1. Introduction
Due to seasonal variations in forage quality and production, Brazilian beef females reach puberty from 2 and 3 years of age [
1,
2], a common trait for
Bos Indicus females, which are later than
Bos Taurus. According to the Brazilian Association of Zebu Breeders, in 2020 the average age at first calving in Nellore animals was 39 ± 6 months [
3], confirming the onset of puberty is still late.
However, heifers can be managed to start their reproductive life at 14 months of age with genetic selection and nutritional management strategies [
2,
4]. The reduction in the puberty implies a reduction in the first calving age, in addition to allowing the identification and removal of unproductive young females from the herd [
5].
The age at first birth is linked to the concept of “permanence”, which indicates the probability that the female will remain in the herd until 76 months old, giving birth at least 3 times. In other words, the sooner this animal gives birth, the greater the chance of achieving this goal. In 2020, this rate was 33.9% in Nellore females in Brazil [
3]. Decreasing the age at first calving from 3 to 2 years can generate an increase of 0.5 to 0.8 calves per cow during its productive life, and about a 6% increase in the profitability of the cow-calf system [
6].
However, improving the reproductive aspects of primiparous cows represents one of the biggest challenges for a better efficiency in beef herds, as they generally have lower reproductive rates than multiparous cows. One of the main causes of this pattern in primiparous cows, is that the heifers are not physically or physiologically mature at the first calving [
7]. Consequently, in addition to growth requirements, maintenance and lactation requirements are added [
8,
9,
10] after the first calving. Thus, due to the greater proportional energy demand, primiparous cows tend to return to ovarian function, on average, 20 to 40 days later than multiparous cows [
11,
12].
Heifers that begin their reproductive life at 24 months have more time to grow and reach the necessary weight to be ready for calving than heifers that become pregnant at 14 months. However, as long as they are able to maintain growth, heifers that become pregnant at 14 or 24 months may have similar reproductive rates [
13], if managed to achieve pre- and postpartum weight gain to maintain lactation, return to cyclic activity and wean a healthy calf.
Therefore, the objective of this study was to evaluate the performance and metabolic characteristics of primiparous Nellore beef cows under grazing, which became pregnant at 14 (young) or 24 (conventional) months of age, and the performance of their respective calves from birth to weaning.
2. Materials and Methods
The experiment was conducted at the Research, Teaching and Extension Unit in Beef Cattle of the Federal University of Viçosa, Viçosa, Minas Gerais State.
2.1. Animals, facilities, and management
Thirty-eight primiparous Nellore beef cows at 202 ± 5 days of gestation were used, including 20 cows that were bred for their first pregnancy at 14 months of age (body weight [BW] = 403 ± 6.6 kg), and 18 animals that became pregnant at 24 months of age (BW = 505 ± 7.5 kg). Cows were randomly divided into seven groups, each group containing 2-3 animals of each breeding age.
The experimental area covered with
Uruchloa decumbens grass was divided into 7 paddocks of 3.2-ha for continuous grazing, equipped with water dispensers and feeders. Every 28 days, cows were rotated between paddocks to control any paddock effects on the treatments. All animals received 1.5 kg/d of a protein-energy supplement (
Table 1), which was provided to the animals 3 times a week as follows: Monday—3 kg, Wednesday—3 kg, and Friday—4.5 kg at 11:00 h, from 90 days before expected calving (last third of gestation) to the last day of the breeding season (final pregnancy diagnosis). From the end of breeding until weaning (approximately 240 days postpartum), cow-calf pairs had only ad libitum access to a complete mineral mixture (
Table 1).
2.2. Experimental procedures and sampling
Primiparous cows were weighed at 08:00 h at the beginning of the experiment (90 days before expected calving), at calving, and at weaning. Calves’ BW was recorded at birth, at 45, 95, 160, 220, and 240 days. The BW was measured without fasting; except on the day of parturition. The body condition score (BCS) was recorded at prepartum, calving, and weaning by three experienced evaluators on a scale ranging from 1 to 9 [
14] and averaged across evaluators.
At 15 days before calving and at weaning, the carcass ultrasound measurements were performed. The Longissimus muscle area and Longissimus muscle depth were taken between the 12th and 13th ribs. The subcutaneous fat thickness was taken over Longissimus muscle between the 12th and 13th rib and over the gluteus biceps (rump fat). The carcass images were recorded using ultrasound an Aloka SSD500II, equipped with an 18 cm long linear probe and the BioSoft Toolbox® II for Beef software (Biotronics Inc., Ames, Iowa, USA).
Forage samples were taken every 28 days by hand-plucking sampling to evaluate the chemical composition of the forage consumed by the animals (
Table 2). All samples were oven-dried (55 °C) and ground in a Wiley mill (model 3; Arthur H. Thomas, Philadelphia, Pennsylvania, USA) to pass through a 2-mm sieve. After that, half of each ground sample was ground again to pass through a 1-mm sieve.
Blood samples were collected by jugular venous puncture using vacuum tubes with clot activator and serum separation gel (BD Vacutainer SST II Plus, São Paulo, Brazil) and with ethylenediamine tetraacetic acid (EDTA) and sodium fluoride (BD Vacutainer Fluoreto/EDTA, São Paulo, Brazil) 15 days before expected calving, and 30, 60, 120, and 240 days postpartum, at 08:00 h, to quantity the blood concentrations of glucose, non-esterified fatty acids (NEFA), β-hydroxybutyrate (β-OHB), total protein, albumin, and urea. At 30 and 45 days postpartum, blood was collected for progesterone analysis; and 15 days before calving, 60, and 240 days postpartum for insulin-like growth factor-1 (IGF-1) analysis. After collection, samples were centrifuged at 3,600 × g for 15 min and the serum or plasma was frozen (-20 °C) for further analysis.
At 60, 120 and 240 days after calving, milk yield was estimated by collecting milk with a milking machine. Calves were separated from their mothers at 15:00 h on the previous day. At 17:30 h the calves were again placed with their mothers to suck all the milk, being separated again at 18:00 h. After that, the cows returned to the paddocks whereas the calves remained in the cattle shed with free access to water. At 06:00 h of the following day, milking was performed mechanically. Milk secretion was stimulated with 1 mL of oxytocin (10 UI/ mL, Lactocina
®, Patrocínio Paulista, São Paulo, Brazil) in the mammary artery. After each milking, milk was weighed and recorded. The milk produced was corrected for production on a 24-h basis [
15].
All cows underwent fixed-time artificial insemination (FTAI) protocol 45 days after calving. For this, all cows received a single-dose intravaginal implant (Primer®, Agener União, Embu-Gauçu, São Paulo, Brazil) containing 1.9 g of progesterone (CIDR-B®, Pfizer Animal Health, São Paulo, Brazil) plus 2 mg of estradiol benzoate (Estrogin®, Farmavet, São Paulo, Brazil). Nine days after the beginning of the FTAI protocol, the implant was removed and 1.5 mL of equine chorionic gonadotropin (Ecegon®, Biogeneses Bago, Curitiba, Paraná, Brazil) plus 2.0 mL of prostaglandin F2 alpha (Estron®, Agener União, Embu-Guaçu, São Paulo, Brazil) were administered. Twenty-four hours after the implant removal, 1 mL of estradiol benzoate (RIC-BE®, Agener União, Embu-Guaçu, São Paulo, Brazil) was administrated. Pre-ovulatory follicle diameter was measured by ultrasonography (DP-2200Vet® with a 7.5-MHz linear-array transrectal transducer; Mindray) 48h after implant removal and cows that presented pre-ovulatory follicle diameter greater than 11 mm were artificial inseminated. Conception and pregnancy rates were measured 30 days after the artificial insemination. A second FTAI protocol was performed in cows that did not present a positive pregnancy diagnosis in the first FTAI.
2.3. Laboratory analyses and calculations
Supplement and forage samples processed to pass a 1-mm sieve were analyzed for DM, ash, crude protein (CP), ether extract (EE), and neutral detergent fiber corrected for ash and protein (apNDF) [
16]. Indigestible neutral detergent fiber (iNDF) content was evaluated in samples processed to pass through a 2-mm sieve using a 288-h in situ procedure [
17].
The blood NEFA concentration was quantified by a colorimetric method, whereas blood β-OHB was analyzed by an enzymatic-kinetic method (FA115 and RB1007 respectively, Randox, Ireland, UK). Glucose (K082) and urea (K056) were quantified by the enzymatic-colorimetric method (Bioclin Quibasa®, Belo Horizonte, Minas Gerais, Brazil); and total protein and albumin were analyzed by the colorimetric method (K031 and K040 respectively, Biopclin Quibasa®, Belo Horizonte, Minas Gerais, Brazil). An automated biochemical analyzer (Mindray BS 200E, Shenzhen, China) determined all the analyses previously mentioned. Serum concentrations of IGF- 1 were quantified by chemiluminescent assay in commercial laboratory (Vicosalab®, Viçosa, Minas Gerais, Brazil). Progesterone concentrations were measure by radioimmunoassay kit (125/RIA, ICN Pharmaceuticals, Inc, Costa Mesa, California, USA) and quantified by the automatic PerkinElmer-wizard -1470- gamma counter (Laboratory of Endocrinology/Physiology of Domestic Animals—UNESP, Araçatuba, São Paulo, Brazil).
2.4. Statistical analyses
The statistical analysis of the data followed the basic model:
where Y
ijk is the response measured in the animal k with the breeding age j and belonging to the group i, µ is the general constant, P
i is the random effect of the paddock or group of animals i, A
j is the fixed effect of the breeding age j (i.e., 14 or 24 months), and ε
ijk is the random error, to be NIID (0, σ
2ε).
Most response variables were analyzed as repeated measures. The choice of the best structure for the residual (co)variance matrix was based on the Akaike information criterion with correction. All variables were analyzed using the GLIMMIX procedure of SAS (Statistical Analysis System, version 9.4). Differences were declared significant at p < 0.05, and trends were declared when 0.05 ≤ p < 0.10.
3. Results
The BW of the cows showed an interaction between breeding age and time (
p < 0.01), where on average, 24-mo cows were heavier (
p < 0.01). Interaction slicing indicated that both categories had BW loss at parturition, which was reinforced by the concomitant decrease in the BCS (
Figure 1a,b). However, following their first calving, BW of 24-mo cows remained stable (
p > 0.05), whereas 14-mo cows showed a gradual BW recovery after parturition (
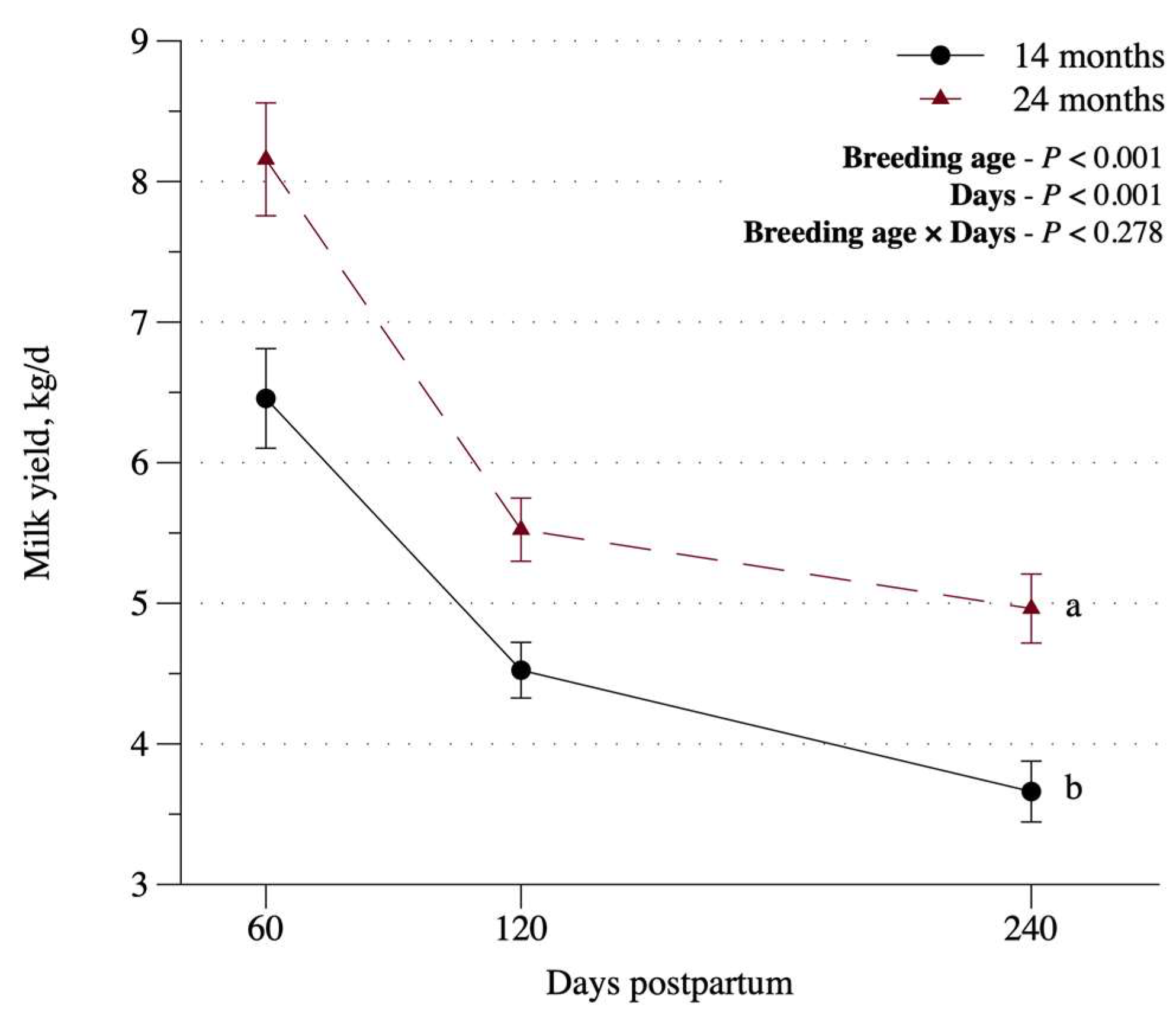
p < 0.05), due to the higher average daily gain (ADG) of these animals. Milk yield was higher in 24-mo cows (
p < 0.01), but decreased with increasing milking days (
p < 0.01) for both cow ages (
Figure 2).
The ADG of calves born from the cows bred at 24-mo was higher (P < 0.01), which reflected in greater BW (P < 0.05) from 160 days of age until weaning at 240 days (
Figure 1C).
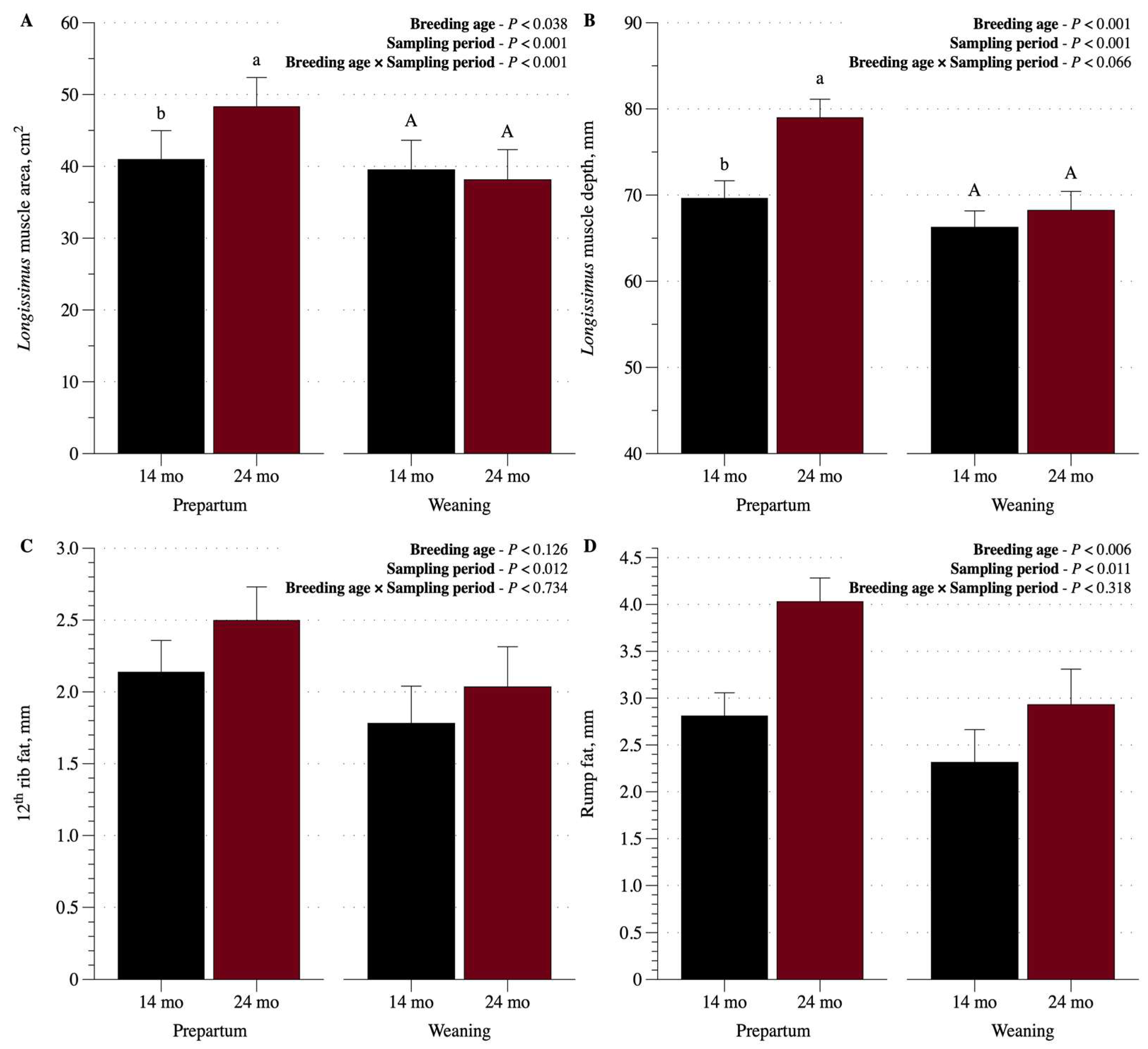
The 12th rib fat was not affected by breeding age (
p > 0.12), but was greater before calving (
Figure 3). The rump fat was also greater before calving (
p < 0.01), but was, on average, greater in 24-mo cows when compared to the 14-mo cows (
p < 0.01). An interaction between time and breeding age was observed for
Longissimus muscle area (
p < 0.01) and tended to occur for
Longissimus muscle depth (
p = 0.066). Both variables were, on average, greater before calving. Differences between ages were observed only before calving (
p < 0.05), where 24-mo cows had greater values.
Amongst blood characteristics, glucose, total protein, albumin, and NEFA were affect only by sampling period (
p < 0.01,
Table 3). Blood concentrations of glucose, protein, and urea increased, whereas albumin decreased as cows moved from prepartum to lactation period. The blood urea was higher in 24-mo cows (
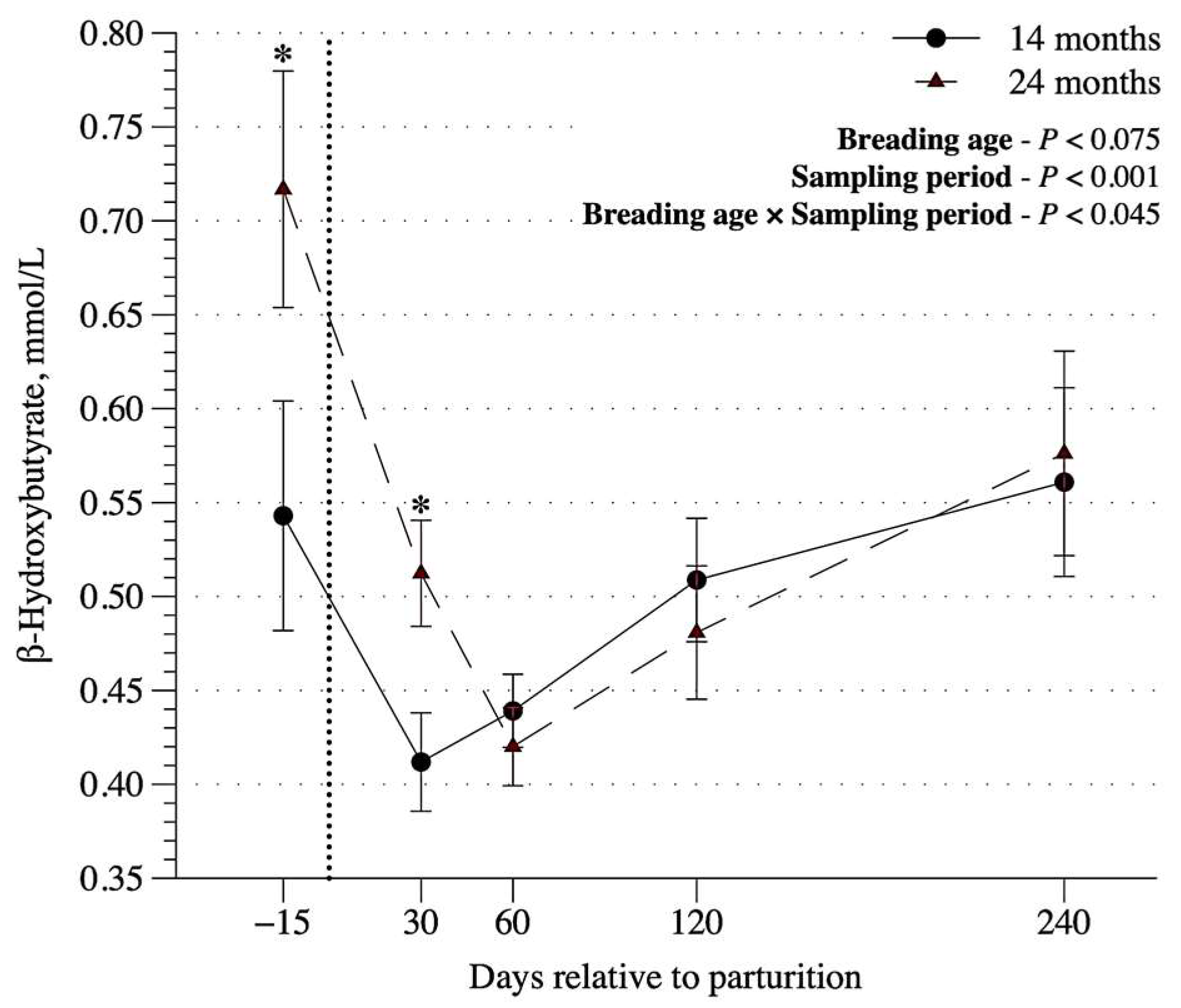
p < 0.01) and its concentration increased after parturition compared to prepartum period, but decreased as the lactation progressed. An interaction between sampling period and breeding age was observed for blood β-OHB (
p < 0.05,
Table 3). The β-OHB concentration was greater before calving and a marked decrease after parturition (i.e, 30 days). After that, there was a less pronounced increase until weaning. The 24-mo cows had greater blood β-OHB compared to 14-mo cows (
p < 0.05) only at prepartum and 30 days after calving (
Figure 4). Progesterone concentration in blood was greater in 24-mo cows, without any effect of time (
p > 0.11) or interaction between sampling period and breeding age (
Table 3). No effect was observed (
p ≥ 0.11) on blood IGF-1.
4. Discussion
Primiparous cows bred to be pregnant at 24-mo were heavier throughout the experiment, which was expected since these cows were at a more advanced development stage [
18]. The cows bred to be pregnant at 14 months reached roughly 80% of adult weight at parturition, which is the recommended target weight for the animal to be able to sustain postpartum nutritional requirements and, consequently, provide an adequate return to reproduction [
19]. The continuous weight loss after parturition of the 24-mo cows, associated with the greater milk yield (21.5% greater than 14-mo cows) demonstrate the greater energy demand of these animals for lactation.
Although our study focused on milk production after 60 days postpartum, a greater milk production before this period can be indirectly inferred from the greater ADG presented by calves born to 24-mo cows (
Figure 1C) and may be related to a greater persistence of lactation in these cows when compared to younger cows.
In beef cows, BCS is a determining factor for the return of cows to estrus, improving conception rates. Cows that kept their BCS between 5 and 6 in the last trimester of pregnancy tend to lose less BW and BCS after parturition, and show greater pregnancy rates in the next breeding season when compared to cows that had a BCS of 4 to 5 [
20]. In our study, all animals kept adequate BCS (i.e., above 5) which indicates an adequate nutritional status to support lactation and reproduction. An adequate cow’s BCS has been directly associated with circulating concentrations of metabolic hormones synthetized by hepatic, adipose, and gut tissues that serve as nutritional mediators of reproductive function [
21]. Despite negatives changes in BW followed parturition, when cows showed an average decrease of 1 point in the score, the BCS was still above 5, a value considered as threshold for an adequate level of body reserves in beef cows [
8]. Even 24-mo cows having a lower ADG, the fact that they had an adequate BCS may have guaranteed sufficient body reserves to not compromise their reproduction [
22]. These results demonstrate the importance of ensuring proper nutrition during pregnancy, so that the cows may maintain an adequate BCS, and improve their reproductive performance [
23].
Ultrasonography measurements of muscle and body fat reserves can be used as indicators of how the animal’s body reserves are and can even be more sensitive to small changes in body composition than BCS [
24]. The reduction in
Longissimus muscle area and
Longissimus muscle depth values at weaning compared to prepartum probably suggest that these animals had protein mobilization, justified by the greater fetal demand for amino acids at the end of gestation and early lactation [
25]. When protein demand is greater than or close to the amount ingested, muscle tissue can be mobilized to maintain the necessary levels of circulating amino acids [
26].
The NEFA concentrations were highest 15 days before calving, indicating an increase in the adipose tissue lipolysis [
27]. However, they decreased throughout the experiment, suggesting that the cows recovered their nutritional status.
Indicators of the mobilization of lipid reserves, β-OHB, and NEFA have being related to the energy deficit [
28]. High plasma levels of β-OHB are linked to energy deficit at the end of pregnancy and early lactation [
29]. The high levels of β-OHB, together with the greater milk yield of the 24-mo cows can be justified by the lactation peak of Nellore cows, which occurs in the first postpartum month [
30], suggesting greater tissue mobilization to meet the higher energy demands.
The carcass ultrasonography results, added to the β-OHB results and the non-recovery of body weight and BCS after calving, demonstrate that the 24-mo cows had a greater protein and energy demand. This can be explained by its greater milk production, also associate with higher maintenance requirements, which account for 50% of energy required for beef production [
31]. The 14-mo cows still require more additional energy for their own growth compared to more mature cows [
10], and this may also contribute to their lower milk production.
The progesterone concentration was higher for 24-mo cows (
Table 3), which could indicate the earlier return of cyclical activity after postpartum anestrus, that’s probably resulted in higher pregnancy rates for these animals in the first FTAI (35% in 14-mo versus 52,9% in 24-mo cows). The pregnancy rate in 14-mo cows increased by 50% after the second FTAI protocol, while in 24-mo cows this difference was 30%, resulting in final pregnancy rate of 70% and 82% for 14-mo and 24-mo, respectively. Even though they were not evaluated in this study, it is important to mention these probable differences in pregnancy rates, as they can help in the implementation of nutritional plans that specifically help young animals in the second breeding season, improving their reproductive indexes.
Younger cows took longer to cycle again, showing less sexual maturity, indicating that the combined effect of growth and first lactation increases nutritional requirements and reduces pregnancy change in precocious primiparous cows. Additionally, this study did not evaluate embryonic and fetal loss after conception, nor the effects of heifer age at first gestation on fetal and postnatal growth. This condition may have to be considered in further studies involving maternal nutritional requirements and response in calves.
In general, calves from primiparous cows are lighter at weaning than the calves from multiparous cows [
32]. In this study, calves born to 14-mo cows had a weaning weight about 20 kg lower than those born to 24-mo cows due to lower milk production by their mothers.
In summary, our data support that even cows considered to be of the same productive category, that is, primiparous beef cows, but that become pregnant at 14 or 24 months of age, have different nutritional requirements and metabolic profile, especially in the first two reproductive years. Therefore, they must be handled in different ways. In the first year, the target body weight at the beginning of the breeding season is 55 to 60% of mature body weight is used as parameters [
11,
33]. In the second year, the challenges are lactation and continued weight gain.
At this point, it is necessary to evaluate cow-calf production efficiency using several indexes, one the most used is kg of weaned calf/cow exposed to breeding, which reflects reproductive efficiency of cows and developmental responses of the offspring [
21] and represent one of the main sources of income in the cow-calf operations.
Author Contributions
M.N.F.V.: investigation, formal analysis, writing—original draft, J.J.S.P.: investigation, formal analysis, R.T.P.: investigation, formal analysis, M.I.M.: formal analysis, writing—review and editing, J.M.S.J.: writing—review and editing, M.O.F.: writing—review and editing, E.D.: formal analysis, writing—review and editing, C.B.S.: writing—review and editing, conceptualization, formal analysis, supervision, funding acquisition, and project administration. All authors have read and agreed to the published version of the manuscript.