Jasminum (Oleaceae) is a genus with over 200 species, which are native to Asia, Australia, Africa, and the southern Pacific Islands. [
1] The phytochemical investigations on some of Jasminum species have revealed the presence of secoiridoids, lignans, triterpenoids, flavonoids, sesquiterpenoids. [2-9] The roots of
Jasminum sambac (L.) Ait. is a traditional Chinese medicine with anesthetic and analgesic effects and used for the treatment of insomnia, headache, decayed tooth, and injuries from falls. It was recorded that the roots is thought to be one important ingredient of “Ma-Fei-San”, which was created by Tuo Hua and used for surgeries due to its significant anesthetic and analgesic effects. So it is very essential to deeply study the ingredients of the roots of
Jasminum sambac (L.) Ait.
In our previous work, we have isolated and confirmed some compounds (triterpenoid, sesquiterpenoids, lignans and glycoside) from the roots of
Jasminum sambac (L.) Ait. [
10,
11]. Here, we report the isolation and elucidation of another four new unusual pentacyclic triterpenoids (
1-4) (Structures were shown in
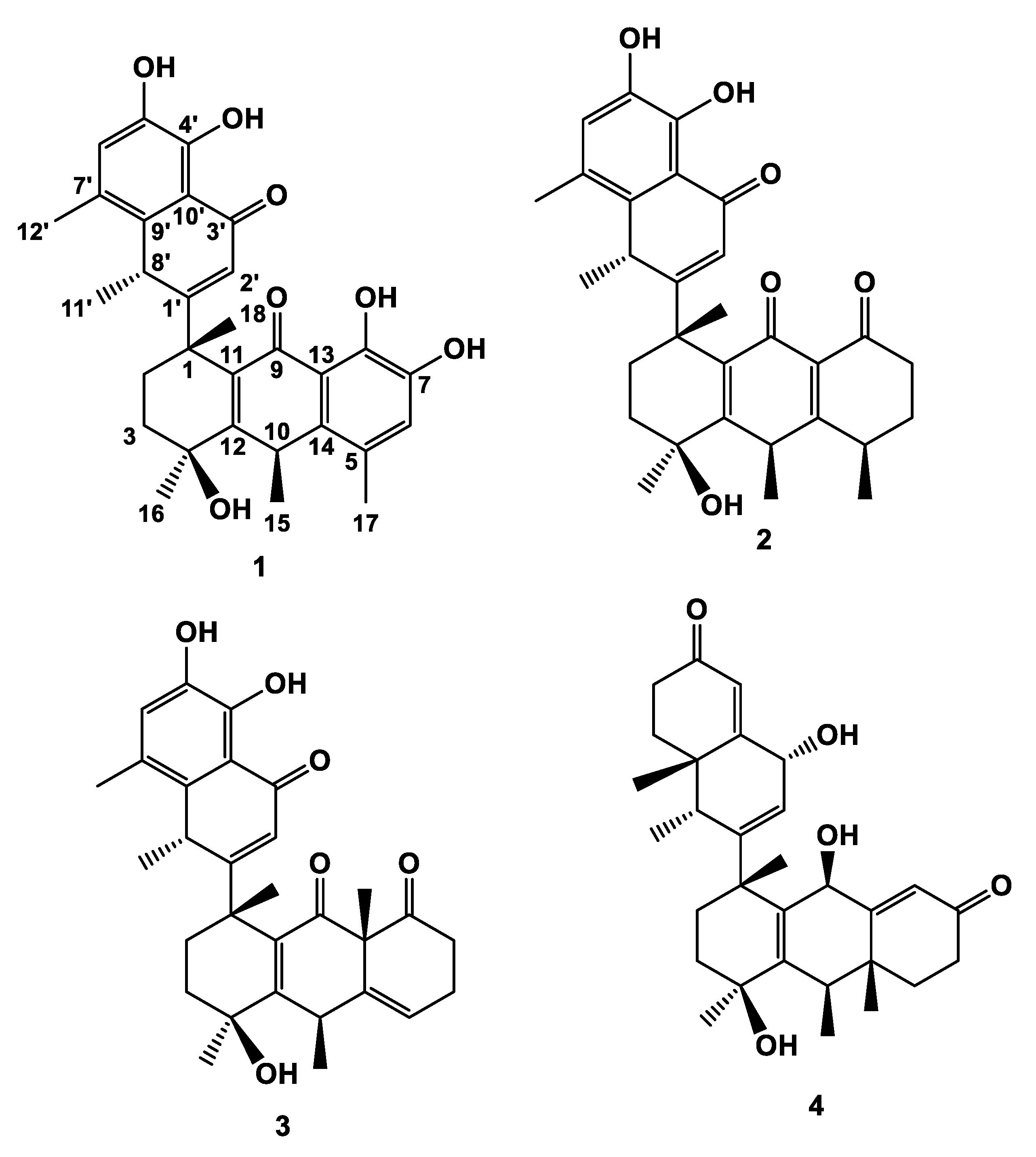
Figure 1) from the roots of
Jasminum sambac (L.) Ait.
Figure 1.
Structures of compounds (1-4).
Figure 1.
Structures of compounds (1-4).
Compound 1: Golden yellow solid, M.p 61-62 oC, HPLC purity: 96.596 %, retention time:17.067 min. Crystal Data: orthorhombic, space group P212121(no. 19), a=7.8148(3)Å, b=13.1614(6)Å, c=25.3786(11)Å, V=2610.28(19)Å3, Z=4, T=272.00 K, μ(Mo Kα)= 0.091 mm-1, Dcalc=1.284 g/cm3, 22104 reflections measured (5.454° ≤ 2θ ≤ 54.36°), 5766 unique (Rint=0.0704, Rsigma=0.0650) which were used in all calculations. The final R1 was 0.0536 (I > 2σ(I)) and wR2 was 0.1512.
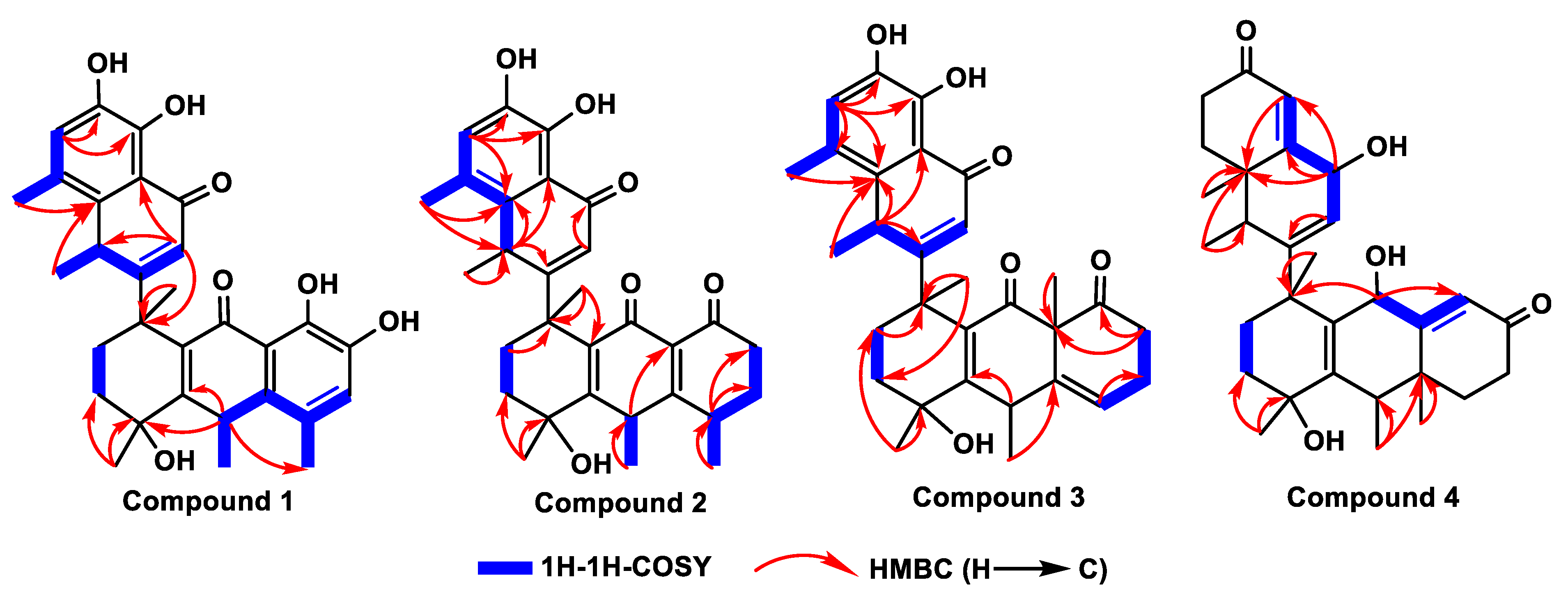
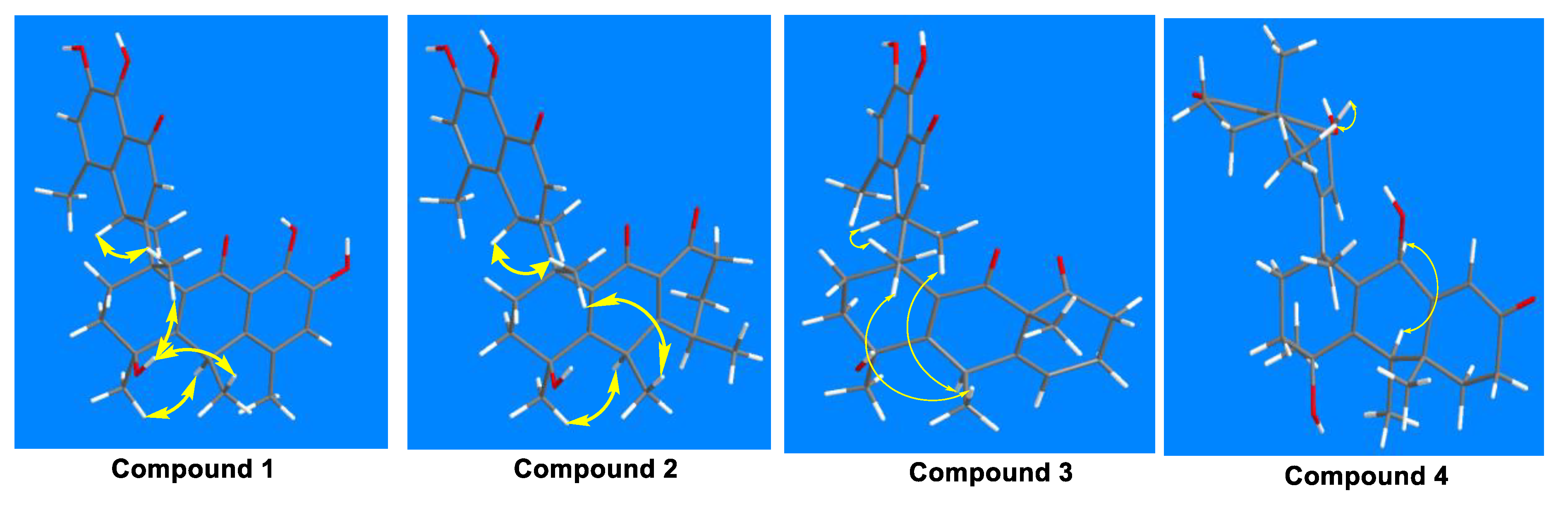
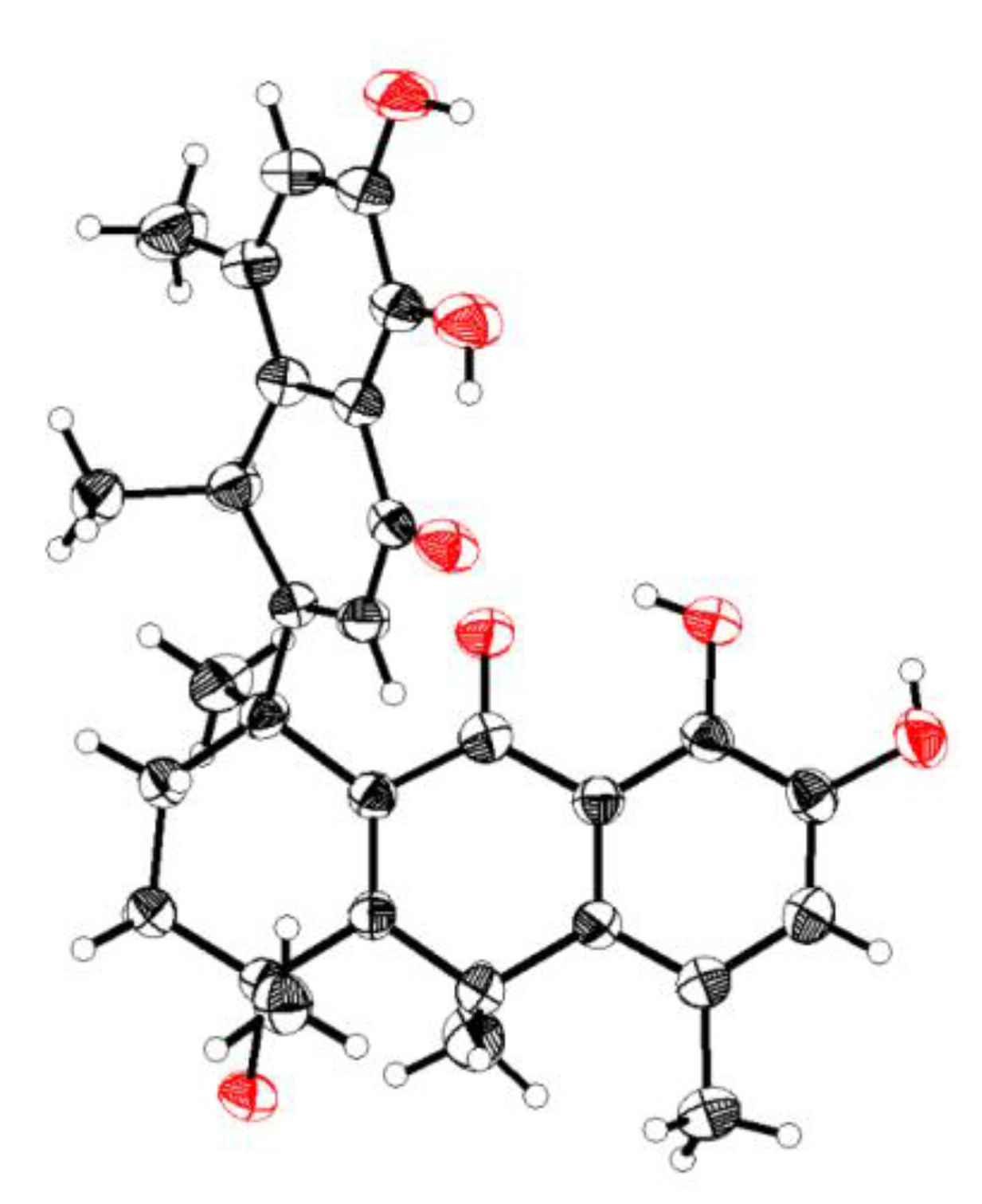
The molecular formula was confirmed as “C30H32O7” through HRESIMS (m/z: found: 527.2040 [M+Na]+, calcd.: 527.2040). The 1H NMR spectrum showed six methyl groups at [δH: 1.26 (d, J=4.4 Hz, H-12'), 1.32 (d, J=4.4 Hz, H-17), 1.45 (s, H-16), 1.72 (s, H-18), 2.24 (s, H-15), and2.31 (s, H-11')] ppm; two aromatic protons at [δH: 6.84 (s, H-6) and 6.93 (s, H-6')] ppm; five hydroxyl protons at [δH: 5.35 (s, 4-OH), 8.99 (s, 4'-OH), 9.10 (s, 8-OH), 11.78 (s, 7-OH), and 12.53 (s, 5'-OH)] ppm. The 13C NMR toghether with DEPT spectra revealed the thirty signals, including: six methyls (C-15, C-16, C-17, C-18, C-11', and C-12'); two methylenes (C-2 and C-3); two methines (C-10 and C-8'); three olefinic methine (C-6, C-2', and C-6'), and seventeen quaternary carbons (C-1, C-4, C-5, C-7, C-8, C-9, C-11, C-12, C-13, C-14, C-1', C-3', C-4', C-5', C-7, C-9' and C-10). The HMBC correlations (Figure 2): from 4-OH (δH 5.35) to C-16 (δC 28.9), C-3 (δC 34.8), and C-4 (δC 71.1); from 8-OH (δH 9.10) to C-6 (δC 124.0) and C-8 (δC 148.3); from 7-OH (δH 11.78) to C-13 (δC 116.1), C-7 (δC 143.4), and C-8 (δC 148.3); from 4'-OH (δH 8.99) to C-6' (δC 123.9) and C-4' (δC 148.4); and from 5'-OH (δH 12.53) to C-10' (δC 114.8), C-5' (δC 143.6), and C-4' (δC 148.4), supported the “hydroxyl groups” at C-4, C-7, C-8, C-5' and C-4', respectively. 1H-1H COSY correlations (Figure 2): from H-6'/H-12' and H-8'/H-2'/H-11' coupled with the guidance of HMBC correlations: from H-2' (δH 5.69) to C-8' (δC 35.5) and C-10' (δC 114.8); from H-6' (δH 6.93) to C-9' (δC 137.2), C-5' (δC 143.5), and C-4' (δC 148.4); from H-11' (δH 2.31) to C-10' (δC 114.8), C-7' (δC 124.5), C-9' (δC 137.2), and C-4' (δC 148.4); and from H-12' (δH 1.26) to C-8' (δC 35.5) and C-9' (δC 137.2) indicated the presence of methylnaphthalen-1onyl group. Likewise, the correlations from H-10 (δH 4.01) to C-17 (δC 28.1), C-4 (δC 71.1), and C-12 (δC 137.1); from H-15 (δH 2.24) to C-13 (δC 116.1), C-5 (δC 123.8), and C-14 (δC 136.9); from H-17 (δH 1.32) to C-10 (δC 33.1) and C-14 (δC 136.9); from H-16 (δH 1.45) to C-3 (δC 34.8) and C-4 (δC 71.1), accompanying with 1H-1H COSY correlations of H-6/H-17 and H-10/H-15/H-17, revealed the existence of hydroanthracen-9-onyl moiety. The methylnaphthalen-1-onyl and hydroanthracen-9-onyl groups of compound 1 were connected by analyzing HMBC correlation from H-2' (δH 5.69) to C-1 (δC 43.9), which was also supported by 1H-1H COSY correlation of H-2'/H-2/H-3. The ROESY correlations (Figure 3) showed that H-10α (δH 4.01) correlates with Me-16 (δH 1.45), Me-11' (δH 2.31), and H-8'β (δH 4.17) correlates with Me-15 (δH 2.24), Me-18 (δH 1.72) and 4-OH (δH 5.35), which indicated its relative configuration. The detailed 1H and 13C NMR are shown in Table 1. The single-crystal X-ray diffraction analysis confidently confirmed its absolute configuration (CCDC 2259478, XRD structure is shown in Figure 4).
Figure 1.
Structures with key 1H-1H COSY and HMBC correlations of the compounds 1 to 4.
Figure 1.
Structures with key 1H-1H COSY and HMBC correlations of the compounds 1 to 4.
Figure 2.
Key ROESY correlations of compounds 1 to 4.
Figure 2.
Key ROESY correlations of compounds 1 to 4.
Figure 3.
X-ray crystal structure for compound 1.
Figure 3.
X-ray crystal structure for compound 1.
Compound 2: Yellow solid, M.p 58-59 oC, HPLC purity: 92.29%, retention time:18.807min. The molecular formula was confirmed as “C30H34O6” through HRESIMS (m/z: 513.2247 [M+Na]+, calcd.: 513.2248). The 1H and 13C NMR data (Table 1) revealed six methyls at [δH 0.94 (d, J=4.4 Hz, H-17), δH 0.99 (s, H-15), δH 1.26 (d, J=4.5 Hz, H-12'), δH 1.51 (s, H-16), δH 1.58 (s, H-18), and δH 2.25 (s, H-11')] ppm; one aromatic proton at [δH 6.89 (s, H-6')] ppm; three hydroxyl protons at [δH 5.40 (s, 4-OH), δH 9.07 (1H, s, 4'-OH) and δH 12.23 (s, 5'-OH)] ppm; which are identical with those of compound 1.
The 1H-1H COSY of H-17/H-5/H-6/H-7, H-15/H-10, H-16/H-15/H-3, H-18/H-11'/H-2, H-12'/H-6'/H-8 of compound 2 (Figure 2), guiding with HMBC correlations (Figure 2) from H-17 (δH 0.94) to C-5 (δC29.6) and C-7 (δC 42.4); from H-15 (δH 0.99) to C-6 (δC 38.3) and C-10 (δC 39.6); from H-12' (δH 1.26) to C-8' (δC 31.7) and C-9' (δC 137.4); from H-16 (δH 1.51) to C-3 (δC 35.0) and C-4 (δC 70.1); from H-18 (δH 1.58) to C-2 (δC 33.1), C-1 (δC 43.8), C-11 (δC 135.5); and from H-11' (δH 2.25) to C-8' (δC 31.7), C-7' (δC 123.6), C-9' (δC 137.4) are aided in assigning the positions of methyl groups. Also, the HMBC correlations from 4-OH (δH 5.40) to C-16 (δC 27.8), C-3 (δC 35.0) and C-4 (δC 70.1); from 4'-OH (δH 9.07) to C-6' (δC 123.9), C-5' (δC 143.5), and C-4' (δC 148.7), and from 5'-OH (δH 12.23) to C-10' (δC 115.8), C-5' (δC 143.5), C-4' (δC 148.7), indicated the positions of hydroxyl groups. The correlations from H-6' (δH 6.89) to C-11' (δC 17.7), C-9' (δC 137.4), C-5' (δC 143.5), C-4' (δC 148.7) revealed the position of aromatic protons. Its relative configuration of H-8β/Me-18/Me-15 and H-10α/H-5α/Me-16 is supported by ROSY correlations (Figure 3).
Compound 3: Yellow solid, M.p 73-74
oC, HPLC purity: 96.67%, retention time:12.407min. The molecular formula was confirmed as “C
30H
34O
6” through HRESIMS (m/z: 513.2247 [M+Na]+, calcd.: 513.2248). The
1H and
13C NMR data are closely similar to those of compound 2, expect that the position of olefinic bond between C-13 and C-14 of compound 2 shifted to C-6 and C-14 of compound 3, which revealed the appearance of two peaks at δC 126.4 and δC 158.1 ppm for C-6 and C-14, respectively. This was also supported on the basis of HMBC correlations from H-5 (δH 6.47) to C-10 (δC 39.6), C-14 (δC 158.1), and C-9 (δC 187.8). The position of Me-17 of
compound 3 was assigned with the guidance of HMBC correlation from H-17 (δH 0.84) to C-13 (δC 39.6) and C-7 (δC 41.7) (
Figure 2). The relative configuration of
compound 3 was similar to compound 2, which was determined by ROESY (
Figure 3). The detailed
1H and
13C NMR are shown in
Table 1.
Compound 4: Yellow solid, M.p 96-98
oC, HPLC purity: 98.83%, retention time:21.400min. The molecular formula was confirmed as “C
30H
40O
5” through HRESIMS (m/z: 503.2771 [M+Na]+, calcd.: 503.2768). The
1H NMR data showed four tertiary methyls at [δH 0.86 (s, H-17), δH 1.49 (s, H-16), δH 1.44 (s, H-18) and δH 0.93 (s, H-12')] ppm and two secondary methyls at [δH 0.97 (d, J=4.6 Hz, H-15) and δH 0.99 (d, J=2.9 Hz, H-11')] ppm. The
13C NMR together with DEPT revealed 30 carbon signals, including: six methyls (C-15, C-16, C-17, C-18, C-11', and C-12'); six methylenes (C-2, C-3, C-5, C-6, C-6', and C-7'); two oxygenated methines (C-9 and C-3'); three olefinic methines (C-8, C-2', and C-4'); five sp
2 quaternary carbons (C-11, C-12, C-13, C-1', and C-10'); and six sp
3 quaternary carbons (C-1, C-4, C-14, C-9', C-7 and C-5'). The
1H and
13C NMR data(
Table 1) were resembled with those of (1S*,5S*,10aR*)-1-[(8',8a'-dimethyl-4'-oxo-1',4',6',7',8',8a'-hexahydronaphthalene-2'-yl]-4-hydroxy-1,4,5,10atetramethyl-1,2,3,4,5,6,7,9,10,10a-decahydroanthracen-9-one [
4] (which was isolated from the J. sambac roots), except that the two carbonyl groups of (1S*,5S*,10aR*)-1-[(8',8a'-dimethyl-4'-oxo-1',4',6',7',8',8a'-hexahydronaphthalene-2'-yl]-4-hydroxy-1,4,5,10a tetramethyl-1,2,3,4,5,6,7,9,10,10a-decahydroanthracen-9-one were replaced with hydroxyl groups of compound 4, the hydroxyl groups of compound 4 were assigned according to HMBC correlations (
Figure 2): from H-9 (δH 4.17) to C-17 (δC 18.1), C-1 (δC 41.9), C-4 (δC 68.8), C-13 (δC 139.1); and from H-3' (δH 4.51) to C-12' (δC 18.4), C-8' (δC 30.6), C-9' (δC 41.6), C-10' (δC 136.6), and C-4' (δC 139.2), which are accompanied by
1H-
1H COSY correlations of H-9/H-15/H-8 and H-3'/H-2'/H-11'/H-4'. Also, the
13C and DEPT spectra indicated the presence of two carbonyl groups, unlike those of (1S*,5S*,10aR*)-1-[(8',8a'-dimethyl-4'-oxo-1',4',6',7',8',8a'-hexahydronaphthalene-2'-yl]-4-hydroxy-1,4,5,10a tetramethyl-1,2,3,4,5,6,7,9,10,10a-decahydroanthracen-9-one. The ROESY correlations of H-3'β/H-8'β/Me-15 and Me-11'/10α/H-9α/Me-16 (
Figure 3) established its relative configuration.
In conclusion, we have isolated and confirmed four new unusual pentacyclic triterpenoids from the roots of Jasminum sambac (L.) Ait.. This work discovers new compounds from the roots of Jasminum sambac (L.) Ait., and also enriches the types of triterpenoids.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (21875252), and Science and Technology Planning Program of Xiamen (No. 2022CXY0702).
References
- Guo, Z.Y.; Li, P.; Huang, W.; Wang, J.J.; Liu, Y.J.; Liu, B.; Wang, Y.L.; Wu, S.B.; Kennelly, E.J.; Long, C.L. Antioxidant and anti-inflammatory caffeoyl phenylpro-panoid and secoiridoid glycosides from Jasminum nervosum stems, a Chinese folk medicine. Phytochem. 2014, 106, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Lopez, H.; Perez, J. A.; Hernandez, J. M.; Trujillo, J. Secoiridoids from Jasminum odoratissimum. J. Nat. Prod. 1997, 60, 1334–1337. [Google Scholar] [CrossRef]
- Gallo, F.R.; Palazzino, G.; Federici, E.; Iurilli, R.; Monache, F. D.; Chifundera, K.; Galeffi, C. Oligomeric secoiridoid glucosides from Jasminum abyssinicum. Phytochemistry. 2006, 67, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.H.; Hu, M.; Yan, Y.M.; Lu, Q.; Cheng, Y.X. Compounds from the roots of Jasminum sambac. Journal of Asian Natural Products Research. 2012, 14, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.L; Han, L.F.; Meng, D.L.; Li, N.; Li, X. Janceolaroside A and Janceoside A, Two New Compounds from the Stems and Roots of Jasminum lanceolarium. Natural Product Communications. 2011, 6, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Han, Z.Z.; Zhang, C.G.; Ye, Z.; Wu, L.L.; Xu, H. Four new sesquiterpenoids with anti-inflammatory activity from the stems of Jasminum officinale. Fitoterapia. 2019, 135, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.G.; Qin, H.; Li, Y.H.; Sun, Y.; Wang, Z.P.; Yang, T.H.; Liu, L.; Wang, M.C.; Feng, F.; Mei, Q.B. Chemical Constituents of the Root of Jasminum giraldii. Molecules. 2013, 18, 4766–4775. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Yang, J.S.; Zhang, H. Two New Flavanone Glycosides of Jasminum lanceolarium and Their Anti-oxidant Activities. Chem. Pharm. Bull. 2007, 55, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Tomassinia, L.; Ventronea, A.; Frezzaa, C.; Serafinib, I.; Biancob, A.; Cometa, M.F. Lignans and secoiridoid glycosides from the stem barks of Jasminum tortuosum. Natural Product Research, 2018, 32, 1853–1857. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.Z.; Yong, JP.; Lu, C.Z. Isolation, Structural Elucidation of a New Triterpenoid from the Roots of Jasminum sambac (L. ) Ait. with Potent Cytotoxicity against MCF 7 Cell Lines. ACS Omega. 2023, 8, 14662–14664. [Google Scholar] [PubMed]
- Olatunde, O.Z.; Yong, J.P.; Lu, C.Z. Chemical Constituents from the roots of Jasminum sambac (L.) Ait. and cytotoxicity to the cancer cell lines. Anticancer Agents in Medicinal Chemistry. [CrossRef]
Table 1.
1H(400 MHz) and 13C(100 MHz) NMR data for compounds 1, 2 in DMSO-d6 and compounds 3 ,4 in CD3OD (δ in ppm and J in Hz)
Table 1.
1H(400 MHz) and 13C(100 MHz) NMR data for compounds 1, 2 in DMSO-d6 and compounds 3 ,4 in CD3OD (δ in ppm and J in Hz)
| No. |
Compound 1 |
Compound 2 |
Compound 3 |
Compound 4 |
| |
1H |
13C |
1H |
13C |
1H |
13C |
1H |
13C |
| 1 |
|
43.9 C |
|
43.8 |
|
44.4 C |
|
41.9 C |
| 2α |
1.98, m |
39.2 CH2 |
1.90, m |
33.1 CH2
|
1.85, dt(3.4, 2.5) |
34.0 CH2
|
1.24, m |
29.4 CH2
|
| 2β |
2.12, m |
|
1.53, m |
|
2.18, m |
|
|
|
| 3α |
1.80, m |
34.8 CH2
|
1.61, m |
35.0 CH2
|
2.02, m |
35.5 CH2
|
1.76, m |
33.7 CH2
|
| 3β |
2.15, m |
|
|
|
|
|
|
|
| 4 |
|
71.1 C |
|
70.1 C |
|
71.3 C |
|
68.8 C |
| 5 |
|
123.8 C |
1.22, m |
29.2 CH2
|
6.47, s |
126.4 CH |
2.30, m |
25.6 CH2
|
| 6α |
6.84 |
123.9 CH |
2.56, m |
38.3 CH2
|
2.58, d(11.8) |
38.5 CH2
|
1.60, m |
26.0 CH2
|
| 6β |
|
|
2.46 |
|
|
|
|
|
| 7α |
|
143.4 C |
2.43, d(1.8) |
42.4 CH2
|
2.39, d(2.1) |
41.7 CH2
|
|
188.7 C |
| 7β |
|
|
2.21, m |
|
2.27, m |
|
|
|
| 8 |
|
148.3 C |
|
199.6 C |
|
200.4 C |
7.01, s |
139.9 CH |
| 9 |
|
191.9 C |
|
191.9 C |
|
187.8 C |
4.17, s |
67.9 CH |
| 10 |
4.01, q(4.4) |
33.1 CH |
3.26, m |
39.6 CH |
3.76, m |
39.9 CH |
2.24 |
30.7 CH |
| 11 |
|
137.1 C |
|
135.5 C |
|
136.7 C |
|
|
| 12 |
|
148.34 |
|
148.8 C |
|
142.9 C |
|
|
| 13 |
|
116.1 C |
|
135.6 C |
|
39.6 C |
|
139.1 C |
| 14 |
|
136.9 C |
|
157.7 C |
|
158.1 C |
|
42.8 C |
| 15 |
2.24, s |
17.7 CH3
|
0.99, s |
17.6 CH3 |
1.04, s |
16.8 CH3
|
0.97, d(4.6) |
14.0 CH3
|
| 16 |
1.45, s |
28.9 CH3
|
1.51, s |
27.8 CH3
|
1.45, s |
27.2 CH3
|
1.49, s |
26.3 CH3
|
| 17 |
1.32, d(4.4) |
28.1 CH3
|
0.94, d(4.4) |
15.3 CH3
|
0.84, s |
13.7 CH3
|
0.86, s |
18.1 CH3
|
| 18 |
1.72, s |
23.2 CH3
|
1.58, s |
25.6 CH3
|
1.73, s |
23.4 CH3
|
1.44, s |
23.1 CH3
|
| 1' |
|
168.9 C |
|
167.5 C |
|
168.8 C |
|
138.5 C |
| 2' |
5.69, s |
121.9 CH |
6.27, d(0.7) |
126.8 CH |
6.89, s |
122.9 CH |
5.79, s |
125.7 CH |
| 3' |
|
191.8 C |
|
186.9 C |
|
191.2 C |
4.51, s |
66.2 CH |
| 4' |
|
148.4 C |
|
148.7 C |
|
147.9 C |
6.96, s |
139.2 CH |
| 5' |
|
143.6 C |
|
143.5 C |
|
142.9 C |
|
187.2 C |
| 6' |
6.93, s |
124.0 CH |
6.89, s |
123.9 CH |
6.90, s |
122.4 CH |
2.28, m |
25.9 CH2
|
| 7' |
|
124.5 C |
|
123.6 C |
|
123.7 C |
1.58, m |
25.4 CH2
|
| 8' |
4.17, q(4.8) |
35.5 CH |
4.29, q(4.5) |
31.7 CH |
4.09, q(4.4) |
33.1 CH |
2.30 |
30.6 CH |
| 9' |
|
137.2 C |
|
137.4 C |
|
136.8 C |
|
41.6 C |
| 10' |
|
114.8 C |
|
115.8 C |
|
115.2 C |
|
136.6 C |
| 11' |
2.31, s |
18.1 CH3
|
2.25, s |
17.7 CH3
|
2.32, s |
16.3 CH3
|
0.99, d(2.9) |
13.9 CH3
|
| 12' |
1.26, d(4.4) |
24.4 CH3
|
1.26, d(4.5) |
27.9 CH3
|
1.42, d(4.4) |
26.7 CH3
|
0.93, s |
18.4 CH3
|
| 4-OH |
5.35, s |
|
5.40, s |
|
|
|
|
|
| 7-OH |
11.78, |
|
|
|
|
|
|
|
| 8-OH |
9.10, s |
|
|
|
|
|
|
|
| 4'-OH |
8.99, s |
|
9.07, s |
|
|
|
|
|
| 5'-OH |
12.53, s |
|
12.23, s |
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).