 30s Presenting With Shortness of Breath and Productive Cough After a Recent Pneumonia. Chest 2020, 157, e91-e93.
- Takahashi, S.; Ishitsuka, T.; Namatame, K.; Nakamura, M. A false-positive pneumococcal rapid urinary antigen test in Streptococcus intermedius infection. Respirol. Case Rep. 2019, 7, e00466. [CrossRef]
- Dyrhovden, R.; Nygaard, R.M.; Patel, R.; Ulvestad, E.; Kommedal, Ø. The bacterial aetiology of pleural empyema. A descriptive and comparative metagenomic study. Clin. Microbiol. Infect. 2019, 25, 981-986. [CrossRef]
- Cobo, F.; Sampedro, A.; Rodríguez-Granger, J.; Aliaga-Martínez, L.; Navarro-Marí, J.M. Clinical and microbiologic characteristics of pleuro-pulmonary infection due to Streptococcus intermedius. Rev. Esp. Quimioter. 2018, 31, 146-151.
- Crespo Valadés, E.; Fernández Blanco, J.M.; Troya García, J.; Malmierca Corral, M. Absceso subfrencio izquierdo primario por Streptoccocus intermedius [Primary left subphrenic abscess due to Streptococcus intermedius]. An. Med. Interna 2005, 22, 202-3.
- Noguchi, S.; Yatera, K.; Kawanami, T.; Yamasaki, K.; Fukuda, K.; Naito, K.; Akata, K.; Nagata, S.; Ishimoto, H.; Taniguchi, H.; Mukae, H. Pneumonia and empyema caused by Streptococcus intermedius that shows the diagnostic importance of evaluating the microbiota in the lower respiratory tract. Intern. Med. 2014, 53, 47-50. [CrossRef]
- Hannoodi, F.; Ali, I.; Sabbagh, H.; Kumar, S. Streptococcus intermedius Causing Necrotizing Pneumonia in an Immune Competent Female: A Case Report and Literature Review. Case Rep. Pulmonol. 2016, 2016, 7452161. [CrossRef]
- Mautner, G.H.; Lu, I.; Ort, R.J.; Grossman, M.E. Transverse leukonychia with systemic infection. Cutis 2000, 65, 318-20.
- Huang, M.; Li, S.; Wu, X.; Xu, D.; Tang, L.; Chen, Z. An isolated pulmonary nodule secondary to Streptococcus intermedius infection in an otherwise healthy 10-year-old boy: A case report and literature review. Front. Pediatr. 2022, 10, 921258. [CrossRef]
- Kurkowski, S.C.; Thimmesch, M.J.; Jha, P.; Abdelgadir, Y.H. Streptococcus intermedius Bacteremia and Pyogenic Liver Abscess in a Patient With No Risk Factors. Cureus 2022, 14, e26786. [CrossRef]
- Jud, P.; Fink-Neuboeck, N.; Lindenmann, J. Massive ventilator-associated pleural empyema. Korean J. Intern. Med. 2019, 34, 942-943. [CrossRef]
- Lescan, M.; Lepski, G.; Steger, V.; Schlensak, C.; Walker, T. Rapidly progressive paraplegia and pleural empyema: how does that correlate? Gen. Thorac. Cardiovasc. Surg. 2013, 61, 640-2.
- Stelzmueller, I.; Berger, N.; Wiesmayr, S.; Eller, M.; Tabarelli, W.; Fille, M.; Margreiter, R.; Bonatti, H. Group milleri streptococci: significant pathogens in solid organ recipients. Transpl. Int. 2007, 20, 51-6. [CrossRef]
- Iskandar, S.B.; Al Hasan, M.A.; Roy, T.M.; Byrd, R.P. Jr. Streptococcus intermedius: an unusual cause of a primary empyema. Tenn. Med. 2006, 99, 37-9.
- Lau, S.K.; Woo, P.C.; Chan, B.Y.; Fung, A.M.; Que, T.L.; Yuen, K.Y. Haemophilus segnis polymicrobial and monomicrobial bacteraemia identified by 16S ribosomal RNA gene sequencing. J. Med. Microbiol. 2002, 51, 635-640. [CrossRef]
- Kawanami, T.; Fukuda, K.; Yatera, K.; Kido, M.; Mukae, H.; Taniguchi, H. A higher significance of anaerobes: the clone library analysis of bacterial pleurisy. Chest 2011, 139, 600-608.
- Yamasaki, K.; Kawanami, T.; Yatera, K.; Fukuda, K.; Noguchi, S.; Nagata, S.; Nishida, C.; Kido, T.; Ishimoto, H.; Taniguchi, H.; Mukae, H. Significance of anaerobes and oral bacteria in community-acquired pneumonia. PLoS One 2013, 8, :e63103. [CrossRef]
- Teramoto, S.; Fukuchi, Y.; Sasaki, H.; Sato, K.; Sekizawa, K.; Matsuse, T.; Japanese Study Group on Aspiration Pulmonary Disease. High incidence of aspiration pneumonia in community- and hospital-acquired pneumonia in hospitalized patients: a multicenter, prospective study in Japan. J. Am. Geriatr. Soc. 2008, 56, 577-9.
- Issa, E.; Salloum, T.; Tokajian, S. From Normal Flora to Brain Abscesses: A Review of Streptococcus intermedius. Front. Microbiol. 2020, 11, 826.
| Article | n. of patients |
Gender | Age (y) | Infection site | Outcome |
|---|---|---|---|---|---|
| Shinzato et al.1, 1995 | 9 | M=8 F=1 |
Mean 61.3 | Lung, pleura | NA |
| Noguchi et al.2, 2015 | 14 | M=10 F=4 |
Mean 77.3 | Lung/pleura | Recovered=12 Death=2 |
| Bueno et al.3, 2023 | 1 | F | 25 | Lung | Recovered |
| Erne et al.4, 2010 | 1 | M | 61 | Lung, brain | Recovered |
| Jerng et al.5, 1997 | 17 | NA | NA | Lung/pleura | NA |
| Chandy et al.6, 2001 | 1 | M | 21 | Blood, lung, frontal sinus, epidural abscess | Recovered |
| May et al.7, 2010 | 1 | M | 53 | Lung | Death |
| Van Laren et al.8, 2011 | 1 | F | 29 | Blood, lung, genital | Recovered |
| De Cruif et al.9, 2012 | 1 | F | 63 | Lung, dental abscess | Recovered |
| Trabue et al.10, 2014 | 1 | M | 36 | Lung, brain | NA |
| Maeda et al.11, 2012 | 1 | F | 46 | Chest wall abscess, pleura | Recovered |
| Armendariz-Guezala et al.12, 2017 | 1 | M | 33 | Brain, lung | Recovered |
| Sakurai et al.13, 2020 | 1 | M | 80 | Pleura, iliopsoas abscess | Recovered |
| Carrena et al.14, 2018 | 1 | M | 61 | Lung, brain, Chiari network endocarditis | Recovered |
| Fujihara et al.15, 2021 | 1 | M | 64 | Lung, pleura | Death |
| Tasleem et al. 16, 2021 | 1 | M | 54 | Lung, pleura | Death |
| Manasrah et al.17, 2021 | 1 | M | 54 | Lung, pleura, vertebrae and discitis | Recovered |
| Nakagawa et al.18, 2022 | 1 | M | 6 mo | Lung, pleura | Recovered |
| Christensen et al.19, 1993 | 1 | M | 56 | Lung | Recovered |
| Patail et al.20, 2020 | 1 | M | 30 | Lung, pleura | NA |
| Takahashi et al.21, 2019 | 1 | F | 83 | Pleura | Recovered |
| Dyrhovden et al.22, 2019 | 16 | NA | NA | Pleura | NA |
| Cobo et al.23, 2018 | 9 | M=7 F=2 |
Mean 63.9 | Lung, pleura | Recovered=8 Death=1 |
| Crespo Valades et al.24, 2005 | 1 | M | 60 | Pleural effusion, subphrenic abscess | Recovered |
| Noguchi et al.25, 2014 | 1 | M | 79 | Lung, pleura | Recovered |
| Hannoodi et al.26, 2016 | 1 | F | 52 | Lung, pleura | Recovered |
| Mautner et al.27, 2000 | 1 | M | 80 | Lung, pleura | Recovered |
| Huang et al.28, 2022 | 1 | M | 10 | Lung, pleura | Recovered |
| Kurkowski et al.29, 2022 | 1 | M | 39 | Liver, pleura, blood | Recovered |
| Jud et al.30, 2019 | 1 | M | 59 | Lung, pleura | Recovered |
| Lescan et al.31, 2013 | 1 | M | 74 | Lung, pleura, epidural abscess | Recovered |
| Stelzmueller et al.32, 2006 | 2 | NA | NA | Pleural empyema | Recovered |
| Iskandar et al.33, 2006 | 1 | NA | NA | Pleural empyema | NA |
| Lau et al.34, 2002 | 1 | M | 32 | Pleural empyema, blood | Recovered |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





