Submitted:
03 May 2023
Posted:
11 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
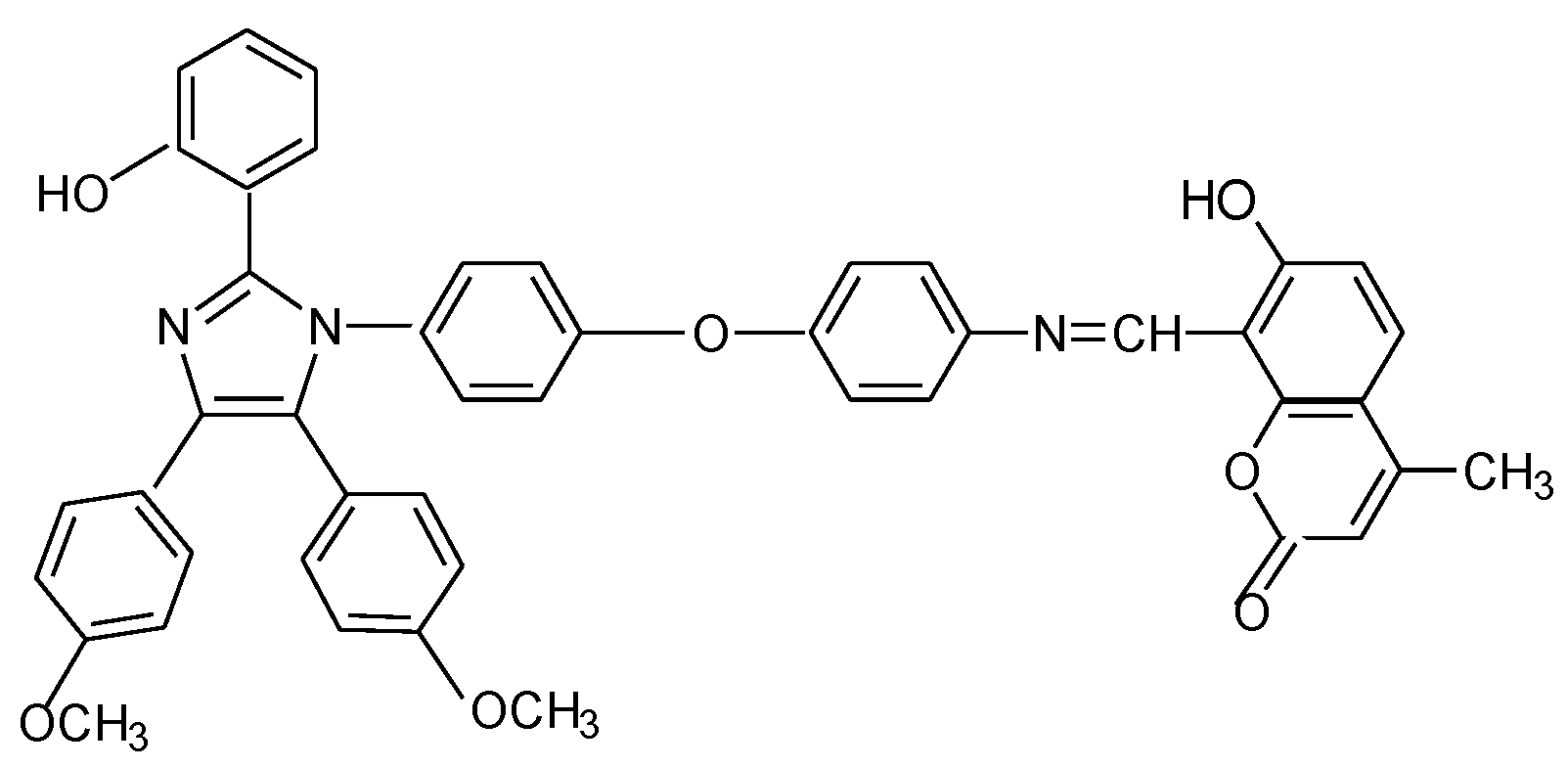
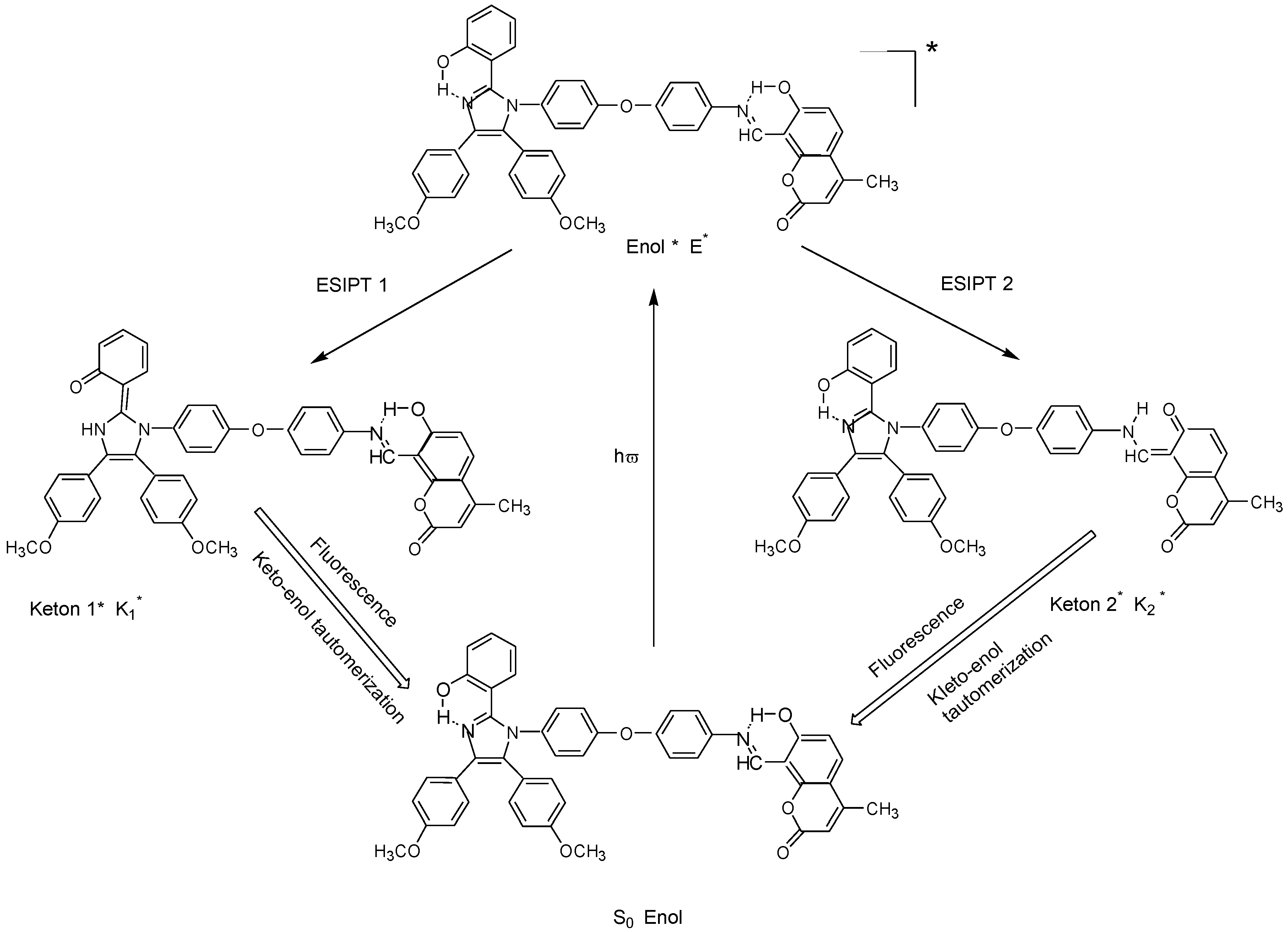
2. Materials and methods
3. Results and Discussion
4. Conclusions
Funding
Conflicts of Interest
References
- Berlin, A.A.; Korolyov, H.V.; Kefeli, T.Y.; et al. . Acrylic oligomers and materials based on them. 1983, Khimiya, Moscow, [In Russian].
- Berlin, A.A.; Sivergin, Yu.V.; Kefeli, T.Y.; Marshavin, N.L.; Sokolovskaya, N.B.; Alimpieva, Ye.S. Effect of the structure of oligo(carbonate-methacrylates) on the mechanical properties of their polymers. Polymer science USSR 1971, 13, 3006–3016. [Google Scholar] [CrossRef]
- Berlin, A.A.; Kefeli, T.Y.; Sukhareva, L.A. Investigation of the structure and properties of crosslinked polymers based on oligocarbonate methacrylates in film formation. Journal of macromolecular science – chemistry 1977, 11, 977–997. [Google Scholar] [CrossRef]
- Prishcheva, N.D.; Khoromskaya, V.A.; Shiryayeva, G.V.; Kefeli, N.Ya.; Berlin, A.A. Radiation hardening of oligoester acrylate and oligocarbonate methacrylate systems. Polymer scienece USSR, 1980, 22, 2536–2541. [Google Scholar] [CrossRef]
- Zapadinskii, B.I. The development of Al. An. Berlin’s ideas in the synthesis of polymerizable oligomers. Polymer Science Series D 2007, 49, 222–234. [Google Scholar] [CrossRef]
- Avramenko, V.L.; Podhornaya, L.F.; Karandashov, O.N. Photocurable polymer composite materials with an improved combination of strength and service properties. Functional materials. 2020, 27, 587–594. [Google Scholar]
- Florjanczyk, Z,; Rokicki, G,; Parzuchowski, P.G.; Mazure-Budzynska, M.; Debowski, M. Polymer materials based on carbone dioxide: a brief review of studied carried out at the faculty of chemistry, Warsaw university of technology. Polymers 2022, 14, 718. [CrossRef] [PubMed]
- Evseev, A.V.; Markov, M.A. Layer-by-layer manufacture of parts from liquid photopolymerisable compositions by XeCl laser radiation. Quantum Electronics. 1994, 24, 458–461. [Google Scholar] [CrossRef]
- Rot, A.; Zaks, I.; Wielgosz, Z. Photopolymer coatings for optical discs. Progress in Organic coatings 1993, 21, 285–294. [Google Scholar] [CrossRef]
- Rocheva, V.V.; Koroleva, A.A., Savelyev, A.G.; Khaydukov, K.V.; Generalova, A.N.; Nechaev, A.V.; Guller, A.E; Semchishen, V.A.; Chichkov, B.N.; Khaydukov, E.V. High-resolution 3D photopolymerization assisted by upconversion nanoparticles for rapid prototyping applications. Scientific reports 2018, 8, 3663.
- Savelyev, A.G.; Semchishev, V.A.; Nechaev, A.V.; Khaydukov, K.V.; Demina, P.A.; Generalova, A.N.; Khaydukov, E.V. Near-infrared photopolymerization assisted by upconversion nanophosphores for biomedical applications. EPJ Web of Conferences 2018, 190, 04018. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, S. , Chen, Y.; Guo, H.; Yang, P. Excited state intramolecular proton transfer (ESIPT): from principal photophysics to the development of new chromophores and applications in fluorescent molecular probes and luminescent materials. Phys Chem Chem Phys. 2012, 14, 8803–8817. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, W.; Ge, J.; Zhang, H.; Wang, P. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem Soc Rev. 2011, 40, 3483–3495. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Thilagar, P. Organic white-light emitting materials. Dyes and Pigments. 2014, 110, 2–27. [Google Scholar] [CrossRef]
- Park, S.Y.; Won, M.; Kang, C.; Kim, J. S/; Lee, M.H. A coumarin-naphthalimide hybrid as a dual emissive fluorescent probe for hNQO1. Dyes and Pigments. 2019, 164, 341–345. [Google Scholar] [CrossRef]
- Kwon, J.E.; Park, S.; Park, S.Y. Realizing molecular pixel system for full-color fluorescence reproduction: RGB-emitting molecular mixture free from energy transfer crosstalk. J.Am. Chem. Soc. 2013, 135, 11239–11246. [Google Scholar] [CrossRef] [PubMed]
- Levin, P.P.; Liubimov, A.V.; Shashkov, A.S.; Mardaleishvili, I.R.; Venidiktova, O.V.; Shienok, A.I.; Koltsova, L.S.; Astafiev, A.A.; Barachevsky, V.A.; Zaichenko, N.L. Multiple fluorescence of tetraarylimidazole and azomethinocoumarin dyad with dual excited-state intramolecular proton transfer. Dyes and pigments 2020, 183, 108716. [Google Scholar] [CrossRef]
- Park, S.; Kwon, J.E.; Kim, S.H.; Seo, J.; Chung, K.; Jang, D.J.; Medina, B.W.; Giecher, J.; Park, S.Y. A white-light-emitting molecule: frustrated energy transfer between constituent emitting centers. J. Am. Chem. Soc. 2009, 131, 14043–14049. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, I.A.; Shashkova, V.T.; Liubimov, A.V.; Liubimova, G.V.; Koltsova, L.S.; Shienok, A.I.; Zaichenko, N.L. Peculiarities of the Manifestation of Multiple Luminescence of Organic Compounds in Photocured Acrylic Polymers. Russian Journal of Physical Chemistry B. 2019, 13, 803–811. [Google Scholar] [CrossRef]
- Shashkova, V.T.; Koval’chuk, O.S.; Tseitlin, G.H.; Zapadinski, B.I. The mechanism of formation of carborane- containing oligo(carbonate methacrylates). Polymer Sci.A. 1997, 39, 737–743. [Google Scholar]
- Zubov, P.I.; Sukhareva, L.A. Structure and properties of polymer coatings. 1982, Khimiya, Moscow. (In Russian).
- Korolev, G.V.; Mogilevich, M.M.; Golikov, I.V. Network polyacrylates. 1995., Khimiya, Moscow. (In Russian).
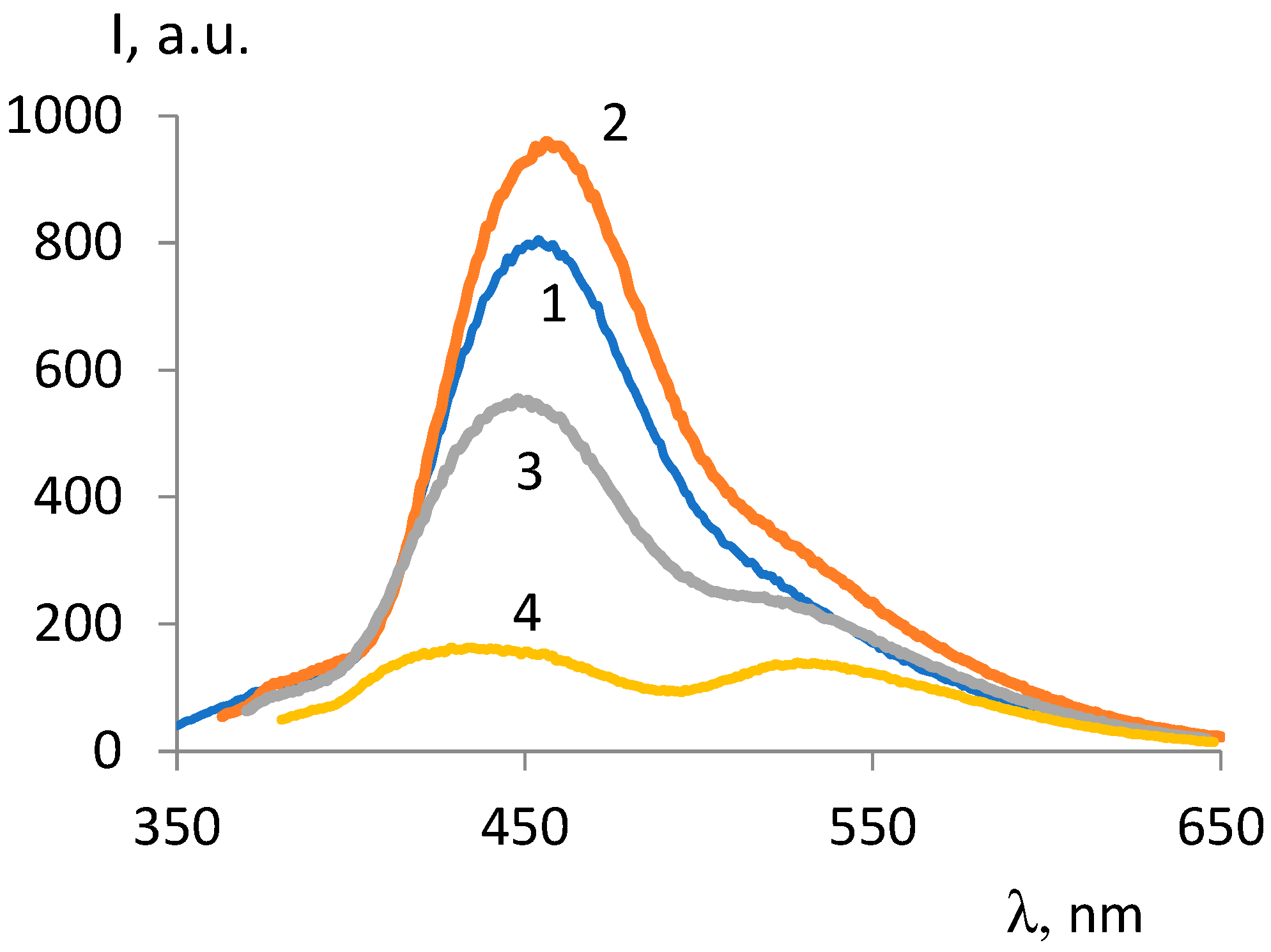
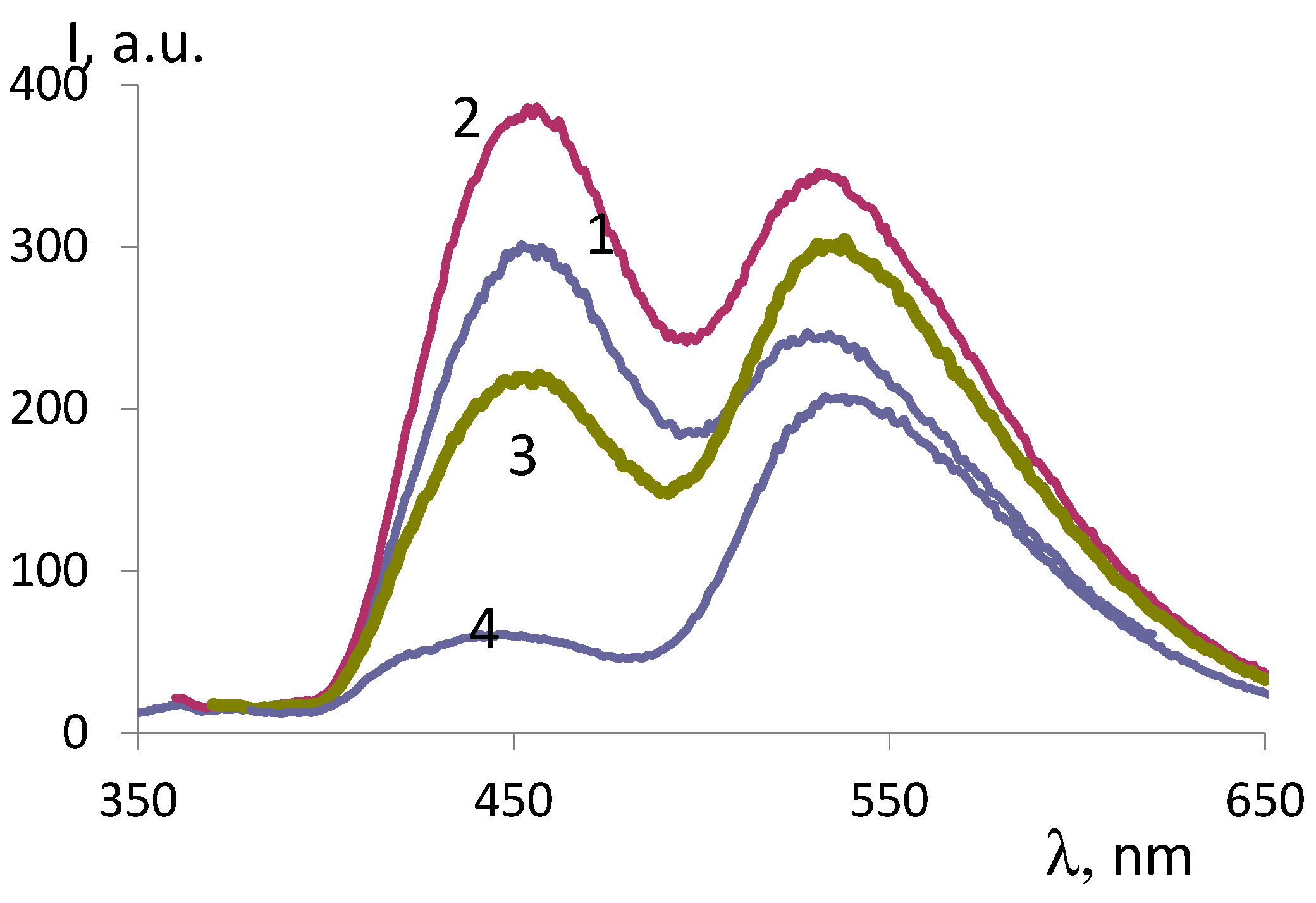
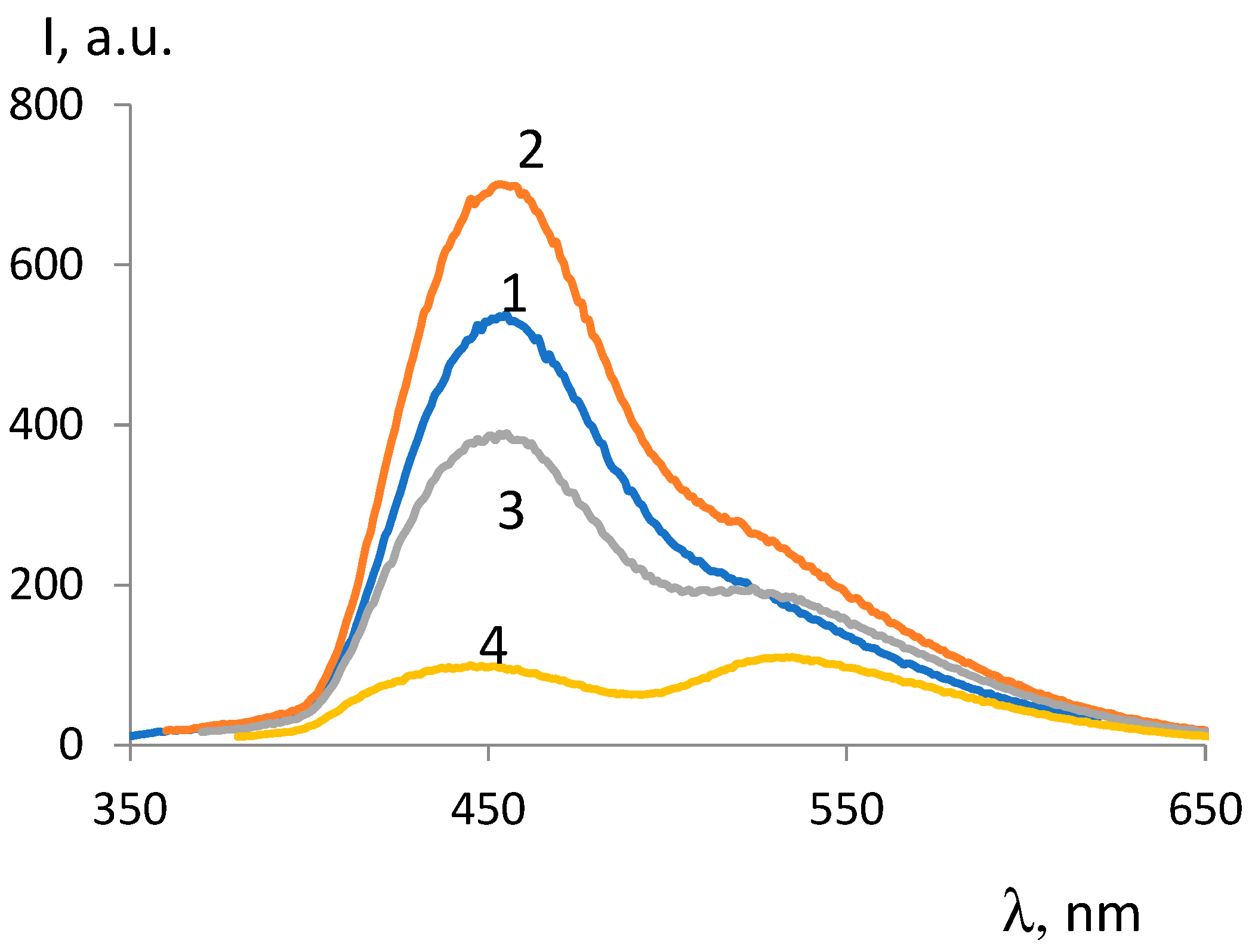
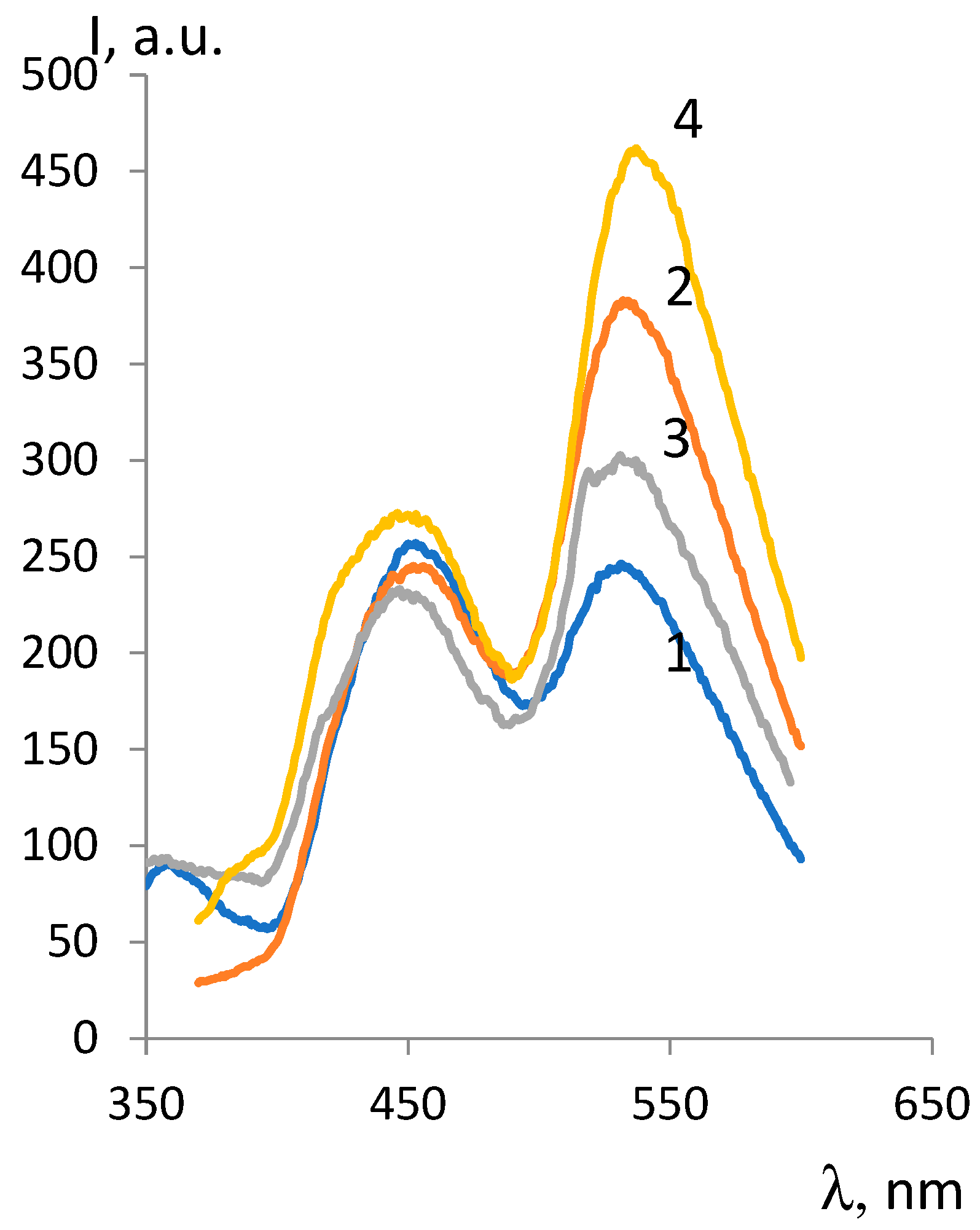
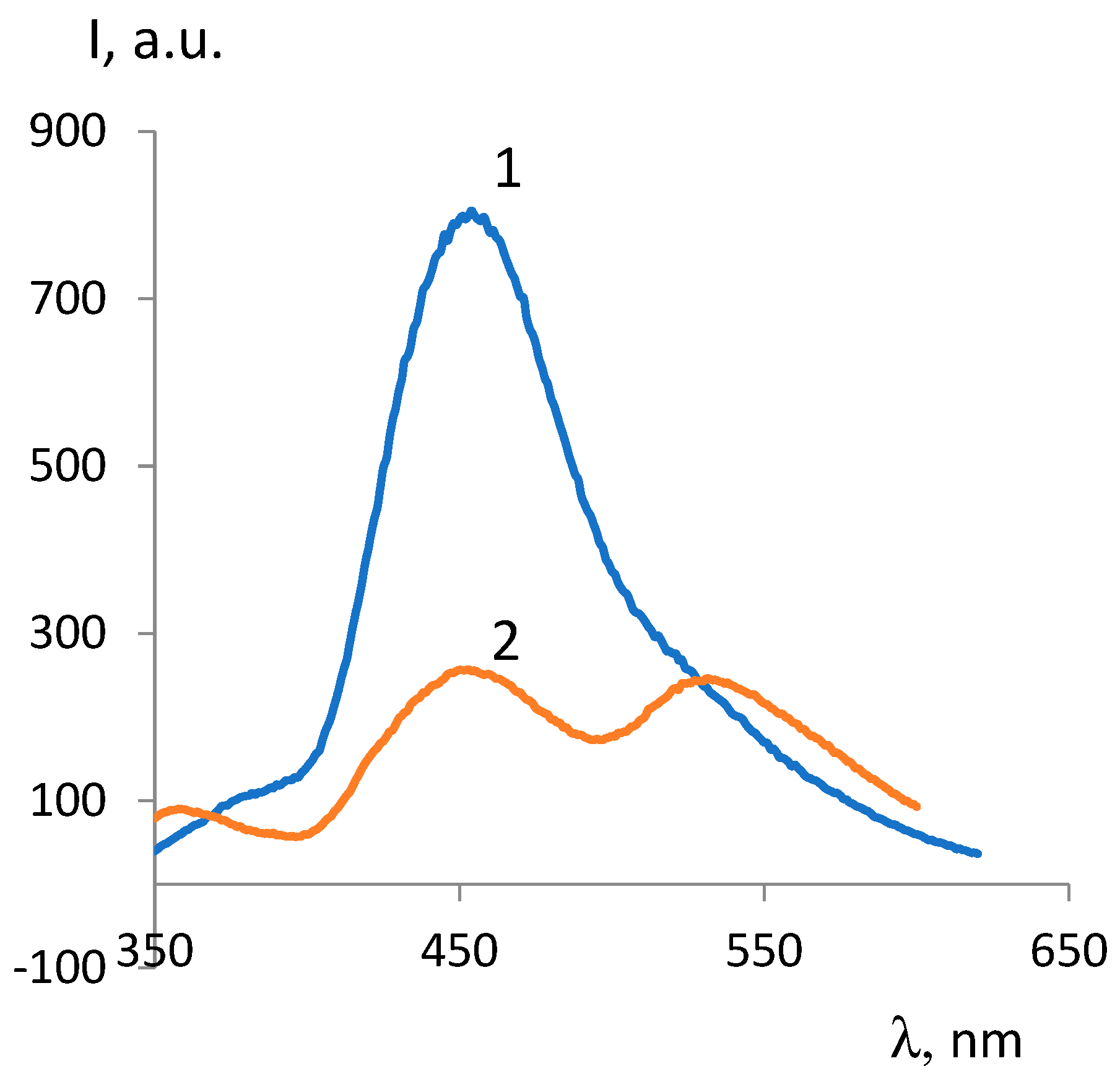
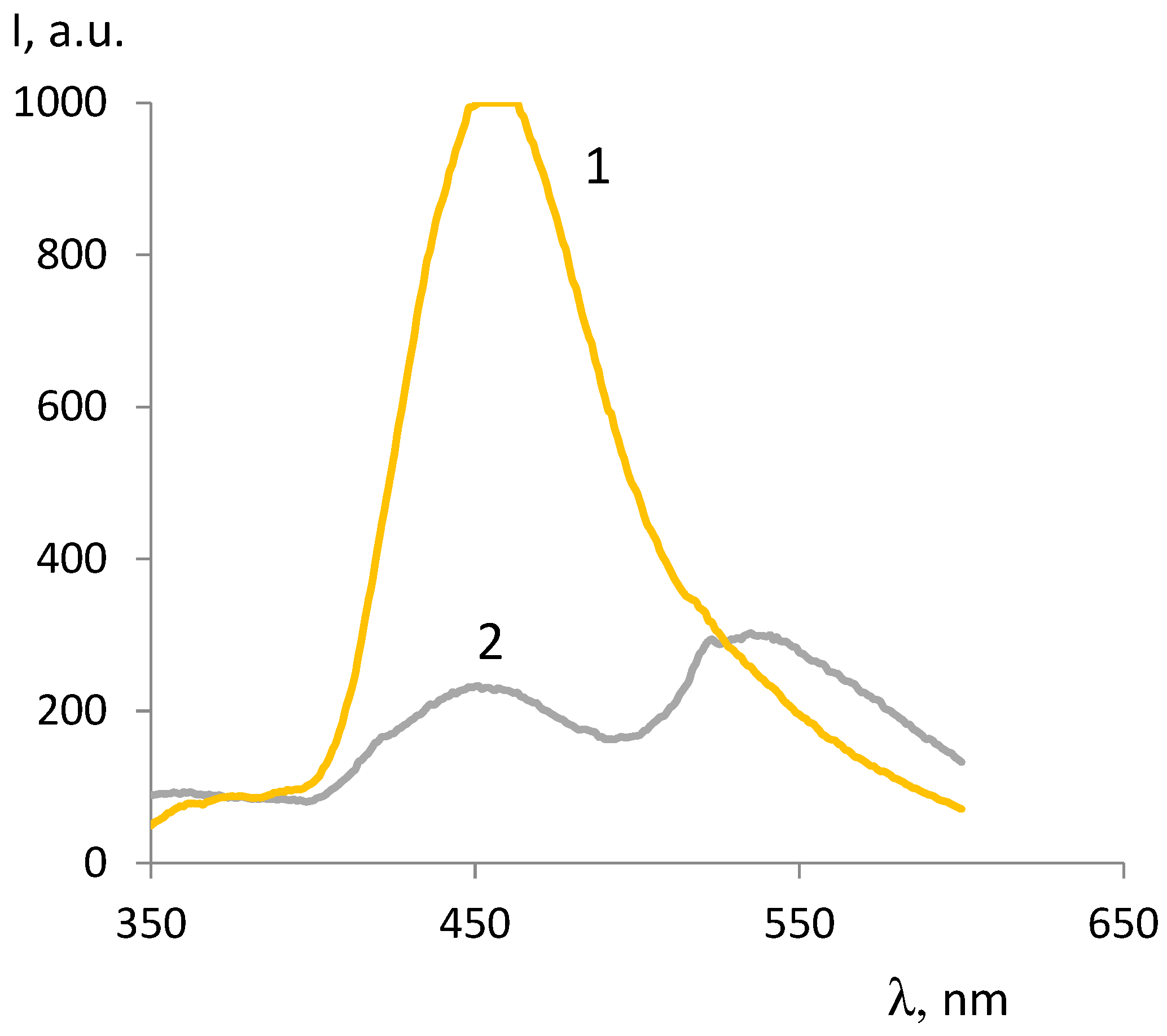
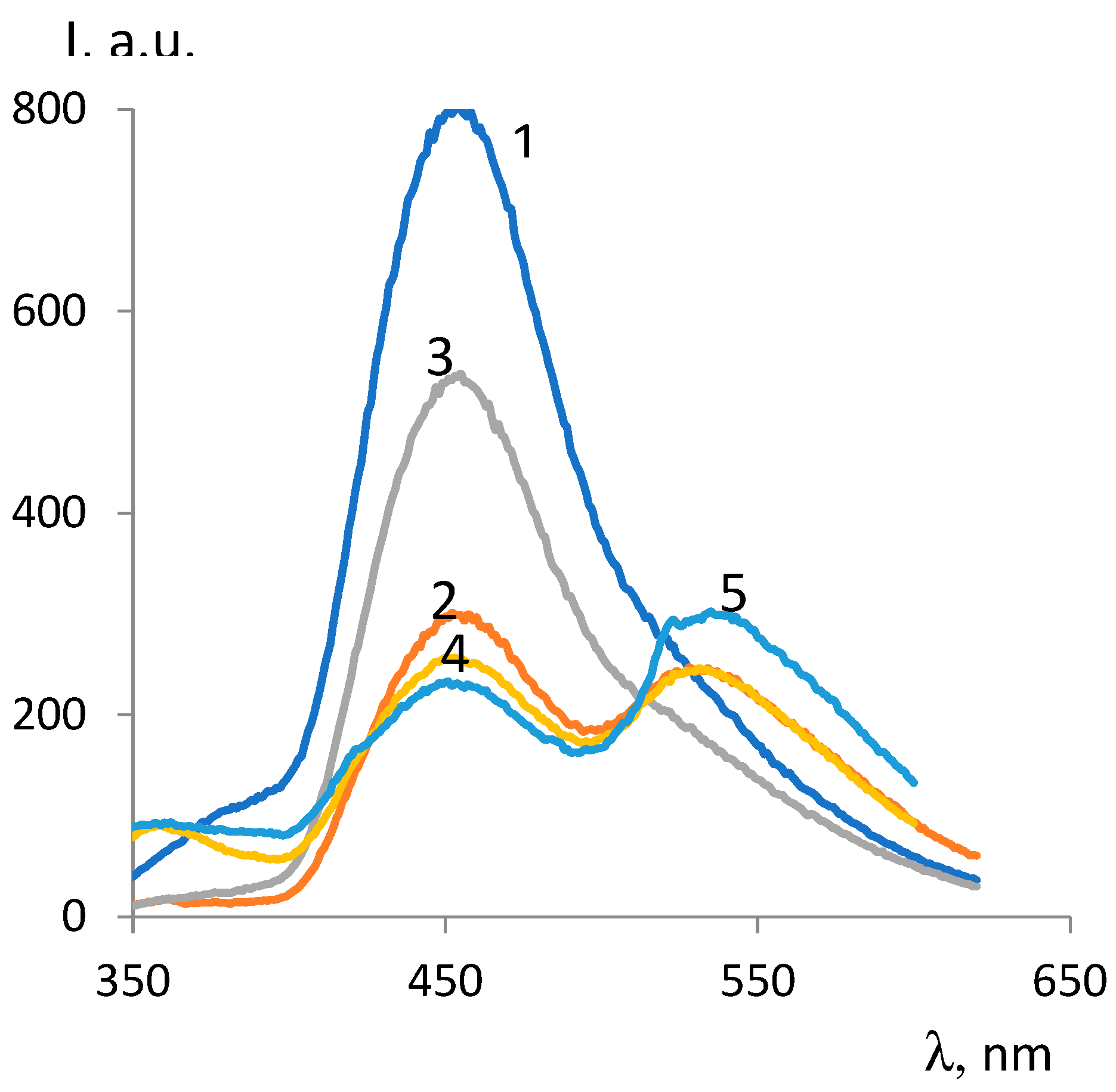
| ОCМ | ММ | Fluorescence intensity, λex =330 nm |
Fluorescence intensity,, λex=350 nm |
||
|---|---|---|---|---|---|
| 450 nm band | 535 nm band | 450 nm band | 535 nm band | ||
| ОCМ-1 | 374 | 802 | - | 534 | 249 |
| ОCМ-2 | 418 | 296 | 240 | 217 | 296 |
| ОCМ-7 | 420 | 257 | 246 | 245 | 383 |
| ОCМ-5 | 540 | 239 | 303 | 273 | 462 |
| ОCМ2/1 | 683 | 529 | - | 383 | 193 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





