Submitted:
15 May 2023
Posted:
18 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Obtaining the Epididymal Sperm
2.3. Sperm Cryopreservation and Maca Supplementation
2.4. Evaluation of Sperm Parameters
2.4.1. Sperm Motility
2.4.2. Sperm Viability
2.4.3. Plasma Membrane Integrity
2.5.1. Sperm Mitochondrial Activity
2.5.2. DNA Fragmentation Analysis
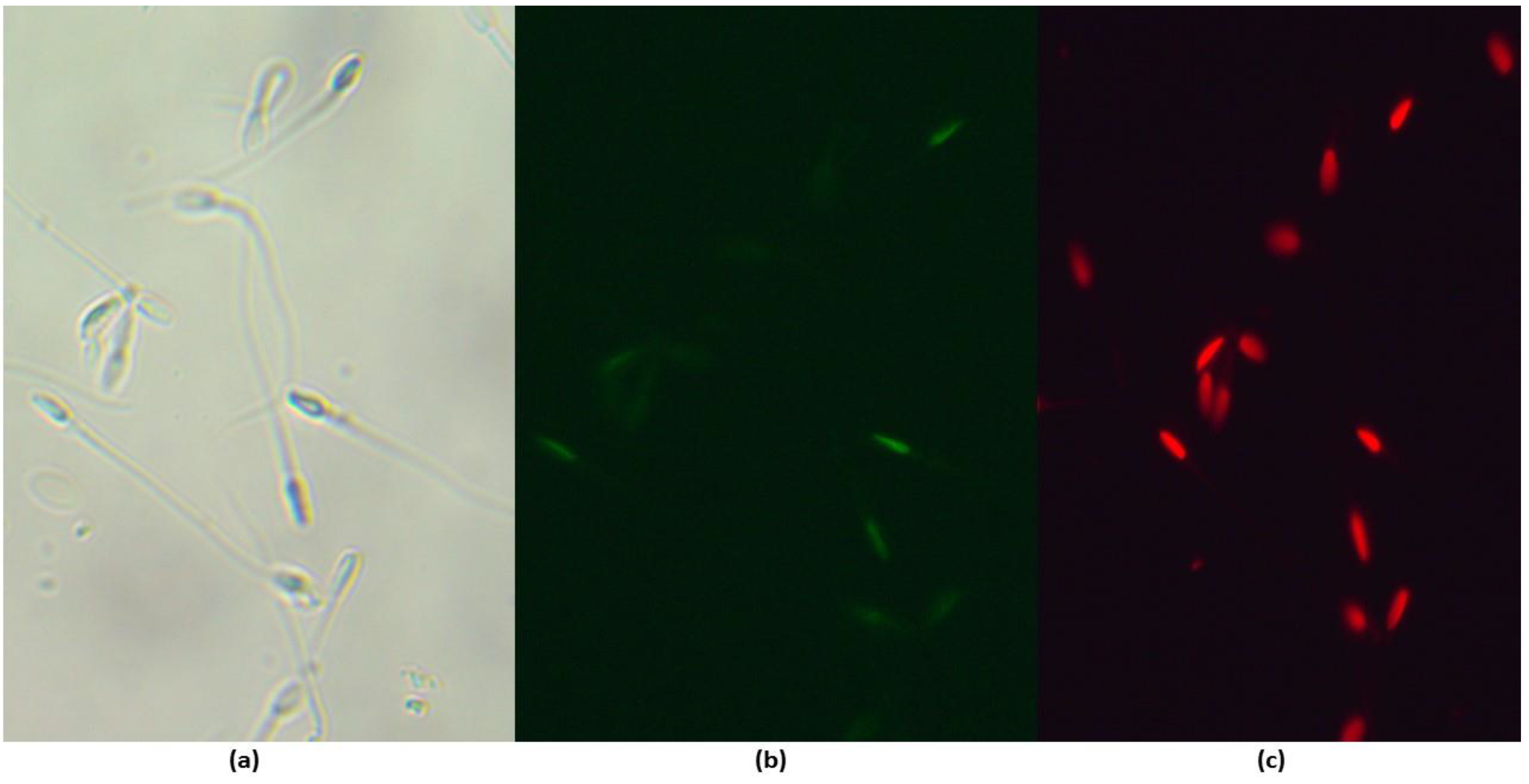
2.5.3. Evaluation of ROS Production
2.6. Cryopreservation Method
2.7. Statistical Analysis
Results
3.1. Effect of Maca on Sperm Parameters
3.2. Effect of Maca on Sperm Motility
3.3. Effect of Maca on the plasma membrane integrity
3.4. Effect of Maca on Sperm Viability
3.5. Effect of Maca on Sperm Oxidative Stress Activity
3.6. Effect of Maca on Sperm DNA Fragmentation
3.7. Effect of Maca on Lipid Peroxidation
3.8. Effect of Maca on Cytochemical Activity of Oxidoreductases
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quina, E. Inseminación artificial de alpacas en un contexto de crianza campesina. Descosur 2017.
- Landeo L, Zuñiga M, Gastelu T, Artica M, Ruiz J, Silva M, Ratto MH. Oocyte Quality, In Vitro Fertilization and Embryo Development of Alpaca Oocytes Collected by Ultrasound-Guided Follicular Aspiration or from Slaughterhouse Ovaries. Animals 2022, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- O'Flaherty, C. , Beorlegui, N., & Beconi, M. T. Participation of superoxide anion in the capacitation of cryopreserved bovine sperm. International journal of andrology 2003, 26, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Santiani A, Huanca W, Sapana R, Huanca T, Sepúlveda N, Sánchez R. Effects on the quality of frozen-thawed alpaca (Lama pacos) semen using two different cryoprotectants and extenders. Asian J. Androl. 2005, 7, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Casaretto, C. , Martínez Sarrasague, M., Giuliano, S., Rubin de Celis, E., Gambarotta, M., Carretero, I., & Miragaya, M. Evaluation of Lama glama semen viscosity with a cone-plate rotational viscometer. Andrologia, 2012, 44, 335–341. [Google Scholar] [CrossRef]
- O’Neill, H. C. , Nikoloska, M., Ho, H., Doshi, A., & Maalouf, W.Improved cryopreservation of spermatozoa using vitrification: comparison of cryoprotectants and a novel device for long-term storage. Journal of assisted reproduction and genetics, 2019, 36, 1713–1720. [Google Scholar] [CrossRef]
- Rosato, M. & Laffaldano, N.. Cryopreservation of rabbit semen: comparing the effects of different cryoprotectants, cryoprotectant-free vitrification, and the use of albumin plus osmoprotectants on sperm survival and fertility after standard vapor freezing and vitrification. Theriogenology, 2013, 79, 508–516. [Google Scholar] [CrossRef]
- Agarwal, A. , Makker, K., Sharma, R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol 2008, 59, 2–11. [Google Scholar] [CrossRef]
- Du Plessis, S. , Makker K., Desai N.& Agarwal A. The impact of oxidative stress on in vitro fertilization. Expert Review Of Obstetrics & Gynecology. 2008, 3, 539–554. [Google Scholar] [CrossRef]
- Aitken, R. J. , Gibb, Z., Baker, M. A., Drevet, J., & Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reproduction, Fertility and Development 2016, 28, 1–10. [Google Scholar] [CrossRef]
- Aitken, R.J. , 2017. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev 2017, 84, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Treulen F, Arias ME, Aguila L, Uribe P, Felmer R. Cryopreservation induces mitochondrial permeability transition in a bovine sperm model. Cryobiology. 2018, 83, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Treulen F, Aguila L, Arias ME, Jofré I, Felmer R. Impact of post-thaw supplementation of semen extender with antioxidants on the quality and function variables of stallion spermatozoa. Anim Reprod Sci. 2019, 201, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Shojaeian, K. , Nouri, H., Kohram, H. Does MnTBAP ameliorate DNA fragmentation and in vivo fertility of frozen-thawed Arabian stallion sperm? Theriogenology 2018, 108, 16–21. [Google Scholar] [CrossRef]
- Amidi, F. , Pazhohan, A., Shabani Nashtaei, M., Khodarahmian, M., & Nekoonam, S. The role of antioxidants in sperm freezing: a review. Cell and tissue banking 2016, 17, 745–756. [Google Scholar] [CrossRef]
- Ros-Santaella JL, Pintus E. Plant Extracts as Alternative Additives for Sperm Preservation. Antioxidants (Basel). 2021, 10, 772. [Google Scholar] [CrossRef]
- Pasquariello R, Verdile N, Brevini TAL, Gandolfi F, Boiti C, Zerani M, Maranesi M. The Role of Resveratrol in Mammalian Reproduction. Molecules. 2020, 25, 4554. [Google Scholar] [CrossRef]
- Wang S, Zhu F. Chemical composition and health effects of maca (Lepidium meyenii). Food Chem. 2019, 288, 422–443. [Google Scholar] [CrossRef]
- León, J. (1964). The" Maca"(Lepidium meyenii), a little known food Plant of Peru. Economic botany 1964, 18, 122–127. [Google Scholar] [CrossRef]
- Piacente, S. , Carbone, V., Plaza, A., Zampelli, A., & Pizza, C. Investigation of the tuber constituents of maca (Lepidium meyenii Walp. ). Journal of agricultural and food chemistry 2022, 50, 5621–5625. [Google Scholar] [CrossRef]
- Gonzales GF, Cordova A, Vega K, Chung A, Villena A, Góñez C. Effect of Lepidium meyenii (Maca), a root with aphrodisiac and fertility-enhancing properties, on serum reproductive hormone levels in adult healthy men. J Endocrinol. 2003, 176, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Zheng BL, He K, Kim CH, Rogers L, Shao Y, Huang ZY, et al. . Effect of a lipidic extract from Lepidium meyenii on sexual behavior in mice and rats. Urology. 2000, 55, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Zenico T, Cicero AF, Valmorri L, Mercuriali M, Bercovich E. Subjective effects of Lepidium meyenii (Maca) extract on well-being and sexual performances in patients with mild erectile dysfunction: a randomised, double-blind clinical trial. Andrologia., 2009, 41, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Gonzales C, Rubio J, Gasco M, Nieto J, Yucra S, Gonzales GF. Effect of short-term and long-term treatments with three ecotypes of Lepidium meyenii (MACA) on spermatogenesis in rats. J Ethnopharm. 2006, 103, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Sandoval M, Okuhama NN, Angeles FM, Melchor VV, Condezo LA, Lao J, et al. . Antioxidant activity of the cruciferous vegetable Maca (Lepidium meyenii). Food Chem. 2002, 79, 207–213. [Google Scholar] [CrossRef]
- Lee K-J, Dabrowski K, Sandoval M, Miller MJ. Activity-guided fractionation of phytochemicals of maca meal, their antioxidant activities and effects on growth, feed utilization, and survival in rainbow trout (Oncorhynchus mykiss) juveniles. Aquaculture. 2005, 244, 293–301. [Google Scholar] [CrossRef]
- Zha S, Zhao Q, Chen J, Wang L, Zhang G, Zhang H, et al. . Extraction, purification and antioxidant activities of the polysaccharides from maca (Lepidium meyenii). Carbohydrate polymers. 2014, 111, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Valdivia M, Yarasca K, Lévano G, Vásquez J, Temoche H, Torres L, et al. Effect of Lepidium meyenii (maca) on testicular function of mice with chemically and physically induced subfertility. Andrologia. 2016, 48, 927–934. [Google Scholar] [CrossRef]
- Rubio, J. , Riqueros, M.I., Gasco, M., Yucra, S., Miranda, S. and Gonzales, G.F. Lepidium meyenii (Maca) reversed the lead acetate induced-damage on reproductive function in male rats. Food Chem. Toxicol. 2006, 44, 1114–1122. [Google Scholar] [CrossRef]
- Gonzales-Castañeda C, Rivera V, Chirinos AL, Evelson P, Gonzales GF. Photoprotection against the UVB-induced oxidative stress and epidermal damage in mice using leaves of three different varieties of Lepidium meyenii (maca). Int J Dermatol. 2011, 50, 928–938. [Google Scholar] [CrossRef]
- Dzięcioł, M. , Wróblewska, A., & Janda-Milczarek, K. Comparative Studies of DPPH Radical Scavenging Activity and Content of Bioactive Compounds in Maca (Lepidium meyenii) Root Extracts Obtained by Various Techniques. Applied Sciences. 2023, 13, 4827. [Google Scholar] [CrossRef]
- N. Inoue, C. Farfan and G. F. Gonzales, Effect of butanolic fraction of yellow and black maca (Lepidium meyenii) on the sperm count of adult mice, Andrology, 2016, 48, 915–921. [CrossRef] [PubMed]
- World Health Organization "WHO Laboratory Manual for the examination and processing of human semen”, 2010 ,5ta edición.
- Hrudka, F. Cytochemistry of oxidoreductases in spermatozoa: The technique revisited. Andrologia, 1979, 11, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Santiani, A. , Evangelista, S., Cheuquemán, C., von Baer, A., Risopatrón, J., & Sánchez, R.. Evaluación de la integridad de ADN mediante citometría de flujo en espermatozoides de alpaca criopreservados con análogos de superóxido dismutasa. Revista de Investigaciones Veterinarias del Perú, 2012, 23, 182–191. [Google Scholar]
- Nova, Z. , Skovierova, H., Strnadel, J., Halasova, E., & Calkovska, A. Short-term versus long-term culture of A549 cells for evaluating the effects of lipopolysaccharide on oxidative stress, surfactant proteins and cathelicidin LL-37. International Journal of Molecular Sciences, 2020, 21, 1148. [Google Scholar] [CrossRef]
- Canorio Pariona, N. , Paredes Arnedo, F., & Valdivia Cuya, M. Agentes crioprotectores alternativos para el congelamiento lento de espermatozoides epididimarios de alpaca (Vicugna pacos). Revista de Investigaciones Veterinarias del Perú 2015, 26, 434–443. [Google Scholar] [CrossRef]
- Buyanbadrakh, E. , Hong, H.S., Lee, K.W., Huang, W.Y. and Oh, J.H. Anti-oxidant activity, macamide B content and muscle cell protection of Maca (Lepidium meyenii) extracted using ultrasonication-assisted extraction. Microbiol. Biotechnol. Lett. 2020, 48, 129–13. [Google Scholar] [CrossRef]
- Ezzati, M. , Shanehbandi, D., Hamdi, K., Rahbar, S., & Pashaiasl, M. Influence of cryopreservation on structure and function of mammalian spermatozoa: An overview. Cell and Tissue Banking. 2020, 21, 1–15. [Google Scholar] [CrossRef]
- Grötter, L. G. , Cattaneo, L., Marini, P. E., Kjelland, M. E., & Ferré, L. B.. Recent advances in bovine sperm cryopreservation techniques with a focus on sperm post-thaw quality optimization. Reproduction in Domestic Animals. 2019, 54, 655–665. [Google Scholar] [CrossRef]
- Ciani F, Cocchia N, Del Prete C, Palumbo V, Carotenuto D, Pasolini MP, Tafuri S. Sperm cromatin integrity in stallions with Lepidium meyenii (Maca) dietary supplementation. Reprod Domest Anim. 2017, 52, 7. [Google Scholar]
- Tafuri, S.; Cocchia, N.; Carotenuto, D.; Vassetti, A.; Staropoli, A.; Mastellone, V.; Peretti, V.; Ciotola, F.; Albarella, S.; Del Prete, C.; et al. Chemical analysis of Lepidium meyenii (Maca) and its effects on redox status and on reproductive biology in stallions. Molecules 2019, 24, 1981. [Google Scholar] [CrossRef] [PubMed]
- Mamani-Mango, G. , Moina Gonzales, M., Ramos Hidalgo, M., Mendoza Mallma, J., Ruiz Bejar, J., Rivas Palma, V., & Mellisho Salas, E. Effect of extender and freezing rate on quality parameters and in vitro fertilization capacity of alpaca spermatozoa recovered from cauda epididymis. Biopreservation and biobanking, 2019, 17, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Juárez, J. , & Santiani, A. Determinación del porcentaje de viabilidad espermática mediante citometría de flujo durante el proceso de criopreservación en espermatozoides obtenidos de epidídimo de alpaca. Revista de Investigaciones Veterinarias del Perú, 2019, 30, 1175–1183. [Google Scholar] [CrossRef]
- Naresh, S. , & Atreja, S. K.. The protein tyrosine phosphorylation during in vitro capacitation and cryopreservation of mammalian spermatozoa. Cryobiology, 2015, 70, 211–216. [Google Scholar] [CrossRef]
- Leiva-Revilla, J. , Rolón, M., Siyadatpanah, A., de Lourdes Pereira, M., & Nissapatorn, V. First study of in vitro protective effect of Lepidium meyenii (Maca) on frozen–thawed bovine spermatozoa. Veterinary World 2022, 15, 1481. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. , Mattson, M.P. and Calabrese, V. Resveratrol commonly displays hormesis: Occurrence and biomedical significance. Hum. Exp. Toxicol., 29(12): 980–1015 fragmentation. Journal of Assisted Reproduction and Genetics. 2010, 24, 561–569. [Google Scholar] [CrossRef]
- Rashki Ghaleno, L. , Alizadeh, A., Drevet, J. R., Shahverdi, A., & Valojerdi, M. R. Oxidation of sperm DNA and male infertility. Antioxidants, 2021, 10, 97. [Google Scholar] [CrossRef]
- Del Prete, C. , Tafuri, S., Ciani, F., Pasolini, M. P., Ciotola, F., Albarella, S.,... & Cocchia, N. Influences of dietary supplementation with Lepidium meyenii (Maca) on stallion sperm production and on preservation of sperm quality during storage at 5 C. Andrology. 2018, 6, 351–361. [Google Scholar] [CrossRef]
- Restrepo, G. , Varela, E., Duque, J. E., Gómez, J. E., & Rojas, M. Freezing, vitrification, and freeze-drying of equine spermatozoa: Impact on mitochondrial membrane potential, lipid peroxidation, and DNA integrity. Journal of equine veterinary science, 2019, 72, 8–15. [Google Scholar] [CrossRef]
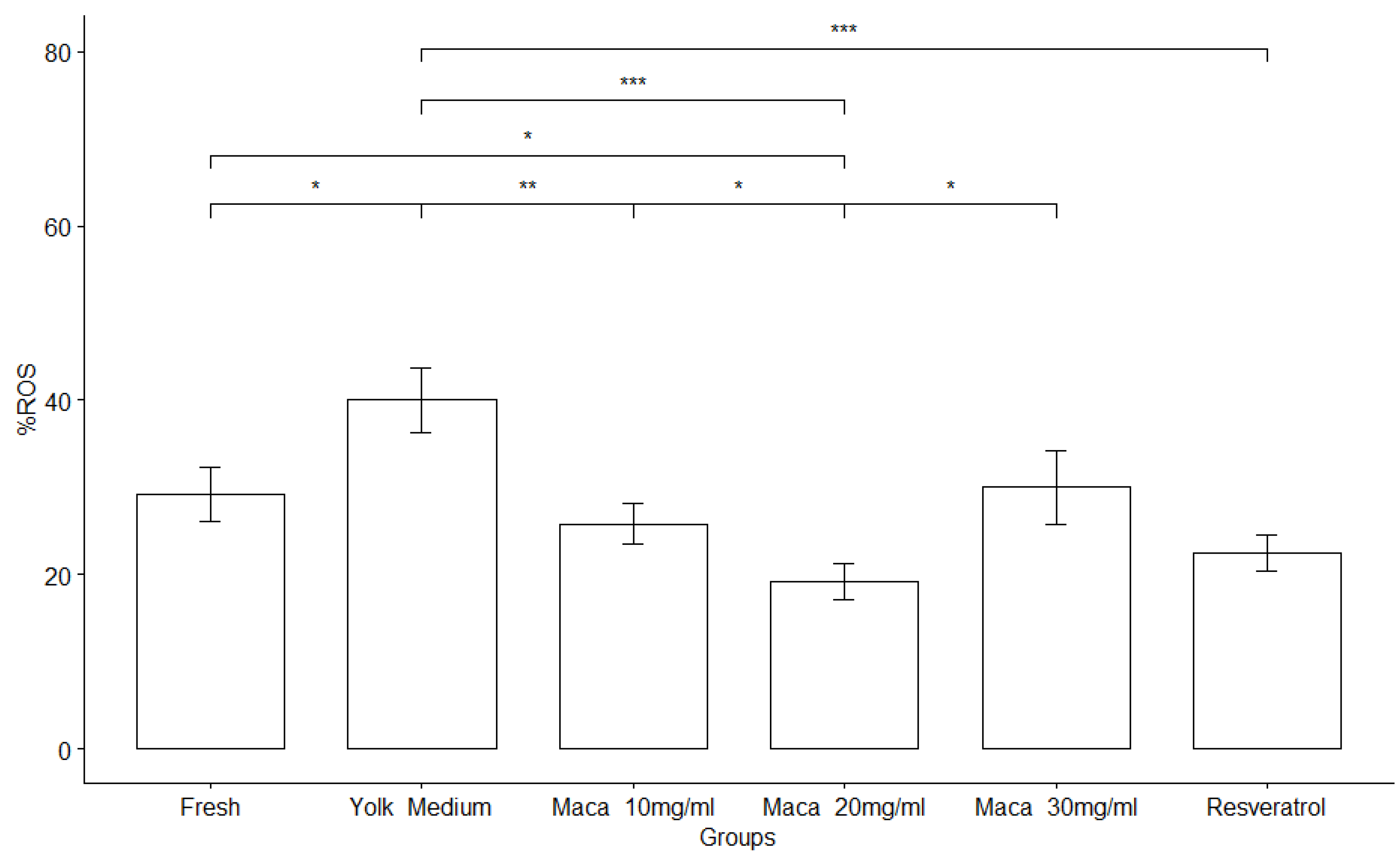
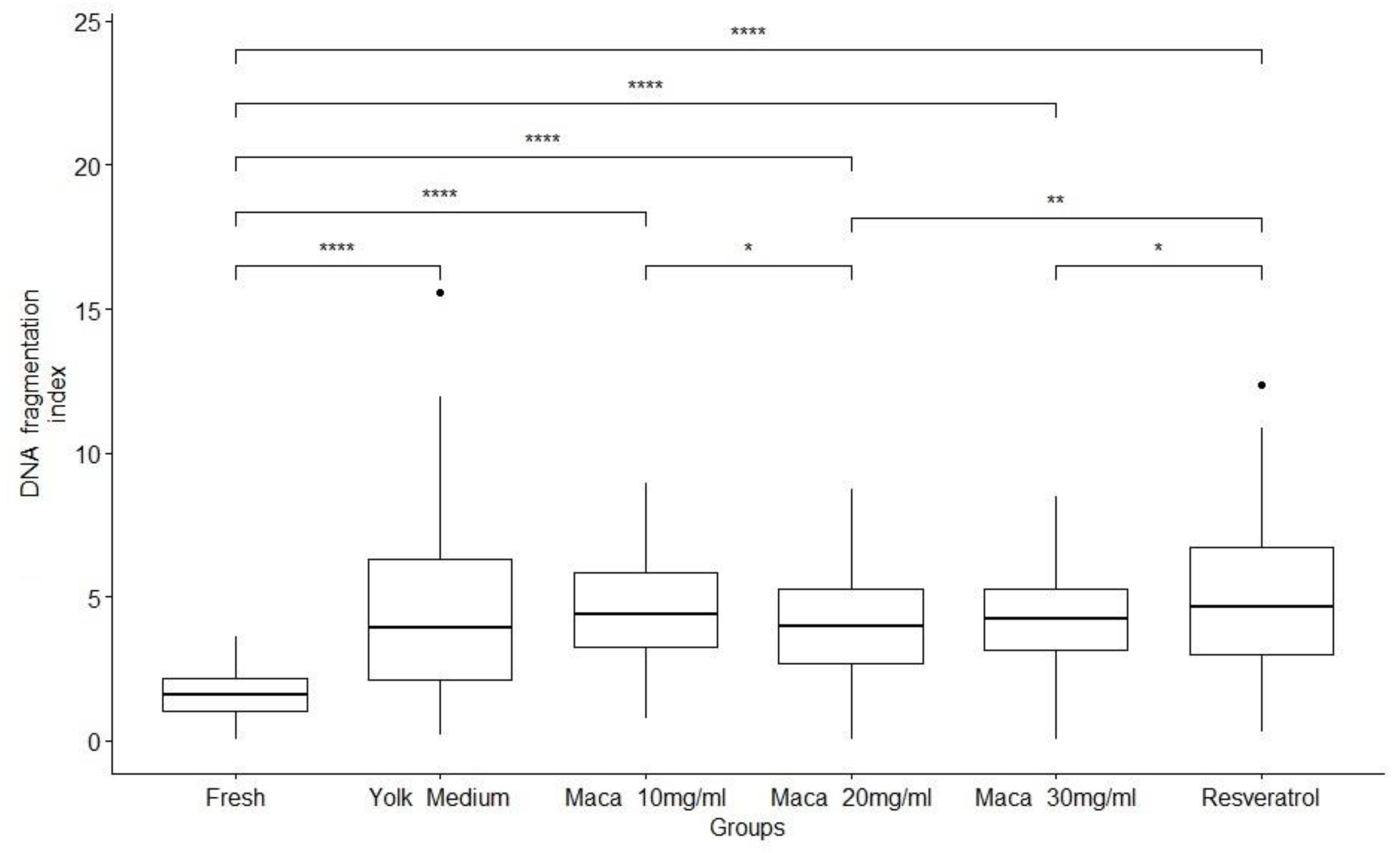

| Groups | Motility % | Viability % | HOST% | DNA fragmentation index |
ROS % | Mitochondrial activity index |
|---|---|---|---|---|---|---|
| Fresh | 75 ± 2.67a | 61.25 ± 8.29a | 69.16 ± 8.21a | 1.64 ± 0.83a | 29.2 ± 10.7a |
0.892±0.255a |
| *YM | 25 ± 1.49b | 46.25 ± 5.69b | 51.25 ± 7.72b | 4.47 ± 3.10a | 40 ± 12.6b | 0,910 ± 0,277a |
| *Maca 10mg/ml | 32.5±1.71c | 47.08 ± 6.89b | 50.08 ± 6.20b | 4.55 ± 1.91a | 25.8 ±7.93c | 0,896 ± 0,255a |
| *Maca 20mg/ml | 40 ± 2.45d | 54.58 ± 5.41c | 58.33 ± 7.78c | 3.95 ± 1.88a | 19.2 ± 7.33c | 0,882 ± 0,171ª |
| *Maca 30mg/ml | 22.5 ±1.83b | 48.33 ±6.85b | 52.91 ± 3.34b | 4.16 ± 1.77a | 30 ± 14.8c | 0,765± 0,188a |
| *Resveratrol | 27 ± 1.20b | 55.83 ±8.75c | 57.08 ± 2.57c | 4.90 ± 2.65a | 22.5 ± 7.23c | 0.872 ± 0.098a |
| Rate of Activity (%) | ||||||
|---|---|---|---|---|---|---|
| Standar | Sub-standar | Low | Residual | Intensive Reaction (St + Sub-st) |
Reduced Reaction (Low + Res) |
|
| Fresh | 38 ± 19.4 | 13.5± 9.42 | 0.900 ±0.65 | 0 | 51.5a | 0.9a |
| *TYM | 44.2± 32.8 | 26.0 ± 19.2 | 0.500 ±0.79 | 0.150± 0.28 | 70.2b | 0.65b |
| *Maca 10mg/ml | 47.9 ± 32.1 | 13.7 ± 9.50 | 0.550 ±1.50 | 0.008 ±0.03 | 61.6b | 0.55c |
| *Maca 20mg/ml | 21.3 ± 5.61 | 16.9 ± 22.4 | 0.375 ±0.45 | 0.033± 0.12 | 38.2c | 0.408c |
| *Maca 30mg/ml | 40.3 ± 21.2 | 11.0 ± 8.44 | 0.525 ±0.73 | 0.083±0.15 | 51.3a | 0.608d |
| *Resveratrol | 23.5 ± 12.4 | 11.5 ± 9.66 | 2.22 ±0.46 | 0.017±0.06 | 35c | 2.217e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





