1. Introduction
The practical use of these materials as phosphors, dosimeters, detectors, etc. is connected to the research of the mechanism of formation of electron and hole trapping centers in irradiated sulfates of alkaline earth metals [
1,
2,
3]. The produced electronic excitations at trapping sites [
4,
5,
6] in irradiation sulfates of alkaline earth metals relax as intrinsic and recombination emissions.
The creation of electron and hole trapping centers is related to the practical use of these crystals as dosimeters and detectors. The concentration of accumulated electron and hole trapping centers in TLD dosimeters is used to quantify the absorbed dosage in crystals [
7,
8,
9,
10]. Local levels below the conduction band and above the top of the valence band correspond to intrinsic trapping centers in the matrix transparency region. Special impurities are added to concentrate accumulated defects and radiate the energy of recombination processes [
11,
12,
13].
Experimental evidence has demonstrated that accumulated defects in practically all sulfates are associated with long-wavelength recombination emission bands at 3.0-3.1 eV, 2.6-2.7 eV, and 2.3-2.4 eV. At photon energies between 6 and 12.4 eV, free electron-hole pairs are formed, which results in the formation of these recombination emissions. It has been experimentally shown that, upon excitation in the recombination emission bands at 3.0–3.1 eV and 2.6–2.7 eV, excitations appear corresponding to 3.9–4.0 eV and 4.45–4.5 eV [
14,
15,
16,
17] in the transparency region of the main matrix. These excitation energies must correspond to the local levels of electron
and hole
trapping centers.
When impurities capture electrons in irradiated K
2SO
4-Tl
+ and Na
2SO
4-Cu
+ crystals, it leads to the formation of electron trapping centers such as Tl
0[
18] and Cu
0[
19]. These centers are associated with
and as a result create a hole trapping centers located under the conduction band. Within the 2.9-3.0 eV spectral range, the recombination emission bands that correspond to the impurity trapping centers are located below the conduction band. They are closely situated to the recombination emission of a pure matrix which is observed at 3.0-3.1 eV. In contrast to the emission band of the electronic impurity trapping centers, the emission centers of these impurities in sulfates, Tl
+ (4.2 eV), and Cu
+ (2.6-2.7 eV), are in distinct spectral ranges (
Figure 8).
The fundamental goal of this research is to understand how electronic Mn+ and hole trapping centers are formed, as well as their sensitizing function in the energy transfer from electron-hole couples to emitters, or impurities, in CaSO4-Mn and BaSO4-Mn dosimetric crystals. By measuring its intensity, dosimeters can gauge how much dosage has been absorbed.
3. Results
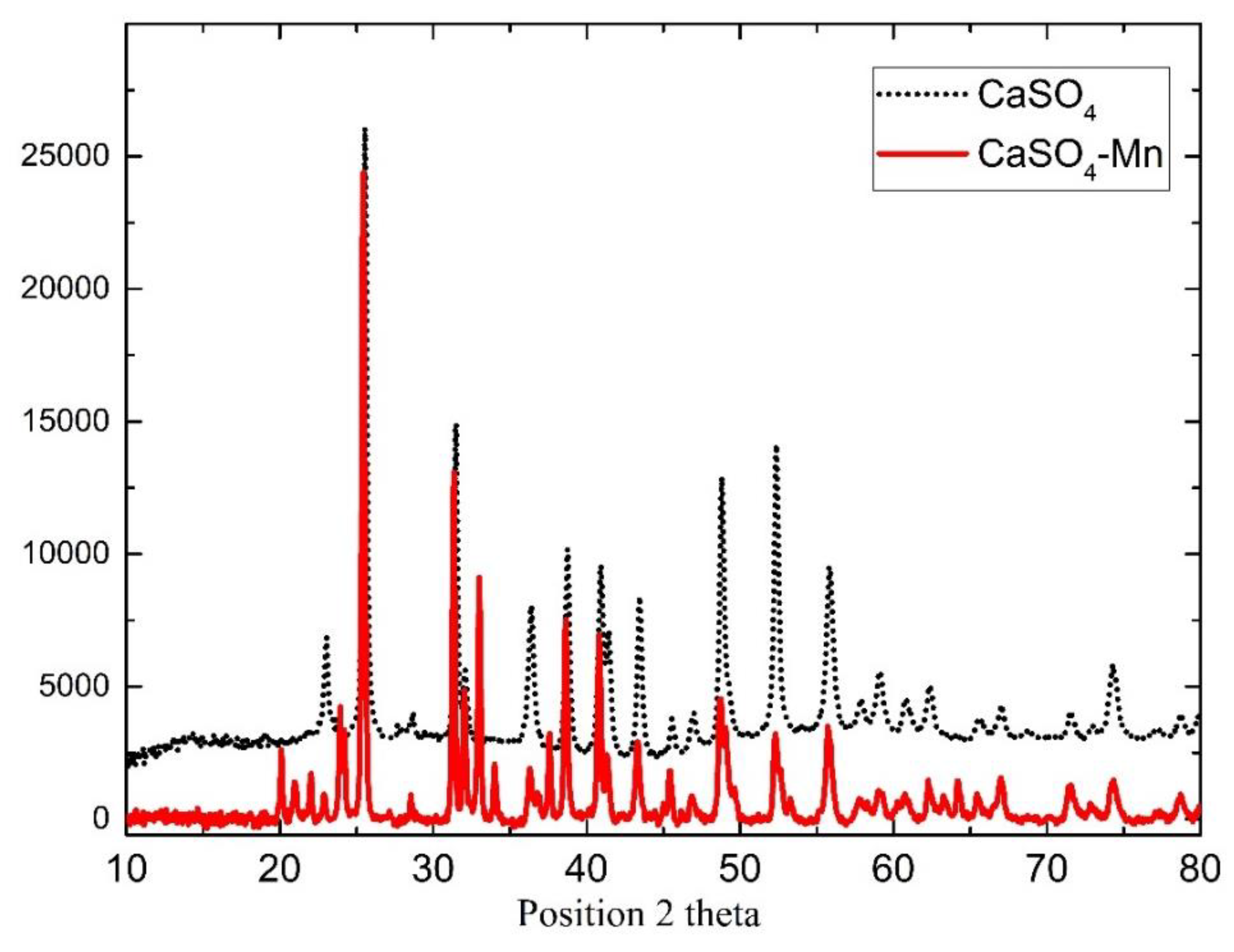
Figure 1 illustrates the sample’s XRD pattern. The spectrum data shows that the sample is CaSO
4-Mn with orthorhombic structure and corresponds to JCPDS card no. 06-0226. Obtained results confirm the purity and existence of Mn in investigated samples. Similar results were also obtained for BaSO
4-Mn.
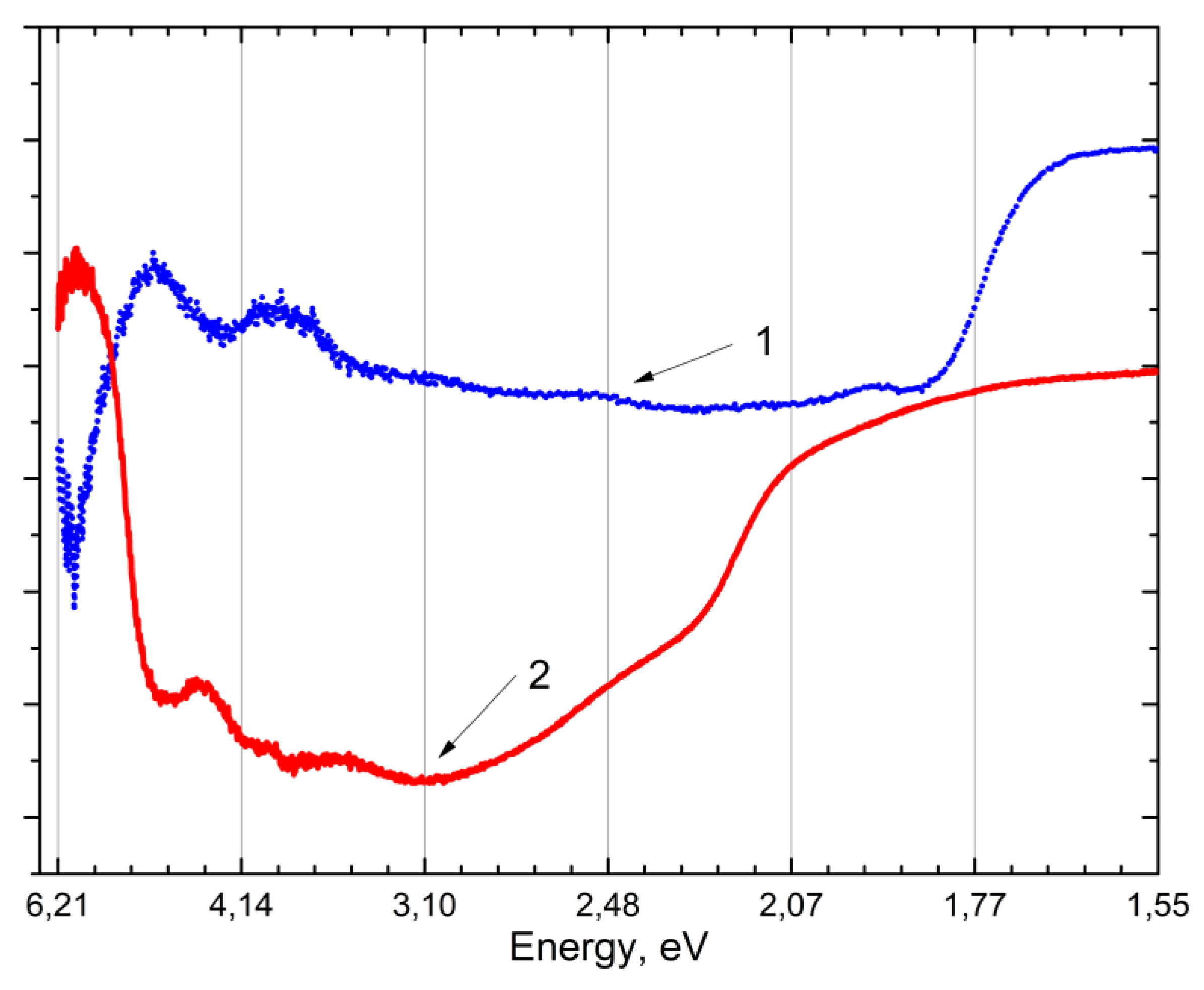
Based on the measurement of the absorption reflection spectrum (
Figure 2) and the creation of intrinsic emission [
14,
17] in
and other sulfates, the band gap is estimated about 5.5-6.2 eV.
In accordance with the main objective of this work, we investigated the mechanism of energy transfer of electronic excitations to Mn2+ impurities in CaSO4-Mn and BaSO4-Mn dosimetric crystals.
In our previous works [
15,
16,
17] devoted to study of energy transfer to impurities in alkali metal sulfates Na
2SO
4 and LiKSO
4, it was shown that during irradiation by UV photons of 6.2–12.4 eV, recombination or tunneling emission at 3.1 eV and 2.7 eV appears at electron-hole trapping centers. It is shown that these recombination emissions are excited in the transparency region of the Na
2SO
4 and LiKSO
4 matrices at photon energies of 4.0 eV and 4.5 eV. Similar studies were carried out on pure dosimetric CaSO
4 crystals and BaSO
4 powders. First, electron-hole trapping centers were created in CaSO
4 and BaSO
4 by irradiation with photons of 7.3–7.75 eV at 15 K, then they were irradiated with photons of 4.0 eV and 4.5 eV.
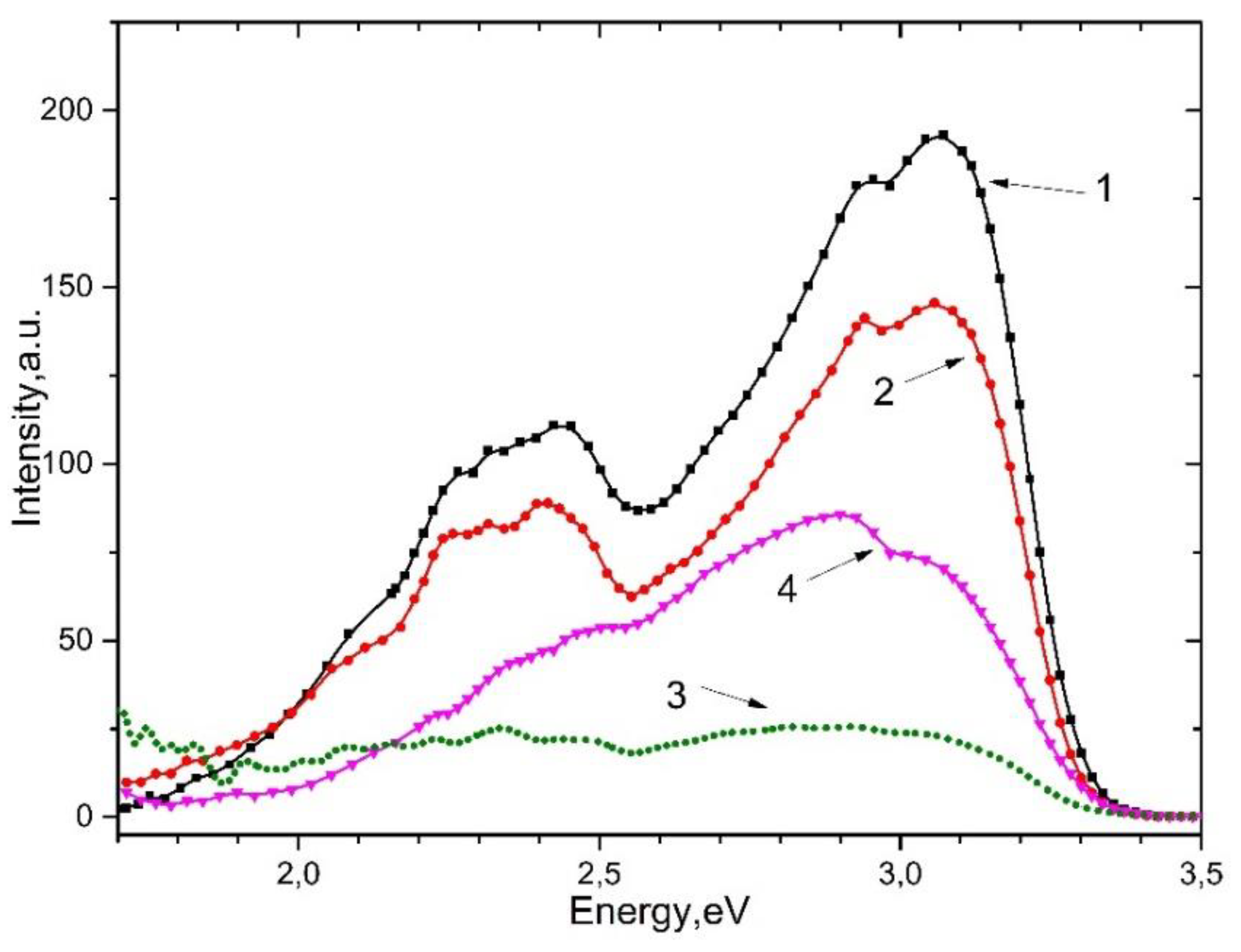
Figure 3. shows the emission spectrum of a CaSO
4 crystal and a BaSO
4 powder with induced defects upon excitation by photons of 4.5 eV (curves 1.2) and 4.0 eV (curves 3.4), respectively. It can be seen that, as in alkali metal sulfates, emission appears at 2.95 eV, 3.1 eV, 2.7 eV, and 2.3–2.4 eV.
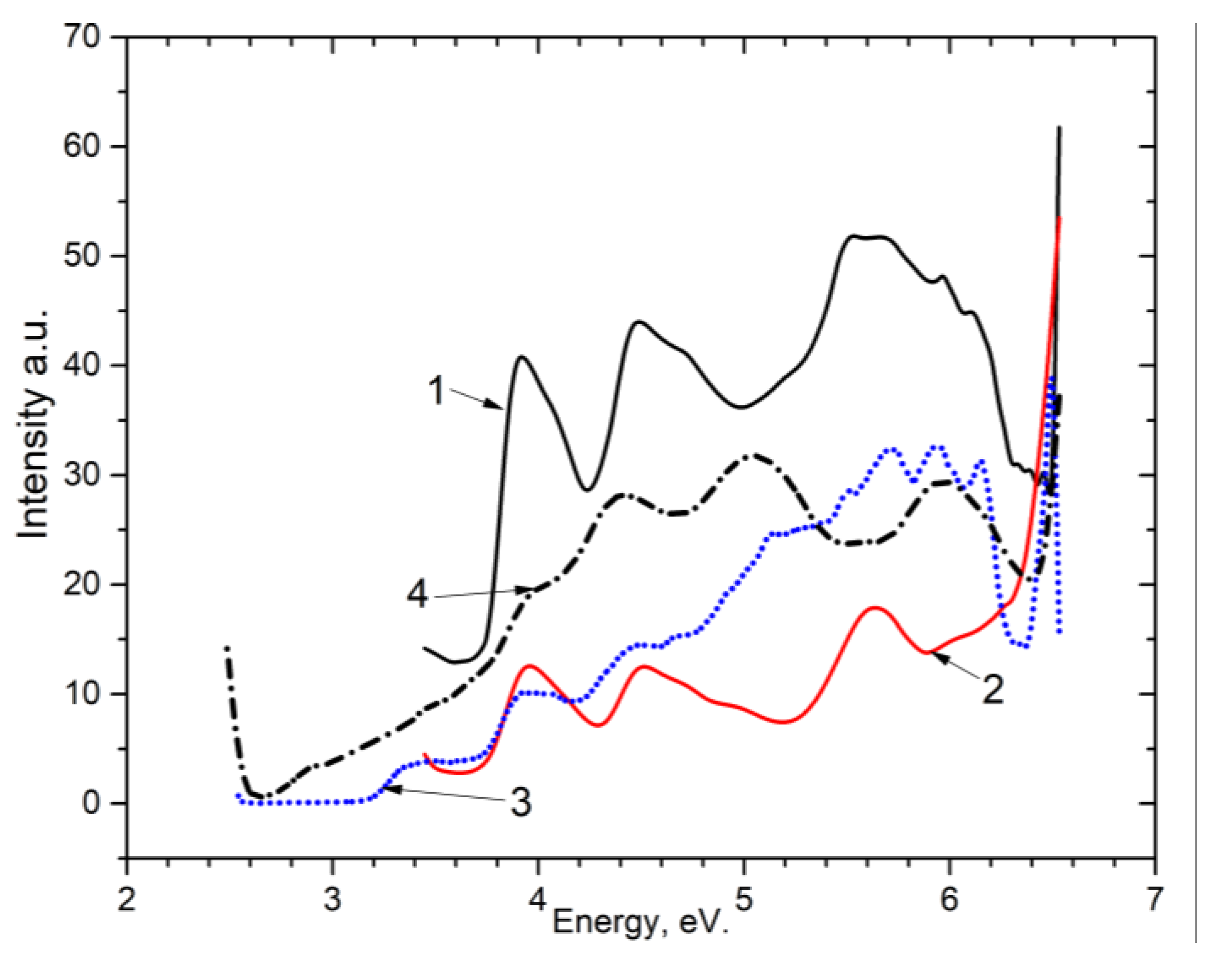
By measuring the excitation spectra for these recombination emissions (
Figure 4) at 80 K, the following were obtained: for CaSO
4, the 2.95–3.1 eV band (curve 1) and the 2.7 eV band (curve 2), the excitation spectrum for BaSO
4 for band 3, 1 eV (curve 3) and 2.7 eV (curve 4). It can be seen that for both matrices an excitation spectrum appears at 4.45–4.6 eV and 3.9–4.0 eV.
Thus, both in alkali metal sulfates and in CaSO4 and BaSO4 dosimetric samples, electron-hole trapping centers are created that emit recombination or tunneling emission at 2.95–3.1 eV and 2.7 eV. The intensity of recombination emission in dosimetric materials is proportional to the concentration of accumulated defects, i.e., concentration of electron-hole trapping centers, which is proportional to the absorbed dose.
In CaSO4-Mn and BaSO4-Mn crystals, Mn2+ impurities should significantly increase the concentration of electron-hole trapping centers, respectively, the intensity of recombination emission, which is proportional to the absorbed dose.
The objective of the study is to reveal the process of energy transfer of the host recombination emission to Mn2+ impurities.
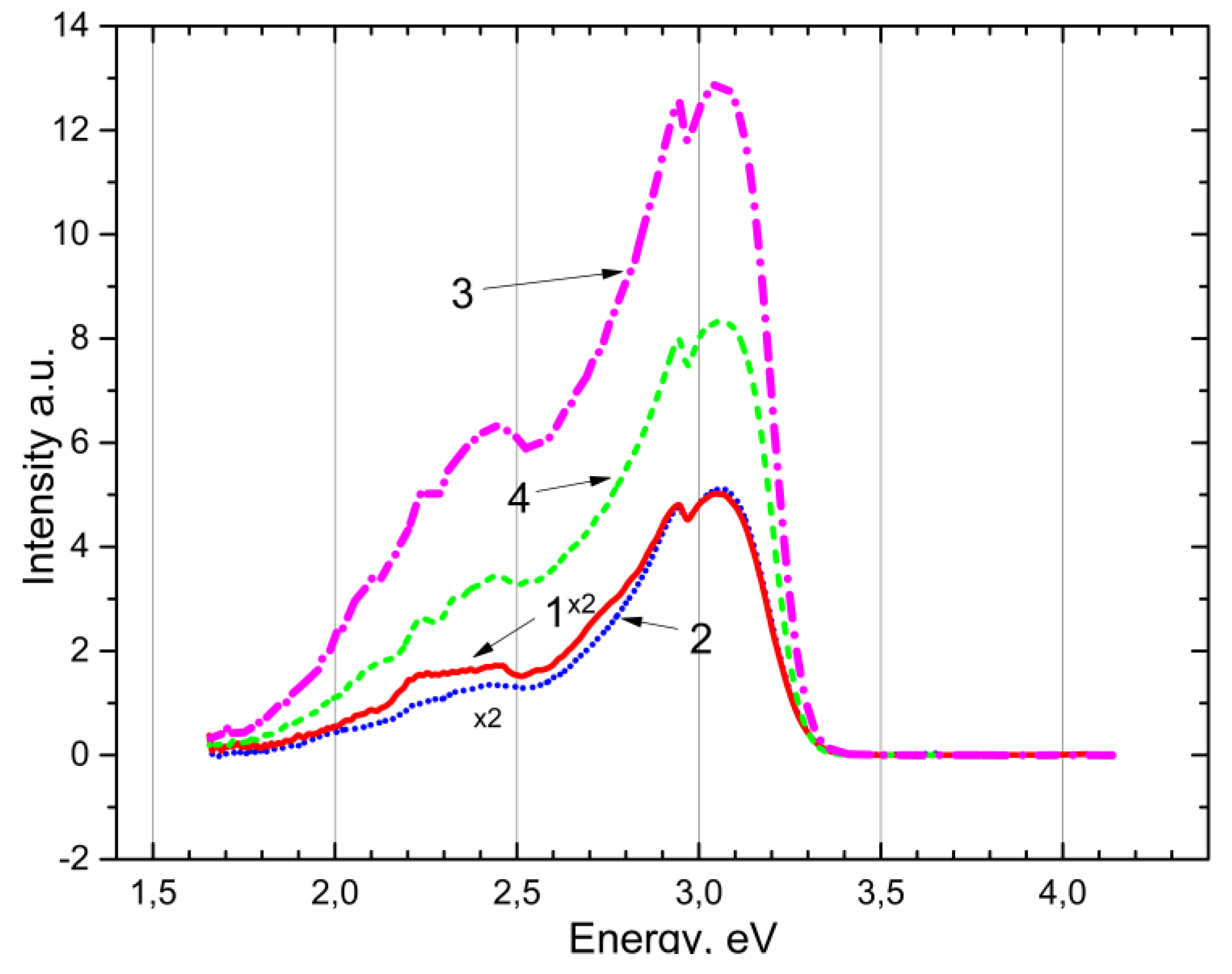
Figure 5 demonstrate photoluminescence under photon excitation at 5.6 eV, pre-irradiated with x-rays and unirradiated at 80 K in CaSO
4-Mn and BaSO
4-Mn. It is evidently that in both samples the emission associated with the Mn
2+ impurity appears at 2.3-2.4 eV and new emission bands appear at 2.95-3.1 eV. The emission band 2.95-3.1 eV refers to intrinsic and impurity electron-hole trapping centers. Preliminary irradiation reveals the same emission bands with a pronounced cumulative effect (curve 1, 4).
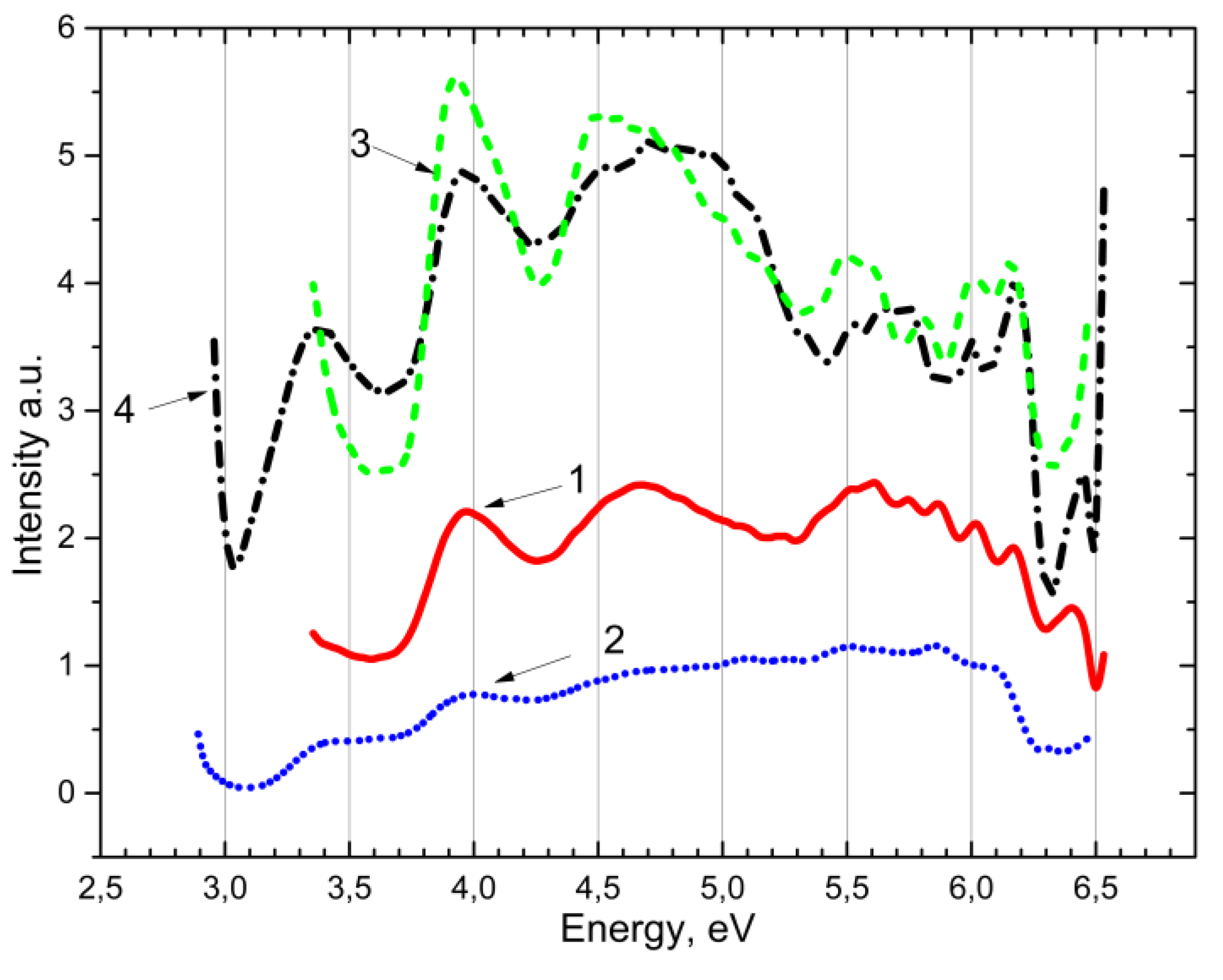
Following this, an analysis was conducted on the excitation spectra (as shown in
Figure 6) of the emission center of Mn
2+ impurity for the 2.3 eV band at 80 K, for both CaSO
4-Mn (curve 2) and BaSO
4-Mn (curve 1). As can be observed, excitation occurs in three spectral ranges: 3.35 eV, 4.0 eV, 4.5 eV, and 5.0-6.2 eV. The fundamental spectral area of the matrix is defined as 5.0–6.2 eV. New electron-hole trapping centers are formed in this region.
The same
Figure 6 displays the BaSO
4-Mn powder’s recombination emission excitation spectra at 3.1 eV (curve 3) and 2.75 eV (curve 4). The excitation bands observed in the ~4.0 eV and ~4.5 eV were found to be analogous to those of the pure CaSO
4 and BaSO
4 samples (as shown in
Figure 3), albeit with a greater intensity of the bands.
Experimental evidence demonstrates that the excitation energies of the Mn2+ impurity in the matrices of CaSO4 and BaSO4 align with the excitation of the recombination emission associated with the electron-hole trapping center at 4.0 eV and 4.5 eV.
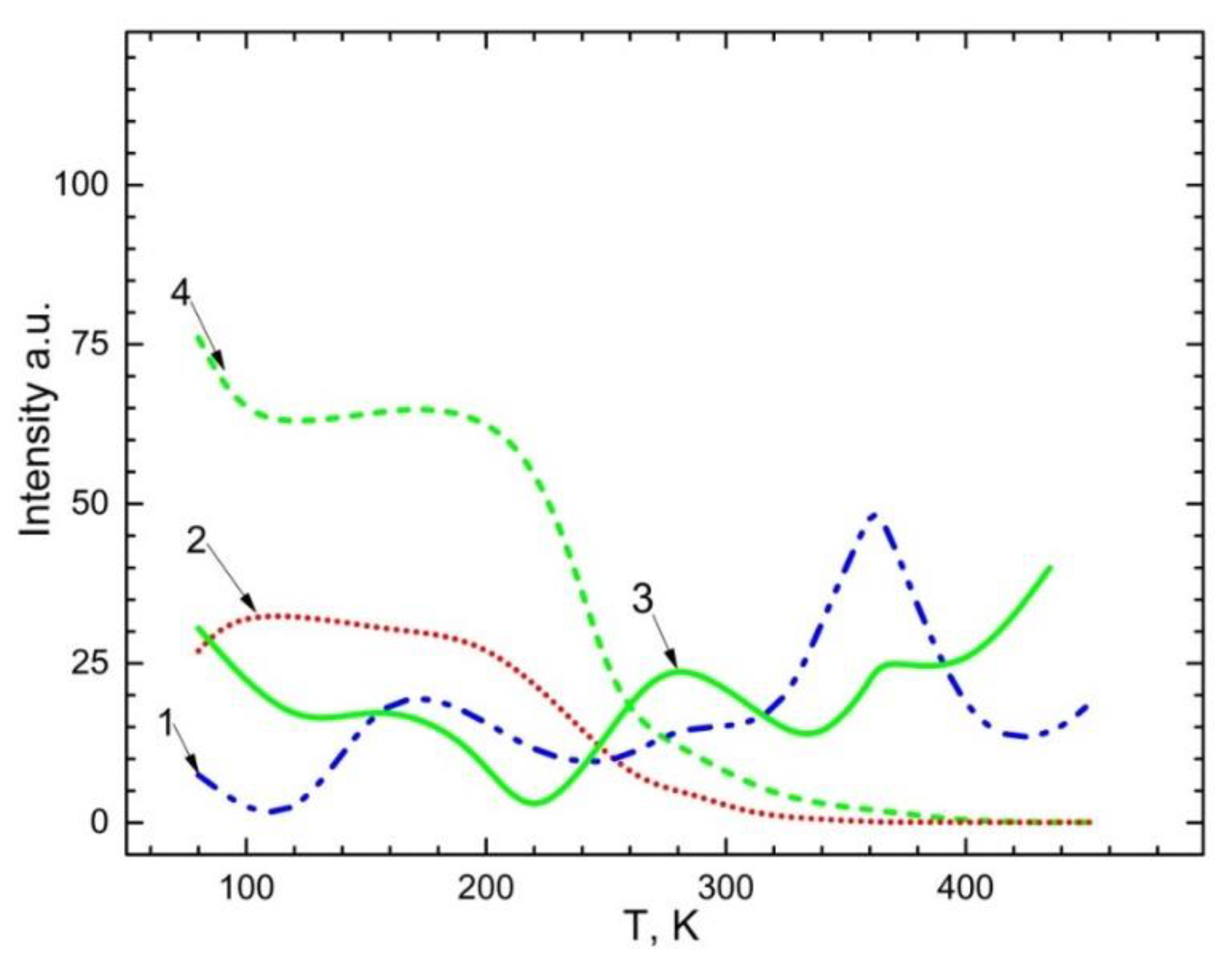
Figure 7 illustrates the temperature dependency of the emission spectra of 2.3 eV Mn
2+ impurities as well as 2.95 eV and 3.1 eV recombination emissions. The exciting energy of bands was 4.5 eV and 5.6-5.9 eV for CaSO
4-Mn and BaSO
4-Mn. You can observe from the graph that:
a) In CaSO4-Mn and BaSO4-Mn crystals, emission 3.1 eV and 2.95 eV are steady up to 200–220 K (curve 4.2). The band’s intensity starts to decline at a temperature of 200–220 K. It is presumable that after the electron delocalizes from the Mn+ trapping centers at this temperature, the intensity of the recombination emission band gradually diminishes until it reaches a minimum value.
b) the electron is ionized from the trapping centers in accordance with the following reaction: ; the Mn2+ impurity is restored (curves 1,3); the intensity of the emission band ~2.3 eV corresponding to the emission of Mn2+ increases. The delocalization of holes from centers (), which occurs in the temperature range of 350–360 K, is linked to an increase in the Mn2+ impurity’s emission intensity.
4. Discussion
The excitation spectra of the long-wavelength recombination emissions at 3.0–3.1 eV and 2.6–2.7 eV indicated that these emissions can be stimulated by photon energies of 4.5 eV, 4.0 eV, and 3.35 eV. Long-wavelength recombination emissions are once more detected upon reverse excitation of CaSO
4 and BaSO
4 samples with induced trapping centers at 4.5 eV and 4.0 eV. Based on experimental facts, a formation mechanism, and a band scheme for the arrangement of local states for electron and hole trapping centers are proposed. Electron trapping centers are produced in accordance with the reaction
when electrons are trapped by anionic complexes or during charge transfer
during excitation of the anionic complex
. The hole excitation component is localized in the form of the radical
. The formation of the radical
in irradiation sulfates was established by the authors of [
20] using the EPR technique. This is how electron and hole trapping centers are formed in the form
. The trapping centers correspond to recombination emission.
Based on theoretical calculations by the authors of [
21], it was predicted that holes would exist in various local states from the top of the valence band. These calculations revealed that the ground state of the unpaired electron in the
radical will differ in each of the three crystallographic directions (a, b, and c). Additionally, experimental evidence shows that the thermal decollation of a
hole of two types in CaSO
4 occurs at various temperatures and activation energies [
22]. All these data indicate the existence of three local states from the top of the valence band, corresponding to localized holes
- different crystallographic directions in the transparency region of the crystal. As a result, the produced holes are localized at distinct distances of 3.35 eV, 4.0 eV, and 4.5 eV from the local level of electronic trapping centers.
The authors of [
15,
16,
17] studied the mechanisms of energy transfer to impurities in alkali metal sulfates activated by Mn
2+ and Cu
+ ions and in a CaSO
4-Mn crystal. The excitation spectra of impurities and intrinsic recombination emissions of the matrix were measured. In these and our previous works, the relation between the excitation spectra of the recombination emission of the matrix and impurities was not specified.
It is assumed that in the irradiated crystals and powders of CaSO
4-Mn and BaSO
4-Mn in the spectral region of 2.95–3.1 eV, corresponding to the recombination emission of the matrix, a hybrid band appears, including the emission of its intrinsic recombination emission and the emission arising on impurity electron-hole trapping centers.
Figure 5 shows that the hybrid emission band 2.95–3.1 eV is excited in the same way at photon energies of 3.9–4.0 eV and 4.5–4.6 eV. It is assumed that in the CaSO
4-Mn and BaSO
4-Mn powders irradiated with UV photons, upon excitation of the
anionic complex, a hybrid radiative state of 2.95–3.1 eV is created by two mechanisms:
- -
during charge transfer from oxygen (O2- Mn2+) to impurities;
- -
when electron-hole pairs are trapped by Mn2+ impurities.
In both cases, an impurity electron-hole state Mn+- is created.
Parallel in the host:
- -
when charge is transferred from oxygen (O2-) to the next to anionic complex , intrinsic electron-hole trapping centers are created near the impurity;
- -
when an electron is captured by an anionic complex and a hole is localized in the form of , similar capture centers can be created.
Recombination decays of emerging trapping centers occur:
during the decay of , emission of 2.95-3.1 eV occurs;
during the decay of an electron recombines with a hole located near the impurity and the energy of the recombination process excite impurities, emission of impurities is observed at 2.3 eV.
The formation of hybrid states 2.95-3.1 eV appear during the measurement of the temperature dependence of the recombination emission band and the intracenter emission of . At a temperature of 220–250 K, where electron delocalization from centers occurs, an increase in the intensity of the intracenter emission band corresponding to ions () is observed.
The exhibition of hybrid states is also characteristic of other alkali metal sulfates activated by Cu
+ and Tl
+ impurities. We have shown the formation of such states in the band diagram in
Figure 8.
We have experimentally shown that impurity emission at 2.3 eV and recombination emission of 2.95-3.1 eV are excited at the same energies 4.0 eV and 4.5 eV. The unique manner in which electron and hole trapping centers are created, involving localized states in the transparency region of the matrix, should be a distinguishing characteristic of alkali and alkaline earth metal sulfates. A distinctive characteristic of these matrices is the creation of Tl
0, Cu
0,
and Mn
+ electronic trapping centers in both pure and doped sulfates, which possess local energy states of approximately 2.95-3.17 eV.
Figure 8.
Band scheme of impurity ) and intrinsic electron and hole trapping centers.
Figure 8.
Band scheme of impurity ) and intrinsic electron and hole trapping centers.