Submitted:
18 May 2023
Posted:
19 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis of Principal Components (PCA)
2.2. Margarines and Plant Material
2.3. Study of Oxidative Stability
2.4. Ferric Reducing Ability of Power (FRAP) Assay in Plants
2.5. Total Phenolic Content (TPC) of Plants
2.6. Statistical Analysis
3. Results and Discussion
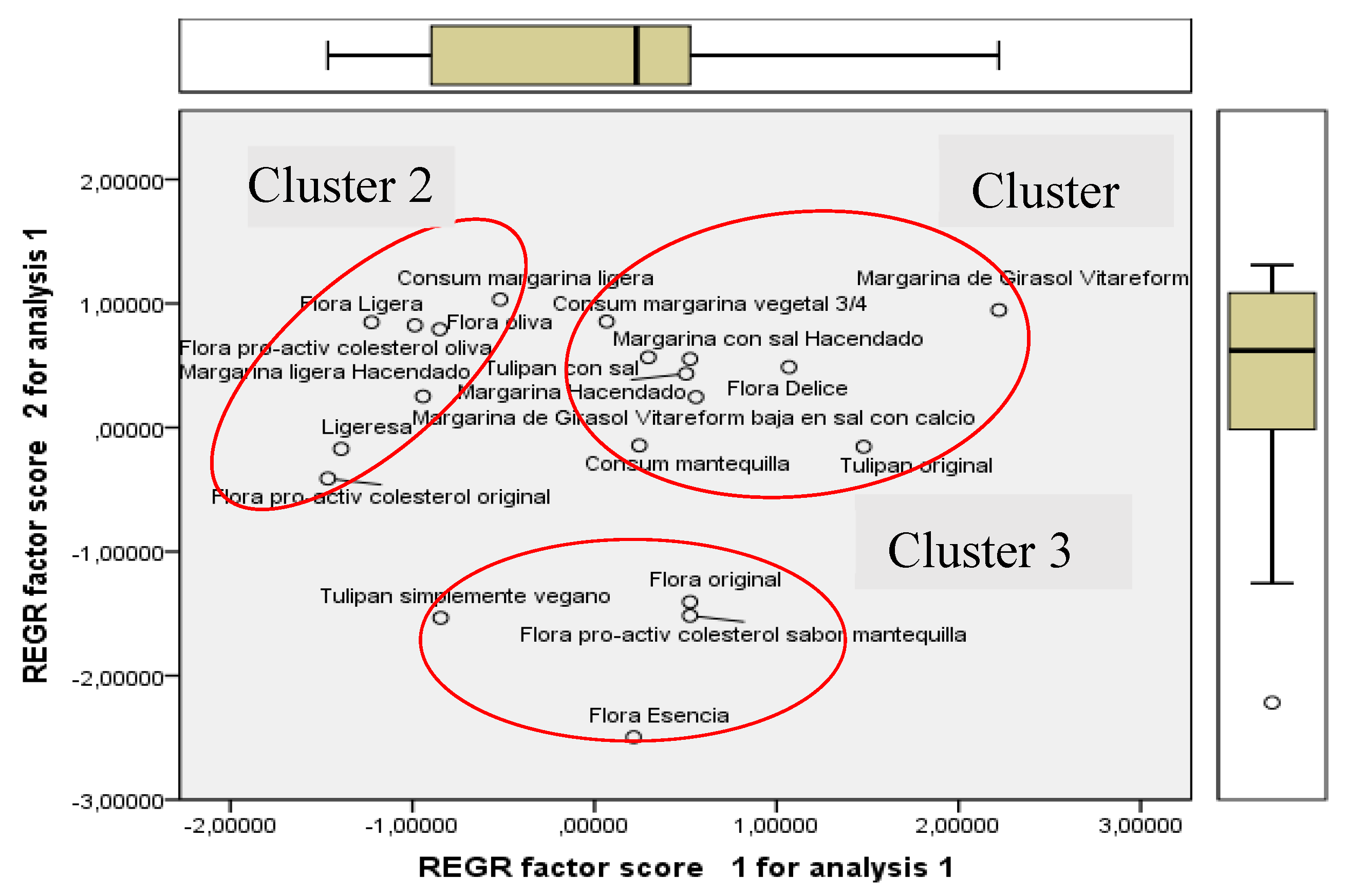
3.1. Preliminary Selection of Margarines for Use in Subsequent Experimental Trials
- 1) They are extracted in decaying order of importance. First PC F1 always contains more information than the second F2 does, F2 more than the third F3, etc.
- 2) Every PC is orthogonal to one another. There is no correlation between the information contained in different PCs.
3.2. Effect of the Fatty Acid Composition of the Different Margarines on the Induction Period Obtained
3.3. Natural Antioxidants of Herbs and Spices Improve the Oxidative Stability of Margarines of Different Composition
3.3.1. Antioxidant Capacity of the Plant Species Used in the Tests
3.3.2. Effect of the addition of different plant species on the oxidative stability of tested margarines
3.3.3. Statistical Analysis of the Eight Margarines Together with the Plant Species
Principal Component Analysis (PCA)
- LOG IP = (0.399±0.093) + (0.952 ±0.150) ω-3 – (0.010±0.003) PUFA + (0.0010±0.0001) AA + (0.021±0.002) SFA – (0.366±0.051) MUFA/PUFA
- R2 = 0.815
- F = 36.910
- Std. Error of the estimate= 0.87212484
- LOG IP = 0.314647 + 0.942967 ω-3 – 0.006232 PUFA + 0.000691 AA + 0.019548 SFA – 0.330934 MUFA/PUFA
- R2 = 0.8083
- Prediction Error Sum Of Squares (PESS)= 0.2090
- Mean Absolute Percentage Error= 9.87 %
- Approximation Error Variance= 0.1917
4. Conclusions
Acknowledgments
References
- Anand, S.P.; Sati, N. Artificial Preservatives and Their Harmful Effects: Looking Toward Nature for Safer Alternatives. Int. J. Pharm. Sci. Res. 2013, 4, 2496–2501. [Google Scholar] [CrossRef]
- Gould, G.W. Preservation: Past, Present and Future. Br. Med. Bull. 2000, 56, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Paulus, K. Physikalische Konservierung von Lebensmitteln [Physical Preservation of Food]. Zentralbl Bakteriol Mikrobiol Hyg B. 1985, 180, 299–310. [Google Scholar]
- Ida Del Greco, N. Estudio Sobre La Tendencia de Consumo de Alimentos. Available online: http://bvs.minsa.gob.pe/local/MINSA/2603.pdf (accessed on 19 June 2021).
- Moure, A.; Cruz, J.M.; Franco, D.; Manuel Domínguez, J.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Carlos Parajó, J. Natural Antioxidants from Residual Sources. Food Chem. 2001, 72, 155–171. [Google Scholar] [CrossRef]
- European Food Safety Authority. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/721 (accessed on 19 June 2021).
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant Activity of Rosemary (Rosmarinus officinalis L.) Essential Oil and Its Hepatoprotective Potential. BMC Complement. Altern. Med. 2014, 14, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C. Some Strategies for the Stabilization of Long Chain N-3 PUFA Enriched Foods: A Review. Eur. J. Lipid Sci. Technol. 2015, 117, 1853–1866. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Misawa, R. Effect of Emulsifier Concentration on the Oxidation of an O/W Emulsion Prepared from Canola Oil. Food Nutr. Sci. 2018, 09, 683–692. [Google Scholar] [CrossRef]
- Redondo-Cuevas, L.; Castellano, G.; Raikos, V. Natural Antioxidants from Herbs and Spices Improve the Oxidative Stability and Frying Performance of Vegetable Oils. Int. J. Food Sci. Technol. 2017, 52, 2422–2428. [Google Scholar] [CrossRef]
- Redondo Cuevas, L.; Castellano, G.; Torrens, F.; Raikos, V. Revealing the Relationship between Vegetable Oil Composition and Oxidative Stability: A Multifactorial Approach. J. Food Compos. Anal. 2018, 66, 221–229. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sable, S.S.; Gaikwad, S.G.; Sonawane, S.H.; Saini, D.R.; Gogate, P.R. Intensification of Extraction of Curcumin from Curcuma Amada Using Ultrasound Assisted Approach: Effect of Different Operating Parameters. Ultrason. Sonochem. 2017, 38, 437–445. [Google Scholar] [CrossRef]
- Ayati Z, Ramezani M, Amiri MS, Moghadam AT, Rahimi H, Abdollahzade A, Sahebkar A, E. S. Ethnobotany, Phytochemistry and Traditional Uses of Curcuma Spp. and Pharmacological Profile of Two Important Species (C. Longa and C. Zedoaria): A Review. Curr. Pharm. Des. Pharm. Des. 2019, 25, 871–901. [Google Scholar] [CrossRef] [PubMed]
- Erkan, N.; Ayranci, G.; Ayranci, E.; Akter, J.; Hossain, M.A.; Takara, K.; Islam, M.Z.; Hou, D.X. Antioxidant Activities of Rosemary (Rosmarinus officinalis L.) Extract, Blackseed (Nigella sativa L.) Essential Oil, Carnosic Acid, Rosmarinic Acid and Sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef]
- Ivanov, I.G. Polyphenols Content and Antioxidant Activities of Taraxacum officinale F.H. Wigg (Dandelion) Leaves. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 889–893. [Google Scholar]
- Aly, S.E.; Sabry, B.A.; Shaheen, M.S.; Hathout, A.S. Assessment of Antimycotoxigenic and Antioxidant Activity of Star Anise (Illicium verum) in Vitro. J. Saudi Soc. Agric. Sci. 2016, 15, 20–27. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In Vitro Antibacterial and Antioxidant Properties of Chitosan Edible Films Incorporated with Thymus Moroderi or Thymus piperella Essential Oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Grigore, A.; Paraschiv, I.; Colceru-Mihul, S.; Bubueanu, C.; Draghici, E.; Ichim, M. Chemical Composition and Antioxidant Activity of Thymus vulgaris L. Volatile Oil Obtained by Two Different Methods. Rom. Biotechnol. Lett. 2010, 15, 5436–5443. [Google Scholar]
- Manual 892 Professional Rancimat. Available online: https://www.metrohm.com/es_es/products/stability-measurement/Stability-measurement-Rancimat-Thermomat.html (accessed on 19 June 2021).
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Parry, J.; Su, L.; Luther, M.; Zhou, K.; Peter Yurawecz, M.; Whittaker, P.; Yu, L. Fatty Acid Composition and Antioxidant Properties of Cold-Pressed Marionberry, Boysenberry, Red Raspberry, and Blueberry Seed Oils. J. Agric. Food Chem. 2005, 53, 566–573. [Google Scholar] [CrossRef]
- Hu, M.; Jacobsen, C. Oxidative Stability and Shelf Life of Foods Containing Oils and Fats, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands; AOCS Press: London, UK, 2016; ISBN 9781630670566. [Google Scholar]
- Sahraee, S.; Milani, J.M.; Regenstein, J.M.; Kafil, H.S. Protection of Foods against Oxidative Deterioration Using Edible Films and Coatings: A Review. Food Biosci. 2019, 32, 429–451. [Google Scholar] [CrossRef]
- Lis, B.; Olas, B. Pro-Health Activity of Dandelion (Taraxacum officinale L.) and Its Food Products – History and Present. J. Funct. Foods 2019, 59, 40–48. [Google Scholar] [CrossRef]
- Akter, J.; Hossain, M.A.; Takara, K.; Islam, M.Z.; Hou, D.X. Antioxidant Activity of Different Species and Varieties of Turmeric (Curcuma Spp): Isolation of Active Compounds. Comp. Biochem. Physiol. Part - C Toxicol. Pharmacol. 2019, 215, 9–17. [Google Scholar] [CrossRef]
- Chizzola, R.; Michitsch, H.; Franz, C. Antioxidative Properties of Thymus vulgaris Leaves: Comparison of Different Extracts and Essential Oil Chemotypes. J. Agric. Food Chem. 2008, 56, 6897–6904. [Google Scholar] [CrossRef]
- Nickavar, B.; Esbati, N. Evaluation of the Antioxidant Capacity and Phenolic Content of Three Thymus Species. J. Acupunct. Meridian Stud. 2012, 5, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. Chemical Characterization and Antibacterial Activity of Thymus Moroderi and Thymus piperella Essential Oils, Two Thymus Endemic Species from Southeast of Spain. Food Control 2012, 27, 294–299. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Cheng, L.Z.; Zhang, T.; Yaron, S.; Jiang, H.X.; Sui, Z.Q.; Corke, H. Phenolic Profiles, Antioxidant, and Antiproliferative Activities of Turmeric (Curcuma longa). Ind. Crops Prod. 2020, 152, 112561. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Nartop, P.; Gurel, A.; Bedir, E.; Vardar-Sukan, F. Determination of Phenolic Content and Antioxidant Activity of Extracts Obtained from Rosmarinus officinalis Calli. J. Plant Physiol. 2007, 164, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Löliger, J. The Use of Antioxidants in Foods. In Free Radicals and Food Additives. In Science and education; Edition, H.B., Ed.; Springer: London, UK, 1991; Volume 35, pp. 121–150. [Google Scholar]
- Nieto, G.; Huvaere, K.; Skibsted, L.H. Antioxidant Activity of Rosemary and Thyme By-Products and Synergism with Added Antioxidant in a Liposome System. Eur. Food Res. Technol. 2011, 233, 11–18. [Google Scholar] [CrossRef]
- Rege, S.A.; Momin, S.A.; Wadekar, S.D.; Bhowmick, D.N. Formulation of a Functional Fat Spread Stabilised by Natural Antioxidants and Emulsifiers. Malays. J. Nutr. 2013, 19, 121–130. [Google Scholar]
- Ungureanu, C.R.M.; Poiana, M.A.; Cocan, I.; Lupitu, A.I.; Alexa, E.; Moigradean, D. Strategies to Improve the Thermo-Oxidative Stability of Sunflower Oil by Exploiting the Antioxidant Potential of Blueberries Processing by Products. Molecules 2020, 25, 1–22. [Google Scholar] [CrossRef]
- Cuvelier, M.E.; Richard, H.; Berset, C. Antioxidative Activity and Phenolic Composition of Pilot-Plant and Commercial Extracts of Sage and Rosemary. J. Am. Oil Chem. Soc. 1996, 73, 645–652. [Google Scholar] [CrossRef]
- Cedeño-Pinos, C.; Martínez-Tomé, M.; Murcia, M.A.; Jordán, M.J.; Bañón, S. Assessment of Rosemary (Rosmarinus officinalis L.) Extract as Antioxidant in Jelly Candies Made with Fructan Fibres and Stevia. Antioxidants 2020, 9, 1–16. [Google Scholar] [CrossRef]
- Etter, S.C. Rosmarinus officinalis as an Antioxidant. J. Herbs, Spices Med. Plants 2004, 11, 121–159. [Google Scholar] [CrossRef]
- Chen, C.; Pearson, A.M.; Gray, J.I. Effects of Synthetic Antioxidants (BHA, BHT and PG) on the Mutagenicity of IQ-like Compounds. Food Chem. 1992, 43, 177–183. [Google Scholar] [CrossRef]
- Aruoma, O.I. Commentary: Antioxidant Actions of Plant Foods: Use of Oxidative DNA Damage as a Tool for Studying Antioxidant Efficacy. Free Radic. Res. 1999, 30, 419–427. [Google Scholar] [CrossRef]
- Nieto, G. A Review on Applications and Uses of Thymus in the Food Industry. Plants 2020, 9, 3–29. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods - A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of Antioxidant Activity, Total Phenols and Phenolic Compounds in Thyme (Thymus vulgaris L.), Sage (Salvia officinalis L.), and Marjoram (Origanum majorana L.) Extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial Activity and Chemical Composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis Essential Oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Youdim, K.A.; Deans, S.G.; Finlayson, H.J. The Antioxidant Properties of Thyme (Thymus Zygis L.) Essential Oil: An Inhibitor of Lipid Peroxidation and a Free Radical Scavenger. J. Essent. Oil Res. 2002, 14, 210–215. [Google Scholar] [CrossRef]
- Guillén, M.D.; Manzanos, M.J. Study of the Composition of the Different Parts of a Spanish Thymus vulgaris L. Plant. Food Chem. 1998, 63, 373–383. [Google Scholar] [CrossRef]
- Boira, H.; Blanquer, A. Environmental Factors Affecting Chemical Variability of Essential Oils in Thymus piperella L. Biochem. Syst. Ecol. 1998, 26, 811–822. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Gruzdiene, D.; Tirzite, D.; Tirzitis, G. Assessment of Antioxidant Activity of Plant Extracts by Different Methods. Wocmap Perspect. Nat. Prod. Chem. 2005, 3, 99–107. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical Profile, Antioxidant and Antibacterial Activity of Thyme and Oregano Essential Oils, Thymol and Carvacrol and Their Possible Synergism. J. Essent. Oil-Bearing Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, E.M.; Hossen, M.S.; Hossain, M.F.; Afroz, R.; Gan, S.H.; Khalil, M.I.; Karim, N. Antioxidant Properties of Popular Turmeric (Curcuma longa) Varieties from Bangladesh. J. Food Qual. 2017, 2017, 97–105. [Google Scholar] [CrossRef]
- Kim, I.S.; Yang, M.R.; Lee, O.H.; Kang, S.N. Antioxidant Activities of Hot Water Extracts from Various Spices. Int. J. Mol. Sci. 2011, 12, 4120–4131. [Google Scholar] [CrossRef]
- Gonzalez-Albadalejo, J.; Sanz, D.; Claramunt, R.M.; Lavandera, J.L.; Alkorta, I.; Elguero, J. Curcumin and Curcuminoids: Chemistry, Structural Studies and Biological Properties | Curcumina y Curcuminoides: Quimica, Estudios Estructurales y propiedades Biologicas. An. la Real Acad. Nac. Farm. 2015, 81, 278–310. [Google Scholar]
- Somparn, P.; Phisalaphong, C.; Nakornchai, S.; Unchern, S.; Morales, N.P. Comparative Antioxidant Activities of Curcumin and Its Demethoxy and Hydrogenated Derivatives. Biol. Pharm. Bull. 2007, 30, 74–78. [Google Scholar] [CrossRef]
- Jha, N.S.; Mishra, S.; Jha, S.K.; Surolia, A. Antioxidant Activity and Electrochemical Elucidation of the Enigmatic Redox Behavior of Curcumin and Its Structurally Modified Analogues. Electrochim. Acta 2015, 151, 574–583. [Google Scholar] [CrossRef]
- Cheng Hong, Y.; Fang Rong, C.; Hsueh Wei, C.; Shao Ming, W.; Ming Che, H.; Li Yeh, C. Investigation of the Antioxidant Activity of Illicium verum Extracts. J. Med. Plants Res. 2012, 6, 314–324. [Google Scholar] [CrossRef]
- Fulga, A.; Pantea, V.; Andronache, L.; Tagadiuc, O.; Todiras, M.; Gudumac, V. Antioxidant Activity of Taraxacum officinale. Inter Conf 2021, 46, 264–280. [Google Scholar] [CrossRef]




| Margarine Commercial brand |
TFA (g) | SFA (g) | MUFA (g) | PUFA (g) | UFA (g) | MUFA/PUFA | Carbo- hydrates (g) |
Proteins (g) | Vit. A (µg) | Vit. D (µg) | Vit. E (µg) | Salt (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tulipan original* | 80 | 34 | 23 | 23 | 46 | 0.50 | 0.5 | 0.5 | 800 | 7.5 | 17000 | 0.31 |
|
Tulipan con sal |
60 | 18 | 15 | 26 | 41 | 0.63 | 0.5 | 0.5 | 800 | 7.5 | 16000 | 0.91 |
| Tulipan simplemente vegano | 50 | 12 | 20 | 17 | 37 | 0.46 | 0.5 | 0.5 | 0 | 7.5 | 10000 | 0.71 |
| Flora original* | 70 | 18 | 19 | 32 | 51 | 0.63 | 0.5 | 0 | 800 | 7.5 | 25000 | 0.20 |
|
Flora pro-activ colesterol original* |
35 | 8 | 11 | 16 | 27 | 0.59 | 0.5 | 0.5 | 120 | 7.5 | 10000 | 0.3 |
|
Flora pro-activ colesterol oliva |
35 | 8.1 | 12 | 15 | 27 | 0.56 | 0.5 | 0.5 | 800 | 7.5 | 10000 | 0.33 |
|
Flora pro-activ colesterol sabor mantequilla |
70 | 16 | 23 | 31 | 54 | 0.57 | 0.5 | 0.5 | 120 | 7.5 | 18000 | 0.5 |
| Flora Esencia | 70 | 15 | 33 | 22 | 55 | 0.40 | 0.5 | 0.5 | 120 | 7.5 | 13000 | 0.4 |
| Flora oliva* | 40 | 11 | 13 | 17 | 30 | 0.57 | 0.5 | 0.5 | 800 | 7.5 | 12000 | 0.34 |
| Flora Ligera | 39 | 12 | 12 | 15 | 27 | 0.56 | 0.5 | 0.5 | 800 | 7.5 | 9700 | 0.37 |
| Flora Delice | 70 | 18 | 18 | 33 | 27 | 1.22 | 0.5 | 0.5 | 804 | 7.5 | 24700 | 0.21 |
|
Consum margarina vegetal ¾* |
60 | 15 | 17 | 27 | 51 | 0.25 | 0.5 | 0.5 | 650 | 5.4 | 24000 | 0.46 |
|
Consum margarina ligera* |
40 | 12 | 17 | 11 | 28 | 0.39 | 0.5 | 0.5 | 853 | 7.5 | 22000 | 0.4 |
| Margarina Hacendado | 60 | 17 | 17 | 26 | 43 | 0.60 | 0.5 | 0.5 | 700 | 5.9 | 23000 | 0.3 |
| Margarina con sal Hacendado | 60 | 17 | 17 | 26 | 43 | 0.60 | 0.5 | 0.5 | 760 | 5.9 | 23000 | 0.9 |
| Margarina ligera Hacendado | 40 | 12 | 20 | 8.2 | 28 | 0.29 | 0.5 | 0.5 | 800 | 6.8 | 12000 | 0.4 |
|
Margarina de girasol Vitareform |
80 | 23 | 20 | 37 | 57 | 0.65 | 0 | 0 | 900 | 2.5 | 40000 | 0.2 |
|
Margarina de girasol Vitareform baja en sal con calcio |
60 | 13 | 23 | 24 | 47 | 0.51 | 0.5 | 0.5 | 800 | 7.5 | 27000 | 0.2 |
| Ligeresa* | 40 | 11 | 20 | 9 | 29 | 0.31 | 0.1 | 0.1 | 800 | 7.5 | 0 | 0.76 |
|
Consum mantequilla* |
60 | 25 | 24 | 11 | 35 | 0.31 | 1 | 1 | 800 | 7.5 | 14000 | 0.4 |
| Plant species | Batch number | Manufacturer name / Location |
|---|---|---|
| Curcuma longa | F12747 | Celeplame S.L. / Barbate (Cádiz) |
| Rosmarinus officinalis | F12449 | Celeplame S.L./ Barbate (Cádiz) |
| Taraxacum officinale | F12598 | Celeplame S.L./ Barbate (Cádiz) |
| Illicium verum | 3489 | Celeplame S.L./ Barbate (Cádiz) |
| Thymus piperella | 5049 | Celeplame S.L./ Barbate (Cádiz) |
| Thymus vulgaris | 11110.11110 | Jesús Navarro S.A./ Monforte del Cid (Alicante) |
| Cluster number |
Margarine Brand |
TFA (g) |
SFA (g) |
MUFA (g) |
PUFA (g) |
UFA (g) |
MUFA/ PUFA |
UFA/ SFA |
ω 3 (g) |
ω 6 (g) |
IP value (h) |
|---|---|---|---|---|---|---|---|---|---|---|---|
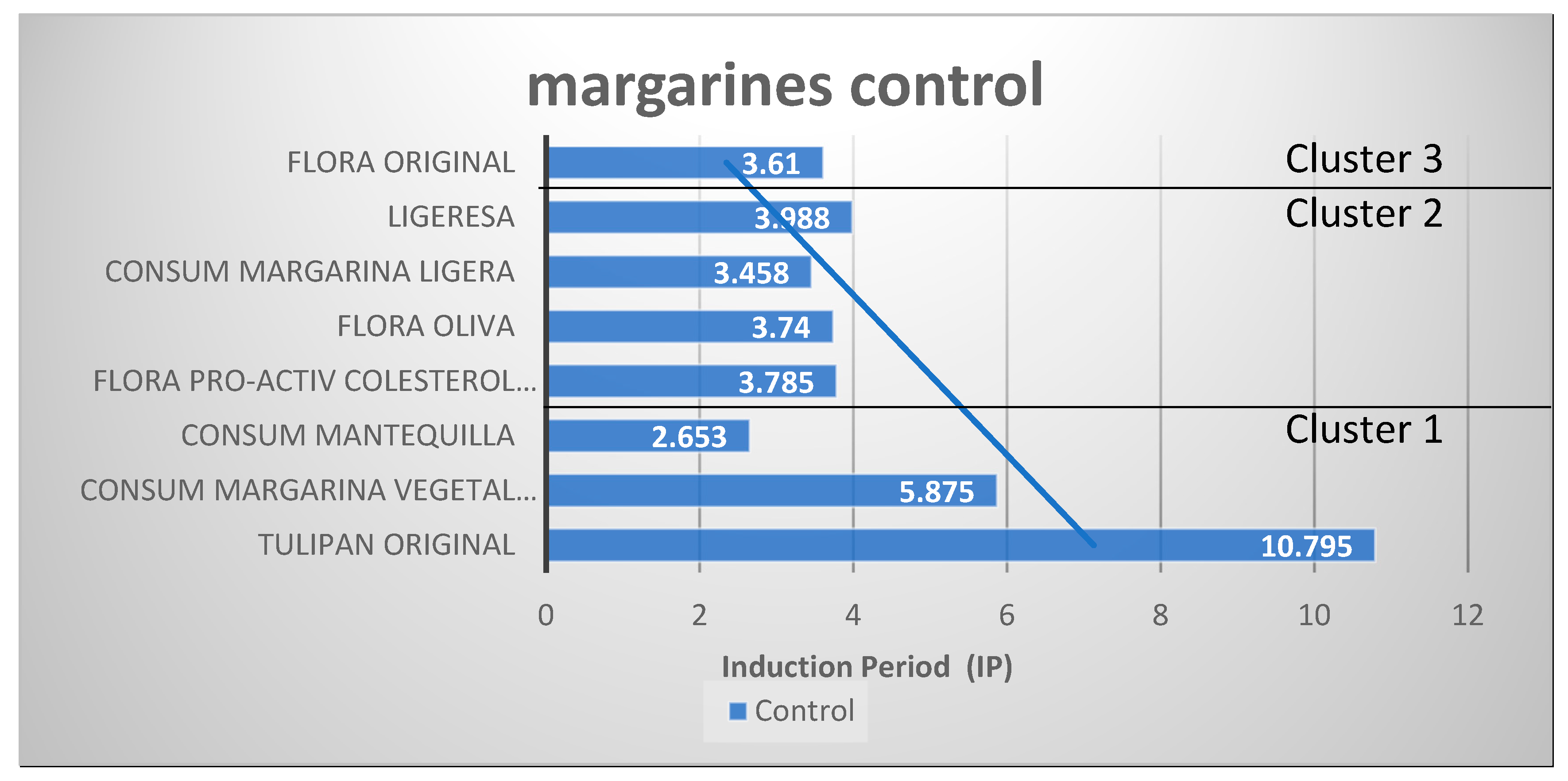
| 1 | Tulipan original | 80 | 34 | 23 | 23 | 46 | 1.00 | 1.35 | 0 | 0 | 10.8 ± 0.3 |
| Consum margarina vegetal 3/4 | 60 | 15 | 17 | 27 | 51 | 0.63 | 3.40 | 2.90 | 10 | 5.88 ± 0.21 | |
| Consum mantequilla | 60 | 25 | 24 | 11 | 35 | 2.18 | 1.40 | 0.00 | 11 | 2.65 ± 0.24 | |
| 2 | Flora proactiv colesterol original | 35 | 8 | 11 | 16 | 27 | 0.69 | 3.38 | 3.20 | 12 | 3.79 ± 0.09 |
| Flora oliva | 40 | 11 | 13 | 17 | 30 | 0.76 | 2.73 | 1.90 | 15 | 3.74 ± 0.19 | |
| Ligeresa | 40 | 11 | 20 | 9 | 29 | 2.22 | 2.64 | 0 | 0 | 4.0 ± 0.3 | |
| Consum margarina ligera | 40 | 12 | 17 | 11 | 28 | 1.55 | 2.33 | 0 | 11 | 3.46 ± 0.17 | |
| 3 | Flora original | 70 | 18 | 19 | 32 | 51 | 0.59 | 2.83 | 3.60 | 29 | 3.6 ± 0.3 |
| Plant species | Phenol content (mg GAE/g) |
Antioxidant capacity (μM Fe2+/g) |
|---|---|---|
| Curcuma longa | 240 ± 30 | 166.0 ± 0.7 |
| Rosmarinus officinalis | 410 ± 22 | 570 ± 70 |
| Taraxacum officinale | 33.90 ± 0.18 | 131.5 ± 0.9 |
| Illicium verum | 96.3 ± 0.5 | 88.9 ± 0.5 |
| Thymus piperella | 57.4 ± 0.4 | 213.5 ± 1.4 |
| Thymus vulgaris | 172.6 ± 1.4 | 242.3 ± 1.9 |
| 8 | CLUSTER No. |
ADDED HERBS | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | Rosmarinus officinalis |
Curcuma longa |
Illicium verum |
Taraxacum officinale | Thymus piperella |
Thymus vulgaris |
||
| Tulipan original |
1 | 10.8±0.3 | 13.49±0.23 (+24.97%) |
11.87±0.13 (+9.94%) |
5.9±0.5 (-45.21%) |
6.41±0.22 (-40.62%) |
11.8±0.8 (+9.03%) |
11.87±0.17 (+9.96%) |
| Consum margarina vegetal 3/4 |
5.88±0.21 | 8.64±0.24 (+47.06%) |
6.77±0.18 (+15.15%) |
3.7±0.8 (-36.89%) |
4.20±0.18 (-28.55%) |
6.2±0.6 (+6.04%) |
6.6±0.3 (+12.73%) |
|
| Consum mantequilla |
2.65±0.24 | 6.1±0.6 (+131.25%) |
3.6±0.6 (+37.32%) |
1.9±0.3 (-28.94%) |
2.31±0.16 (-12.81%) |
4.1±0.5 (+55.41%) |
5.4±0.5 (+104.67%) |
|
| Flora pro-activ colesterol original | 2 | 3.79±0.09 | 6.43±0.07 (+69.83%) |
4.2±0.03 (+10.91%) |
3.1±0.4 (-17.44%) |
3.8±0.3 (-0.92%) |
4.47±0.12 (+17.97%) |
5.5±0.3 (+44.07%) |
| Flora oliva | 3.74±0.19 | 6.32±0.17 (+68.98%) |
3.81±0.14 (+1.87%) |
3.4±0.3 (-8.82%) |
3.6±0.4 (-2.67%) |
3.9±0.4 (+4.01%) |
3.94±0.17 (+5.35%) |
|
| Consum margarina ligera |
3.46±0.17 | 6.7±0.5 (+94.48%) |
3.77±0.13 (+9.02%) |
2.2±0.7 (-35.57%) |
2.75±0.10 (-20.39%) |
3.76±0.16 (+8.59%) |
4.79±0.10 (+38.43%) |
|
| Ligeresa | 4.0±0.3 | 7.0±0.9 (+75.60%) |
4.8±0.3 (+20.56%) |
2.37±0.18 (-40.70%) |
3.5±0.3 (-12.78%) |
4.0±0.5 (+1.18%) |
4.2±0.5 (+4,19%) |
|
| Flora original | 3 | 3.6±0.3 | 5.28±0.13 (+46.26%) |
3.84±0.18 (+6.37%) |
3.0±0.4 (-17.17%) |
3.57±0.09 (-1.11%) |
4.07±0.19 (+12.74%) |
4.4±0.6 (+21.05%) |
| 1. Tulipan original + Rosmarinus officinalis | 17. Tulipan original+ Illicium verum | 33. Tulipan original + Thymus piperella |
| 2. Flora original + Rosmarinus officinalis | 18. Flora original + Illicium verum | 34. Flora original + Thymus piperella |
| 3. Flora pro-activ colesterol original + Rosmarinus officinalis | 19. Flora pro-activ colesterol original + Illicium verum | 35. Flora pro-activ colesterol original + Thymus piperella |
| 4. Flora oliva + Rosmarinus officinalis | 20. Flora oliva + Illicium verum | 36. Flora oliva + Thymus piperella |
| 5. Consum margarina vegetal ¾ + Rosmarinus officinalis | 21. Consum margarina vegetal ¾ + Illicium verum | 37. Consum margarina vegetal ¾ + Thymus piperella |
| 6. Consum margarina ligera + Rosmarinus officinalis | 22. Consum margarina ligera + Illicium verum | 38. Consum margarina ligera + Thymus piperella |
| 7. Ligeresa + Rosmarinus officinalis | 23. Ligeresa + Illicium verum | 39. Ligeresa + Thymus piperella |
| 8. Consum mantequilla + Rosmarinus officinalis | 24. Consum mantequilla + Illicium verum | 40. Consum mantequilla + Thymus piperella |
| 9. Tulipan original + Curcuma longa | 25. Tulipan original + Taraxacum officinale | 41. Tulipan original + Thymus vulgaris |
| 10. Flora original + Curcuma longa | 26. Flora original + Taraxacum officinale | 42. Flora original + Thymus vulgaris |
| 11. Flora pro-activ colesterol original + Curcuma longa | 27. Flora pro-activ colesterol original + Taraxacum officinale | 43. Flora pro-activ colesterol original + Thymus vulgaris |
| 12. Flora oliva + Curcuma longa | 28. Flora oliva + Taraxacum officinale | 44. Flora oliva + Thymus vulgaris |
| 13 .Consum margarina vegetal ¾ + Curcuma longa | 29. Consum margarina vegetal ¾ + Taraxacum officinale | 45 Consum margarina vegetal ¾ + Thymus vulgaris |
| 14.Consum margarina ligera + Curcuma longa | 30. Consum margarina ligera + Taraxacum officinale | 46. Consum margarina ligera + Thymus vulgaris |
| 15.Ligeresa + Curcuma longa | 31. Ligeresa + Taraxacum officinale | 47. Ligeresa + Thymus vulgaris |
| 16.Consum mantequilla + Curcuma longa | 32. Consum mantequilla + Taraxacum officinale | 48. Consum mantequilla + Thymus vulgaris |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





