Submitted:
24 May 2023
Posted:
25 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. TTSPs: General Background
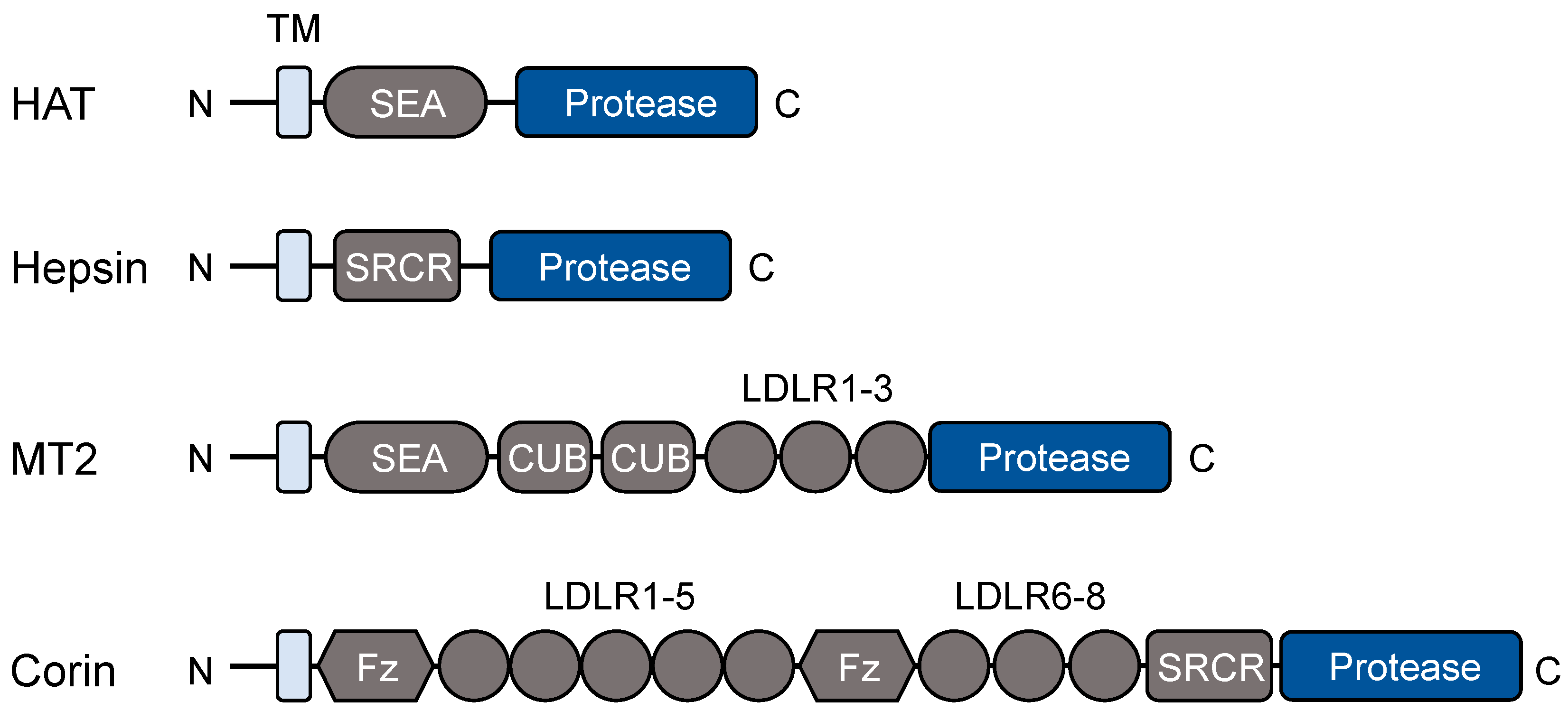
2.1. Protein Domains and Post-Translational Modifications
2.2. Physiological Functions
3. TTSP Expression in Adipose Tissues
4. Hepsin in Adipose Tissue Differentiation
4.1. Hepsin Protein and Function
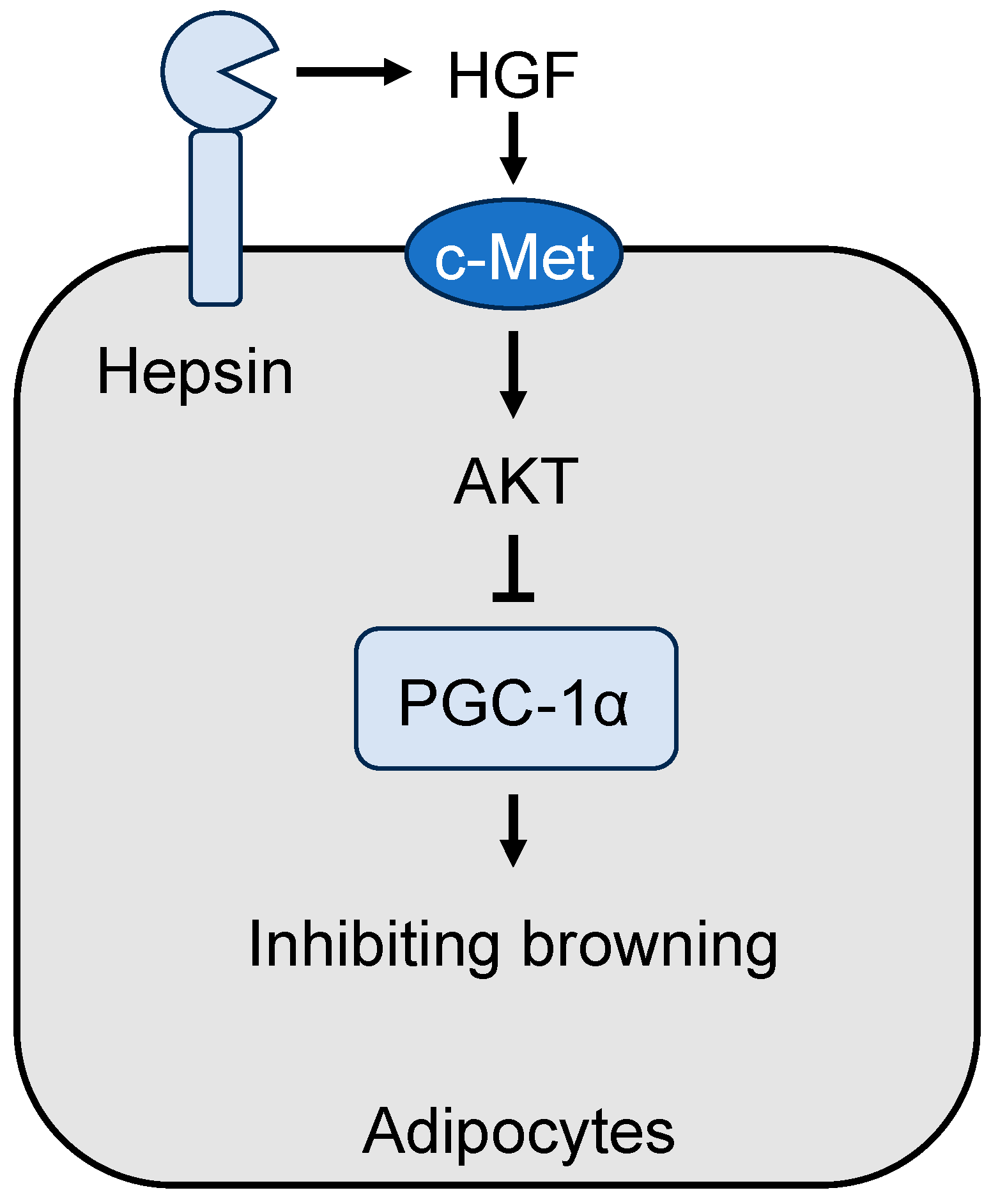
4.2. Role of Hepsin in Adipose Tissue Browning
4.3. Regulation of Hepsin Expression in Adipose Tissue
5. Matriptase-2 in Iron Metabolism and Adiposity
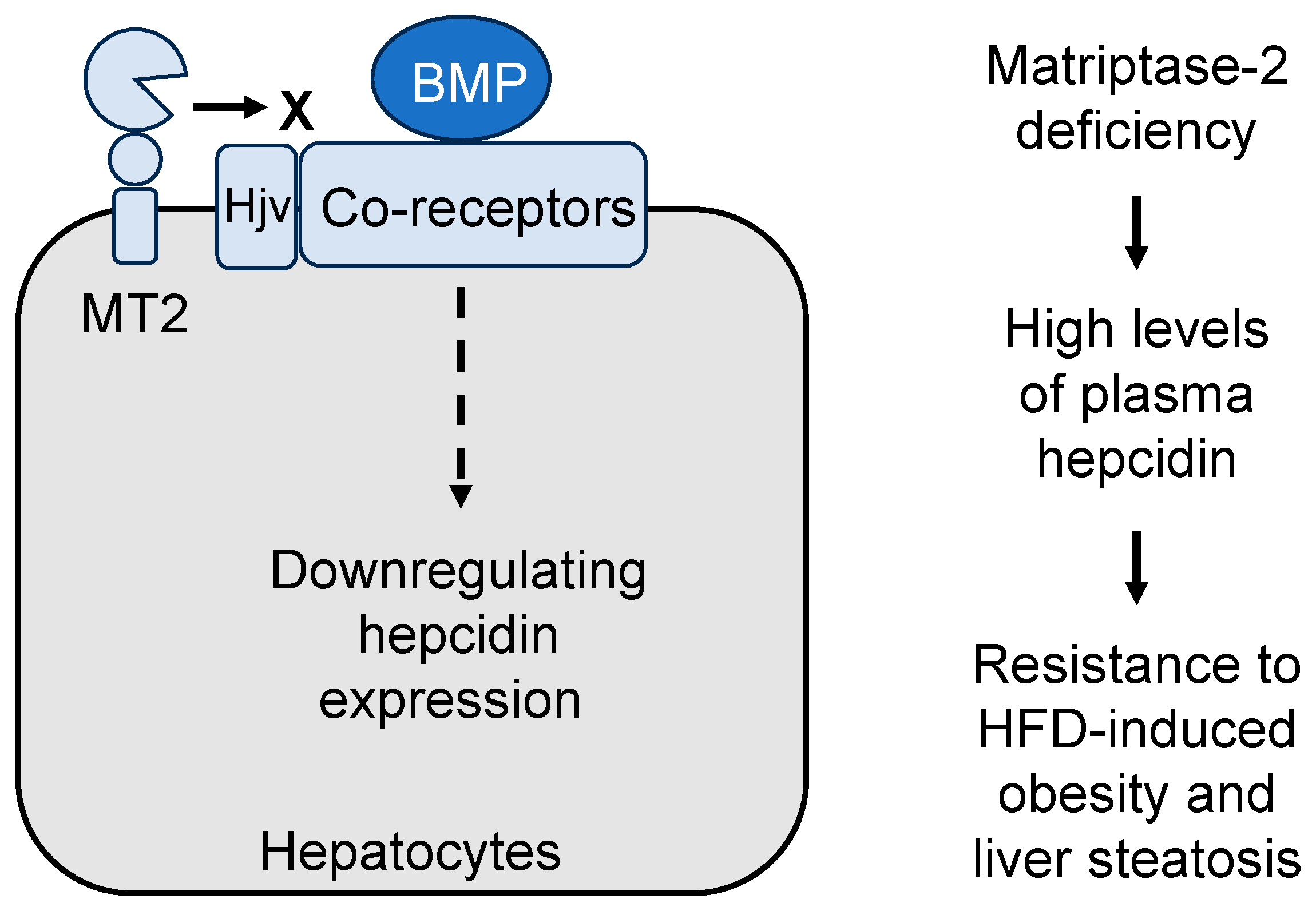
5.1. Matriptase-2 in Iron Metabolism
5.2. Matriptase-2 in Lipolysis and Obesity
6. Corin in Adipose Tissue Phenotype and Thermogenesis
6.1. Corin in Pro-ANP Processing
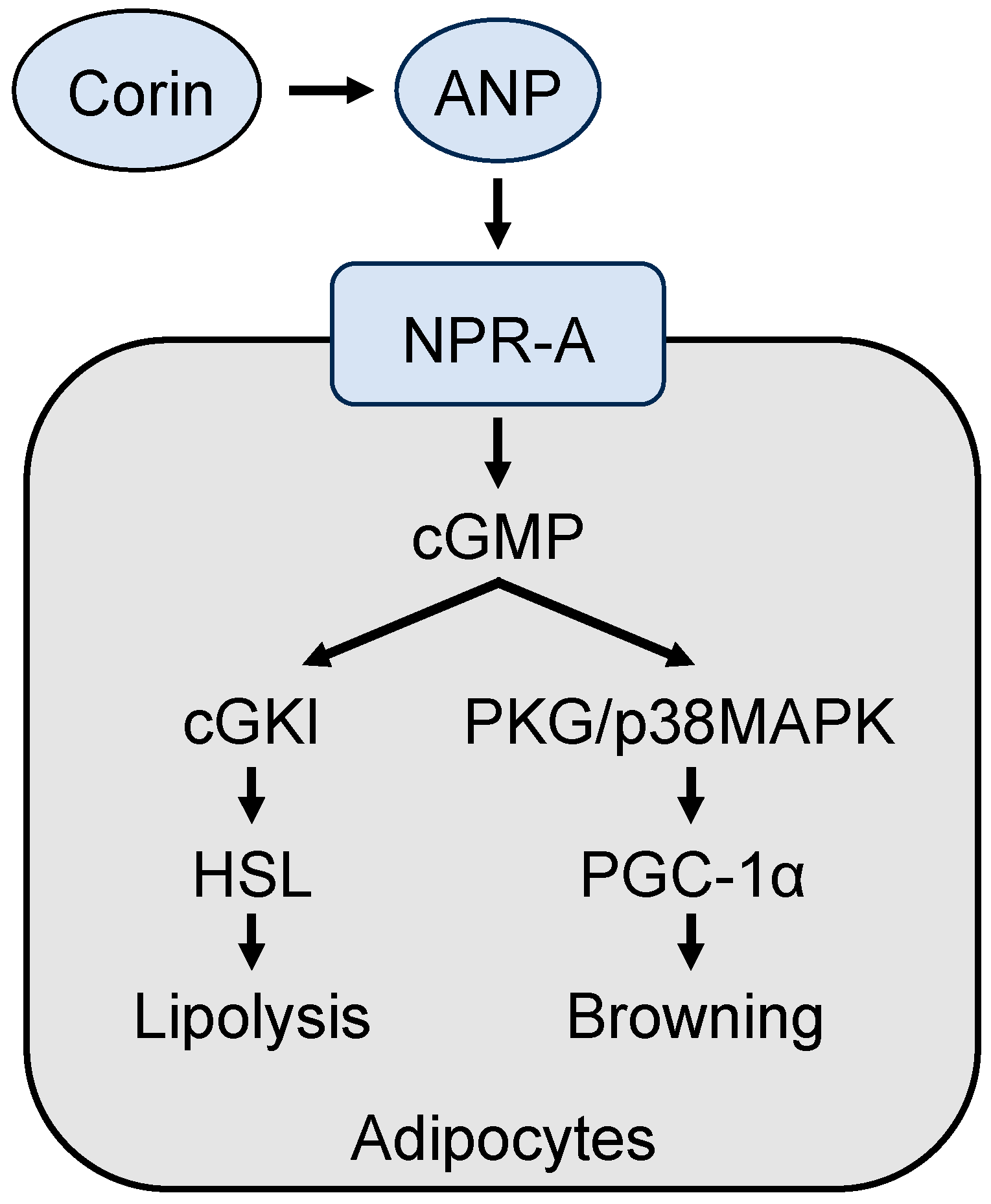
6.2. Role of ANP in Lipid Metabolism in Adipose Tissue
6.3. Role of ANP in Adipose Tissue Browning and Thermogenesis
6.4. Role of ANP in Adipose Tissue Inflammation
6.5. Impaired Adipose Tissue Browning and Thermogenesis in Corin KO Mice
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419-446. [CrossRef]
- Perona, J.J.; Craik, C.S. Structural basis of substrate specificity in the serine proteases. Protein Sci 1995, 4, 337-360. [CrossRef]
- Bugge, T.H.; Antalis, T.M.; Wu, Q. Type II transmembrane serine proteases. J Biol Chem 2009, 284, 23177-23181. [CrossRef]
- Wu, Q. Type II transmembrane serine proteases. Curr Top Dev Biol 2003, 54, 167-206. [CrossRef]
- Overall, C.M.; Blobel, C.P. In search of partners: linking extracellular proteases to substrates. Nat Rev Mol Cell Biol 2007, 8, 245-257. [CrossRef]
- Zhang, Y.; Sun, S.; Du, C.; Hu, K.; Zhang, C.; Liu, M.; Wu, Q.; Dong, N. Transmembrane serine protease TMPRSS2 implicated in SARS-CoV-2 infection is autoactivated intracellularly and requires N-glycosylation for regulation. J Biol Chem 2022, 298, 102643. [CrossRef]
- Afar, D.E.; Vivanco, I.; Hubert, R.S.; Kuo, J.; Chen, E.; Saffran, D.C.; Raitano, A.B.; Jakobovits, A. Catalytic cleavage of the androgen-regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia. Cancer Res 2001, 61, 1686-1692.
- Zhang, C.; Zhang, Y.; Zhang, S.; Wang, Z.; Sun, S.; Liu, M.; Chen, Y.; Dong, N.; Wu, Q. Intracellular autoactivation of TMPRSS11A, an airway epithelial transmembrane serine protease. J Biol Chem 2020, 295, 12686-12696. [CrossRef]
- Murray, A.S.; Varela, F.A.; Hyland, T.E.; Schoenbeck, A.J.; White, J.M.; Tanabe, L.M.; Todi, S.V.; List, K. Phosphorylation of the type II transmembrane serine protease, TMPRSS13, in hepatocyte growth factor activator inhibitor-1 and -2-mediated cell-surface localization. J Biol Chem 2017, 292, 14867-14884. [CrossRef]
- Wang, L.; Zhang, C.; Sun, S.; Chen, Y.; Hu, Y.; Wang, H.; Liu, M.; Dong, N.; Wu, Q. Autoactivation and calpain-1-mediated shedding of hepsin in human hepatoma cells. Biochem J 2019, 476, 2355-2369. [CrossRef]
- Stirnberg, M.; Maurer, E.; Horstmeyer, A.; Kolp, S.; Frank, S.; Bald, T.; Arenz, K.; Janzer, A.; Prager, K.; Wunderlich, P.; et al. Proteolytic processing of the serine protease matriptase-2: identification of the cleavage sites required for its autocatalytic release from the cell surface. Biochem J 2010, 430, 87-95. [CrossRef]
- Jiang, J.; Yang, J.; Feng, P.; Zuo, B.; Dong, N.; Wu, Q.; He, Y. N-glycosylation is required for matriptase-2 autoactivation and ectodomain shedding. J Biol Chem 2014, 289, 19500-19507. [CrossRef]
- Lu, D.; Yuan, X.; Zheng, X.; Sadler, J.E. Bovine proenteropeptidase is activated by trypsin, and the specificity of enteropeptidase depends on the heavy chain. J Biol Chem 1997, 272, 31293-31300. [CrossRef]
- Chen, S.; Cao, P.; Dong, N.; Peng, J.; Zhang, C.; Wang, H.; Zhou, T.; Yang, J.; Zhang, Y.; Martelli, E.E.; et al. PCSK6-mediated corin activation is essential for normal blood pressure. Nat Med 2015, 21, 1048-1053. [CrossRef]
- Wang, J.K.; Teng, I.J.; Lo, T.J.; Moore, S.; Yeo, Y.H.; Teng, Y.C.; Kaul, M.; Chen, C.C.; Zuo, A.H.; Chou, F.P.; et al. Matriptase autoactivation is tightly regulated by the cellular chemical environments. PLoS One 2014, 9, e93899. [CrossRef]
- Jia, B.; Thompson, H.A.; Barndt, R.B.; Chiu, Y.L.; Lee, M.J.; Lee, S.C.; Wang, J.K.; Tang, H.J.; Lin, C.Y.; Johnson, M.D. Mild acidity likely accelerates the physiological matriptase autoactivation process: a comparative study between spontaneous and acid-induced matriptase zymogen activation. Hum Cell 2020, 33, 1068-1080. [CrossRef]
- Friis, S.; Uzzun Sales, K.; Godiksen, S.; Peters, D.E.; Lin, C.Y.; Vogel, L.K.; Bugge, T.H. A matriptase-prostasin reciprocal zymogen activation complex with unique features: prostasin as a non-enzymatic co-factor for matriptase activation. J Biol Chem 2013, 288, 19028-19039. [CrossRef]
- Szabo, R.; Lantsman, T.; Peters, D.E.; Bugge, T.H. Delineation of proteolytic and non-proteolytic functions of the membrane-anchored serine protease prostasin. Development 2016, 143, 2818-2828. [CrossRef]
- Chen, C.Y.; Chen, C.J.; Lai, C.H.; Wu, B.Y.; Lee, S.P.; Johnson, M.D.; Lin, C.Y.; Wang, J.K. Increased matriptase zymogen activation by UV irradiation protects keratinocyte from cell death. J Dermatol Sci 2016, 83, 34-44. [CrossRef]
- Wilkinson, D.J.; Desilets, A.; Lin, H.; Charlton, S.; Del Carmen Arques, M.; Falconer, A.; Bullock, C.; Hsu, Y.C.; Birchall, K.; Hawkins, A.; et al. The serine proteinase hepsin is an activator of pro-matrix metalloproteinases: molecular mechanisms and implications for extracellular matrix turnover. Sci Rep 2017, 7, 16693. [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat Rev Nephrol 2019, 15, 346-366. [CrossRef]
- Wang, H.; Li, S.; Wang, J.; Chen, S.; Sun, X.L.; Wu, Q. N-glycosylation in the protease domain of trypsin-like serine proteases mediates calnexin-assisted protein folding. eLife 2018, 7, e35672. [CrossRef]
- Sun, S.; Hu, K.; Wang, L.; Liu, M.; Zhang, Y.; Dong, N.; Wu, Q. Spatial position is a key determinant of N-glycan functionality of the scavenger receptor cysteine-rich domain of human hepsin. Febs j 2023, 10.1111/febs.16757. [CrossRef]
- Sun, S.; Wang, L.; Zhang, S.; Zhang, C.; Chen, Y.; Wu, Q.; Dong, N. N-glycan in the scavenger receptor cysteine-rich domain of hepsin promotes intracellular trafficking and cell surface expression. Int J Biol Macromol 2020, 161, 818-827. [CrossRef]
- Kozlov, G.; Gehring, K. Calnexin cycle - structural features of the ER chaperone system. Febs j 2020, 287, 4322-4340. [CrossRef]
- Zhang, C.; Chen, Y.; Sun, S.; Zhang, Y.; Wang, L.; Luo, Z.; Liu, M.; Dong, L.; Dong, N.; Wu, Q. A conserved LDL-receptor motif regulates corin and CD320 membrane targeting in polarized renal epithelial cells. eLife 2020, 9, e56059. [CrossRef]
- Gladysheva, I.P.; Robinson, B.R.; Houng, A.K.; Kováts, T.; King, S.M. Corin is co-expressed with pro-ANP and localized on the cardiomyocyte surface in both zymogen and catalytically active forms. J Mol Cell Cardiol 2008, 44, 131-142. [CrossRef]
- Lichtenthaler, S.F.; Lemberg, M.K.; Fluhrer, R. Proteolytic ectodomain shedding of membrane proteins in mammals-hardware, concepts, and recent developments. Embo j 2018, 37. [CrossRef]
- Tseng, C.C.; Jia, B.; Barndt, R.; Gu, Y.; Chen, C.Y.; Tseng, I.C.; Su, S.F.; Wang, J.K.; Johnson, M.D.; Lin, C.Y. Matriptase shedding is closely coupled with matriptase zymogen activation and requires de novo proteolytic cleavage likely involving its own activity. PLoS One 2017, 12, e0183507. [CrossRef]
- Yoon, J.; Cho, Y.; Kim, K.Y.; Yoon, M.J.; Lee, H.S.; Jeon, S.D.; Kim, C.; Kim, M.G. A JUN N-terminal kinase inhibitor induces ectodomain shedding of the cancer-associated membrane protease Prss14/epithin via protein kinase CβII. J Biol Chem 2020, 295, 7168-7177. [CrossRef]
- Najy, A.J.; Dyson, G.; Jena, B.P.; Lin, C.Y.; Kim, H.R. Matriptase activation and shedding through PDGF-D-mediated extracellular acidosis. Am J Physiol Cell Physiol 2016, 310, C293-304. [CrossRef]
- Friis, S.; Sales, K.U.; Schafer, J.M.; Vogel, L.K.; Kataoka, H.; Bugge, T.H. The protease inhibitor HAI-2, but not HAI-1, regulates matriptase activation and shedding through prostasin. J Biol Chem 2014, 289, 22319-22332. [CrossRef]
- Larsen, B.R.; Steffensen, S.D.; Nielsen, N.V.; Friis, S.; Godiksen, S.; Bornholdt, J.; Soendergaard, C.; Nonboe, A.W.; Andersen, M.N.; Poulsen, S.S.; et al. Hepatocyte growth factor activator inhibitor-2 prevents shedding of matriptase. Exp Cell Res 2013, 319, 918-929. [CrossRef]
- Zhao, N.; Nizzi, C.P.; Anderson, S.A.; Wang, J.; Ueno, A.; Tsukamoto, H.; Eisenstein, R.S.; Enns, C.A.; Zhang, A.S. Low intracellular iron increases the stability of matriptase-2. J Biol Chem 2015, 290, 4432-4446. [CrossRef]
- Martin, C.E.; Murray, A.S.; Mackinder, J.R.; Sala-Hamrick, K.E.; Flynn, M.G.; Lundgren, J.G.; Varela, F.A.; List, K. TMPRSS13 zymogen activation, surface localization, and shedding is regulated by proteolytic cleavage within the non-catalytic stem region. Biol Chem 2022, 403, 969-982. [CrossRef]
- Jiang, J.; Wu, S.; Wang, W.; Chen, S.; Peng, J.; Zhang, X.; Wu, Q. Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J Biol Chem 2011, 286, 10066-10072. [CrossRef]
- Wang, H.; Zhou, T.; Peng, J.; Xu, P.; Dong, N.; Chen, S.; Wu, Q. Distinct roles of N-glycosylation at different sites of corin in cell membrane targeting and ectodomain shedding. J Biol Chem 2015, 290, 1654-1663. [CrossRef]
- Béliveau, F.; Brulé, C.; Désilets, A.; Zimmerman, B.; Laporte, S.A.; Lavoie, C.L.; Leduc, R. Essential role of endocytosis of the type II transmembrane serine protease TMPRSS6 in regulating its functionality. J Biol Chem 2011, 286, 29035-29043. [CrossRef]
- Zheng, X.L.; Kitamoto, Y.; Sadler, J.E. Enteropeptidase, a type II transmembrane serine protease. Front Biosci (Elite Ed) 2009, 1, 242-249. [CrossRef]
- List, K.; Kosa, P.; Szabo, R.; Bey, A.L.; Wang, C.B.; Molinolo, A.; Bugge, T.H. Epithelial integrity is maintained by a matriptase-dependent proteolytic pathway. Am J Pathol 2009, 175, 1453-1463. [CrossRef]
- Buzza, M.S.; Netzel-Arnett, S.; Shea-Donohue, T.; Zhao, A.; Lin, C.Y.; List, K.; Szabo, R.; Fasano, A.; Bugge, T.H.; Antalis, T.M. Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc Natl Acad Sci U S A 2010, 107, 4200-4205. [CrossRef]
- Szabo, R.; Bugge, T.H. Membrane-anchored serine proteases as regulators of epithelial function. Biochem Soc Trans 2020, 48, 517-528. [CrossRef]
- Wu, C.J.; Feng, X.; Lu, M.; Morimura, S.; Udey, M.C. Matriptase-mediated cleavage of EpCAM destabilizes claudins and dysregulates intestinal epithelial homeostasis. J Clin Invest 2017, 127, 623-634. [CrossRef]
- Szabo, R.; Callies, L.K.; Bugge, T.H. Matriptase drives early-onset intestinal failure in a mouse model of congenital tufting enteropathy. Development 2019, 146. [CrossRef]
- Fasquelle, L.; Scott, H.S.; Lenoir, M.; Wang, J.; Rebillard, G.; Gaboyard, S.; Venteo, S.; François, F.; Mausset-Bonnefont, A.L.; Antonarakis, S.E.; et al. Tmprss3, a transmembrane serine protease deficient in human DFNB8/10 deafness, is critical for cochlear hair cell survival at the onset of hearing. J Biol Chem 2011, 286, 17383-17397. [CrossRef]
- Molina, L.; Fasquelle, L.; Nouvian, R.; Salvetat, N.; Scott, H.S.; Guipponi, M.; Molina, F.; Puel, J.L.; Delprat, B. Tmprss3 loss of function impairs cochlear inner hair cell Kcnma1 channel membrane expression. Hum Mol Genet 2013, 22, 1289-1299. [CrossRef]
- Battelino, S.; Klancar, G.; Kovac, J.; Battelino, T.; Trebusak Podkrajsek, K. TMPRSS3 mutations in autosomal recessive nonsyndromic hearing loss. Eur Arch Otorhinolaryngol 2016, 273, 1151-1154. [CrossRef]
- Menou, A.; Duitman, J.; Flajolet, P.; Sallenave, J.M.; Mailleux, A.A.; Crestani, B. Human airway trypsin-like protease, a serine protease involved in respiratory diseases. Am J Physiol Lung Cell Mol Physiol 2017, 312, L657-l668. [CrossRef]
- Matsushima, R.; Takahashi, A.; Nakaya, Y.; Maezawa, H.; Miki, M.; Nakamura, Y.; Ohgushi, F.; Yasuoka, S. Human airway trypsin-like protease stimulates human bronchial fibroblast proliferation in a protease-activated receptor-2-dependent pathway. Am J Physiol Lung Cell Mol Physiol 2006, 290, L385-395. [CrossRef]
- Miki, M.; Nakamura, Y.; Takahashi, A.; Nakaya, Y.; Eguchi, H.; Masegi, T.; Yoneda, K.; Yasuoka, S.; Sone, S. Effect of human airway trypsin-like protease on intracellular free Ca2+ concentration in human bronchial epithelial cells. J Med Invest 2003, 50, 95-107.
- Beaufort, N.; Leduc, D.; Eguchi, H.; Mengele, K.; Hellmann, D.; Masegi, T.; Kamimura, T.; Yasuoka, S.; Fend, F.; Chignard, M.; et al. The human airway trypsin-like protease modulates the urokinase receptor (uPAR, CD87) structure and functions. Am J Physiol Lung Cell Mol Physiol 2007, 292, L1263-1272. [CrossRef]
- Fernandez, C.; Burgos, A.; Morales, D.; Rosales-Rojas, R.; Canelo, J.; Vergara-Jaque, A.; Vieira, G.V.; da Silva, R.A.A.; Sales, K.U.; Conboy, M.J.; et al. TMPRSS11a is a novel age-altered, tissue specific regulator of migration and wound healing. Faseb j 2021, 35, e21597. [CrossRef]
- Zhang, Z.; Hu, Y.; Yan, R.; Dong, L.; Jiang, Y.; Zhou, Z.; Liu, M.; Zhou, T.; Dong, N.; Wu, Q. The Transmembrane Serine Protease HAT-like 4 Is Important for Epidermal Barrier Function to Prevent Body Fluid Loss. Sci Rep 2017, 7, 45262. [CrossRef]
- Callies, L.K.; Tadeo, D.; Simper, J.; Bugge, T.H.; Szabo, R. Iterative, multiplexed CRISPR-mediated gene editing for functional analysis of complex protease gene clusters. J Biol Chem 2019, 294, 15987-15996. [CrossRef]
- Madsen, D.H.; Szabo, R.; Molinolo, A.A.; Bugge, T.H. TMPRSS13 deficiency impairs stratum corneum formation and epidermal barrier acquisition. Biochem J 2014, 461, 487-495. [CrossRef]
- Li, S.; Wang, L.; Sun, S.; Wu, Q. Hepsin: a multifunctional transmembrane serine protease in pathobiology. FEBS J 2021, 288, 5252-5264. [CrossRef]
- Hsu, Y.C.; Huang, H.P.; Yu, I.S.; Su, K.Y.; Lin, S.R.; Lin, W.C.; Wu, H.L.; Shi, G.Y.; Tao, M.H.; Kao, C.H.; et al. Serine protease hepsin regulates hepatocyte size and hemodynamic retention of tumor cells by hepatocyte growth factor signaling in mice. Hepatology 2012, 56, 1913-1923. [CrossRef]
- Stirnberg, M.; Gütschow, M. Matriptase-2, a regulatory protease of iron homeostasis: possible substrates, cleavage sites and inhibitors. Curr Pharm Des 2013, 19, 1052-1061. [CrossRef]
- Wahedi, M.; Wortham, A.M.; Kleven, M.D.; Zhao, N.; Jue, S.; Enns, C.A.; Zhang, A.S. Matriptase-2 suppresses hepcidin expression by cleaving multiple components of the hepcidin induction pathway. J Biol Chem 2017, 292, 18354-18371. [CrossRef]
- Zhou, Y.; Wu, Q. Corin in natriuretic peptide processing and hypertension. Curr Hypertens Rep 2014, 16, 415. [CrossRef]
- Zhang, X.; Gu, X.; Zhang, Y.; Dong, N.; Wu, Q. Corin: A Key Mediator in Sodium Homeostasis, Vascular Remodeling, and Heart Failure. Biology (Basel) 2022, 11. [CrossRef]
- Sullivan, R.D.; Houng, A.K.; Gladysheva, I.P.; Fan, T.M.; Tripathi, R.; Reed, G.L.; Wang, D. Corin Overexpression Reduces Myocardial Infarct Size and Modulates Cardiomyocyte Apoptotic Cell Death. Int J Mol Sci 2020, 21. [CrossRef]
- Kim, T.S.; Heinlein, C.; Hackman, R.C.; Nelson, P.S. Phenotypic analysis of mice lacking the Tmprss2-encoded protease. Mol Cell Biol 2006, 26, 965-975. [CrossRef]
- Sales, K.U.; Hobson, J.P.; Wagenaar-Miller, R.; Szabo, R.; Rasmussen, A.L.; Bey, A.; Shah, M.F.; Molinolo, A.A.; Bugge, T.H. Expression and genetic loss of function analysis of the HAT/DESC cluster proteases TMPRSS11A and HAT. PLoS One 2011, 6, e23261. [CrossRef]
- Martin, C.E.; List, K. Cell surface-anchored serine proteases in cancer progression and metastasis. Cancer Metastasis Rev 2019, 38, 357-387. [CrossRef]
- Wu, Q.; Parry, G. Hepsin and prostate cancer. Front Biosci 2007, 12, 5052-5059. [CrossRef]
- Murray, A.S.; Hyland, T.E.; Sala-Hamrick, K.E.; Mackinder, J.R.; Martin, C.E.; Tanabe, L.M.; Varela, F.A.; List, K. The cell-surface anchored serine protease TMPRSS13 promotes breast cancer progression and resistance to chemotherapy. Oncogene 2020, 39, 6421-6436. [CrossRef]
- Mukai, S.; Yamasaki, K.; Fujii, M.; Nagai, T.; Terada, N.; Kataoka, H.; Kamoto, T. Dysregulation of Type II Transmembrane Serine Proteases and Ligand-Dependent Activation of MET in Urological Cancers. Int J Mol Sci 2020, 21. [CrossRef]
- Fuentes-Prior, P. Priming of SARS-CoV-2 S protein by several membrane-bound serine proteinases could explain enhanced viral infectivity and systemic COVID-19 infection. J Biol Chem 2021, 296, 100135. [CrossRef]
- Harbig, A.; Mernberger, M.; Bittel, L.; Pleschka, S.; Schughart, K.; Steinmetzer, T.; Stiewe, T.; Nist, A.; Böttcher-Friebertshäuser, E. Transcriptome profiling and protease inhibition experiments identify proteases that activate H3N2 influenza A and influenza B viruses in murine airways. J Biol Chem 2020, 295, 11388-11407. [CrossRef]
- Murza, A.; Dion, S.P.; Boudreault, P.L.; Désilets, A.; Leduc, R.; Marsault, É. Inhibitors of type II transmembrane serine proteases in the treatment of diseases of the respiratory tract - A review of patent literature. Expert Opin Ther Pat 2020, 30, 807-824. [CrossRef]
- Forni, D.; Sironi, M.; Cagliani, R. Evolutionary history of type II transmembrane serine proteases involved in viral priming. Hum Genet 2022, 141, 1705-1722. [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271-280.e278. [CrossRef]
- Shapira, T.; Monreal, I.A.; Dion, S.P.; Buchholz, D.W.; Imbiakha, B.; Olmstead, A.D.; Jager, M.; Désilets, A.; Gao, G.; Martins, M.; et al. A TMPRSS2 inhibitor acts as a pan-SARS-CoV-2 prophylactic and therapeutic. Nature 2022, 605, 340-348. [CrossRef]
- Hsin, F.; Hsu, Y.C.; Tsai, Y.F.; Lin, S.W.; Liu, H.M. The transmembrane serine protease hepsin suppresses type I interferon induction by cleaving STING. Sci Signal 2021, 14. [CrossRef]
- Scheja, L.; Heeren, J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol 2019, 15, 507-524. [CrossRef]
- Massier, L.; Jalkanen, J.; Elmastas, M.; Zhong, J.; Wang, T.; Nono Nankam, P.A.; Frendo-Cumbo, S.; Bäckdahl, J.; Subramanian, N.; Sekine, T.; et al. An integrated single cell and spatial transcriptomic map of human white adipose tissue. Nat Commun 2023, 14, 1438. [CrossRef]
- Hildreth, A.D.; Ma, F.; Wong, Y.Y.; Sun, R.; Pellegrini, M.; O'Sullivan, T.E. Single-cell sequencing of human white adipose tissue identifies new cell states in health and obesity. Nat Immunol 2021, 22, 639-653. [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [CrossRef]
- Karlsson, M.; Zhang, C.; Méar, L.; Zhong, W.; Digre, A.; Katona, B.; Sjöstedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A single-cell type transcriptomics map of human tissues. Sci Adv 2021, 7. [CrossRef]
- Leytus, S.P.; Loeb, K.R.; Hagen, F.S.; Kurachi, K.; Davie, E.W. A novel trypsin-like serine protease (hepsin) with a putative transmembrane domain expressed by human liver and hepatoma cells. Biochemistry 1988, 27, 1067-1074. [CrossRef]
- Xuan, J.A.; Schneider, D.; Toy, P.; Lin, R.; Newton, A.; Zhu, Y.; Finster, S.; Vogel, D.; Mintzer, B.; Dinter, H.; et al. Antibodies neutralizing hepsin protease activity do not impact cell growth but inhibit invasion of prostate and ovarian tumor cells in culture. Cancer Res 2006, 66, 3611-3619. [CrossRef]
- Herter, S.; Piper, D.E.; Aaron, W.; Gabriele, T.; Cutler, G.; Cao, P.; Bhatt, A.S.; Choe, Y.; Craik, C.S.; Walker, N.; et al. Hepatocyte growth factor is a preferred in vitro substrate for human hepsin, a membrane-anchored serine protease implicated in prostate and ovarian cancers. Biochem J 2005, 390, 125-136. [CrossRef]
- Hsu, Y.C.; Yu, I.S.; Tsai, Y.F.; Wu, Y.M.; Chen, Y.T.; Sheu, J.C.; Lin, S.W. A Preconditioning Strategy to Augment Retention and Engraftment Rate of Donor Cells During Hepatocyte Transplantation. Transplantation 2021, 105, 785-795. [CrossRef]
- Li, S.; Peng, J.; Wang, H.; Zhang, W.; Brown, J.M.; Zhou, Y.; Wu, Q. Hepsin enhances liver metabolism and inhibits adipocyte browning in mice. Proc Natl Acad Sci U S A 2020, 117, 12359-12367. [CrossRef]
- Aljakna, A.; Choi, S.; Savage, H.; Hageman Blair, R.; Gu, T.; Svenson, K.L.; Churchill, G.A.; Hibbs, M.; Korstanje, R. Pla2g12b and Hpn are genes identified by mouse ENU mutagenesis that affect HDL cholesterol. PLoS One 2012, 7, e43139. [CrossRef]
- Franceschini, N.; van Rooij, F.J.; Prins, B.P.; Feitosa, M.F.; Karakas, M.; Eckfeldt, J.H.; Folsom, A.R.; Kopp, J.; Vaez, A.; Andrews, J.S.; et al. Discovery and fine mapping of serum protein loci through transethnic meta-analysis. Am J Hum Genet 2012, 91, 744-753. [CrossRef]
- Koch, J.P.; Aebersold, D.M.; Zimmer, Y.; Medová, M. MET targeting: time for a rematch. Oncogene 2020, 39, 2845-2862. [CrossRef]
- Tervonen, T.A.; Belitškin, D.; Pant, S.M.; Englund, J.I.; Marques, E.; Ala-Hongisto, H.; Nevalaita, L.; Sihto, H.; Heikkilä, P.; Leidenius, M.; et al. Deregulated hepsin protease activity confers oncogenicity by concomitantly augmenting HGF/MET signalling and disrupting epithelial cohesion. Oncogene 2016, 35, 1832-1846. [CrossRef]
- Brunati, M.; Perucca, S.; Han, L.; Cattaneo, A.; Consolato, F.; Andolfo, A.; Schaeffer, C.; Olinger, E.; Peng, J.; Santambrogio, S.; et al. The serine protease hepsin mediates urinary secretion and polymerisation of Zona Pellucida domain protein uromodulin. Elife 2015, 4, e08887. [CrossRef]
- Olinger, E.; Lake, J.; Sheehan, S.; Schiano, G.; Takata, T.; Tokonami, N.; Debaix, H.; Consolato, F.; Rampoldi, L.; Korstanje, R.; et al. Hepsin-mediated Processing of Uromodulin is Crucial for Salt-sensitivity and Thick Ascending Limb Homeostasis. Sci Rep 2019, 9, 12287. [CrossRef]
- Guipponi, M.; Tan, J.; Cannon, P.Z.; Donley, L.; Crewther, P.; Clarke, M.; Wu, Q.; Shepherd, R.K.; Scott, H.S. Mice deficient for the type II transmembrane serine protease, TMPRSS1/hepsin, exhibit profound hearing loss. Am J Pathol 2007, 171, 608-616. [CrossRef]
- Naz, S. Molecular genetic landscape of hereditary hearing loss in Pakistan. Hum Genet 2022, 141, 633-648. [CrossRef]
- Belitškin, D.; Pant, S.M.; Munne, P.; Suleymanova, I.; Belitškina, K.; Hongisto, H.A.; Englund, J.; Raatikainen, T.; Klezovitch, O.; Vasioukhin, V.; et al. Hepsin regulates TGFβ signaling via fibronectin proteolysis. EMBO Rep 2021, 22, e52532. [CrossRef]
- Puigserver, P.; Spiegelman, B.M. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 2003, 24, 78-90. [CrossRef]
- Tervonen, T.A.; Pant, S.M.; Belitškin, D.; Englund, J.I.; Närhi, K.; Haglund, C.; Kovanen, P.E.; Verschuren, E.W.; Klefström, J. Oncogenic Ras Disrupts Epithelial Integrity by Activating the Transmembrane Serine Protease Hepsin. Cancer Res 2021, 81, 1513-1527. [CrossRef]
- Ramsay, A.J.; Hooper, J.D.; Folgueras, A.R.; Velasco, G.; López-Otín, C. Matriptase-2 (TMPRSS6): a proteolytic regulator of iron homeostasis. Haematologica 2009, 94, 840-849. [CrossRef]
- Ramsay, A.J.; Reid, J.C.; Velasco, G.; Quigley, J.P.; Hooper, J.D. The type II transmembrane serine protease matriptase-2--identification, structural features, enzymology, expression pattern and potential roles. Front Biosci 2008, 13, 569-579. [CrossRef]
- Finberg, K.E.; Heeney, M.M.; Campagna, D.R.; Aydinok, Y.; Pearson, H.A.; Hartman, K.R.; Mayo, M.M.; Samuel, S.M.; Strouse, J.J.; Markianos, K.; et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet 2008, 40, 569-571. [CrossRef]
- Du, X.; She, E.; Gelbart, T.; Truksa, J.; Lee, P.; Xia, Y.; Khovananth, K.; Mudd, S.; Mann, N.; Moresco, E.M.; et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science 2008, 320, 1088-1092. [CrossRef]
- Cui, Y.; Wu, Q.; Zhou, Y. Iron-refractory iron deficiency anemia: new molecular mechanisms. Kidney Int 2009, 76, 1137-1141. [CrossRef]
- Babitt, J.L.; Huang, F.W.; Wrighting, D.M.; Xia, Y.; Sidis, Y.; Samad, T.A.; Campagna, J.A.; Chung, R.T.; Schneyer, A.L.; Woolf, C.J.; et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet 2006, 38, 531-539. [CrossRef]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344-361. [CrossRef]
- Silvestri, L.; Pagani, A.; Nai, A.; De Domenico, I.; Kaplan, J.; Camaschella, C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab 2008, 8, 502-511. [CrossRef]
- Enns, C.A.; Jue, S.; Zhang, A.S. The ectodomain of matriptase-2 plays an important nonproteolytic role in suppressing hepcidin expression in mice. Blood 2020, 136, 989-1001. [CrossRef]
- Friis, S.; Tadeo, D.; Le-Gall, S.M.; Jürgensen, H.J.; Sales, K.U.; Camerer, E.; Bugge, T.H. Matriptase zymogen supports epithelial development, homeostasis and regeneration. BMC Biol 2017, 15, 46. [CrossRef]
- Tripathi, R.; Sullivan, R.D.; Fan, T.M.; Houng, A.K.; Mehta, R.M.; Reed, G.L.; Gladysheva, I.P. Cardiac-Specific Overexpression of Catalytically Inactive Corin Reduces Edema, Contractile Dysfunction, and Death in Mice with Dilated Cardiomyopathy. Int J Mol Sci 2019, 21, 203. [CrossRef]
- Beckmann, A.M.; Maurer, E.; Lülsdorff, V.; Wilms, A.; Furtmann, N.; Bajorath, J.; Gütschow, M.; Stirnberg, M. En Route to New Therapeutic Options for Iron Overload Diseases: Matriptase-2 as a Target for Kunitz-Type Inhibitors. Chembiochem 2016, 17, 595-604. [CrossRef]
- McClung, J.P.; Karl, J.P. Iron deficiency and obesity: the contribution of inflammation and diminished iron absorption. Nutr Rev 2009, 67, 100-104. [CrossRef]
- del Giudice, E.M.; Santoro, N.; Amato, A.; Brienza, C.; Calabrò, P.; Wiegerinck, E.T.; Cirillo, G.; Tartaglione, N.; Grandone, A.; Swinkels, D.W.; et al. Hepcidin in obese children as a potential mediator of the association between obesity and iron deficiency. J Clin Endocrinol Metab 2009, 94, 5102-5107. [CrossRef]
- Stoffel, N.U.; El-Mallah, C.; Herter-Aeberli, I.; Bissani, N.; Wehbe, N.; Obeid, O.; Zimmermann, M.B. The effect of central obesity on inflammation, hepcidin, and iron metabolism in young women. Int J Obes (Lond) 2020, 44, 1291-1300. [CrossRef]
- Bekri, S.; Gual, P.; Anty, R.; Luciani, N.; Dahman, M.; Ramesh, B.; Iannelli, A.; Staccini-Myx, A.; Casanova, D.; Ben Amor, I.; et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology 2006, 131, 788-796. [CrossRef]
- Dongiovanni, P.; Ruscica, M.; Rametta, R.; Recalcati, S.; Steffani, L.; Gatti, S.; Girelli, D.; Cairo, G.; Magni, P.; Fargion, S.; et al. Dietary iron overload induces visceral adipose tissue insulin resistance. Am J Pathol 2013, 182, 2254-2263. [CrossRef]
- Folgueras, A.R.; Freitas-Rodríguez, S.; Ramsay, A.J.; Garabaya, C.; Rodríguez, F.; Velasco, G.; López-Otín, C. Matriptase-2 deficiency protects from obesity by modulating iron homeostasis. Nat Commun 2018, 9, 1350. [CrossRef]
- Yan, W.; Sheng, N.; Seto, M.; Morser, J.; Wu, Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem 1999, 274, 14926-14935. [CrossRef]
- Yan, W.; Wu, F.; Morser, J.; Wu, Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A 2000, 97, 8525-8529. [CrossRef]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat Rev Cardiol 2020, 17, 698-717. [CrossRef]
- Kuhn, M. Cardiac actions of atrial natriuretic peptide: new visions of an old friend. Circ Res 2015, 116, 1278-1280. [CrossRef]
- Zhan, W.; Chen, L.; Liu, H.; Long, C.; Liu, J.; Ding, S.; Wu, Q.; Chen, S. Pcsk6 Deficiency Promotes Cardiomyocyte Senescence by Modulating Ddit3-Mediated ER Stress. Genes (Basel) 2022, 13. [CrossRef]
- Cui, Y.; Wang, W.; Dong, N.; Lou, J.; Srinivasan, D.K.; Cheng, W.; Huang, X.; Liu, M.; Fang, C.; Peng, J.; et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature 2012, 484, 246-250. [CrossRef]
- Zhang, W.; Li, S.; Lou, J.; Li, H.; Liu, M.; Dong, N.; Wu, Q. Atrial natriuretic peptide promotes uterine decidualization and a TRAIL-dependent mechanism in spiral artery remodeling. J Clin Invest 2021, 131, e151053. [CrossRef]
- Song, W.; Wang, H.; Wu, Q. Atrial natriuretic peptide in cardiovascular biology and disease (NPPA). Gene 2015, 569, 1-6. [CrossRef]
- Dong, N.; Niu, Y.; Chen, Y.; Sun, S.; Wu, Q. Function and regulation of corin in physiology and disease. Biochem Soc Trans 2020, 48, 1905-1916. [CrossRef]
- Zhou, T.; Zhang, S.; Du, C.; Wang, K.; Gu, X.; Sun, S.; Zhang, X.; Niu, Y.; Wang, C.; Liu, M.; et al. Renal Corin Is Essential for Normal Blood Pressure and Sodium Homeostasis. Int J Mol Sci 2022, 23, 11251. [CrossRef]
- He, M.; Zhou, T.; Niu, Y.; Feng, W.; Gu, X.; Xu, W.; Zhang, S.; Wang, Z.; Zhang, Y.; Wang, C.; et al. The protease corin regulates electrolyte homeostasis in eccrine sweat glands. PLoS Biol 2021, 19, e3001090. [CrossRef]
- Chan, J.C.; Knudson, O.; Wu, F.; Morser, J.; Dole, W.P.; Wu, Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci U S A 2005, 102, 785-790. [CrossRef]
- Wang, W.; Shen, J.; Cui, Y.; Jiang, J.; Chen, S.; Peng, J.; Wu, Q. Impaired sodium excretion and salt-sensitive hypertension in corin-deficient mice. Kidney Int 2012, 82, 26-33. [CrossRef]
- Dong, N.; Fang, C.; Jiang, Y.; Zhou, T.; Liu, M.; Zhou, J.; Shen, J.; Fukuda, K.; Qin, J.; Wu, Q. Corin mutation R539C from hypertensive patients impairs zymogen activation and generates an inactive alternative ectodomain fragment. J Biol Chem 2013, 288, 7867-7874. [CrossRef]
- Zhang, Y.; Zhou, T.; Niu, Y.; He, M.; Wang, C.; Liu, M.; Yang, J.; Zhou, J.; Fukuda, K.; Qin, J.; et al. Identification and functional analysis of CORIN variants in hypertensive patients. Hum Mutat 2017, 38, 1700-1710. [CrossRef]
- Rame, J.E.; Tam, S.W.; McNamara, D.; Worcel, M.; Sabolinski, M.L.; Wu, A.H.; Dries, D.L. Dysfunctional corin i555(p568) allele is associated with impaired brain natriuretic peptide processing and adverse outcomes in blacks with systolic heart failure: results from the Genetic Risk Assessment in Heart Failure substudy. Circ Heart Fail 2009, 2, 541-548. [CrossRef]
- Dong, N.; Zhou, T.; Zhang, Y.; Liu, M.; Li, H.; Huang, X.; Liu, Z.; Wu, Y.; Fukuda, K.; Qin, J.; et al. Corin mutations K317E and S472G from preeclamptic patients alter zymogen activation and cell surface targeting. J Biol Chem 2014, 289, 17909-17916. [CrossRef]
- Zhao, Y.; Yuan, X.; Zhong, Y.; Zhang, Y.; Zhang, S.; Li, S.; Zheng, W.; Liu, J.; Xia, Y.; Yang, Y.; et al. Single-Nucleotide Polymorphisms in the 3' Untranslated Region of CORIN Associated With Cardiovascular Diseases in a Chinese Han Population: A Case-Control Study. Front Cardiovasc Med 2021, 8, 625072. [CrossRef]
- Sengenes, C.; Bouloumie, A.; Hauner, H.; Berlan, M.; Busse, R.; Lafontan, M.; Galitzky, J. Involvement of a cGMP-dependent pathway in the natriuretic peptide-mediated hormone-sensitive lipase phosphorylation in human adipocytes. J Biol Chem 2003, 278, 48617-48626. [CrossRef]
- Birkenfeld, A.L.; Budziarek, P.; Boschmann, M.; Moro, C.; Adams, F.; Franke, G.; Berlan, M.; Marques, M.A.; Sweep, F.C.; Luft, F.C.; et al. Atrial natriuretic peptide induces postprandial lipid oxidation in humans. Diabetes 2008, 57, 3199-3204. [CrossRef]
- Wu, W.; Shi, F.; Liu, D.; Ceddia, R.P.; Gaffin, R.; Wei, W.; Fang, H.; Lewandowski, E.D.; Collins, S. Enhancing natriuretic peptide signaling in adipose tissue, but not in muscle, protects against diet-induced obesity and insulin resistance. Sci Signal 2017, 10, eaam6870. [CrossRef]
- Cabiati, M.; Raucci, S.; Liistro, T.; Belcastro, E.; Prescimone, T.; Caselli, C.; Matteucci, M.; Iozzo, P.; Mattii, L.; Giannessi, D.; et al. Impact of obesity on the expression profile of natriuretic peptide system in a rat experimental model. PLoS One 2013, 8, e72959. [CrossRef]
- Bartels, E.D.; Nielsen, J.M.; Bisgaard, L.S.; Goetze, J.P.; Nielsen, L.B. Decreased expression of natriuretic peptides associated with lipid accumulation in cardiac ventricle of obese mice. Endocrinology 2010, 151, 5218-5225. [CrossRef]
- Galitzky, J.; Sengenès, C.; Thalamas, C.; Marques, M.A.; Senard, J.M.; Lafontan, M.; Berlan, M. The lipid-mobilizing effect of atrial natriuretic peptide is unrelated to sympathetic nervous system activation or obesity in young men. J Lipid Res 2001, 42, 536-544.
- Engeli, S.; Birkenfeld, A.L.; Badin, P.M.; Bourlier, V.; Louche, K.; Viguerie, N.; Thalamas, C.; Montastier, E.; Larrouy, D.; Harant, I.; et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest 2012, 122, 4675-4679. [CrossRef]
- Bordicchia, M.; Liu, D.; Amri, E.Z.; Ailhaud, G.; Dessì-Fulgheri, P.; Zhang, C.; Takahashi, N.; Sarzani, R.; Collins, S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 2012, 122, 1022-1036. [CrossRef]
- Coué, M.; Moro, C. Natriuretic peptide control of energy balance and glucose homeostasis. Biochimie 2016, 124, 84-91. [CrossRef]
- Kimura, H.; Nagoshi, T.; Oi, Y.; Yoshii, A.; Tanaka, Y.; Takahashi, H.; Kashiwagi, Y.; Tanaka, T.D.; Yoshimura, M. Treatment with atrial natriuretic peptide induces adipose tissue browning and exerts thermogenic actions in vivo. Sci Rep 2021, 11, 17466. [CrossRef]
- Liu, D.; Ceddia, R.P.; Collins, S. Cardiac natriuretic peptides promote adipose 'browning' through mTOR complex-1. Mol Metab 2018, 9, 192-198. [CrossRef]
- Carper, D.; Coué, M.; Nascimento, E.B.M.; Barquissau, V.; Lagarde, D.; Pestourie, C.; Laurens, C.; Petit, J.V.; Soty, M.; Monbrun, L.; et al. Atrial Natriuretic Peptide Orchestrates a Coordinated Physiological Response to Fuel Non-shivering Thermogenesis. Cell Rep 2020, 32, 108075. [CrossRef]
- Inoue, K.; Sakamoto, T.; Yuge, S.; Iwatani, H.; Yamagami, S.; Tsutsumi, M.; Hori, H.; Cerra, M.C.; Tota, B.; Suzuki, N.; et al. Structural and functional evolution of three cardiac natriuretic peptides. Mol Biol Evol 2005, 22, 2428-2434. [CrossRef]
- Loretz, C.A.; Pollina, C. Natriuretic peptides in fish physiology. Comp Biochem Physiol A Mol Integr Physiol 2000, 125, 169-187. [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front Cardiovasc Med 2020, 7, 22. [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front Physiol 2019, 10, 1607. [CrossRef]
- Staedtke, V.; Bai, R.Y.; Kim, K.; Darvas, M.; Davila, M.L.; Riggins, G.J.; Rothman, P.B.; Papadopoulos, N.; Kinzler, K.W.; Vogelstein, B.; et al. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome. Nature 2018, 564, 273-277. [CrossRef]
- Mezzasoma, L.; Antognelli, C.; Talesa, V.N. Atrial natriuretic peptide down-regulates LPS/ATP-mediated IL-1β release by inhibiting NF-kB, NLRP3 inflammasome and caspase-1 activation in THP-1 cells. Immunol Res 2016, 64, 303-312. [CrossRef]
- Moro, C.; Klimcakova, E.; Lolmède, K.; Berlan, M.; Lafontan, M.; Stich, V.; Bouloumié, A.; Galitzky, J.; Arner, P.; Langin, D. Atrial natriuretic peptide inhibits the production of adipokines and cytokines linked to inflammation and insulin resistance in human subcutaneous adipose tissue. Diabetologia 2007, 50, 1038-1047. [CrossRef]
- Cheng, C.; Xue, F.; Sui, W.; Meng, L.; Xie, L.; Zhang, C.; Yang, J.; Zhang, Y. Deletion of natriuretic peptide receptor C alleviates adipose tissue inflammation in hypercholesterolemic Apolipoprotein E knockout mice. J Cell Mol Med 2021, 25, 9837-9850. [CrossRef]
- Sarzani, R.; Strazzullo, P.; Salvi, F.; Iacone, R.; Pietrucci, F.; Siani, A.; Barba, G.; Gerardi, M.C.; Dessì-Fulgheri, P.; Rappelli, A. Natriuretic peptide clearance receptor alleles and susceptibility to abdominal adiposity. Obes Res 2004, 12, 351-356. [CrossRef]
- Zhang, X.; Li, W.; Zhou, T.; Liu, M.; Wu, Q.; Dong, N. Corin Deficiency Alters Adipose Tissue Phenotype and Impairs Thermogenesis in Mice. Biology (Basel) 2022, 11, 1101. [CrossRef]
- Garruti, G.; Giusti, V.; Nussberger, J.; Darimont, C.; Verdumo, C.; Amstutz, C.; Puglisi, F.; Giorgino, F.; Giorgino, R.; Cotecchia, S. Expression and secretion of the atrial natriuretic peptide in human adipose tissue and preadipocytes. Obesity (Silver Spring) 2007, 15, 2181-2189. [CrossRef]
- Kamberov, Y.G.; Karlsson, E.K.; Kamberova, G.L.; Lieberman, D.E.; Sabeti, P.C.; Morgan, B.A.; Tabin, C.J. A genetic basis of variation in eccrine sweat gland and hair follicle density. Proc Natl Acad Sci U S A 2015, 112, 9932-9937. [CrossRef]
- Best, A.; Kamilar, J.M. The evolution of eccrine sweat glands in human and nonhuman primates. J Hum Evol 2018, 117, 33-43. [CrossRef]
- Bramble, D.M.; Lieberman, D.E. Endurance running and the evolution of Homo. Nature 2004, 432, 345-352. [CrossRef]
- Hooper, J.D.; Clements, J.A.; Quigley, J.P.; Antalis, T.M. Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. J Biol Chem 2001, 276, 857-860. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




