1. Introduction
Photodynamic therapy (PDT) is long-standing curative method with fast response after the local, non-invasive light-treatment procedure [
1,
2]. PDT is based on the proper interaction between a photosensitive compound and irradiation within red visible or near infrared spectra (630 - 850 nm) in oxygen surrounding [
3,
4]. The procedure starts with a light absorption from a photosensitizer (PS) which have to uptake high amount PS rather in tumor then the surrounding normal cells. The proper irradiation induces triplet excited state PS which further initiates the photochemical reactions with formation of different reactive oxygen species [
5].
Phthalocyanine metal complexes (MPcs) participate in the photosensitization process mainly through Type II mechanism with singlet oxygen generation [
6,
7]. MPcs as photosensitizers have intensive absorption in the far-red spectral region (670 - 730 nm) and far- red shifted fluorescence which do not overlap the spectra of the endogenous natural cellular chromophores [
8]. Presently, there are a few clinically approved phthalocyanine complexes for treatment of different tumor localizations [
9]. Though, the promising photo- properties of MPcs, the research and development of novel MPcs derivatives is still actual which aim to improve low selectivity, high cytotoxicity and harsh phototoxicity with undesirable effects on the normal adjacent cells [
10].
The interaction of photosensitive drugs with native bioorganic molecules as part of circulating body fluids, is known as valuable approach for medication delivery and with high impact on the therapeutic outcomes [
11,
12]. Collagen is known to accomplish the carriage and distribution of many inborn physiological substances such as growth factors, genes, proteins, etc. [
13]. Collagen has also good in vivo adsorption, biocompatibility with bioactive substances, hemostatic characteristics, and low immunogenicity. It can form the natural associates with significant impacts on the biological functionality as a transporter with many advantageous over other natural and synthetic compositions because of the promising degradability, with a high contact surface, low toxicity and high compactness of cationic-charged amino groups [
14].
PDT process may influence on collagen cross-linkage and the acceleration of collagen biosynthesis at sufficient UV-visible light [
15]. There are additional profits of collagen for PDT viewing its adsorptive ability for the hydrophobic compounds, the minimal risk of developing resistance and not the last the expand uptake, stability and retention in physiological conditions. There are many studies about effects of native collagens of different origin for drug delivery [
16,
17], but a few are related to photosensitizers and PDT [
18]. Moreover, the impact of collagen hydrolysate is not yet known in respect to phthalocyanine photosensitizer for PDT method. Furthermore, it is expected that some negative effects on healthy tissues because of the photosensitization reactions may minimized by the presence of collagen.
The identified photophysical parameters of collagen are characterized with a very intensive absorption band in UVC region (190-260 nm) and a noticeable, characteristic fluorescence in the wide spectral range (300-700 nm) which depends on the substantial collagen modifications and the excitation wavelength [
19,
20]. These characteristics are not overlapping the optical properties of the studied phthalocyanines. In addition, these exogenous photosensitizers cannot induce some unfavorable photochemical processes of collagen photobleaching for example in the skin exposed to therapeutic red light. For example, in 2001, Yova and coauthors showed that the photosensitization of collagen with natural dye hypericin tend to increase the photobleaching of collagen under UV-visible spectrum of irradiation [
21]. In the same study was reported that an addition of hypericin was resulted in fluorescence to longer wavelengths. This early study reported also the generation of the matrix protein cross-links due to PDT, which is hindering an invasive cellular migration. The denatured collagen (gelatin) showed the spectra which was demonstrated the breaking of crosslinks in collagen under photointeraction. The similar observations were reported for hypocreline after the addition to collagen solution which resulted in a quenching of collagen fluorescence and slight shifting of its maximum. The changes in collagens’ fluorescence are used in diagnosis such as optical or fluorescence diagnosis of superficial tumors and other disorders [
22,
23]. The phenomenon is supposed to referred to fluorescence quenching of collagen by the mechanism of charge transfer between chromophores [
23]. The mechanism includes the singlet oxygen interaction with amino acid residues from collagen which tend the generation of other reactive species. Further on, the newly generated free radicals during PDT can react with other biomolecules to form cross-linkages and inactivate the growth factors, increasing the resistance of enzymatic degradation of collagen [
24].
The study presents synthesis, characterization, and photodynamic efficacy of a new Ga (III)-phthalocyanine (GaPc) and after its conjugation with bovine collagen hydrolysate (GaPc-Clg). The main absorption and fluorescence properties of GaPc and the conjugate were investigated in buffered solution. The photo- and dark cytotoxicity of GaPc and GaPc-Clg were studied in comparison on melanoma cell line (SH-4) versus two normal model cell lines (human keratocytes cells, HaCaT and fibroblasts cells, BJ). The photo-safety of GaPc, Clg, and their conjugate (GaPc-Clg) were evaluated on embryonal mouse cell line (BALB/c 3T3, clone A31 cells). The related parameters such as selectivity index (SI), phototherapeutic index (PI) and photo-irritation factor (PIF) were determined for both structures in comparison. PDT capacity of GaPc and after conjugation with collagen hydrolysate were evaluated.
2. Materials and Methods
2.1. Chemicals and Synthesis
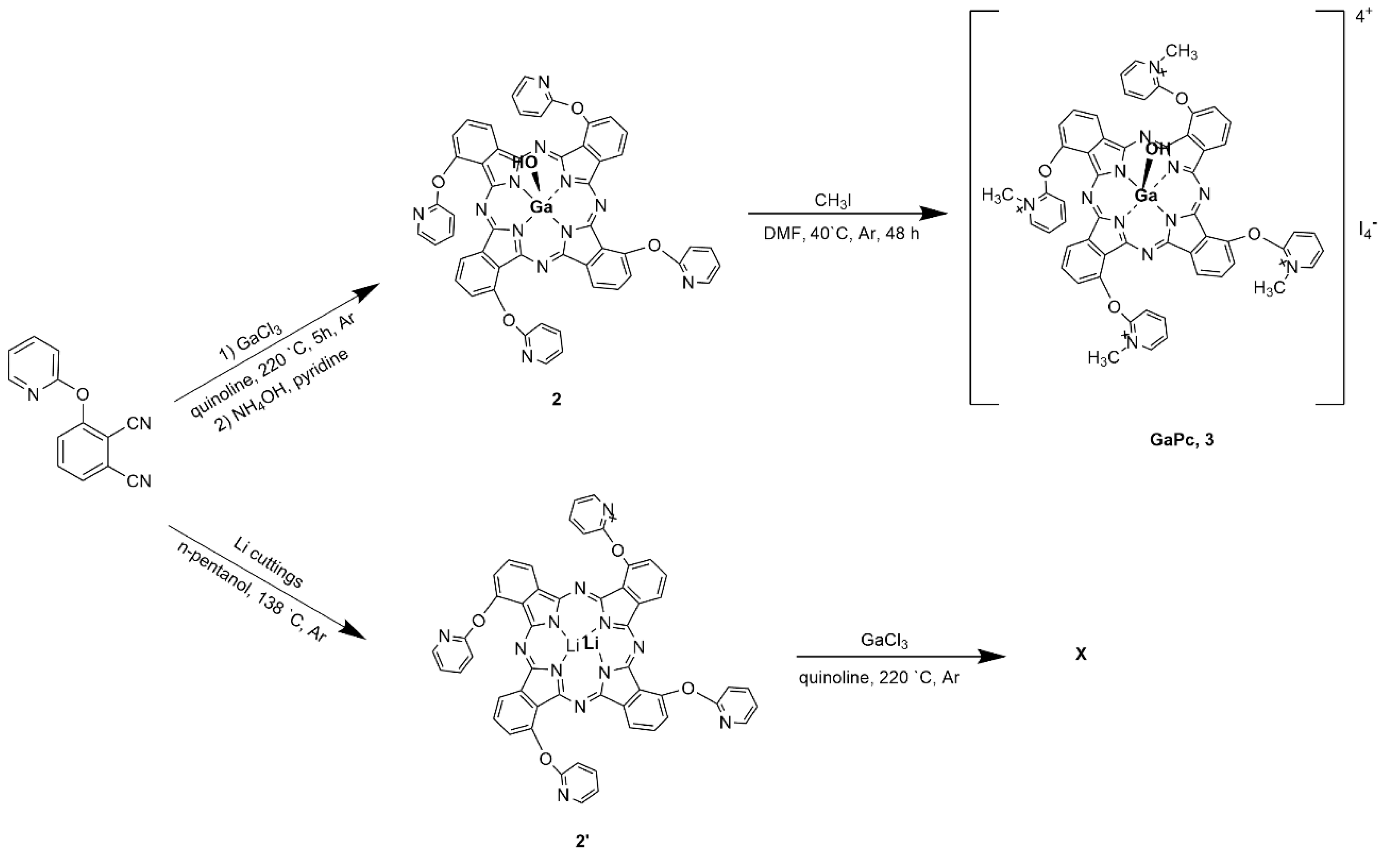
Gallium (III) phthalocyanine with methylpyridiloxy groups (GaPc) was synthesized following modification of the previously developed scheme [
25,
26]. All used solids were dried before usage. The solvents were additionally purified. A bovine collagen hydrolysate (Clg) has a certificate for human consumption as a food supplementary. It has characteristics of type III collagen (with traces of type I collagen). It was use as received from the vendor (The Netherlands, DCP B.V., Ter Apelkanaal, Article Coll B
TM 3000 SE).
2.1.1. 1(4),8(11),15(18),22(25)-. Tetrakis-[(2-pyridyloxy) Phthalocyaninato] Hydroxygallium(III) (2)
The reaction mixture of 3-(2-pyridyloxy)phthalonitrile (1.0 g, 4.52 mmol) and anhydrous gallium(III) chloride (0.200 g, 2.26 mmol) was stirred in freshly distilled quinoline (3 mL) under argon and heated at 220 ◦C for 5 h. The mixture was cooled to room temperature. Then a mixture of pyridine and ammonia hydroxide (1:1, v/v) was added. The reaction continued by stirring under argon at 25 ◦C. The product was determined with increase solubility. After cooling, the reaction mixture was poured into hexane to obtain a fine dark greenish precipitate. The isolation of the solid was done by several steps of centrifugation. The initial purification was carried out with hexane until the indication of transparency of supernatant. The product was purified on SiO2 using the mixture DCM-MeOH (9:1). Yield: 0.110 g (53%). IR [νmax/cm−1]: 3091 (Ar-CH), 1648 (C=C), 1562, 1532, 1520, 1397, 1327, 1287, 1258, 1132, 1112 (C-O-C), 1045, 805, 744. 1H-NMR (300 MHz, CDCl3): δ, ppm 8.64–7.24 (14H, m, Pc-H and Pyridyl-H), 7.68–6.81 (14H, m, benzene H). MALDI-TOF-MS m/z: Calc. 971.61 for C52H29N12O5Ga; Found [M]+ 971.16; [M + Na]+ 993.12.
2.1.2. 1(4),8(11),15(18),22(25)-. Tetrakis-[(2-pyridyloxy) Phthalocyaninato] Hydroxygallium(III) iodide (GaPc, 3)
Phthalocyanine, 2 (0.100 g, 0.1 mmol) was dissolved in dry dimethylformamide (5 mL) and an excess of methyliodide (2 mL) was added stepwise. The reaction mixture was stirred under argon at temperature 40 °C for 48 h. The product was isolated by precipitation in chloroform. The formed fine water-soluble solid was washed with chloroform, dichloromethane, ethyl acetate, acetone, n-hexane, hot ethanol and isolated by centrifugation. The green solid was dried under vacuum over phosphorous pentoxide at 80 °C overnight. Yield: 0.80 g (78%). UV-Vis (DMSO) λmax/nm (log ε): 358 (3.43), 615 (3.28), 686 (4.26). Yield: 0.080 g (67%). IR [νmax/cm−1]: 3076 (Ar-CH), 1635, 1572 (C=C), 1531, 1471, 1326, 1223 (S=O), 1178, 1105 (S=O), 1026 (C-O-C), 931, 835, 764, 661 (S-O). 1H-NMR (300 MHz, DMSO-d6): δ, ppm 7.99–7.24 (28H, m, Pc-H and Pyridyl-H), 4.39 (12H, m, CH3). MALDI-TOF-MS m/z: Calc. 1539.34 for C56H41N12O5I4Ga; Found 385.83 [(M + 4)/4]+.
2.2. Photo-Physicochemical Study
The absorption spectra were measured using a spectrophotometer (Evolution 300 UV-VIS) with quartz cuvettes with 1.0 cm optical pathway. A stock solution of GaPc (~ 2 mM) in Dimethyl Sulfoxide (DMSO) was freshly prepared. Clg was dissolved to concentration between 2 – 10 mg/mL in phosphate buffered saline (PBS) with pH7.2 just before the measurements. The absorption spectra of GaPc alone were recorded in a range 250 – 750 nm. The spectra of Clg were recorded in UV region (190 – 350 nm) in PBS. The study of interaction between GaPc and Clg was registered by titration of 6 µM GaPc in PBS, pH7.2 with increase of Clg concentrations or the opposite titration. After short incubation time, the full spectrum (190 – 750 nm) was recorded. Fluorescence measurements were carried out using a fluorimeter Perkin-Elmer LS 55 (Switzerland). The spectra of GaPc were recorded at excitation wavelength 615 nm or 365 nm and emission between 660 – 750 nm for constant concentration in DMSO or PBS. The emission spectra of GaPc-Clg were recorded at different excitation wavelengths (226 nm; 260 nm; 365 nm and 615 nm).
2.3. Photobiology Study
Phototoxicity studies were performed on four model cell lines. The solution of GaPc in DMSO was prepared as a stock: 2 mM. The cells were incubated after serial dilution with culture medium to final concentrations between 0.0025 µM - 40 µM GaPc. The solutions of Clg and the conjugate GaPc-Clg were prepared in PBS (10 mg/ mL Clg).
2.3.1. Cell Lines and Cultivation
Cell lines BALB/c 3T3 clone A31 (ATCC® CСL-163TM) – mouse embryo fibroblasts, BJ (ATCC® CRL-2522™) – normal skin fibroblasts and SH-4 (ATCC® CRL-7724™) – human skin melanoma, all were obtained from American Type Cultures Collection (ATCC, Manassas, Virginia, USA). Keratinocyte cell line HaCaT (CLS, cat. № 300493) was obtained from CLS Cell Lines Service GmbH (CLS, Eppelheim, Deutschland). The cells were cultured in 25 cm2 and 75 cm2 tissue culture flasks in DMEM - high glucose (4.5 g/L), 10% FBS, 2 mM glutamine and antibiotics (penicillin 100 U/ml and streptomycin 100 µg/ml) at 37°C, 5% CO2 and 90% relative humidity. Cells were plated in a 96-well microtiter plate at a density of 1 × 104 cells / 100 µl / well and were incubated for 24 h. Before treatment with test compounds, dry compounds dissolved in DMSO and further diluted in culture medium, so that the final concentration of DMSO was less than 1% (v/v). The incubation was carried out for a concentrations’ range (0.0025 – 40 µM) GaPc as well as for the conjugate.
2.3.2. Light Source and Parameters
A light-emitting diode LED 660-nm used as light source is a product of ELO Ltd., Sofia, Bulgaria. The light source was fixed for a power density of 100 mW/cm
2 and a light dose 50 J/cm
2 which is optimal for PDT studies. The photo- safety study was carried out with a solar simulator of a light-emitting diode (LED) Helios-iO (SERIC Ltd., Tokyo, Japan). The fluence rate was fixed by measurements of the power (power- meter PM 100D with a sensor S120VC, Thorlabs Inc., North Newton, Kansas, USA). The working dimension in the range 50 nW - 50 mW was for the spectrum 200 nm - 1100 nm. The spectrum was measured within the range 360-850 nm [
27]. The intensity was achieved for the distance of 25 cm with a normal diffuse in experimental zone of ≈ 1,16 W/m
2.
2.3.3. Photo- and Cytotoxicity Study
The complex GaPc and its conjugate GaPc-Clg were tested on the basis of Neutral Red Uptake in vitro test (NRU-assay). This color method for determination of the cells’ viability in vitro is based on the capability of the alive cells to accumulate the Neutral Red dye in their lysosomes. The mouse embryonal fibroblast cells (BALB/c 3T3, clone А31) were cultivated in dishes with an area 75 cm
2 as monolayer cells’ cultures at the standard conditions. The cells with density 1 × 10
4 cells in 100 μL culture medium per well were cultivated using a standard 96 well plate. The standard conditions were applied for the cell incubation for 24 hours to reach a good adhesion. The cells were incubated after application of double increase of the treatment concentrations for each compound. The study was performed in two plates at the same time in order to have the dark control in identical conditions as the light irradiated for comparison of photo and dark cytotoxicity. One plate with cells containing GaPc or GaPc-Clg was kept in the dark place (covered with Al-foil) and was used for evaluation of dark toxicity. The second plate was irradiated with LED 660 nm with light dose of 50 J/cm
2 and was used for evaluation of photo- toxicity. After irradiation, the culture medium was changed with medium contained the NR dye. The incubation continued for 24 hours. Three hours later the wells were washed with phosphate buffered saline (PBS, pH 7.4) and a mixture of distil water/ ethanol/ acetic acid (50: 49: 1) was added. The measurements of optical density were carried out on TECAN microplate reader at λ = 570 nm. The cellular toxicity was calculated by the Equation (1):
Other parameters calculated based on the photo- and dark cytotoxicity studies are following the Equations reported before [
28,
29,
30,
31]. Briefly, these are the phototherapeutic index (PI), toxicity value (TC50), photodynamic activity (TC50), selectivity index (SI), and photo-irritation factor (PIF) calculated using the formula (2):
where −Irr is the absence of light and +Irr is the presence of light.
Phototherapeutic index (PI) is defined as the ratio of dark to light EC50 values and is used as a determine of light-induced potency [
32,
33]. The ratio between the IC50 value (half maximal inhibitory concentration) of the resting and the IC50 value of the activated compound must be as high as probable. Phototherapeutic index was defined as the dark EC
50 value divided by the light EC
50 value and calculated using the Equation (3).
The anticancer activity of GaPc and its cytotoxicity towards normal cell lines were used to calculate the SI value. Rashidi et al. [
34] pointed SI values > 2 for a high selectivity. The SI value was calculated by equation (4).
2.4. Statistics
The experiments were carried out in triplicate and the data are presented as a mean value ± standard deviation (SD) by the Student’s test and the difference between two means was compared by an unpaired Student’s test. The values of p < 0.05 were considered as significant.
3. Results
Gallium (III) phthalocyanine complex (GaPc) was successfully synthesized starting from a phthalonitrile (
Scheme 1). Additional attempts were made with lithium or metal-free phthalocyanine, but this pathway was not successful.
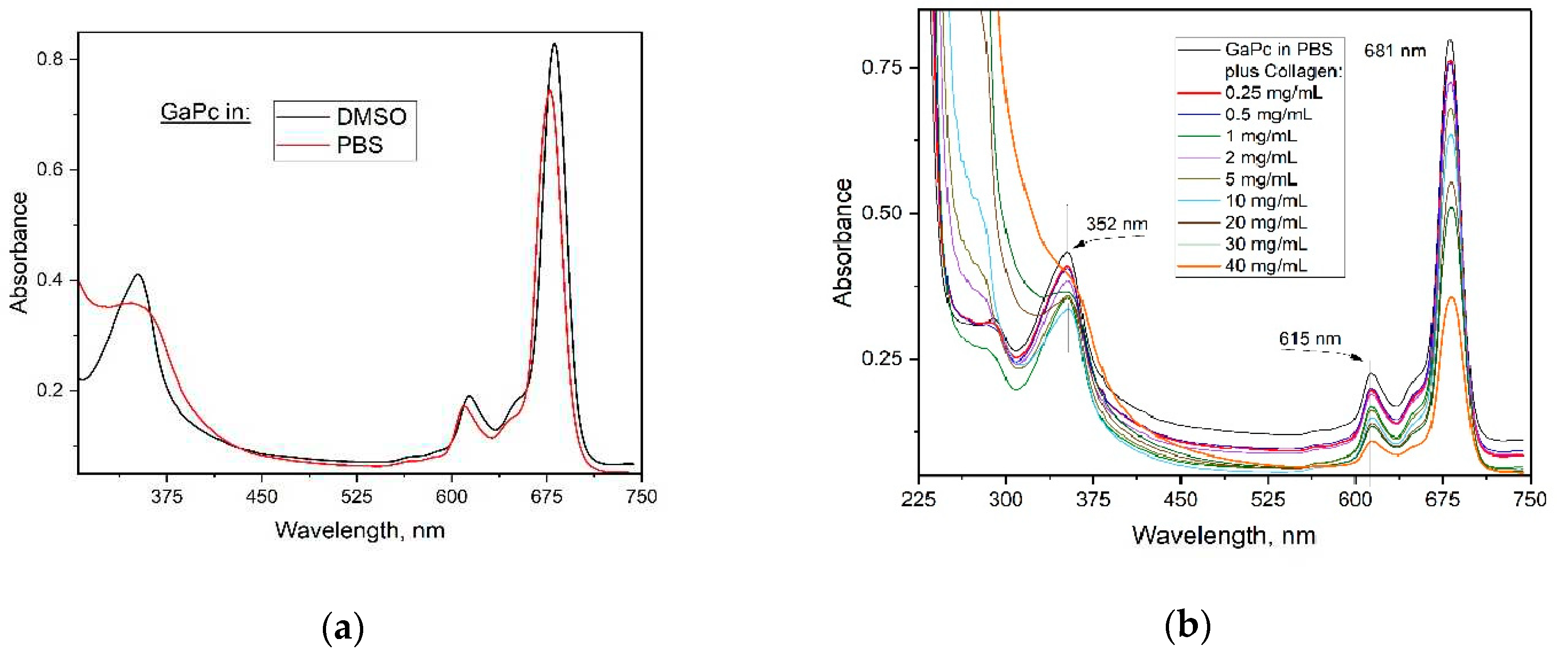
The absorption spectra of GaPc were recorded in dimethylsulfoxide (DMSO) and buffered solution (PBS, pH 7.2), (
Figure 1a). A characteristic Q band for non-aggregated phthalocyanines was observed in both media. The band in UVA region was shown at 352 nm in water solutions and 358 nm in DMSO. Two characteristic bands were observed in the visible region (615 nm and 686 nm, DMSO). The low intensity band at 610 nm or 615 nm and a strong sharp Q-band at 681 nm to 686 nm depending on the solvents ‘polarity. All spectra strictly followed the Lambert–Beer law. The absorption maxima showed a small red shift of approx. 5-6 nm as compared to similar peripherally substituted phthalocyanines [
32]. The absorption spectra showed that the addition of collagen hydrolysate (Clg) to GaPc (6 µM) diminish Q-band and increase the Soret band for a range of Clg concentrations (0.25 mg/mL – 40 mg/mL) (
Figure 1b). UV-band was disappeared by addition of Clg with increase of Clg concentration. The peak was transformed to the shoulder as seen for the highest concentration (40 mg/ mL) of Clg linear macromolecules.
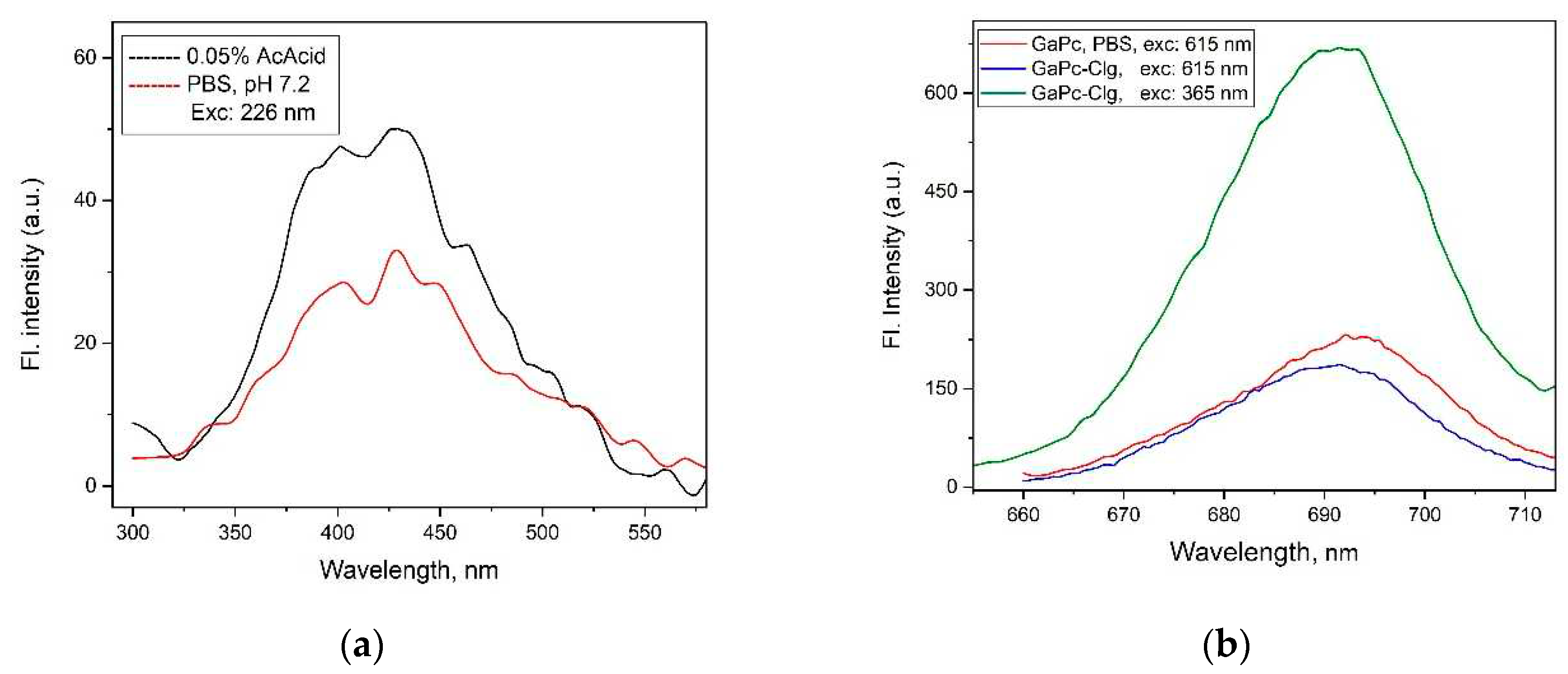
Fluorescence study was carried out upon excitation at 365 nm and 615 nm for GaPc and its conjugate with Clg. The emission peak of GaPc was registered with maximum at 704 nm (DMSO) and with a relatively high fluorescence quantum yield (0.23). The novelty is a fluorescence spectrum of Clg which was evaluated with wide non-uniform band at 400 nm – 450 nm for exc: 226 nm (
Figure 2a). This band is typical for phenylalanine which is may be the main fluorescent amino acid in the studied hydrolysate. The emission band of GaPc in PBS was recorded at 694 nm and by addition of Clg the maximum was slightly moved to the blue region (
Figure 2b). At excitation wavelength 365 nm, an increase of the emission was shown for GaPc in conjugate. This was suggested a contribution of Clg in fluorescence emission of GaPc. The fluorescence quantum yield of GaPc in conjugate GaPc-Clg was determined with low value (0.012) at typical for phthalocyanine excitation wavelength (615 nm). The photophysical studies showed that GaPc characterized with red shifted both absorption and fluorescence wavelengths (686 nm and 704 nm, DMSO) due to non-peripheral substitutions. In addition, GaPc showed the lower intensity and blue shifted spectra in water medium and by addition of Clg.
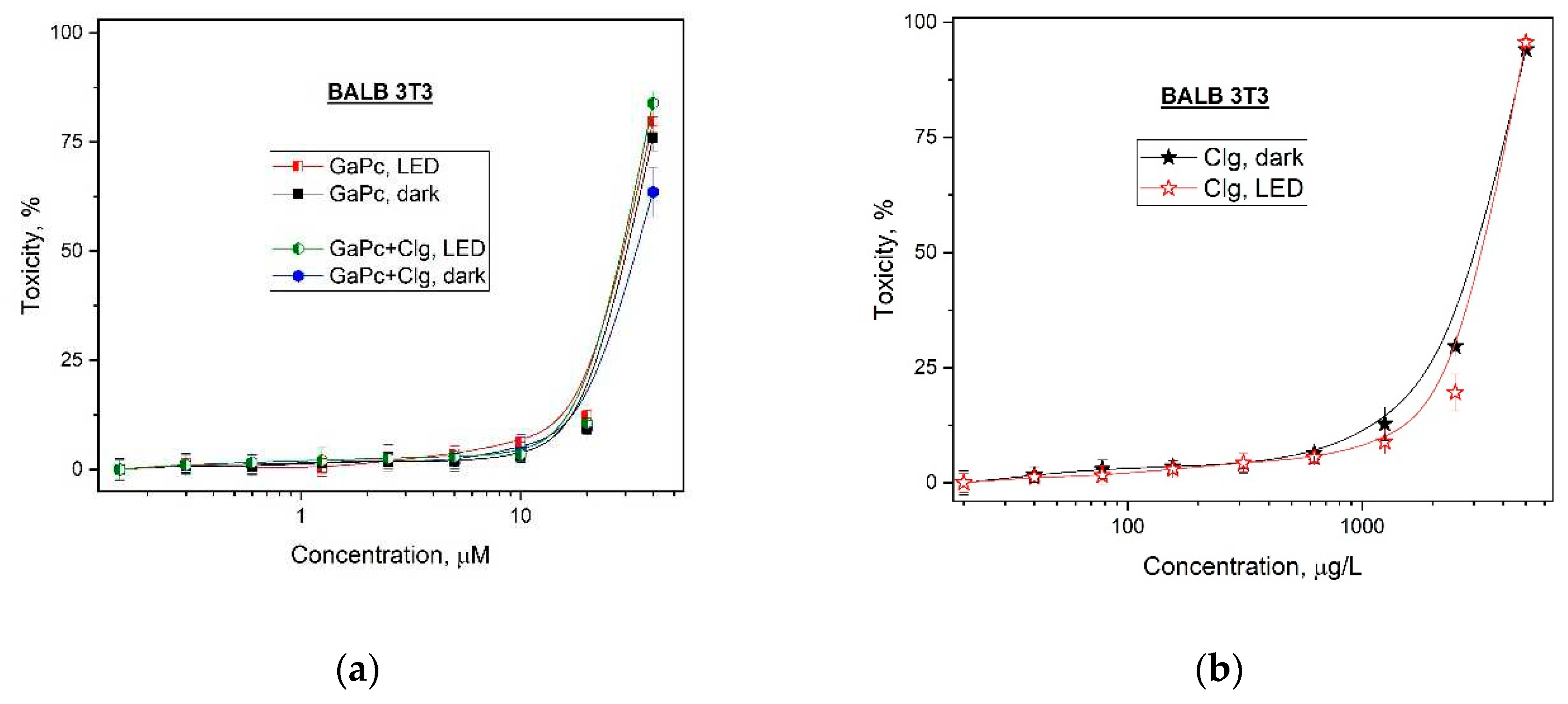
The evaluation of photo- and dark cytotoxicity of Ga (III)-phthalocyanine (GaPc) and hydrolyzed collagen (Clg), and the freshly formed conjugate (GaPc-Clg) was carried out in dark conditions and with specific light irradiation (LED 660-nm). The photo-safety study was carried out on a model mouse embryo fibroblasts normal cell line BALB 3T3 (
Figure 3). The results showed no difference in the obtained curves for GaPc for both treatment conditions (dark or light), (
Figure 3a). These results suggested low photo-safety respectively a high toxicity for GaPc. The lack of dark and phototoxicity was shown for Clg for the same concentration range. Similar results for photo- and dark toxicity of Clg were determined for higher concentrations (
Figure 3b). As can be seen the cytotoxic effect was observed for concentrations higher than 10 mg/mL which are usually taken as supplements. The dark toxicity of GaPc was registered with a slight decrease after irradiation of the tested cells. This observation suggests the contribution of collagen hydrolysate in toxicity of the studied GaPc as a photosensitizer for PDT. The results for the normal cell line BALB 3T3 showed a similar behavior for concentrations up to 10 µM for the samples with and without 660-nm light exposure. A lack of cytotoxic effect on BALB 3T3 cells was observed for PDT concentrations of GaPc and GaPc-Clg as well as for Clg (x 100 times higher) without influence of the applied light (
Figure 3b).
Table 1.
Toxicity and photo-irritation factor (PIF) of Ga(III)-phthalocyanine (GaPc), bovine collagen hydrolysate (Clg) and the conjugate (GaPc-Clg) expressed by CC50 values ±SD (μM) for exposure of embryonal BALB 3T3 cell line with solar light-emitting diode (LED) with a dose of 10 J/cm2.
Table 1.
Toxicity and photo-irritation factor (PIF) of Ga(III)-phthalocyanine (GaPc), bovine collagen hydrolysate (Clg) and the conjugate (GaPc-Clg) expressed by CC50 values ±SD (μM) for exposure of embryonal BALB 3T3 cell line with solar light-emitting diode (LED) with a dose of 10 J/cm2.
| Compounds |
Cytotoxicity |
Phototoxicity |
PIF* |
| Clg** |
3148.63 ± 80.48 |
3299.40 ± 67.79 |
0.95 |
| GaPc |
33.90 ± 1.78 |
29.51 ± 0.44 |
1.15 |
| GaPc-Clg |
30.55 ± 0.63 |
29.11 ± 0.38 |
1.05 |
Table 2 summarized the calculated CC
50 values for the photo- and dark cytotoxicity for evaluation of the photo-safety properties and the photo-irritation factor (PIF) of three tested photosensitive substances on embryonal BALB 3T3 cell line. The photosensitizer GaPc was evaluated with relatively high values of 33.90 μM and 29.51 μM, and with PIF=1.15 which is typical for non-toxic on solar light exposure compounds. The data for the conjugate GaPc-Clg showed similar values (30.55 μM and 29.11 μM) and PIF=1.05 which also in in favor and suggested the high photo- safety impact of collagen (0.95) as well as the conjugate (1.05). The results showed that the conjugation with a collagen hydrolysate with spectrum in UV region did not lower the high photo- safety of compound.
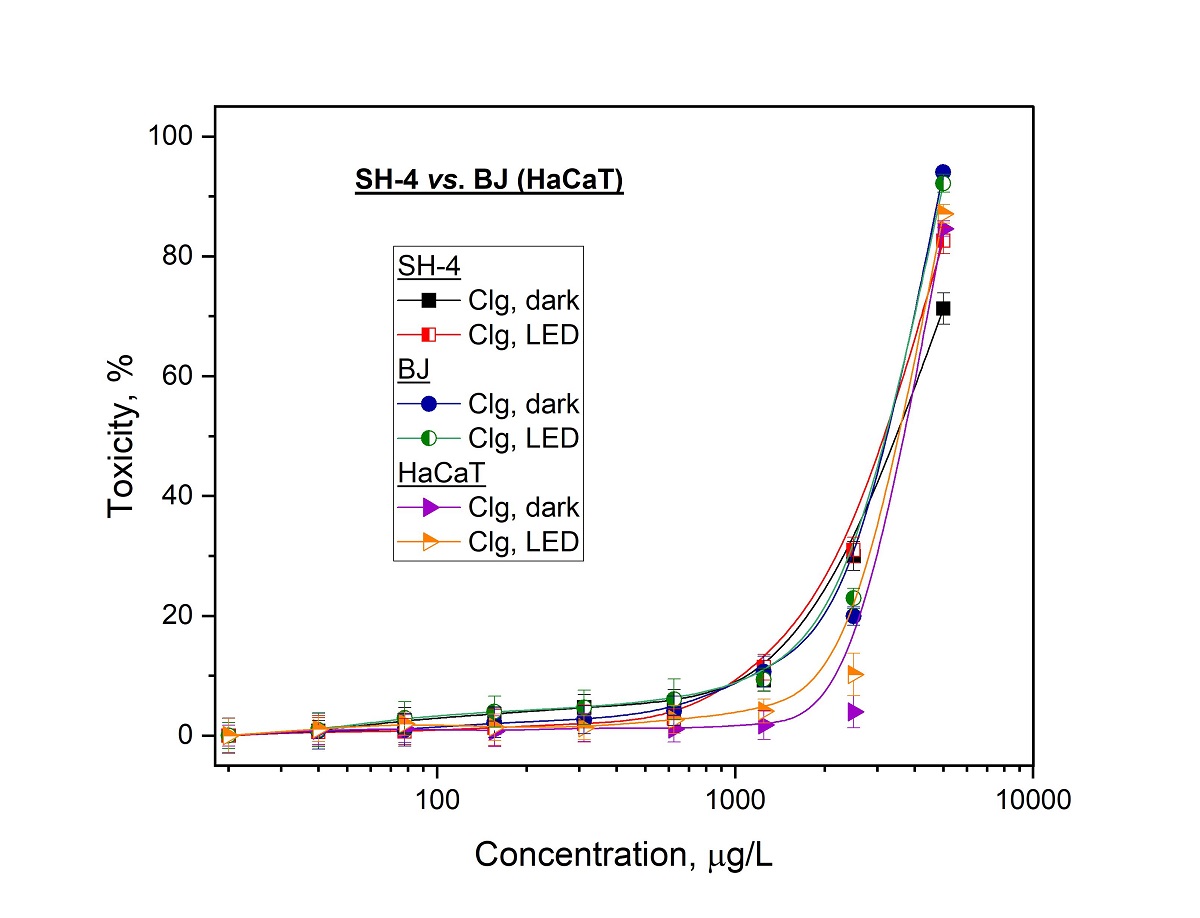
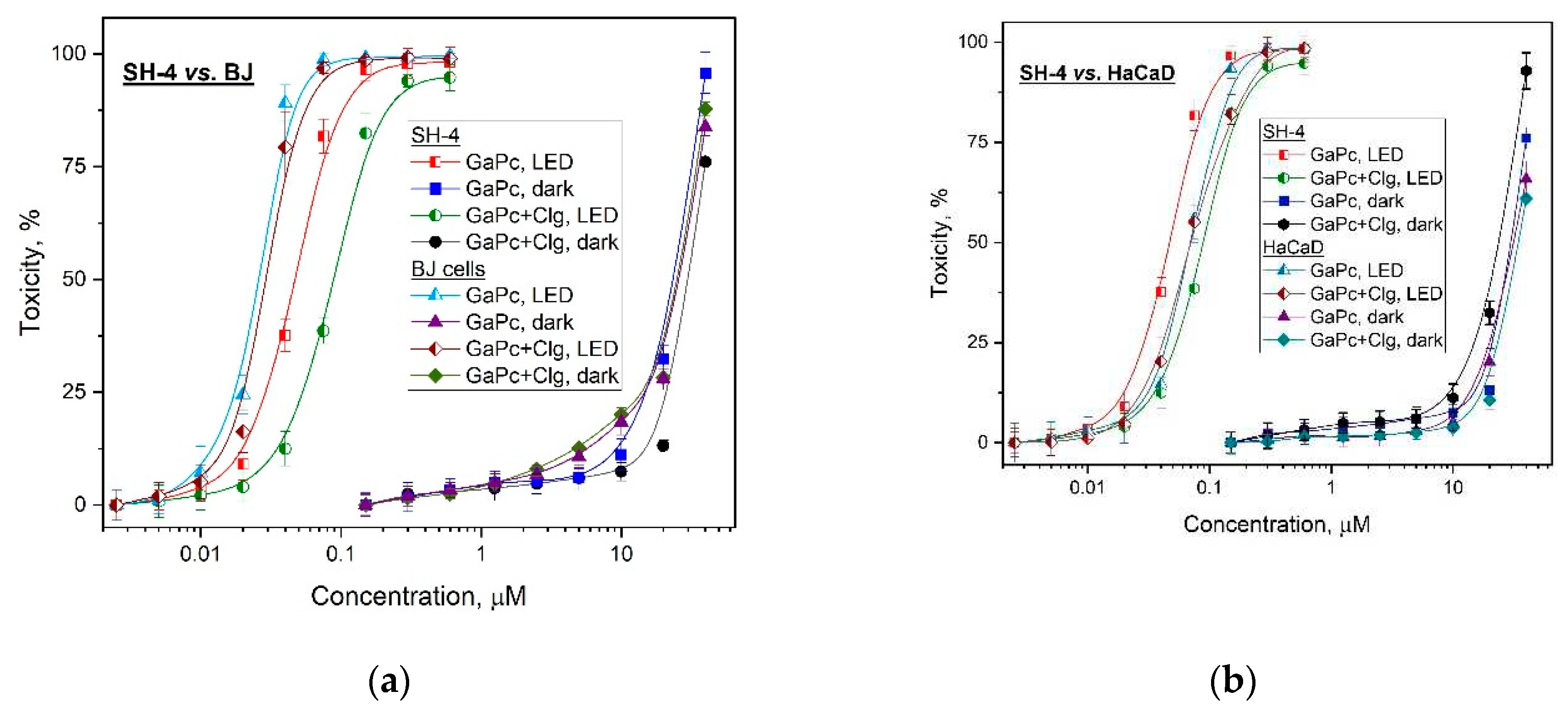
The photodynamic activity studies were carried out in comparison with GaPc and its conjugate (GaPc-Clg) on human melanoma cancer cells (SH-4). In addition, two normal cell lines with different origin namely the skin keratocytes (HaCaT) and the fibroblasts (BJ) were investigated. The same treatment conditions were applied in order to evaluate the selectivity index (SI) of both structures. A relatively high phototoxicity of GaPc was observed for low concentrations (0.01–0.1 µM) and for this concentration range GaPc was evaluated without dark toxicity for SH-4 tumor cell line as well as for normal BJ cell line (
Figure 4a). The phototoxicity results were obtained upon irradiation with PDT specific parameters of a light emission diode (LED) with maximum at 660 nm, the power density of 60 mW/cm
2 and light dose of 50 J/cm
2, which were previously accepted as proper [
29]. The photosensitizer GaPc was evaluated with a high phototoxic effect but also for the normal cell line HaCaT (
Figure 4b). It was observed > 50% cellular phototoxicity for very low concentrations (0.05-0.1 µM). The dark toxicity of GaPc was observed for concentrations almost ten times higher than a needed dose for phototoxicity towards SH-4 pigmented melanoma cells.
The main photo- biological parameters were calculated on the base of the obtained cytotoxicity results (
Table 2). As seen, the characteristic values for a photosensitizer were evaluated for phototherapeutic index (PI=819) for melanoma tumor cell line (SH-4). In case of normal cell lines (HaCaT and BJ) this index (PI) showed diverse values namely 580 and 1208. The results for a conjugate GaPc-Clg showed value of PI for tumor cells which are approx. two times lower (PI=360) as compared to the values for GaPc alone (PI=819). The normal cell line HaCaT and BJ cell line showed the same tendency for PI values of GaPc-Clg. This suggests the high photodynamic efficiency of GaPc as a photoactive agent. However, GaPc-Clg showed low PI=360 which reduced the general toxicity. There was no significant difference in the PI values for both normal cell lines with less than 10 % lower PI for GaPc-Clg as compared to GaPc. The selectivity index (SI) was double for GaPc vs. GaPc-Clg towards HaCaT cells (1.49 vs. 0.71) as well as for BJ cells (0.54 vs. 0.28).
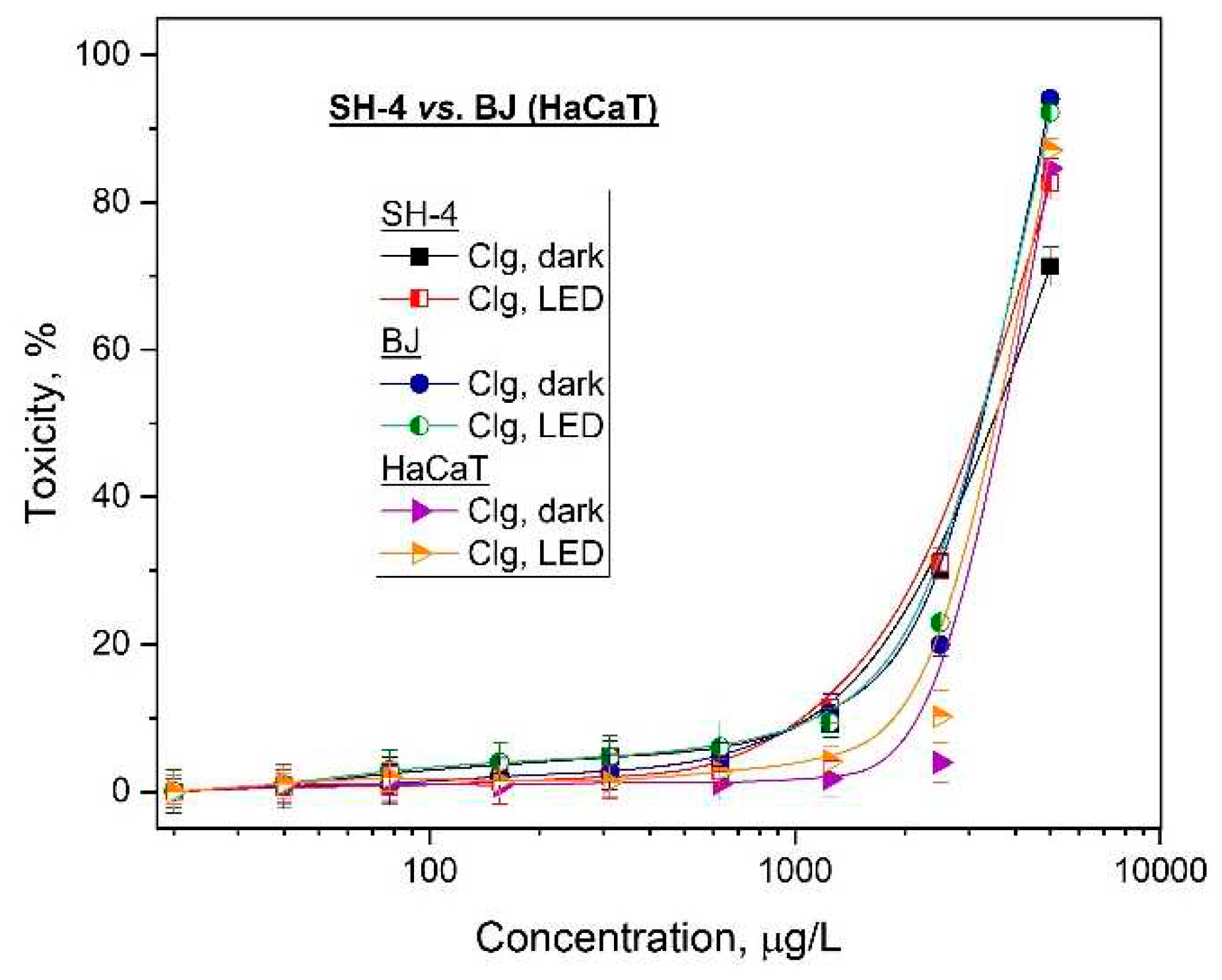
These studies showed decrease of photo-safety and a high photocytotoxicity for the conjugate in case of normal keratinocytes and fibroblasts cells. The data suggests that the used collagen decreases the cytotoxicity of GaPc but also the important factors such as selectivity of the photosensitizer. Further, this can cause the photosensitivity of the normal cells located in the light spot of irradiation. Furthermore, the photodynamic effect of GaPc was determined as relatively strong for the human melanoma tumor cell line (PI = 819) which was reduced two times after conjugation for GaPc-Clg (PI=360). Although GaPc-Clg was evaluated with low value of selectivity index because of Clg which tent to lower cellular toxicity. The used hydrolysate (Clg) was also determined with high photo-safety (low phototoxicity) towards the tumor as well as for the both normal cell lines (
Figure 5).
4. Discussion
The present study focused the influence of collagen hydrolysate (Clg) used such as food supplementary for human consumption on the main photophysical properties and photodynamic activity of a novel Ga(III)-phthalocyanine (GaPc) towards human pigmented melanoma cells. The photosensitizer was designed as cationic, water-soluble, and with non-peripheral substitution groups GaPc owning to our previous efforts with metallo-phthalocyanines (MPcs), [
33,
34]. The applied synthetic procedure followed the synthetic pathway which was previously used (
Scheme 1) for analogous peripherally substituted GaPc1 and GaPc2 [
25]. The attempts were made to prepare GaPc using a substituted lithium phthalocyanine complex or metal-free phthalocyanine as starting compounds but this pathway was not productive. This may be because of the big atom size of gallium which needs high energy for coordination in the closed ring molecule. The studies on photophysical properties of GaPc in dimethyl sulfoxide (DMSO) and water buffer solutions (PBS and 0.05% acetic acid) showed bathochromic shift of the absorption bands (686 nm to 681 nm in DMSO and PBS) as compared to the similar peripherally substituted GaPc1 [
25]. These observations were expected because of the non-peripheral substitution groups of GaPc as was well documented for other phthalocyanines [
35]. The formation of the conjugate with collagen (GaPc-Clg) was registered with decrease of absorption Q-band of GaPc and the increase with loss of shape of Soret band (
Figure 1b). The fluorescence study was carried out in organic and water solutions (
Figure 2). The emission spectra showed the band with lower intensity maximum with a hypochromic shift to 694 nm because of the water media and the presence of collagen. In addition, a quenching of fluorescence of GaPc because of Clg was observed as compared to the emission of GaPc as pure compound (fluorescence quantum yield of 0.012 and 0.23).
The studied GaPc was evaluated with a high photodynamic activity as compared to similar phthalocyanine complexes studied on pigmented melanoma cells [
36,
37,
38]. A high photo-safety (low cytotoxicity) was determined for GaPc and for its conjugate on the tested model embryonal cell line BALB 3T3 for the concentrations which related to PDT usage (
Figure 3a). A low cytotoxic effect was also observed for collagen used as the food supplementary at concentration range which is higher than used for human consumption (
Figure 3b). The studies showed no dependence on the applied irradiation (LED 660 nm) as seen from the overlapping of the curves for both treatment conditions. This observation can be explained by the suitable spectrum of the light source with maximum at 660 nm. The effects of collagen hydrolysate on the selectivity index (SI) and the phototherapeutic index (PI) showed a photo-safety of GaPc-Clg with no influence on the model normal cell line BALB 3T3 (
Table 1).
The photo- and dark toxicity of GaPc showed the high phototoxicity for the tested melanoma tumor cell line (SH-4) with a significant concentration gap between results for the normal cell lines (BJ and HaCaT) as is shown in
Figure 4a,b. The conjugate GaPc-Clg was evaluated to decrease the both dark and photo- cytotoxicity and antitumor efficacy, respectively. However, this observation was determined only for tumor cells but not for normal cell lines. The cytotoxic effect was determined with no influence of collagen addition (
Figure 4a,b). The positive effect of collagen as seen is the lower dark toxicity of GaPc-Clg than for GaPc alone, which is an undesirable property for the compounds used as photosensitizers (
Table 2). On the other hand, this tend to provoke a reduction of harsh toxicity action of GaPc for the studied concentrations minimizing the cytotoxicity to the normal cells as well. The results obtained for the second normal cell line followed the same tendency. These observations can be explained with the quenching behavior of collagen to the main photo- properties of GaPc as well as some limitation of the light absorption due to large linear structure of macromolecules.
A study of photosensitivity effects on collagen with a polycyclic quinone (hypericin) at 355 nm and 532 nm laser irradiation showed the bleaching of collagen fluorescence which is connected with irreversible decomposition of collagen chromophores for doses of 9 J/cm
2 and 18 J/cm
2. The harsh effect was observed at both wavelength which should be taken in consideration for the actions on human skin of the sun light irradiation [
21]. PDT has an advantage of limited overall tissue toxicity and minimal side-effects through a local appeal of the procedure, and it also has a fast response with low ability for the development of resistance to drug light exposure. Melanomas as highly metastatic tumors have critical outcome by application of the well-defined clinical route of therapeutic procedures has been reported with several limitations [
39]. Previous studies with different photosensitizers including metallophthalocyanines (MPcs) suggested their potential on highly metastatic melanoma cells treated with PDT methodology [
40]. The early stage treatment of pigmented melanoma can be treated with high efficiency with PDT [
41]. The present study suggested that these properties can diminish by the addition of a biomolecules such as collagen hydrolysate with well-approved therapeutic impact on human health and in common usage as a food supplementary.
5. Conclusions
A novel non-peripheral tetra- methylpyridiloxy substituted Ga(III)-phthalocyanine (GaPc) and the freshly formed physical conjugate with a bovine hydrolyzed collagen (GaPc-Clg) were studied for photodynamic therapy (PDT) of pigmented melanoma cells. GaPc was successfully synthesized using the pathway from a monomer. The main photophysical properties showed that collagen lower the main absorption and fluorescence characteristics of GaPc as a photosensitizer. For example, the conjugation was observed with a blue shifted absorption (678 nm), the low intensity Q-band at the maximum of PDT irradiation, and the fluorescence showed a lower quantum yield (0.012). The photo- and dark cytotoxicities study on tumor cells were observed with higher values for GaPc than for its Clg conjugate. The photo-safety was examined as optimal (0.95, 1.05 and 1.15) on BALB 3T3 cells as well as approx. > 100 times concentration gap between the dark and photo- cytotoxicity was observed on the both tested normal cell lines (keratocytes HaCaT and fibroblast BJ). The hydrolyzed collagen was not influenced by the light exposure (LED 660 nm) for all tested cell lines showing identical results in the dark condition as after red LED irradiation. A diminish dark toxicity was observed for the conjugate GaPc-Clg with the selectivity index twice higher for GaPc alone than for the conjugate. As general can be concluded that the used as a supplementary collagen hydrolysate tends to reduce the photo- properties and the efficiency of a strong photosensitizer such as the tested new Ga(III)-phthalocyanine but it has the positive effect of diminish harsh cytotoxicity on the normal cells which can be damaged during the light exposure.
Author Contributions
Conceptualization, V.M.; methodology, V.M. and I.I.; software, V.M. and I.I.; validation, I.I. and I.S.; investigation, V.M., I.I. and I.S.; resources, V.M. and I.I.; data curation, V.M. and I.I.; writing—original draft preparation, V.M.; writing—review and editing, V.M., I.I. and M.D.; supervision, V.M. and I.I.; project administration, V.M. and Ts.G.; funding acquisition, Ts.G. All authors have read and agreed to the published version of the manuscript.
Funding
The experimental research was funded by the Bulgarian National Science Fund with the project KP-06-H28/11.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable.
Data Availability Statement
The experimental data of the present study are available at the request of the corresponding author.
Acknowledgments
V.M. thanks Prof. Dieter Wöhrle, Bremen University, Germany for the valuable knowledge in the synthesis of gallium phthalocyanine complexes. Thanks to Plamen Hristov, MSc for collagen hydrolysate and the National Science Fund, Sofia, Bulgaria for supporting our research.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Huis in ‘t Veld, R.V.; Heuts, J.; Ma, S.; Cruz, L.J.; Ossendorp, F.A.; Jager, M.J. Current Challenges and Opportunities of Photodynamic Therapy against Cancer. Pharmaceutics 2023, 15, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, H.; Fu, L.; Cui, J.; Yang, Y. Global Trends and Research Progress of Photodynamic Therapy in Skin Cancer: A Bibliometric Analysis and Literature Review. Clinical, Cosmetic and Investigational Dermatology 2023, 16, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, S.; Kumar, P.; Jain, G.K.; Aggarwal, G.; Almalki, W.H.; Kesharwani, P. 2 - Mechanisms of photodynamic therapy, Editor(s): Prashant Kesharwani, Nanomaterials for Photodynamic Therapy, In: Woodhead Publishing Series in Biomaterials, Woodhead Publishing 2023, pp. 41–54. 54. [CrossRef]
- Maharjan, P.S.; Bhattarai, H.K. Singlet Oxygen, Photodynamic Therapy, and Mechanisms of Cancer Cell Death. Journal of Oncology 2022, 2022, 7211485. [Google Scholar] [CrossRef] [PubMed]
- Bacellar, I.; Tsubone, T.; Pavani, C.; Baptista, M. Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. International Journal of Molecular Sciences 2015, 16, 20523–20559. [Google Scholar] [CrossRef]
- Ogura, S.-I.; Tabata, K.; Fukushima, K.; Kamachi, T.; Okura, I. Development of phthalocyanines for photodynamic therapy. Journal of Porphyrins and Phthalocyanines 2006, 10, 1116–1124. [Google Scholar] [CrossRef]
- Nyokong, T. Desired properties of new phthalocyanines for photodynamic therapy. Pure and Applied Chemistry 2011, 83, 1643–1799. [Google Scholar] [CrossRef]
- Dias, L.M.; de Keijzer, M.J.; Ernst, D.E.; Sharifi, F.; de Klerk, D.J.; Kleijn, D.G.; et al. Metallated phthalocyanines and their hydrophilic derivatives for multi-targeted oncological photodynamic therapy. J. Photochem. Photobiol. B: Biol 2022, 234, 112500. [Google Scholar] [CrossRef]
- Li, X.; Zheng, B.-D.; Peng, X.-H.; Li, S.-Z.; Ying, J.-W.; Zhao, Y.; Huang, J.-D.; Yoon, J. Phthalocyanines as medicinal photosensitizers: Developments in the last five years. Coord. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Chen, D.; Song, M.; Huang, J.; Chen, N.; Xue, J.; Huang, M. Photocyanine: A novel and effective phthalocyanine-based photosensitizer for cancer treatment. J. Innov. Opt. Health Sci. 2020, 13, 2030009. [Google Scholar] [CrossRef]
- Yewale, C.; Baradia, D.; Vhora, I.; Misra, A. Proteins: emerging carrier for delivery of cancer therapeutics. Expert Opin. Drug Deliv. 2013, 10, 1429–1448. [Google Scholar] [CrossRef]
- Kaltbeitzel, J.; Wich, P.R. Protein-based Nanoparticles: From Drug Delivery to Imaging, Nanocatalysis and Protein Therapy. Angewandte Chemie Int. Edition 2023. [Google Scholar] [CrossRef] [PubMed]
- Arun, A.; Malrautu, P.; Laha, A.; Luo, H.; Ramakrishna, S. Collagen Nanoparticles in Drug Delivery Systems and Tissue Engineering. Appl. Sci. 2021, 11, 11369. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Sun, J.; Cecic, I. Photodynamic Therapy-Induced Cell Surface Expression and Release of Heat Shock Proteins: Relevance for Tumor Response. Cancer Res. 2005, 65, 1018–1026. [Google Scholar] [CrossRef]
- Kasoju, N.; Ali, S.S.; Dubey, V.K.; Bora, U. Exploiting the Potential of Collagen as a Natural Biomaterial in Drug Delivery. J. Proteins and Proteomics 2013, 1, 9–14. [Google Scholar]
- Sánchez-Cid Bueno, P., Jiménez Rosado, M., Romero García, A. y Pérez-Puyana, V.M. Novel Trends in Hydrogel Development for Biomedical Applications: A Review. Polymers 2022, 14, 3023. [CrossRef]
- Verrico, A.K.; Moore, J.V. Expression of the collagen-related head shock protein HSP47 in fibroblasts treated with hyperthermia or photodynamic therapy. Br. J. Cancer 1997, 76, 719–724. [Google Scholar] [CrossRef]
- Deyl, Z.; Praus, P.; Lcova, H.&J.; Goldman, J.N. Fluorescence of collagen – properties of tyrosine residues and another fluorescent element in calf skin collagen. FEBS Letters 1969, 5, 187–191.
- Chan, B.P.; Chan, O.C.M.; So, K.-F. Effects of photochemical crosslinking on the microstructure of collagen and a feasibility study on controlled protein release. Acta Biomaterialia 2008, 4, 1627–1636. [Google Scholar] [CrossRef]
- Yova, D.M.; Hovhannisyan, V.A.; Theodossiou, T. Photochemical effects and hypericin photosensitized processes in collagen. J. Biomed. Opt. 2001, 6, 52–57. [Google Scholar] [CrossRef]
- Katz, A.; Alfano, R.R. Optical Biopsy - Detecting Cancer with Light. Editors: Sevick-Muraca and D. Benaron, In Biomedical Optical Spectroscopy and Diagnostics, Trends in Optics and Photonics Series, 1996, Optica Publishing Group, paper FT1.
- Marcu, L.; Grundfest, W.S.; Maarek, J.-M. I. Photobleaching of arterial fluorescent compounds: characterization of elastin, collagen and cholesterol time-resolved spectra during prolonged ultraviolet irradiation. Photochem. Photobiol. 1999, 69, 713–721. [Google Scholar] [PubMed]
- Foote, C.S. Definition of type I and type l photosensitised oxidation. Photochem Photobiol 1991, 54, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Mantareva, V.; Kussovski, V.; Angelov, I.; Wöhrle, D.; Dimitrov, R.; Popova, E.; Dimitrov, S. Non-aggregated Ga (III)-phthalocyanines in the photodynamic inactivatio planktonic and biofilm cultures of pathogenic microorganisms. Photochemical & Photobiological Sciences 2011, 10, 92–102. [Google Scholar]
- Mantareva, V.; Iliev, I.; Sulikovska, I.; Durmuş, M.; Angelov, I. Cobalamin (Vitamin B12) in Anticancer Photodynamic Therapy with Zn(II) Phthalocyanines. Int. J. Mol. Sci. 2023, 24, 4400. [Google Scholar] [CrossRef]
- Mantareva, V.; Iliev, I.; Sulikovska, I. Cyanocobalamin (vitamin B12) as insensitive porphyrinoid to solar LED irradiation. J. Phys. Conf. Ser. 2023, 2487. [Google Scholar] [CrossRef]
- Sen, P.; Managa, M.; Nyokong, T. New Type of Metal-Free and Zn (II), In (III), Ga (III) Phthalocyanines Carrying Biologically Active Substituents: Synthesis and Photophysicochemical Properties and Photodynamic Therapy Activity. Inorg. Chim. Acta 2019, 491, 1–8. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar]
- Peña-Morán, O.A.; Villarreal, M.L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V.; Rodríguez-López, V. Cytotoxicity Post-Treat. Recovery, and selectivity analysis of naturally occurring podophyllotoxins from Bursera fagaroides var. fagaroides on breast Cancer cell lines. Molecules 2016, 21, 1013. [Google Scholar]
- Weerapreeyakul, N.; Nonpunya, A.; Barusrux, S.; Thitimetharoch, T.; Sripanidkulchai, B. Evaluation of the anticancer potential of six herbs against a hepatoma cell line. Chin. Med. 2012, 7, 15. [Google Scholar] [CrossRef]
- Yalazan, H.; Kantekin, H.; Durmuş, M. Peripherally, non-peripherally and axially pyrazoline-fused phthalocyanines: synthesis, aggregation behaviour, fluorescence, singlet oxygen generation, and photodegradation studies. New J. Chemistry, 2023, 47, 7849–7861. [Google Scholar] [CrossRef]
- Dogandzhiyska, V. , Dimitrov, Sl., Angelov, I., Mantareva, V., Gueorgieva, Tz. Investigation of biocompatibility of Zn- and Ga-based Metal phthalocyanine and FotoSan ™ Photosensitizers, activated by laser light. SYLWAN 2021, 165, 151–163. [Google Scholar]
- Mantareva, V.; Angelov, I.; Syuleyman, M.; Kussovski, V.; Eneva, I.; Avramov, L.; Borisova, E. Phthalocyanines Structure Versus Photodynamic Effectiveness towards Pathogenic Microorganisms: Our Recent Experience. Journal of Biomedical Photonics & Engineering 2021, 7, 1–15. [Google Scholar] [CrossRef]
- Pişkin, M. Phthalocyanine photosensitizers with bathochromic shift, of suitable brightness, capable of producing singlet oxygen with effective efficiency. J. Photochem. Photobiol. Chem. 2023, 2023, 114325. [Google Scholar] [CrossRef]
- Baldea, I.; Ion, R.M.; Olteanu, D.E.; Nenu, I.; Tudor, D.; Filip, A.G. Photodynamic therapy of melanoma using new, synthetic porphyrins and phthalocyanines as photosensitisers - a comparative study. Clujul Med. 2015, 88, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Castro, K.A.D.F.; Prandini, J.A.; Biazzotto, J.C.; Tomé, J.P.C.; da Silva, R.S.; Lourenço, L.M.O. The Surprisingly Positive Effect of Zinc-Phthalocyanines With High Photodynamic Therapy Efficacy of Melanoma Cancer. Front Chem. 2022, 10, 825716. [Google Scholar] [CrossRef] [PubMed]
- Nkune, N.W.; Matlou, G.G.; Abrahamse, H. Photodynamic Therapy Efficacy of Novel Zinc Phthalocyanine Tetra Sodium 2-Mercaptoacetate Combined with Cannabidiol on Metastatic Melanoma. Pharmaceutics 2022, 14, 2418. [Google Scholar] [CrossRef]
- Lopes, J.; Rodrigues, C.M.P.; Gaspar, M.M.; Reis, C.P. Melanoma Management: From Epidemiology to Treatment and Latest Advances. Cancers 2022, 14, 4652. [Google Scholar] [CrossRef]
- Naidoo, C.; Kruger, C.A.; Abrahamse, H. Photodynamic Therapy for Metastatic Melanoma Treatment: A Review. Technology in Cancer Research & Treatment 2018, 17. [Google Scholar] [CrossRef]
- Lopes, J.; Rodrigues, C.M.P.; Gaspar, M.M.; Reis, C.P. How to Treat Melanoma? The Current Status of Innovative Nanotechnological Strategies and the Role of Minimally Invasive Approaches like PTT and PDT. Pharmaceutics 2022, 14, 1817. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).