Submitted:
11 May 2023
Posted:
12 May 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Methods
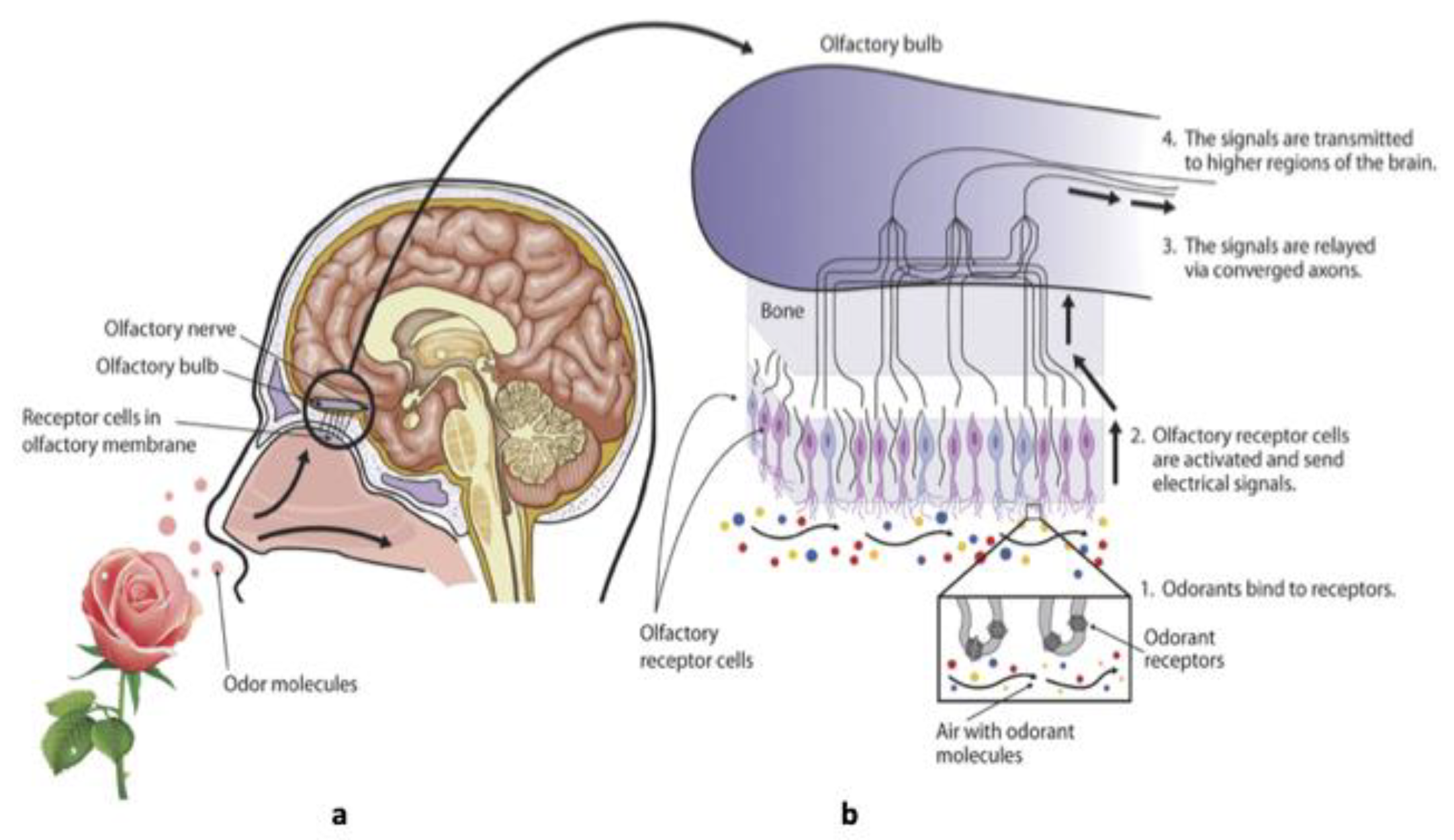
3. Olfactory Receptor and Odorant Detection
4. Viral infection Causing Loss of Smell
5. Viruses Impacting Respiratory System
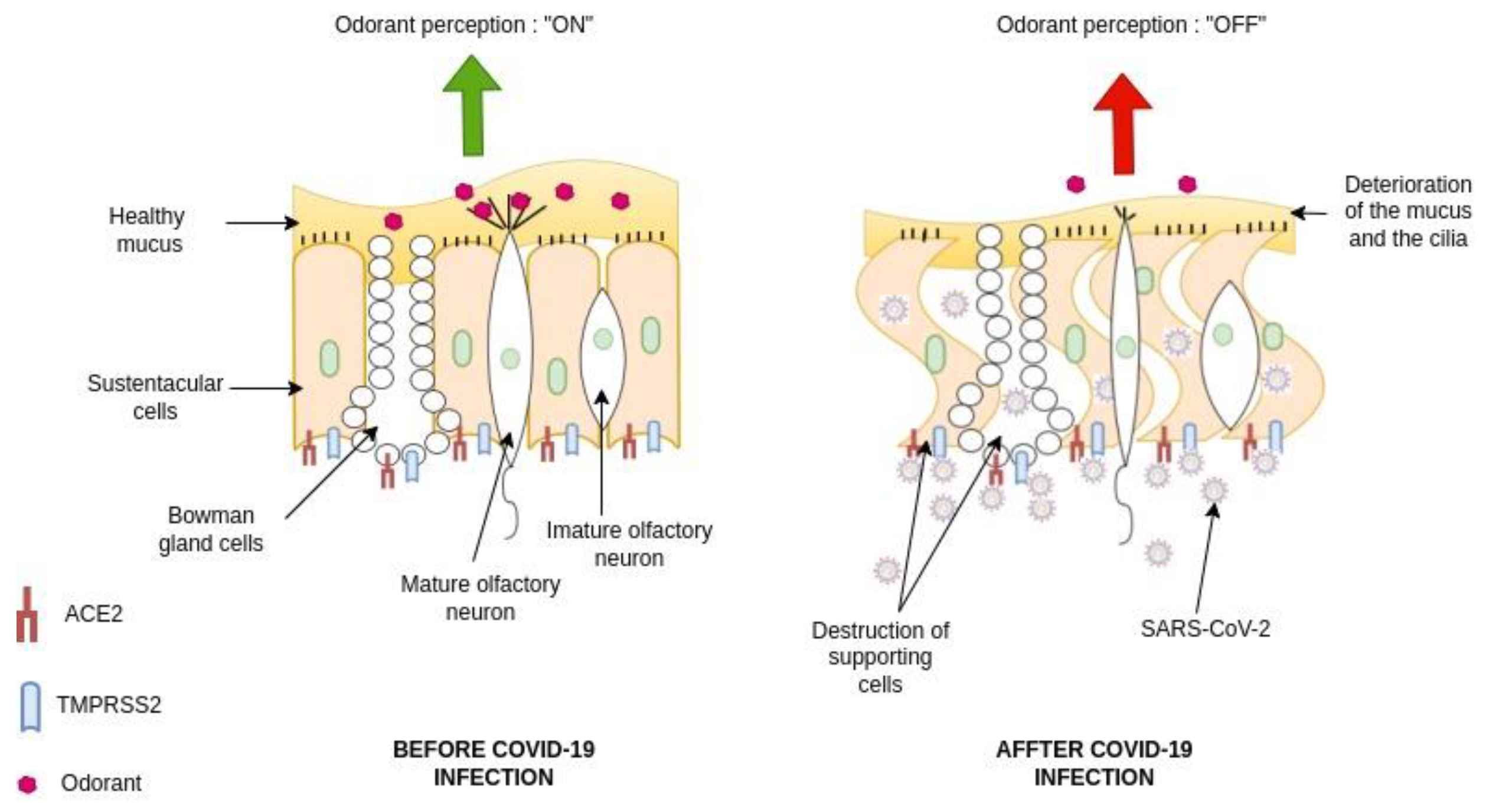
6. Mechanisms of SARS-CoV-2 Mediating the Loss of Smell
7. Conclusions and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689-97. Epub 2020/02/06. PubMed PMID: 32014114; PubMed Central PMCID: PMCPMC7159271. [CrossRef]
- Hui DS, E IA, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264-6. Epub 2020/01/19. PubMed PMID: 31953166; PubMed Central PMCID: PMCPMC7128332. [CrossRef]
- Fisher D, Heymann D. Q&A: The novel coronavirus outbreak causing COVID-19. BMC Med. 2020;18(1):57. Epub 2020/02/29. PubMed PMID: 32106852; PubMed Central PMCID: PMCPMC7047369. [CrossRef]
- World Heath Organization. Situation report – 51. Available online at : https://wwwwhoint/docs/default- source/coronaviruse/situation- reports/20200311-sitrep-51-covid-19pdf?sfvrsn=1ba62e57_10 (2020). (accessed on April 15, 2023).
- World Health Organization. Coronavirus disease (COVID-2019) situation reports. Available online at: www.who.int/emergencies/diseases/novelcoronavirus-2019/situation-reports/. (accessed on April 15, 2023).
- Gandhi M, Yokoe DS, Havlir DV. Asymptomatic Transmission, the Achilles' Heel of Current Strategies to Control Covid-19. N Engl J Med. 2020;382(22):2158-60. Epub 2020/04/25. PubMed PMID: 32329972; PubMed Central PMCID: PMCPMC7200054. [CrossRef]
- Wolfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Muller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465-9. Epub 2020/04/03. [CrossRef] [PubMed]
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-81. Epub 2020/02/28. PubMed PMID: 32105632; PubMed Central PMCID: PMCPMC7102538. [CrossRef]
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708-20. Epub 2020/02/29. PubMed PMID: 32109013; PubMed Central PMCID: PMCPMC7092819. [CrossRef]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. Epub 2020/01/28. PubMed PMID: 31986264; PubMed Central PMCID: PMCPMC7159299. [CrossRef]
- Iacobucci, G. Covid-19: Runny nose, headache, and fatigue are commonest symptoms of omicron, early data show. BMJ. 2021;375:n3103. Epub 2021/12/18. [CrossRef] [PubMed]
- Bagheri SH, Asghari A, Farhadi M, Shamshiri AR, Kabir A, Kamrava SK, et al. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak in Iran. Med J Islam Repub Iran. 2020;34:62. Epub 2020/09/26. PubMed PMID: 32974228; PubMed Central PMCID: PMCPMC7500422. [CrossRef]
- Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251-61. Epub 2020/04/08. PubMed PMID: 32253535; PubMed Central PMCID: PMCPMC7134551. [CrossRef]
- Rebholz H, Braun RJ, Ladage D, Knoll W, Kleber C, Hassel AW. Loss of Olfactory Function-Early Indicator for Covid-19, Other Viral Infections and Neurodegenerative Disorders. Front Neurol. 2020;11:569333. Epub 2020/11/17. PubMed PMID: 33193009; PubMed Central PMCID: PMCPMC7649754. [CrossRef]
- Maniaci A, Ferlito S, Bubbico L, Ledda C, Rapisarda V, Iannella G, et al. Comfort rules for face masks among healthcare workers during COVID-19 spread. Ann Ig. 2021;33(6):615-27. Epub 2021/04/03. [CrossRef] [PubMed]
- de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F, et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021;13(596). Epub 2021/05/05. PubMed PMID: 33941622; PubMed Central PMCID: PMCPMC8158965. [CrossRef]
- Zazhytska M, Kodra A, Hoagland DA, Frere J, Fullard JF, Shayya H, et al. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell. 2022;185(6):1052-64 e12. Epub 2022/02/19. PubMed PMID: 35180380; PubMed Central PMCID: PMCPMC8808699. [CrossRef]
- Bilinska K, Butowt R. Anosmia in COVID-19: A Bumpy Road to Establishing a Cellular Mechanism. ACS Chem Neurosci. 2020;11(15):2152-5. Epub 2020/07/17. PubMed PMID: 32673476; PubMed Central PMCID: PMCPMC7467568. [CrossRef]
- Samaranayake LP, Fakhruddin KS, Panduwawala C. Sudden onset, acute loss of taste and smell in coronavirus disease 2019 (COVID-19): a systematic review. Acta Odontol Scand. 2020;78(6):467-73. Epub 2020/08/09. [CrossRef] [PubMed]
- Kirchdoerfer RN, Wang N, Pallesen J, Wrapp D, Turner HL, Cottrell CA, et al. Stabilized coronavirus spikes are resistant to conformational changes induced by receptor recognition or proteolysis. Sci Rep. 2018;8(1):15701. Epub 2018/10/26. PubMed PMID: 30356097; PubMed Central PMCID: PMCPMC6200764. [CrossRef]
- Othman BA, Maulud SQ, Jalal PJ, Abdulkareem SM, Ahmed JQ, Dhawan M, et al. Olfactory dysfunction as a post-infectious symptom of SARS-CoV-2 infection. Ann Med Surg (Lond). 2022;75:103352. Epub 2022/02/17. PubMed PMID: 35169465; PubMed Central PMCID: PMCPMC8830927. [CrossRef]
- Firestein, S. How the olfactory system makes sense of scents. Nature. 2001;413(6852):211-8. Epub 2001/09/15. [CrossRef] [PubMed]
- Olender T, Lancet D, Nebert DW. Update on the olfactory receptor (OR) gene superfamily. Hum Genomics. 2008;3(1):87-97. Epub 2009/01/09. PubMed PMID: 19129093; PubMed Central PMCID: PMCPMC2752031. [CrossRef]
- Reed, RR. After the holy grail: establishing a molecular basis for Mammalian olfaction. Cell. 2004;116(2):329-36. Epub 2004/01/28. [CrossRef] [PubMed]
- Dammalli M, Dey G, Madugundu AK, Kumar M, Rodrigues B, Gowda H, et al. Proteomic Analysis of the Human Olfactory Bulb. OMICS. 2017;21(8):440-53. Epub 2017/08/18. [CrossRef] [PubMed]
- Huttenbrink KB, Hummel T, Berg D, Gasser T, Hahner A. Olfactory dysfunction: common in later life and early warning of neurodegenerative disease. Dtsch Arztebl Int. 2013;110(1-2):1-7, e1. Epub 2013/03/02. PubMed PMID: 23450985; PubMed Central PMCID: PMCPMC3561743. [CrossRef]
- Dammalli M, Dey G, Kumar M, Madugundu AK, Gopalakrishnan L, Gowrishankar BS, et al. Proteomics of the Human Olfactory Tract. OMICS. 2018;22(1):77-87. Epub 2018/01/23. [CrossRef] [PubMed]
- Oboti L, Peretto P, Marchis SD, Fasolo A. From chemical neuroanatomy to an understanding of the olfactory system. Eur J Histochem. 2011;55(4):e35. Epub 2012/02/03. PubMed PMID: 22297441; PubMed Central PMCID: PMCPMC3284237. [CrossRef]
- Barral-Arca R, Gomez-Carballa A, Cebey-Lopez M, Bello X, Martinon-Torres F, Salas A. A Meta-Analysis of Multiple Whole Blood Gene Expression Data Unveils a Diagnostic Host-Response Transcript Signature for Respiratory Syncytial Virus. Int J Mol Sci. 2020;21(5). Epub 2020/03/12. PubMed PMID: 32155831; PubMed Central PMCID: PMCPMC7084441. [CrossRef]
- Boesveldt S, Postma EM, Boak D, Welge-Luessen A, Schopf V, Mainland JD, et al. Anosmia-A Clinical Review. Chem Senses. 2017;42(7):513-23. Epub 2017/05/23. PubMed PMID: 28531300; PubMed Central PMCID: PMCPMC5863566. [CrossRef]
- Brennan PA, Keverne EB. Something in the air? New insights into mammalian pheromones. Curr Biol. 2004;14(2):R81-9. Epub 2004/01/24. [CrossRef] [PubMed]
- Buck, LB. The molecular architecture of odor and pheromone sensing in mammals. Cell. 2000;100(6):611-8. Epub 2000/04/13. [CrossRef] [PubMed]
- Mombaerts, P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci. 2004;5(4):263-78. Epub 2004/03/23. [CrossRef] [PubMed]
- Restrepo D, Arellano J, Oliva AM, Schaefer ML, Lin W. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm Behav. 2004;46(3):247-56. Epub 2004/08/25. [CrossRef] [PubMed]
- Lavoie J, Gasso Astorga P, Segal-Gavish H, Wu YC, Chung Y, Cascella NG, et al. The Olfactory Neural Epithelium As a Tool in Neuroscience. Trends Mol Med. 2017;23(2):100-3. Epub 2017/01/22. PubMed PMID: 28108112; PubMed Central PMCID: PMCPMC5399677. [CrossRef]
- Liang F, Wang Y. COVID-19 Anosmia: High Prevalence, Plural Neuropathogenic Mechanisms, and Scarce Neurotropism of SARS-CoV-2? Viruses. 2021;13(11). Epub 2021/11/28. PubMed PMID: 34835030; PubMed Central PMCID: PMCPMC8625547. [CrossRef]
- Malnic B, Godfrey PA, Buck LB. The human olfactory receptor gene family. Proc Natl Acad Sci U S A. 2004;101(8):2584-9. Epub 2004/02/26. PubMed PMID: 14983052; PubMed Central PMCID: PMCPMC356993. [CrossRef]
- Sosinsky A, Glusman G, Lancet D. The genomic structure of human olfactory receptor genes. Genomics. 2000;70(1):49-61. Epub 2000/11/23. [CrossRef] [PubMed]
- Zozulya S, Echeverri F, Nguyen T. The human olfactory receptor repertoire. Genome Biol. 2001;2(6):RESEARCH0018. Epub 2001/06/26. PubMed PMID: 11423007; PubMed Central PMCID: PMCPMC33394. [CrossRef]
- Young JM, Trask BJ. The sense of smell: genomics of vertebrate odorant receptors. Hum Mol Genet. 2002;11(10):1153-60. Epub 2002/05/17. [CrossRef] [PubMed]
- Zhang X, Firestein S. The olfactory receptor gene superfamily of the mouse. Nat Neurosci. 2002;5(2):124-33. Epub 2002/01/22. [CrossRef] [PubMed]
- Breer H, Raming K, Krieger J, Boekhoff I, Strotmann J. Towards an identification of odorant receptors. J Recept Res. 1993;13(1-4):527-40. Epub 1993/01/01. [CrossRef] [PubMed]
- Amoore, JE. Stereochemical and vibrational theories of odour. Nature. 1971;233(5317):270-1. Epub 1971/09/24. [CrossRef] [PubMed]
- Araneda RC, Peterlin Z, Zhang X, Chesler A, Firestein S. A pharmacological profile of the aldehyde receptor repertoire in rat olfactory epithelium. J Physiol. 2004;555(Pt 3):743-56. Epub 2004/01/16. PubMed PMID: 14724183; PubMed Central PMCID: PMCPMC1664868. [CrossRef]
- Sanz G, Schlegel C, Pernollet JC, Briand L. Comparison of odorant specificity of two human olfactory receptors from different phylogenetic classes and evidence for antagonism. Chem Senses. 2005;30(1):69-80. Epub 2005/01/14. [CrossRef] [PubMed]
- Ma M, Shepherd GM. Functional mosaic organization of mouse olfactory receptor neurons. Proc Natl Acad Sci U S A. 2000;97(23):12869-74. Epub 2000/10/26. PubMed PMID: 11050155; PubMed Central PMCID: PMCPMC18856. [CrossRef]
- Wade F, Espagne A, Persuy MA, Vidic J, Monnerie R, Merola F, et al. Relationship between homo-oligomerization of a mammalian olfactory receptor and its activation state demonstrated by bioluminescence resonance energy transfer. J Biol Chem. 2011;286(17):15252-9. Epub 2011/04/02. PubMed PMID: 21454689; PubMed Central PMCID: PMCPMC3083150. [CrossRef]
- Stewart WB, Kauer JS, Shepherd GM. Functional organization of rat olfactory bulb analysed by the 2-deoxyglucose method. J Comp Neurol. 1979;185(4):715-34. Epub 1979/06/15. [CrossRef] [PubMed]
- Minic J, Persuy MA, Godel E, Aioun J, Connerton I, Salesse R, et al. Functional expression of olfactory receptors in yeast and development of a bioassay for odorant screening. FEBS J. 2005;272(2):524-37. Epub 2005/01/19. [CrossRef] [PubMed]
- Johnson BA, Leon M. Odorant molecular length: one aspect of the olfactory code. J Comp Neurol. 2000;426(2):330-8. Epub 2000/09/12. [CrossRef] [PubMed]
- Kurian SM, Gordon S, Barrick B, Dadlani MN, Fanelli B, Cornell JB, et al. Feasibility and Comparison Study of Fecal Sample Collection Methods in Healthy Volunteers and Solid Organ Transplant Recipients Using 16S rRNA and Metagenomics Approaches. Biopreserv Biobank. 2020;18(5):425-40. Epub 2020/08/25. [CrossRef] [PubMed]
- Glezer I, Malnic B. Olfactory receptor function. Handb Clin Neurol. 2019;164:67-78. Epub 2019/10/13. [CrossRef] [PubMed]
- Schwob, JE. Neural regeneration and the peripheral olfactory system. Anat Rec. 2002;269(1):33-49. Epub 2002/03/14. [CrossRef] [PubMed]
- Graziadei PP, Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8(1):1-18. Epub 1979/02/01. [CrossRef] [PubMed]
- Urata S, Maruyama J, Kishimoto-Urata M, Sattler RA, Cook R, Lin N, et al. Regeneration Profiles of Olfactory Epithelium after SARS-CoV-2 Infection in Golden Syrian Hamsters. ACS Chem Neurosci. 2021;12(4):589-95. Epub 2021/02/02. PubMed PMID: 33522795; PubMed Central PMCID: PMCPMC7874468. [CrossRef]
- Dicpinigaitis, PV. Post-viral Anosmia (Loss of Sensation of Smell) Did Not Begin with COVID-19! Lung. 2021;199(3):237-8. Epub 2021/04/25. PubMed PMID: 33893845; PubMed Central PMCID: PMCPMC8067782. [CrossRef]
- Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272-7. Epub 2007/02/06. PubMed PMID: 17277621; PubMed Central PMCID: PMCPMC7165544. [CrossRef]
- Lee DY, Lee WH, Wee JH, Kim JW. Prognosis of postviral olfactory loss: follow-up study for longer than one year. Am J Rhinol Allergy. 2014;28(5):419-22. Epub 2014/09/10. [CrossRef] [PubMed]
- Welge-Lussen A, Wolfensberger M. Olfactory disorders following upper respiratory tract infections. Adv Otorhinolaryngol. 2006;63:125-32. Epub 2006/05/31. [CrossRef] [PubMed]
- Seiden, AM. Postviral olfactory loss. Otolaryngol Clin North Am. 2004;37(6):1159-66. Epub 2004/11/27. [CrossRef] [PubMed]
- Moran DT, Jafek BW, Eller PM, Rowley JC, 3rd. Ultrastructural histopathology of human olfactory dysfunction. Microsc Res Tech. 1992;23(2):103-10. Epub 1992/10/15. [CrossRef] [PubMed]
- Doty RL, Hawkes CH. Chemosensory dysfunction in neurodegenerative diseases. Handb Clin Neurol. 2019;164:325-60. Epub 2019/10/13. [CrossRef] [PubMed]
- Lazarini F, Katsimpardi L, Levivien S, Wagner S, Gressens P, Teissier N, et al. Congenital Cytomegalovirus Infection Alters Olfaction Before Hearing Deterioration In Mice. J Neurosci. 2018;38(49):10424-37. Epub 2018/10/21. PubMed PMID: 30341181; PubMed Central PMCID: PMCPMC6596252. [CrossRef]
- Teissier N, Fallet-Bianco C, Delezoide AL, Laquerriere A, Marcorelles P, Khung-Savatovsky S, et al. Cytomegalovirus-induced brain malformations in fetuses. J Neuropathol Exp Neurol. 2014;73(2):143-58. Epub 2014/01/16. [CrossRef] [PubMed]
- Tian J, Liu L, Zhou K, Hong Z, Chen Q, Jiang F, et al. Metal-organic tube or layered assembly: reversible sheet-to-tube transformation and adaptive recognition. Chem Sci. 2020;11(36):9818-26. Epub 2021/06/08. PubMed PMID: 34094242; PubMed Central PMCID: PMCPMC8162108. [CrossRef]
- Mori I, Komatsu T, Takeuchi K, Nakakuki K, Sudo M, Kimura Y. Parainfluenza virus type 1 infects olfactory neurons and establishes long-term persistence in the nerve tissue. J Gen Virol. 1995;76 ( Pt 5):1251-4. Epub 1995/05/01. [CrossRef] [PubMed]
- Tian J, Pinto JM, Cui X, Zhang H, Li L, Liu Y, et al. Sendai Virus Induces Persistent Olfactory Dysfunction in a Murine Model of PVOD via Effects on Apoptosis, Cell Proliferation, and Response to Odorants. PLoS One. 2016;11(7):e0159033. Epub 2016/07/20. PubMed PMID: 27428110; PubMed Central PMCID: PMCPMC4948916. [CrossRef]
- Nakashima T, Suzuki H, Teranishi M. Olfactory and gustatory dysfunction caused by SARS-CoV-2: Comparison with cases of infection with influenza and other viruses. Infect Control Hosp Epidemiol. 2021;42(1):113-4. Epub 2020/05/06. PubMed PMID: 32366336; PubMed Central PMCID: PMCPMC7298085. [CrossRef]
- van Riel D, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015;235(2):277-87. Epub 2014/10/09. [CrossRef] [PubMed]
- Mori I, Goshima F, Imai Y, Kohsaka S, Sugiyama T, Yoshida T, et al. Olfactory receptor neurons prevent dissemination of neurovirulent influenza A virus into the brain by undergoing virus-induced apoptosis. J Gen Virol. 2002;83(Pt 9):2109-16. Epub 2002/08/20. [CrossRef] [PubMed]
- Chen Z, Ni D, Gao Y, Lin J. [Apoptosis related genes--Bcl-2, bax and iNOS, expressed in the olfactory epithelium of mice infected with influenza virus]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;21(11):510-2. Epub 2007/08/07. [PubMed]
- Siegers JY, van den Brand JM, Leijten LM, van de Bildt MM, van Run PR, van Amerongen G, et al. Vaccination Is More Effective Than Prophylactic Oseltamivir in Preventing CNS Invasion by H5N1 Virus via the Olfactory Nerve. J Infect Dis. 2016;214(4):516-24. Epub 2016/07/23. [CrossRef] [PubMed]
- Khani E, Khiali S, Beheshtirouy S, Entezari-Maleki T. Potential pharmacologic treatments for COVID-19 smell and taste loss: A comprehensive review. Eur J Pharmacol. 2021;912:174582. Epub 2021/10/23. PubMed PMID: 34678243; PubMed Central PMCID: PMCPMC8524700. [CrossRef]
- Silva Andrade B, Siqueira S, de Assis Soares WR, de Souza Rangel F, Santos NO, Dos Santos Freitas A, et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses. 2021;13(4). Epub 2021/05/01. PubMed PMID: 33919537; PubMed Central PMCID: PMCPMC8072585. [CrossRef]
- Fernandez-de-Las-Penas C, Cancela-Cilleruelo I, Rodriguez-Jimenez J, Gomez-Mayordomo V, Pellicer-Valero OJ, Martin-Guerrero JD, et al. Associated-Onset Symptoms and Post-COVID-19 Symptoms in Hospitalized COVID-19 Survivors Infected with Wuhan, Alpha or Delta SARS-CoV-2 Variant. Pathogens. 2022;11(7). Epub 2022/07/28. PubMed PMID: 35889971; PubMed Central PMCID: PMCPMC9320021. [CrossRef]
- von Bartheld CS, Hagen MM, Butowt R. The D614G Virus Mutation Enhances Anosmia in COVID-19 Patients: Evidence from a Systematic Review and Meta-analysis of Studies from South Asia. ACS Chem Neurosci. 2021;12(19):3535-49. Epub 2021/09/18. PubMed PMID: 34533304; PubMed Central PMCID: PMCPMC8482322. [CrossRef]
- von Bartheld CS, Wang L. Prevalence of Olfactory Dysfunction with the Omicron Variant of SARS-CoV-2: A Systematic Review and Meta-Analysis. Cells. 2023;12(3). Epub 2023/02/12. PubMed PMID: 36766771; PubMed Central PMCID: PMCPMC9913864. [CrossRef]
- Rodriguez-Sevilla JJ, Guerri-Fernadez R, Bertran Recasens B. Is There Less Alteration of Smell Sensation in Patients With Omicron SARS-CoV-2 Variant Infection? Front Med (Lausanne). 2022;9:852998. Epub 2022/05/03. PubMed PMID: 35492353; PubMed Central PMCID: PMCPMC9039252. [CrossRef]
- Chee J, Chern B, Loh WS, Mullol J, Wang Y. Pathophysiology of SARS-CoV-2 Infection of Nasal Respiratory and Olfactory Epithelia and Its Clinical Impact. Curr Allergy Asthma Rep. 2023;23(2):121-31. Epub 2023/01/05. PubMed PMID: 36598732; PubMed Central PMCID: PMCPMC9811886. [CrossRef]
- Mutiawati E, Fahriani M, Mamada SS, Fajar JK, Frediansyah A, Maliga HA, et al. Anosmia and dysgeusia in SARS-CoV-2 infection: incidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms - a systematic review and meta-analysis. F1000Res. 2021;10:40. Epub 2021/04/08. PubMed PMID: 33824716; PubMed Central PMCID: PMCPMC7993408. [CrossRef]
- Butowt R, von Bartheld CS. Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist. 2021;27(6):582-603. Epub 2020/09/12. PubMed PMID: 32914699; PubMed Central PMCID: PMCPMC7488171. [CrossRef]
- Fantin F, Frosolini A, Tundo I, Inches I, Fabbris C, Spinato G, et al. A singular case of hyposmia and transient audiovestibular post-vaccine disorders: case report and literature review. Transl Neurosci. 2022;13(1):349-53. Epub 2022/10/29. PubMed PMID: 36304095; PubMed Central PMCID: PMCPMC9547348. [CrossRef]
- Lechien JR, Diallo AO, Dachy B, Le Bon SD, Maniaci A, Vaira LA, et al. COVID-19: Post-vaccine Smell and Taste Disorders: Report of 6 Cases. Ear Nose Throat J. 2021:1455613211033125. Epub 2021/09/02. [CrossRef] [PubMed]
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-90. Epub 2020/04/11. PubMed PMID: 32275288; PubMed Central PMCID: PMCPMC7149362. [CrossRef]
- Butowt R, Bilinska K, von Bartheld CS. Olfactory dysfunction in COVID-19: new insights into the underlying mechanisms. Trends Neurosci. 2023;46(1):75-90. Epub 2022/12/06. PubMed PMID: 36470705; PubMed Central PMCID: PMCPMC9666374. [CrossRef]
- Buqaileh R, Saternos H, Ley S, Aranda A, Forero K, AbouAlaiwi WA. Can cilia provide an entry gateway for SARS-CoV-2 to human ciliated cells? Physiol Genomics. 2021;53(6):249-58. Epub 2021/04/16. PubMed PMID: 33855870; PubMed Central PMCID: PMCPMC8213509. [CrossRef]
- Butowt R, Meunier N, Bryche B, von Bartheld CS. The olfactory nerve is not a likely route to brain infection in COVID-19: a critical review of data from humans and animal models. Acta Neuropathol. 2021;141(6):809-22. Epub 2021/04/28. PubMed PMID: 33903954; PubMed Central PMCID: PMCPMC8075028. [CrossRef]
- Bilinska K, Jakubowska P, Von Bartheld CS, Butowt R. Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem Neurosci. 2020;11(11):1555-62. Epub 2020/05/08. PubMed PMID: 32379417; PubMed Central PMCID: PMCPMC7241737. [CrossRef]
- Bryche B, St Albin A, Murri S, Lacote S, Pulido C, Ar Gouilh M, et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav Immun. 2020;89:579-86. Epub 2020/07/07. PubMed PMID: 32629042; PubMed Central PMCID: PMCPMC7332942. [CrossRef]
- Sia SF, Yan LM, Chin AWH, Fung K, Choy KT, Wong AYL, et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583(7818):834-8. Epub 2020/05/15. PubMed PMID: 32408338; PubMed Central PMCID: PMCPMC7394720. [CrossRef]
- Lee Y, Min P, Lee S, Kim SW. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J Korean Med Sci. 2020;35(18):e174. Epub 2020/05/10. PubMed PMID: 32383370; PubMed Central PMCID: PMCPMC7211515. [CrossRef]
- Fodoulian L, Tuberosa J, Rossier D, Boillat M, Kan C, Pauli V, et al. SARS-CoV-2 Receptors and Entry Genes Are Expressed in the Human Olfactory Neuroepithelium and Brain. iScience. 2020;23(12):101839. Epub 2020/12/01. PubMed PMID: 33251489; PubMed Central PMCID: PMCPMC7685946. [CrossRef]
- Chen M, Shen W, Rowan NR, Kulaga H, Hillel A, Ramanathan M, Jr., et al. Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur Respir J. 2020;56(3). Epub 2020/08/21. PubMed PMID: 32817004; PubMed Central PMCID: PMCPMC7439429. [CrossRef]
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-80 e8. Epub 2020/03/07. PubMed PMID: 32142651; PubMed Central PMCID: PMCPMC7102627. [CrossRef]
- Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181(5):1016-35 e19. Epub 2020/05/16. PubMed PMID: 32413319; PubMed Central PMCID: PMCPMC7252096. [CrossRef]
- Gkogkou E, Barnasas G, Vougas K, Trougakos IP. Expression profiling meta-analysis of ACE2 and TMPRSS2, the putative anti-inflammatory receptor and priming protease of SARS-CoV-2 in human cells, and identification of putative modulators. Redox Biol. 2020;36:101615. Epub 2020/08/31. PubMed PMID: 32863223; PubMed Central PMCID: PMCPMC7311357. [CrossRef]
- Sungnak W, Huang N, Becavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681-7. Epub 2020/04/25. PubMed PMID: 32327758; PubMed Central PMCID: PMCPMC8637938. [CrossRef]
- Li W, Li M, Ou G. COVID-19, cilia, and smell. FEBS J. 2020;287(17):3672-6. Epub 2020/07/22. PubMed PMID: 32692465; PubMed Central PMCID: PMCPMC7426555. [CrossRef]
- Sternberg A, Naujokat C. Structural features of coronavirus SARS-CoV-2 spike protein: Targets for vaccination. Life Sci. 2020;257:118056. Epub 2020/07/10. PubMed PMID: 32645344; PubMed Central PMCID: PMCPMC7336130. [CrossRef]
- Chen M, Pekosz A, Villano JS, Shen W, Zhou R, Kulaga H, et al. Evolution of nasal and olfactory infection characteristics of SARS-CoV-2 variants. bioRxiv. 2022. Epub 2022/04/21. PubMed PMID: 35441175; PubMed Central PMCID: PMCPMC9016639. [CrossRef]
- Torabi A, Mohammadbagheri E, Akbari Dilmaghani N, Bayat AH, Fathi M, Vakili K, et al. Proinflammatory Cytokines in the Olfactory Mucosa Result in COVID-19 Induced Anosmia. ACS Chem Neurosci. 2020;11(13):1909-13. Epub 2020/06/12. PubMed PMID: 32525657; PubMed Central PMCID: PMCPMC7299394. [CrossRef]
- Seo JS, Yoon SW, Hwang SH, Nam SM, Nahm SS, Jeong JH, et al. The Microvillar and Solitary Chemosensory Cells as the Novel Targets of Infection of SARS-CoV-2 in Syrian Golden Hamsters. Viruses. 2021;13(8). Epub 2021/08/29. PubMed PMID: 34452517; PubMed Central PMCID: PMCPMC8402700. [CrossRef]
- Chen CR, Kachramanoglou C, Li D, Andrews P, Choi D. Anatomy and cellular constituents of the human olfactory mucosa: a review. J Neurol Surg B Skull Base. 2014;75(5):293-300. Epub 2014/10/11. PubMed PMID: 25302141; PubMed Central PMCID: PMCPMC4176544. [CrossRef]
- Klingenstein M, Klingenstein S, Neckel PH, Mack AF, Wagner AP, Kleger A, et al. Evidence of SARS-CoV2 Entry Protein ACE2 in the Human Nose and Olfactory Bulb. Cells Tissues Organs. 2020;209(4-6):155-64. Epub 2021/01/25. PubMed PMID: 33486479; PubMed Central PMCID: PMCPMC7900466. [CrossRef]
- Cooper KW, Brann DH, Farruggia MC, Bhutani S, Pellegrino R, Tsukahara T, et al. COVID-19 and the Chemical Senses: Supporting Players Take Center Stage. Neuron. 2020;107(2):219-33. Epub 2020/07/09. PubMed PMID: 32640192; PubMed Central PMCID: PMCPMC7328585. [CrossRef]
- Solbu TT, Holen T. Aquaporin pathways and mucin secretion of Bowman's glands might protect the olfactory mucosa. Chem Senses. 2012;37(1):35-46. Epub 2011/07/13. [CrossRef] [PubMed]
- Doty, RL. Olfactory dysfunction in COVID-19: pathology and long-term implications for brain health. Trends Mol Med. 2022;28(9):781-94. Epub 2022/07/10. PubMed PMID: 35810128; PubMed Central PMCID: PMCPMC9212891. [CrossRef]
- Villar PS, Delgado R, Vergara C, Reyes JG, Bacigalupo J. Energy Requirements of Odor Transduction in the Chemosensory Cilia of Olfactory Sensory Neurons Rely on Oxidative Phosphorylation and Glycolytic Processing of Extracellular Glucose. J Neurosci. 2017;37(23):5736-43. Epub 2017/05/14. PubMed PMID: 28500222; PubMed Central PMCID: PMCPMC6596473. [CrossRef]
- Acevedo C, Blanchard K, Bacigalupo J, Vergara C. Possible ATP trafficking by ATP-shuttles in the olfactory cilia and glucose transfer across the olfactory mucosa. FEBS Lett. 2019;593(6):601-10. Epub 2019/02/26. [CrossRef] [PubMed]
- Villar PS, Vergara C, Bacigalupo J. Energy sources that fuel metabolic processes in protruding finger-like organelles. FEBS J. 2021;288(12):3799-812. Epub 2020/11/04. [CrossRef] [PubMed]
- Krishnan S, Nordqvist H, Ambikan AT, Gupta S, Sperk M, Svensson-Akusjarvi S, et al. Metabolic Perturbation Associated With COVID-19 Disease Severity and SARS-CoV-2 Replication. Mol Cell Proteomics. 2021;20:100159. Epub 2021/10/08. PubMed PMID: 34619366; PubMed Central PMCID: PMCPMC8490130. [CrossRef]
- Khan M, Yoo SJ, Clijsters M, Backaert W, Vanstapel A, Speleman K, et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell. 2021;184(24):5932-49 e15. Epub 2021/11/20. PubMed PMID: 34798069; PubMed Central PMCID: PMCPMC8564600. [CrossRef]
- Seehusen F, Clark JJ, Sharma P, Bentley EG, Kirby A, Subramaniam K, et al. Neuroinvasion and Neurotropism by SARS-CoV-2 Variants in the K18-hACE2 Mouse. Viruses. 2022;14(5). Epub 2022/05/29. PubMed PMID: 35632761; PubMed Central PMCID: PMCPMC9146514. [CrossRef]
- Zheng J, Wong LR, Li K, Verma AK, Ortiz ME, Wohlford-Lenane C, et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature. 2021;589(7843):603-7. Epub 2020/11/10. PubMed PMID: 33166988; PubMed Central PMCID: PMCPMC7855185. [CrossRef]
- Davies J, Randeva HS, Chatha K, Hall M, Spandidos DA, Karteris E, et al. Neuropilin-1 as a new potential SARS-CoV-2 infection mediator implicated in the neurologic features and central nervous system involvement of COVID-19. Mol Med Rep. 2020;22(5):4221-6. Epub 2020/10/02. PubMed PMID: 33000221; PubMed Central PMCID: PMCPMC7533503. [CrossRef]
- Kang YL, Chou YY, Rothlauf PW, Liu Z, Soh TK, Cureton D, et al. Inhibition of PIKfyve kinase prevents infection by Zaire ebolavirus and SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117(34):20803-13. Epub 2020/08/09. PubMed PMID: 32764148; PubMed Central PMCID: PMCPMC7456157. [CrossRef]
- Mayi BS, Leibowitz JA, Woods AT, Ammon KA, Liu AE, Raja A. The role of Neuropilin-1 in COVID-19. PLoS Pathog. 2021;17(1):e1009153. Epub 2021/01/05. PubMed PMID: 33395426; PubMed Central PMCID: PMCPMC7781380. [CrossRef]
- Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370(6518):856-60. Epub 2020/10/22. PubMed PMID: 33082293; PubMed Central PMCID: PMCPMC7857391. [CrossRef]
- Daly JL, Simonetti B, Klein K, Chen KE, Williamson MK, Anton-Plagaro C, et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370(6518):861-5. Epub 2020/10/22. PubMed PMID: 33082294; PubMed Central PMCID: PMCPMC7612957. [CrossRef]
| Effect of SARS-CoV-2 Infection on Cells in the OE | References |
|
[102] [103] [88] [104] [105] [106] [89] [107] |
|
[108] [109] [110] [110] [85] [111] [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





