Introduction
Prostate cancer (PCa) is the most common cancer in men in the Western world [
1]. Despite a definitive treatment in form of either Radical Prostatectomy (RP) or radiation, many men later develop recurrence of prostate-specific antigen (PSA) with no evidence of disease on conventional imaging; called as biochemical recurrence (BCR) [
2]. In literature, up to 53% men with prostate cancer, have biochemical recurrence of PSA after radical treatments [
3].
Although, an increase of PSA-level can indicate the progression of PCa, it cannot localize the clinical recurrence. Early detection of a recurrent disease provides possibility of treatment with curative intent. Morphological imaging modalities (e.g. computed tomography (CT), magnetic resonance imaging (MRI) have limited value in detection of PCa lesions (metastases or recurrences) due to their low sensitivity and specificity [
4].
Recently, PSMA-PET imaging is replacing conventional imaging for evaluation of biochemically recurrent PCa based on its superior sensitivity and specificity. At present, several PSMA tracers are available for clinical use, including tracers labelled with
68Ga or
18F.
18F labelled tracers are considered more beneficial as compared to
68Ga, especially due to the lower kinetic properties of emitted positron and longer half-life. Nevertheless, high detection rates for recurrent lesions are documented for both
68Ga and
18F labelled tracers [
5]. As a result, PET imaging with PSMA tracers for prostate cancer has found its way into standard clinical practice and is already incorporated in European and national guidelines.
However, the studies till now have focused on the diagnostic performance of PSMA PET CT and not their effect on care pathways. There are no trials indicating that early detection of recurrence by PSMA PET CT has changed the management and improved patient care[
6].
Ours is the first evaluation of PSMA PET CT scans being done to study its impact on clinical management and its implications which can lead to possible changes in referral criteria.
Results
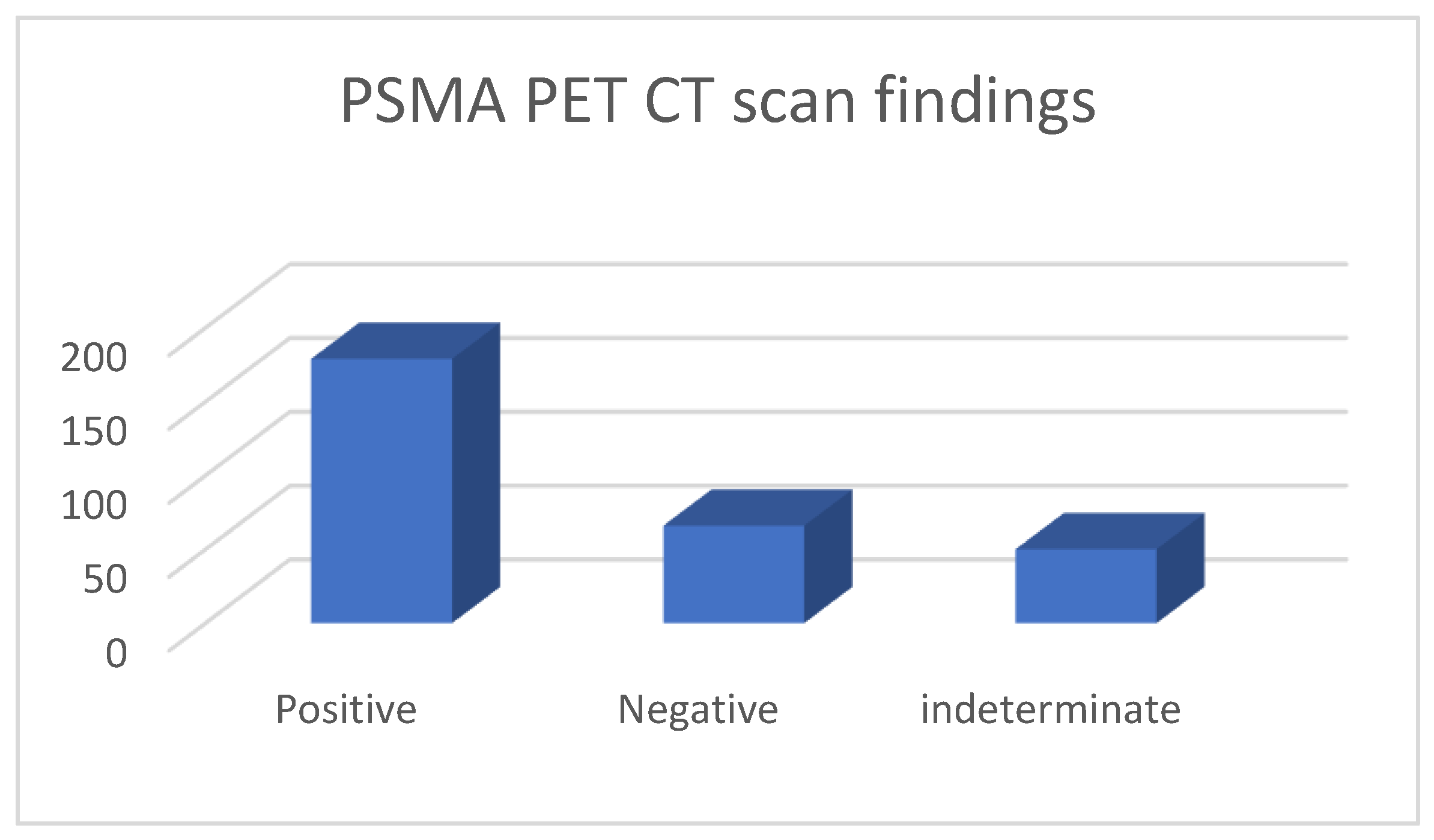
These 295 PSMA PET CT were done for 229 patients; 57 patients had PSMA PET CT done twice, 3 had it thrice and 1 patient had it four times; due to various reasons (indeterminate findings on a prior scan or further rise in PSA levels following prior negative PSMA scan and imaging with MRI, CT). Of the 295 PET CT scans, 179 were considered positive, 66 were negative and 50 had indeterminate findings (graph 1).
In these scans being evaluated for BCR, the most common T stage; either post operatively in case of Radical Prostatectomy or Radiologically was T3 (
Table 1) among all groups. Among the positive group of 179 scan, PSA range at the time of scan request was 0.18-99.7 ng/ml, most common Gleason’s score was 7, 67 (37.3%) scans had Radical Prostatectomy status (RP) and rest received Radiotherapy. Among the negative and indeterminate group, the PSA range was understandably lower than the positive group with PSA range 0.08-3.5 ng/ml among negative group and 0.14 to 6.5 among the indeterminate group. In the negative group 92.4 % had RP status, 5 received only Radiotherapy while in 22 scans, patents had received additional salvage radiotherapy to prostate bed, pelvic nodes after RP. In the indeterminate group 28 had RP status, 22 received only radiotherapy, while 12 received salvage radiotherapy following RP.
In 179 positive scans, highest number of positive findings were seen in lymph nodes with pelvic lymph nodes (112/179 scans) being most involved; 35 only had pelvic lymph nodes, 6 had only pelvic and retroperitoneal lymph nodes, and in rest pelvic lymph node were involved in various combinations along with involvement of prostate bed, bones and soft tissue. Among this positive PET CT group (
Table 3); 67 had radical prostatectomy and in these PSMA avid lesions were seen most in pelvic lymph nodes with only pelvic lymph node in 33, prostate bed and pelvic lymph nodes in 4, pelvic, retroperitoneal lymph nodes along with bones in 13 each, only bone involvement in 1 and only soft tissue involvement (lungs) in followed by pelvic, retroperitoneal lymph nodes along with bones (5 scans), prostate bed along with pelvic nodes (4 scans), only bones (2 scans) and bone with soft tissue involvement (1 scan). In 112 positive PET CT scans, in non-radical prostatectomy group, 25 had recurrence only in prostate, 17 had recurrence involving prostate and seminal vesicles (i.e. prostate bed) only; 28 had no recurrence in prostate gland and only pelvic lymph nodal (2 scan), pelvic and retroperitoneal lymph nodal (6 scans) or systemic recurrence were present (20 scans, bone, visceral metastases); while 42 patients had recurrence in prostate as well as extra prostatic sites. Overall, in this non-prostatectomy recurrence group, in 75 % population (84/112 scans) prostate gland was found to harbor a PSMA avid lesion while in 25% population recurrence did not involve prostate gland.
In 50 PET CT scans with indeterminate findings, majority (41/50 scans) of indeterminate findings were seen in small pelvic or retroperitoneal lymph nodes or skeletal regions (ribs/others) and only in 9 patients indeterminate focus was seen in the prostate bed only.
We analysed our scan findings based on PSA levels at the time of request for scan. We found that PSA levels ≥ 0.5ng/ml resulted in higher number of PSMA avid lesions (both positive or indeterminate uptake) on scan (184/229 times); while PSA <0.5 ng/ml was more associated with negative scan findings (48/66 times), however this observation were not found to be statistically significant. Similar observations were also noted for PSA ≥ 1ng/ml vs PSA <1 ng/ml (p value 0.05,
Table 2).
On separate analysis of patients having prostate gland at the time of scan (i.e non Radical Prostatectomy group), we found that in patients having prostate gland, PSMA PET CT showed a tracer avid lesion (positive or indeterminate) if PSA level ≥ 0.71. Further, in this group majority were having tracer avid focus in prostate gland either alone or in various combinations of seminal vesicle, lymph nodal and systemic involvements.
We did a separate subgroup analysis of 61 patients (
Table 4) who had repeat imaging. 30 had indeterminate findings on initial PSMA PET CT and the findings changed to positive lesion in 14 (10 with known small indeterminate pelvic lymph node and 4 with known indeterminate prostate focus, PSA increase of 0.7 to 3.0 in 6 months), negative in 8 (4 with pelvic lymph node, 4 with uptake in bones on prior PET CT, PSA increase of 0.13 - 2), 6 patient had same indeterminate small pelvic lymph node, 2 had same indeterminate retroperitoneal lymph node (PSA increase of 0.4 to 2.5). Overall, follow up PSMA PET CT was able to conclude in 24/30 patients on follow up. 31 patients who had repeat PSMA PET CT scans done for further rise in PSA levels (PSA rise range 0.3-10) 24 had new lesions (prostate lesion in 4, prostate and seminal vesicle involvement in 4, pelvic/retroperitoneal lymph nodes in 12, new bone lesion in 4), 8 had negative scan even on repeat imaging (PSA rise 0.3-2.3).
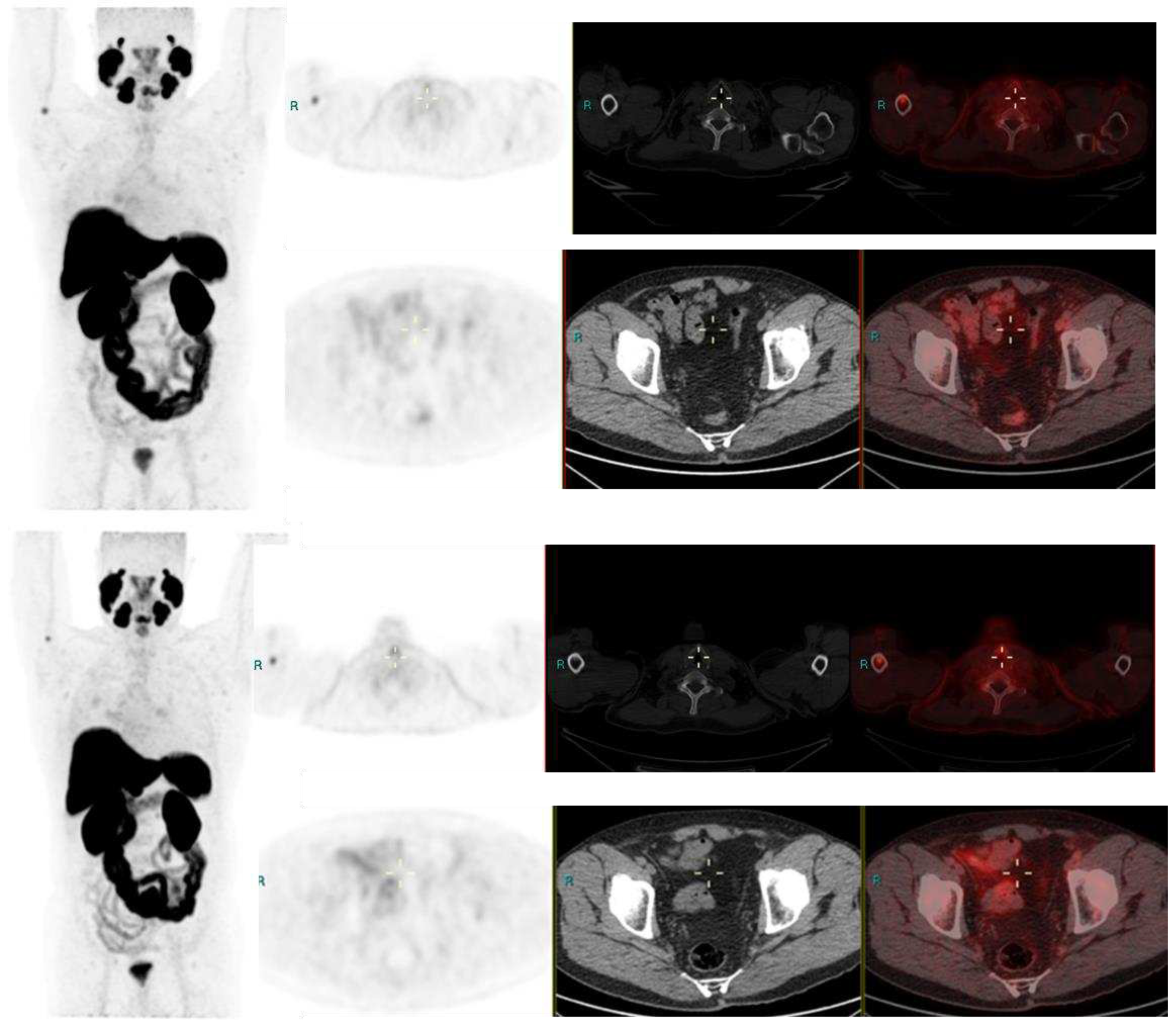
Image 1.
Radical Prostatectomy - Negative scan at PSA 2.1 and 3.4 ng/ml, PSA rise of 1.3 ng/ml in after 6 months. PSMA RADS 1 B, due to uptake in prior known humeral fracture.
Image 1.
Radical Prostatectomy - Negative scan at PSA 2.1 and 3.4 ng/ml, PSA rise of 1.3 ng/ml in after 6 months. PSMA RADS 1 B, due to uptake in prior known humeral fracture.
Image 2.
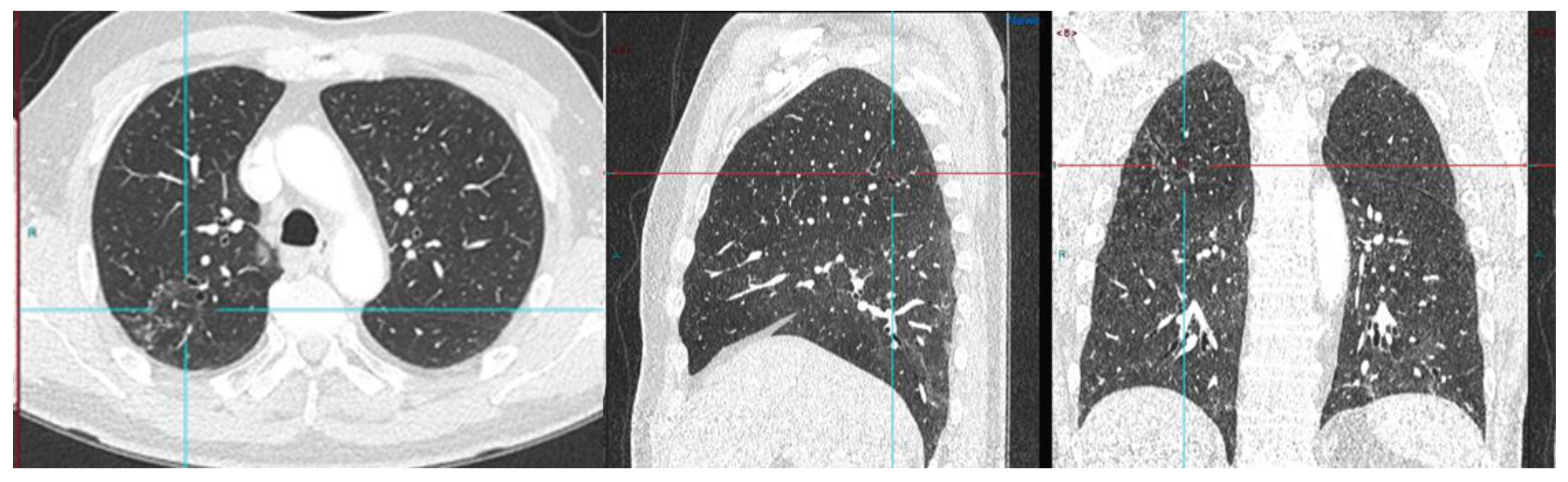
Radical Prostatectomy negative scan at PSA 3.5 ng/ml, indeterminate lymph node at PSA 6.5, rise of 3ng/ml in 2 years RADS 3A – RP status. Consolidation in right upper lobe. Resolved on follow up CT after 2 months.
Image 2.
Radical Prostatectomy negative scan at PSA 3.5 ng/ml, indeterminate lymph node at PSA 6.5, rise of 3ng/ml in 2 years RADS 3A – RP status. Consolidation in right upper lobe. Resolved on follow up CT after 2 months.
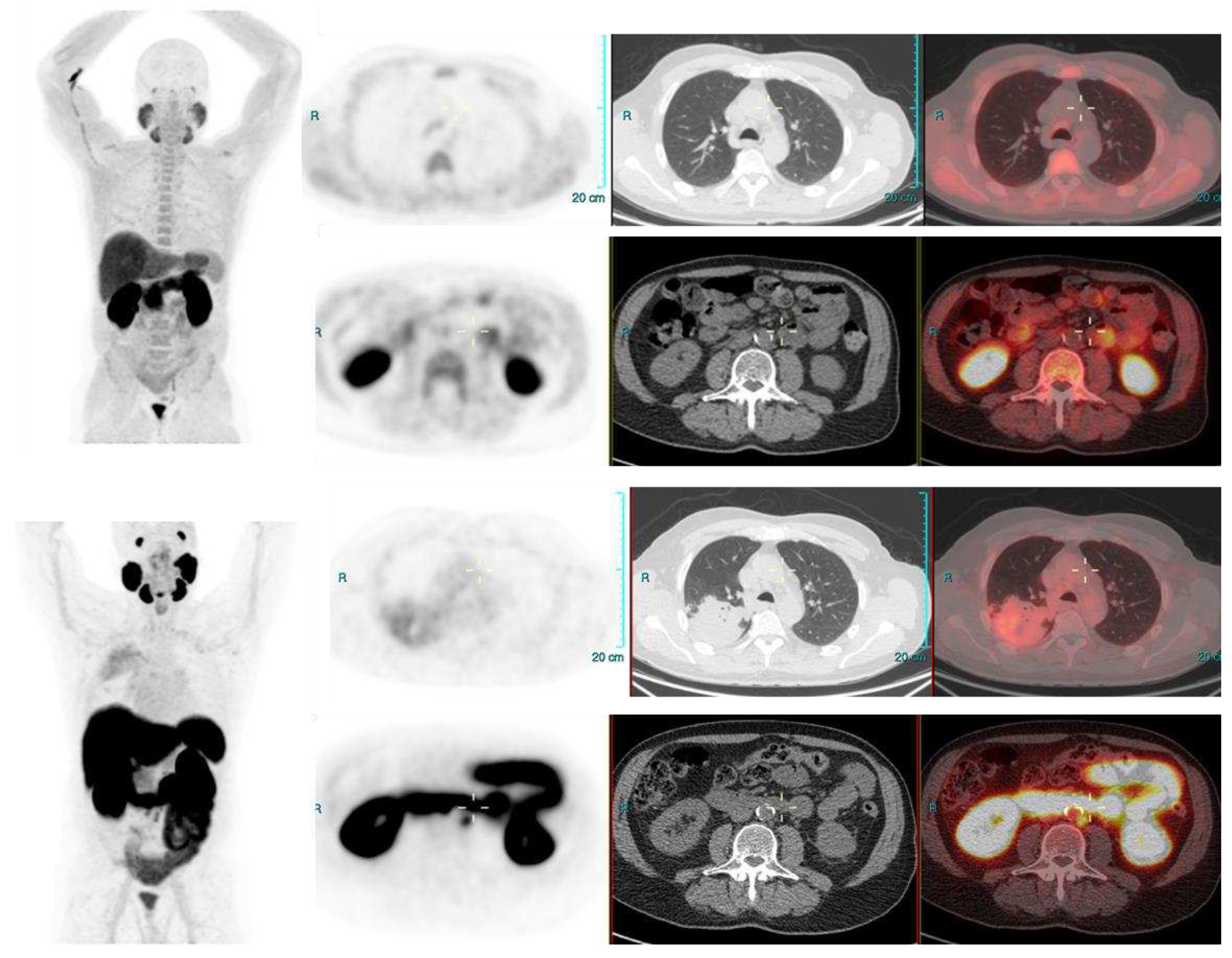
Image 3.
PSA 0.9 ng/ml. Positive lymph node metastasis and suspicious uptake in prostate.
Image 3.
PSA 0.9 ng/ml. Positive lymph node metastasis and suspicious uptake in prostate.
Discussion
PSMA-PET CT is being increasing used in clinical practice and is slowly becoming indispensable in care of many PCa patients by overcoming the challenges of low sensitivity and specificity of conventional imaging modalities. Studies focusing on diagnostic performances of PSMA PET CT in BCR have reported high detection rates (75-81%) in detecting PSMA positive lesions. Xing Zhoe et al, while evaluating BCR in patient following RP have found high detection rate for patients after radical prostatectomy. In their study 79% patients showed at least one pathological finding on
18F-PSMA-1007 PET/CT [
8]. Treglia G et al, performed a systematic review and meta-analysis for detection rate of
18F-labeled PSMA PET/CT in BCR of PCa and found a pooled diagnostic rate of 81% for
18F-labeled PSMA PET/CT [
9]. Similarly, in our study we were able to detect PSMA avid lesions in 229/295 scans (77%) which included both positive and indeterminate lesions as per PSMA RADS criteria.
In our study, the mean PSA levels in positive PSMA group was higher than the indeterminate group and negative group, indicating higher detection rates at high PSA levels. The available literature also suggests higher detection rated at higher PSA levels. Xing Zhoe et al, reported higher detection rates in their population group with rising PSA levels, 50% detection rates at PSA levels ≤0.5 ng/ml and 10%% at >2.0 ng/mL. Similarly, the pooled diagnostic rate for detecting recurrent lesion in systemic review by Treglia G et al for 18 FSMA PET CT was 86% for PSA ≥ 0.5 ng/mL (95% CI: 78-93%) and 49% for PSA < 0.5 ng/mL (95% CI: 23-74%). Geisel et al in their studies for BCR of PCa found good diagnostic rate of 18 F-PSMA-1007 PET CT and also found it to be related to serum PSA values. They concluded that higher PSA values were associated with higher diagnostic rate of 18 F PSMA PET CT. [
10,
11].
A few articles have evaluated the detection rate of 18 F PSMA PET CT at different serum PSA levels. A metanalysis by Ferrari et al, showed pooled diagnostic rate was 51% (95% CI: 29–73%) for PSA values <0.5ng/mL and 88% (95% CI: 77–96%) for PSA values ≥0.5ng/mL, and the difference among these subgroups was statistically significant [
12]. Similar findings have also been observed by Calais J et al while evaluating BCR of PCa with 68Ga-PSMA-11PET CT also [
13]. In our study also, 18F PSMA PET CT was able to detect tracer avid lesions even at very low PSA levels; a tracer avid lesion could be seen in indeterminate group at low PSA levels of 0.14 ng/ml and a definite positive lesion could be found on PSA level as low as 0.18 ng/ml. The high detection rates even at very low PSA levels, seen in our study are in sync with the available literature.
Further, in our analysis a PSA value of even ≥1ng/mL, was not found to be statistically significant for finding a tracer avid lesion in patients with BCR. We attribute this observation as true reflection of real world scenario where a mixture of patient populations with various T,N stages are referred and the PSA levels might not be most recent. Nevertheless, we still could draw some inferences of practical importance from our analysis; we observed that following radical prostatectomy, a PSA of less than 0.08 ng/ml would result in a negative scan and a negative PSMA PET CT could be seen upto PSA 3.5ng/ml while evaluating biochemical recurrence. Similarly indeterminate findings could be seen in patients with PSA levels of upto 6.5 ng/ml while evaluating BCR, which would most commonly be seen in lymph nodes. In patients having prostate gland, PSMA PET CT was positive if PSA level ≥ 0.71. Further, in this group majority would have positive focus in prostate gland either alone or in various combinations of seminal vesicle, lymph nodal and systemic involvements.
In our study, most commonly positive findings were seen in lymph nodes with pelvic lymph nodes being most commonly involved with rest of structural involvement being less in other combinations. Similar observations have been seen in the previous studies [
14,
15,
16]. Giesel FL et al while evaluating lymph nodal recurrence of prostate cancer found mean volume of lymph node was 0.5 ml. In 33% of their study population at least one node was larger than conventional criteria for morphological positivity. In their study 36 % patients had lymph nodes ≥8 mm and in patients with PSMA positive lymph nodes (67%) none of the PSMA-positive lymph nodes met the morphological criteria for positivity. In their study due to subcm, PSMA avid lymph nodes, the N stage was changed from N0 to N1 in 67 % of cohort on
68Ga-PSMA ligand PET CT [
14]. Rahbar K et al [
15], in their study while evaluating diagnostic performance of
18F-PSMA-1007 in patients with biochemical recurrent prostate cancer found, the patient-based sensitivity to be 95%. In their analysis also maximum number of recurrences were found in lymph nodes. They found a total, 213 lesions characteristic of PCa of which 37 were local relapses, 107 lymph node metastases, 67 bone metastases and 2 soft tissue metastases. In 29 patients’ relapse was seen exclusively in lymph node metastases. Sprute K et al, in their study of staging of prostate carcinoma in primary and biochemical recurrence found that in 34.4% of the cohort, positive lymph nodes were present on imaging. In their study the patient-based analysis showed a sensitivity of 85.9% and a specificity of 99.5% for lymph nodes larger than 3 mm [
16]. We did not evaluate further the pattern of distant spread, correlation with pathology, PSA dynamics, levels in our current study as these have been studied in earlier studies with few showing their significances and few not, and further as this was not the aim of our study.
In our study we found that approximately one third of time (31.5 %) a tracer avid lesion was seen in the prostate gland in case of BCR. Multiple other studies performed for biochemical recurrences have suggested local recurrence from 23% to as high as more than 65% involving various patient populations. Ahmadi et al, while evaluating biochemical recurrence in a mixed population post brachytherapy, high-intensity focused ultrasound (HIFU), and RP found 80 % positivity rate. In their study group 23 % showed local recurrence, 43 % showed involvement of lymph nodes and 33%, 11 % showed bone, soft tissue lesions. No multivariable model could be constructed for predictors of overall scan positivity; their findings were in concordance with our observations [
17]. Mingels, C et al, while evaluating biochemical recurrence, reported high positivity rate of 91% and they also observed increased PET positivity with rising PSA levels similar to our study. On a region basis in their study, PSMA avid lesions were detected in the prostatic fossa in 51%, in pelvic LN in 48%, in retroperitoneal LN in 23%, in supradiaphragmatic LN in 16%, in bones in 53%, and in other metastasis (soft tissue lesions) in 7% population [
18].
Further in non-prostatectomy group with biochemical recurrence, 75% of scans showed definite recurrence involving prostate. A few studies focussing on BCR in post radiotherapy setting have also reported similar high local recurrence detection rate of ranging from 48 to about 64 % following radiation therapy [
19,
20,
21]. Hruby et al, in their study observed 31% men had recurrence within the gland, 11 % in lymph node, 3 % in bone and 1% in both. On further analysis, they have found 17% population had an isolated located recurrence and interestingly it represented 2% of patients managed who were manged with definitive EBRT and followed for at least 2 years[
19]. Einspieler I et al, in their study of BCR evaluation using hybrid 68 Ga PSMA PET CT in post radiotherapy patient found approximately 91 % patients showed tumor recurrence, with higher positivity rate on higher PSA levels in this population. They found approximately 64 % population had local recurrence, distant lesions were seen in approximately 60 % population while approximately one fourth of population in their study group had both local recurrence as well as distant lesions [
20].
Unfortunately, the high local recurrence have not yet translated into directed interventions towards reducing local recurrences. We feel that it should be evaluated and discussed in further studies. A higher recurrence in prostate bed while evaluating biochemical recurrence provokes us to think if prostatectomy be offered more proactively?
Further, our study reflects the importance of follow up PSMA PET CT for characterization of indeterminate findings of PSMA PET CT. In follow up, findings turned definite in 22/30 patients (73.3 %) with 14 patients having definite positive uptake (PSA rise 0.7 -3.0 ng/ml in 6 months) and 8 patients having definite negative uptake (PSA rise 0.13-2 ng/ml in 6 months) in prior indeterminate lesions. The PSA velocity was not considered statistically significant in our study, and this could be due to small number of this group in our study population. Our study findings of few patients PSMA PET CT scan turning positive later on with further rise of PSA levels, can be explained based on observations of Jia J et al and Bashir et al. In their study Jia J et al found negative 18 F PSMA PET CT results in 22/114 patient. 7 of these received ADT before and after 18 F PSMA PET CT scan. 15 patients were followed on PSA value and did not receive any treatment. The patients, who were treated with ADT, showed an early response of PSA; but PSA levels further increased in patients who were followed up without receiving any treatment [
21].
Similarly, Bashir et.al found 11 of 28 BCR patients had negative
18F-PSMA PET CT results after the initial PCa surgery. 3 patients in their study group received prostate bed salvage radiotherapy and 8 patients did not receive any treatment and were only followed up PSA. The PSA level of all patients receiving treatment decreased, and the PSA level of follow-up patients increased. From their studies it can be understood that that a negative PSMA PET CT in cases of BCR following radical treatment, can lead to underestimation of local recurrence. This is further proven by the fact that few patients who received further treatment despite negative PSMA PET CT had reduction in PSA levels on follow up [
22]. Similar significances of a negative PSMA-PET/CT have been suggested by Emmett et al while evaluating treatment outcomes of salvage radiation treatment in men with rising PSA after radical prostatectomy; their study showed high PSA-response rates of about 85% to salvage radiotherapy in patients who received stereotactic radiotherapy to pelvis despite negative PSMA-PET CT, suggesting pelvis-confined disease in the majority of patients with a negative PSMA-PET/CT, especially within the prostatectomy bed. They further commented that a false negative PSMA PET CT, could be due to small lesions getting obscured in very close proximity to high urinary PSMA activity. In our study, the urinary activity was not considered a significant factor due to use of 18 F labelled PSMA tracer which is considered superior in localising local disease[
23].
In 30 patients who had repeat PSMA PET CT to monitor small volume positive disease, 24 patients had new lesions with 4 patients having new uptake in prostate (average PSA rise 2.1), 4 having new lesion in prostate bed (average PSA rise 3), 12 having new pelvic lymph node (average PSA rise 3.8) and 4 having new bone lesions (average PSA rise 5). Eight patients had negative PSMA PET CT on repeat imaging also (PSA rise 0.3-2.3). Although, a higher PSA rise was seen in patients with new PSMA avid lesion, we were unable to draw a statistical significance of this small number.
Our present study has few limitations. One of the limitations is the retrospective nature of analysis and another is lack of histological proof of our scan findings as most of the findings, particularly the lymph nodes could not be histologically confirmed. Unfortunately, this problem is commonly observed in radiological studies evaluating BCR. As most of the lesions detected on scans are located deep in pelvis and are not easily accessible, pathological confirmation of most of the scan findings is difficult. However, due to use of standardized PSMA RADS criteria, we seem to have at least partially overcome this limitation as compared to earlier studies evaluating BCR. PSMA RADS criteria, reflect the likelihood of the presence of PCa; PSMA RADS-1, 2 lesions are either certainly or almost certainly benign, PSMA-RADS-4 indicates a high likelihood PCa presence and PSMA-RADS-5 lesions almost certainly represent PCa, we seem to have achieved a fair amount of certainty towards our findings.
Overall, our findings are significant as they reflect a real-world scenario of care in a tertiary care centre and we believe our findings can lead to definite changes in management and referral criteria. We believe further that prospective studies would be helpful.