Submitted:
18 May 2023
Posted:
18 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
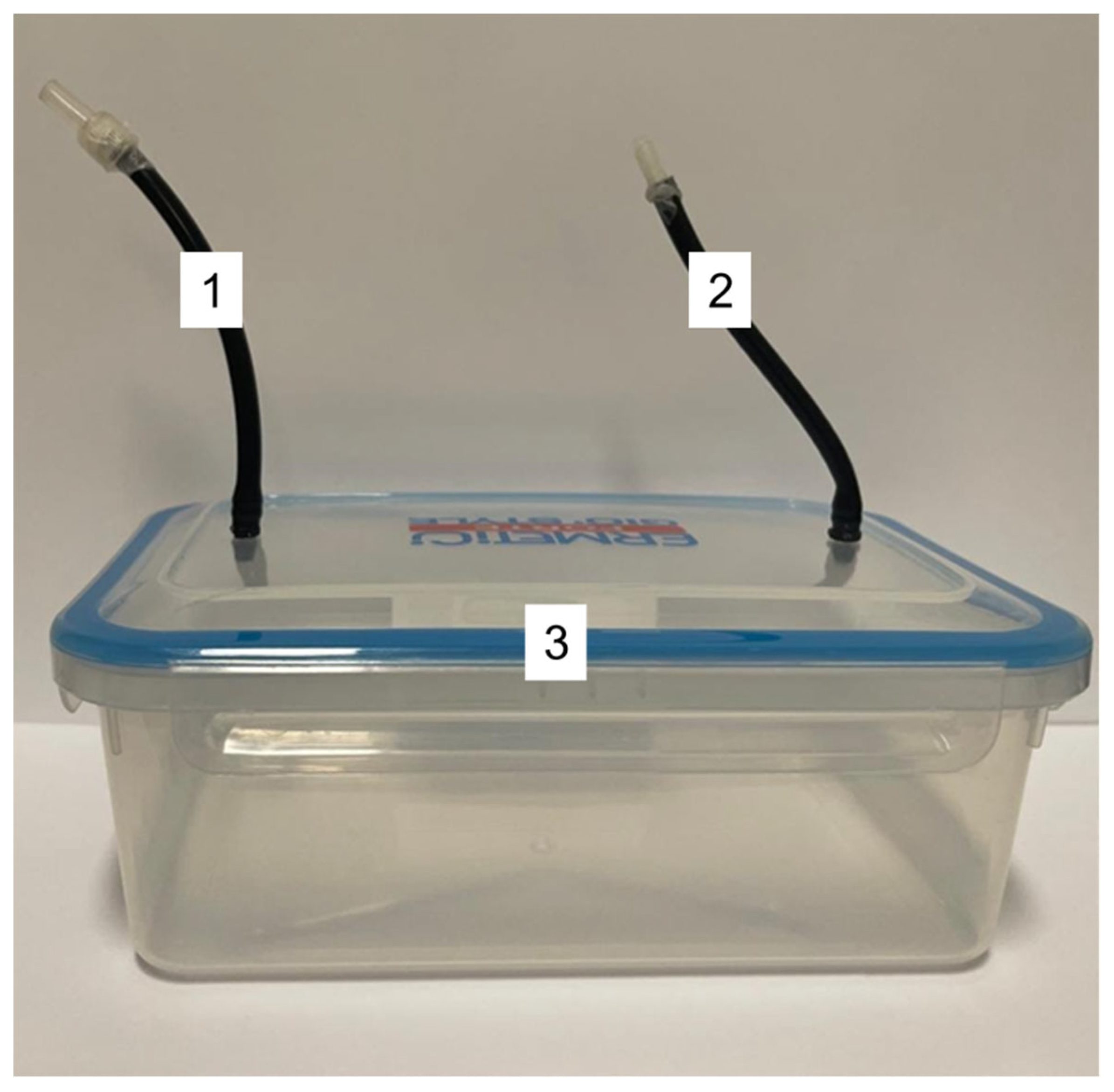
2.1. O3 Generator
2.2. Hermetic Box for Gas Flow
2.3. Cells and Virus
2.4. Cytotoxicity Assay
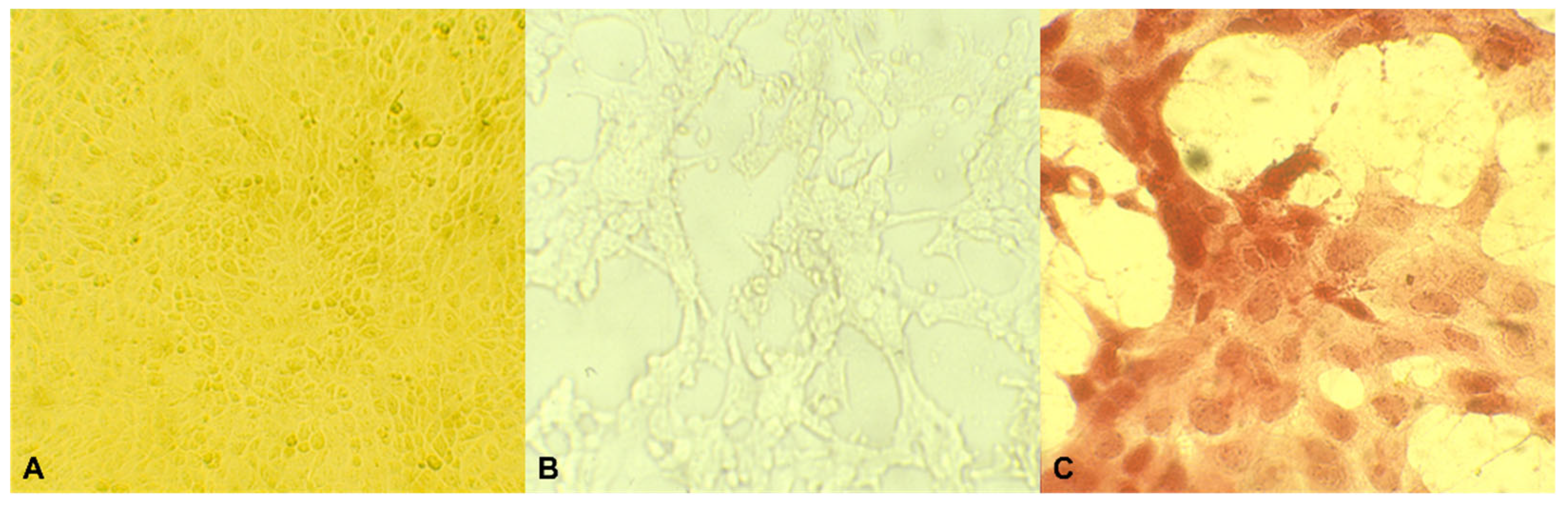
2.5. Cytophatic Effect
2.6. Virucidal Activity Assay
2.7. Antiviral Assays
2.7.1. Protocol A: Virus Infection of Cell Monolayers before Treatment with O3
2.7.2. Protocol B: Virus Infection of Cell Monolayers after Treatment with O3
2.8. Viral Titration
2.9. Data Analysis
3. Results
3.1. Cytotoxicity Assay
3.2. Cytophatic Effect
3.3. Virucidal Activity Assay
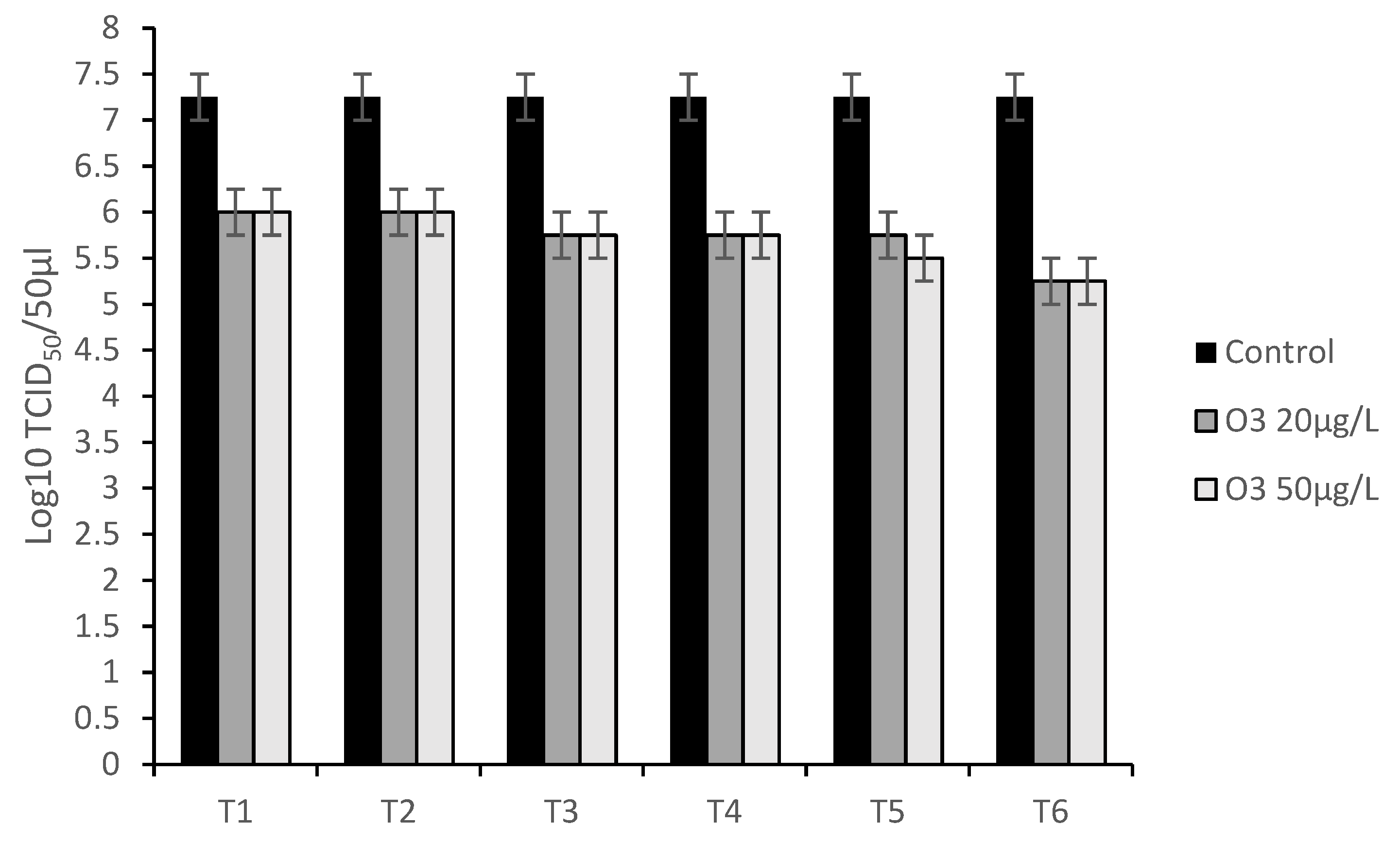
3.4. Antiviral Assays
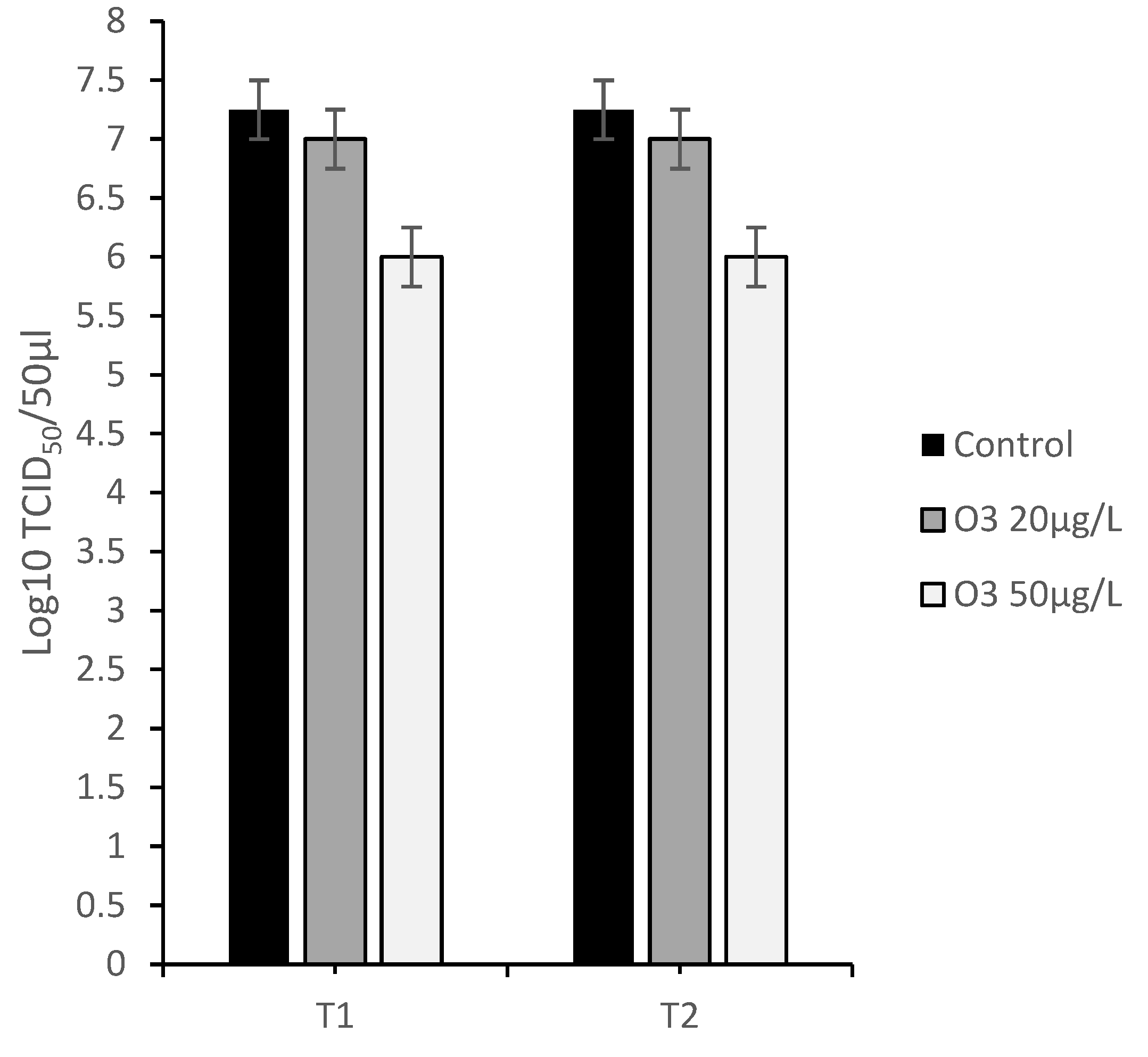
3.4.1. Protocol A: Virus Infection of Cell Monolayers before Treatment with O3
3.4.2. Protocol B: Virus Infection of Cell Monolayers after Treatment with O3
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, J.R.; Didlick, S. Epidemiology, Disease and Control of Infections in Ruminants by Herpesviruses - an Overview : Review Article. J S Afr Vet Assoc 2008, 79, 8–14. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, M.E.; Abu-raddad, L.J. WHO-Bulletin HSV-2 2016. Bull World Health Organ 2020, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Engels, M.; Ackermann, M. Pathogenesis of Ruminant Herpesvirus Infections. Vet Microbiol 1996, 53, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Warren, T.; Wald, A. Genital Herpes. The Lancet 2007, 370, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.E.; Weiss, H.A.; Glynn, J.R.; Cross, P.L.; Whitworth, J.A.; Hayes, R.J. Herpes Simplex Virus 2 Infection Increases HIV Acquisition in Men and Women: Systematic Review and Meta-Analysis of Longitudinal Studies. AIDS 2006, 20, 73–83. [Google Scholar] [CrossRef]
- Sadowski, L.A.; Upadhyay, R.; Greeley, Z.W.; Margulies, B.J. Current Drugs to Treat Infections with Herpes Simplex Viruses-1 and -2. Viruses 2021, 13, 1228. [Google Scholar] [CrossRef]
- Schalkwijk, H.H.; Snoeck, R.; Andrei, G. Acyclovir Resistance in Herpes Simplex Viruses: Prevalence and Therapeutic Alternatives. Biochem Pharmacol 2022, 206, 115322. [Google Scholar] [CrossRef]
- Tempesta, M.; Pratelli, A.; Greco, G.; Martella, V.; Buonavoglia, C. Detection of Caprine Herpesvirus 1 in Sacral Ganglia of Latently Infected Goats by PCR. J Clin Microbiol 1999, 37, 1598–1599. [Google Scholar] [CrossRef]
- Tempesta, M.; Camero, M.; Sciorsci, R.L.; Greco, G.; Minoia, R.; Martella, V.; Pratelli, A.; Buonavoglia, C. Experimental Infection of Goats at Different Stages of Pregnancy with Caprine Herpesvirus 1. Comp Immunol Microbiol Infect Dis 2004, 27, 25–32. [Google Scholar] [CrossRef]
- Thiry, J.; Keuser, V.; Muylkens, B.; Meurens, F.; Gogev, S.; Vanderplasschen, A.; Thiry, E. Ruminant Alphaherpesviruses Related to Bovine Herpesvirus 1. Vet Res 2006, 37, 169–190. [Google Scholar] [CrossRef]
- Camero, M.; Marinaro, M.; Losurdo, M.; Larocca, V.; Bodnar, L.; Patruno, G.; Buonavoglia, C.; Tempesta, M. Caprine Herpesvirus 1 (CpHV-1) Vaginal Infection of Goats: Clinical Efficacy of Fig Latex. Nat Prod Res 2016, 30, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Tempesta, M.; Crescenzo, G.; Camero, M.; Bellacicco, A.L.; Tarsitano, E.; Decaro, N.; Neyts, J.; Martella, V.; Buonavoglia, C. Assessing the Efficacy of Cidofovir against Herpesvirus-Induced Genital Lesions in Goats Using Different Therapeutic Regimens. Antimicrob Agents Chemother 2008, 52, 4064–4068. [Google Scholar] [CrossRef]
- Camero, M.; Buonavoglia, D.; Lucente, M.S.; Losurdo, M.; Crescenzo, G.; Trerotoli, P.; Casalino, E.; Martella, V.; Elia, G.; Tempesta, M. Enhancement of the Antiviral Activity against Caprine Herpesvirus Type 1 of Acyclovir in Association with Mizoribine. Res Vet Sci 2017, 111, 120–123. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, Z.; Huang, Q.; Yan, J.; Zhang, X.; Yu, X.; Tan, G.; Zheng, C.; Xu, F.; He, S. Antiviral Activity of PHA767491 against Human Herpes Simplex Virus in Vitro and in Vivo. BMC Infect Dis 2017, 17, 217. [Google Scholar] [CrossRef] [PubMed]
- Lanave, G.; Lucente, M.S.; Siciliano, P.; Zizzadoro, C.; Trerotoli, P.; Martella, V.; Buonavoglia, C.; Tempesta, M.; Camero, M. Antiviral Activity of PHA767491 on Caprine Alphaherpesvirus 1 in Vitro. Res Vet Sci 2019, 126, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyev, A.; Duran, N.; Ozguven, M.; Koltas, S. Antiviral Activity of the Volatile Oils of Melissa Officinalis L. against Herpes Simplex Virus Type-2. Phytomedicine 2004, 11, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Camero, M.; Lanave, G.; Catella, C.; Capozza, P.; Gentile, A.; Fracchiolla, G.; Britti, D.; Martella, V.; Buonavoglia, C.; Tempesta, M. Virucidal Activity of Ginger Essential Oil against Caprine Alphaherpesvirus-1. Vet Microbiol 2019, 230, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Camero, M.; Marinaro, M.; Losurdo, M.; Larocca, V.; Bodnar, L.; Patruno, G.; Buonavoglia, C.; Tempesta, M. Caprine Herpesvirus 1 (CpHV-1) Vaginal Infection of Goats: Clinical Efficacy of Fig Latex. Nat Prod Res 2016, 30, 605–607. [Google Scholar] [CrossRef]
- Schnitzler, P.; Schön, K.; Reichling, J. Antiviral Activity of Australian Tea Tree Oil and Eucalyptus Oil against Herpes Simplex Virus in Cell Culture. Pharmazie 2001, 56, 343–347. [Google Scholar]
- Sciorsci, R.L.; Lillo, E.; Occhiogrosso, L.; Rizzo, A. Ozone Therapy in Veterinary Medicine: A Review. Res Vet Sci 2020, 130, 240–246. [Google Scholar] [CrossRef]
- Braidy, N.; Izadi, M.; Sureda, A.; Jonaidi-Jafari, N.; Banki, A.; Nabavi, S.F.; Nabavi, S.M. Therapeutic Relevance of Ozone Therapy in Degenerative Diseases: Focus on Diabetes and Spinal Pain. J Cell Physiol 2018, 233, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Azarpazhooh, A.; Limeback, H. The Application of Ozone in Dentistry: A Systematic Review of Literature. J Dent 2008, 36, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Lillo, E.; Cordisco, M.; Trotta, A.; Greco, G.; Carbonari, A.; Rizzo, A.; Sciorsci, R.L.; Corrente, M. Evaluation of Antibacterial Oxygen/Ozone Mixture in Vitro Activity on Bacteria Isolated from Cervico-Vaginal Mucus of Cows with Acute Metritis. Theriogenology 2023, 196, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.K.; Ohmine, S.; Tomer, D.P.; Jensen, K.J.; Johnson, F.B.; Kirsi, J.J.; Robison, R.A.; O’Neill, K.L. Virion Disruption by Ozone-Mediated Reactive Oxygen Species. J Virol Methods 2008, 153, 74–77. [Google Scholar] [CrossRef]
- Jiang, H.J.; Chen, N.; Shen, Z.Q.; Yin, J.; Qiu, Z.G.; Miao, J.; Yang, Z.W.; Shi, D.Y.; Wang, H.R.; Wang, X.W.; et al. Inactivation of Poliovirus by Ozone and the Impact of Ozone on the Viral Genome. Biomed Environ Sci 2019, 32, 324–333. [Google Scholar] [CrossRef]
- Thurston-Enriquez, J.A.; Haas, C.N.; Jacangelo, J.; Gerba, C.P. Inactivation of Enteric Adenovirus and Feline Calicivirus by Ozone. Water Res 2005, 39, 3650–3656. [Google Scholar] [CrossRef]
- Dubuis, M.-E.; Dumont-Leblond, N.; Laliberté, C.; Veillette, M.; Turgeon, N.; Jean, J.; Duchaine, C. Ozone Efficacy for the Control of Airborne Viruses: Bacteriophage and Norovirus Models. PLoS One 2020, 15, e0231164. [Google Scholar] [CrossRef]
- Wells, K.; Latino, J.; Gavalchin, J.; Poiesz, B. Inactivation of Human Immunodeficiency Virus Type 1 by Ozone in Vitro. Blood 1991, 78, 1882–1890. [Google Scholar] [CrossRef]
- Criscuolo, E.; Diotti, R.A.; Ferrarese, R.; Alippi, C.; Viscardi, G.; Signorelli, C.; Mancini, N.; Clementi, M.; Clementi, N. Fast Inactivation of SARS-CoV-2 by UV-C and Ozone Exposure on Different Materials. Emerg Microbes Infect 2021, 10, 206–209. [Google Scholar] [CrossRef]
- Dubuis, M.-E.; Racine, É.; Vyskocil, J.M.; Turgeon, N.; Tremblay, C.; Mukawera, E.; Boivin, G.; Grandvaux, N.; Duchaine, C. Ozone Inactivation of Airborne Influenza and Lack of Resistance of Respiratory Syncytial Virus to Aerosolization and Sampling Processes. PLoS One 2021, 16, e0253022. [Google Scholar] [CrossRef]
- Petry, G.; Rossato, L.G.; Nespolo, J.; Kreutz, L.C.; Bertol, C.D. In Vitro Inactivation of Herpes Virus by Ozone. Ozone Sci Eng 2014, 36, 249–252. [Google Scholar] [CrossRef]
- OHTSUKA, H.; OGATA, A.; TERASAKI, N.; KOIWA, M.; KAWAMURA, S. Changes in Leukocyte Population after Ozonated Autohemoadministration in Cows with Inflammatory Diseases. Journal of Veterinary Medical Science 2006, 68, 175–178. [Google Scholar] [CrossRef] [PubMed]
- TERASAKI, N.; OGATA, A.; OHTSUKA, H.; TAMURA, K.; HOSHI, F.; KOIWA, M.; KAWAMURA, S. Changes of Immunological Response after Experimentally Ozonated Autohemoadministration in Calves. Journal of Veterinary Medical Science 2001, 63, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Đuričić, D.; Valpotić, H.; Samardžija, M. Prophylaxis and Therapeutic Potential of Ozone in Buiatrics: Current Knowledge. Anim Reprod Sci 2015, 159, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, D.; Valpotic, H.; Samardzija, M. The Intrauterine Treatment of the Retained Foetal Membrane in Dairy Goats by Ozone: Novel Alternative to Antibiotic Therapy. Reproduction in Domestic Animals 2015, 50, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Escandón, B.M.; Espinoza, J.S.; Perea, F.P.; Quito, F.; Ochoa, R.; López, G.E.; Galarza, D.A.; Garzón, J.P. Intrauterine Therapy with Ozone Reduces Subclinical Endometritis and Improves Reproductive Performance in Postpartum Dairy Cows Managed in Pasture-Based Systems. Trop Anim Health Prod 2020, 52, 2523–2528. [Google Scholar] [CrossRef] [PubMed]
- Zobel, R.; Tkalčić, S.; Štoković, I.; Pipal, I.; Buić, V. Efficacy of Ozone as a Novel Treatment Option for Urovagina in Dairy Cows. Reproduction in Domestic Animals 2012, 47, 293–298. [Google Scholar] [CrossRef] [PubMed]
- OGATA, A.; NAGAHATA, H. Intramammary Application of Ozone Therapy to Acute Clinical Mastitis in Dairy Cows. Journal of Veterinary Medical Science 2000, 62, 681–686. [Google Scholar] [CrossRef]
- Suzuki, N.; Hirano, M.; Shinozuka, Y.; Kawai, K.; Okamoto, Y.; Isobe, N. Effects of Ozonized Glycerin on Inflammation of Mammary Glands Induced by Intramammary Lipopolysaccharide Infusion in Goats. Animal Science Journal 2022, 93. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Disinfection, Sterilization, and Antisepsis: An Overview. Am J Infect Control 2019, 47, A3–A9. [Google Scholar] [CrossRef]
- Lanave, G.; Cavalli, A.; Martella, V.; Fontana, T.; Losappio, R.; Tempesta, M.; Decaro, N.; Buonavoglia, D.; Camero, M. Ribavirin and Boceprevir Are Able to Reduce Canine Distemper Virus Growth in Vitro. J Virol Methods 2017, 248, 207–211. [Google Scholar] [CrossRef] [PubMed]
- REED, L.J.; MUENCH, H. A SIMPLE METHOD OF ESTIMATING FIFTY PER CENT ENDPOINTS12. Am J Epidemiol 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Sawadaishi, K.; Miura, K.; Ohtsuka, E.; Ueda, T.; Shinriki, N.; Ishizaki, K. Structure- and Sequence-Specificity of Ozone Degradation of Supercoiled Plasmid DNA 1. Nucleic Acids Res 1986, 14, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Chian, E.S.K.; Engelbrecht, R.S. Kinetics of Enteroviral Inactivation by Ozone. Journal of the Environmental Engineering Division 1981, 107, 887–901. [Google Scholar] [CrossRef]
- Costanzo, M.; Cisterna, B.; Vella, A.; Cestari, T.; Covi, V.; Tabaracci, G.; Malatesta, M. Low Ozone Concentrations Stimulate Cytoskeletal Organization, Mitochondrial Activity and Nuclear Transcription. European Journal of Histochemistry 2015, 59. [Google Scholar] [CrossRef] [PubMed]
- Scassellati, C.; Costanzo, M.; Cisterna, B.; Nodari, A.; Galiè, M.; Cattaneo, A.; Covi, V.; Tabaracci, G.; Bonvicini, C.; Malatesta, M. Effects of Mild Ozonisation on Gene Expression and Nuclear Domains Organization in Vitro. Toxicology in Vitro 2017, 44, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.; Borrelli, E.; Travagli,, Valter; Zanardi, I. The Ozone Paradox: Ozone Is a Strong Oxidant as Well as a Medical Drug. Med Res Rev 2009, 29, 646–682. [Google Scholar] [CrossRef]
- Mustafa, M.G. Biochemical Basis of Ozone Toxicity. Free Radic Biol Med 1990, 9, 245–265. [Google Scholar] [CrossRef]
- Dubuis, M.-E.; Racine, É.; Vyskocil, J.M.; Turgeon, N.; Tremblay, C.; Mukawera, E.; Boivin, G.; Grandvaux, N.; Duchaine, C. Ozone Inactivation of Airborne Influenza and Lack of Resistance of Respiratory Syncytial Virus to Aerosolization and Sampling Processes. PLoS One 2021, 16, e0253022. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




