Submitted:
29 May 2023
Posted:
31 May 2023
You are already at the latest version
Abstract
Keywords:
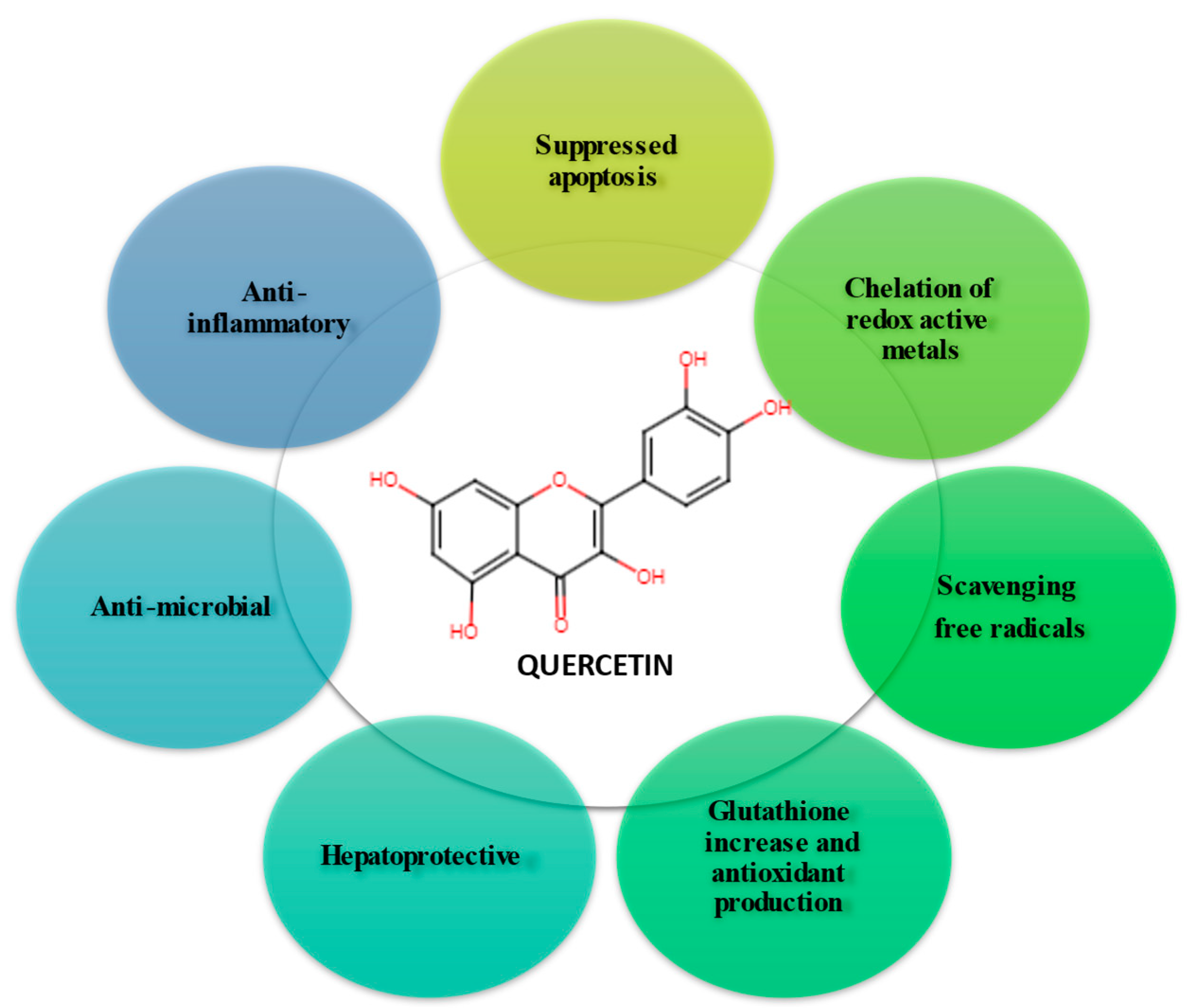
1. Introduction
2. Methodology
3. Oxidative stress
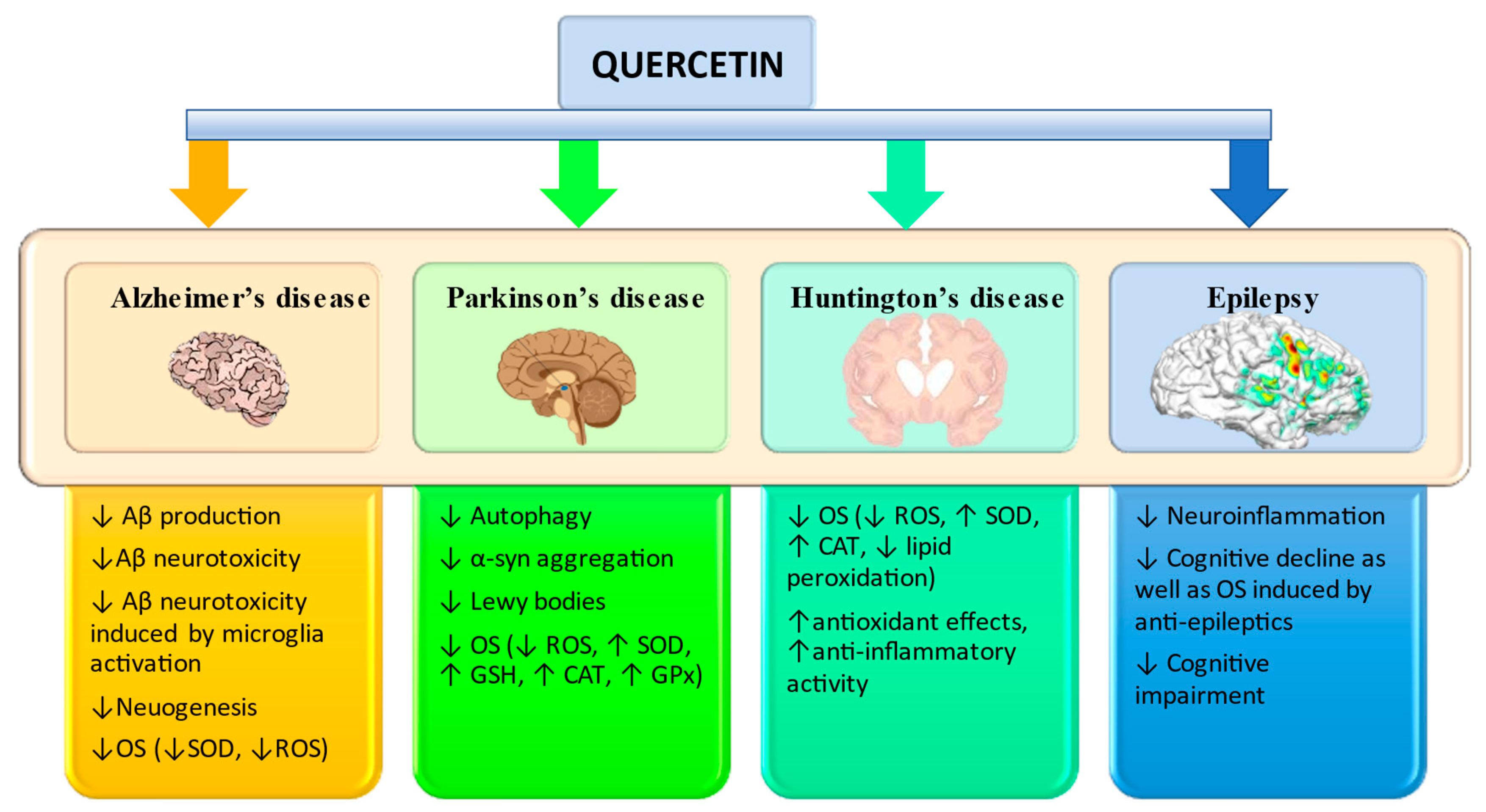
3.1. Alzheimer’s disease
| Types of quercetin | Concentration of quercetin | Model | Exposure | Effects | Ref. |
|---|---|---|---|---|---|
| Quercetin | Dosage: 2.2 μM Duration: 24 h. |
HT-22 mouse hippocampal cell | H2O2 | ↓lipid peroxidation, ↑intracellular GSH, ↓ROS | [54] |
| Dosage: 10-100 µmol L−1 Duration: 10 min |
PC12 cells | H2O2 | ↓lipid peroxidation, ↓GSH, mitochondrial protection mechanisms. | [55] | |
| Dosage: 10 μM | APP695-transfected SH-SY5Y cells | Aβ25–35 | ↓ROS, ↓BACE, ↓Aβ, ↓GSH, ↓lipid peroxidation | [57] | |
| Dosage: 10 and 50 μM Duration: 7 days |
HT-22 mouse hippocampal cells | Aβ1–42 or Aβ1–40 | ↓Aβ peptides, ↓the performed mature fibrils | [58] | |
| Dosage: 2.4 µg mL−1 | HT-22 murine neuroblastoma cells | Aβ25–35 | ↓amyloidogenic Aβ peptides, inhibited Aβ fibril formation. |
[59] | |
| Quercetin-3′-glucoside | Dosage: 10, 20, 40, and 80 μmol L−1 Duration: 24 h, 48 h, and 72 h |
PC12 cells | Aβ25–35 | ↑ the survival rate of PC12 injured by Aβ25-35, promote cell proliferation, and antagonize the toxicity of Aβ, ↑ CREB/BDNF signaling pathway, ↓ROS | [61] |
| Q3G | Dosage: 50 mg kg−1 Ad: gavage Duration: 24 h, 48 h, and 72 h |
APP695-transfected SH-SY5Y cells | Aβ1–42 | ↓Aβ peptides, ↑CREB signaling, ↓Aβ aggregation, ↑mitogen-activated protein | [64] |
| Dosage: 50 mg kg −1 Ad: gavage Duration: 4 months |
Tg2576 AD primary neuron cultures | ↑neuronal survival, ↑c-Jun N-terminal kinases, ↓stress-induced impairments | |||
| Quercetin/Ginkgo biloba | Dosage: 1.5-6 μg mL−1 Ad: i.p. |
SHSY5Y human neuroblastoma cells | Aβ1–42 | ↓ Akt signaling pathways, ↓Aβ toxicity, ↓platelet-activating factor | [65] |
| Quercetin/ Acanthopanax henryi |
Dosage: 2.5, 5, 10, 20, and 40 μg mL−1 | Cell free system | ↓ AchE activity, ↑antioxidant activity |
[66] |
| Types of quercetin | Concentration | Model | Exposure | Effects | Ref. | |
|---|---|---|---|---|---|---|
| Quercetin | Dosage: 5 or 10 mg kg−1 b.w.; Ad: p.o.; Duration: once daily; |
hBMECs | fAβ1–40 | ↓SOD, ↓LDH | [68] | |
| Dosage: 25 mg kg−1 b.w.; Ad: i.p.; Duration: every 2 days for 3 months; |
3xTg-AD mice | ↓tauopathy, ↓β-amyloidosis, ↑memory, ↑learning↓ microgliosis, ↓astrogliosis | [69] | |||
| Dosage: 100 mg kg−1 b.w.; Ad: gavage; Duration: every 48 h for 12 months; |
3xTg-AD mice | ↓neurodegeneration, ↓β-amyloidosis | [70] | |||
| Dosage: 20 and 40 mg kg−1 b.w.; Ad: p.o.; Duration: 16 weeks; |
adult male C57BL mice | ↑ MMP, ↑ATP levels, ↓ROS | [71] | |||
| Dosage: 20 mg; Ad: p.o.; Duration: 5 weeks; |
APP23 AD mice model | Aβ | ↓eIF2α, ↓ATF4, ↓GADD34, ↑memory in aged mice, ↓memory deterioration in the early stage of AD, ↓memory dysfunction, ↓OS | [72] | ||
| Dosage: 1% in mouse chow; Ad: p.o.; Duration: from 3 to 13 months; |
double transgenic female mice | ↓neuroinflammation, ↓neurodegeneration, ↓ IL-1β |
[73] | |||
| Dosage: 50 mg kg−1 b.w.; Duration: 2 times a week for 4 weeks; |
homozygotic transgenic mouse line B6.129S7-Sod2tm1Leb/J | H2O2 and Aβ | ↓ROS levels, improved the typical morphology of mitochondria, prevented mitochondrial dysfunction | [74] | ||
| Dosage: 100 mg kg−1 b.w.; Ad: p.o.; Duration: 22 days; |
adult male Sprague-Dawley rats | Aβ1–42 | ↑expression of Nrf2/HO-1 in rat brain, ↓Aβ1-42 level, ↓antioxidant activity | [75] | ||
| Dosage: 25 mg kg−1; Ad: p.o.; Duration: 2 times a week for 2 months; |
SAMP8 mice | ↑ the cognition and memory impairments, ↓ astrogliosis | [77] | |||
| Dosage: 12.5 and 25 mg kg−1; | mice | Scopolamine | ↓OS, ↓AchE activity | [78] | ||
| Dosage: 30 mg kg−1 b.w.; Ad: i.p.; Duration: every day for 8 days; |
male albino Wistar rats | Scopolamine | abridged transfer latency, ↓avoidance response, ↓3,4-methylenedioxyamphetamine, acetylcholinesterase levels, ↑CAT, ↑ GSH levels | [79] | ||
| Dosage: 10 mg kg −1 b.w.; Ad: p.o.; Duration: every day for 12 weeks; |
male albino Wistar rats | aluminum | ↓ROS production, ↑mitochondrial superoxide dismutase activity |
[80] | ||
| Quercetin/ ginkgo flavonols | Dosage: 4.8% in extract, all based on weight; |
Double Transgenic (TgAPP/PS1) mice | - | Reversed the spatial learning deficit | [82] | |
3.2. Parkinson’s disease
| Types of quercetin | Concentration | Model | Exposure | Effects | Ref. |
|---|---|---|---|---|---|
| Quercetin | Dosage: 0.1 μM |
Microglial (N9)-neuronal (PC12) cells | MPP | ↓iNOS gene expression, ↓ROS, ↓ cellular death, ↓DNA fragmentation, ↑apoptosis, ↓nuclear translocation of apoptosis-inducing factor, ↓caspase-3activation | [92] |
| Dosage:10 mM |
PC12 cells | α-Synuclein | ↓Aβ fibrillation | [95] | |
| Isoquercetin | Dosage: 10, 50, and 100 μM |
PC12 cells | 6-OHDA | ↓ROS, ↑SOD, ↑GSH, ↑CAT, ↑GPx | [96] |
| Quercetin glycoside | Dosage: 10, 50, and 100 μM | PC12 cells | 6-OHDA | ↑antioxidant activity, ↑GSH, ↑GPx | [97] |
| Types of quercetin | Concentration | Model | Exposure | Effects | Ref. |
|---|---|---|---|---|---|
| Quercetin | Dosage: 25 mg kg−1 Ad: p.o. |
Wistar rats | Haloperidol MPTP | ↓cataleptic score, ↑actophotometer activity score, ↑GSH, ↓lipid peroxidation, ↓ROS | [98] |
| Dosage: 25 and 50 mg kg−1, Ad: intragastrically Duration: 14 days |
Wistar rats | MPTP | ↓TNF-α, ↓IL-1β and ↓IL-6, ↓glutamate level, | [99] | |
| Dosage: 50, 100 and 200 mg kg−1 Ad: p.o. Duration: 14 days. |
adult male C57BL/6 mice | MPTP | ↓striatal dopamine depletion, ↓level of acetylcholine, ↑AchE activity, ↑motor deficits, ↑GPx, ↑SOD | [100] | |
| Dosage: 100, 200 and 300 mg kg −1 Duration: 14 days |
Wistar rats | 6-OHDA | ↑spatial memory, ↓OS, ↓AchE activity, ↑antioxidant activity, ↓neuronal damage | [101] | |
| Dosage: 20 mg kg−1 Ad: i.p. Duration: 1 month. |
Wistar rats | 6-OHDA | ↓neuroplastic changes in neural circuits, ↓excitability in neurons involved in epilepsy, ↓NMDA receptor functionality | [102] | |
| Dosage: 25-75 mg kg−1 Duration: 12 h intervals for 4 days |
Wistar rats | Rotenone | ↓nigral GSH depletion, ↓ROS, ↓striatal DA loss, ↑mitochondrial complex, ↓neuronal death | [103] | |
| Dosage: 50 mg kg−1, Ad: p.o. Duration: 14 days |
Wistar rats | Rotenone | ↑AchE activity, ↑SOD, ↓GPx, ↓CAT | [104] | |
| Quercetin + fish oil | Dosage: 25 mg kg−1 Ad: p.o. Duration: 28 days |
Wistar rats | Rotenone | ↑mitochondrial functions, ↑GSH, ↑antioxidant defenses | [105] |
3.3. Huntington’s disease
| Types of quercetin | Concentration | Model | Exposure | Effects | Ref. |
|---|---|---|---|---|---|
| Quercetin | Dosage: 25 mg kg−1 Ad: p.o. Duration: 21 days; |
Wistar rats | 3-NPA | ↑ATP, ↑activity of complex II and V enzyme of respiratory chain complex, ↓ROS, ↑SOD, ↑CAT, ↓lipid peroxidation | [107] |
| Dosage: 25–50 mg kg−1 Ap: i.p.; Duration: 4 days |
Sprague Dawley rats |
3-NPA | ↓gait despair, ↓microglial proliferation, ↓anxiety, ↑astrocyte numbers in the lesion core, ↓motor coordination deficits, ↓serotonin metabolism | [108] | |
| Quercetin + lycopene | Dosage: 50 mg kg−1, Duration: 14 days |
Wistar rats | 3-NPA |
↓anxiety, ↓depression | [109] |
| Quercetin + fish oil | Dosage: 25 mg kg−1 | Wistar rats | 3-NPA | ↓OS, ↑motor function | [110] |
| Quercetin + sesamol | Dosage: 25, 50, and 100 mg kg−1, Ad: i.p. Duration: 14 days before and 14 days after quinolinic acid administration |
Wistar rats | QA | ↓behavioral, biochemical, and neurochemical alterations in the rat brain, ↑antioxidant effects, ↑anti-inflammatory activity | [111] |
3.4. Epilepsy
| Types of quercetin | Concentration | Model | Type of test | Exposure | Effects | Ref. |
|---|---|---|---|---|---|---|
| Quercetin | Dosage: 5, 10, 20, 40 mg kg−1 | Albino rats | in vivo | PTZ | ↑antiseizure effect, ↑anticonvulsant effect | [113] |
| Dosage: 25, 50, and 100. mg kg−1 Ad: i.p. |
Wistar rats | in vivo | PTZ | ↑anticonvulsant effects, ↓seizure severity, ↓lipid peroxidation, ↑antioxidant effect, ↑memory retrieval in the passive avoidance task | [115] | |
| Quercetin/ Anisomelesma labarica |
Dosage: 25 and 50 mg kg−1 Ad: i.p. |
Wistar rat | in vivo | PTZ |
↓ locomotor activity and motor activity performance |
[114] |
| Dosage: 6.25 and 12.5 mg kg−1 Ad: i.p. Duration: 1 week |
Wistar rat | in vivo | PTZ |
potentiating the GABAergic system, inhibition of the NMDA receptor and Na+ channels. |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.M.; Zhang, Z.Y.; Wang, R.X. Protective Mechanisms of Quercetin Against Myocardial Ischemia Reperfusion Injury. Front Physiol 2020. [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Mishra, S.K.; Pant, H.C. Oxidative Stress in Neurodegeneration. Adv Pharmacol Sci 2011, 1–13. [Google Scholar] [CrossRef]
- Gkekas, I.; Gioran, A.; Boziki, M.K.; Grigoriadis, N.; Chondrogianni, N.; Petrakis, S. Oxidative Stress and Neurodegeneration: Interconnected Processes in Polyq Diseases. Antioxidants 2021, 1450. [Google Scholar] [CrossRef] [PubMed]
- Bandiwadekar, A.; Jose, J.; Khayatkashani, M.; Habtemariam, S.; Khayat Kashani, H.R.; Nabavi, S.M. Emerging Novel Approaches for the Enhanced Delivery of Natural Products for the Management of Neurodegenerative Diseases. Journal of Molecular Neuroscience 2021, 72, 653–676. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.M.; Muday, G.K. Flavonols Modulate Lateral Root Emergence by Scavenging Reactive Oxygen Species in Arabidopsis Thaliana. Journal of Biological Chemistry 2021, 296. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, A.; Ogun, M. Biochemistry of Reactive Oxygen and Nitrogen Species. In Basic Principles and Clinical Significance of Oxidative Stress; 2015.
- Klebanoff, S.J. Oxygen Metabolism and the Toxic Properties of Phagocytes. Ann Intern Med 1980, 93, 391–398. [Google Scholar] [CrossRef]
- Sheikh, S. ; Safia; Haque, E.; Mir, S.S. Neurodegenerative Diseases: Multifactorial Conformational Diseases and Their Therapeutic Interventions. J Neurodegener Dis, 2013; 1–8. [Google Scholar] [CrossRef]
- Erden Inal, M.; Kahraman, A.; Köken, T. Beneficial Effects of Quercetin on Oxidative Stress Induced by Ultraviolet A. Clin Exp Dermatol 2001, 26, 536–539. [Google Scholar] [CrossRef]
- Elumalai, P.; Lakshmi, S. Role of Quercetin Benefits in Neurodegeneration. In Advances in Neurobiology; 2016; Vol. 12, pp. 229–245.
- Halliwell, B. Oxidants and Human Disease: Some New Concepts 1. The FASEB Journal 1987, 1, 358–364. [Google Scholar] [CrossRef]
- Ansari, M.A.; Abdul, H.M.; Joshi, G.; Opii, W.O.; Butterfield, D.A. Protective Effect of Quercetin in Primary Neurons against Aβ(1-42): Relevance to Alzheimer’s Disease. Journal of Nutritional Biochemistry 2009, 20, 269–275. [Google Scholar] [CrossRef]
- Islam, M.S.; Quispe, C.; Hossain, R.; Torequl Islam, M.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N.; et al. Neuropharmacological Effects of Quercetin: A Literature-Based Review. [CrossRef]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, Antioxidants, and the Degenerative Diseases of Aging. Proc Natl Acad Sci U S A 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Zalpoor, H.; Nabi-Afjadi, M.; Forghaniesfidvajani, R.; Tavakol, C.; Farahighasreaboonasr, F.; Pakizeh, F.; Dana, V.G.; Seif, F. Quercetin as a JAK–STAT Inhibitor: A Potential Role in Solid Tumors and Neurodegenerative Diseases. Cell Mol Biol Lett 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Huang, Y.; Tu, S. Potential Implications of Quercetin in Autoimmune Diseases. Front Immunol 2021, 12. [Google Scholar] [CrossRef]
- Haytowitz, D.; Wu, X.; Bhagwat, S. USDA Database for the Flavonoid Content of Selected Foods, Release 3.3. USDA Agricultureal Research Service 2018. [Google Scholar]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A Critical Review of the Data Related to the Safety of Quercetin and Lack of Evidence of in Vivo Toxicity, Including Lack of Genotoxic/Carcinogenic Properties. Food and Chemical Toxicology 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Mishra, D.; Flora, S.J.S. Quercetin Administration during Chelation Therapy Protects Arsenic-Induced Oxidative Stress in Mice. Biol Trace Elem Res 2008, 122, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and Anti-Inflammatory Activities of Quercetin and Its Derivatives. J Funct Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Dhaouadi, Z.; Nsangou, M.; Garrab, N.; Anouar, E.H.; Marakchi, K.; Lahmar, S. DFT Study of the Reaction of Quercetin with · O2- and · OH Radicals. Journal of Molecular Structure: THEOCHEM, 2009; 35–42. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and Cancer: New Insights into Its Therapeutic Effects on Ovarian Cancer Cells. Cell Biosci 2020, 10. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Asemi, Z. Quercetin: A Natural Compound for Ovarian Cancer Treatment. J Ovarian Res 2019, 12. [Google Scholar] [CrossRef]
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological Basis and New Insights of Quercetin Action in Respect to Its Anti-Cancer Effects. Biomedicine and Pharmacotherapy 2020, 121. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A Review on Anti-Cancer Properties of Quercetin in Breast Cancer. Life Sci 2020. [CrossRef] [PubMed]
- Hou, D.D.; Zhang, W.; Gao, Y.L.; Sun, Y. zhe; Wang, H.X.; Qi, R.Q.; Chen, H.D.; Gao, X.H. Anti-Inflammatory Effects of Quercetin in a Mouse Model of MC903-Induced Atopic Dermatitis. Int Immunopharmacol 2019, 74. [Google Scholar] [CrossRef]
- Kawabata, K.; Baba, N.; Sakano, T.; Hamano, Y.; Taira, S.; Tamura, A.; Baba, S.; Natsume, M.; Ishii, T.; Murakami, S.; et al. Functional Properties of Anti-Inflammatory Substances from Quercetin-Treated Bifidobacterium Adolescentis. Biosci Biotechnol Biochem 2018, 82, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.R. Anti-Inflammatory Potential of Quercetin in COVID-19 Treatment. Journal of Inflammation (United Kingdom) 2021, 18. [Google Scholar] [CrossRef]
- Liu, M.; Yu, Q.; Xiao, H.; Li, M.; Huang, Y.; Zhang, Q.; Li, P. The Inhibitory Activities and Antiviral Mechanism of Medicinal Plant Ingredient Quercetin Against Grouper Iridovirus Infection. Front Microbiol 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and Its Derivates as Antiviral Potentials: A Comprehensive Review. Phytotherapy Research 2022, 36, 266–278. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, J.E.; Song, Y.J. Antiviral Activities of Quercetin and Isoquercitrin against Human Herpesviruses. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic Effect of Quercetin as an Antibiotic Alternative in Vivo and Its Antibacterial Mechanism in Vitro. J Food Prot 2018, 81, 69–78. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Richert, A.; Grabska-Zielińska, S.; Rudawska, A.; Bouaziz, M. Antibacterial Films Based on Polylactide with the Addition of Quercetin and Poly(Ethylene Glycol). Materials 2021, 14. [Google Scholar] [CrossRef]
- Júnior, S.D. da C.; Santos, J.V. de O.; Campos, L.A. de A.; Pereira, M.A.; Magalhães, N.S.S.; Cavalcanti, I.M.F. Antibacterial and Antibiofilm Activities of Quercetin against Clinical Isolates of Staphyloccocus Aureus and Staphylococcus Saprophyticus with Resistance Profile. International Journal of Environment, Agriculture and Biotechnology 2018, 3. [Google Scholar] [CrossRef]
- Jaisinghani, R.N. Antibacterial Properties of Quercetin. Microbiol Res (Pavia) 2017, 8. [Google Scholar] [CrossRef]
- Aljadaan, S.A.N.; Elias, R.S.; Al-Anssari, R.A. Investigation of the Antioxidant and Antibacterial Activity of Novel Quercetin Derivatives. Biointerface Res Appl Chem 2020, 10. [Google Scholar] [CrossRef]
- Patel, R. V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.S. Therapeutic Potential of Quercetin as a Cardiovascular Agent. Eur J Med Chem 2018, 115, 889–904. [Google Scholar] [CrossRef]
- Lei, X.; Chao, H.; Zhang, Z.; Lv, J.; Li, S.; Wei, H.; Xue, R.; Li, F.; Li, Z. Neuroprotective Effects of Quercetin in a Mouse Model of Brain Ischemic/Reperfusion Injury via Anti-Apoptotic Mechanisms Based on the Akt Pathway. Mol Med Rep 2015, 12, 3688–3696. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement; 2018; Vol. 62; ISBN 4930184126.
- Harishkumar, R.; Reddy, L.P.K.; Karadkar, S.H.; Murad, M. Al; Karthik, S.S.; Manigandan, S.; Selvaraj, C.I.; Christopher, J.S.G. Toxicity and Selective Biochemical Assessment of Quercetin, Gallic Acid, and Curcumin in Zebrafish. Biol Pharm Bull 2019, 42, 1969–1976. [Google Scholar] [CrossRef]
- Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Jomova, K.; Kollar, V.; Rusko, M.; Valko, M. Management of Oxidative Stress and Other Pathologies in Alzheimer’s Disease. Arch Toxicol 2019, 93, 2491–2513. [Google Scholar] [CrossRef]
- Kook, D.; Wolf, A.H.; Yu, A.L.; Neubauer, A.S.; Priglinger, S.G.; Kampik, A.; Welge-Lüssen, U.C. The Protective Effect of Quercetin against Oxidative Stress in the Human RPE in Vitro. Invest Ophthalmol Vis Sci 2008, 49, 1712–1720. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Valério, D.A.; Georgetti, S.R.; Magro, D.A.; Casagrande, R.; Cunha, T.M.; Vicentini, F.T.M.C.; Vieira, S.M.; Fonseca, M.J.V.; Ferreira, S.H.; Cunha, F.Q.; et al. Quercetin Reduces Inflammatory Pain: Inhibition of Oxidative Stress and Cytokine Production. J Nat Prod 2009, 72. [Google Scholar] [CrossRef]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic Insights and Perspectives Involved in Nfeuroprotective Action of Quercetin. Biomedicine and Pharmacotherapy 2021, 140, 111729. [Google Scholar] [CrossRef] [PubMed]
- Deture, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol Neurodegener 2019, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kommaddi, R.P.; Das, D.; Karunakaran, S.; Nanguneri, S.; Bapat, D.; Ray, A.; Shaw, E.; Bennett, D.A.; Nair, D.; Ravindranath, V. Aβ Mediates F-Actin Disassembly in Dendritic Spines Leading to Cognitive Deficits in Alzheimer’s Disease. Journal of Neuroscience 2018, 38, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Parent, M.J.; Zimmer, E.R.; Shin, M.; Kang, M.S.; Fonov, V.S.; Mathieu, A.; Aliaga, A.; Kostikov, A.; do Carmo, S.; Dea, D.; et al. Multimodal Imaging in Rat Model Recapitulates Alzheimer’s Disease Biomarkers Abnormalities. Journal of Neuroscience 2017, 37, 12263–12271. [Google Scholar] [CrossRef]
- Wallace, R.A.; Dalton, A.J. What Can We Learn from Study of Alzheimer’s Disease in Patients with Down Syndrome for Early-Onset Alzheimer’s Disease in the General Population? Alzheimers Res Ther 2011, 3. [Google Scholar] [CrossRef]
- Hollingworth, P.; Harold, D.; Jones, L.; Owen, M.J.; Williams, J. Alzheimer’s Disease Genetics: Current Knowledge and Future Challenges. Int J Geriatr Psychiatry 2011, 26, 793–802. [Google Scholar] [CrossRef]
- Mayeux, R.; Stern, Y. Epidemiology of Alzheimer Disease. Cold Spring Harb Perspect Med 2012, 2. [Google Scholar] [CrossRef]
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids Protect Neuronal Cells from Oxidative Stress by Three Distinct Mechanisms. Free Radic Biol Med 2001, 30, 433–446. [Google Scholar] [CrossRef]
- Ho, J.H.; Chang, Y.L. Protective Effects of Quercetin and Vitamin C against Oxidative Stress-Induced Neurodegeneration. J Agric Food Chem 2004, 52, 7514–7517. [Google Scholar] [CrossRef]
- Porat, Y.; Abramowitz, A.; Gazit, E. Inhibition of Amyloid Fibril Formation by Polyphenols: Structural Similarity and Aromatic Interactions as a Common Inhibition Mechanism. Chemical Biology Drug Design 2006, 67, 27–37. [Google Scholar] [CrossRef]
- Jiménez-Aliaga, K.; Bermejo-Bescós, P.; Benedí, J.; Martín-Aragón, S. Quercetin and Rutin Exhibit Antiamyloidogenic and Fibril-Disaggregating Effects in Vitro and Potent Antioxidant Activity in APPswe Cells. Life Sci 2011, 89, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Yoshiike, Y.; Takashima, A.; Hasegawa, K.; Naiki, H.; Yamada, M. Potent Anti-Amyloidogenic and Fibril-Destabilizing Effects of Polyphenols in Vitro: Implications for the Prevention and Therapeutics of Alzheimer’s Disease. J Neurochem 2003, 87, 172–181. [Google Scholar] [CrossRef]
- Kim, H.; Park, B.S.; Lee, K.G.; Cheol, Y.C.; Sung, S.J.; Kim, Y.H.; Lee, S.E. Effects of Naturally Occurring Compounds on Fibril Formation and Oxidative Stress of β-Amyloid. J Agric Food Chem 2005, 53, 8537–8541. [Google Scholar] [CrossRef]
- Rattanajarasroj, S.; Unchern, S. Comparable Attenuation of Aβ25-35-Induced Neurotoxicity by Quercitrin and 17β-Estradiol in Cultured Rat Hippocampal Neurons. Neurochem Res 2010, 35, 1196–1205. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Mu, X. Effect of Quercetin on PC12 Alzheimer’s Disease Cell Model Induced by Aβ 25-35 and Its Mechanism Based on Sirtuin1/Nrf2/HO-1 Pathway. Biomed Res Int 2020, 1–10. [Google Scholar] [CrossRef]
- Ishisaka, A.; Mukai, R.; Terao, J.; Shibata, N.; Kawai, Y. Specific Localization of Quercetin-3-O-Glucuronide in Human Brain. Arch Biochem Biophys 2014, 557, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Ferruzzi, M.G.; Janle, E.M.; Wang, J.; Gong, B.; Chen, T.Y.; Lobo, J.; Cooper, B.; Wu, Q.L.; Talcott, S.T.; et al. Identification of Brain-Targeted Bioactive Dietary Quercetin-3-O- Glucuronide as a Novel Intervention for Alzheimer’s Disease. FASEB Journal 2013, 27, 769–781. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, L.; Zhu, B.; Li, Q.; Yew, D.T.; Yao, Z.; Xu, J. Protective Effects of Ginkgo Biloba Extract (EGb761) and Its Constituents Quercetin and Ginkgolide B against β-Amyloid Peptide-Induced Toxicity in SH-SY5Y Cells. Chem Biol Interact 2009, 181, 115–123. [Google Scholar] [CrossRef]
- Zhang, X.D.; Liu, X.Q.; Kim, Y.H.; Whang, W.K. Chemical Constituents and Their Acetyl Cholinesterase Inhibitory and Antioxidant Activities from Leaves of Acanthopanax Henryi: Potential Complementary Source against Alzheimer’s Disease. Arch Pharm Res 2014, 37, 606–616. [Google Scholar] [CrossRef]
- Miriyala, S.; K. Holley, A.; St Clair, D.K. Mitochondrial Superoxide Dismutase—Signals of Distinction. Anticancer Agents Med Chem 2012, 11, 181–190. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, S.; Li, J.; Sun, Y.; Hasimu, H.; Liu, R.; Zhang, T. Quercetin Protects Human Brain Microvascular Endothelial Cells from Fibrillar β-Amyloid1-40-Induced Toxicity. Acta Pharm Sin B 2015, 5, 47–54. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Muñoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The Flavonoid Quercetin Ameliorates Alzheimer’s Disease Pathology and Protects Cognitive and Emotional Function in Aged Triple Transgenic Alzheimer’s Disease Model Mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Paula, P.C.; Maria, S.G.A.; Luis, C.H.; Patricia, C.G.G. Preventive Effect of Quercetin in a Triple Transgenic Alzheimer’s Disease Mice Model. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Li, S.Q.; Wu, W.L.; Zhu, X.Y.; Wang, Y.; Yuan, H.Y. Effects of Long-Term Treatment with Quercetin on Cognition and Mitochondrial Function in a Mouse Model of Alzheimer’s Disease. Neurochem Res 2014, 39, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, M.; Itoh, M.; Ohta, K.; Li, S.; Ueda, M.; Wang, M.X.; Nishida, E.; Islam, S.; Suzuki, C.; Ohzawa, K.; et al. Quercetin Reduces EIF2α Phosphorylation by GADD34 Induction. Neurobiol Aging 2015, 36, 2509–2518. [Google Scholar] [CrossRef]
- JUNG, S.H.; Murphy, E.A.; McClellan, J.L.; Carmichael, M.D.; Davis, J.M. The Dietary Flavonoid Quercetin Decreases Neuroinflammation in a Mouse Model of Alzheimer’s Disease. The FASEB Journal 2010, 24. [Google Scholar] [CrossRef]
- Godoy, J.A.; Lindsay, C.B.; Quintanilla, R.A.; Carvajal, F.J.; Cerpa, W.; Inestrosa, N.C. Quercetin Exerts Differential Neuroprotective Effects Against H2O2 and Aβ Aggregates in Hippocampal Neurons: The Role of Mitochondria. Mol Neurobiol 2017, 54, 7116–7128. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Q.; Li, Z.; Dang, M.; Lin, Y.; Hou, X. Activation of Nrf2 Signaling by Sitagliptin and Quercetin Combination against β-Amyloid Induced Alzheimer’s Disease in Rats. Drug Dev Res 2019, 80, 837–845. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.; Zhong, L.; Wang, N.; Yang, L.; Liu, C.C.; Li, H.; Wang, X.; Zhou, Y.; Zhang, Y.; et al. Quercetin Stabilizes Apolipoprotein e and Reduces Brain Aβ Levels in Amyloid Model Mice. Neuropharmacology 2016, 179–192. [Google Scholar] [CrossRef]
- Moreno, L.C.G. e. I.; Puerta, E.; Suárez-Santiago, J.E.; Santos-Magalhães, N.S.; Ramirez, M.J.; Irache, J.M. Effect of the Oral Administration of Nanoencapsulated Quercetin on a Mouse Model of Alzheimer’s Disease. Int J Pharm 2017, 14, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Olayinka, J.N.; Eduviere, A.; Adeoluwa, O.; Akinluyi, E.; Obisesan, A.; Akawa, O.; Adebanjo, A. Quercetin Mitigates Scopolamine-Induced Memory Dysfunction: Impact on Oxidative Stress and Cholinergic Mechanisms. Metab Brain Dis 2022, 37, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Palle, S.; Neerati, P. Quercetin Nanoparticles Attenuates Scopolamine Induced Spatial Memory Deficits and Pathological Damages in Rats. Bulletin of Faculty of Pharmacy, Cairo University 2017, 55, 101–106. [Google Scholar] [CrossRef]
- Sharma, D.R.; Wani, W.Y.; Sunkaria, A.; Kandimalla, R.J.; Sharma, R.K.; Verma, D.; Bal, A.; Gill, K.D. Quercetin Attenuates Neuronal Death against Aluminum-Induced Neurodegeneration in the Rat Hippocampus. Neuroscience 2016, 324, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.Y.; Gudup, S.; Sunkaria, A.; Bal, A.; Singh, P.P.; Kandimalla, R.J.L.; Sharma, D.R.; Gill, K.D. Protective Efficacy of Mitochondrial Targeted Antioxidant MitoQ against Dichlorvos Induced Oxidative Stress and Cell Death in Rat Brain. Neuropharmacology 2011, 61, 1193–1201. [Google Scholar] [CrossRef]
- Hou, Y.; Aboukhatwa, M.A.; Lei, D.L.; Manaye, K.; Khan, I.; Luo, Y. Anti-Depressant Natural Flavonols Modulate BDNF and Beta Amyloid in Neurons and Hippocampus of Double TgAD Mice. Neuropharmacology 2010, 58, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M. Type 2 Diabetes Mellitus and Alzheimer’s Disease. World J Diabetes 2014, 5, 889–893. [Google Scholar] [CrossRef]
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Frittitta, L. Type 2 Diabetes Mellitus and Alzheimer’s Disease: Role of Insulin Signalling and Therapeutic Implications. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef]
- Xu, W.L.; Von Strauss, E.; Qiu, C.X.; Winblad, B.; Fratiglioni, L. Uncontrolled Diabetes Increases the Risk of Alzheimer’s Disease: A Population-Based Cohort Study. Diabetologia 2009. [Google Scholar] [CrossRef]
- Michailidis, M.; Moraitou, D.; Tata, D.A.; Kalinderi, K.; Papamitsou, T.; Papaliagkas, V. Alzheimer’s Disease as Type 3 Diabetes: Common Pathophysiological Mechanisms between Alzheimer’s Disease and Type 2 Diabetes. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Kandimalla, R.; Thirumala, V.; Reddy, P.H. Is Alzheimer’s Disease a Type 3 Diabetes? A Critical Appraisal. Biochim Biophys Acta Mol Basis Dis 2017, 1863, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Giau, V. Van Type 3 Diabetes and Its Role Implications in Alzheimer’s Disease. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Halli-Tierney, A.D.; Luker, J.; Carroll, D.G. Parkinson Disease. Am Fam Physician 2020. [Google Scholar] [CrossRef]
- Vargas-Restrepo, F.; Sabogal-Guáqueta, A.M.; Cardona-Gómez, G.P. Quercetin Ameliorates Inflammation in CA1 Hippocampal Region in Aged Triple Transgenic Alzheimer’s Disease Mice Model. Biomedica 2018, 38, 1–23. [Google Scholar] [CrossRef]
- Benameur, T.; Soleti, R.; Porro, C. The Potential Neuroprotective Role of Free and Encapsulated Quercetin Mediated by Mirna against Neurological Diseases. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Bournival, J.; Plouffe, M.; Renaud, J.; Provencher, C.; Martinoli, M.G. Quercetin and Sesamin Protect Dopaminergic Cells from MPP +-Induced Neuroinflammation in a Microglial (N9)-Neuronal (PC12) Coculture System. Oxid Med Cell Longev 2012. [Google Scholar] [CrossRef]
- Bureau, G.; Longpré, F.; Martinoli, M.G. Resveratrol and Quercetin, Two Natural Polyphenols, Reduce Apoptotic Neuronal Cell Death Induced by Neuroinflammation. J Neurosci Res 2008, 86, 403–410. [Google Scholar] [CrossRef]
- Amanzadeh, E.; Esmaeili, A.; Rahgozar, S.; Nourbakhshnia, M. Application of Quercetin in Neurological Disorders: From Nutrition to Nanomedicine. Rev Neurosci 2019, 30, 555–572. [Google Scholar] [CrossRef]
- Zhu, M.; Han, S.; Fink, A.L. Oxidized Quercetin Inhibits α-Synuclein Fibrillization. Biochim Biophys Acta Gen Subj 2013, 1830, 2872–2881. [Google Scholar] [CrossRef]
- Magalingam, K.B.; Radhakrishnan, A.; Haleagrahara, N. Protective Effects of Flavonol Isoquercitrin, against 6-Hydroxy Dopamine (6-OHDA)—Induced Toxicity in PC12 Cells. BMC Res Notes 2014, 7. [Google Scholar] [CrossRef]
- Magalingam, K.B.; Radhakrishnan, A.; Haleagrahara, N. Protective Effects of Quercetin Glycosides, Rutin, and Isoquercetrin against 6-Hydroxydopamine (6-OHDA)-Induced Neurotoxicity in Rat Pheochromocytoma (PC-12) Cells. Int J Immunopathol Pharmacol 2016, 29, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Pany, S.; Pal, A.; Sahu, P.K. Neuroprotective Effect of Quercetin in Neurotoxicity Induced Rats: Role of Neuroinflammation in Neurodegeneration. Asian Journal of Pharmaceutical and Clinical Research 2014, 7, 152–156. [Google Scholar]
- Singh, S.; Jamwal, S.; Kumar, P. Neuroprotective Potential of Quercetin in Combination with Piperine against 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Neurotoxicity. Neural Regen Res 2017, 12, 1137–1144. [Google Scholar] [CrossRef]
- Lv, C.; Hong, T.; Yang, Z.; Zhang, Y.; Wang, L.; Dong, M.; Zhao, J.; Mu, J.; Meng, Y. Effect of Quercetin in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine- Induced Mouse Model of Parkinson’s Disease. Evidence-based Complementary and Alternative Medicine, 2016; 1–6. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Sriraksa, N.; Muchimapura, S.; Tiamkao, S.; Brown, K.; Chaisiwamongkol, K. Cognitive-Enhancing Effect of Quercetin in a Rat Model of Parkinson’s Disease Induced by 6-Hydroxydopamine. Evidence-based Complementary and Alternative Medicine, 2016. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Joghataei, M.T.; Nobakht, M.; Aryanpour, R. Neuroprotective Effect of Quercetin in a Model of Parkinson’s Disease in Rat: A Histochemical Analysis. Basic Clin Neurosci 2009, 1, 3–6. [Google Scholar]
- Karuppagounder, S.S.; Madathil, S.K.; Pandey, M.; Haobam, R.; Rajamma, U.; Mohanakumar, K.P. Quercetin Up-Regulates Mitochondrial Complex-I Activity to Protect against Programmed Cell Death in Rotenone Model of Parkinson’s Disease in Rats. Neuroscience 2013, 236, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Madiha, S.; Batool, Z.; Tabassum, S.; Liaquat, L.; Sadir, S.; Shahzad, S.; Naqvi, F.; Saleem, S.; Yousuf, S.; Nawaz, A.; et al. Quercetin Exhibits Potent Antioxidant Activity, Restores Motor and Non-Motor Deficits Induced by Rotenone Toxicity. PLoS One 2021, 16. [Google Scholar] [CrossRef]
- Denny Joseph, K.M. ; Muralidhara Combined Oral Supplementation of Fish Oil and Quercetin Enhances Neuroprotection in a Chronic Rotenone Rat Model: Relevance to Parkinson’s Disease. Neurochem Res 2015, 40, 894–905. [Google Scholar] [CrossRef]
- Bombardi Duarte, A.C.; Santana, M.G.; di Camilo Orfali, G.; de Oliveira, C.T.P.; Priolli, D.G. Literature Evidence and ARRIVE Assessment on Neuroprotective Effects of Flavonols in Neurodegenerative Diseases’ Models. CNS Neurol Disord Drug Targets 2017, 17, 34–42. [Google Scholar] [CrossRef]
- Sandhir, R.; Mehrotra, A. Quercetin Supplementation Is Effective in Improving Mitochondrial Dysfunctions Induced by 3-Nitropropionic Acid: Implications in Huntington’s Disease. Biochim Biophys Acta Mol Basis Dis 2013, 1832, 421–430. [Google Scholar] [CrossRef]
- Chakraborty, J.; Singh, R.; Dutta, D.; Naskar, A.; Rajamma, U.; Mohanakumar, K.P. Quercetin Improves Behavioral Deficiencies, Restores Astrocytes and Microglia, and Reduces Serotonin Metabolism in 3-Nitropropionic Acid-Induced Rat Model of Huntington’s Disease. CNS Neurosci Ther 2014, 20, 10–19. [Google Scholar] [CrossRef]
- Jain, D.; Gangshettiwar, A. Combination of Lycopene, Quercetin and Poloxamer188 Alleviates Anxiety and Depression in 3-Nitropropionic Acid-Induced Huntingtons Disease in Rats. J Intercult Ethnopharmacol 2014. [Google Scholar] [CrossRef] [PubMed]
- Denny Joseph, K.M. ; Muralidhara Enhanced Neuroprotective Effect of Fish Oil in Combination with Quercetin against 3-Nitropropionic Acid Induced Oxidative Stress in Rat Brain. Prog Neuropsychopharmacol Biol Psychiatry 2013. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, A.; Singla, S.; Arora, V.; Chopra, K. Neuroprotective Effect of Sesamol and Quercetin against QA Induced Neurotoxicity: An Experimental Paradigm of Huntington’s Disease. J Neurol Sci 2013, 333. [Google Scholar] [CrossRef]
- Patel, D.C.; Tewari, B.P.; Chaunsali, L.; Sontheimer, H. Neuron–Glia Interactions in the Pathophysiology of Epilepsy. Nat Rev Neurosci 2019, 20, 282–297. [Google Scholar] [CrossRef]
- Sefil, F.; Kahraman, I.; Dokuyucu, R.; Gokce, H.; Ozturk, A.; Tutuk, O.; Aydin, M.; Ozkan, U.; Pinar, N. Ameliorating Effect of Quercetin on Acute Pentylenetetrazole Induced Seizures in Rats. Int J Clin Exp Med 2014, 7, 2471–2477. [Google Scholar]
- Choudhary, N.; Bijjem, K.R. V.; Kalia, A.N. Antiepileptic Potential of Flavonoids Fraction from the Leaves of Anisomeles Malabarica. J Ethnopharmacol 2011, 135, 238–242. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Moghbelinejad, S.; Abbasi, E.; Yonesi, F.; Haghighi, M.R.; Lotfizadeh, M.; Bazahang, P. Effects of Quercetin on Oxidative Stress and Memory Retrieval in Kindled Rats. Epilepsy and Behavior 2013, 28, 151–155. [Google Scholar] [CrossRef]
| Source | Quercetin | |||
|---|---|---|---|---|
| Food | Common Name | Scientific Name | Active Portions | mg 100 g−1 weight |
| Fruits | Acerola | Malpighia emarginata | Fruits | 4.74 |
| Apple | Malus domestica | Fruits | 19.36 | |
| Cranberry | Vaccinium oxycoccus | Fruits | 25.0 | |
| Apricots | Prunus armeniaca | Fruits | 1.63 | |
| Blackberries | Rubus spp. | Fruits | 3.58 | |
| Blueberries | Vaccinium spp. | Fruits | 7.67 | |
| Cherries | Prunus avium | Fruits | 17.44 | |
| Cranberries | Vaccinium macrocarpon | Fruits | 14.84 | |
| Grapefruit | Citrus paradisi | Fruits | 0.50 | |
| Grapes | Vitis vinifera | Fruits | 1.04 | |
| Vegetables | Capers, raw | Capparis spinosa | Flower buds | 233.84 |
| Onions, raw | Allium cepa | Bulbs | 20.30 | |
| Dill weed, fresh | Anethum graveolens | Leaves | 55.15 | |
| Oregano | Origanum vulgare | Leaves | 42.00 | |
| Tarragon, fresh | Artemisia dracunculus | Leaves | 10.00 | |
| Chicory | Cichorium intybus | Leaves | 6.49 | |
| Beverage | mg 100 mL−1 | |||
| Black tea | 2.50 | |||
| Red wine | 3.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).