1. Introduction
Prostate tumor is the first most commonly diagnosed tumor in male population with a estimated 1.4 million new diagnoses and 300,000 related deaths worldwide in 2020. Nowadays, the gold standard for its diagnosis is prostate biopsy, which is usually performed after clinical suspicion, initially based on PSA value and digital-rectal examination (DRE). However, the reduced sensitivity of the DRE and the low specificity of the PSA have led to the research of new methods of early and non-invasive diagnosis for prostate cancer (PC) [
1]
Exosomes are extracellular nanovesicles (30-100 nm), carriers of different biomolecules such as lipids, proteins, nucleic acids, secreted from all mammalian cells under normal and pathological conditions. Their role of extracellular messengers is similar to that of a message in a bottle traveling in our body [
2]. Their composition and the fact that their release dramatically increases in case of tumorigenesis, opens different scenarios on the possible application in the research for new biomarkers. The ability of exosomes to transmit their content of lipids, proteins, DNA, mRNA and other metabolites into the target cells, confers a crucial role in intercellular communication and modulation of physiological or pathological processes, including tumor progression. For these reasons exosomes can be consider an ideal source of new and more specific tumor biomarkers. From an analytical point of view, exosomes are not easy to study mainly due to their size. Tumor-derived exosomes can be detected in our fluids and can provide diagnostic or prognostic information [
3]. Pre-clinical evidences showed that the increased levels of plasmatic exosomes are directly related to the presence of a neoplastic mass and that the surgical treatment of the primary tumor led to a significant reduction of their plasmatic levels [
3]. Our group finalized different research on exosome analysis in patients with prostate cancer, obtaining significant results. The purpose of the present editorial is to summarize the methodology used, results obtained and limits found in our personal experience. We will try to underline how research on exosome could allow to obtain markers with higher specificity for neoplastic disease in the prostate but the complexity of the analytical methodology required is an obstacle to overcome. In particular, differently to previous reviews: 1.methodology to be used for exosomes analysis in PC is specifically described; 2. we specifically analyzed how exosome analysis can improve specificity and sensitivity of the major marker used in PC diagnosis, PSA (PSA expressing exosomes); 3. we specifically analyzed the relationship between exosomes and carbonic anhydrase expression in PC.
2. Biomarkers in Prostate Cancer
A biomarker is defined as an indicator of the normal or pathological biological process of a cell or tissue, able to differentiate between the two conditions [
4] The main characteristics of a biomarker are its specificity and sensitivity for the pathologic condition, its usefulness as regards early diagnosis, prognosis and therapeutic indication.
Tumor biomarkers for PC can be obtained from serum, urine, and tissue samples. To date, urine samples are the simplest to obtain from the patient and the most abundant, containing important amounts of proteins, cellular DNA and RNA, as well as cellular metabolites and exosomes, which can represent the basis to obtain a reliable biomarker for PC [
5]. Urine is not associated with proteolytic processes, as it does not cross tissue barriers, and this can represent a relevant advantage in the isolation process when compared to blood [
6].
2.1. Serum biomarkers
Prostate specific antigen (PSA) is a glycoprotein produced mainly by the prostate glandular tissue and it is secreted in the seminal fluid. It is an enzyme belonging to the hydrolase class, structurally similar to kallikrein (also called kallikrein 3). PSA is not a cancer-specific marker but an organ-specific marker, and its levels can be affected by non-neoplastic conditions such as prostatitis and benign prostatic hyperplasia (BPH) [
7]. PSA is considered a continuous parameter, so that elevated PSA levels indicate a higher likelihood of PC and a higher risk of high-grade malignancy. In evaluating PSA for the early diagnosis of PC, the Area Under the Curve (AUC) was found to be 0.68, while the AUC of PSA in discriminating between a clinically significant and non-significant PC is 0.78 [
8].
The 4K score (OPKO Lab, Nashville, TN, USA) considers the serum levels of four kallikrein proteins, including PSA, free PSA, intact PSA and human kallikrein 2 (hK2), combining them with data from the digital rectal examination, age and previous prostate biopsy, through an algorithm that therefore allows to calculate the risk of high-grade PC [
9]. It is recommended for patients who have already performed a previous prostate biopsy and the AUC for the initial diagnosis of PC was between 0.69 and 0.83 [
9].
2.2. Urine biomarkers
The Select MDx test (MDx Health, Irvine, CA, USA) is a Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR) that evaluates the
HOXC6 and
DLX1 mRNA genes levels in urine samples after prostate massage, in correlation with the risk of PC and high grade PC. The AUC for the early diagnosis of high grade PC ranged between 0.86 and 0.90 when combined with clinical parameters [
10].
A biomarker measured in urine after prostate massage is the
Fusion trans-membrane serine protease 2 (TMPRSS2) plus v-ets erythroblastosis virus E26 oncogene homolog (ERG) fusion gene, evaluated by reverse transcription polymerase chain reaction (RT-PCR) and fluorescence in situ hybridization (FISH). Both are located on chromosome 21; TMPRSS2 is a prostate-specific and androgen-response gene expressing a serine protease protein, operating in prostate carcinogenesis and based on gene fusion with ETS transcription factors (ERG and ETV1) [
11]. ERG is an oncogene that encodes for a transcription factor, member of the erythroblast transformation-specific family, which is a key regulator of cell proliferation, differentiation, angiogenesis, inflammation and apoptosis [
11].
The Michigan Prostate Score (MiPS) (University of Michigan, MLabs) is a predictive model that incorporates serum PSA, urinary PCA3 mRNA and urinary
TMPRSS2-
ERG. The sample is collected immediately before prostate biopsy and requires 20-30 ml of urine, obtained after 3 prostate massages. The AUC in PC risk prediction was 0.75 and 0.77 for high-risk PC [
12].
ExoDx Prostate IntelliScore (EPI) urine exosome test (Exosome Diagnostics, Inc., Waltham, MA, USA) analyzes exosomes derived from normalized
PCA3 and ERG RNA in urine specimens without the need of a previous prostate massage [
13]. When EPI is combined with other clinical parameters, reduced the number of useless biopsies by 27% and it provided additional predictive accuracy to detect clinically significant PC, with an AUC of 0.80 [
14].
2.3. Tissue biomarkers
The ConfirmMDx test (MDxHealth, Inc, Irvine, CA, USA) determines the level of methylation of the promoter regions of three genes involved in cell regulation (
APC – adenomatous polyposis coli, RASSF1 – ras association (RalGDS/AF-6) domain family member 1 and GSTP1 – glutathiones-transferase PI1) in prostate tissue. DNA methylation occurs early during the oncogenic cycle and in the presence of cancer lesion, the perilesional tissue undergoes epigenetic modifications, which are detected by the test [
33]. The test is performed using a multiplexed quantitative DNA methylation-specific polymerase chain reaction assay for the 3 genes considered [
15]. The AUC for the prediction of clinically significant PC reached 0.76 [
15].
Prolaris score (Myriad Genetics, Inc., Salt Lake City, UT, USA) analyzes 46 genes (31 genes involved in cellular biology and 15 housekeeping genes by RT-PCR) in order to predict the risk of 10-years disease specific mortality and distant metastasis in PC cases. It is performed on both formalin-fixed paraffin-embedded (FFPE) needle biopsy and FFPE radical prostatectomy tissue samples. The test showed an AUC of 0.878 as regards the prediction of PC, when combined with clinical parameters [
16].
2.4. Exosome-based biomarkers
Extracellular vesicles (EV) include apoptotic bodies, exosomes, microvesicles. To date, many studies are focusing on the development of exosome-based diagnostic tests. Exosomes are small extracellular vesicles originating as cytoplasmic invaginations of the endosomes, produced by a wide range of normal and neoplastic cells and are spilled-over into body fluids, so they are easily detectable in serum, urine or semen samples [
17]. They are subsequently expelled after a mechanism of multivesicular body fusion with the plasma membrane [
18], showing specific markers derived from endosomes, such as tetraspanins, heat shock proteins and compounds of the Rab family [
19]. It is possible to find nucleic acid derivatives (DNA, mRNA, microRNA) in the exosomes and their function is probably a mediation of cell-to-cell communication or microenvironment modulation.
A characteristic phenotype of cancer cells is the development of an acidic microenvironment. This trait, closely linked to a hyperfunction of the proton pumps, guarantees increased survival for cancer cells since normal cells do not survive in an acidic environment. The acidity of the microenvironment significantly increases the release of exosomes by cancer cells. Consequently, in the oncogenic process, there is a significant increase in the spill-over of exosomes by the neoplastic cells, regardless of the histotype of the tumor [
20]. These concepts led to postulate that exosomes may be considered as tumor biomarkers, with potential use in screening, diagnosis and prognosis of the disease.
3. How to measure exosome for prostate cancer characterization
A crucial point in the study of exosomes is represented by their correct isolation. The most widely applied methodologies include ultracentrifugation techniques, size-based techniques, immunoaffinity capture-based techniques (mainly ELISA) and precipitation. To optimize the acquisition of exosomes from the reference sample, more techniques can be applied in sequence for a single extraction, determining an increase in labor time and costs.
The analysis of the obtained EV can be performed by physical analysis techniques (mainly nanoparticle tracking analysis - NTA, and flow cytometry) and compositional analysis techniques (mainly Western blotting) (
Table 1) [
21].
Nanoparticle tracking analysis (NTA), immune captured based technologies, and nanoscale flowcytometry (NFC) represent valid technologies to analyze extracellular vesicles and exosome in samples with quantitative and qualitative information [
2,
3,
22,
23].
Moreover EV at plasma level can be characterized and quantified by an immunocapture-based ELISA (IC-ELISA) test [
2,
22]. For example, the association of these techniques well described the role of PSA-expressing exosomes and they showed that acidic conditions stimulate exosome release from tumor cells [
22,
23].
Comparing the different techniques for detecting plasmatic EV in human fluids, several factors have made IC-ELISA a very interesting tool, including the following:
(a) it is non-invasive; (b) it is rapid, specific, and quantitative; (c) it requires a small quantity of sample, and it is reproducible; (d), it is affordable with reasonable costs in laboratories.
Preparation of plasma to obtain EV requires EDTA-treated blood, extraction of plasma and collection at -80°C, centrifugation in order to eliminate cell debris, organelles and microvesicles. In the last step, plasma samples are centrifuged for 1 h 30 min at 110,000 x g using a Fiberlite™ F50L-24 x 1.5 Fixed-Angle Rotor, K-Factor: 33 in the Sorvall WX Ultracentrifuge Series, to obtain the exosomal pellet, which are then washed in PBS and resuspended in the appropriate buffer for subsequent analyzes.
Nanoparticle Tracking Analysis (NTA) can be used for the measurement of size distribution and concentration of extracellular vesicles samples in liquids [
22]. It allows to capture, detect, characterize and quantify extracellular vesicles in both human body fluids and cell culture supernatants. It is based on the use of two antibodies directed one against a typical exosomal housekeeping protein and the second against either another exosomal housekeeping protein or a potential disease marker: the first antibody is used for the capture of exosomes, the second for the quantification and characterization of the captured vesicles. In fact, with this method it is possible both to characterize and count exosomes and to detect the presence of disease, including tumor, biomarkers. This needs to preliminary obtain an extracellular vesicles purification from the clinical sample and the most recommended method is the repeated rounds of ultracentrifugation, that is the methodological approach allowing to not exclude extracellular vesicles subpopulation from the separation procedure and to analyze a full range of them from both qualitative and quantitative point of view.
Following laser beam illumination, the light scattering allowed to visualize, record and track the particles with a CCD or CMOS camera.
The NTA 3.0 software can first identify and then track each particle on a frame-by-frame basis. NTA is based on the phenomenon of the random movement (diffusion) of small particles when they are dispersed in a liquid, allowing direct and precise measurement of the concentration and size of the particles. The Brownian motion of each particle is tracked using the Stokes–Einstein equation: D= kT/6πηr where D is the diffusion coefficient, kT/6πηr is the frictional coefficient of the particle and T is the absolute temperature.
The
Immnocapture IC-
ELISA test demonstrates clinical potentiality for PSA-exosome evaluation. An antibody specific for a typical exosome antigen (CD81) is used to identify exosomes within the pool of extracellular vesicles, and an antibody for PSA isused for the detection of plasmatic exosomes expressing PSA. Nanovesicles purified from plasma are quantified by Bradford assay and then suspended in a final volume of 50 μL and incubated overnight at 37 °C. After 3 washes with PBS, a mouse anti-PSA HRP-conjugated is added, the reaction is developed with Blue POD for 15 min and blocked with 4N H2SO4 stop solution. Optical densities is recorded at 450 nm and a PSA calibration curve allows to convert the optical densities of each sample into micrograms of Exo-PSA [
22,
23].
In the
Flow Cytometry Analysis of Exosomes, EV purified from plasma are diluted in PBS in a final volume of 50 μL. Anti-human CD81 allophycocyanin (APC) conjugated,anti-human PSA fluorescein (FITC) conjugated and IgG1 FITC are added to the exosome preparation at optimal pre-tittered concentrations. PBS is added to samples before the acquisition on the CytoFLEX flow cytometer [
22,
23]. Also in this analysis, an antibody specific for a typical exosome antigen (CD81) is used to identify exosomes within the pool of extracellular vesicles, and an antibody for PSA is used for the detection of plasmatic exosomes expressing PSA.
The cytometer is calibrated using a mixture of nonfluorescent silica beads and fluorescent (green) latex beads with sizes ranging from 110 nm to 1300 nm. This calibration step enables the determination of the sensitivity and resolution of the flow cytometer (fluorescent latex beads) and the size of extracellular vesicles (silica beads). All samples are acquired at low flow rate for the same amount of time in order to obtain an estimate of absolute counts of exosomes comparable between various samples.
4. PSA-expressing exosomes in PC
In recent years, a critical concept in the field of oncological diseases has been represented by liquid biopsy. Growing interest in this approach is due to the potential to address some of the problems associated with the acquisition of a histological diagnosis, such as the invasiveness of the biopsy procedure and the risk of missing pathological tissue.
In PC cases, exosomes analysis represents an ideal model for liquid biopsy due to their ability to provide valuable information that may overcome some limitations of the commonly used biomarkers.
In two consecutive experiences, Logozzi et al demonstrated that analyzing PSA-expressing exosomes provides an incredibly accurate way to discriminate between healthy patients and those with prostate disease, and even within the latter group, differentiates between patients affected by BPH and PC [
22,
23]. The study enrolled a total of 240 patients, divided into three groups of 80 patients (control, BPH, and PC). For each patient, exosomes were extracted from an EDTA-treated blood sample using centrifugation, and their characterization and quantification were performed using a multiple techniques approach.
Size distribution and concentration of extracellular vesicles in liquid suspension were measured using Nanoparticle Tracking Analysis (NTA) from Malvern. Western Blotting was performed using anti-Tsg101 and anti-CD81 monoclonal antibodies. ELISA for PSA was carried out using rabbit polyclonal anti-CD81 and mouse anti-PSA HRP-conjugated antibodies. Flow Cytometry Analysis of exosomes was performed using anti-human CD81 allophycocyanin-conjugated and anti-human PSA fluorescein-conjugated or anti-IgG2a APC and IgG1 FITC antibodies (
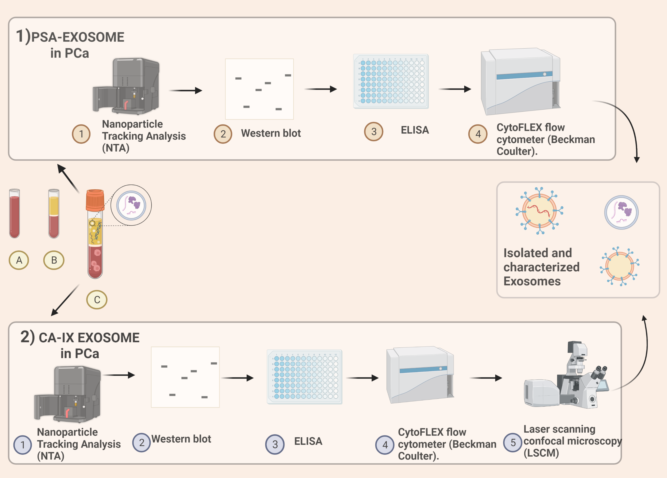
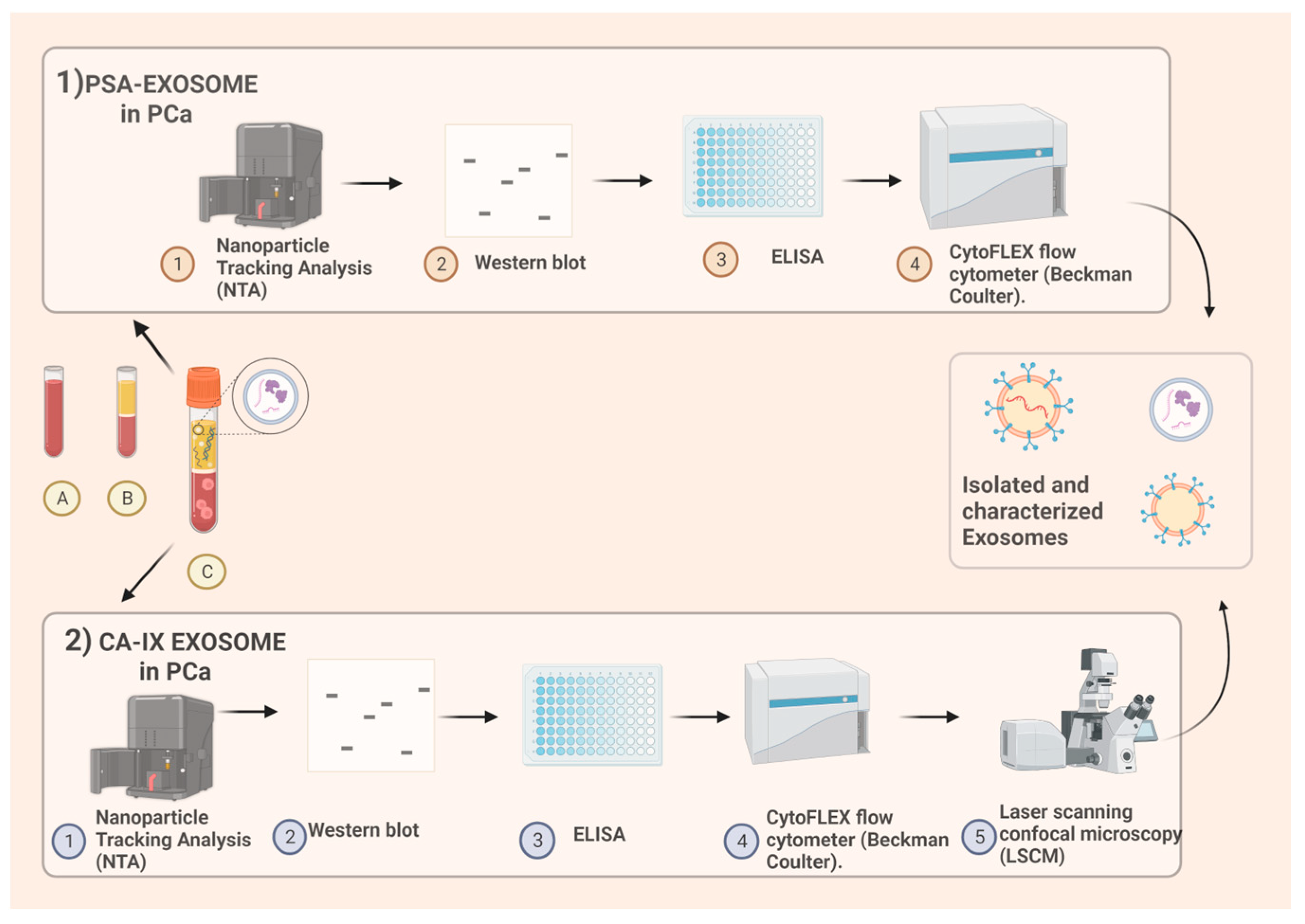
Figure 1).
1) PSA exosomes extraction. From blood samples, after centrifugation, plasma is obtained. Protocol includes nanoparticle tracking analysis (NTA) for quality control of plasmatic samples after the ultracentrifugation, afterwards the use of both nanoscale flow-cytometry and immunocapture-based ELISA for extracellular vesicles characterization and quantification. Immunocapture-based ELISA (IC-ELISA) and NanoScale Flow Cytometry (NSFC), In both the analyses an antibody specific for a typical exosome antigen (CD81) is exploited to identify exosomes within the pool of extracellular vesicles, and an antibody for PSA is used for the detection of plasmatic exosomes expressing PSA.
2) Carbonic-anhydrase IX exosomes extraction. Human plasma samples are collected from EDTA-treated whole blood. Nanoparticle tracking analysis (NTA) is used for size distribution and concentration measurements of exosomes samples in liquid suspension. Western blot analysis is performed. ELISA for CA is obtained and then Exosomal pH is evaluated by Nanoscale Flow Cytometry. Intracellular acidity is analysed by Confocal Microscopy using fluorescent tracers. Figure “Created with BioRender.com”.
The level of specific PSA-exosomes was able to discriminate between PC patients and non-PC patients (BPH and healthy controls), outperforming the conventional serum PSA test. Specifically, IC-ELISA alone method achieved 98.57% sensitivity and 80.28% specificity in discriminating PC from BPH. The combination of IC-ELISA and NFSC led to an increase up to 96% in sensitivity and 100% in specificity [
23].
Moreover, in the same populations, non-specific exosomes were characterized either in terms of number or size distribution by NTA and we showed a significant difference between controls and PC patients exosome plasma samples for both the non-specific concentration and the size parameters (p<0.0001). We detected a significant increase in number of exosomes in PC as well as the shrinking in their size. The standard deviation (SD) of the size distributions were substantially identical in the two population, suggesting a general rigid shift of distribution going from controls to PCs. In general, PC exosomes were not only more numerous but also smaller than the control cases exosomes [
24].
The ROC analysis performed on the combination of the number and size of plasmatic exosomes showed a maximal sensitivity (89%) and specificity (71%) at cut-off = -0.544. This method consents to significantly discriminate (AUROC = 0.86, p < 0.0001) PC patients from healthy cases. At the end, we analyzed the correlation between non-specific and specific exosome-based markers. The specific use based on PSA-expressing exosomes (NSFC-exo) had a statistically significant (but relatively weak) correlation with exosomes number, suggesting that the kind of ‘cancer related’ information provided by both size and number of exosomes is widely independent to the specific (prostate) cancer type. Despite the “non-specific” (no consideration of PSA expression) predictivity is lower than the specific one of PSA exosome, it allows for a very considerable predictive power [
24].
In the context of PC, miRNa-exosomes have been studied extensively since they could play a critical role in the development and even progression of the disease. Different cancer-specific miRNAs have been identified in exosomes obtained from biological fluid samples of PC cases [
25].
Wang et al. provided a comprehensive review of the utility of exosomal miRNA analysis [
25]. They highlighted promising results on blood and urine samples considering miRNAs such as Mir141, Mir-375 and Mir-21, but also Mir-200, Mir574, Mir196A-5P, Mir-501-3p, Mir-2909, Mir-19, Mir-1246 and Lit-7 as potential biomarkers. However, some studies included in this review have yielded inconsistent results, possibly due to a lack of standardization in the exosomal miRNA analysis procedure [
26].
Prostate-specific membrane antigen (PSMA) is a zinc metalloprotein encoded by the folate hydrolase 1 gene (FOLH1) and predominantly expressed on the surface of prostate cells.
In a study conducted by Wang et al., exosome-PSMA derived from urine samples of 247 patients, (194 individuals with BPH and 80 with PC), was analyzed. Exosomes were isolated by centrifugation and subsequently subjected to enzyme-linked immunosorbent assay (ELISA) using a human glutamate carboxypeptidase 2 (FOLH1)/PSMA ELISA Kit. The analysis of urinary PSMA-exosomes revealed a higher diagnostic potential in differentiating PC from BPH , when compared to serum PSA [
27].
5. Exosomes and Carbonic Anhydrase expression in PC
Carbonic anhydrase (CA) IX is a zinc-metalloenzyme, included in the family of α-carbonic anhydrases (αCA) that catalyze the reversible hydration of carbon dioxide to bicarbonate ions and protons [
28]. To date, sixteen human isoforms of αCA have been isolated: five of them have been discovered in cellular cytosol (CA I, II, III, VII, XIII), five are “membrane-related proteins” (CA IV, IX, XII, XIV and XV), two belong to the mitochondria (CA VA and VB), one is secreted in milk and saliva (CA VI), and the least three non-catalytic isoforms have been classify as CA-related proteins (CARP VIII, X and XI). The catalytic- isoforms accomplish many biological functions, including pH regulation and ion transport in many organs [
28]. Of all the isoforms, CA IX is almost uniquely expressed in solid tumors, its presence in non-neoplastic tissues is appreciable exclusively in the gastro-intestinal tract, where it is part of the cell proliferation and differentiation pathways. Cancers are highly active tissues that often contain hypoxic regions and produce large quantities of metabolic acids, so that the overexpression of CA IX in solid tumors is regulated by the hypoxia-inducible factor (HIF)-1 [
29]. Through the constant release of acid molecules, tumor cells create a hostile environment which on the one hand favors tumor growth, on the other it is fatal to the host cells. Increased formation and discharge of protons, lead to severe alterations in intracellular and extracellular pH, with important repercussion for tumour growing and progression. This swap in the pH gradient has been shown to occur at an early stage in malignant transformation pathway [
30]. Extracellular pH acidity seems to support tumor progression through alteration of the “pH-dependent modulation” of adhesion between cell and matrix, degrading the matrix itself by the activation of cathepsins and metalloproteases[
29]. Moreover, an acidic pH has been shown to suppress immunity function, either by a local strong inhibition of chemotaxis or preventing the T-cell activation [
30]. It has been widely demonstrated through in vitro-studies that the cell lines culture exposed to lower pH microenvironment – (6.5) leads to a greater release of exosomes, compared to the same cells cultured at physiological pH (7.4), independently from the tumor histotype [
29,
30].
In the last 10 years there has been an exponential increase of in-vitro studies regarding the possible use of CA IX in multiple tumors. Kengo Horie et al.suggested that CA IX contained in exosomes from cell culture of human renal cell carcinoma (RCC - Caki-1 (JCRB0801), KMRC-, OSRC-2 and 786-O cells) and analyzed with Western Blot and angiogenesis assays, promotes cell migration and tube formation [
31]. PC shares with other solid tumors different biochemical features: hypoxia, acidity, low nutrient supply and low pH microenvironment, all related to CA IX expression. Therefore, the use of this metalloenzyme as a biomarker or target of possible new therapies represents a concrete objective. In a preliminary study of Logozzi et al. [
32], men with histologically confirmed PC and healthy donors (CTR) were compared. They aimed to evaluate the level of CA IX expression in exosomes purified from PC and controls. Exosomes were isolated from plasma samples of PC patients and CTR using ultracentrifugation and characterized through Nanoparticle Tracking Analysis (NTA) (
Figure 1). A subsequent immunocapture-based ELISA assay was performed to quantify and characterize CA IX expression in exosomes. PC patients showed higher plasmatic levels of exosomes than the controls (p<0.1) and exosomes from PC were more homogeneous in size distribution when compared to plasmatic exosomes from controls. CA IX expression was up-regulated in exosomal purification lysates from PC plasma when compared to the exosomal fractions of control plasma. Moreover, authors showed that the CA-activity/mg protein found in exosomes isolated from PC plasma (2.9 ± 0.4) was 2.4-fold higher as compared to exosomes purified from CTR plasma (1.2 ± 0.2) (p<.0001). The results revealed that CA IX positive exosomes were 25-fold higher in plasma samples from PC patients (558 ± 90) than in those from CTR (22 ± 2),(p<.0001) [
32]. This study aimed to demonstrate not only the increased levels of CA IX in exosomes of PC patients but also the direct correlation between the acidic microenvironment, the release of exosomal particles in the extracellular compartment, and the upregulation of CA IX expression and activity.
6. Conclusions
The main limit in the current use of PSA as a routine marker for the early diagnosis of PC, is its lack of specificity in distinguishing between PC and BPH . The organ specificity and not disease specificity of serum PSA, reduces the possibility to differentiate two diseases such as PC and BPH that often coexist in the same patient. In our clinical practice we very often evaluate patients who have significant BPH related PSA elevations in the absence of a PC. On this point, the use of exosome detection can improve the specificity of PSA as marker for the early diagnosis of PC. The analysis of PC-linked EV through liquid biopsy represents a promising diagnostic model that can effectively distinguish patients with PC from those with non-malignant prostatic disease or healthy individuals. Various biomolecules characteristic of different exosomes have emerged as potential biomarkers for PC, sustaining their potential use in clinical practice. Notably, the analysis of PSA-exosomes obtained from blood samples has demonstrated remarkable sensitivity and specificity when compared to traditional serum PSA analysis. Quantitative and qualitative analysis, defined as the search for EV expressing specific antigens, represents a promising frontier in the search for new and reliable diagnostic and disease progression biomarkers that could innovate the current clinical practice. We also suggest that more simply, plasmatic exosomes from PC cases are more numerous and smaller in size as compared to thos in healthy subjects. The NTA analysis performed on the combination of the size and number of plasmatic exosomes can significantly distinguish between PC and BPH cases. However, to date, there are only a few studies conducted on isolated cohorts of patients. The use of exosome analysis in clinical practice is currently limited by several issues, including a lack of standardization in the analytical process and high costs, which are still too high for a large-scale use. In the presence of absolutely promising results, the real obstacle for the diffusion of this analysis in clinical practice is a methodology that requires high costs and prolonged times. The characteristics of exosomes make analytical simplification difficult. It is reasonable to anticipate that future research will continue to explore this new area of medicine, leading to the development of new methodologies that will enable liquid biopsy through exosomes analysis to become a routine diagnostic tool in PC diagnosis.
Author Contributions
Stefano Salciccia, Marco Frisenda, Giulio Bevilacqua, Luca Gobbi, Bruno Bucca, Martina Moriconi, Pietro Viscuso, Alessandro Gentilucci, Gianna Mariotti, Susanna Cattarino, Flavio Forte , Stefano Fais, Mariantonia Logozzi , Beatrice Sciarra, Alessandro Sciarra: all authors contribute in the collection of data and development of the manuscript. In particular: Stefano Fais, Mariantonia Logozzi, Alessandro Sciarra and Alessandro Gentilucci performed the previous clinical trials referred as References [
2,
22,
23,
31,
32].
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All the authors declare no conflict of interest.
References
- Dall’Era, M.A.; Albertsen, P.C.; Bangma, C.; Carroll, P.R.; Carter, H.B.; Cooperberg, M.R. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012, 62, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Salciccia, S.; Capriotti, A.L.; Laganà, A.; Fais, S.; Logozzi, M.; De Berardinis, E.; Sciarra, A. Biomarkers in Prostate Cancer Diagnosis: From Current Knowledge to the Role of Metabolomics and Exosomes. Int J Mol Sci. 2021, 22, 4367. [Google Scholar] [CrossRef] [PubMed]
- Properzi, F.; Logozzi, M.; Fais, S. Exosomes: the future of biomarkers in medicine. Biomark Med. 2013, 7, 769–778. [Google Scholar] [CrossRef] [PubMed]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005, 93, 387–391. [Google Scholar] [CrossRef]
- Eskra, J.N.; Rabizadeh, D.; Pavlovich, C.P.; Catalona, W.J.; Luo, J. Approaches to urinary detection of prostate cancer. Prostate Cancer Prostatic Dis. 2019, 22, 362–381. [Google Scholar] [CrossRef]
- Harpole, M.; Davis, J.; Espina, V. Current state of the art for enhancing urine biomarker discovery. Expert Rev Proteomics. 2016, 13, 609–626. [Google Scholar] [CrossRef]
- Sarwar, S.; Adil, M.A.M.; Nyamath, P.; Ishaq, M. Biomarkers of Prostatic Cancer: An Attempt to Categorize Patients into Prostatic Carcinoma, Benign Prostatic Hyperplasia, or Prostatitis Based on Serum Prostate Specific Antigen, Prostatic Acid Phosphatase, Calcium, and Phosphorus. Prostate Cancer. 2017, 20, 125–134. [Google Scholar] [CrossRef]
- Semjonow, A.; Brandt, B.; Oberpenning, F.; Roth, S.; Hertle, L. Discordance of assay methods creates pitfalls for the interpretation of prostate-specific antigen values. Prostate Suppl. 1996, 7, 3–16. [Google Scholar] [CrossRef]
- Zappala, S.M.; Scardino, P.T.; Okrongly, D.; Linder, V.; Dong, Y. Clinical performance of the 4Kscore Test to predict high-grade prostate cancer at biopsy: A meta-analysis of us and European clinical validation study results. Rev Urol. 2017, 19, 149–155. [Google Scholar]
- Farha, M.W.; Salami, S.S. Biomarkers for prostate cancer detection and risk stratification. Ther Adv Urol. 2022, 14, 174–186. [Google Scholar] [CrossRef]
- Yu, J.; Yu, J.; Mani, R.S.; Cao, Q.; Brenner, C.J.; Cao, X. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010, 17, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Porzycki, P.; Ciszkowicz, E. Modern biomarkers in prostate cancer diagnosis. Cent European J Urol. 2020, 73, 300–306. [Google Scholar] [PubMed]
- Nilsson, J.; Skog, J.; Nordstrand, A.; Baranov, V.; Mincheva-Nilsson, L.; Breakefield, X.O. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009, 100, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Shaba, E.; Vantaggiato, L.; Governini, L.; Haxhiu, A.; Sebastiani, G.; Fignani, D. Multi-Omics Integrative Approach of Extracellular Vesicles: A Future Challenging Milestone. Proteomes. 2022, 10, 125–136. [Google Scholar] [CrossRef] [PubMed]
- McKiernan, J.; Donovan, M.J.; O’Neill, V.; Bentink, S.; Noerholm, M.; Belzer, S.; et al. A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA Oncol. 2016, 2, 882–889. [Google Scholar] [CrossRef]
- Stewart, G.D.; Van Neste, L.; Delvenne, P.; Delrée, P.; Delga, A.; McNeill, S.A.; et al. Clinical utility of an epigenetic assay to detect occult prostate cancer in histopathologically negative biopsies: results of the MATLOC study. J Urol. 2013, 189, 1110–1116. [Google Scholar] [CrossRef]
- Li, M.; Zeringer, E.; Barta, T.; Schageman, J.; Cheng, A.; Vlassov, A.V. Analysis of the RNA content of the exosomes derived from blood serum and urine and its potential as biomarkers. Philos Trans R Soc Lond B Biol Sci. 2014, 369, 275–288. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015, 4, 270–276. [Google Scholar] [CrossRef]
- van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer. 2022, 21, 56–64. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019, 8, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. Immunocapture-based ELISA to characterize and quantify exosomes in both cell culture supernatants and body fluids. Methods Enzymol. 2020, 645, 155–180. [Google Scholar]
- Logozzi, M.; Angelini, D.F.; Giuliani, A.; Mizzoni, D.; Di Raimo, R. Increased Plasmatic Levels of PSA-Expressing Exosomes Distinguish Prostate Cancer Patients from Benign Prostatic Hyperplasia: A Prospective Study. Cancers. 2019, 11, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Mizzoni, D.; Di Raimo, R.; Giuliani, A.; Maggi, M.; Sciarra, A.; Fais, S. Plasmatic Exosome Number and Size Distinguish Prostate Cancer Patients From Healthy Individuals: A Prospective Clinical Study. Front Oncol. 2021, 11, 727317. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal miRNAs as Biomarkers for Prostate Cancer. Front Genet. 2013, 4, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ni, J.; Beretov, J.; Thompson, J.; Graham, P.; Li, Y. Exosomal microRNAs as liquid biopsy biomarkers in prostate cancer. Crit Rev Oncol Hematol. 2020, 145, 102860. [Google Scholar] [CrossRef]
- Wang, C.B.; Chen, S.H.; Zhao, L.; Jin, X.; Chen, X.; Ji, J. Urine-derived exosomal PSMA is a promising diagnostic biomarker for the detection of prostate cancer on initial biopsy. Clin Transl Oncol. 2023, 25, 758–767. [Google Scholar] [CrossRef]
- Ambrosio, M.R.; Di Serio, C.; Danza, G.; Rocca, B.J.; Ginori, A.; Prudovsky, I. Carbonic anhydrase IX is a marker of hypoxia and correlates with higher Gleason scores and ISUP grading in prostate cancer. Diagn Pathol. 2016, 11, 45–49. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Beasley, N.J.; Watson, P.H.; Turner, K.J.; Pastorek, J.; Sibtain, A. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000, 60, 7075–7083. [Google Scholar]
- Becker, H.M. Carbonic anhydrase IX and acid transport in cancer. Br J Cancer. 2020, 122, 157–167. [Google Scholar] [CrossRef]
- Horie, K.; Kawakami, K.; Fujita, Y.; Sugaya, M.; Kameyama, K.; Mizutani, K. Exosomes expressing carbonic anhydrase 9 promote angiogenesis. Biochem Biophys Res Commun. 2017, 492, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Mizzoni, D.; Capasso, C.; Del Prete, S.; Di Raimo, R.; Falchi, M.; Sciarra, A. Plasmatic exosomes from prostate cancer patients show increased carbonic anhydrase IX expression and activity and low pH. J Enzyme Inhib Med Chem. 2020, 35, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Spugnini, E.; Mizzoni, D.; Di Raimo, R.; Fais, S. Extracellular acidity and increased exosome release as key phenotypes of malignant tumors. Cancer Metastasis Rev. 2019, 38, 93–101. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).