Submitted:
23 May 2023
Posted:
24 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
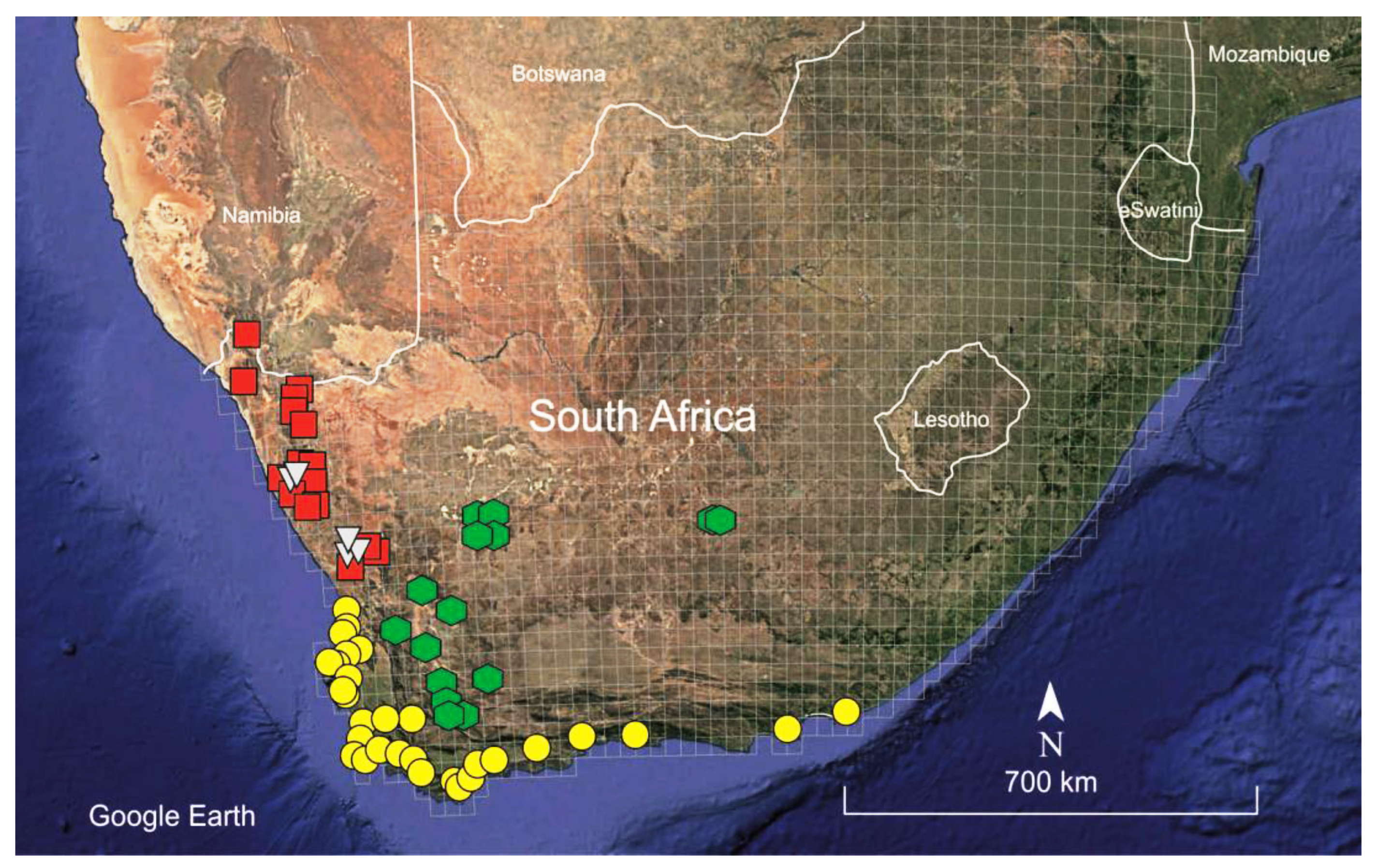
2.1. Morphological and habitat studies
| Locality | Voucher |
|---|---|
| ZA: Northern Cape, Rietfontein to Brandvlei, G.Germishuizen 4022 | PRE0694503 |
| ZA: Northern Cape, Farm Grootfontein, G.Germishuizen 6524 | PRE0791338 |
| ZA: Northern Cape, Calvinia, A.A.Schmidt 408 | PRE0405924 |
| ZA: Northern Cape, Hanover, J.P.H.Acocks 18797 | PRE0405795 |
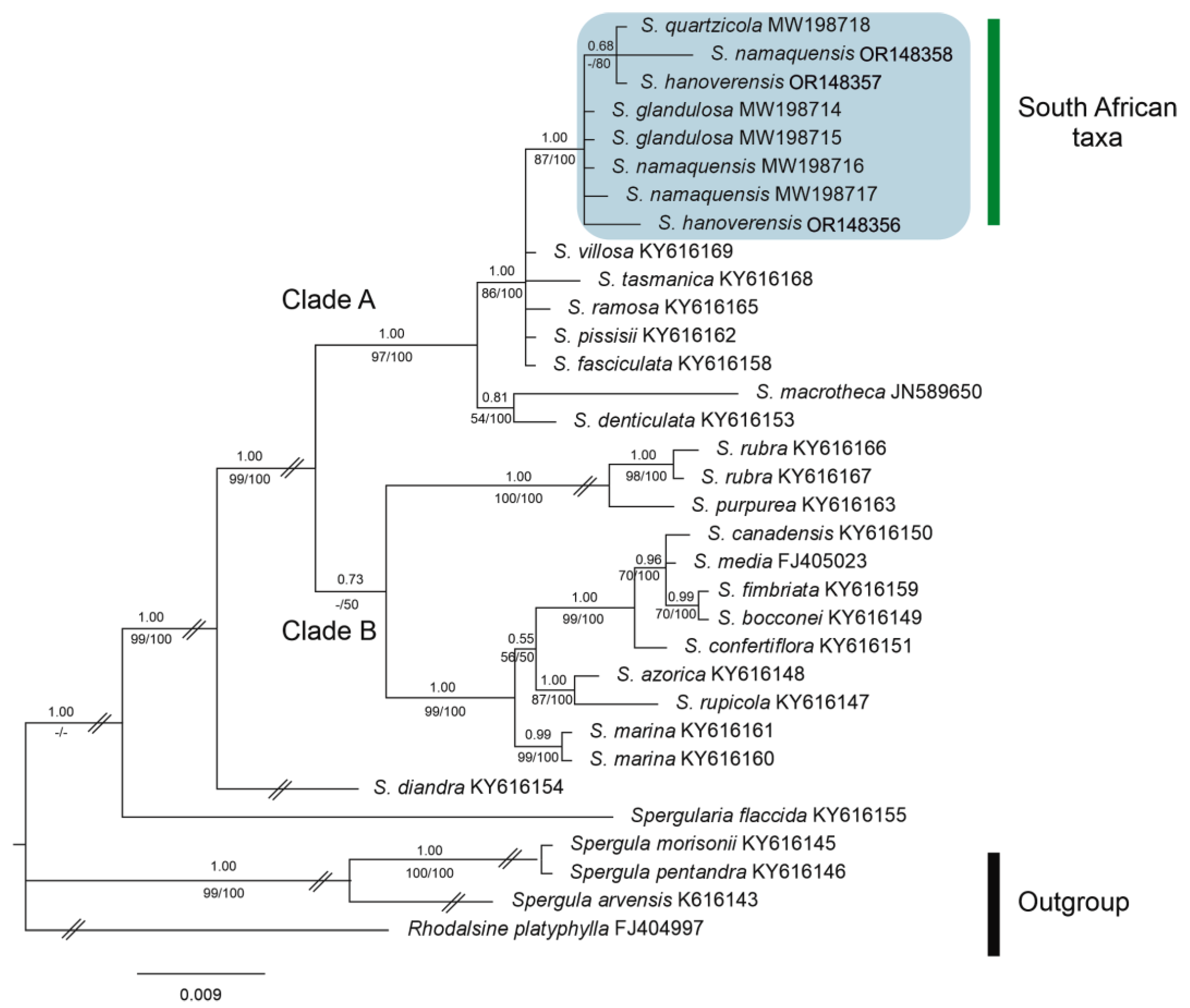
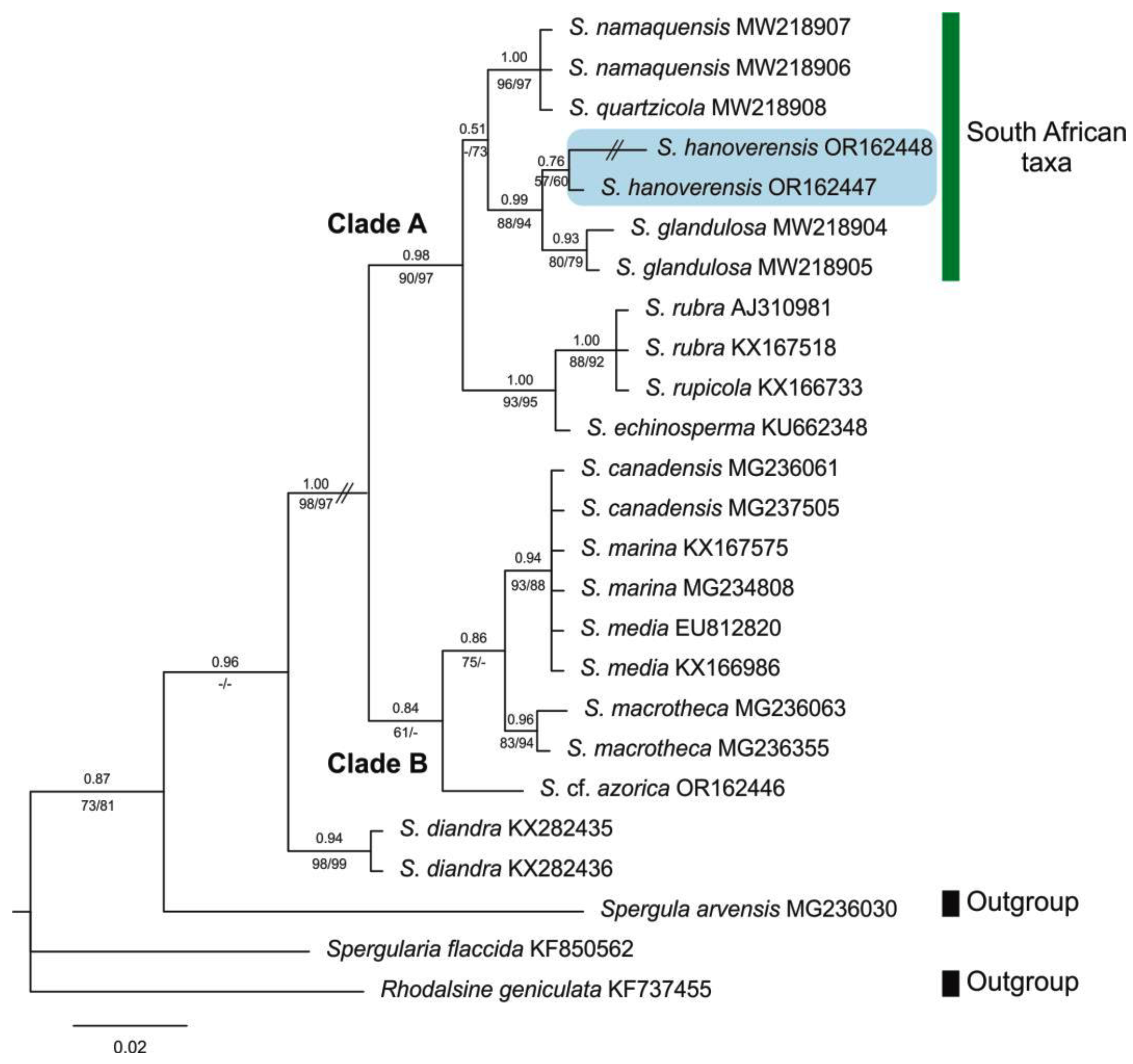
2.2. Molecular analyses
3. Results and discussion
3.1. On the trail of Spergularia hanoverensis: a brief story of the name and its application
- i)
- a subshrubby perennial plant, mostly glabrous, with a compact, often many-branched woody underground base;
- ii)
- stems often prostrate-ascendent;
- iii)
- leaves somewhat glaucous, with large whitish stipules, very apparent and showy, long-lasting, giving a Paronychia-like aspect;
- iv)
- inflorescence glanduliferous, broadly subdichasial, with inconspicuous bracts;
- v)
- flowers numerous, small, with white petals;
- vi)
- capsule small, many-seeded;
- vii)
- seeds small, triangular, blackish-brown, matte, densely papillate, unwinged or with a discolorous, vestigial to poorly developed wing.
3.2. Taxonomic treatment
3.2.1. Spergularia hanoverensis E.Simon ex M.Á.Alonso, M.B.Crespo, Mart.-Azorín & Mucina, sp. nov. (Figure 1)
3.3. Taxonomic and phylogenetic relationships of Spergularia hanoverensis
4. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Identification key for taxa in the South African group of Spergularia.
- 1
- Petals 1.8–5 mm long, about equalling or slightly shorter than sepals; styles 0.5–0.7 mm long, free from the base; seed disk densely tuberculate or papillate ............................................................................................................................................................. 2
- -
- Petals 6–9 mm long, ca. 1.5 times longer than sepals; styles 1–2 mm long, fused up to the middle or beyond; seed disk smooth or with scattered conical tubercles or granular protuberances on edge .................................................................................................................................................................... 3
- 2
- Plant diffuse, suffruticose at base; leaves often densely hairy-glanduliferous (at least at base), with a short mucro 0.2–0.5 mm long; flowers with leaf-like bracts; stamens 10; seeds monomorphic, broadly winged, with disk loosely covered with conical tubercles ...................................................................................................................................... S. glandulosa
- -
- Plant compact, strongly woody at base; leaves entirely glabrous, with a long mucro up to 1 mm long; flowers with inconspicuous bracts, much shorter than leaves; stamens 7–8; seeds dimorphic, unwinged to broadly winged, with disk densely covered with stalked globose papillae ..................................................................................................................................... S. hanoverensis
- 3
- Branchlets and leaves densely glanduliferous at least when young (sometimes leaves glabrescent when withering); sepals 4–5 mm wide, patent to slightly deflexed at anthesis, with broad scarious margins; seed wing entire to slightly eroded ....................................................................................................................................... S. namaquensis
- -
- Branchlets and leaves entirely glabrous (sometimes branchlets sparsely glanduliferous when young); sepals 1.5–3 mm wide, strongly deflexed at anthesis, with narrow scarious margins; seed wing deeply and irregularly lacerate ............................................................................................................... S. quartzicola
References
- Hernández-Ledesma. , P, Berendsohn, W.G., Borsch, T., von Mering, S., Akhani, H., Arias, S., Castañeda-Noa, I., Eggli, U., Eriksson, R., Flores-Olvera, H., Fuentes-Bazán, S., Kadereit, G., Klak, C., Korotkova, N., Nyffeler, R., Ocampo, G., Ochoterena, H., Oxelman, B., Rabeler, R.K., Sánchez, A., Schlumpberger, B.O., Uotila, P. A taxonomic backbone for the global synthesis of species diversity in the angiosperm order Caryophyllales. Willdenowia 2015, 45, 281–383. [Google Scholar] [CrossRef]
- Bittrich, V. Caryophyllaceae. In The families and genera of vascular plants; Kubitzki, K., Rohwer, J.C., Bittrich, V., Eds.; Springer, Berlin, 1993; Vol. 2 (Magnoliid, Hamamelid, and Caryophyllid families). pp. 206–236.
- Harbaugh, D.T. , Nepokroeff, M., Rabeler, R.K., McNeill, J., Zimmer, E.A., Wagner, W.L. A new lineage-based tribal classification of the family Caryophyllaceae. Int. J. Pl. Sci. 2010, 171, 185–198. [Google Scholar] [CrossRef]
- Greenberg, A.K. , Donoghue, M.J. Molecular systematics and character evolution in Caryophyllaceae. Taxon 2011, 60, 1637–1652. [Google Scholar] [CrossRef]
- Pax, F. , Hoffmann, K. Caryophyllaceae. In Die naturlichen Pflanzenfamilien, 2nd edition; Engler, A., Harms, H., Eds.; Wilhelm Engelmann, Leipzig, 1934; Vol. 16c; pp. 275–364.
- López González, G. Sobre el género Spergula L. [incl. Spergularia (Pers.) Pers. ex J. Presl & C. Presl, nom. cons.] (Caryophyllaceae) y sus especies en la península ibérica e Islas Baleares. Lagascalia 2010, 30, 7–18. [Google Scholar]
- Kool, A. , Thulin, M. A giant spurrey on a tiny island: on the phylogenetic position of Sanctambrosia manicata (Caryophyllaceae) and the generic circumscriptions of Spergula, Spergularia and Rhodalsine. Taxon 2017, 66, 615–622. [Google Scholar] [CrossRef]
- Alonso, M.Á. , Crespo, M.B., Martínez-Azorín, M., Mucina, L. Taxonomic identity and evolutionary relationships of South African taxa related to the Spergularia media group (Caryophyllaceae). Plant Syst. Evol. 2021, 307, 24. [Google Scholar] [CrossRef]
- Alonso, M.Á. , Crespo, M.B., Martínez-Azorín, M., Mucina, L. Morphological and molecular data support recognition of Spergularia quartzicola (Caryophyllaceae) as a new species endemic to South Africa. Plant Biosyst. 2022, 156, 506–514. [Google Scholar] [CrossRef]
- Goldblatt, P. , Manning, J.C., Eds. Cape plants: a conspectus of the Cape Flora of South Africa; National botanical institute, Cape Town and Missouri Botanical Garden, St Louis, USA, 2000.
- Manning, J.C. , Goldblatt, P., Eds. Plants of the greater cape floristic region, 1: the core cape flora; South African National Biodiversity Institute, Pretoria, South Africa, 2012.
- Snijman, D.A. Caryophyllaceae (= Illicebraceae). In Plants of the greater cape floristic region, 2: the extra cape flora; Snijman, D.A.., Ed.; South African National Biodiversity Institute, Pretoria, South Africa, 2013, pp. 346–348.
- POWO. Plants of the World Online (continuously updated). Available online: https://powo.science.kew.org/ (accessed on 2 May 2023).
- Nkonki, T. Caryophyllacee. In Plants of southern Africa: an annotated checklist; Germishuizen, G., Meyer, N.L., Eds.; South African National Biodiversity Institute, Pretoria, South Africa, 2003 [Strelitzia 14].
- Mucina, L., Rutherford, M.C., Eds. The vegetation of South Africa, Lesotho and Swaziland. South African National Biodiversity Institute: Pretoria, South Africa, 2006. [Strelitzia 19].
- Thiers, B. Index Herbariorum: a global directory of public herbaria and associated staff (continuously updated). Available online: http://sweetgum.nybg.org/ih/ (accessed on 3 May 2023).
- Rasband, W.S.; ImageJ. U.S. National Institutes of Health, Bethesda, Maryland. Available online: https://imagej.nih.gov/ij/ (accessed on 3 May 2023).
- IPNI. The International Plant Names Index. Available online: http://www.ipni.org (accessed on 3 May 2023).
- Turland, N.J. , Wiersema, J.H., Barrie, F.R., Greuter, W., Hawksworth, D.L., Herendeen, P.S., Knapp, S., Kusber, W.-H., Li, D.-Z., Marhold, K., May, T.W., McNeill, J., Monro, A.M., Prado, J., Price, M.J., Smith G.F., Eds.; International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. − Koeltz Scientific Books: Königstein, Germany, 2018. [Google Scholar] [CrossRef]
- Leistner, O.A. , Morris, J.W. Southern African place names. Ann. Cape Prov. Mus. Nat. Hist. 1976, 12, 1–565. [Google Scholar]
- National Geospatial Information. SA mapsheet referencing. Available online: http://www.ngi.gov.za/indexphp/what-we-do/maps-and-geospatial-information/41-sa-mapsheet-referencing (accessed on 3 May 2023).
- Doyle, J.J. , Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Oxelman B, Kornhall P, Olmstead RG, Bremer B Further disintegration of Scrophulariaceae. Taxon 2005, 54, 411–425. [CrossRef]
- White, T.J. , T.D., Lee, S.B., Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR protocols: a guide to methods and applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, USA, 1990. [Google Scholar]
- Edgar, R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. , Stecher, G., Li, M., Knyaz, C., Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. , Kumar, S. Molecular evolution and phylogenetics, Oxford University Press: New York, USA, 2000. [Google Scholar]
- Felsenstein, J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef]
- Saitou, N. , Nei, M. The neighbour-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1874, 19, 716–723. [Google Scholar] [CrossRef]
- Darriba, D. , Taboada, G.L., Doallo, R., Posada, D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Method. 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G + C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [CrossRef]
- Ronquist, F. , Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Hohna, S., Larget, B., Liu, L., Suchard, M.A., Huelsenbeck, J.P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Olivier, P.M. , Germishuizen, G. Caryophyllaceae. In List of species of southern African plants; Gibbs Russell, G.E., Germishuizen, G., Herman, P., Olivier, P.M., Perold, S.M., Reid, C. Retief,E., Smook, L., van Rooy, J, Welman, W.G., Eds.; Botanical Research Institute, Pretoria, South Africa, 1984, pp. 64–65. [Mem. Bot. Surv. South Africa 48].
- Plug, C. Sim, Dr Thomas Robertson (botany, forestry). S2A3 Biographical Database of Southern African Science. Available on line: https://www.s2a3.org.za/bio/Biograph_final.php?serial=2584 (accessed on 2 May 2023).
- TROPICOS. Tropicos.org. Missouri Botanical Garden. Available online: https://tropicos.org/ (accessed on 2 May 2023).
- Guédès, M. Eugène Ernest Simon (1871–1967). Bull. Soc. Bot. France, 1967, 114, 357–359. [Google Scholar] [CrossRef]
- Monnier, P. Ratter, J.A. Spergularia. In Flora europaea, 2nd edition; Tutin, T.G., Burges, N.A., Chater, A.O., Edmondson, J.R., Heywood, V.H., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds; Cambridge University Press, Cambridge, UK, 1993; Vol. 1, pp. 186–188.
- Cholo, F.; Spergularia hanoverensis Simon. National Assessment (2006/05/31): Red List of South African Plants, version 2020.1. Available online: http://redlist.sanbi.org/species.php?species=3967-6 (accessed on 3 May 2023).
- IUCN. IUCN Red List Categories and Criteria: Version 3.1, 2nd ed.; UCN Species Survival Commission: Gland, Switzerland, and Cambridge, UK, 2012. [Google Scholar]
- APD. 2008. The African Plant Database. Available on line: https://www.ville-ge.ch/musinfo/bd/cjb/africa/recherche.php (accessed 3 May 2023). 3 May.
- GBIF. The Global Biodiversity Information Facility. Available on line: https://www.gbif.org/species/8231915 (accessed 3 May 2023). 3 May.
- Rossbach, R.P. El género Spergularia (Caryophyllaceae) en Chile. Darwiniana 1943, 6, 211–256. [Google Scholar]
- Salisbury, E.J. Spergularia salina and Spergularia marginata and their heteromorphic seeds. Kew Bull. 1958, 1, 41–51. [Google Scholar]
- Maire, R. Flore de l’Afrique du Nord, vol. 9. Paul Lechevalier, Paris, France, 1963.
- Ratter, J.A. Spergularia. In Flora iberica; Castroviejo, S., Laínz, M., López González, G., Montserrat, P., Muñoz Garmendia, F., Paiva, J., Villar, L., Eds.; Real Jardín Botánico, CSIC, Madrid, Spain, 1990; vol, 2, pp. 149–161.
- Hartman, R.L., Rabeler, R.K. Spergularia. In Flora of North America north of Mexico; Flora of North America Editorial Committee, Eds.; Oxford University Press, Oxford, UK, 2005; vol. 5(2), pp. 16–23.
- Adams, L.G. , West, J.G., Cowley, K.J. Revision of Spergularia (Caryophyllaceae) in Australia. Austr. Syst. Bot. 2008, 21, 251–270. [Google Scholar] [CrossRef]
- Lebel, M.E. Morphologie du genre Spergularia. Bull. Soc. Bot. France 1868, 15, 50–64. [Google Scholar] [CrossRef]
- Sterk, A.A. Biosystematic studies on Spergularia media and S. marina in the Netherlands II. The morphological variation of S. marina. Acta. Bot. Neerl. 1969, 18, 467–476. [Google Scholar] [CrossRef]
- Telenius, A. , Torstensson, P. Seed type and seed size variation in the heteromorphic saltmarsh annual Spergularia salina along the coast of Sweden. Plant Biol. 1999, 1, 585–593. [Google Scholar] [CrossRef]
- Memon, R.A. , Bhatti, G.R., Khalid, S., Arshad, M.G., Mirbahar, A.A., Qureshi, A. Microstructural features of seeds of Spergularia marina (L.) Griseb. (Caryophyllaceae). Pak. J. Bot. 2010, 42, 1423–1427. [Google Scholar]
- Telenius, A. , Torstensson, P. Seed wings in relation to seeds size in the genus Spergularia. Oikos 1991, 61, 216–222. [Google Scholar] [CrossRef]


| Taxon | Provenance (herbarium voucher) | Source | GenBank accession code | |
|---|---|---|---|---|
| trnL-trnF | 5.8S-ITS2 | |||
| S. cf. azorica Lebel | Spain: Canary Isl., Tenerife (ABH79973) | This paper | ||
| S. hanoverensis E.Simon ex M.Á.Alonso et al. | South Africa: Zoekop Farm (ABH83276) | This paper | ||
| South Africa: Karreekop Farm (ABH83288) | This paper | |||
| S. namaquensis Schltr. Ex M.Á.Alonso et al. | South Africa: Skoverfontein (ABH83197) | This paper | ||
| S. glandulosa | S. namaquensis | S. media | S. quartzicola | S. hanoverensis | |
|---|---|---|---|---|---|
| Height (cm) | up to 15 | up to 20 | 5–50 (–65) | up to 30 | up to 30 |
| Habit | subshrub | subshrub | perennial herbs, weakly lignified at base | subshrub | subshrub |
| Stems | caespitose, strongly nodose, procumbent to prostrate | erect, nodose, with ascending branches | diffuse, procumbent to prostrate | erect, nodose below, with subfastigiate branches | compact, slightly nodose, with ascending branches |
| Young branches indumentum | densely covered all over with gladuliferous, pluricellular hairs | densely covered all over with glanduliferous, pluricellular hairs | glabrous | glabrous (occasionally sparsely glanduliferous when young) | glabrous |
| Leaves (mm) | 8–20 × 0.5–1 | 8–25 × 0.8–1.2 | 10–35 × 0.3–2.5 | 8–45 × 0.5–1.2 | 4–10 (–1.7) × 0.5–0.7 |
| Indumentum of leaves | covered with glanduliferous hairs, occasionally glabrescent | covered with glanduliferous hairs, occasionally glabrescent | glabrous | glabrous | glabrous |
| Leaf mucro (mm) | 0.2–0.4 | 0.4–0.5 | 0–0.2 | 0.2–0.5 | 0.5-1.0 |
| Stipules (mm) | 3–6 × 3–4 | 2–4 × 2–4 | 2.3–2.5 × 1.3–2 | 3–5 × 2–2.6 | 4–6 × 1–2 |
| Stipules shape and connation | triangular to broadly triangular, those of young stems fused up to 1/3 of their length | triangular to broadly triangular, those of young stems fused up to 1/3 of their length | triangular, not acuminate, those of young stems fused up to 1/2 of their length | narrowly triangular, long acuminate, those of young stems fused up to 1/3 of their length | triangular-acuminate, those of young stems fused up to 1/3 of their length |
| Stipules indumentum | whitish-scarious, with glanduliferous hairs in the basal part | whitish-scarious, with glanduliferous hairs in the basal part | whitish-scarious, glabrous | whitish-scarious, glabrous | whitish-scarious, glabrous |
| Floral bracts/ Stipules | bracts longer to slightly longer than stipules | bracts slightly longer than stipules | bracts slightly longer than stipules | bracts longer than stipules | bracts longer than stipules |
| Inflorescence | monochasial to dichasial cyme, narrowly branched | dichasial cyme, broadly branched | dichasial cyme, broadly branched | dichasial cyme, broadly branched. rarely monochasial | dichasial cyme, broadly branched |
| Bract length (mm) | (2–)6–10 | (2–)5–9 | 1.7–2 | (1.5–)3–7 | (1–)1.5–2.5 |
| Indumentum of bracts | glandular-pubescent | glandular-pubescent | glabrescent to glandular-pubescent | glandular-pubescent | glandular-pubescent |
| Sepals (mm) | 2–6 × 2–3, with narrowly scarious margins, erect-patent at anthesis | 5–7.5 × 4–5, with broadly scarious margins, patent to slightly deflexed at anthesis | 4–6 × 2, with broadly scarious margins, erect-patent at anthesis | 4.6–6 × 1.5–3, with narrowly scarious margins, strongly deflexed at anthesis | 2–3 × 1–1.5 with narrowly scarious margins, patent to slightly deflexed at anthesis |
| Indumentum of sepals | glandular-pubescent | glandular-pubescent | glandular-pubescent | glandular-pubescent | glandular-pubescent |
| Petal colour | white | white | white or sometimes pink at apex | white | white |
| Sepals/petals | slightly shorter than to equalling sepals | up to 1.5 times longer than sepals | up to 1.2 longer than sepals | up to 1.5 times longer than sepals | slightly shorter than to equalling sepals |
| Petals (mm) | 2–5 × 1.5–3 | 6–9 × 4–5 | 4–5 × 1.8–2 | 6–8 × 4–5 | 1.8–2.8 × 1–1.2 |
| Stamens | 10, widened at bade | 10, not widened at base | (7–9)10, widened at base | 10, dissimilar (5 widened at base) | 7–8, slightly widened at the base |
| Stamens/petals | shorter than petals | shorter than petals | shorter than petals | shorter than petals | shorter than petals |
| Anther | 0.5–0.8 | 0.6–1 | 0.9–1 | 0.8–1 | 0.3–0.4 |
| Styles | 3, free from base | 3, fused in column to half or beyond | 3, free from base | 3, fused in column to half or beyond | 3, free from base |
| Style length (mm) | 0.5–0.7 | 1–1.1 | 0.5–0.6 | 1.5–2 | 1 |
| Capsule (mm) | 4–6.5 × 1.5–3.5 | 6–9 × 3–4 | (5–)6–9 × (3.5–)4–6 | 5.5–6.5 × 3–3.5 | 3–4 × 2.5–3.5 |
| Capsule/sepals | slightly longer than sepals | equalling to r slightly longer than sepals | 1/3 longer than sepals | equalling to slightly longer than sepals | slightly longer than sepals |
| Seed | winged, discolour | winged, discolour | winged, discolour | winged, discolour | winged, discolour, to unwinged |
| Seed colour | scarious whitish wing and dull brown to blackish disk | scarious whitish wing and blackish disk | scarious whitish wing and blackish disk | scarious whitish wing and blackish disk | scarious whitish wing and blackish-brown and matte |
| Size of seed (mm) | 0.9–1.3 × 1.2–1.3 | 1.1–1.3 × 1.2–1.4 | 1.2–1.5 × 1.3–1.6 | 1.1–1.2 × 1.1–1.2 | 0.5–0.75 × 0.3–0.6 and 0.7–1.1(–1.4) × 0.6–1.2(–1.4) |
| Seed disk (mm) | 0.6–0.7 × 0.6–0.9, loosely covered with conical tubercles | 0.6–0.7 × 0.6–0.8, smooth or with conical tubercles only on edges | 0.6–0.8 × 0.5–0.7 smooth | 0.6–0.7 × 0.6–0.7, smooth or with granular protuberances, mostly on edges | 0.5–0.9 × 0.4–0.9 mm, densely covered with globose, stalked papillae |
| Width of seed wing (mm) | 0.3–0.4 | 0.4–0.6 | 0.3–0.5 | 0.5–0.6 | 0–0.5 |
| Wing edge | entire to slightly eroded | entire to slightly eroded | entire to slightly eroded | deeply and irregularly lacerate | eroded, when present |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





