Submitted:
21 May 2023
Posted:
22 May 2023
You are already at the latest version
Abstract
Keywords:
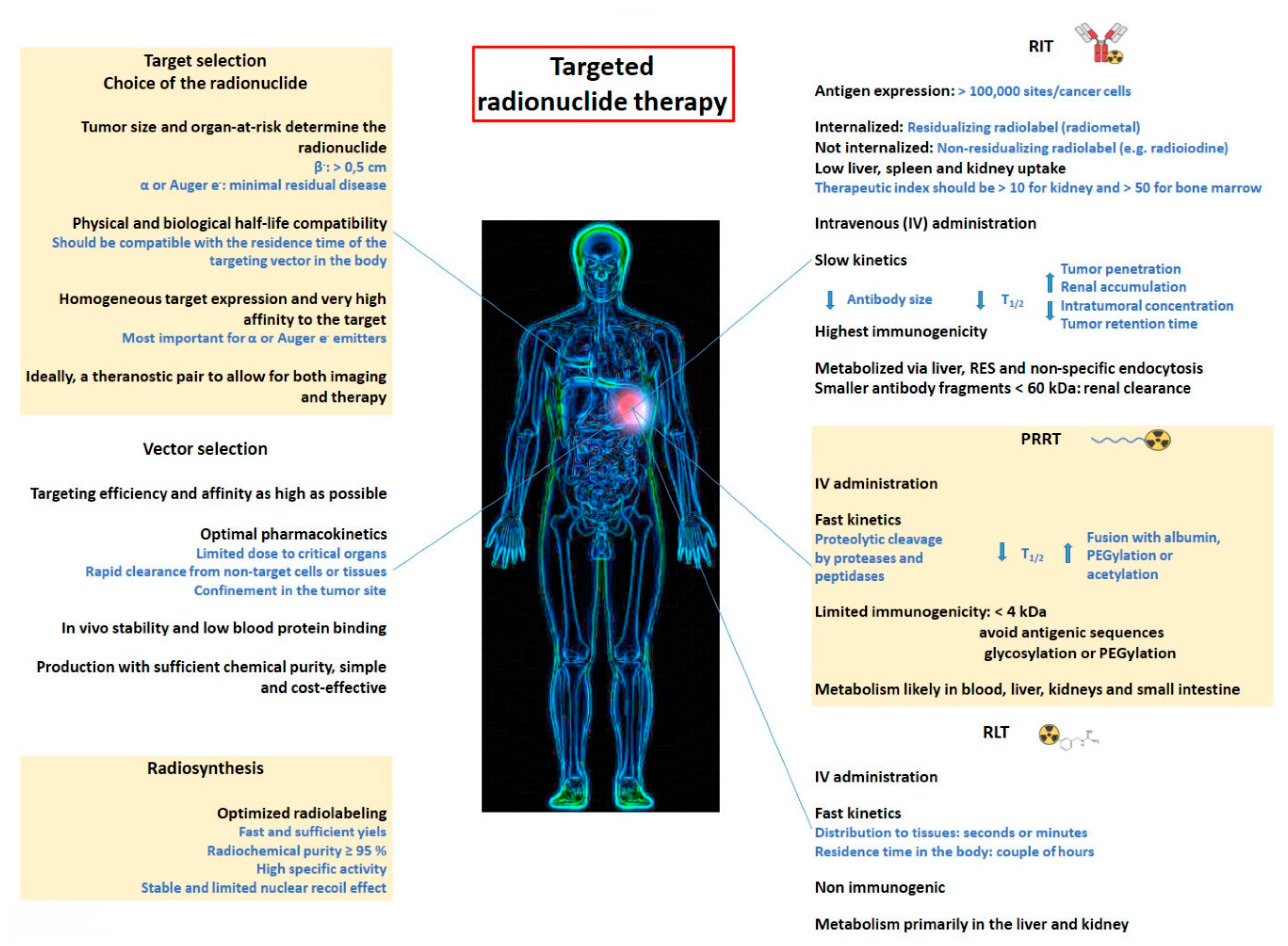
1. Introduction
2. Radioimmunotherapy (RIT)
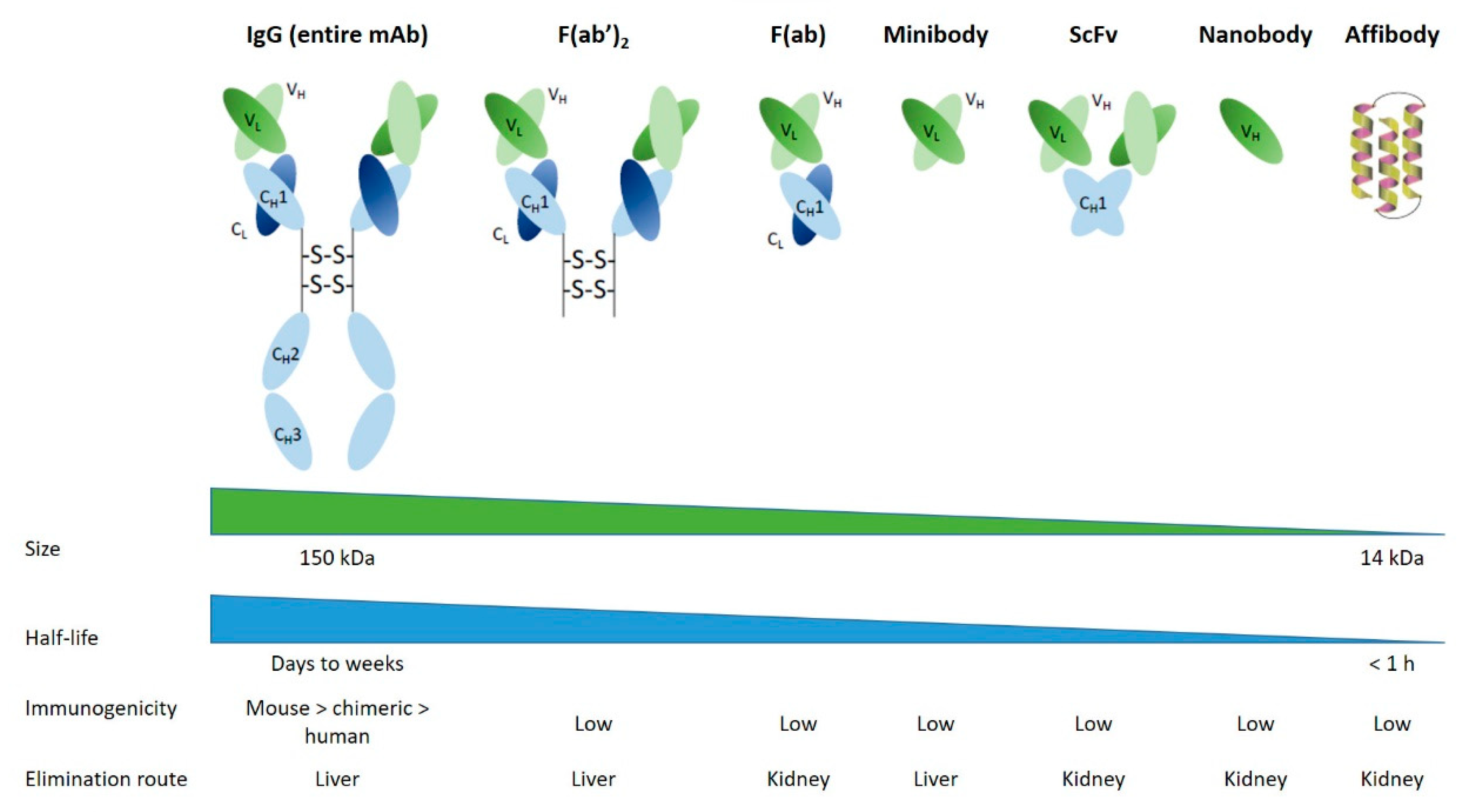
2.1. Antibodies and derivatives
2.2. Pretargeting approach
2.3. Dose fractionation approach
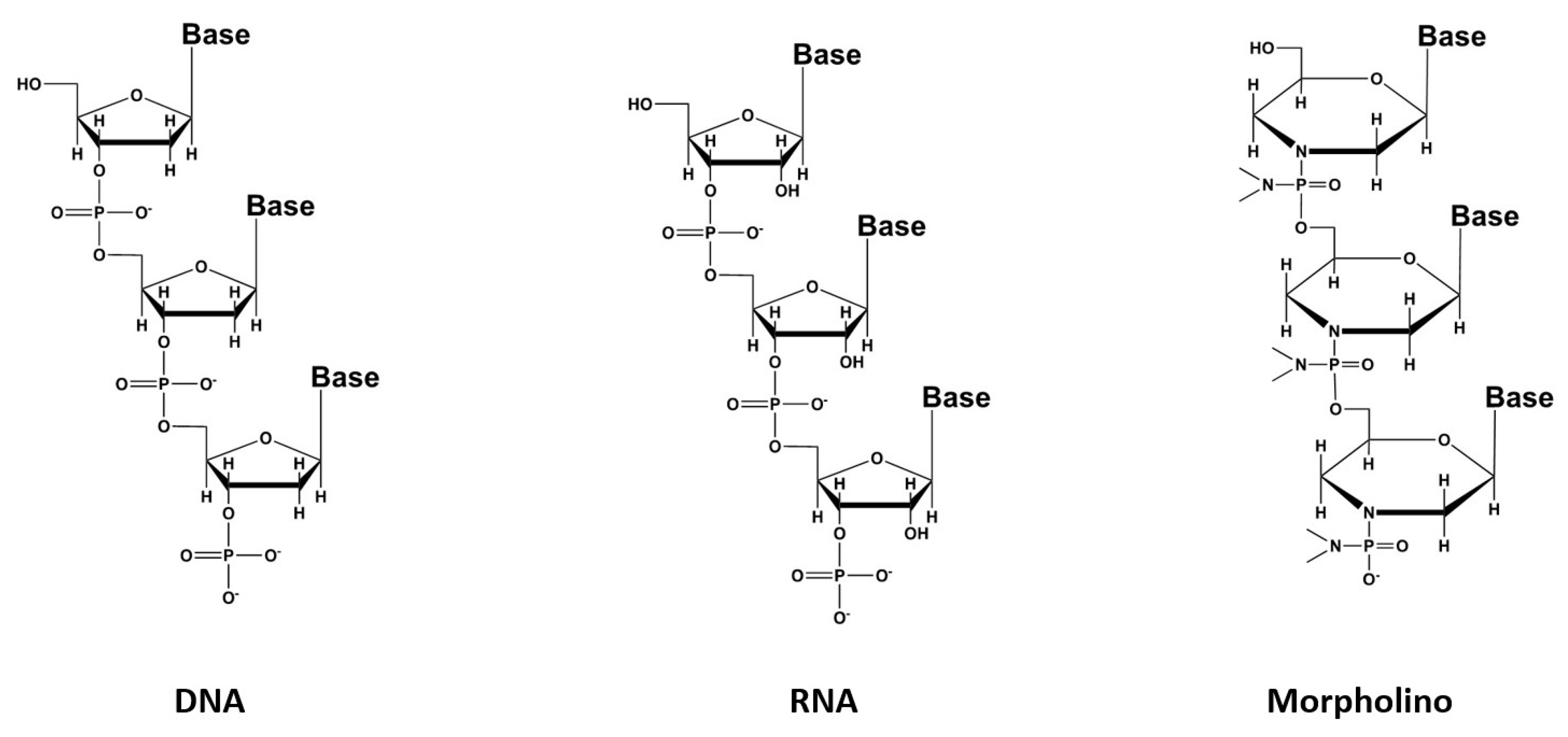
3. Oligonucleotides
4. Peptide Receptor Radionuclide Therapy (PRRT)
4.1. The G protein-coupled receptors family
4.1.1. Somatostatin analogs
4.1.2. Bombesin analogs
4.1.3. Substance P
4.1.4. Other analogs
4.2. C-X-C chemokine receptor type 4 (CXCR-4)
4.3. Other peptide derivatives
5. Radioligand Therapy (RLT)
5.1. Bone-seeking agents
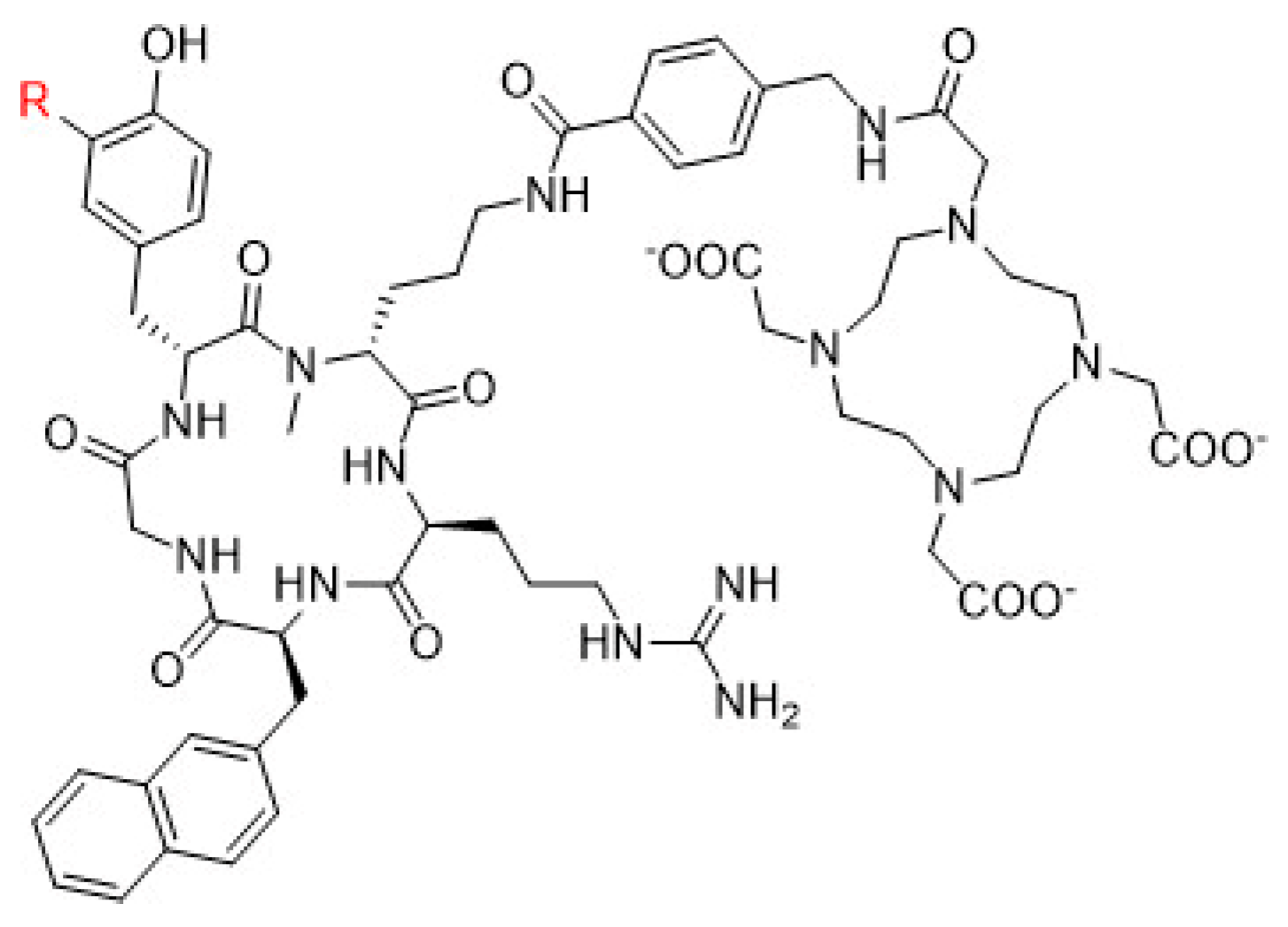
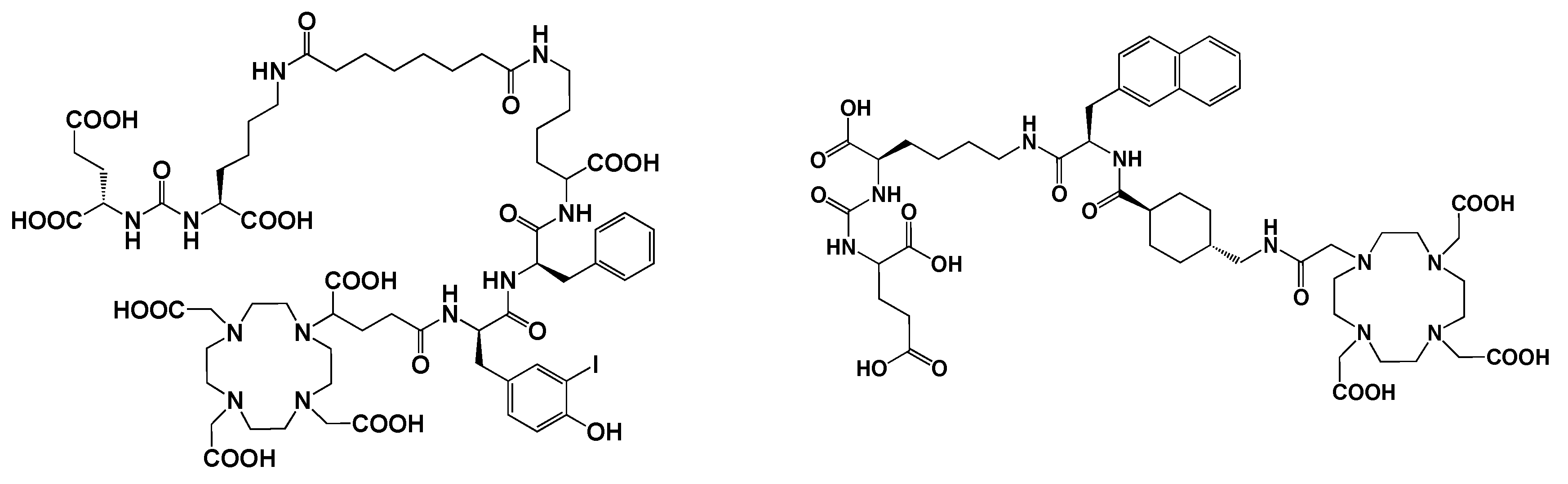
5.3. Prostate-specific membrane antigen (PSMA) inhibitors
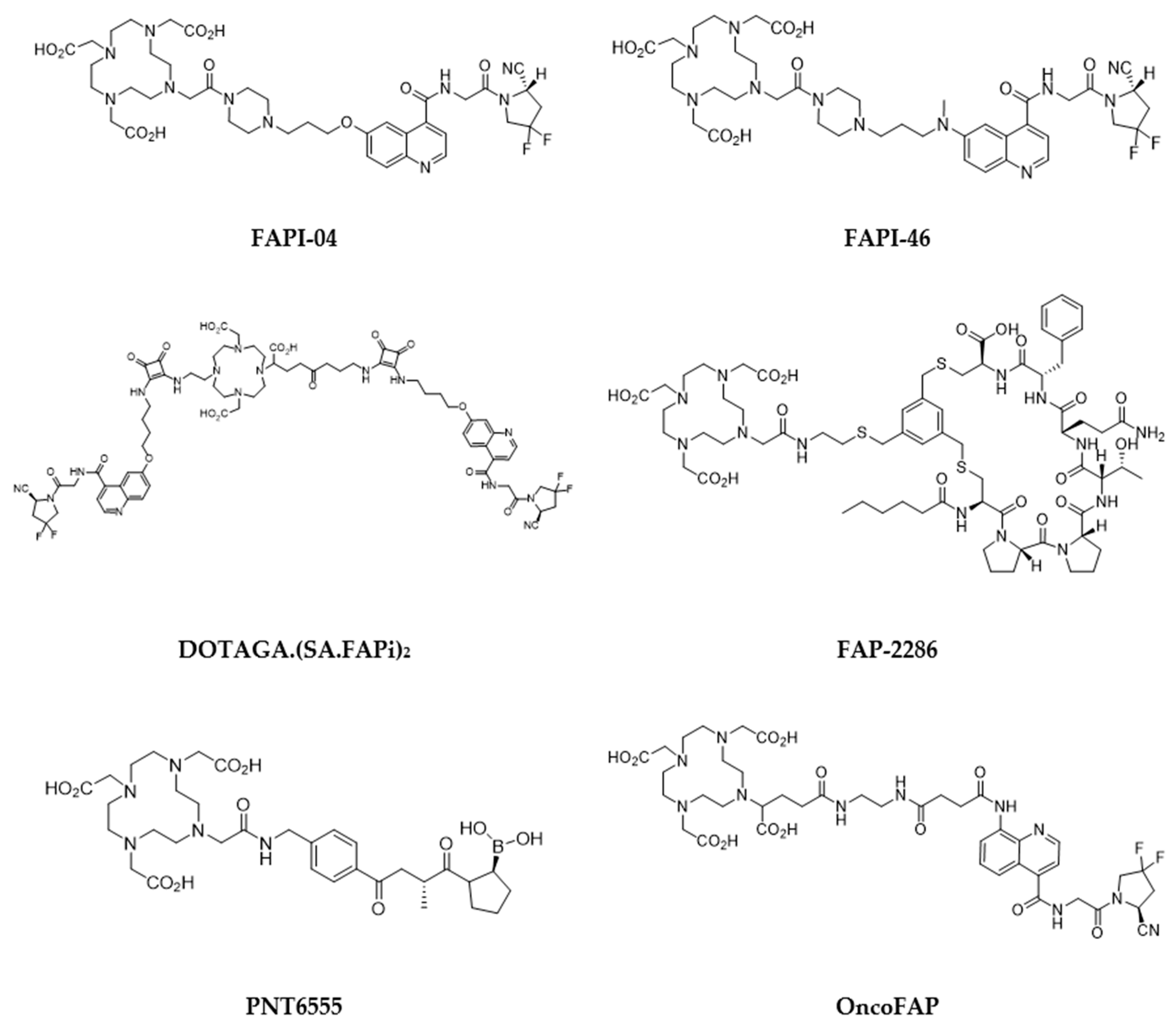
5.4. Fibroblast-activation protein (FAP) inhibitors
5.5. Poly(ADP-Ribose)Polymerase (PARP) inhibitors
5.6. Carbonic Anhydrase IX (CA IX) inhibitors
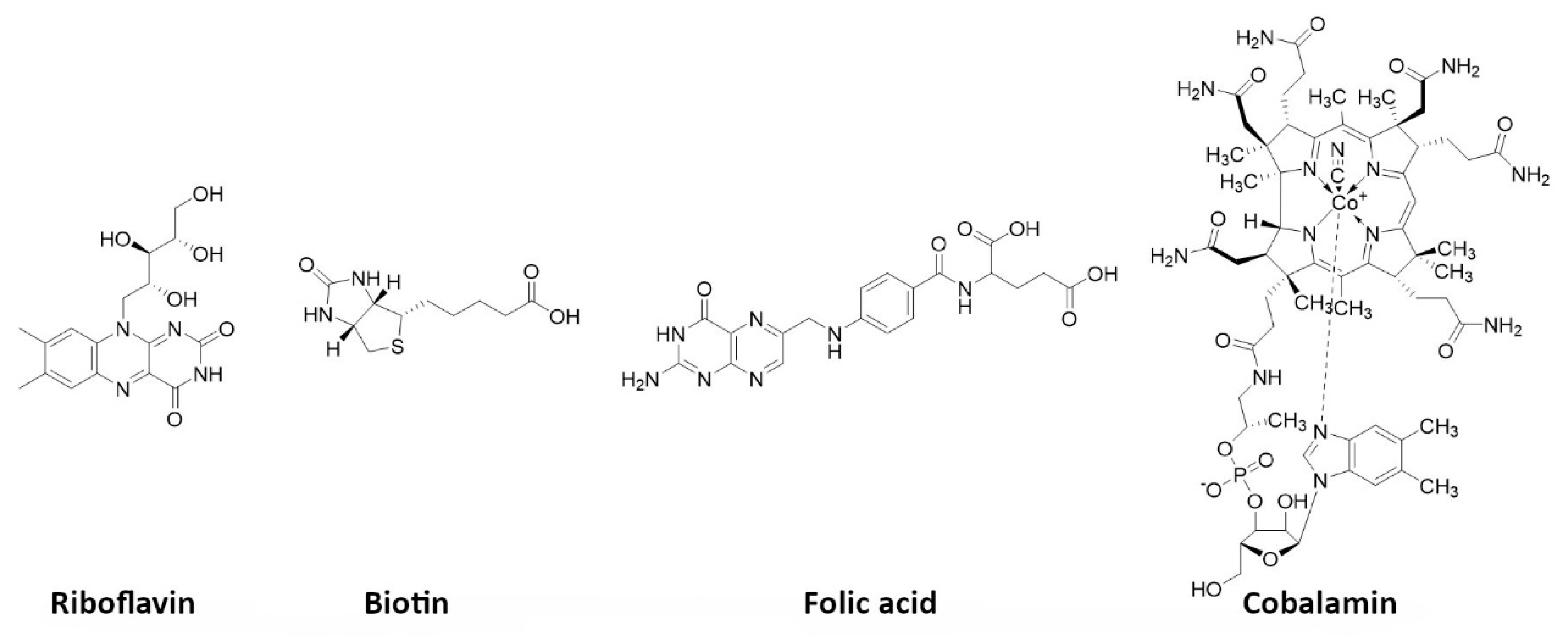
5.7. Vitamins
5.8. Phospholipid ether analogues
5.9. Melanin targeting agents
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hertz, B.; Watabe, T.; Baum, R.P. Celebrating 80 years anniversary of radioiodine for use in thyroid cancer and perspectives for theranostics. Ann. Nucl. Med. 2022, 36, 1007–1009. [Google Scholar] [CrossRef] [PubMed]
- Volkert, W.A.; Hoffman, T.J. Therapeutic Radiopharmaceuticals. Chem. Rev. 1999, 99, 2269–2292. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M. Radionuclide therapy beyond radioiodine. Wien. Med. Wochenschr. 2012, 162, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef] [PubMed]
- James, S.S.; Bednarz, B.; Benedict, S.; Buchsbaum, J.C.; Dewaraja, Y.; Frey, E.; Hobbs, R.; Grudzinski, J.; Roncali, E.; Sgouros, G.; et al. Current Status of Radiopharmaceutical Therapy. Int. J. Radiat. Oncol. 2021, 109, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Pouget, J.-P.; Santoro, L.; Raymond, L.; Chouin, N.; Bardiès, M.; Bascoul-Mollevi, C.; Huguet, H.; Azria, D.; Kotzki, P.-O.; Pèlegrin, M.; et al. Cell Membrane is a More Sensitive Target than Cytoplasm to Dense Ionization Produced by Auger Electrons. Radiat. Res. 2008, 170, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Palma, E.; Silva, F.; Belchior, A.; Pinto, C.I.G.; Guerreiro, J.F.; Botelho, H.M.; Mendes, F.; Raposinho, P.; Paulo, A. Searching for a Paradigm Shift in Auger-Electron Cancer Therapy with Tumor-Specific Radiopeptides Targeting the Mitochondria and/or the Cell Nucleus. Int. J. Mol. Sci. 2022, 23, 7238. [Google Scholar] [CrossRef] [PubMed]
- Pirovano, G.; Wilson, T.C.; Reiner, T. Auger: The future of precision medicine. Nucl. Med. Biol. 2021, 96-97, 50–53. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O'Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; Assenat, E.; Tacher, V.; Terroir-Cassou-Mounat, M.; Mariano-Goulart, D.; Amaddeo, G.; et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29. [Google Scholar] [CrossRef]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The Prioritization of Cancer Antigens: A National Cancer Institute Pilot Project for the Acceleration of Translational Research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, M.C.; De Geus, S.W.; Prevoo, H.A.; Hawinkels, L.J.; Van De Velde, C.J.; Kuppen, P.J.; Vahrmeijer, A.L.; Sier, C.F. Selecting Targets for Tumor Imaging: An Overview of Cancer-Associated Membrane Proteins. Biomarkers Cancer 2016, 8, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Aloj, L.; Attili, B.; Lau, D.; Caraco, C.; Lechermann, L.M.; Mendichovszky, I.A.; Harper, I.; Cheow, H.; Casey, R.T.; Sala, E.; et al. The emerging role of cell surface receptor and protein binding radiopharmaceuticals in cancer diagnostics and therapy. Nucl Med Biol. 2021, 92, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Das, A.; Bal, C.S. Tumor-targeting agents. In: S. Harsini et al. (eds), Nuclear Medicine and Immunology. Springer Nature. 2022; 217-236.
- Liolios, C.; Sachpekidis, C.; Schäfer, M.; Kopka, K. Bispecific radioligands targeting prostate-specific membrane antigen and gastrin-releasing peptide receptors on the surface of prostate cancer cells. J. Label. Compd. Radiopharm. 2019, 62, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Qian, J.; Olbrecht, S.; Boeckx, B.; Vos, H.; Laoui, D.; Etlioglu, E.; Wauters, E.; Pomella, V.; Verbandt, S.; Busschaert, P.; et al. A pan-cancer blueprint of the heterogeneous tumor microenvironment revealed by single-cell profiling. Cell Res. 2020, 30, 745–762. [Google Scholar] [CrossRef]
- Poltavets, V.; Kochetkova, M.; Pitson, S.M.; Samuel, M.S. The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Front. Oncol. 2018, 8, 431. [Google Scholar] [CrossRef]
- Khalaf, K.; Hana, D.; Chou, J.T.-T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Ni, Y.; Zhou, X.; Yang, J.; Shi, H.; Li, H.; Zhao, X.; Ma, X. The Role of Tumor-Stroma Interactions in Drug Resistance Within Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- van der Heide, C.D.; Dalm, S.U. Radionuclide imaging and therapy directed towards the tumor microenvironment: a multi-cancer approach for personalized medicine. Eur J Nucl Med Mol Imaging. 2022, 49, 4616–4641. [Google Scholar] [CrossRef]
- Kharaishvili, G.; Simkova, D.; Bouchalova, K.; Gachechiladze, M.; Narsia, N.; Bouchal, J. The role of cancer-associated fibroblasts, solid stress and other microenvironmental factors in tumor progression and therapy resistance. Cancer Cell Int. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Corroyer-Dulmont, A.; Jaudet, C.; Frelin, A.-M.; Fantin, J.; Weyts, K.; Vallis, K.A.; Falzone, N.; Sibson, N.R.; Chérel, M.; Kraeber-Bodéré, F.; et al. Radioimmunotherapy for Brain Metastases: The Potential for Inflammation as a Target of Choice. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sonanini, D.; Maurer, A.; Daldrup-Link, H.E. The yin and yang of imaging tumor associated macrophages with PET and MRI. Theranostics 2019, 9, 7730–7748. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.; Herhaus, P.; Schottelius, M.; Buck, A.K.; Werner, R.A.; Wester, H.-J.; Keller, U.; Lapa, C. CXCR4-directed theranostics in oncology and inflammation. Ann. Nucl. Med. 2018, 32, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Masłowska, K.; Halik, P.K.; Tymecka, D.; Misicka, A.; Gniazdowska, E. The Role of VEGF Receptors as Molecular Target in Nuclear Medicine for Cancer Diagnosis and Combination Therapy. Cancers 2021, 13, 1072. [Google Scholar] [CrossRef] [PubMed]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Burvenich, I.; Scott, A. Novel Target Selection for Nuclear Medicine Studies. Semin. Nucl. Med. 2019, 49, 357–368. [Google Scholar] [CrossRef]
- Turck, R. Radio-pharmaceuticals for cancer treatment: are they ready for prime time yet? Ann Oncol. 2018, 29, 1594–1597. [Google Scholar] [CrossRef]
- Herrmann, K.; Schwaiger, M.; Lewis, J.S.; Solomon, S.B.; McNeil, B.J.; Baumann, M.; Gambhir, S.S.; Hricak, H.; Weissleder, R. Radiotheranostics: a roadmap for future development. Lancet Oncol. 2020, 21, e146–e156. [Google Scholar] [CrossRef]
- Srivastava, S.C.; Buraggi, G.L. NATO Advanced Study Institute on "radiolabeled monoclonal antibodies for imaging and therapy--potential, problems, and prospects". Int J Biol Markers. 1987, 2, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, S.J.; DeNardo, G.L.; O'Grady, L.F.; Macey, D.J.; Mills, S.L.; Epstein, A.L.; Peng, J.-S.; McGahan, J.P. Treatment of a Patient with b Cell Lymphoma by 1-131 Lym-1 Monoclonal Antibodies. Int. J. Biol. Markers 1987, 2, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, F.; Radford, J.; Van Hoof, A.; Botto, B.; Rohatiner, A.Z.; Salles, G.; Soubeyran, P.; Tilly, H.; Bischof-Delaloye, A.; van Putten, W.L.; et al. 90Yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-Hodgkin lymphoma: updated results after a median follow-up of 7.3 years from the International, Randomized, Phase III First-Line Indolent trial. J Clin Oncol. 2013, 31, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, F.; Kraeber-Bodéré, F.; Wegener, W.A.; Harousseau, J.-L.; Petillon, M.-O.; Huglo, D.; Trümper, L.H.; Meller, J.; Pfreundschuh, M.; Kirsch, C.-M.; et al. High Rates of Durable Responses With Anti-CD22 Fractionated Radioimmunotherapy: Results of a Multicenter, Phase I/II Study in Non-Hodgkin's Lymphoma. J. Clin. Oncol. 2010, 28, 3709–3716. [Google Scholar] [CrossRef] [PubMed]
- Blakkisrud, J.; Løndalen, A.; Martinsen, A.C.T.; Dahle, J.; Holtedahl, J.E.; Bach-Gansmo, T.; Holte, H.; Kolstad, A.; Stokke, C. Tumor-Absorbed Dose for Non-Hodgkin Lymphoma Patients Treated with the Anti-CD37 Antibody Radionuclide Conjugate 177Lu-Lilotomab Satetraxetan. J. Nucl. Med. 2017, 58, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Stokke, C.; Blakkisrud, J.; Løndalen, A.; Dahle, J.; Martinsen, A.C.T.; Holte, H.; Kolstad, A. Pre-dosing with lilotomab prior to therapy with 177Lu-lilotomab satetraxetan significantly increases the ratio of tumor to red marrow absorbed dose in non-Hodgkin lymphoma patients. Eur. J. Nucl. Med. 2018, 45, 1233–1241. [Google Scholar] [CrossRef]
- Gritti, G.; Gianatti, A.; Petronzelli, F.; De Santis, R.; Pavoni, C.; Rossi, R.L.; Cattaneo, L.; Spagnoli, L.G.; Ferrari, S.; Rossi, A.; et al. Evaluation of tenascin-C by tenatumomab in T-cell non-Hodgkin lymphomas identifies a new target for radioimmunotherapy. Oncotarget 2018, 9, 9766–9775. [Google Scholar] [CrossRef]
- Bäck, T.; Haraldsson, B.; Hultborn, R.; Jensen, H.; Johansson, M.E.; Lindegren, S.; Jacobsson, L. Glomerular filtration rate after alpha-radioimmunotherapy with 211At-MX35-F(ab')2: a long-term study of renal function in nude mice. Cancer Biother Radiopharm. 2009, 24, 649–658. [Google Scholar]
- Adams, G.; Shaller, C.; Chappell, L.; Wu, C.; Horak, E.; Simmons, H.; Litwin, S.; Marks, J.; Weiner, L.; Brechbiel, M. Delivery of the α-emitting radioisotope bismuth-213 to solid tumors via single-chain Fv and diabody molecules. Nucl. Med. Biol. 2000, 27, 339–346. [Google Scholar] [CrossRef]
- Diebolder, P.; Mpoy, C.; Scott, J.; Huynh, T.T.; Fields, R.; Spitzer, D.; Bandara, N.; Rogers, B.E. Preclinical Evaluation of an Engineered Single-Chain Fragment Variable-Fragment Crystallizable Targeting Human CDJ. Nucl. Med. 2021, 62, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Altunay, B.; Morgenroth, A.; Beheshti, M.; Vogg, A.; Wong, N.C.L.; Ting, H.H.; Biersack, H.-J.; Stickeler, E.; Mottaghy, F.M. HER2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging. Eur. J. Nucl. Med. 2021, 48, 1371–1389. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Orlova, A.; Pehrson, R.; Galli, J.; Baastrup, B.; Andersson, K.; Sandström, M.; Rosik, D.; Carlsson, J.; Lundqvist, H.; et al. Radionuclide therapy of HER2-positive microxenografts using a 177Lu-labeled HER2-specific Affibody molecule. Cancer Res. 2007, 67, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Barbet, J.; Kraeber-Bodéré, F.; Vuillez, J.-P.; Gautherot, E.; Rouvier. ; Chatal, J.-F.; Bodet-Milin, C.; Ferrer, L.; Rauscher, A.; Masson, D.; et al. Pretargeting with the Affinity Enhancement System for Radioimmunotherapy. Cancer Biotherapy Radiopharm. 1999, 14, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Kraeber-Bodéré, F.; Rousseau, C.; Bodet-Milin, C.; Ferrer, L.; Faivre-Chauvet, A.; Campion, L.; Vuillez, J.-P.; Devillers, A.; Chang, C.-H.; Goldenberg, D.M.; et al. Targeting, toxicity, and efficacy of 2-step, pretargeted radioimmunotherapy using a chimeric bispecific antibody and 131I-labeled bivalent hapten in a phase I optimization clinical trial. J. Nucl. Med. 2006, 47, 247–55. [Google Scholar] [PubMed]
- Salaun, P.Y.; Campion, L.; Bournaud, C.; Faivre-Chauvet, A.; Vuillez, J.P.; Taieb, D.; Ansquer, C.; Rousseau, C.; Borson-Chazot, F.; Bardet, S.; et al. Phase II trial of anticarcinoembryonic antigen pretargeted radioimmunotherapy in progressive metastatic medullary thyroid carcinoma: biomarker response and survival improvement. J Nucl Med. 2012, 53, 1185–1192. [Google Scholar] [CrossRef]
- Batra, J.S.; Karir, B.; Pinto-Chengot, K.; Jhanwar, Y.S.; Vallabhajosula, S.; Christos, P.J.; Hodes, G.; Lam, L.; Molina, A.; Beltran, H.; et al. MP50-19 DOSE-FRACTIONATED ANTI-PSMA RADIOIMMUNOTHERAPY ( 177 LU-J591) FOR MCRPC. J. Urol. 2016, 195. [Google Scholar] [CrossRef]
- Kraeber-Bodere, F.; Pallardy, A.; Maisonneuve, H.; Campion, L.; Moreau, A.; Soubeyran, I.; Le Gouill, S.; Tournilhac, O.; Daguindau, E.; Jardel, H.; et al. Consolidation anti-CD22 fractionated radioimmunotherapy with 90 Y-epratuzumab tetraxetan following R-CHOP in elderly patients with diffuse large B-cell lymphoma: a prospective, single group, phase 2 trial. Lancet Haematol. 2017, 4, e35–e45. [Google Scholar] [CrossRef]
- Filippi, L.; Bagni, O.; Nervi, C. Aptamer-based technology for radionuclide targeted imaging and therapy: a promising weapon against cancer. Expert Rev. Med Devices 2020, 17, 751–758. [Google Scholar] [CrossRef]
- Gijs, M.; Aerts, A.; Impens, N.; Baatout, S.; Luxen, A. Aptamers as radiopharmaceuticals for nuclear imaging and therapy. Nucl. Med. Biol. 2016, 43, 253–271. [Google Scholar] [CrossRef]
- Varmira, K.; Hosseinimehr, S.J.; Noaparast, Z.; Abedi, S.M. An improved radiolabelled RNA aptamer molecule for HER2 imaging in cancers. J. Drug Target. 2014, 22, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Summerton, J.; Weller, D.D.; Tunç, C.U.; Öztaş, D.Y.; Uzunoğlu, D.; Bayrak. F.; Çulha, M.; Cleaves, H.J.; Meringer, M.; Goodwin, J.; et al. Morpholino Antisense Oligomers: Design, Preparation, and Properties. Antisense Nucleic Acid Drug Dev. 1997, 7, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wu, P.; He, J.; Zhong, L.; Zhao, Y. Advances of Aptamer-based Clinical Applications for the Diagnosis and Therapy of Cancer. Discov Med. 2020, 29, 169–180. [Google Scholar] [PubMed]
- de Visser, M.; Verwijnen, S.M.; de Jong, M. Update:Improvement Strategies for Peptide Receptor Scintigraphy and Radionuclide Therapy. Cancer Biotherapy Radiopharm. 2008, 23, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Tornesello, A.L.; Buonaguro, L.; Tornesello, M.L.; Buonaguro, F.M. New Insights in the Design of Bioactive Peptides and Chelating Agents for Imaging and Therapy in Oncology. Molecules 2017, 22, 1282. [Google Scholar] [CrossRef]
- Gharibkandi, N.A.; Conlon, J.M.; Hosseinimehr, S.J. Strategies for improving stability and pharmacokinetic characteristics of radiolabeled peptides for imaging and therapy. Peptides 2020, 133, 170385. [Google Scholar] [CrossRef]
- Qu, T.; Wang, Y.; Zhu, Z.; Rusckowski, M.; Hnatowich, D.J. Different chelators and different peptides together influence the in vitro and mouse in vivo properties of 99Tcm. Nucl. Med. Commun. 2001, 22, 203–215. [Google Scholar] [CrossRef]
- Fani, M.; Del Pozzo, L.; Abiraj, K.; Mansi, R.; Tamma, M.L.; Cescato, R.; Waser, B.; Weber, W.A.; Reubi, J.C.; Maecke, H.R. PET of somatostatin receptor-positive tumors using 64Cu- and 68Ga-somatostatin antagonists: the chelate makes the difference. J Nucl Med. 2011, 52, 1110–1118. [Google Scholar] [CrossRef]
- E Weiner, R.; Thakur, M.L. Radiolabeled peptides in the diagnosis and therapy of oncological diseases. Appl. Radiat. Isot. 2002, 57, 749–763. [Google Scholar] [CrossRef]
- Morgat, C.; Mishra, A.K.; Varshney, R.; Allard, M.; Fernandez, P.; Hindié, E. Targeting Neuropeptide Receptors for Cancer Imaging and Therapy: Perspectives with Bombesin, Neurotensin, and Neuropeptide-Y Receptors. 2014, 55, 1650–1657. [CrossRef]
- Tornesello, A.L.; Tornesello, M.L.; Buonaguro, F.M. An Overview of Bioactive Peptides for in vivo Imaging and Therapy in Human Diseases. Mini-Reviews Med. Chem. 2017, 17, 758–770. [Google Scholar] [CrossRef]
- Reubi, J.; Waser, B.; Schaer, J.-C.; Laissue, J.A. Somatostatin receptor sst1–sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur. J. Nucl. Med. 2001, 28, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Eychenne, R.; Bouvry, C.; Bourgeois, M.; Loyer, P.; Benoist, E.; Lepareur, N. Overview of Radiolabeled Somatostatin Analogs for Cancer Imaging and Therapy. Molecules 2020, 25, 4012. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Benešová, M. [68Ga]Ga-DOTA-TOC: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging. Pharmaceuticals (Basel) 2020, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Kopka, K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- A Kaltsas, G.; Papadogias, D.; Makras, P.; Grossman, A.B. Treatment of advanced neuroendocrine tumours with radiolabelled somatostatin analogues. Endocrine-Related Cancer 2005, 12, 683–699. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Das, S.; Al-Toubah, T.; Pelle, E.; El-Haddad, G.; Strosberg, J. Somatostatin receptor radionuclide therapy in neuroendocrine tumors. Endocrine-Related Cancer 2021, 28, R81–R93. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, L.; Boschi, A.; Cittanti, C.; Martini, P.; Panareo, S.; Tonini, E.; Nieri, A.; Urso, L.; Caracciolo, M.; Lodi, L.; et al. 90Y/177Lu-DOTATOC: From Preclinical Studies to Application in Humans. Pharmaceutics 2021, 13, 1463. [Google Scholar] [CrossRef] [PubMed]
- Puliani, G.; Chiefari, A.; Mormando, M.; Bianchini, M.; Lauretta, R.; Appetecchia, M. New Insights in PRRT: Lessons From 2021. Front. Endocrinol. 2022, 13, 861434. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Bidakhvidi, N.A.; Goffin, K.; Dekervel, J.; Baete, K.; Nackaerts, K.; Clement, P.; Van Cutsem, E.; Verslype, C.; Deroose, C.M. Peptide Receptor Radionuclide Therapy Targeting the Somatostatin Receptor: Basic Principles, Clinical Applications and Optimization Strategies. Cancers 2021, 14, 129. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur. J. Nucl. Med. 2014, 41, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: first clinical experience on the efficacy and safety. Eur. J. Nucl. Med. 2020, 47, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.; Bezak, E.; Chan, D.; Cehic, G. Targeted alpha-particle therapy in neuroendocrine neoplasms: A systematic review. World J. Nucl. Med. 2021, 20, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Ginj, M.; Zhang, H.; Waser, B.; Cescato, R.; Wild, D.; Wang, X.; Erchegyi, J.; Rivier, J.; Mäcke, H.R.; Reubi, J.C. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc Natl Acad Sci USA 2006, 103, 16436–16441. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Peitl, P.K.; Velikyan, I. Current Status of Radiopharmaceuticals for the Theranostics of Neuroendocrine Neoplasms. Pharmaceuticals 2017, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Fani, M.; Behe, M.; Brink, I.; Rivier, J.E.; Reubi, J.C.; Maecke, H.R.; Weber, W.A. First Clinical Evidence That Imaging with Somatostatin Receptor Antagonists Is Feasible. J. Nucl. Med. 2011, 52, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Nicolas, G.P.; Wild, D. Somatostatin Receptor Antagonists for Imaging and Therapy. J. Nucl. Med. 2017, 58, 61S–66S. [Google Scholar] [CrossRef]
- Wild, D.; Fani, M.; Fischer, R.; Del Pozzo, L.; Kaul, F.; Krebs, S.; Rivier, J.E.; Reubi, J.C.; Maecke, H.R.; Weber, W.A. Comparison of Somatostatin Receptor Agonist and Antagonist for Peptide Receptor Radionuclide Therapy: A Pilot Study. J. Nucl. Med. 2014, 55, 1248–1252. [Google Scholar] [CrossRef]
- Reidy-Lagunes, D.; Pandit-Taskar, N.; O'Donoghue, J.A.; Krebs, S.; Staton, K.D.; Lyashchenko, S.K.; Lewis, J.S.; Raj, N.; Gönen, M.; Lohrmann, C.; et al. Phase I Trial of Well-Differentiated Neuroendocrine Tumors (NETs) with Radiolabeled Somatostatin Antagonist 177Lu-Satoreotide Tetraxetan. Clin. Cancer Res. 2019, 25, 6939–6947. [Google Scholar] [CrossRef]
- Baum, R.P.; Zhang, J.; Schuchardt, C.; Mueller, D.; Maecke, H. First-in-Humans Study of the SSTR Antagonist 177Lu-DOTA-LM3 for Peptide Receptor Radionuclide Therapy in Patients with Metastatic Neuroendocrine Neoplasms: Dosimetry, Safety, and Efficacy. J. Nucl. Med. 2021, 62, 1571–1581. [Google Scholar] [CrossRef]
- Fani, M.; Mansi, R.; Nicolas, G.P.; Wild, D. Radiolabeled Somatostatin Analogs—A Continuously Evolving Class of Radiopharmaceuticals. Cancers 2022, 14, 1172. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.E.; Zhernosekov, K. The evolution of PRRT for the treatment of neuroendocrine tumors; What comes next? Front Endocrinol 2022, 13, 941832. [Google Scholar] [CrossRef]
- Van de Wiele C, Dumont F, van Belle S, Slegers G, Peers SH, Dierckx RA. Is there a role for agonist gastrin-releasing peptide receptor radioligands in tumour imaging? Nucl Med Commun. 2001, 22, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Volkert, W.A.; Hoffman, T.J. Radiolabeled peptide conjugates for targeting of the bombesin receptor superfamily subtypes. Nucl. Med. Biol. 2005, 32, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Mansi, R.; Nock, B.A.; Dalm, S.U.; Busstra, M.B.; van Weerden, W.M.; Maina, T. Radiolabeled Bombesin Analogs. Cancers 2021, 13, 5766. [Google Scholar] [CrossRef] [PubMed]
- Bodei L, Ferrari M, Nunn A, Llull J, Cremonesi M, Martano L, Laurora G, Scardino E, Tiberini S, Bufi G, Eaton S, de Cobelli O, Paganelli G. . 177Lu-AMBA bombesin analogue in hormone refractory prostate cancer patients: A phase I escalation study with single-cycle administrations. Eur. J. Nucl. Med. Mol. Imaging. 2007, 34, S221. [Google Scholar]
- Cescato, R.; Maina, T.; Nock, B.; Nikolopoulou, A.; Charalambidis, D.; Piccand, V.; Reubi, J.C. Bombesin Receptor Antagonists May Be Preferable to Agonists for Tumor Targeting. J. Nucl. Med. 2008, 49, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Maina, T.; Nock, B.A.; Kulkarni, H.; Singh, A.; Baum, R.P. Theranostic Prospects of Gastrin-Releasing Peptide Receptor–Radioantagonists in Oncology. PET Clin. 2017, 12, 297–309. [Google Scholar] [CrossRef]
- Kurth, J.; Krause, B.J.; Schwarzenböck, S.M.; Bergner, C.; Hakenberg, O.W.; Heuschkel, M. First-in-human dosimetry of gastrin-releasing peptide receptor antagonist [177Lu]Lu-RM2: a radiopharmaceutical for the treatment of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. 2020, 47, 123–135. [Google Scholar] [CrossRef]
- Guenther, T.; Deiser, S.; Felber, V.; Beck, R.; Wester, H.-J. Substitution of l-Tryptophan by α-Methyl-l-Tryptophan in 177Lu-RM2 Results in 177Lu-AMTG, a High-Affinity Gastrin-Releasing Peptide Receptor Ligand with Improved In Vivo Stability. J. Nucl. Med. 2022, 63, 1364–1370. [Google Scholar] [CrossRef]
- Montemagno, C.; Raes, F.; Ahmadi, M.; Bacot, S.; Debiossat, M.; Leenhardt, J.; Boutonnat, J.; Orlandi, F.; Barbato, D.; Tedesco, M.; et al. In Vivo Biodistribution and Efficacy Evaluation of NeoB, a Radiotracer Targeted to GRPR, in Mice Bearing Gastrointestinal Stromal Tumor. Cancers 2021, 13, 1051. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok EAM, Verhoeven M, Konijnenberg MW, de Blois E, de Ridder CMA, Stuurman DC, Bertarione L, Rolfo K, de Jong M, Dalm SU. Safety of [177Lu]Lu-NeoB treatment: a preclinical study characterizing absorbed dose and acute, early, and late organ toxicity. Eur J Nucl Med Mol Imaging. 2022, 49, 4440–4451. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.T.; van Dam, E.M.; Sreekumar, S.; Mpoy, C.; Blyth, B.J.; Muntz, F.; Harris, M.J.; Rogers, B.E. Copper-67-Labeled Bombesin Peptide for Targeted Radionuclide Therapy of Prostate Cancer. Pharmaceuticals 2022, 15, 728. [Google Scholar] [CrossRef] [PubMed]
- Majkowska-Pilip, A.; Halik, P.K.; Gniazdowska, E. The Significance of NK1 Receptor Ligands and Their Application in Targeted Radionuclide Tumour Therapy. Pharmaceutics 2019, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Kneifel, S.; Cordier, D.; Good, S.; Ionescu, M.C.; Ghaffari, A.; Hofer, S.; Kretzschmar, M.; Tolnay, M.; Apostolidis, C.; Waser, B.; et al. Local Targeting of Malignant Gliomas by the Diffusible Peptidic Vector 1,4,7,10-Tetraazacyclododecane-1-Glutaric Acid-4,7,10-Triacetic Acid-Substance P. Clin. Cancer Res. 2006, 12, 3843–3850. [Google Scholar] [CrossRef] [PubMed]
- Cordier, D.; Krolicki, L.; Morgenstern, A.; Merlo, A. Targeted Radiolabeled Compounds in Glioma Therapy. Semin. Nucl. Med. 2016, 46, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Krolicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Apostolidis, C.; Mirzadeh, S.; Rola, R.; et al. Prolonged survival in secondary glioblastoma following local injection of targeted alpha therapy with 213Bi-substance P analogue. Eur. J. Nucl. Med. 2018, 45, 1636–1644. [Google Scholar] [CrossRef]
- Królicki, L.; Kunikowska, J.; Bruchertseifer, F.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Rola, R.; Morgenstern, A.; Rosiak, E.; et al. 225Ac- and 213Bi-Substance P Analogues for Glioma Therapy. Semin. Nucl. Med. 2020, 50, 141–151. [Google Scholar] [CrossRef]
- Halik, P.K.; Lipiński, P.F.J.; Matalińska, J.; Koźmiński, P.; Misicka, A.; Gniazdowska, E. Radiochemical Synthesis and Evaluation of Novel Radioconjugates of Neurokinin 1 Receptor Antagonist Aprepitant Dedicated for NK1R-Positive Tumors. Molecules 2020, 25, 3756. [Google Scholar] [CrossRef]
- Matalińska J, Kosińska K, Halik PK, Koźmiński P, Lipiński PFJ, Gniazdowska E, Misicka A. Novel NK1R-Targeted 68Ga-/177Lu-Radioconjugates with Potential Application against Glioblastoma Multiforme: Preliminary Exploration of Structure-Activity Relationships. Int J Mol Sci. 2022, 23, 1214. [Google Scholar] [CrossRef]
- Davenport AP, Scully CCG, de Graaf C, Brown AJH, Maguire JJ. Advances in therapeutic peptides targeting G protein-coupled receptors. Nat Rev Drug Discov. 2020, 19, 389–413. [Google Scholar] [CrossRef] [PubMed]
- Ansquer, C.; Kraeber-Bodere, F.; Chatal, J.F. Current Status and Perspectives in Peptide Receptor Radiation Therapy. Curr. Pharm. Des. 2009, 15, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Franco Machado J, Silva RD, Melo R, G Correia JD. Less Exploited GPCRs in Precision Medicine: Targets for Molecular Imaging and Theranostics. Molecules 2018, 24, 49. [Google Scholar] [CrossRef]
- Thakur, M.L.; Tripathi, S.K.; Gomella, L.G.; Salmanoglu, E.; Kim, S.; Kelly, W.K.; Keith, S.W.; Intenzo, C.; McCue, P.; Hoffman-Censits, J.; et al. Imaging urothelial bladder cancer: A VPAC PET targeted approach. Can J Urol. 2021, 28, 10596–10602. [Google Scholar] [PubMed]
- von Guggenberg, E.; Kolenc, P.; Rottenburger, C.; Mikołajczak, R.; Hubalewska-Dydejczyk, A. Update on Preclinical Development and Clinical Translation of Cholecystokinin-2 Receptor Targeting Radiopharmaceuticals. Cancers 2021, 13, 5776. [Google Scholar] [CrossRef]
- Rottenburger, C.; Nicolas, G.P.; McDougall, L.; Kaul, F.; Cachovan, M.; Vija, A.H.; Schibli, R.; Geistlich, S.; Schumann, A.; Rau, T.; et al. Cholecystokinin 2 Receptor Agonist 177Lu-PP-F11N for Radionuclide Therapy of Medullary Thyroid Carcinoma: Results of the Lumed Phase 0a Study. J. Nucl. Med. 2020, 61, 520–526. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Zhang, T.-T.; Nakanishi, T. Involvement of CXCR4 in Normal and Abnormal Development. Cells 2019, 8, 185. [Google Scholar] [CrossRef]
- Chatterjee, S.; Azad, B.B.; Nimmagadda, S. The Intricate Role of CXCR4 in Cancer. Adv. Cancer Res. 2014, 124, 31–82. [Google Scholar] [CrossRef]
- Werner, R.A.; Kircher, S.; Higuchi, T.; Kircher, M.; Schirbel, A.; Wester, H.-J.; Buck, A.K.; Pomper, M.G.; Rowe, S.P.; Lapa, C. CXCR4-Directed Imaging in Solid Tumors. Front. Oncol. 2019, 9, 770. [Google Scholar] [CrossRef]
- Kircher, M.; Herhaus, P.; Schottelius, M.; Buck, A.K.; Werner, R.A.; Wester, H.-J.; Keller, U.; Lapa, C. CXCR4-directed theranostics in oncology and inflammation. Ann. Nucl. Med. 2018, 32, 503–511. [Google Scholar] [CrossRef]
- Schottelius, M.; Osl, T.; Poschenrieder, A.; Hoffmann, F.; Beykan, S.; Hänscheid, H.; Schirbel, A.; Buck, A.K.; Kropf, S.; Schwaiger, M.; et al. [177Lu]pentixather: Comprehensive Preclinical Characterization of a First CXCR4-directed Endoradiotherapeutic Agent. Theranostics 2017, 7, 2350–2362. [Google Scholar] [CrossRef] [PubMed]
- Osl T, Schmidt A, Schwaiger M, Schottelius M, Wester HJ. A new class of PentixaFor- and PentixaTher-based theranostic agents with enhanced CXCR4-targeting efficiency. Theranostics. 2020, 10, 8264–8280. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Schottelius, M.; Lapa, C.; Osl, T.; Poschenrieder, A.; Hänscheid, H.; Lückerath, K.; Schreder, M.; Bluemel, C.; Knott, M.; et al. First-in-Human Experience of CXCR4-Directed Endoradiotherapy with 177Lu- and 90Y-Labeled Pentixather in Advanced-Stage Multiple Myeloma with Extensive Intra- and Extramedullary Disease. J. Nucl. Med. 2016, 57, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Maurer, S.; Herhaus, P.; Lippenmeyer, R.; Hänscheid, H.; Kircher, M.; Schirbel, A.; Maurer, H.C.; Buck, A.K.; Wester, H.-J.; Einsele, H.; et al. Side Effects of CXC-Chemokine Receptor 4–Directed Endoradiotherapy with Pentixather Before Hematopoietic Stem Cell Transplantation. J. Nucl. Med. 2019, 60, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Schottelius, M.; Herrmann, K.; Lapa, C. In Vivo Targeting of CXCR4—New Horizons. Cancers 2021, 13, 5920. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.K.; Serfling, S.E.; Lindner, T.; Hänscheid, H.; Schirbel, A.; Hahner, S.; Fassnacht, M.; Einsele, H.; Werner, R.A. CXCR4-targeted theranostics in oncology. Eur. J. Nucl. Med. 2022, 49, 4133–4144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xue, Q.; Yao, S. Nuclear Medicine Application of Pentixafor/Pentixather Targeting CXCR4 for Imaging and Therapy in Related Disease. Mini-Reviews Med. Chem. 2023, 23, 787–803. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Haubner, R.; Decristoforo, C. Radiolabelled RGD peptides and peptidomimetics for tumour targeting. Front. Biosci. 2009, 14, 872–886. [Google Scholar] [CrossRef]
- Notni, J. RGD Forever! — Past, Present, and Future of a 3-Letter-Code in Radiopharmacy and Life Sciences. Pharmaceuticals 2023, 16, 56. [Google Scholar] [CrossRef]
- Kossatz, S.; Beer, A.J.; Notni, J. It’s Time to Shift the Paradigm: Translation and Clinical Application of Non-αvβ3 Integrin Targeting Radiopharmaceuticals. Cancers 2021, 13, 5958. [Google Scholar] [CrossRef] [PubMed]
- Masłowska, K.; Halik, P.K.; Tymecka, D.; Misicka, A.; Gniazdowska, E. The Role of VEGF Receptors as Molecular Target in Nuclear Medicine for Cancer Diagnosis and Combination Therapy. Cancers 2021, 13, 1072. [Google Scholar] [CrossRef] [PubMed]
- Masłowska K, Witkowska E, Tymecka D, Halik PK, Misicka A, Gniazdowska E. Synthesis, Physicochemical and Biological Study of Gallium-68- and Lutetium-177-Labeled VEGF-A165/NRP-1 Complex Inhibitors Based on Peptide A7R and Branched Peptidomimetic. Pharmaceutics. 2022, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Ayo, A.; Laakkonen, P. Peptide-Based Strategies for Targeted Tumor Treatment and Imaging. Pharmaceutics 2021, 13, 481. [Google Scholar] [CrossRef]
- Khalily MP, Soydan M. Peptide-based diagnostic and therapeutic agents: Where we are and where we are heading? Chem Biol Drug Des. 2022, 1–22. [Google Scholar]
- Cao, R.; Liu, H.; Cheng, Z. Radiolabeled Peptide Probes for Liver Cancer Imaging. Curr. Med. Chem. 2020, 27, 6968–6986. [Google Scholar] [CrossRef]
- Ciobanasu, C. Peptides-based therapy and diagnosis. Strategies for non-invasive therapies in cancer. J. Drug Target. 2021, 29, 1063–1079. [Google Scholar] [CrossRef]
- Baum, R.P.; Singh, A.; Schuchardt, C.; Kulkarni, H.R.; Klette, I.; Wiessalla, S.; Osterkamp, F.; Reineke, U.; Smerling, C. 177Lu-3BP-227 for Neurotensin Receptor 1–Targeted Therapy of Metastatic Pancreatic Adenocarcinoma: First Clinical Results. J. Nucl. Med. 2018, 59, 809–814. [Google Scholar] [CrossRef]
- Nock, B.A.; Kanellopoulos, P.; Chepurny, O.G.; Rouchota, M.; Loudos, G.; Holz, G.G.; Krenning, E.P.; Maina, T. Nonpeptidic Z360-Analogs Tagged with Trivalent Radiometals as Anti-CCK2R Cancer Theranostic Agents: A Preclinical Study. Pharmaceutics 2022, 14, 666. [Google Scholar] [CrossRef]
- Gdowski, A.S.; Ranjan, A.; Vishwanatha, J.K. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J. Exp. Clin. Cancer Res. 2017, 36. [Google Scholar] [CrossRef]
- Liepe, K.; Kotzerke, J. Internal radiotherapy of painful bone metastases. Methods 2011, 55, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Potsaid, M.S.; Irwin, R.J.; Castronovo, F.P.; Prout, G.R.; Harvey, W.J.; Francis, M.D.; Tofe, A.J.; Zamenhof, R.G. [32P] diphosphonate dose determination in patients with bone metastases from prostatic carcinoma. J Nucl Med. 1978, 19, 98–104. [Google Scholar] [PubMed]
- Roberts, D.J. 32P-Sodium Phosphate Treatment of Metastatic Malignant Disease. Clin. Nucl. Med. 1979, 4, 92–93. [Google Scholar] [CrossRef]
- Mathieu, L.; Chevalier, P.; Galy, G.; Berger, M. Preparation of rhenium-186 labelled EHDP and its possible use in the treatment of osseous neoplasms. Int. J. Appl. Radiat. Isot. 1979, 30, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Hosain, F.; Spencer, R.P. Radiopharmaceuticals for palliation of metastatic osseous lesions: Biologic and physical background. Semin. Nucl. Med. 1992, 22, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, L.G.; E Bolch, W.; Goddu, S.M.; Howell, R.W.; Rao, D.V. Considerations in the selection of radiopharmaceuticals for palliation of bone pain from metastatic osseous lesions. J Nucl Med. 2000, 41, 682–7. [Google Scholar]
- Pauwels, E.K.; Stokkel, M.P. Radiopharmaceuticals for bone lesions. Imaging and therapy in clinical practice. Q J Nucl Med. 2001, 45, 18–26. [Google Scholar]
- Lange, R.; ter Heine, R.; Knapp, R. (.; de Klerk, J.M.; Bloemendal, H.J.; Hendrikse, N.H. Pharmaceutical and clinical development of phosphonate-based radiopharmaceuticals for the targeted treatment of bone metastases. Bone 2016, 91, 159–179. [Google Scholar] [CrossRef]
- Liepe K, Shinto A. From palliative therapy to prolongation of survival: 223RaCl2 in the treatment of bone metastases. Ther Adv Med Oncol. 2016, 8, 294–304. [Google Scholar] [CrossRef]
- Das, T.; Banerjee, S. Radiopharmaceuticals for metastatic bone pain palliation: available options in the clinical domain and their comparisons. Clin. Exp. Metastasis 2017, 34, 1–10. [Google Scholar] [CrossRef]
- Fernández, R.; Eppard, E.; Lehnert, W.; Jiménez-Franco, L.D.; Soza-Ried, C.; Ceballos, M.; Ribbeck, J.; Kluge, A.; Roesch, F.; Meckel, M.; et al. Evaluation of Safety and Dosimetry of 177Lu-DOTA-ZOL for Therapy of Bone Metastases. J. Nucl. Med. 2021, 62, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Palmedo H, Manka-Waluch A, Albers P, Schmidt-Wolf IG, Reinhardt M, Ezziddin S, Joe A, Roedel R, Fimmers R, Knapp FF Jr, Guhlke S, Biersack HJ. Repeated bone-targeted therapy for hormone-refractory prostate carcinoma: tandomized phase II trial with the new, high-energy radiopharmaceutical rhenium-188 hydroxyethylidenediphosphonate. J Clin Oncol. 2003, 21, 2869–2875. [Google Scholar] [CrossRef] [PubMed]
- Biersack, H.-J.; Palmedo, H.; Andris, A.; Rogenhofer, S.; Knapp, F.F.; Guhlke, S.; Ezziddin, S.; Bucerius, J.; von Mallek, D. Palliation and Survival After Repeated 188Re-HEDP Therapy of Hormone-Refractory Bone Metastases of Prostate Cancer: A Retrospective Analysis. J. Nucl. Med. 2011, 52, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Liberal, F.D.G.; Tavares, A.A.S.; Tavares, J.M.R. Palliative treatment of metastatic bone pain with radiopharmaceuticals: A perspective beyond Strontium-89 and Samarium-153. Appl. Radiat. Isot. 2016, 110, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Liepe, K.; Murray, I.; Flux, G. Dosimetry of Bone Seeking Beta Emitters for Bone Pain Palliation Metastases. Semin. Nucl. Med. 2022, 52, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Askari, E.; Harsini, S.; Vahidfar, N.; Divband, G.; Sadeghi, R. 177Lu-EDTMP for Metastatic Bone Pain Palliation: A Systematic Review and Meta-Analysis. Cancer Biotherapy Radiopharm. 2021, 36, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Wieland, D.M.; Swanson, D.P.; E Brown, L.; Beierwaltes, W.H. Imaging the adrenal medulla with an I-131-labeled antiadrenergic agent. J Nucl Med. 1979, 20, 155–8. [Google Scholar]
- Vallabhajosula, S.; Nikolopoulou, A. Radioiodinated Metaiodobenzylguanidine (MIBG): Radiochemistry, Biology, and Pharmacology. Semin. Nucl. Med. 2011, 41, 324–333. [Google Scholar] [CrossRef]
- Jungels, C.; Karfis, I. 131I-metaiodobenzylguanidine and peptide receptor radionuclide therapy in pheochromocytoma and paraganglioma. Curr. Opin. Oncol. 2021, 33, 33–39. [Google Scholar] [CrossRef]
- Giammarile F, Chiti A, Lassmann M, Brans B, Flux G; EANM. EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy. Eur J Nucl Med Mol Imaging. 2008, 35, 1039–1047. [Google Scholar] [CrossRef]
- Jimenez, C.; Erwin, W.; Chasen, B. Targeted Radionuclide Therapy for Patients with Metastatic Pheochromocytoma and Paraganglioma: From Low-Specific-Activity to High-Specific-Activity Iodine-131 Metaiodobenzylguanidine. Cancers 2019, 11, 1018. [Google Scholar] [CrossRef] [PubMed]
- Prado-Wohlwend, S.; del Olmo-García, M.I.; Bello-Arques, P.; Merino-Torres, J.F. Response to targeted radionuclide therapy with [131I]MIBG AND [177Lu]Lu-DOTA-TATE according to adrenal vs. extra-adrenal primary location in metastatic paragangliomas and pheochromocytomas: A systematic review. Front. Endocrinol. 2022, 13, 957172. [Google Scholar] [CrossRef] [PubMed]
- Sisson, J.C.; Shapiro, B.; Hutchinson, R.J.; Shulkin, B.L.; Zempel, S. Survival of Patients with Neuroblastoma Treated with 125-I MIBG. Am. J. Clin. Oncol. 1996, 19, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, Y.; Sudo, H.; Watanabe, S.; Nagatsu, K.; Tsuji, A.B.; Sakashita, T.; Ito, Y.M.; Yoshinaga, K.; Higashi, T.; Ishioka, N.S. Antitumor effects of radionuclide treatment using α-emitting meta-211At-astato-benzylguanidine in a PC12 pheochromocytoma model. Eur. J. Nucl. Med. 2018, 45, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Ukon, N.; Higashi, T.; Hosono, M.; Kinuya, S.; Yamada, T.; Yanagida, S.; Namba, M.; Nakamura, Y. Manual on the proper use of meta-[211At] astato-benzylguanidine ([211At] MABG) injections in clinical trials for targeted alpha therapy (1st edition). Ann. Nucl. Med. 2022, 36, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar] [PubMed]
- Backhaus, P.; Noto, B.; Avramovic, N.; Grubert, L.S.; Huss, S.; Bögemann, M.; Stegger, L.; Weckesser, M.; Schäfers, M.; Rahbar, K. Targeting PSMA by radioligands in non-prostate disease—current status and future perspectives. Eur. J. Nucl. Med. 2018, 45, 860–877. [Google Scholar] [CrossRef]
- Uijen, M.J.M.; Derks, Y.H.W.; Merkx, R.I.J.; Schilham, M.G.M.; Roosen, J.; Privé, B.M.; van Lith, S.A.M.; van Herpen, C.M.L.; Gotthardt, M.; Heskamp, S.; et al. PSMA radioligand therapy for solid tumors other than prostate cancer: background, opportunities, challenges, and first clinical reports. Eur. J. Nucl. Med. 2021, 48, 4350–4368. [Google Scholar] [CrossRef]
- An, S.; Huang, G.; Liu, J.; Wei, W. PSMA-targeted theranostics of solid tumors: applications beyond prostate cancers. Eur. J. Nucl. Med. 2022, 49, 3973–3976. [Google Scholar] [CrossRef]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. Radionuclide Therapy of Metastatic Prostate Cancer. Semin. Nucl. Med. 2019, 49, 313–325. [Google Scholar] [CrossRef]
- Kulkarni, H.R.; Singh, A.; Schuchardt, C.; Niepsch, K.; Sayeg, M.; Leshch, Y.; Wester, H.-J.; Baum, R.P. PSMA-Based Radioligand Therapy for Metastatic Castration-Resistant Prostate Cancer: The Bad Berka Experience Since 2013. J. Nucl. Med. 2016, 57, 97S–104S. [Google Scholar] [CrossRef] [PubMed]
- Sanli, Y.; Simsek, D.H.; Sanli, O.; Subramaniam, R.M.; Kendi, A.T. 177Lu-PSMA Therapy in Metastatic Castration-Resistant Prostate Cancer. Biomedicines 2021, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Feng, X.; Yang, D.; Lin, M. Advances in PSMA-targeted therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2021, 25, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Huang, G.; Song, H.; Chen, Y.; Chen, L. Cancer associated fibroblasts: An essential role in the tumor microenvironment. Oncol. Lett. 2017, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Calais, J. FAP: The Next Billion Dollar Nuclear Theranostics Target? J Nucl Med. 2020, 61, 163–165. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Pang, Y.; Fu, K.; Shang, Q.; Wu, H.; Sun, L.; Lin, Q.; Chen, H. Fibroblast activation protein-based theranostics in cancer research: A state-of-the-art review. Theranostics 2022, 12, 1557–1569. [Google Scholar] [CrossRef]
- Roustaei H, Kiamanesh Z, Askari E, Sadeghi R, Aryana K, Treglia G. Could Fibroblast Activation Protein (FAP)-Specific Radioligands Be Considered as Pan-Tumor Agents? Contrast Media Mol Imaging. 2022, 2022, 3948873. [Google Scholar]
- Kuyumcu, S.; Kovan, B.M.; Sanli, Y.; Buyukkaya, F.; Simsek, D.H.; Özkan, Z.G.; Isik, E.G.; Ekenel, M.; Turkmen, C. Safety of Fibroblast Activation Protein–Targeted Radionuclide Therapy by a Low-Dose Dosimetric Approach Using 177Lu-FAPI04. Clin. Nucl. Med. 2021, 46, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Rathke H, Fuxius S, Giesel FL, Lindner T, Debus J, Haberkorn U, Kratochwil C. Two Tumors, One Target: Preliminary Experience With 90Y-FAPI Therapy in a Patient With Metastasized Breast and Colorectal Cancer. Clin Nucl Med. 2021, 46, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Assadi M, Rekabpour SJ, Jafari E, Divband G, Nikkholgh B, Amini H, Kamali H, Ebrahimi S, Shakibazad N, Jokar N, Nabipour I, Ahmadzadehfar H. Feasibility and Therapeutic Potential of 177Lu-Fibroblast Activation Protein Inhibitor-46 for Patients With Relapsed or Refractory Cancers: A Preliminary Study. Clin Nucl Med. 2021, 46, e523–e530. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Pang, Y.; Zhao, L.; Lin, L.; Wu, H.; Sun, L.; Lin, Q.; Chen, H. FAP-targeted radionuclide therapy with [177Lu]Lu-FAPI-46 in metastatic nasopharyngeal carcinoma. Eur. J. Nucl. Med. 2021, 49, 1767–1769. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandus, J.; Costa, P.F.; Kessler, L.; Weber, M.; Hirmas, N.; Kostbade, K.; Bauer, S.; Schuler, M.; Ahrens, M.; Schildhaus, H.-U.; et al. Initial clinical experience with 90Y-FAPI-46 radioligand therapy for advanced stage solid tumors: a case series of nine patients. J. Nucl. Med. 2022, 63, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Huang, J.; Sun, L.; Wu, H.; Chen, H. FAP-Targeted Radionuclide Therapy of Advanced Radioiodine-Refractory Differentiated Thyroid Cancer With Multiple Cycles of 177Lu-FAPI-46. Clin. Nucl. Med. 2022, 47, 906–907. [Google Scholar] [CrossRef]
- Fendler, W.P.; Pabst, K.M.; Kessler, L.; Costa, P.F.; Ferdinandus, J.; Weber, M.; Lippert, M.; Lueckerath, K.; Umutlu, L.; Kostbade, K.; et al. Safety and Efficacy of 90Y-FAPI-46 Radioligand Therapy in Patients with Advanced Sarcoma and Other Cancer Entities. Clin. Cancer Res. 2022, 28, 4346–4353. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Rathke, H.; Fink, R.; Dendl, K.; Debus, J.; Mier, W.; Jäger, D.; Lindner, T.; Haberkorn, U. [153Sm]Samarium-labeled FAPI-46 radioligand therapy in a patient with lung metastases of a sarcoma. Eur. J. Nucl. Med. 2021, 48, 3011–3013. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, M.; Ding, J.; Chen, J.; Zhang, T.; Huo, L.; Liu, Z. Fatty acid-conjugated radiopharmaceuticals for fibroblast activation protein-targeted radiotherapy. Eur. J. Nucl. Med. 2022, 49, 1985–1996. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, P.; Ding, J.; Chen, J.; Huo, L.; Liu, Z. Albumin Binder–Conjugated Fibroblast Activation Protein Inhibitor Radiopharmaceuticals for Cancer Therapy. J. Nucl. Med. 2022, 63, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xu, P.; Shi, M.; Liu, J.; Zeng, X.; Zhang, Y.; Shi, C.; Li, J.; Guo, Z.; Zhang, X.; et al. Evans blue-modified radiolabeled fibroblast activation protein inhibitor as long-acting cancer therapeutics. Theranostics 2022, 12, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.S.; Ballal, S.; Yadav, M.P.; Bal, C.; Van Rymenant, Y.; Stephan, S.; Bracke, A.; Van der Veken, P.; De Meester, I.; Roesch, F. Fibroblast Activation Protein (FAP) targeting homodimeric FAP inhibitor radiotheranostics: a step to improve tumor uptake and retention time. Am J Nucl Med Mol Imaging. 2021, 11, 476–491. [Google Scholar] [PubMed]
- Zhao, L.; Chen, J.; Pang, Y.; Fang, J.; Fu, K.; Meng, L.; Zhang, X.; Guo, Z.; Wu, H.; Sun, L.; et al. Development of Fibroblast Activation Protein Inhibitor-Based Dimeric Radiotracers with Improved Tumor Retention and Antitumor Efficacy. Mol. Pharm. 2022, 19, 3640–3651. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Kramer, V.S.; Roesch, F.; Kumari, S.; Bal, C. First-In-Human Results on the Biodistribution, Pharmacokinetics, and Dosimetry of [177Lu]Lu-DOTA.SA.FAPi and [177Lu]Lu-DOTAGA.(SA.FAPi)2. Pharmaceuticals 2021, 14, 1212. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Roesch, F.; Kumari, S.; Agarwal, S.; Tripathi, M.; Sahoo, R.K.; Mangu, B.S.; Tupalli, A.; et al. Novel Fibroblast Activation Protein Inhibitor-Based Targeted Theranostics for Radioiodine-Refractory Differentiated Thyroid Cancer Patients: A Pilot Study. Thyroid® 2022, 32, 65–77. [Google Scholar] [CrossRef]
- Baum RP, Schuchardt C, Singh A, Chantadisai M, Robiller FC, Zhang J, Mueller D, Eismant A, Almaguel F, Zboralski D, Osterkamp F, Hoehne A, Reineke U, Smerling C, Kulkarni HR. Feasibility, Biodistribution and Preliminary Dosimetry in Peptide-Targeted Radionuclide Therapy (PTRT) of Diverse Adenocarcinomas using 177Lu-FAP-2286: First-in-Human Results. J Nucl Med. 2022, 63, 415–423. [Google Scholar] [CrossRef]
- Millul, J.; Bassi, G.; Mock, J.; Elsayed, A.; Pellegrino, C.; Zana, A.; Plaza, S.D.; Nadal, L.; Gloger, A.; Schmidt, E.; et al. An ultra-high-affinity small organic ligand of fibroblast activation protein for tumor-targeting applications. Proc. Natl. Acad. Sci. 2021, 118. [Google Scholar] [CrossRef]
- Bartoli F, Elsinga P, Nazario LR, Zana A, Galbiati A, Millul J, Migliorini F, Cazzamalli S, Neri D, Slart RHJA, Erba PA. Automated Radiosynthesis, Preliminary In Vitro/In Vivo Characterization of OncoFAP-Based Radiopharmaceuticals for Cancer Imaging and Therapy. Pharmaceuticals (Basel). 2022, 15, 958. [Google Scholar] [CrossRef]
- Galbiati, A.; Zana, A.; Bocci, M.; Millul, J.; Elsayed, A.; Mock, J.; Neri, D.; Cazzamalli, S. A Dimeric FAP-Targeting Small-Molecule Radioconjugate with High and Prolonged Tumor Uptake. J. Nucl. Med. 2022, 63, 1852–1858. [Google Scholar] [CrossRef]
- Liu, Y.; Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Naka, S.; Ooe, K.; Toyoshima, A.; Nagata, K.; Haberkorn, U.; Kratochwil, C.; et al. Fibroblast activation protein targeted therapy using [177Lu]FAPI-46 compared with [225Ac]FAPI-46 in a pancreatic cancer model. Eur. J. Nucl. Med. 2022, 49, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Lindner T, Altmann A, Krämer S, Kleist C, Loktev A, Kratochwil C, Giesel F, Mier W, Marme F, Debus J, Haberkorn U. Design and Development of 99mTc-Labeled FAPI Tracers for SPECT Imaging and 188Re Therapy. J Nucl Med. 2020, 61, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Giesel, F.L.; Kratochwil, C.; Serfling, S.E. Radioligands Targeting Fibroblast Activation Protein (FAP). Cancers 2021, 13, 5744. [Google Scholar] [CrossRef] [PubMed]
- Imlimthan, S.; Moon, E.S.; Rathke, H.; Afshar-Oromieh, A.; Rösch, F.; Rominger, A.; Gourni, E. New Frontiers in Cancer Imaging and Therapy Based on Radiolabeled Fibroblast Activation Protein Inhibitors: A Rational Review and Current Progress. Pharmaceuticals 2021, 14, 1023. [Google Scholar] [CrossRef]
- Pazzaglia, S.; Pioli, C. Multifaceted Role of PARP-1 in DNA Repair and Inflammation: Pathological and Therapeutic Implications in Cancer and Non-Cancer Diseases. Cells 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Tan, K.V.; Cornelissen, B. PARP Inhibitors in Cancer Diagnosis and Therapy. Clin. Cancer Res. 2021, 27, 1585–1594. [Google Scholar] [CrossRef]
- Jannetti, S.A.; Zeglis, B.M.; Zalutsky, M.R.; Reiner, T. Poly(ADP-Ribose)Polymerase (PARP) Inhibitors and Radiation Therapy. Front. Pharmacol. 2020, 11, 170. [Google Scholar] [CrossRef]
- Carney, B.; Kossatz, S.; Reiner, T. Molecular Imaging of PARP. J. Nucl. Med. 2017, 58, 1025–1030. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.A.; Kossatz, S.; Weber, W.; Beheshti, M.; Morgenroth, A.; Mottaghy, F.M. Advancements in PARP1 Targeted Nuclear Imaging and Theranostic Probes. J. Clin. Med. 2020, 9, 2130. [Google Scholar] [CrossRef]
- Jannetti, S.A.; Carlucci, G.; Carney, B.; Kossatz, S.; Shenker, L.; Carter, L.M.; Salinas, B.; Brand, C.; Sadique, A.; Donabedian, P.L.; et al. PARP-1–Targeted Radiotherapy in Mouse Models of Glioblastoma. J. Nucl. Med. 2018, 59, 1225–1233. [Google Scholar] [CrossRef]
- Nguyen NT, Pacelli A, Nader M, Kossatz S. DNA Repair Enzyme Poly(ADP-Ribose) Polymerase 1/2 (PARP1/2)-Targeted Nuclear Imaging and Radiotherapy. Cancers (Basel). 2022, 14, 1129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Chen, H.; Mpoy, C.; Afrin, S.; Rogers, B.E.; Garbow, J.R.; Katzenellenbogen, J.A.; Xu, J. Radiosynthesis and Evaluation of Talazoparib and Its Derivatives as PARP-1-Targeting Agents. Biomedicines 2021, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Destro G, Chen Z, Chan CY, Fraser C, Dias G, Mosley M, Guibbal F, Gouverneur V, Cornelissen B. A radioiodinated rucaparib analogue as an Auger electron emitter for cancer therapy. Nucl Med Biol. 2022, 116‒117, 108312. [Google Scholar]
- Makvandi, M.; Lee, H.; Puentes, L.N.; Reilly, S.W.; Rathi, K.S.; Weng, C.-C.; Chan, H.S.; Hou, C.; Raman, P.; Martinez, D.; et al. Targeting PARP-1 with Alpha-Particles Is Potently Cytotoxic to Human Neuroblastoma in Preclinical Models. Mol. Cancer Ther. 2019, 18, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Riad, A.; Martorano, P.; Mansfield, A.; Samanta, M.; Batra, V.; Mach, R.H.; Maris, J.M.; Pryma, D.A.; Makvandi, M. PARP-1–Targeted Auger Emitters Display High-LET Cytotoxic Properties In Vitro but Show Limited Therapeutic Utility in Solid Tumor Models of Human Neuroblastoma. J. Nucl. Med. 2020, 61, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Pirovano, G.; Jannetti, S.A.; Carter, L.M.; Sadique, A.; Kossatz, S.; Guru, N.; De Souza França, P.D.; Maeda, M.; Zeglis, B.M.; Lewis, J.S.; et al. Targeted Brain Tumor Radiotherapy Using an Auger Emitter. Clin. Cancer Res. 2020, 26, 2871–2881. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.A.; Florea, A.; Allekotte, S.; Vogg, A.T.J.; Maurer, J.; Schäfer, L.; Bolm, C.; Terhorst, S.; Classen, A.; Bauwens, M.; et al. PARP targeted Auger emitter therapy with [125I]PARPi-01 for triple-negative breast cancer. EJNMMI Res. 2022, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M. Tumor Hypoxia in Cancer Therapy. Methods Enzymol. 2007, 435, 295–321. [Google Scholar] [CrossRef]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef]
- Benej M, Pastorekova S, Pastorek J. Carbonic Anhydrase IX: Regulation and role in cancer. In Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Singh, S.; Lomelino, C.L.; Mboge, M.Y.; Frost, S.C.; McKenna, R. Cancer Drug Development of Carbonic Anhydrase Inhibitors beyond the Active Site. Molecules 2018, 23, 1045. [Google Scholar] [CrossRef]
- Lau, J.; Lin, K.-S.; Bénard, F. Past, Present, and Future: Development of Theranostic Agents Targeting Carbonic Anhydrase IX. Theranostics 2017, 7, 4322–4339. [Google Scholar] [CrossRef] [PubMed]
- Iikuni, S.; Ono, M.; Watanabe, H.; Shimizu, Y.; Sano, K.; Saji, H. Cancer radiotheranostics targeting carbonic anhydrase-IX with 111In- and 90Y-labeled ureidosulfonamide scaffold for SPECT imaging and radionuclide-based therapy. Theranostics 2018, 8, 2992–3006. [Google Scholar] [CrossRef] [PubMed]
- Janoniene, A.; Petrikaite, V. In Search of Advanced Tumor Diagnostics and Treatment: Achievements and Perspectives of Carbonic Anhydrase IX Targeted Delivery. Mol. Pharm. 2020, 17, 1800–1815. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhi, P.; Farahani, A.M.; Tamaddondar, M. Radiolabeled vitamins as the potential diagnostic probes for targeted tumor imaging. Bioorganic Chem. 2022, 122, 105717. [Google Scholar] [CrossRef] [PubMed]
- Jurczyk, M.; Jelonek, K.; Musiał-Kulik, M.; Beberok, A.; Wrześniok, D.; Kasperczyk, J. Single- versus Dual-Targeted Nanoparticles with Folic Acid and Biotin for Anticancer Drug Delivery. Pharmaceutics 2021, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Jallinoja, V.I.; Houghton, J.L. Current Landscape in Clinical Pretargeted Radioimmunoimaging and Therapy. J. Nucl. Med. 2021, 62, 1200–1206. [Google Scholar] [CrossRef]
- Russell-Jones, G.; McTavish, K.; McEwan, J.; Rice, J.; Nowotnik, D. Vitamin-mediated targeting as a potential mechanism to increase drug uptake by tumours. J. Inorg. Biochem. 2004, 98, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef]
- Xia, W.; Low, P.S. Folate-Targeted Therapies for Cancer. J. Med. Chem. 2010, 53, 6811–6824. [Google Scholar] [CrossRef]
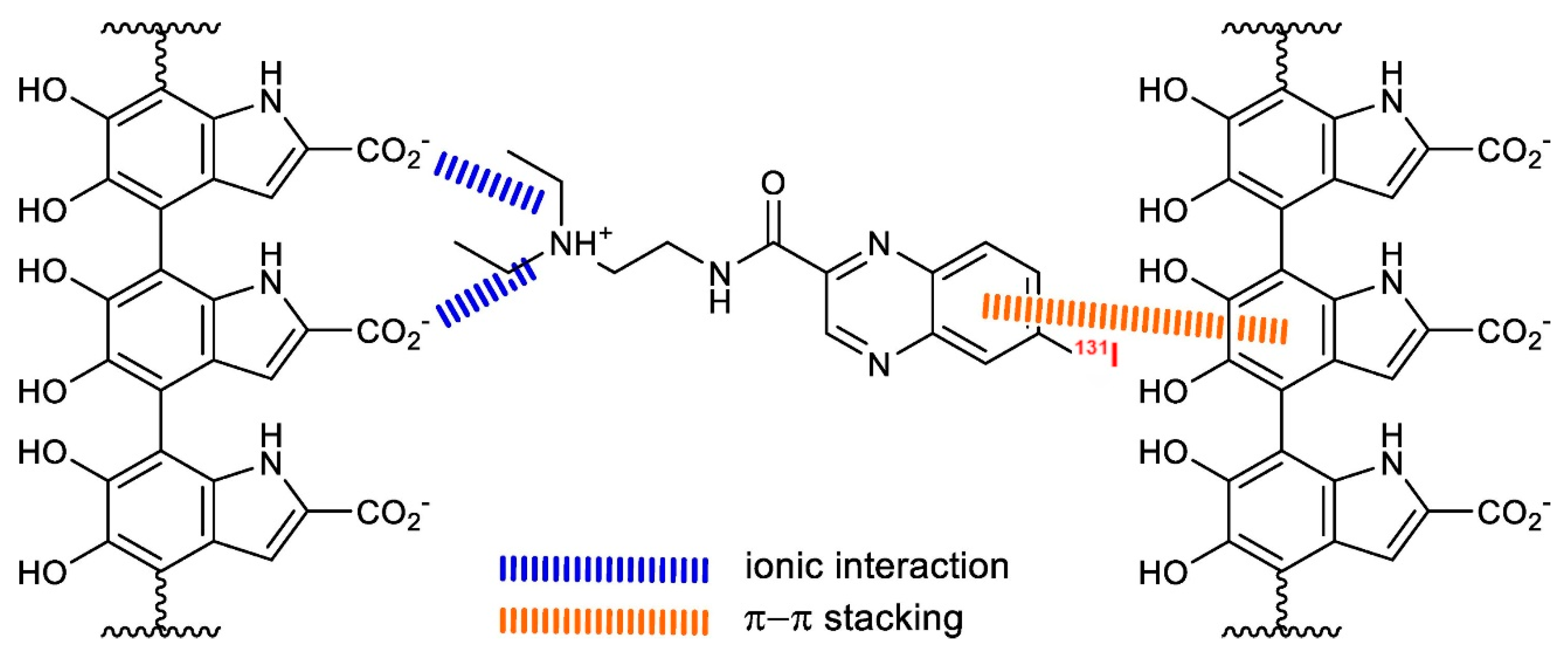
- Muller, C. Folate Based Radiopharmaceuticals for Imaging and Therapy of Cancer and Inflammation. Curr. Pharm. Des. 2012, 18, 1058–1083. [Google Scholar] [CrossRef]
- Müller, C.; Guzik, P.; Siwowska, K.; Cohrs, S.; Schmid, R.M.; Schibli, R. Combining Albumin-Binding Properties and Interaction with Pemetrexed to Improve the Tissue Distribution of Radiofolates. Molecules 2018, 23, 1465. [Google Scholar] [CrossRef] [PubMed]
- Benešová, M.; Guzik, P.; Deberle, L.M.; Busslinger, S.D.; Landolt, T.; Schibli, R.; Müller, C. Design and Evaluation of Novel Albumin-Binding Folate Radioconjugates: Systematic Approach of Varying the Linker Entities. Mol. Pharm. 2022, 19, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Boss, S.D.; Ametamey, S.M. Development of Folate Receptor−Targeted PET Radiopharmaceuticals for Tumor Imaging—A Bench-to-Bedside Journey. Cancers 2020, 12, 1508. [Google Scholar] [CrossRef] [PubMed]
- Müller C, Schibli R. Prospects in folate receptor-targeted radionuclide therapy. Front Oncol. 2013, 3, 249. [Google Scholar]
- Kuda-Wedagedara ANW, Workinger JL, Nexo E, Doyle RP, Viola-Villegas N. 89Zr-Cobalamin PET Tracer: Synthesis, Cellular Uptake, and Use for Tumor Imaging. ACS Omega. 2017, 2, 6314–6320. [Google Scholar] [CrossRef] [PubMed]
- Gendron LN, Zites DC, LaRochelle EPM, Gunn JR, Pogue BW, Shell TA, Shell JR. Tumor targeting vitamin B12 derivatives for X-ray induced treatment of pancreatic adenocarcinoma. Photodiagnosis Photodyn Ther. 2020, 30, 101637. [Google Scholar] [CrossRef]
- Snyder, F.; Wood, R. Alkyl and alk-1-enyl ethers of glycerol in lipids from normal and neoplastic human tissues. Cancer Res. 1969, 29, 251–7. [Google Scholar] [PubMed]
- Vink SR, van Blitterswijk WJ, Schellens JH, Verheij M. Rationale and clinical application of alkylphospholipid analogues in combination with radiotherapy. Cancer Treat Rev. 2007, 33, 191–202. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Lipid rafts and clusters of apoptotic signaling molecule-enriched rafts in cancer therapy. Futur. Oncol. 2010, 6, 811–821. [Google Scholar] [CrossRef]
- Weichert, J.P.; Clark, P.A.; Kandela, I.K.; Vaccaro, A.M.; Clarke, W.; Longino, M.A.; Pinchuk, A.N.; Farhoud, M.; Swanson, K.I.; Floberg, J.M.; et al. Alkylphosphocholine Analogs for Broad-Spectrum Cancer Imaging and Therapy. Sci. Transl. Med. 2014, 6, 240ra75–240ra75. [Google Scholar] [CrossRef]
- Meyer, K.L.; Schwendner, S.W.; Counsell, R.E. Potential tumor or organ-imaging agents. Radioiodinated phospholipid ethers. J. Med. Chem. 1989, 32, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
- Plotzke, K.P.; Haradahira, T.; Stancato, L.; Olken, N.M.; Skinner, S.; Gross, M.D.; Wahl, R.L.; Counsell, R.E. Selective localization of radioiodinated alkylphosphocholine derivatives in tumors. Int. J. Radiat. Appl. Instrumentation. Part B. Nucl. Med. Biol. 1992, 19, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Rampy, M.; Chou, T.; Pinchuk, A.; Skinner, R.; Gross; Fisher, S. ; Wahl, R.; Counsell, R. Synthesis and biological evaluation of radioiodinated phospholipid ether analogs. Nucl. Med. Biol. 1995, 22, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Grudzinski, J.J.; Hall, L.T.; Cho, S.; Liu, G.; Traynor, A.; Lee, M.H.; Longino, M.; Pinchuk, A.; Jaskowiak, C.; Bednarz, B.; et al. Clinical Imaging and Dosimetry of a Pan-Cancer Targeting Alkylphosphocholine Analog, [124I]I-NM404. Radiation 2022, 2, 215–227. [Google Scholar] [CrossRef]
- Pinchuk, A.N.; Rampy, M.A.; Longino, M.A.; Skinner, R.W.S.; Gross, M.D.; Weichert, J.P.; Counsell, R.E. Synthesis and Structure−Activity Relationship Effects on the Tumor Avidity of Radioiodinated Phospholipid Ether Analogues. J. Med. Chem. 2006, 49, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Morris, Z.S.; Weichert, J.P.; Saker, J.; Armstrong, E.A.; Besemer, A.; Bednarz, B.; Kimple, R.J.; Harari, P.M. Therapeutic combination of radiolabeled CLR1404 with external beam radiation in head and neck cancer model systems. Radiother. Oncol. 2015, 116, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Lubner SJ, Mullvain J, Perlman S, Pishvaian M, Mortimer J, Oliver K, Heideman J, Hall L, Weichert J, Liu G. A Phase 1, Multi-Center, Open-Label, Dose-Escalation Study of 131I-CLR1404 in Subjects with Relapsed or Refractory Advanced Solid Malignancies. Cancer Invest. 2015, 33, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.T.; Titz, B.; Robins, H.I.; Bednarz, B.P.; Perlman, S.B.; Weichert, J.P.; Kuo, J.S. PET/CT imaging of the diapeutic alkylphosphocholine analog 124I-CLR1404 in high and low-grade brain tumors. Am J Nucl Med Mol Imaging. 2017, 7, 157–166. [Google Scholar]
- Baiu, D.C.; Marsh, I.R.; Boruch, A.E.; Shahi, A.; Bhattacharya, S.; Jeffery, J.J.; Zhao, Q.; Hall, L.T.; Weichert, J.P.; Bednarz, B.P.; et al. Targeted Molecular Radiotherapy of Pediatric Solid Tumors Using a Radioiodinated Alkyl-Phospholipid Ether Analog. J. Nucl. Med. 2018, 59, 244–250. [Google Scholar] [CrossRef]
- Hall, L.T.; Titz, B.; Baidya, N.; van der Kolk, A.G.; Robins, H.I.; Otto, M.; Perlman, S.B.; Weichert, J.P.; Kuo, J.S. [124I]CLR1404 PET/CT in High-Grade Primary and Metastatic Brain Tumors. Mol. Imaging Biol. 2020, 22, 434–443. [Google Scholar] [CrossRef]
- Shahi, A.; Weiss, G.E.; Bhattacharya, S.; Baiu, D.C.; Marino, R.; Pula, T.; Callander, N.S.; Asimakopoulos, F.; Otto, M. Targeted treatment of multiple myeloma with a radioiodinated small molecule radiopharmaceutical. Leuk. Lymphoma 2021, 62, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Longcor, J.; Callander, N.; Oliver, K.; Chanan-Khan, A.; Ailawadhi, S. Iopofosine I-131 treatment in late-line patients with relapsed/refractory multiple myeloma post anti-BCMA immunotherapy. Blood Cancer J. 2022, 12, 130. [Google Scholar] [CrossRef]
- Grudzinski JJ, Hernandez R, Marsh I, Patel RB, Aluicio-Sarduy E, Engle J, Morris Z, Bednarz B, Weichert J. Preclinical Characterization of 86/90Y-NM600 in a Variety of Murine and Human Cancer Tumor Models. J Nucl Med. 2019, 60, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Grudzinski, J.J.; Aluicio-Sarduy, E.; Massey, C.F.; Pinchuk, A.N.; Bitton, A.N.; Patel, R.; Zhang, R.; Rao, A.V.; Iyer, G.; et al. 177Lu-NM600 Targeted Radionuclide Therapy Extends Survival in Syngeneic Murine Models of Triple-Negative Breast Cancer. J. Nucl. Med. 2020, 61, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Norain, A.; Dadachova, E. Targeted Radionuclide Therapy of Melanoma. Semin. Nucl. Med. 2016, 46, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.H.; Malo, M.E.; Jiao, R.; Dadachova, E. Targeting Melanin in Melanoma with Radionuclide Therapy. Int. J. Mol. Sci. 2022, 23, 9520. [Google Scholar] [CrossRef] [PubMed]
- Thomas NE, Kricker A, Waxweiler WT, Dillon PM, Busman KJ, From L, Groben PA, Armstrong BK, Anton-Culver H, Gruber SB, Marrett LD, Gallagher RP, Zanetti R, Rosso S, Dwyer T, Venn A, Kanetsky PA, Orlow I, Paine S, Ollila DW, Reiner AS, Luo L, Hao H, Frank JS, Begg CB, Berwick M; Genes, Environment, and Melanoma (GEM) Study Group. Comparison of clinicopathologic features and survival of histopathologically amelanotic and pigmented melanomas: a population-based study. JAMA Dermatol. 2014, 150, 1306–1314. [Google Scholar] [CrossRef]
- Klein, M.; Lotem, M.; Peretz, T.; Zwas, S.T.; Mizrachi, S.; Liberman, Y.; Chisin, R.; Schachter, J.; Ron, I.G.; Iosilevsky, G.; et al. Safety and Efficacy of 188-Rhenium-Labeled Antibody to Melanin in Patients with Metastatic Melanoma. J. Ski. Cancer 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Rouanet, J.; Quintana, M.; Auzeloux, P.; Cachin, F.; Degoul, F. Benzamide derivative radiotracers targeting melanin for melanoma imaging and therapy: Preclinical/clinical development and combination with other treatments. Pharmacol. Ther. 2021, 224, 107829. [Google Scholar] [CrossRef]
- Mohammed, A.; Nicholl, C.; Titsch, U.; Eisenhut, M. Radioiodinated N-(alkylaminoalkyl)-substituted 4-methoxy-, 4-hydroxy-, and 4-aminobenzamides: Biological investigations for the improvement of melanoma-imaging agents. Nucl. Med. Biol. 1997, 24, 373–380. [Google Scholar] [CrossRef]
- Eisenhut M, Hull WE, Mohammed A, Mier W, Lay D, Just W, Gorgas K, Lehmann WD, Haberkorn U. Radioiodinated N-(2-diethylaminoethyl)benzamide derivatives with high melanoma uptake: structure-affinity relationships, metabolic fate, and intracellular localization. J Med Chem. 2000, 43, 3913–3922. [Google Scholar] [CrossRef] [PubMed]
- Auzeloux, P.; Papon, J.; Pasqualini, R.; Madelmont, J.-C. Synthesis and Biodistribution of a New Oxo-technetium-99m Bis(aminothiol) Complex as a Potential Melanoma Tracer. J. Med. Chem. 2001, 44, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Oltmanns, D.; Eisenhut, M.; Mier, W.; Haberkorn, U. Benzamides as Melanotropic Carriers for Radioisotopes, Metals, Cytotoxic Agents and as Enzyme Inhibitors. Curr. Med. Chem. 2009, 16, 2086–2094. [Google Scholar] [CrossRef] [PubMed]
- Michelot, J.M.; Moreau, M.F.; Veyre, A.J.; Bonafous, J.F.; Bacin, F.J.; Madelmont, J.C.; Bussiere, F.; A Souteyrand, P.; Mauclaire, L.P.; Chossat, F.M. Phase II scintigraphic clinical trial of malignant melanoma and metastases with iodine-123-N-(2-diethylaminoethyl 4-iodobenzamide). J Nucl Med. 1993, 34, 1260–6. [Google Scholar] [PubMed]
- Link, EM. Targeting melanoma with 211At/131I-methylene blue: preclinical and clinical experience. Hybridoma. 1999, 18, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Joyal, J.L.; Barrett, J.A.; Marquis, J.C.; Chen, J.; Hillier, S.M.; Maresca, K.P.; Boyd, M.; Gage, K.; Nimmagadda, S.; Kronauge, J.F.; et al. Preclinical Evaluation of an 131I-Labeled Benzamide for Targeted Radiotherapy of Metastatic Melanoma. Cancer Res 2010, 70, 4045–4053. [Google Scholar] [CrossRef]
- Degoul F, Borel M, Jacquemot N, Besse S, Communal Y, Mishellany F, Papon J, Penault-Llorca F, Donnarieix D, Doly M, Maigne L, Miot-Noirault E, Cayre A, Cluzel J, Moins N, Chezal JM, Bonnet M. In vivo efficacy of melanoma internal radionuclide therapy with a 131I-labelled melanin-targeting heteroarylcarboxamide molecule. Int J Cancer. 2013, 133, 1042–1053. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, L.; Gai, Y.; Liu, Q.; Yin, L.; Jiang, Y.; Wang, Y.; Zhang, Y.; Lan, X. Targeted radiotherapy of pigmented melanoma with 131I-5-IPN. J. Exp. Clin. Cancer Res. 2018, 37, 1–13. [Google Scholar] [CrossRef]
- Mier, W.; Kratochwil, C.; Hassel, J.C.; Giesel, F.L.; Beijer, B.; Babich, J.W.; Friebe, M.; Eisenhut, M.; Enk, A.; Haberkorn, U. Radiopharmaceutical Therapy of Patients with Metastasized Melanoma with the Melanin-Binding Benzamide 131I-BA52. 2013, 55, 9–14. 14. [CrossRef]
- Thivat, E.; Rouanet, J.; Auzeloux, P.; Sas, N.; Jouberton, E.; Levesque, S.; Billoux, T.; Mansard, S.; Molnar, I.; Chanchou, M.; et al. Phase I study of [131I] ICF01012, a targeted radionuclide therapy, in metastatic melanoma: MELRIV-1 protocol. BMC Cancer 2022, 22, 1–10. [Google Scholar] [CrossRef]
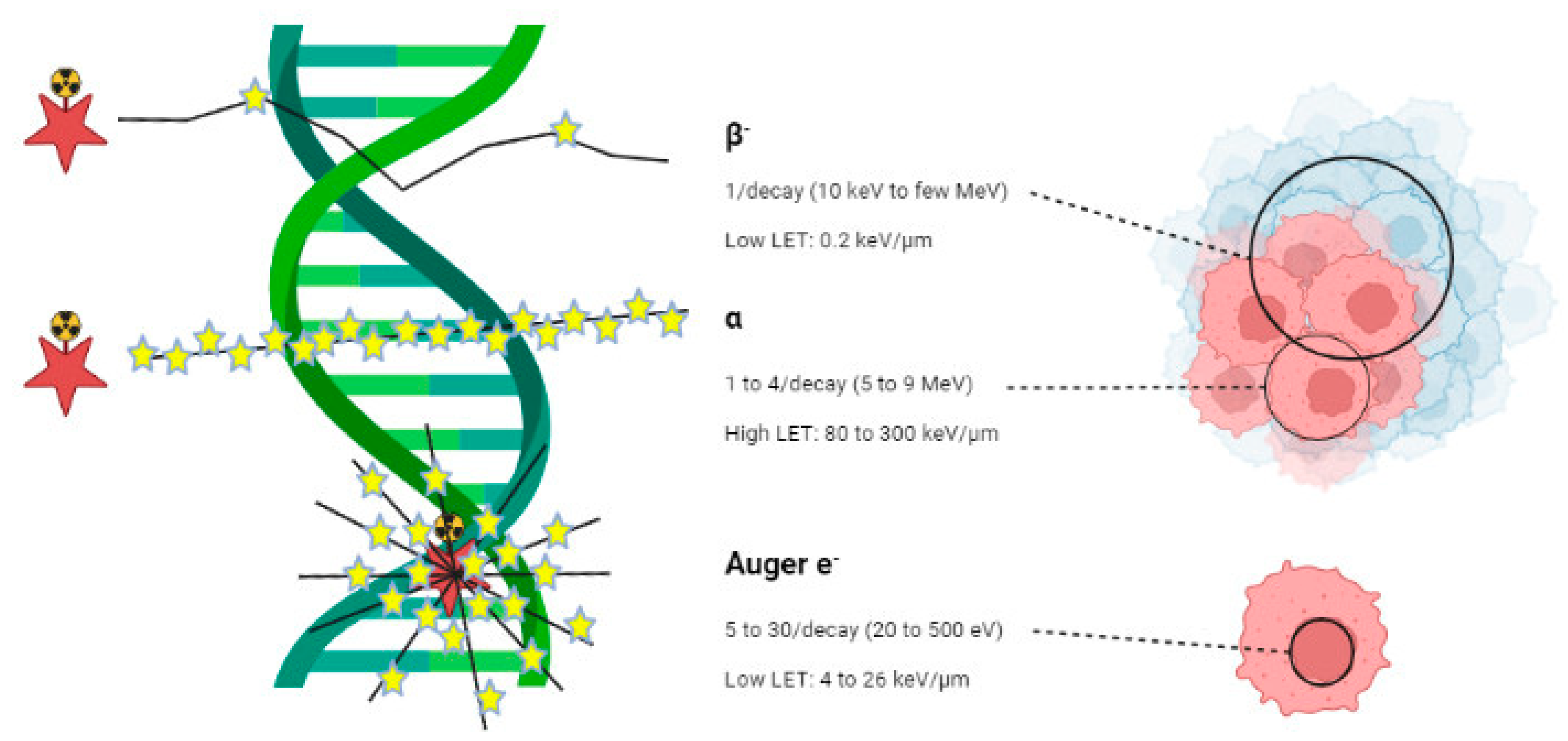
| Radionuclide | Half-life | Energy (MeV) | Eγ (keV) | Tissue penetration range (mm) |
| β-emitter | ||||
| 90Y | 2.7 days | 2.284 | / | 12 |
| 131I | 8 days | 0.81 | 0.364 | 2.4 |
| 161Tb | 6.9 days | 0.593 | 74.6 | 3 |
| 177Lu | 6.7 days | 0.497 | 208113 | 2.2 |
| 188Re | 17 hours | 2.118 | 155 | 11 |
| α-emitter | ||||
| 149Tb | 4.1 hours | 3.97 | Multiple emissions (165-800) | < 100 µm |
| 211At | 7.2 hours | 7.45 | 85 (X-Ray) | |
| 212Pb | 10.6 hours | 8.78 | 238, 300 | |
| 213Bi | 0.8 hours | 8.38 | 440 | |
| 223Ra | 11.4 days | 5.71, 6.82, 7.39, 6.62 | 270 | |
| 225Ac | 10 days | 5.8, 6.3, 7.1, 8.38 | 218, 440 (from daughters) | |
| 227Th | 18.7 days | 6.14, 5.71, 6.82, 7.39, 6.62 | 236 | |
| Auger e- emitter | ||||
| 111In | 2.8 days | 0.007 | 405 | < 1 µm |
| 125I | 60 days | 0.019 | 42 |
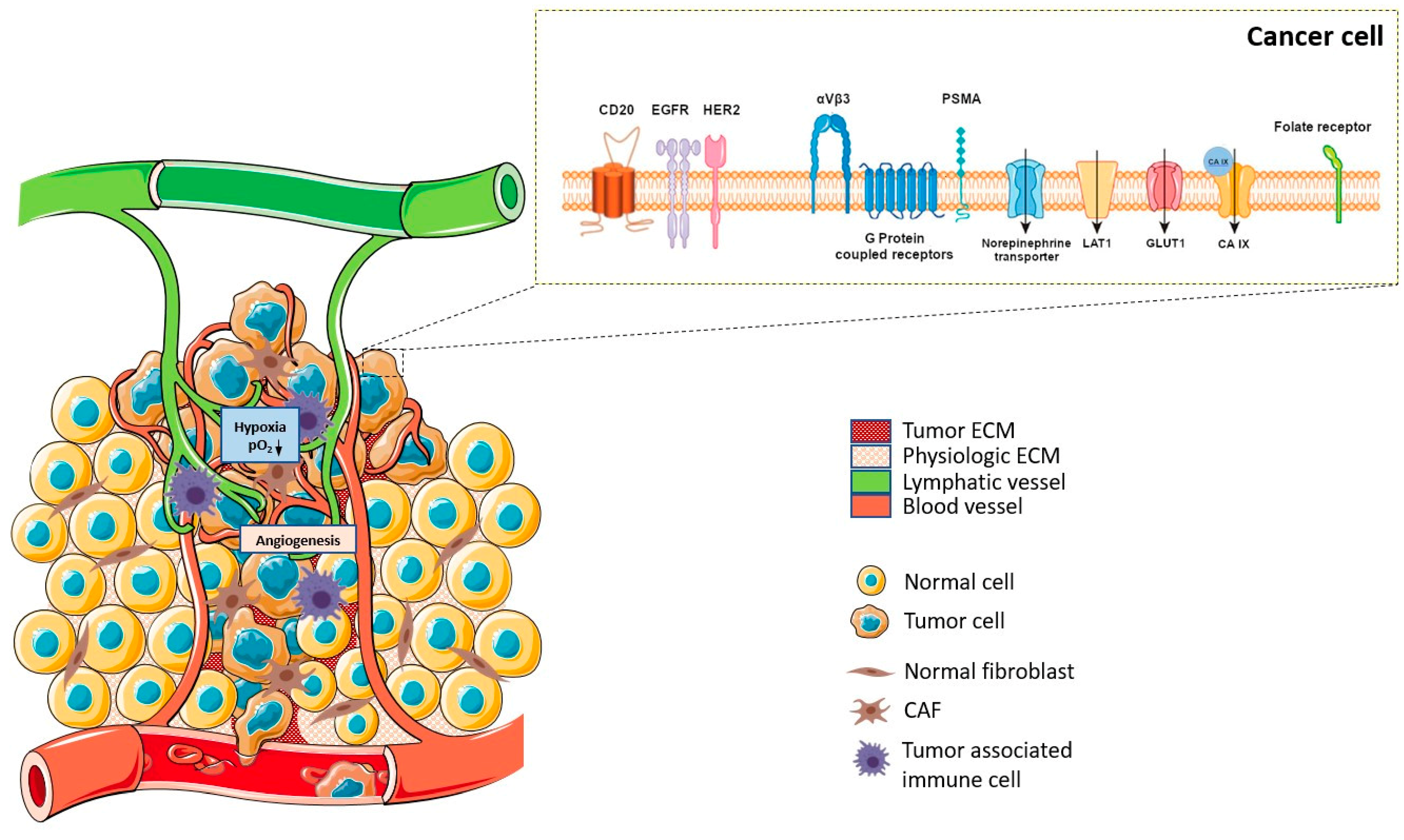
| Peptide | Receptor | Tumor expression |
| α-Melanocyte-stimulating hormone | MCR1, MCR3, MCR5 | Melanomas |
| Bombesin/Gastrin-releasing peptide | BB1, BB2 (GRPR), BB3, BB4 | Glioblastomas, prostate, breast, pancreatic, gastric, colorectal cancers, small cell lung |
| Cholecystokinin/gastrin | CCK1, CCK2 | Adenomas, astrocytomas, gastrointestinal and ovarian stromal tumors, medullary thyroid, pancreatic and small cell lung cancers |
| Epidermal Growth Factor | EGFR | Breast cancer |
| Exendin | GLP-1 | Gastrinomas, insulinomas,medullary thyroid carcinomas, paragangliomas and pheochromocytomas |
| Gonadotropin-releasing hormone | GnRH-R | Breast and prostate cancers |
| Neuropeptide Y | Y1, Y2, Y4, Y5 | Breast, ovary, adrenal, brain, kidney, GI-tract, bone (Ewing’s sarcoma) |
| Neurotensin | NTR1, NTR2, NTR3 | Breast, colon, pancreatic, prostate, small cell lung cancers, meningiomas |
| RGD | αVβ3 integrin | Tumor-induced angiogenesis |
| SDF-1α/CXCL12 | CXCR4, CXCR7 | Leukemias, lymphomas, melanomas, brain, breast, kidney, lung, ovarian, pancreas, prostate tumors |
| Somatostatin | Sstr1, sstr2, sstr3, sstr4, sstr5 | Neuroendocrine tumors, lymphomas, paragangliomas, brain, breast, renal, small cell lung cancers |
| Substance P | NK1, NK2, NK3 | Glial tumors, breast, medullary thyroid, pancreas, small cell lung cancers |
| Vasoactive intestinal peptide | VPAC1, VPAC2 | Bladder, breast, gastrointestinal, non-small cell lung, ovarian, pancreatic, prostate cancers |
| Peptide | Peptidic Sequence |
| OC Octreotide |
d-Phe-cyclo(Cys-Phe-d-Trp-Lys-Thr-Cys)Thr(ol) |
| LAN Lanreotide |
β-d-Nal-cyclo(Cys-Tyr-d-Trp-Lys-Val-Cys)Thr-NH2 |
| VAP Vapreotide |
d-Phe-cyclo(Cys-Phe-d-Trp-Lys-Val-Cys)Trp-NH2 |
| TOC [Tyr3]-Octreotide |
d-Phe-cyclo(Cys-Tyr-d-Trp-Lys-Thr-Cys)Thr(ol) |
| TATE [Tyr3]-Octreotate |
d-Phe-cyclo(Cys-Tyr-d-Trp-Lys-Thr-Cys)Thr |
| NOC [1-Nal3]-Octreotide |
d-Phe-cyclo(Cys-1-Nal-d-Trp-Lys-Thr-Cys)Thr(ol) |
| SOM230 Pasireotide |
Cyclo(Hyp(Unk)-Phg- d-Trp-Lys-Tyr(Bn)-Phe) |
| P2045 Tozaride |
Ser-Thr-Cys(Trt)-Phe(4-NH2)-(β-DAP)-CH2CO-S-cyclo((N-Me)HCy-Phe-Tyr-d-Trp-Lys-Thr) |
| Target | Name | Structure |
|---|---|---|
| SSTR | DOTA-BASS |  |
| DOTA-LM3 |  |
|
| DOTA-JR11 (Satoreotide) | 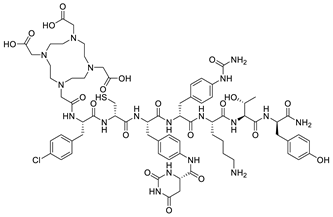 |
|
| GRPR | DOTA-RM2 | 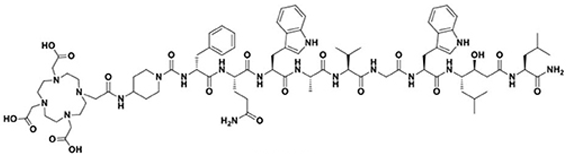 |
| NeoB | 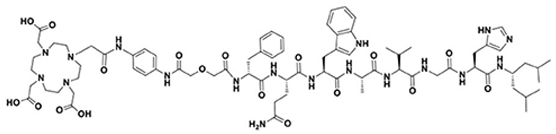 |
|
| NTR | 3BP-227 | 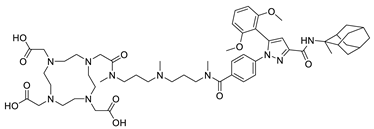 |
| Radionuclide | Agent |
|---|---|
| Approved Agents for Clinical Use | |
| Strontium-89 (β-) (50.5 d) | [89Sr]SrCl2 – Metastron® |
| Samarium-153 (β-) (1.9 d) | EDTMP – Quadramet® |
| Rhenium-186 (β-) (3.7 d) | HEDP |
| Radium-223 (α) (11.4 d) | [223Ra]RaCl2 – Xofigo® |
| Agents in Clinical Trials | |
| Rhenium-188 (β-) (17 h) | HEDP Zoledronic acid |
| Lutetium-177 (β-) (6.8 d) | EDTMP DOTMP* Zoledronic acid (Dotazol) |
| Holmium-166 (β-) (1.1 d) | DOTMP* |
| Tin-117m (CE) (13.6 d) | DTPA** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





