1. Introduction
The Baltic Sea is semi-enclosed water body and its residence time is very high, therefore it is sensitive to external influences [
1]. The water is brackish and because of the many islands, wetlands and lagoons, the Baltic Sea has a specific ecosystem [
1].
At the same time, the Baltic Sea is an inland sea with intense sea traffic in the world. On average, 300,000 ships enter the Baltic Sea every year [
2]. Two-thirds of the ships in the region are cargo ships, with slightly less than half being oil and oil products tankers [
2]. The annual oil transport [
2,
3] in the sea poses a significant risk of oil spills.
Certain coastal locations of the Baltic Sea, such as ports and the vicinity of industrial facilities that use or refine oil, have suffered from frequent small-scale oil discharges and spills for decades [
4]. The port of Klaipeda in Lithuania, with its oil terminal, is a seaport on the eastern Baltic coast and is considered an important node of the East-West transport corridor linking roads and sea routes in that direction [
5]. Analyses of the port’s sediments show that it is polluted with PAH of pyrogenic origins as well as aliphatic hydrocarbons of technogenic origins [
6,
7].
For surface oil spills, sorbents are used effectively in coastal cleanup and for removal of small of oil slicks [
9,
10]. Sorbents are also unsuitable for use on the open sea due to unfavourable weather conditions [
11]. It becomes a pollutant itself when remaining on the water surface for too long. Used sorbent must therefore be removed from the water surface at the right time. Then the best methods for regeneration or recovery of used sorbent (incineration, landfill, biodegradation) must be applied [
10].
A strong focus is therefore on exploring the possibilities of natural organic sorbents. These sorbents are sustainable, cost-effective, biodegradable and their resources are renewable [
10]. However, as described in [
10], natural sorbents must be hydrophobized to stay on the water after sorption process of oil not to sink and not to be able to be caught. Another advantage is the equipment of sorbents with an additional function. Multifunctional Materials [
12,
13] have an expanded range of properties, efficient manufacturing processes and a wide range of design options. The use of natural components makes these materials recyclable.
To enhance the effectiveness of sorbents in removing hydrocarbons from the surface, bioremediation techniques can be employed by introducing materials that encourage microbial biodegradation of oil. These methods involve the addition of microorganisms or nutrients to overcome unfavorable environmental conditions such as low temperatures, insufficient oil-degrading microorganisms, or a lack of inorganic nutrients in the water. Fungi are among the microorganisms used for this purpose [
14], and their ability to break down hydrocarbons has been well-documented [
15]. However, the challenge is that the immobilization of microorganisms might change the effectiveness of sorbent as a buoyancy or the absorption capacity.
This work describes hydrophobic straw as a sorbent with additional oil-degrading filamentous fungi as described in [
16]. The physical properties of the straw without immobilized fungi and its sorption capacity as well as the evaporation rate of crude oil from the straw surface were previously published in [
10]. Here we study if the straw as an oil sorbent is affected by the immobilization of filamentous fungi.
2. Materials and Methods
2.1. Chemicals and Materials
For the hydrocarbon extraction process n-hexane and n-heptane (Honeywell/Riedel-de-Haën, Cromasolv for HPLC ≥ 97%), anhydrous sodium sulphate (Sigma-Aldrich, puriss 99.0–100.5%), aluminum oxide (Sigma-Aldrich, puriss) and acetone (Honeywell, ≥99.5%) were used.
For the gas chromatography analysis standard mixtures of n-alkanes (Shimadzu) and n-hexane (Honeywell/Riedel-de-Haën, Cromasolv for HPLC ≥ 97%) were used.
Oat straw (Klaipeda, Lithuania) was used as a natural sorbent in this study. It was chopped into 1.0 to 1.5 cm length pieces.
Methyltrimethoxysilane (MTMS, C4H12O3Si, 98%; Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) was used for hydrophobization of the straw.
The water was taken from the Baltic Sea next to Karkle [55°48′33″N, 21°4′38″E] (Lithuania). The salinity of the water was 5.76 ‰, and the pH was 8.39.
The crude oil used for the analysis was from SC “ORLEN Lietuva” (Mazeikiai, Lithuania). The density is 867 kg m−3 (T = 20 °C) and the viscosity is 0.0097 Pa s (T = 25 °C).
2.2. Hydrophobization of the Straw
The procedure is identical to what has already been described in [
10]. For each g of straw, 1 g MTMS was poured in a 50 mL glass dish and positioned at the bottom of a 3 L beaker. MTMS was chosen in part because it makes chemical vapour deposition into porous materials readily manageable. The glass dish was topped with a plastic fly screen to prevent straw pieces from falling into the MTMS. The straw was placed evenly on the plastic fly screen. The beaker was closed with foil (aluminium or plastic) and put into the oven at 70 °C for 12 h.
2.3. Immobilization on the Hydrophobic Straw with Fungi
The filamentous fungal species with the ability to degrade petroleum hydrocarbon [
16] was used for straw immobilisation as follows: the hydrophobized straw was dry-heated to 120 °C for 1.5 hours for sterilization. The straw thus sterilized was then placed in the sterile breathing Microsac bag (Sac O2 Nevele, Belgium) and mixed gently with a previously prepared 25 mL fungal inoculum in sterile water Ringer’s solution (NutriSelect
® Basic) and incubated for 72 h at 25 °C. After the fungi overgrow the straw, it was frozen, lyophilized (72 h), and kept at room temperature until use.
2.4. Determination of the Distribution of Total Petroleum Hydrocarbons in the Water and Straw
The total hydrocarbons content (THC) in water and straw was analysed in order to investigate the distribution of hydrocarbons between straw and water.
A 1000 mL volume beaker was half filled with sea water. A plastic fly screen was fitted inside as shown in [
10].
Crude oil (1 mL) and straw (2 g) were placed on the water. After 1, 7 or 14 days, the straw was taken out of the water with the plastic fly screen. The straw was weighed after excess water dripped off for 15 min. All analyses were carried out with and without straw under the same conditions with three replicates.
The hydrocarbon concentrations in the water and straw were determined according to [
10].
The water was stirred with 50 mL of n-hexane at 500 rpm on a magnetic stirrer for 30 min and then poured into a separatory funnel. The straw was added to a sealed 250-mL DURAN glass bottle along with 100 mL of a solvent mixture of heptane and acetone in a 1:2 ratio. The extraction process was carried out on a shaker at 120 rpm for 60 min and then shaken into another sieve. The plastic fly screen was washed several times with small amounts of n-hexane and added to the straw extraction separatory funnel. After the water phase was separated in each case, the hexane extracts of the seawater phase and the straw were purified separately over a short column of 3 g alumina for the water extracts and 6 g for the straw extracts. A paper filter containing 10 g of sodium sulfate was placed on the column in a glass funnel and the hexane fraction from the separating funnel was dried in this way. The respective purified extracts were diluted by a factor of 20 with n-hexane for GC analysis and transferred to labeled glass vials with rubber-crimp caps.
Hydrocarbon concentration analysis was carried out on a GC-2010 Plus gas chromatograph, equipped with flame ionization detector. Helium (purity > 99.999%) was used as the carrier gas at a flow rate of 30 mL min−1. Rxi-1MS type 20 m length capillary column with 0.18 mm inner diameter and 0.18 µm thickness cross bond 100% dimethylpolysiloxane polymer film (Restek, USA) was used for the analysisInstrument control and data evaluation were done with the LabSolution software.
Three series of calibration standard solutions with concentrations of 0.01, 0.05, 0.1, 0.5 and 1.0 mg mL−1 were made of crude oil. 1.0 mg mL−1 standard solution was made by accurately weighing 100 mg of crude oil and dissolving it in 100 mL of n-hexane. Other standard solutions were made by the dilution method.
The total peak area was measured in the range bounded by the n-decane (C10H22) and n-tetracontane (C40H82) standards. Only compounds with a signal-to-noise ratio higher than 5 were considered. The relative proportions of the constituents in the samples were obtained using the percentage area of chromatographic peaks, as is generally done.
Total hydrocarbon concentration in water was calculated as follows [
10]:
Cw is total hydrocarbon concentration in sea water, mg L
−1;
Cex—total hydrocarbon concentration in sea water extract, mg mL
−1;
Vex—volume of sea water extract, mL;
Vw—total volume of sea water sample, mL.
Total hydrocarbon concentration in straw (sorbent) was calculated as follows [
10]:
Cs is total hydrocarbon concentration in straw, g g
−1;
Cex—total hydrocarbon concentration in (straw) extract, mg mL
−1;
Vex—volume of (straw) extract, mL;
F—dilution factor;
Ms—mass of straw, mg.
The crude oil mass of the sorbent was calculated according to [
10]:
wh is the crude oil mass of the straw, mg kg
−1 dry mass;
ρ is the crude oil mass concentration of the extract calculated from the calibration function, mg L
−1;
Vh is the volume of the n-hexane extract, mL;
m is the mass of the sample taken for analysis, g;
ws is the dry mass content of the straw, wt%.
The percentage of water absorbed by the straw during the experiment (1, 7 and 14 days) was calculated as follows [
10]:
m0 is the initial mass of dry straw, g;
mmix is the mass of dry straw with crude oil and sea water, g;
mH2O is the mass of sea water, g.
The amount of non-extractable oil x was calculated from the amount of crude oil and the total amount of extracted oil of the straw and the sea water:
The sorption measurements for crude oil were carried out in three replicates.
2.5. Fungi Quantification with qPCR
For fungal quantification, sorbents were sampled in three stages under two treatments (with and without agitation): at the initial stage (before the start of the experiment (time 0)), after 7 and 14 days. The DNeasy PowerSoil Kit (Qiagen) was used to extract DNA from the sorbents (up to 200 mg of sterile sorbents). Before extraction, sorbents were cut into smaller pieces under sterile conditions. The extracted DNA was used as a template for PCR reactions. β-actin gene, characteristic for fungal genome, was used to quantify fungal cells in the sorbents [
17]. Quantitative real-time PCR was performed using a StepOnePlus
TM Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA) using the SYBR Green PCR Master Mix (Thermo Fisher Scientific). Each 20 μL reaction contained 10 μL SybrGreen, 0.25 μL of a 10 pmol/μL primer solution using the β-actin primers: ACT 512-F (5′ ATG TGC AAG GCC GGT TTC GC 3′) and ACT 783-R (5′ TAC GAG TCC TTC TGG CCC AT 3′) [
18], 8.5 μL H
2O and 2 μL of DNA template. The amplification conditions were 95 °C for 10 min and then 40 cycles of 95 °C 15 s, 61 °C 20 s and 72 °C 15 s. For each sample the analysis was performed in triplicate. Fluorescence measurements were made at the end of each annealing cycle and an additional measuring point at 80 °C (for 1 s) to detect the formation of primer dimers during amplification. A melt curve analysis was made by raising the temperature from 65 to 95 °C in 0.5 °C steps for 5 s each. Standard curves based on threshold cycles were generated by re-amplification of 10-fold dilution series of PCR products from fungal genomic DNA, and an aliquot of each dilution in 3 replicates was used for real-time PCR.
3. Results
The physical properties of the straw without immobilized fungi and its sorption capacity as well as the rate of evaporation of the crude oil from the surface of the straw have already been published in [
10].
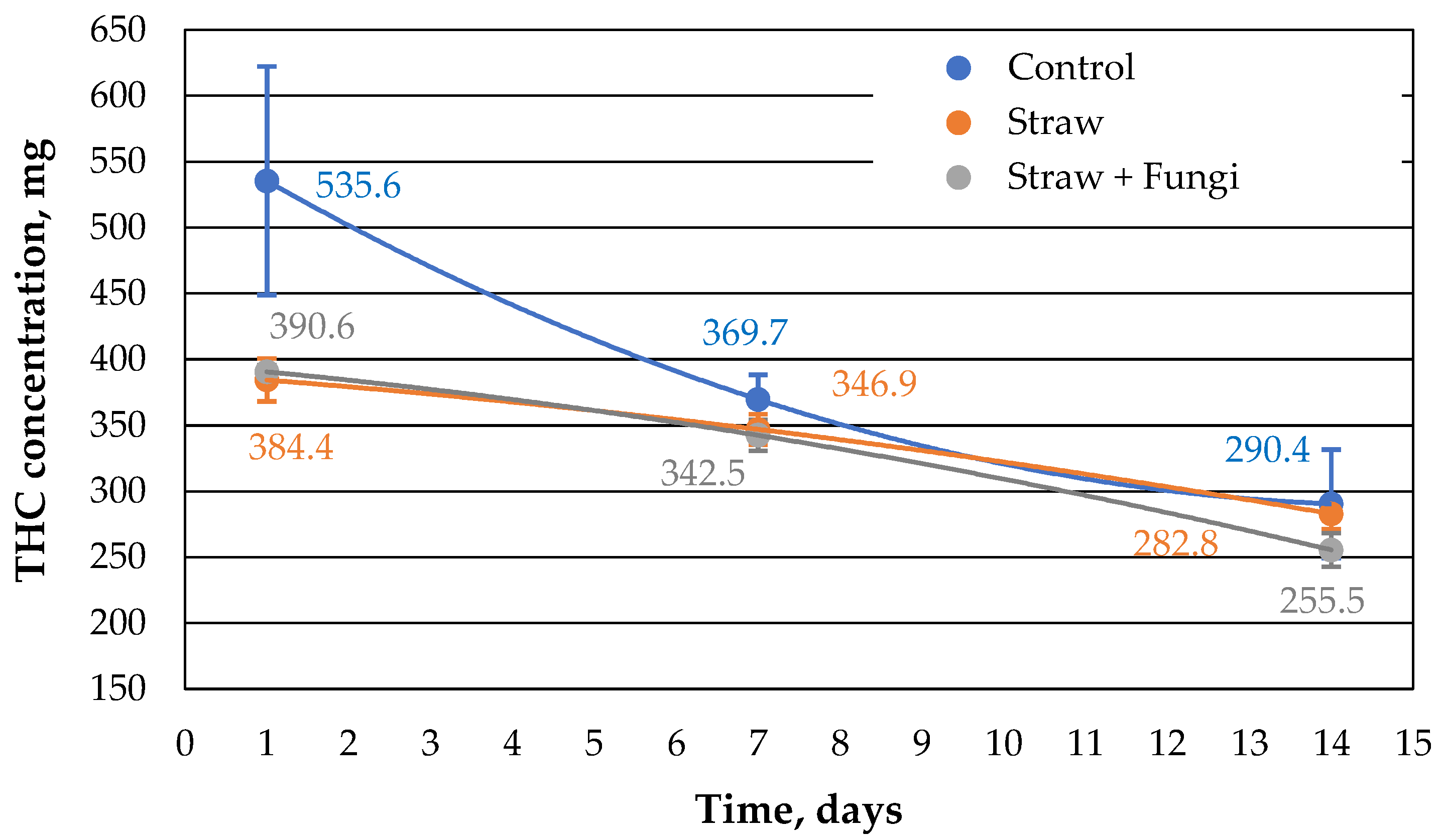
As can be seen in
Figure 1, 2 g of straw with immobilized fungi has a similar sorption capacity as the same amount of straw without immobilized fungi initially and after one week, but lower than the control sample consisting of oil on the surface with no sorbent consists. The sorption capacity of the straw with fungi decreases after 14 days compared to the control sample and the straw without fungi. This could be due to the increased biomass of the fungi occupying part of the porosity of the straw or due to straw decomposition affected by fungi which cannot be decided here.
At the same time,
Figure 1 shows that after 14 days, the amount of extracted oil from the sample with straw without fungi shows the same amount of oil as the control sample, indicating that the straw has completely sorbed the oil at the latest by this point. As already discussed in [
10], the comparative sorption kinetics are probably related to the morphology of the straw.
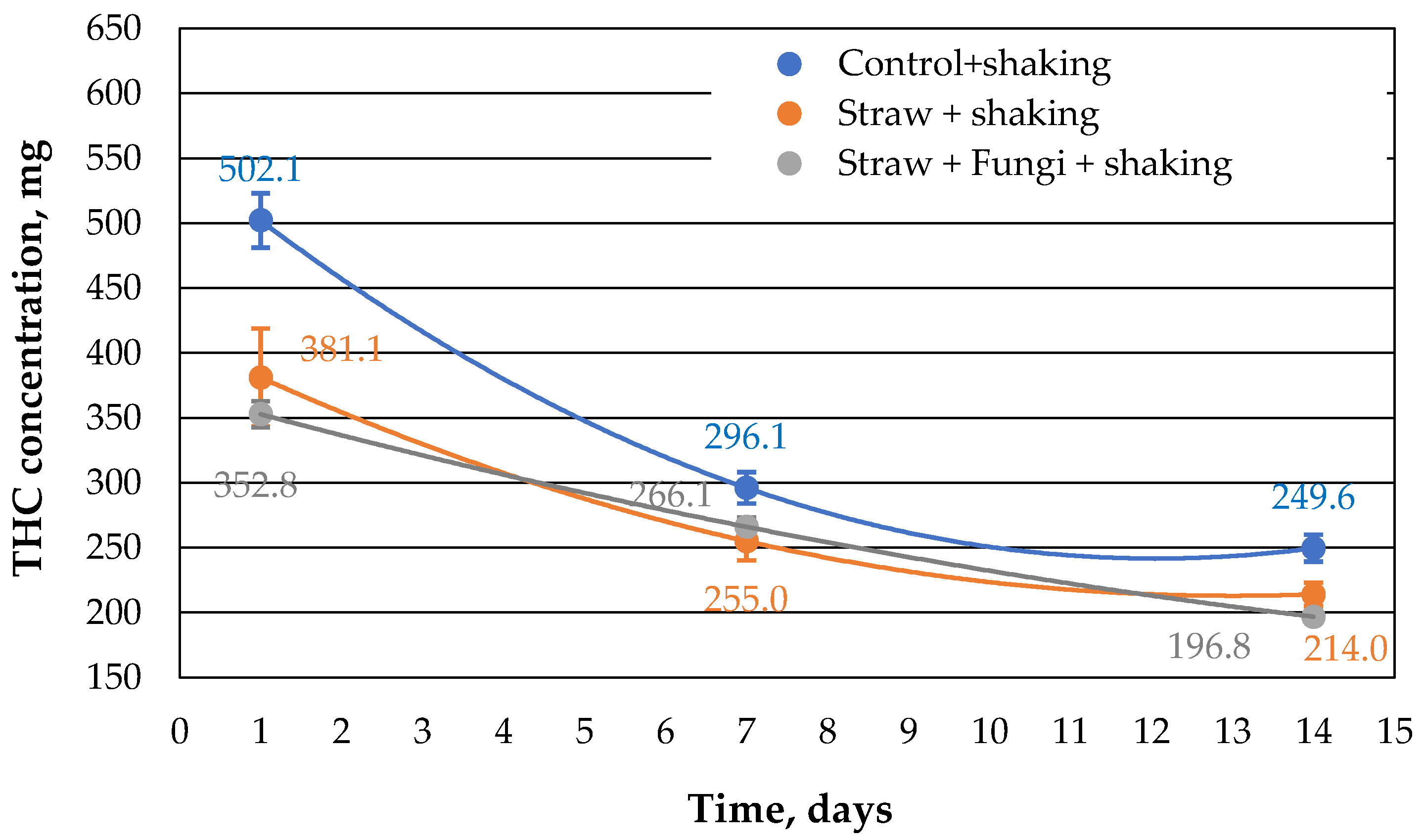
Repeating the same approach with shaking results in a similar trend as in
Figure 1, but with slightly lower values for the extracted THC concentration from the samples (
Figure 2).
Shaking increases the surface area in the beaker track and of course more oil can evaporate from the mixture. This is reflected in the systematically lower values for the extractable THC compared to the control sample in
Figure 2. The values of the sorption capacity for straw with and without microbes differ little within the error, so that one does not have a clear effect on the effectiveness of the microbes can be seen.
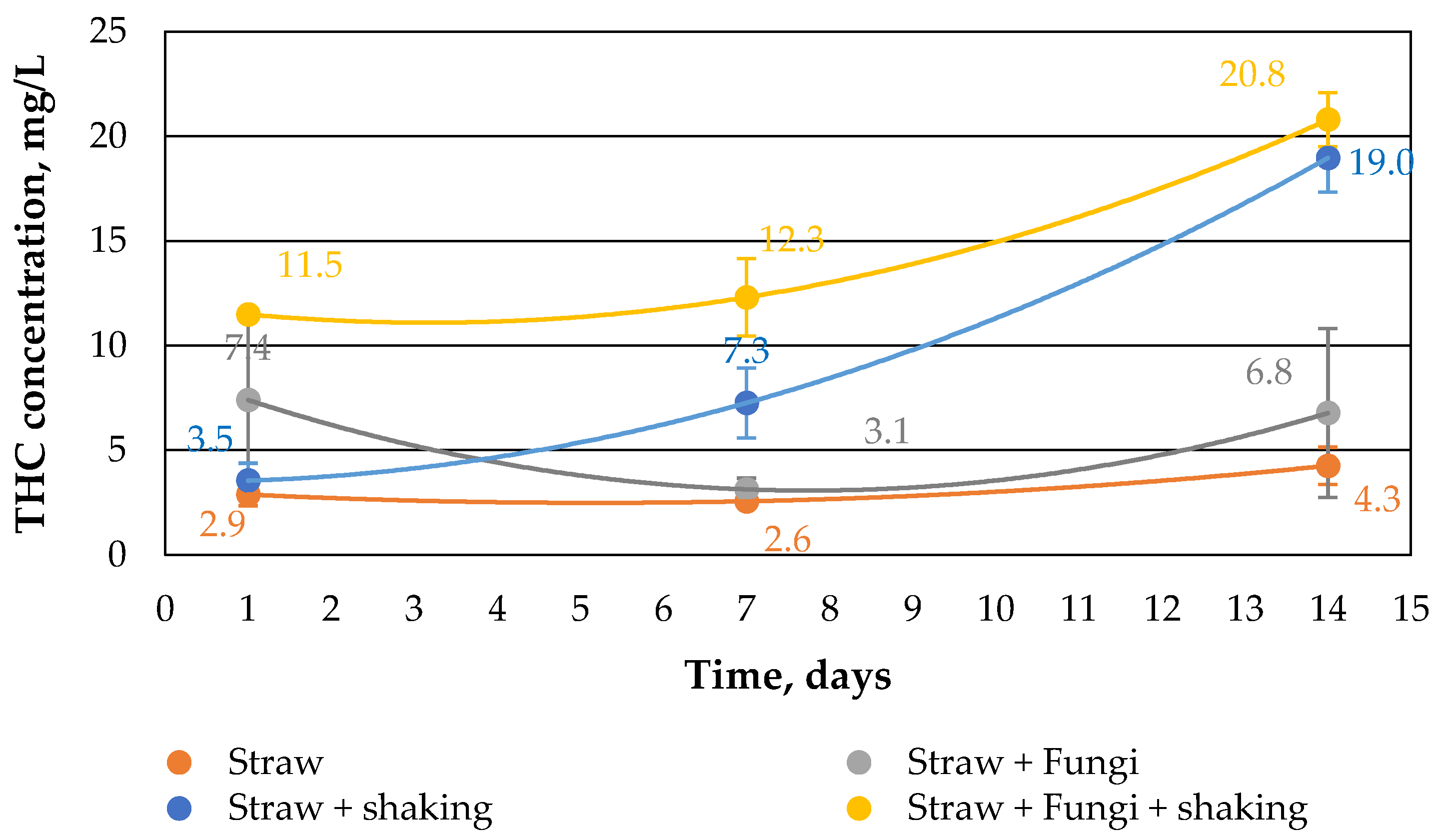
Figure 3 shows the THC concentration in water. It is noteworthy here that the presence of fungi and simultaneous shaking significantly increases the THC concentration. On the one hand, this could be due to the ability of the fungi to form biosurfactants in order to tap the carbon source from the crude oil [
19,
20], and on the other hand others that the water surface always had access to air through the shaking and the straw. As already indicated in [
10], the water volume fell somewhat during the 14-day period. This means that the oil layer on the water was not completely closed and therefore an exchange of substances in both directions was possible.
However, the shaking of the beakers was not so strong that emulsions of the crude oil could form in water. Accordingly, the water was never cloudy during the course of the study, but always clear.
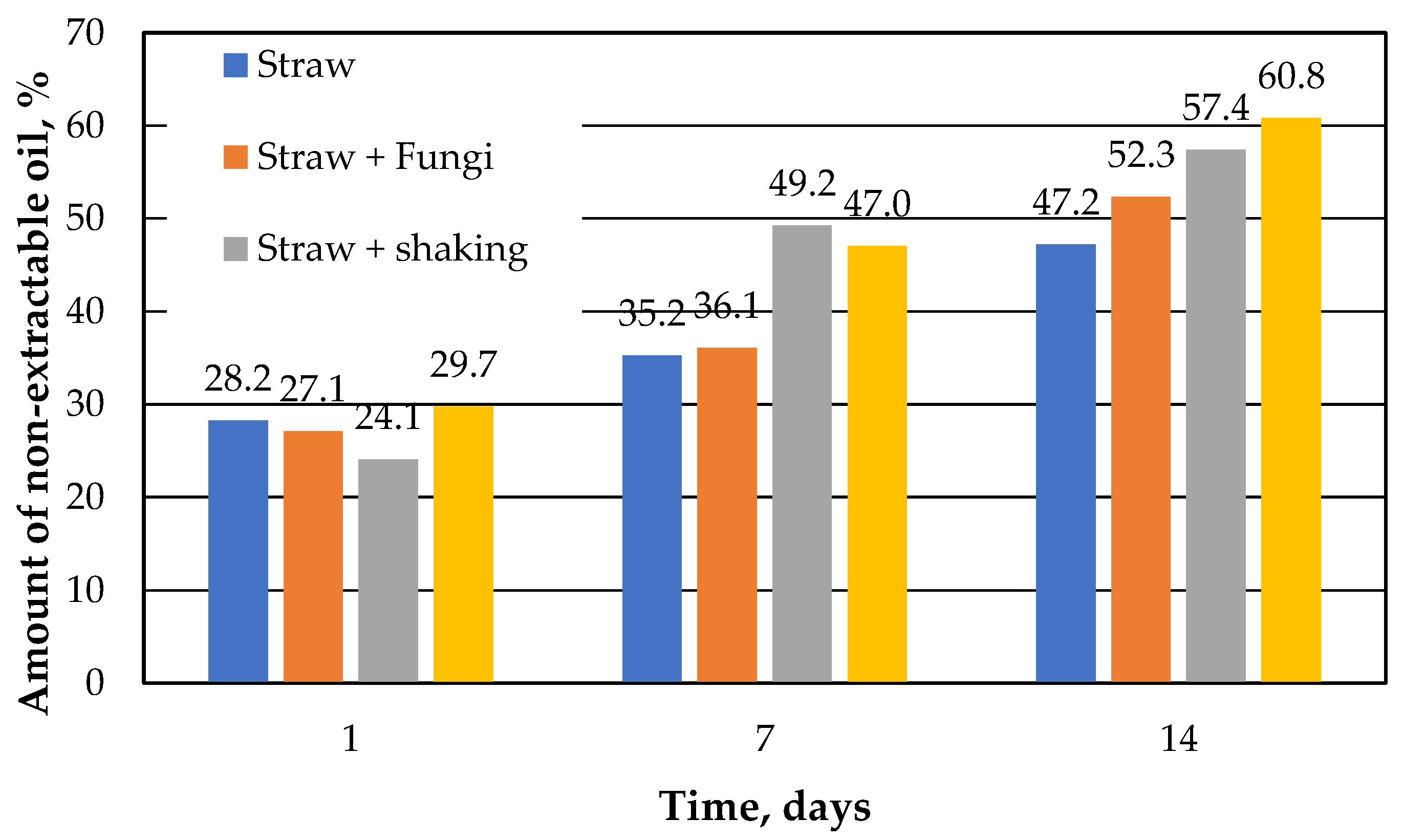
Figure 4 shows a comparison of percent of the non-extractable oil versus the running time. Non-extractable oil is the difference between the theoretical extractable amount of oil, which is identical to that of the control sample, and the amount of oil actually determined. It can be seen that the amount of non-extractable oil in straw increases over the course of the 14 days, which has already been shown [
10]. This can probably also be assumed for the straw samples with immobilized microbes. A slow desorption kinetics due to the inner morphology of the straw was already assumed in [
10] as the cause for the non-extractable amount.
However, it is also noted that after 14 days the amount of non-extractable oil in the straw samples with microbes is a few percent higher than in the respective comparative sample without microbes. Thus, microbes immobilized on straw increase the sorption capacity of straw, but only after 14 days.
Figure 4.
Total efficiency of sorbents and sorbents + fungi (calculated using control results).
Figure 4.
Total efficiency of sorbents and sorbents + fungi (calculated using control results).
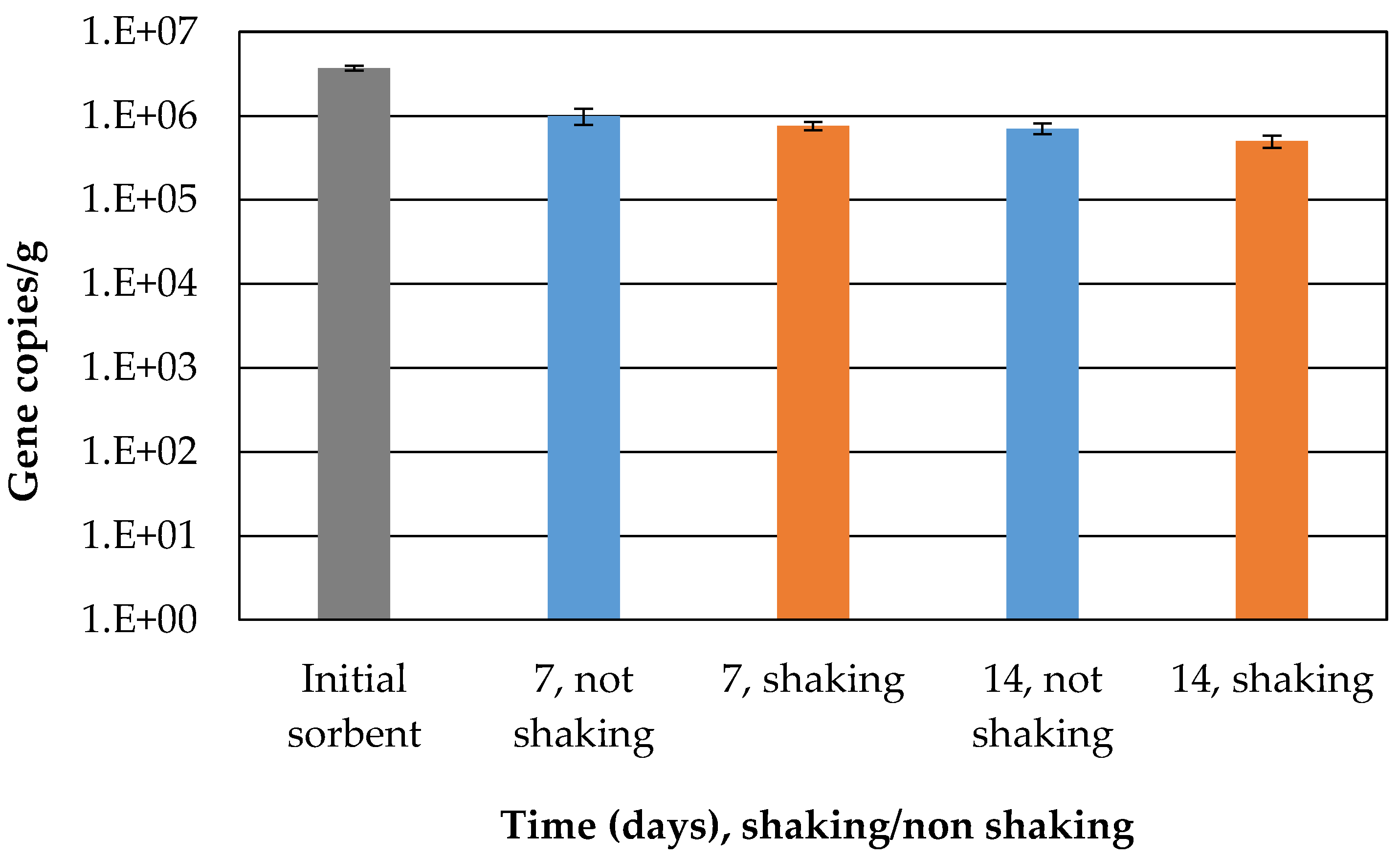
The number of cells of the fungal biomass in the sorbent was determined in order to be able to classify the time delay of the increase in the sorption capacity. The initial number decreased slightly from the initial amount (
Figure 5) but remained stable until the end of experiment, indicating their potential activity in the presence of adsorbed crude oil. The natural microbial community present in the sea water used for the experiment might have oil-degrading microbes that can cooperate or compete with immobilized fungi. Oil degrading bacteria could compete with fungi not only for the petroleum hydrocarbon, but also for other available nutrients [
21].
Despite the decrease in the number of cells, it can also be stated that the fungal community was not extinguished during the course of the experiments.
As already described in [
10], hydrophobization of the straw avoids wetting with and partial sinking into water. In this study, it was found that immobilization of the hydrophobized straw with microbes does not affect this behavior. The following figures show the composition of the extracted oil from the beaker, which apart from the water only contains oil and the hydrophobic straw without fungi (
Figure 6).
As in [
10], it was also found and confirmed here that the light fractions (hydrocarbon with a small number of carbon atoms) in the case of straw without immobilized microbes already evaporate in the first week and this composition hardly changes in the second week. It could be discussed here that the light fraction would have sorbed rather than evaporated, but then it should have reappeared after one or two weeks during the extraction due to the small straw pieces. This could not be confirmed here or in previous published research [
10].
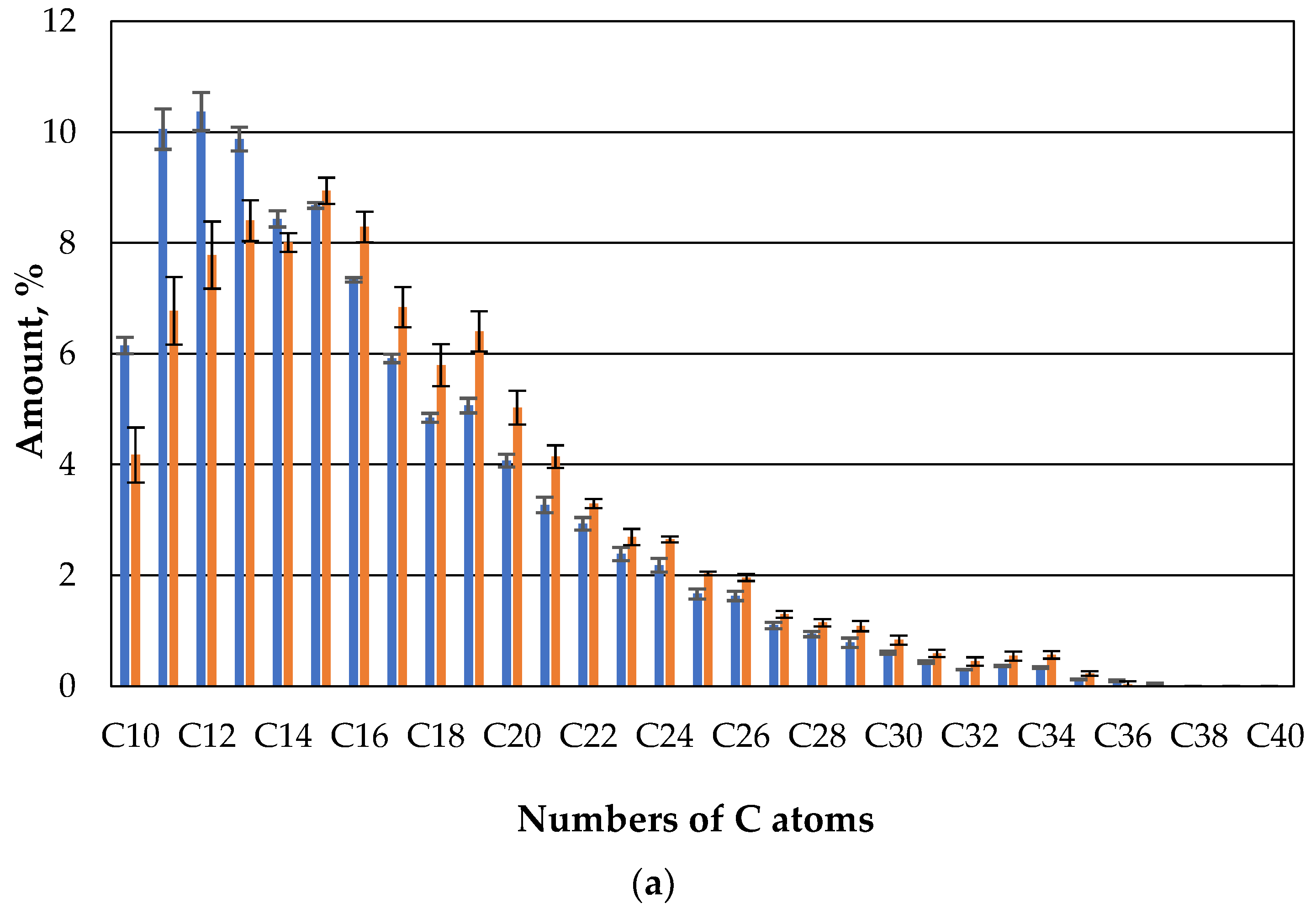
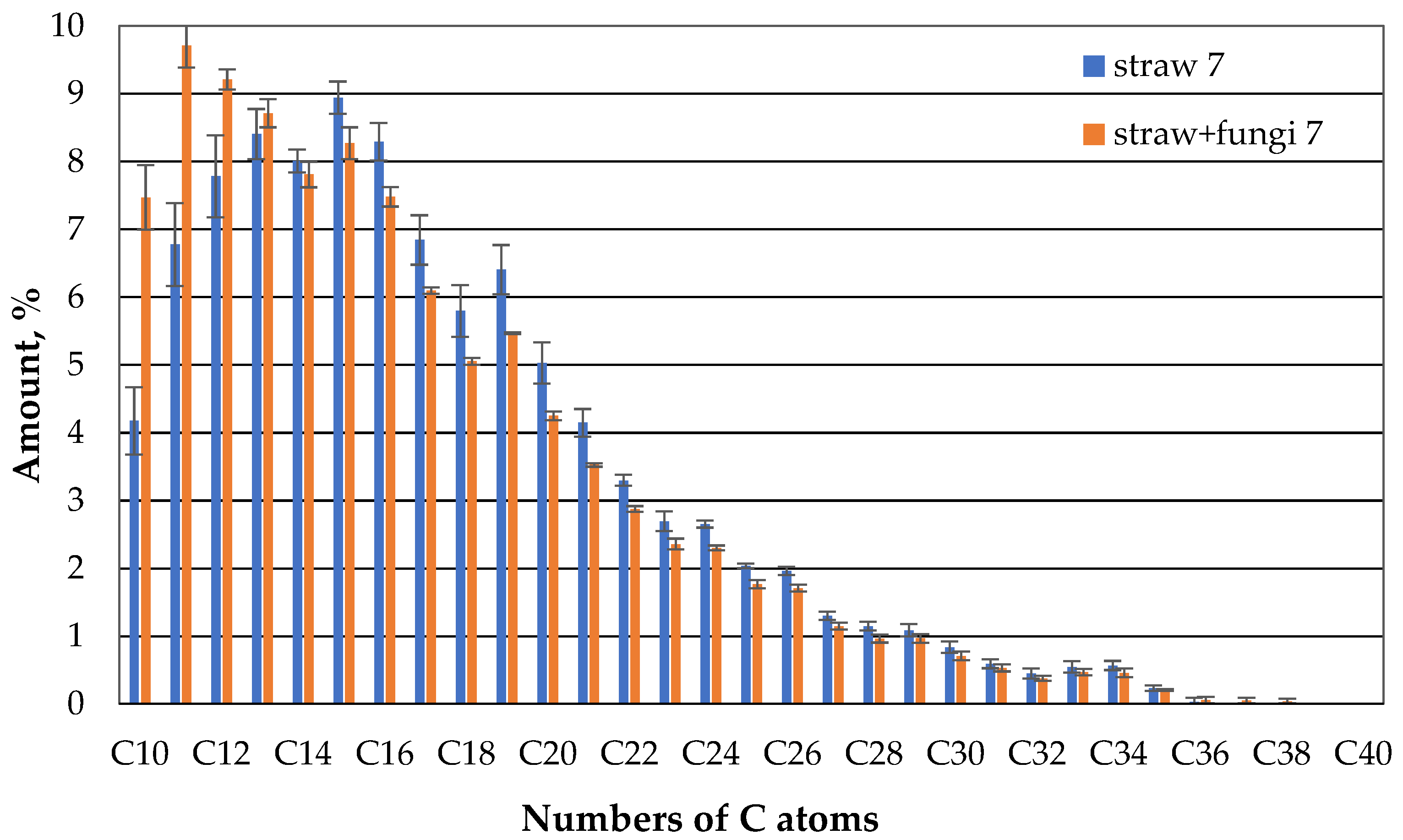
If one now compares the gas-chromatic composition of the oil of the straw with fungi with the straw without microbes after one day, clearly less oil evaporates in the case of the straw with microbes than in the case of straw without microbes in the case of compounds with 10 or 11 carbon atoms (
Figure 7). For the higher hydrocarbons there is no clear difference between the straw with and without microbes.
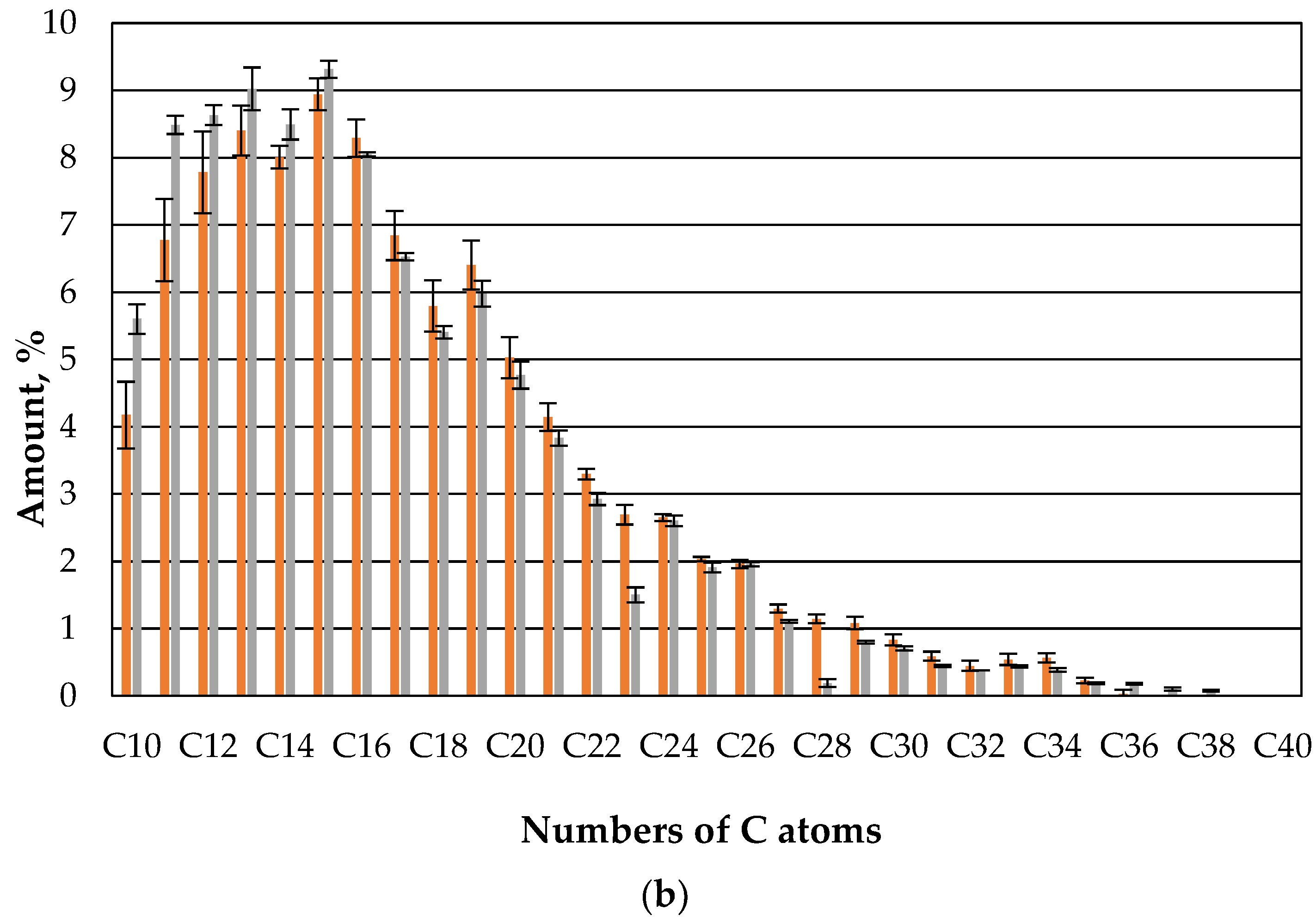
It now appears that straw with microbes retains the fractions with low hydrocarbon chains to a greater extent than straw without microbes, even after 7 days (
Figure 8). In the higher KW chains there is no such clear preference.
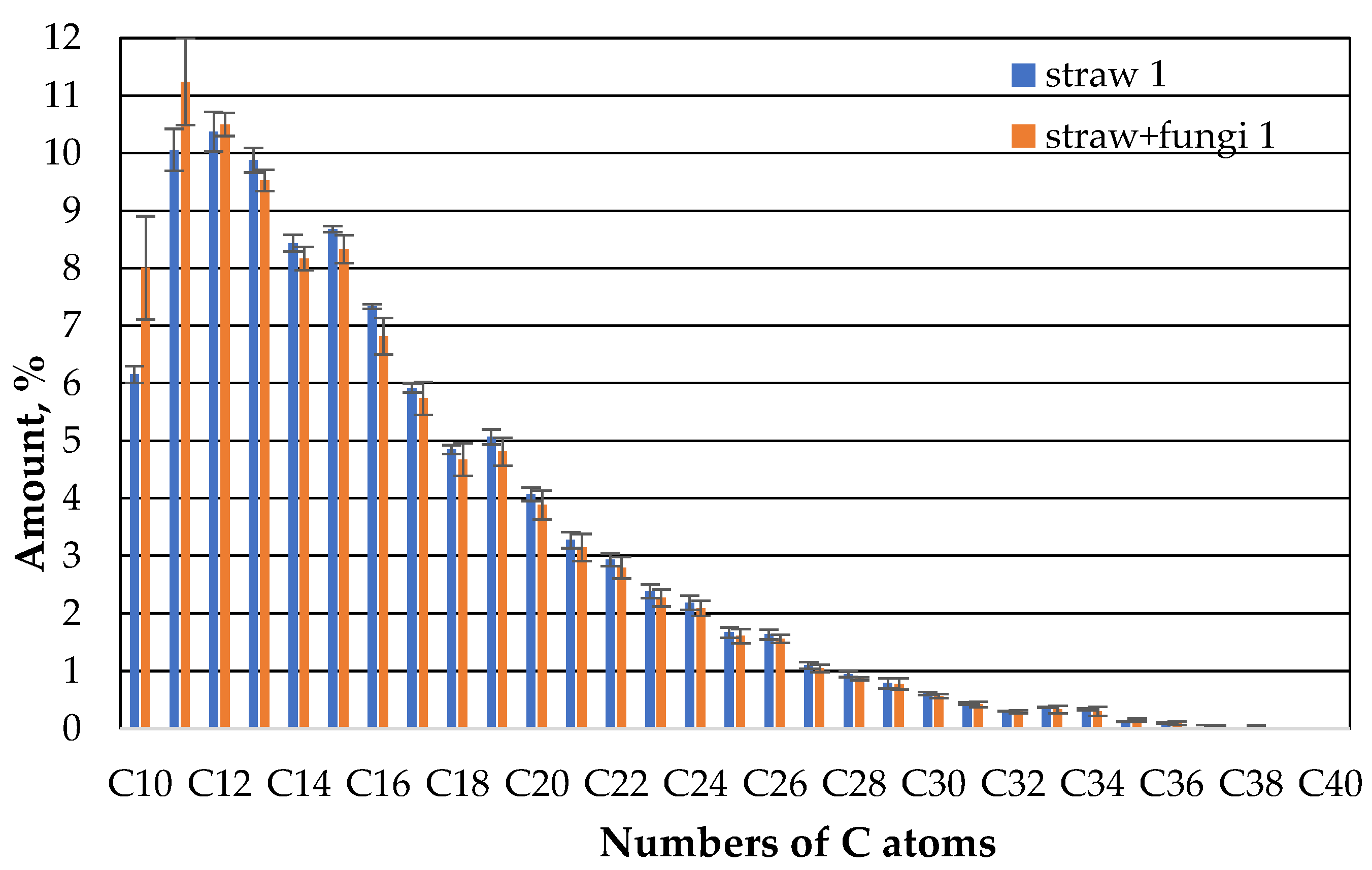
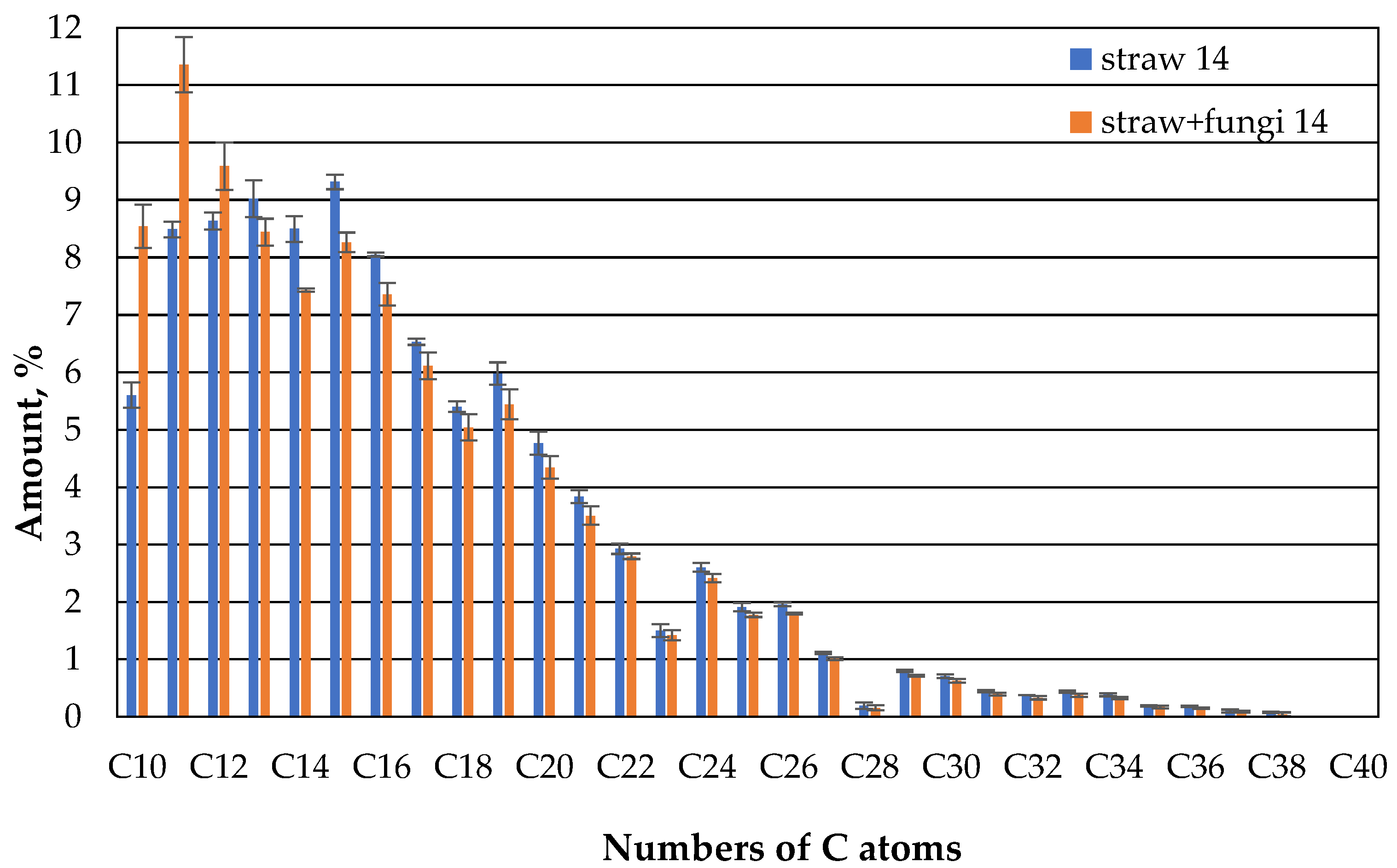
Fungi therefore appear to increase the sorption capacity of straw in relation to the hydrocarbons in the oil in the range of 10 to 13 C atoms. At the same time, the sorption capacity for the higher hydrocarbons is the same as that of straw without fungi. This could have to do with the higher diffusion speed of lower hydrocarbons. And it does not change almost in the second week (
Figure 9).
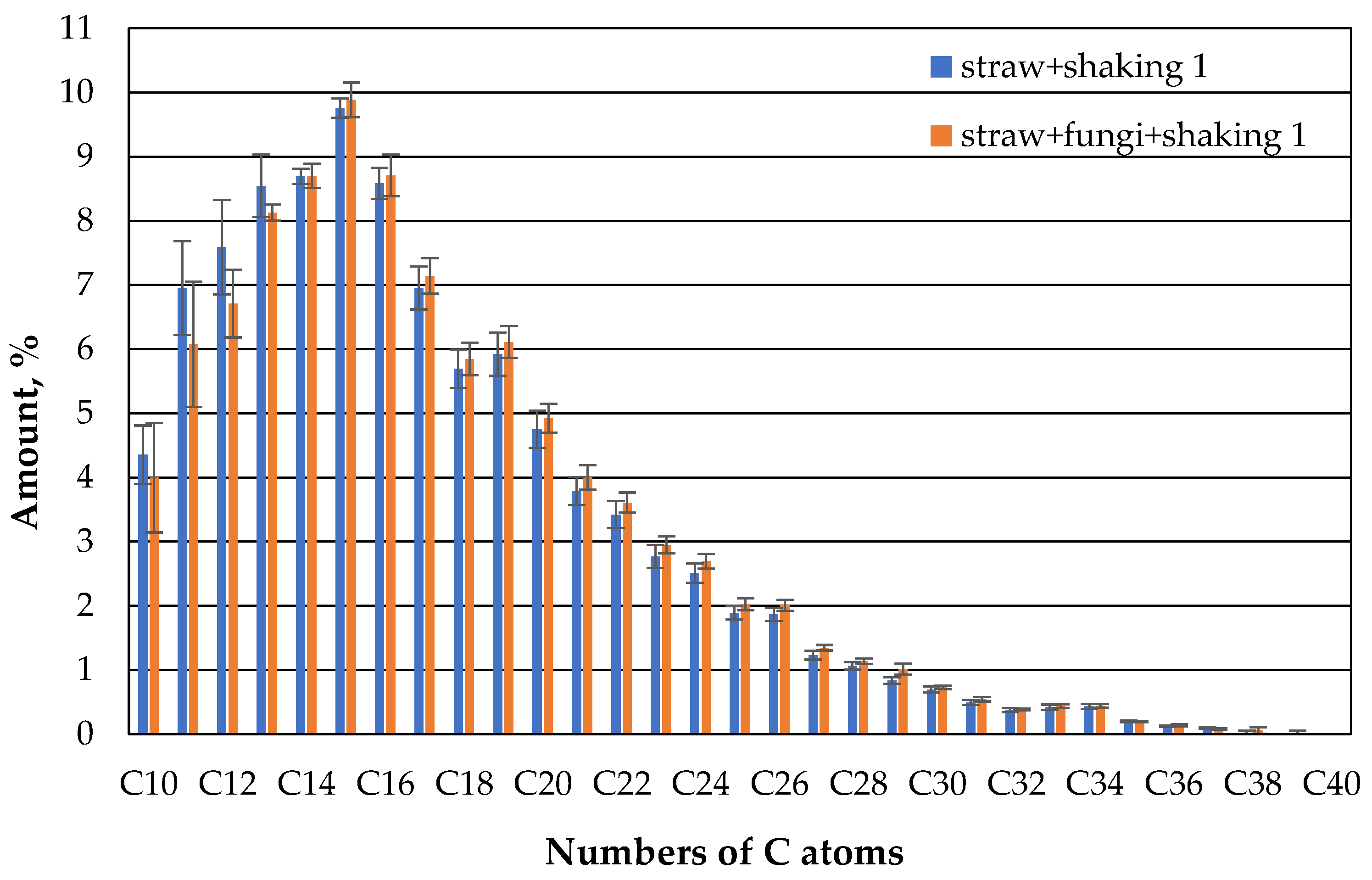
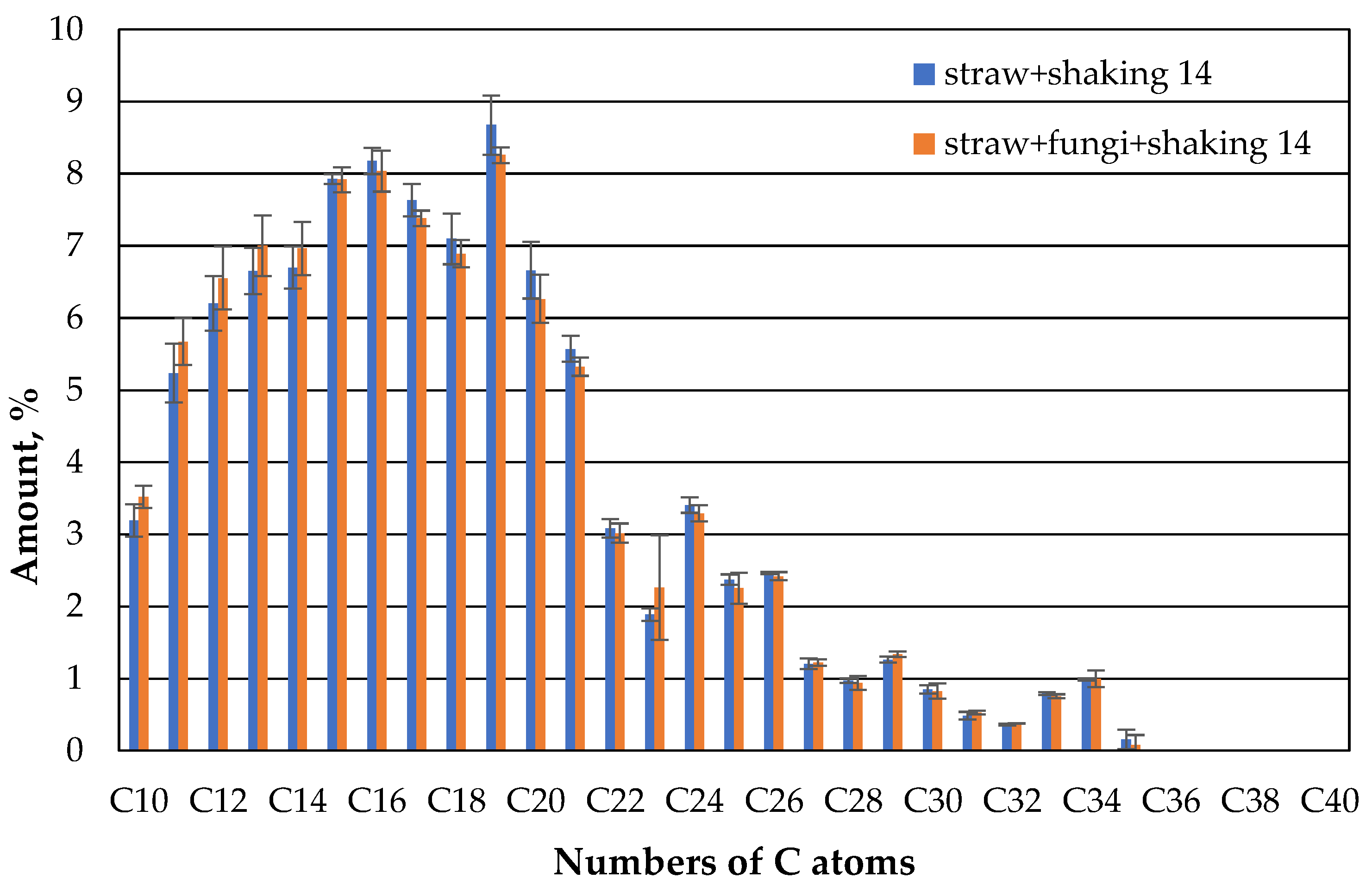
If the beakers are additionally shaken, the behavior changes significantly (
Figure 10). On the first day, about the same amount of oil has evaporated from both the microbe-free and the microbe-containing beaker and the composition of the oil that can still be extracted is about the same.
However, after 7 days, a little more oil in the range of the lower hydrocarbon chains up to about 14 carbon atoms can be extracted from the microbe-containing beakers (
Figure 11).
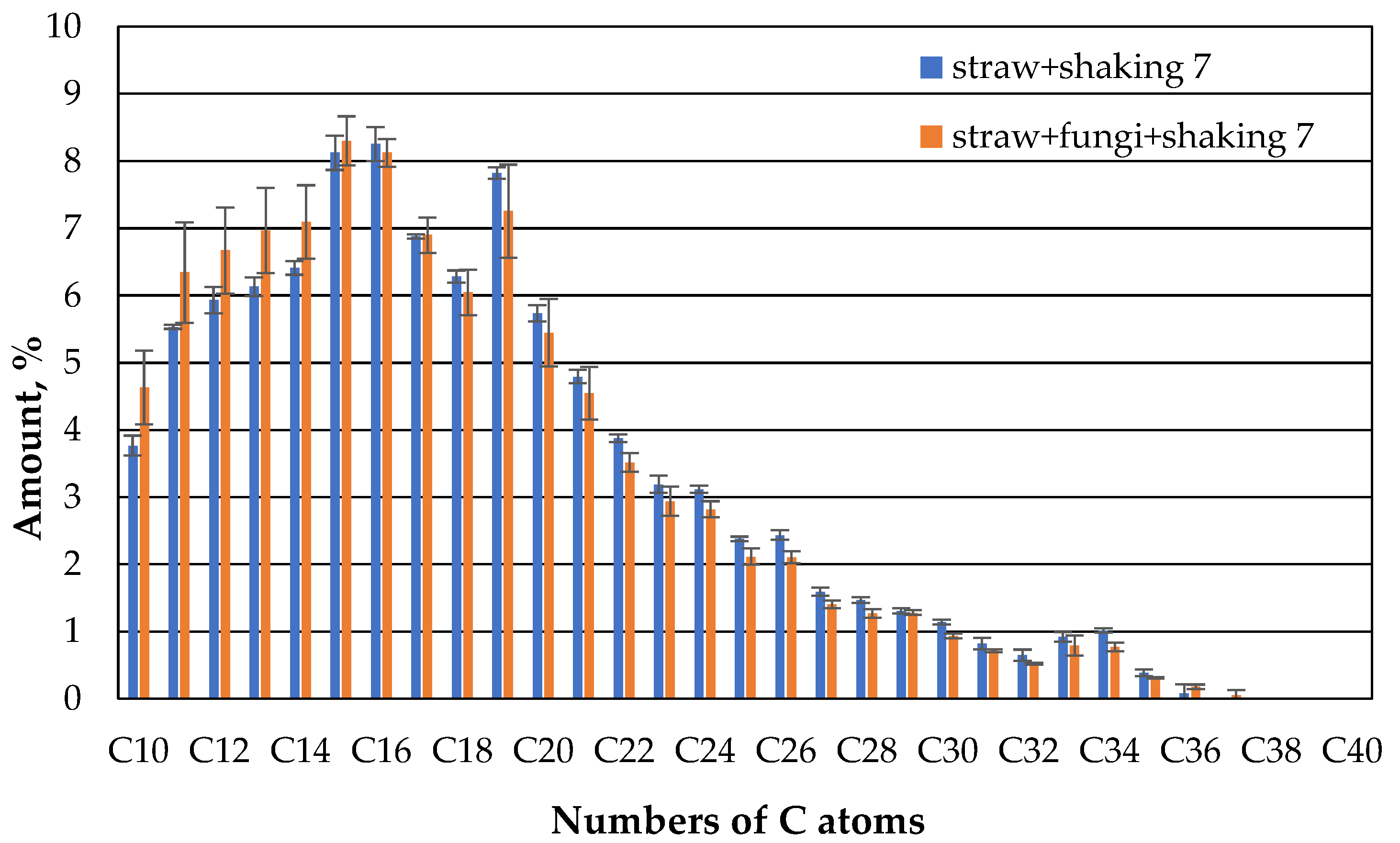
However, this changes again after 14 days (
Figure 12). Here the KW distribution of the shaked straw fungi has matched that of the straw without fungi.
As shown in
Figure 12, the fraction of non-extractable oil is slightly higher in shaked straw fungi. The lower proportion of low HCs in the sample with the shaved straw with fungi compared to the unshaken sample could be attributed to the influence of the microbes. As already described in [
10], the straw enables mass transfer including the transport of oxygen from the environment into the aqueous phase of the beaker. This would support the metabolism of the microbes. In the literature [
22,
23] a reason often cited as preventing microbes from getting into an oil spill is that the microbes lack enough oxygen under the oil spill.
4. Conclusions
The buoyancy of the straw with fungi was not affected by the immobilization of filamentous fungi. Even with sorbed oil, it still floats on the water during the 14-day test period.
The sorption kinetics of the oil appear to be similar in straw with and without immobilized biomass and independent of beaker shaking. However, the fraction of non-extractable oil after 14 days is higher in straw with fungi and shaking than without shaking. This could indicate the beginning of oil mining.
It cannot be ruled out that the fungi will change and even shrink somewhat during the study. Based on this result, instead of leaving the straw with fungi and oil in the water, it might be beneficial to take it out and let the oil degradation and decomposition of the straw proceed on land. It is advantageous here that the straw with fungi and oil is always floating on the water surface during the test.
The shaking and the straw protruding above the oil layer, favored by the sorption of the oil into the straw, allows the microbes to grow unhindered. Growth of the microbes initially immobilized on the straw cannot be confirmed, but a restructuring of the microbial community could also come into question.
Author Contributions
Conceptualization, M.K. and T.P.; methodology, T.P., J.U., Z.K., L.C., M.K. and D.O.; software, T.P. and Z.K.; validation, T.P., J.U., M.K. and D.O.; formal analysis, Z.K. and M.K.; investigation, Z.K., L.S., T.P, M.K. and J.U.; resources, T.P. and M.K.; data curation, T.P.; writing—original draft preparation, J.U., M.K. and T.P.; writing—review and editing, Z.K., M.K. and D.O.; visualization, Z.K.; supervision, T.P.; project administration, T.P.; funding acquisition, T.P. and M.K. All authors have read and agreed to the published version of the manuscript.