Submitted:
09 May 2023
Posted:
11 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
3. Discussion
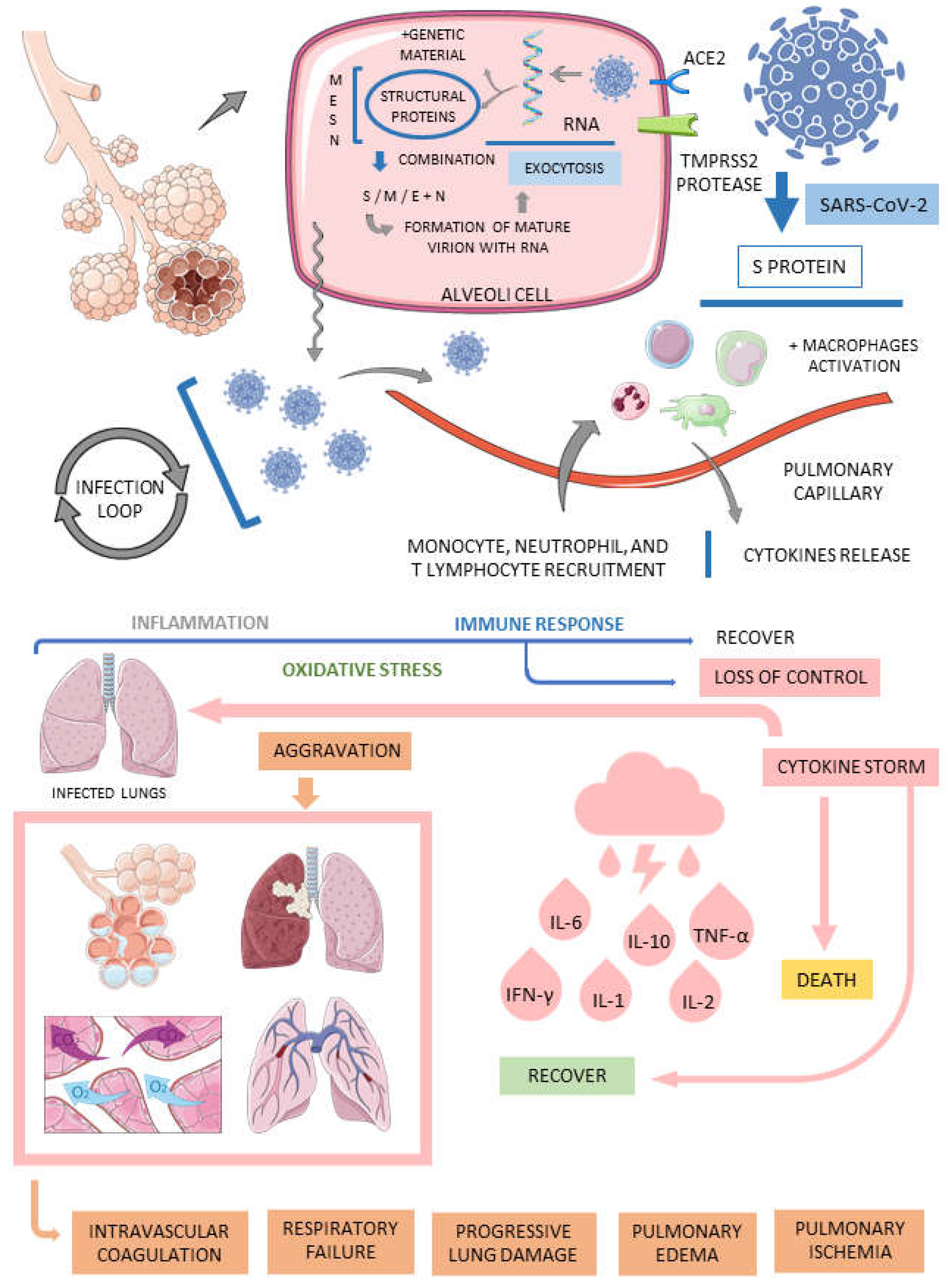
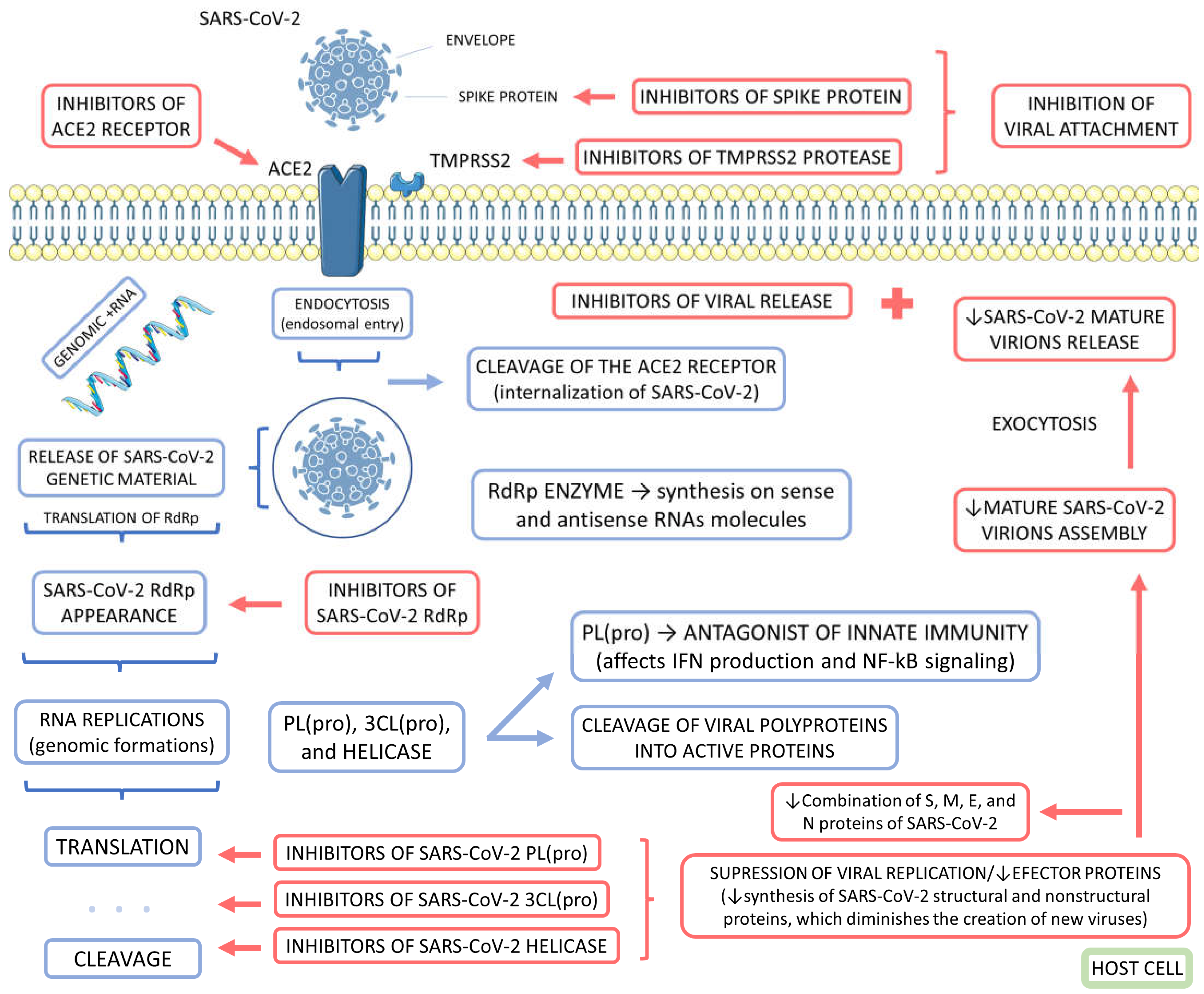
3.1. COVID-19
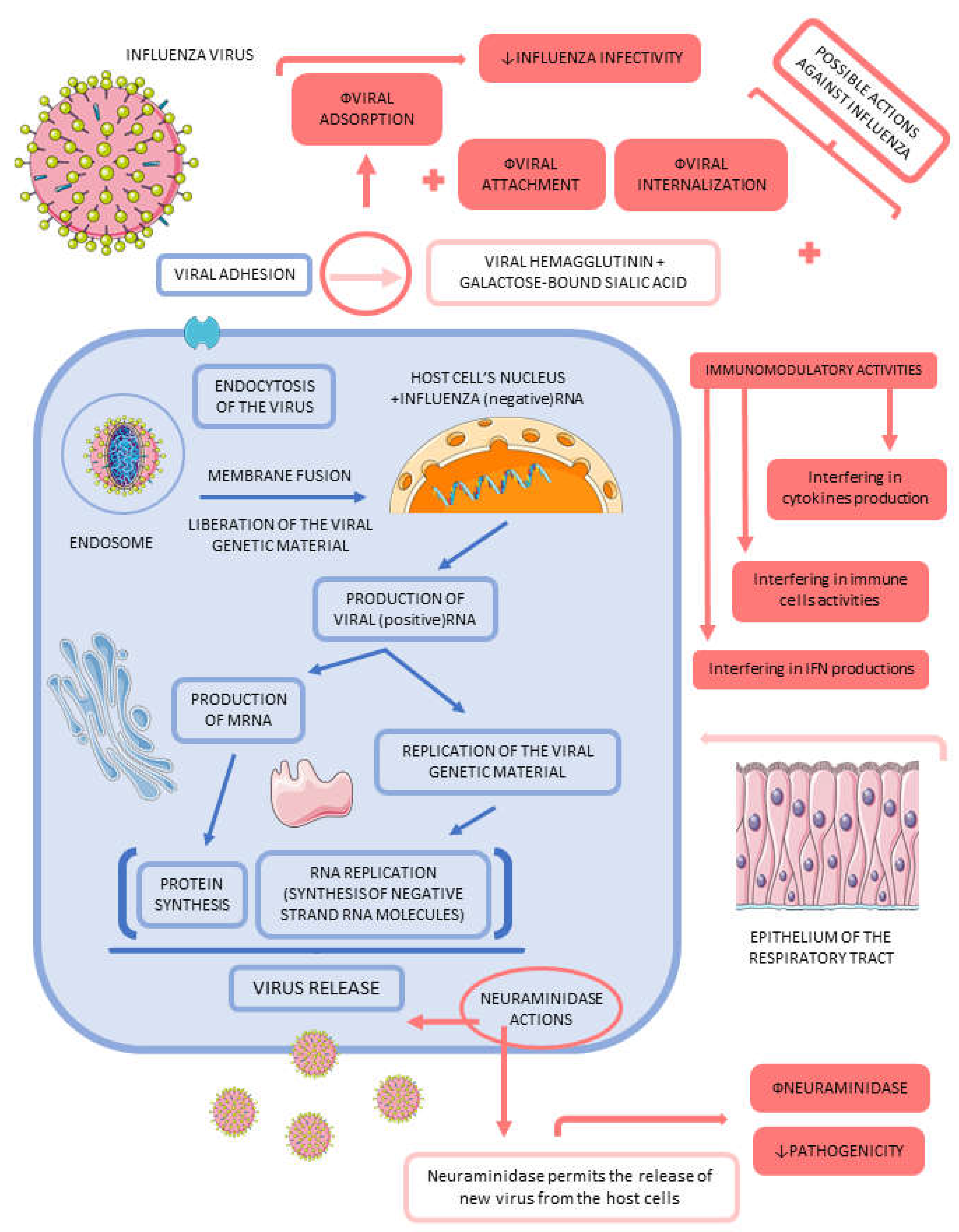
3.2. Influenza
3.3. The Role of Medicinal Plants on COVID-19
3.4. The Role of Medicinal Plants on Influenza
3.5. Medicinal Plants, COVID-19, and Influenza
3.6. Delivery Systems for Medicinal Plants and Their Derivatives against COVID-19 and Influenza
4. Materials and Methods
4.1. Focal Question
4.2. Language
4.3. Databases
4.4. Study Selection
4.5. Data Extraction
4.6. Quality Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flato, U.A.P.; Biteli, P.; Reina, D.O.B.R.; Reina, F.T.R.; Araújo, A.C.; de Souza, G.A.; dos Santos Campanari, G.; Matias, J.N.; Marinho, V.; Lima, T.L.Z.J.I.J.o.A.E.R.; et al. Myocarditis as a serious complication of COVID-19. 2021, 8, 3. [Google Scholar]
- Vallejos, J.; Zoni, R.; Bangher, M.; Villamandos, S.; Bobadilla, A.; Plano, F.; Campias, C.; Chaparro Campias, E.; Medina, M.F.; Achinelli, F.; et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC infectious diseases 2021, 21, 635. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.J.i. Metabolic syndrome and COVID-19. 2020, 8, 10. [Google Scholar]
- Deressa, W.; Worku, A.; Abebe, W.; Gizaw, M.; Amogne, W. Risk perceptions and preventive practices of COVID-19 among healthcare professionals in public hospitals in Addis Ababa, Ethiopia. PloS one 2021, 16, e0242471. [Google Scholar] [CrossRef] [PubMed]
- Durstenfeld, M.S.; Peluso, M.J.; Peyser, N.D.; Lin, F.; Knight, S.J.; Djibo, A.; Khatib, R.; Kitzman, H.; O'Brien, E.; Williams, N.; et al. Factors Associated With Long COVID Symptoms in an Online Cohort Study. Open forum infectious diseases 2023, 10, ofad047. [Google Scholar] [CrossRef] [PubMed]
- Rahier, J.F.; Papay, P.; Salleron, J.; Sebastian, S.; Ellul, P.; Teich, N.; Fiorino, G.; Blaha, B.; Garcia-Sanchez, V.; Haas, T.; et al. Influenza A (H1N1)v infection in patients with inflammatory bowel disease: a case series. Alimentary pharmacology & therapeutics 2011, 33, 499–500. [Google Scholar] [CrossRef]
- Aghaali, M.; Kavousi, A.; Shahsavani, A.; Hashemi Nazari, S.S. Performance of Bayesian Outbreak Detection Algorithm in the Syndromic Surveillance of Influenza-Like Illness in Small Region. Transbound Emerg Dis 2020. [Google Scholar] [CrossRef]
- Cantan, B.; Luyt, C.E.; Martin-Loeches, I. Influenza Infections and Emergent Viral Infections in Intensive Care Unit. Semin Respir Crit Care Med 2019, 40, 488–497. [Google Scholar] [CrossRef]
- Caldera, F.; Hillman, L.; Saha, S.; Wald, A.; Grimes, I.; Zhang, Y.; Sharpe, A.R.; Reichelderfer, M.; Hayney, M.S. Immunogenicity of High Dose Influenza Vaccine for Patients with Inflammatory Bowel Disease on Anti-TNF Monotherapy: A Randomized Clinical Trial. Inflammatory bowel diseases 2020, 26, 593–602. [Google Scholar] [CrossRef]
- Berg, S.H.; O'Hara, J.K.; Shortt, M.T.; Thune, H.; Brønnick, K.K.; Lungu, D.A.; Røislien, J.; Wiig, S. Health authorities' health risk communication with the public during pandemics: a rapid scoping review. BMC public health 2021, 21, 1401. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Blanton, L.H.; Fry, A.M.; Jernigan, D.B.; Atmar, R.L. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2020–2021 Influenza Season. MMWR Recomm Rep 2020, 69, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Duvvuri, V.R.; Hicks, J.T.; Damodaran, L.; Grunnill, M.; Braukmann, T.; Wu, J.; Gubbay, J.B.; Patel, S.N.; Bahl, J. Comparing the transmission potential from sequence and surveillance data of 2009 North American influenza pandemic waves. Infectious Disease Modelling 2023, 8, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, J.; Cheng, L.; Xu, K.; Yang, Y.; Su, X. Deficiency of HIF-1α enhances influenza A virus replication by promoting autophagy in alveolar type II epithelial cells. Emerg Microbes Infect 2020, 9, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Matias, J.N.; Flato, U.A.P.; Pilon, J.P.G.; Bitelli, P.; Junior, M.A.P.; de Carvalho, A.C.A.; dos Santos Haber, J.F.; Reis, C.H.B.; de Alvares Goulart, R.J.G.R. What do influenza and COVID-19 represent for patients with inflammatory bowel disease? 2021, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Jagadesh, A.; Salam, A.A.; Mudgal, P.P.; Arunkumar, G. Influenza virus neuraminidase (NA): a target for antivirals and vaccines. Arch Virol 2016, 161, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Mullender, C.; da Costa, K.A.S.; Alrubayyi, A.; Pett, S.L.; Peppa, D. SARS-CoV-2 immunity and vaccine strategies in people with HIV. Oxford open immunology 2022, 3, iqac005. [Google Scholar] [CrossRef] [PubMed]
- Haunhorst, S.; Bloch, W.; Wagner, H.; Ellert, C.; Krüger, K.; Vilser, D.C.; Finke, K.; Reuken, P.; Pletz, M.W.; Stallmach, A.; et al. Long COVID: a narrative review of the clinical aftermaths of COVID-19 with a focus on the putative pathophysiology and aspects of physical activity. Oxford open immunology 2022, 3, iqac006. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.R.; Gómez, V.; Kislaya, I.; Rodrigues, A.P.; Fernandes Tavares, M.; Pereira, A.C.; Pereira, D.; Côrte-Real, R.; Flores, C.H.; Verdasca, N.; et al. Monitoring COVID-19 and Influenza: The Added Value of a Severe Acute Respiratory Infection Surveillance System in Portugal. The Canadian journal of infectious diseases & medical microbiology = Journal canadien des maladies infectieuses et de la microbiologie medicale 2023, 2023, 6590011. [Google Scholar] [CrossRef]
- Bartoli, A.; Gabrielli, F.; Alicandro, T.; Nascimbeni, F.; Andreone, P.J.I.; medicine, e. COVID-19 treatment options: a difficult journey between failed attempts and experimental drugs. 2021, 1–28. [Google Scholar] [CrossRef]
- Caricchio, R.; Abbate, A.; Gordeev, I.; Meng, J.; Hsue, P.Y.; Neogi, T.; Arduino, R.; Fomina, D.; Bogdanov, R.; Stepanenko, T.; et al. Effect of Canakinumab vs Placebo on Survival Without Invasive Mechanical Ventilation in Patients Hospitalized With Severe COVID-19: A Randomized Clinical Trial. Jama 2021, 326, 230–239. [Google Scholar] [CrossRef]
- Bian, H.; Zheng, Z.H.; Wei, D.; Wen, A.; Zhang, Z.; Lian, J.Q.; Kang, W.Z.; Hao, C.Q.; Wang, J.; Xie, R.H.; et al. Safety and efficacy of meplazumab in healthy volunteers and COVID-19 patients: a randomized phase 1 and an exploratory phase 2 trial. Signal transduction and targeted therapy 2021, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Abiri, R.; Abdul-Hamid, H.; Sytar, O.; Abiri, R.; Bezerra de Almeida, E., Jr.; Sharma, S.K.; Bulgakov, V.P.; Arroo, R.R.J.; Malik, S. A Brief Overview of Potential Treatments for Viral Diseases Using Natural Plant Compounds: The Case of SARS-Cov. Molecules (Basel, Switzerland) 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, D.; Chen, X.; Liu, X.; Xiao, W.; Feng, L. The effect of nirmatrelvir-ritonavir on viral clearance and length of hospital stay in patients infected with SARS-CoV-2 omicron variants. Influenza and other respiratory viruses 2023, 17, e13095. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Shi, Y.; Su, J.; Friedemann, T.; Tao, Z.; Lu, Y.; Ling, Y.; Lv, Y.; Zhao, R.; Geng, Z.; et al. Shufeng Jiedu, a promising herbal therapy for moderate COVID-19:Antiviral and anti-inflammatory properties, pathways of bioactive compounds, and a clinical real-world pragmatic study. Phytomedicine : international journal of phytotherapy and phytopharmacology 2021, 85, 153390. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; He, G.; Huang, N.; Thilakavathy, K.; Lim, J.C.W.; Kumar, S.S.; Xiong, C. Glycyrrhizic Acid: A Natural Plant Ingredient as a Drug Candidate to Treat COVID-19. Frontiers in pharmacology 2021, 12, 707205. [Google Scholar] [CrossRef]
- Rolta, R.; Salaria, D.; Sharma, P.; Sharma, B.; Kumar, V.; Rathi, B.; Verma, M.; Sourirajan, A.; Baumler, D.J.; Dev, K. Phytocompounds of Rheum emodi, Thymus serpyllum, and Artemisia annua Inhibit Spike Protein of SARS-CoV-2 Binding to ACE2 Receptor: In Silico Approach. Current pharmacology reports 2021, 1–15. [Google Scholar] [CrossRef]
- Tegen, D.; Dessie, K.; Damtie, D. Candidate Anti-COVID-19 Medicinal Plants from Ethiopia: A Review of Plants Traditionally Used to Treat Viral Diseases. Evidence-based complementary and alternative medicine : eCAM 2021, 2021, 6622410. [Google Scholar] [CrossRef]
- Tao, Z.; Meng, X.; Han, Y.-q.; Xue, M.-m.; Wu, S.; Wu, P.; Yuan, Y.; Zhu, Q.; Zhang, T.-J.; Wong, C.C.J.J.o.p.r. Therapeutic mechanistic studies of ShuFengJieDu capsule in an acute lung injury animal model using quantitative proteomics technology. 2017, 16, 4009–4019. [Google Scholar] [CrossRef]
- Jiang, F.; Xu, N.; Zhou, Y.; Song, J.; Liu, J.; Zhu, H.; Jiang, J.; Xu, Y.; Li, R. Contribution of traditional Chinese medicine combined with conventional western medicine treatment for the novel coronavirus disease (COVID-19), current evidence with systematic review and meta-analysis. Phytotherapy research : PTR 2021. [Google Scholar] [CrossRef]
- Yu, C.; Huang, Y.; Ren, X.; Sun, L. Plant-derived Ren's oligopeptide has antiviral effects on influenza virus and SARS-CoV-2. Frontiers in veterinary science 2022, 9, 1090372. [Google Scholar] [CrossRef]
- Houeze, E.A.; Wang, Y.; Zhou, Q.; Zhang, H.; Wang, X. Comparison study of Beninese and Chinese herbal medicines in treating COVID-19. Journal of ethnopharmacology 2023, 308, 116172. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.S.; Abd-Elhakeem, M.M.; Abd El Galil, R.M.; Reyad, O.A.; Mohamed, H.A.; Ismail, S.E.S.; Nabil, M.A. Natural Immunomodulators Treat the Cytokine Storm in SARS-CoV-2. Advanced pharmaceutical bulletin 2023, 13, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Witek-Krowiak, A.; Skrzypczak, D.; Mikula, K.; Młynarz, P.J.J.o.F.F. Phytochemicals containing biologically active polyphenols as an effective agent against Covid-19-inducing coronavirus. 2020, 104146. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Chen, X.; Liu, Z. Natural Products and Nanotechnology Against Coronavirus Disease 2019. Front Chem 2022, 10, 819969. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Devi, G.; Nagpal, M.; Singh, M.; Dhingra, G.A.; Aggarwal, G. Antiviral Essential Oils Incorporated in Nanocarriers: Strategy for Prevention from COVID-19 and Future Infectious Pandemics. Pharm Nanotechnol 2020, 8, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Adel Mehraban, M.S.; Shirzad, M.; Mohammad Taghizadeh Kashani, L.; Ahmadian-Attari, M.M.; Safari, A.A.; Ansari, N.; Hatami, H.; Kamalinejad, M. Efficacy and safety of add-on Viola odorata L. in the treatment of COVID-19: A randomized double-blind controlled trial. J Ethnopharmacol 2023, 304, 116058. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Ahmadpoor, M.; Farahi, M.M.; Hadianfar, A.; Sahebkar, A.; Esmaily, H.; Nematy, M.; Rakhshandeh, H. The Effect of Pomegranate Juice and Sumac Consumption in the Treatment of Outpatients with COVID-19. Mediators Inflamm 2022, 2022, 6850342. [Google Scholar] [CrossRef] [PubMed]
- Hasanpour, M.; Safari, H.; Mohammadpour, A.H.; Iranshahy, M.; Dehghan Nayyeri, M.J.; Farhadi, F.; Emami, B.; Iranshahi, M. Efficacy of Covexir® (Ferula foetida oleo-gum) treatment in symptomatic improvement of patients with mild to moderate COVID-19: A randomized, double-blind, placebo-controlled trial. Phytother Res 2022, 36, 4504–4515. [Google Scholar] [CrossRef]
- Borujerdi, R.; Adeli, S.H.; Mohammadbeigi, A.; Aliasl, F.; Asghari, A.; Hormati, A.; Dehnavi, H.M.; Hoseini, F.; Asghari, M. Effects of Iranian Polyherbal Syrup (Zufa syrup) on oxygen saturation and clinical symptoms in suspected patients with COVID-19: a triple-blinded, randomized, placebo-controlled trial. Med Gas Res 2022, 12, 44–50. [Google Scholar] [CrossRef]
- Karimi, M.; Zarei, A.; Soleymani, S.; Jamalimoghadamsiahkali, S.; Asadi, A.; Shati, M.; Jafari, M.; Rezadoost, H.; Kordafshar, G.; Naghizadeh, A.; et al. Efficacy of Persian medicine herbal formulations (capsules and decoction) compared to standard care in patients with COVID-19, a multicenter open-labeled, randomized, controlled clinical trial. Phytother Res 2021, 35, 6295–6309. [Google Scholar] [CrossRef]
- Devpura, G.; Tomar, B.S.; Nathiya, D.; Sharma, A.; Bhandari, D.; Haldar, S.; Balkrishna, A.; Varshney, A. Randomized placebo-controlled pilot clinical trial on the efficacy of ayurvedic treatment regime on COVID-19 positive patients. Phytomedicine : international journal of phytotherapy and phytopharmacology 2021, 84, 153494. [Google Scholar] [CrossRef] [PubMed]
- Margolin, L.; Luchins, J.; Margolin, D.; Margolin, M.; Lefkowitz, S. 20-Week Study of Clinical Outcomes of Over-the-Counter COVID-19 Prophylaxis and Treatment. Journal of evidence-based integrative medicine 2021, 26, 2515690X211026193. [Google Scholar] [CrossRef] [PubMed]
- Vidal, K.; Bucheli, P.; Gao, Q.; Moulin, J.; Shen, L.S.; Wang, J.; Blum, S.; Benyacoub, J. Immunomodulatory effects of dietary supplementation with a milk-based wolfberry formulation in healthy elderly: a randomized, double-blind, placebo-controlled trial. Rejuvenation research 2012, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Nantz, M.P.; Rowe, C.A.; Muller, C.E.; Creasy, R.A.; Stanilka, J.M.; Percival, S.S. Supplementation with aged garlic extract improves both NK and γδ-T cell function and reduces the severity of cold and flu symptoms: a randomized, double-blind, placebo-controlled nutrition intervention. Clinical nutrition (Edinburgh, Scotland) 2012, 31, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, S.; Kashiwagi, K.; Ajisaka, K.; Masui, S.; Takeoka, H.; Ikematsu, H.; Kashiwagi, S. A randomized, controlled trial comparing traditional herbal medicine and neuraminidase inhibitors in the treatment of seasonal influenza. Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy 2012, 18, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Lee, J.H.; Oh, M.; Choi, K.M.; Jeong, M.R.; Park, J.D.; Kwon, D.Y.; Ha, K.C.; Park, E.O.; Lee, N.; et al. Preventive effect of Korean red ginseng for acute respiratory illness: a randomized and double-blind clinical trial. Journal of Korean medical science 2012, 27, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Meyer, M.; Bauer, R.N.; Zhou, H.; Zhang, H.; Jones, S.; Robinette, C.; Noah, T.L.; Jaspers, I. Effect of Broccoli Sprouts and Live Attenuated Influenza Virus on Peripheral Blood Natural Killer Cells: A Randomized, Double-Blind Study. PloS one 2016, 11, e0147742. [Google Scholar] [CrossRef] [PubMed]
- Noah, T.L.; Zhang, H.; Zhou, H.; Glista-Baker, E.; Müller, L.; Bauer, R.N.; Meyer, M.; Murphy, P.C.; Jones, S.; Letang, B.; et al. Effect of broccoli sprouts on nasal response to live attenuated influenza virus in smokers: a randomized, double-blind study. PloS one 2014, 9, e98671. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, H.; Ma, L.; Mu, X.; Wang, Y.; Lu, Y.; Yu, P.; Dai, H. Effect of traditional Chinese medicine Bupleurum in the treatment of influenza A (H1N1). Pakistan journal of pharmaceutical sciences 2018, 31, 1713–1717. [Google Scholar]
- Macknin, M.; Wolski, K.; Negrey, J.; Mace, S. Elderberry Extract Outpatient Influenza Treatment for Emergency Room Patients Ages 5 and Above: a Randomized, Double-Blind, Placebo-Controlled Trial. Journal of general internal medicine 2020, 35, 3271–3277. [Google Scholar] [CrossRef]
- Vanderbeke, L.; Janssen, N.A.F.; Bergmans, D.; Bourgeois, M.; Buil, J.B.; Debaveye, Y.; Depuydt, P.; Feys, S.; Hermans, G.; Hoiting, O.; et al. Posaconazole for prevention of invasive pulmonary aspergillosis in critically ill influenza patients (POSA-FLU): a randomised, open-label, proof-of-concept trial. Intensive care medicine 2021, 47, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Goswami, S.; Paneerselvam, A.; Kabilan, K.; Chowdhury, H.; Roy, A.; Guleria, R.; Soni, K.D.; Baruah, U.; Das, C.J. Imaging of Coronavirus Disease 2019 Infection From Head to Toe: A Primer for the Radiologist. Curr Probl Diagn Radiol 2021. [Google Scholar] [CrossRef]
- Singh, S.P.; Pritam, M.; Pandey, B.; Yadav, T.P. Microstructure, pathophysiology, and potential therapeutics of COVID-19: A comprehensive review. J Med Virol 2021, 93, 275–299. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. Jama 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, X.; Sun, J.; Xie, T.; Lei, Y.; Muhammad, J.; Li, X.; Zeng, X.; Zhou, F.; Qin, H.; et al. Clinical Characteristics and Immune Injury Mechanisms in 71 Patients with COVID-19. mSphere 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Roche, L.; Mesta, F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch Med Res 2020, 51, 384–387. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N Engl J Med 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Fara, A.; Mitrev, Z.; Rosalia, R.A.; Assas, B.M. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol 2020, 10, 200160. [Google Scholar] [CrossRef]
- Gustine, J.N.; Jones, D. Immunopathology of Hyperinflammation in COVID-19. Am J Pathol 2021, 191, 4–17. [Google Scholar] [CrossRef]
- Singh, S.; Pritam, M.; Pandey, B.; Yadav, T. Microstructure, pathophysiology and potential therapeutics of COVID-19: A comprehensive review. Journal of Medical Virology 2020, 93. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Leven, Y.; Bösel, J. Neurological manifestations of COVID-19 - an approach to categories of pathology. Neurol Res Pract 2021, 3, 39–39. [Google Scholar] [CrossRef] [PubMed]
- Gaitonde, D.Y.; Moore, F.C.; Morgan, M.K. Influenza: Diagnosis and Treatment. Am Fam Physician 2019, 100, 751–758. [Google Scholar] [PubMed]
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: pathophysiology and epidemiology. Crit Care 2019, 23, 258. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Ryu, Y. Transmissibility and severity of influenza virus by subtype. Infect Genet Evol 2018, 65, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Govorkova, E.A. Continuing challenges in influenza. Ann N Y Acad Sci 2014, 1323, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: pathophysiology and epidemiology. Critical care (London, England) 2019, 23, 258–258. [Google Scholar] [CrossRef] [PubMed]
- Labella, A.M.; Merel, S.E. Influenza. Med Clin North Am 2013, 97, 621–645. [Google Scholar] [CrossRef]
- Shim, J.M.; Kim, J.; Tenson, T.; Min, J.-Y.; Kainov, D.E. Influenza Virus Infection, Interferon Response, Viral Counter-Response, and Apoptosis. Viruses 2017, 9, 223. [Google Scholar] [CrossRef]
- Adhikari, B.; Marasini, B.P.; Rayamajhee, B.; Bhattarai, B.R.; Lamichhane, G.; Khadayat, K.; Adhikari, A.; Khanal, S.; Parajuli, N. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: A review. Phytother Res 2021, 35, 1298–1312. [Google Scholar] [CrossRef]
- Asif, M.; Saleem, M.; Saadullah, M.; Yaseen, H.S.; Al Zarzour, R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology 2020, 28, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug Deliv Transl Res 2020, 10, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Benarba, B.; Pandiella, A. Medicinal Plants as Sources of Active Molecules Against COVID-19. Front Pharmacol 2020, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, F.R.; Howlader, S.; Raihan, T.; Hasan, M. Plants Metabolites: Possibility of Natural Therapeutics Against the COVID-19 Pandemic. Front Med (Lausanne) 2020, 7, 444–444. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Anderson, D.; Najafzadeh, M. The Antiviral, Anti-Inflammatory Effects of Natural Medicinal Herbs and Mushrooms and SARS-CoV-2 Infection. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Jalali, A.; Dabaghian, F.; Akbrialiabad, H.; Foroughinia, F.; Zarshenas, M.M. A pharmacology-based comprehensive review on medicinal plants and phytoactive constituents possibly effective in the management of COVID-19. Phytother Res 2021, 35, 1925–1938. [Google Scholar] [CrossRef]
- Lim, X.Y.; Teh, B.P.; Tan, T.Y.C. Medicinal Plants in COVID-19: Potential and Limitations. Frontiers in Pharmacology 2021, 12. [Google Scholar] [CrossRef]
- Benarba, B.; Pandiella, A. Medicinal Plants as Sources of Active Molecules Against COVID-19. Frontiers in pharmacology 2020, 11, 1189–1189. [Google Scholar] [CrossRef]
- Balachandar, V.; Mahalaxmi, I.; Kaavya, J.; Vivekanandhan, G.; Ajithkumar, S.; Arul, N.; Singaravelu, G.; Senthil Kumar, N.; Mohana Dev, S. COVID-19: emerging protective measures. Eur Rev Med Pharmacol Sci 2020, 24, 3422–3425. [Google Scholar] [CrossRef]
- Khan, S.A.; Al-Balushi, K. Combating COVID-19: The role of drug repurposing and medicinal plants. J Infect Public Health 2021, 14, 495–503. [Google Scholar] [CrossRef]
- Wong, L.Y.; Lui, P.Y.; Jin, D.Y. A molecular arms race between host innate antiviral response and emerging human coronaviruses. Virol Sin 2016, 31, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Cao, Z.; Xie, X.; Zhang, X.; Chen, J.Y.; Wang, H.; Menachery, V.D.; Rajsbaum, R.; Shi, P.Y. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep 2020, 33, 108234. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Malone, B.; Llewellyn, E.; Grasso, M.; Shelton, P.M.M.; Olinares, P.D.B.; Maruthi, K.; Eng, E.; Vatandaslar, H.; Chait, B.T.; et al. Structural basis for helicase-polymerase coupling in the SARS-CoV-2 replication-transcription complex. bioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Nabavi, S.F.; Banach, M.; Berindan-Neagoe, I.; Sarkar, K.; Sil, P.C.; Nabavi, S.M. Should We Try SARS-CoV-2 Helicase Inhibitors for COVID-19 Therapy? Arch Med Res 2020, 51, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Khare, P.; Sahu, U.; Pandey, S.C.; Samant, M. Current approaches for target-specific drug discovery using natural compounds against SARS-CoV-2 infection. Virus Res 2020, 290, 198169. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Ruggiero, A.; Squeglia, F.; Maga, G.; Berisio, R. A Structural View of SARS-CoV-2 RNA Replication Machinery: RNA Synthesis, Proofreading and Final Capping. Cells 2020, 9. [Google Scholar] [CrossRef]
- Vandelli, A.; Monti, M.; Milanetti, E.; Armaos, A.; Rupert, J.; Zacco, E.; Bechara, E.; Delli Ponti, R.; Tartaglia, G.G. Structural analysis of SARS-CoV-2 genome and predictions of the human interactome. Nucleic Acids Res 2020, 48, 11270–11283. [Google Scholar] [CrossRef]
- Khare, P.; Sahu, U.; Pandey, S.C.; Samant, M. Current approaches for target-specific drug discovery using natural compounds against SARS-CoV-2 infection. Virus Research 2020, 290, 198169. [Google Scholar] [CrossRef]
- Alhazmi, H.A.; Najmi, A.; Javed, S.A.; Sultana, S.; Al Bratty, M.; Makeen, H.A.; Meraya, A.M.; Ahsan, W.; Mohan, S.; Taha, M.M.E.; et al. Medicinal Plants and Isolated Molecules Demonstrating Immunomodulation Activity as Potential Alternative Therapies for Viral Diseases Including COVID-19. Front Immunol 2021, 12. [Google Scholar] [CrossRef]
- Alhazmi, H.A.; Najmi, A.; Javed, S.A.; Sultana, S.; Al Bratty, M.; Makeen, H.A.; Meraya, A.M.; Ahsan, W.; Mohan, S.; Taha, M.M.E.; et al. Medicinal Plants and Isolated Molecules Demonstrating Immunomodulation Activity as Potential Alternative Therapies for Viral Diseases Including COVID-19. Front Immunol 2021, 12, 637553–637553. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Khan, M.A.; Mashwani, Z.U.; Ullah, N.; Nadhman, A. Therapeutic potential of medicinal plants against COVID-19: The role of antiviral medicinal metabolites. Biocatal Agric Biotechnol 2021, 31, 101890. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, V.K.; Singh, R.; Sharma, J.; Rajendran, V.; Purohit, R.; Kumar, S. Identification of bioactive molecules from tea plant as SARS-CoV-2 main protease inhibitors. J Biomol Struct Dyn 2021, 39, 3449–3458. [Google Scholar] [CrossRef] [PubMed]
- Solnier, J.; Fladerer, J.-P. Flavonoids: A complementary approach to conventional therapy of COVID-19? Phytochem Rev 2020, 1–23. [Google Scholar] [CrossRef]
- Hafez Ghoran, S.; El-Shazly, M.; Sekeroglu, N.; Kijjoa, A. Natural Products from Medicinal Plants with Anti-Human Coronavirus Activities. Molecules 2021, 26, 1754. [Google Scholar] [CrossRef] [PubMed]
- Sargin, S.A. Potential anti-influenza effective plants used in Turkish folk medicine: A review. J Ethnopharmacol 2021, 265, 113319. [Google Scholar] [CrossRef]
- Brochot, A.; Guilbot, A.; Haddioui, L.; Roques, C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. MicrobiologyOpen 2017, 6, e00459. [Google Scholar] [CrossRef]
- Hamauzu, Y.; Yasui, H.; Inno, T.; Kume, C.; Omanyuda, M. Phenolic Profile, Antioxidant Property, and Anti-influenza Viral Activity of Chinese Quince (Pseudocydonia sinensis Schneid.), Quince (Cydonia oblonga Mill.), and Apple (Malus domestica Mill.) Fruits. Journal of Agricultural and Food Chemistry 2005, 53, 928–934. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, Z.; Cao, L.; Ding, G.; Wang, Z.; Xiao, W. Antiviral activity of chlorogenic acid against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase. Scientific Reports 2017, 7, 45723. [Google Scholar] [CrossRef]
- Lee, I.-K.; Hwang, B.S.; Kim, D.-W.; Kim, J.-Y.; Woo, E.E.; Lee, Y.-J.; Choi, H.J.; Yun, B.-S. Characterization of Neuraminidase Inhibitors in Korean Papaver rhoeas Bee Pollen Contributing to Anti-Influenza Activities In Vitro. Planta Med 2016, 82, 524–529. [Google Scholar] [CrossRef]
- Chandan, S.; Mohan, B.P.; Chandan, O.C.; Ahmad, R.; Challa, A.; Tummala, H.; Singh, S.; Dhawan, P.; Ponnada, S.; Singh, A.B.; et al. Curcumin use in ulcerative colitis: is it ready for prime time? A systematic review and meta-analysis of clinical trials. Ann Gastroenterol 2020, 33, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Fiore, C.; Eisenhut, M.; Krausse, R.; Ragazzi, E.; Pellati, D.; Armanini, D.; Bielenberg, J. Antiviral effects of Glycyrrhiza species. Phytother Res 2008, 22, 141–148. [Google Scholar] [CrossRef]
- Hu, Z.; Lin, J.; Chen, J.; Cai, T.; Xia, L.; Liu, Y.; Song, X.; He, Z. Overview of Viral Pneumonia Associated With Influenza Virus, Respiratory Syncytial Virus, and Coronavirus, and Therapeutics Based on Natural Products of Medicinal Plants. Frontiers in Pharmacology 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Du, R.; Chen, Z.; Wang, Y.; Zhan, P.; Liu, X.; Kang, D.; Chen, Z.; Zhao, X.; Wang, L.; et al. Punicalagin is a neuraminidase inhibitor of influenza viruses. J Med Virol 2021, 93, 3465–3472. [Google Scholar] [CrossRef]
- Wang, X.; Jia, W.; Zhao, A.; Wang, X. Anti-influenza agents from plants and traditional Chinese medicine. Phytother Res 2006, 20, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Tahir, I.M.; Shah, S.M.A.; Mahmood, Z.; Altaf, A.; Ahmad, K.; Munir, N.; Daniyal, M.; Nasir, S.; Mehboob, H. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: A systematic review. Phytother Res 2018, 32, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Sodagari, H.R.; Farzaei, M.H.; Abdolghaffari, A.H.; Gooshe, M.; Rezaei, N. The preventive and therapeutic potential of natural polyphenols on influenza. Expert Rev Anti Infect Ther 2016, 14, 57–80. [Google Scholar] [CrossRef]
- Mehrbod, P.; Abdalla, M.A.; Njoya, E.M.; Ahmed, A.S.; Fotouhi, F.; Farahmand, B.; Gado, D.A.; Tabatabaian, M.; Fasanmi, O.G.; Eloff, J.N.; et al. South African medicinal plant extracts active against influenza A virus. BMC complementary and alternative medicine 2018, 18, 112–112. [Google Scholar] [CrossRef]
- Zhang, Z.; Morris-Natschke, S.; Yung-Yi, C.; Lee, K.-H.; Li, R.T. Development of anti-influenza agents from natural products. Medicinal Research Reviews 2020, 40. [Google Scholar] [CrossRef]
- Kültür, S. Medicinal plants used in Kirklareli Province (Turkey). J Ethnopharmacol 2007, 111, 341–364. [Google Scholar] [CrossRef]
- Maria John, K.M.; Enkhtaivan, G.; Ayyanar, M.; Jin, K.; Yeon, J.B.; Kim, D.H. Screening of ethnic medicinal plants of South India against influenza (H1N1) and their antioxidant activity. Saudi J Biol Sci 2015, 22, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Elhassan Taha, M.M.; Makeen, H.A.; Alhazmi, H.A.; Al Bratty, M.; Sultana, S.; Ahsan, W.; Najmi, A.; Khalid, A. Bioactive Natural Antivirals: An Updated Review of the Available Plants and Isolated Molecules. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Siew, Y.Y.; Zareisedehizadeh, S.; Seetoh, W.G.; Neo, S.Y.; Tan, C.H.; Koh, H.L. Ethnobotanical survey of usage of fresh medicinal plants in Singapore. J Ethnopharmacol 2014, 155, 1450–1466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-J.; Morris-Natschke, S.L.; Cheng, Y.-Y.; Lee, K.-H.; Li, R.-T. Development of anti-influenza agents from natural products. Medicinal Research Reviews 2020, 40, 2290–2338. [Google Scholar] [CrossRef]
- Chen, F.; Yang, L.; Huang, Y.; Chen, Y.; Sang, H.; Duan, W.; Yang, J. Isocorilagin, isolated from Canarium album (Lour.) Raeusch, as a potent neuraminidase inhibitor against influenza A virus. Biochem Biophys Res Commun 2020, 523, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Wolkerstorfer, A.; Kurz, H.; Bachhofner, N.; Szolar, O.H. Glycyrrhizin inhibits influenza A virus uptake into the cell. Antiviral Res 2009, 83, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.T.; Hung, C.Y.; Hseih, Y.C.; Chang, C.S.; Velu, A.B.; He, Y.C.; Huang, Y.L.; Chen, T.A.; Chen, T.C.; Lin, C.Y.; et al. Effect of aloin on viral neuraminidase and hemagglutinin-specific T cell immunity in acute influenza. Phytomedicine 2019, 64, 152904. [Google Scholar] [CrossRef]
- Yu, S.; Zhu, Y.; Xu, J.; Yao, G.; Zhang, P.; Wang, M.; Zhao, Y.; Lin, G.; Chen, H.; Chen, L.; et al. Glycyrrhizic acid exerts inhibitory activity against the spike protein of SARS-CoV-2. Phytomedicine 2021, 85, 153364. [Google Scholar] [CrossRef]
- Park, J.-Y.; Jeong, H.J.; Kim, J.H.; Kim, Y.M.; Park, S.-J.; Kim, D.; Park, K.H.; Lee, W.S.; Ryu, Y.B. Diarylheptanoids from <i>Alnus japonica</i> Inhibit Papain-Like Protease of Severe Acute Respiratory Syndrome Coronavirus. Biological and Pharmaceutical Bulletin 2012, 35, 2036–2042. [Google Scholar] [CrossRef]
- Laurindo, L.F.; de Carvalho, G.M.; de Oliveira Zanuso, B.; Figueira, M.E.; Direito, R.; de Alvares Goulart, R.; Buglio, D.S.; Barbalho, S.M. Curcumin-Based Nanomedicines in the Treatment of Inflammatory and Immunomodulated Diseases: An Evidence-Based Comprehensive Review. Pharmaceutics 2023, 15. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Malaiya, A.; Mishra, G.; Jain, D.; Kesharwani, P.; Mody, N.; Ahmadi, A.; Paliwal, R.; Jain, A. An exhaustive comprehension of the role of herbal medicines in Pre- and Post-COVID manifestations. J Ethnopharmacol 2022, 296, 115420. [Google Scholar] [CrossRef]
- Derakhshan, M.A.; Amani, A.; Faridi-Majidi, R. State-of-the-Art of Nanodiagnostics and Nanotherapeutics against SARS-CoV-2. ACS Appl Mater Interfaces 2021, 13, 14816–14843. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Prateeksha; Singh, S.P.; Singh, B.N.; Rao, C.V.; Barik, S.K. Nanocurcumin Potently Inhibits SARS-CoV-2 Spike Protein-Induced Cytokine Storm by Deactivation of MAPK/NF-κB Signaling in Epithelial Cells. ACS Appl Bio Mater 2022, 5, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Azimi, M.; Haddadi-Asl, V.; Ahmadi, H.; Gholamzad, M.; Ghorbanpour, S.; Bahador, A. Robust antimicrobial photodynamic therapy with curcumin-poly (lactic-co-glycolic acid) nanoparticles against COVID-19: A preliminary in vitro study in Vero cell line as a model. Photodiagnosis Photodyn Ther 2021, 34, 102286. [Google Scholar] [CrossRef] [PubMed]
- AbouAitah, K.; Allayh, A.K.; Wojnarowicz, J.; Shaker, Y.M.; Swiderska-Sroda, A.; Lojkowski, W. Nanoformulation Composed of Ellagic Acid and Functionalized Zinc Oxide Nanoparticles Inactivates DNA and RNA Viruses. Pharmaceutics 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Pilaquinga, F.; Bosch, R.; Morey, J.; Bastidas-Caldes, C.; Torres, M.; Toscano, F.; Debut, A.; Pazmiño-Viteri, K.; Nieves Piña, M.L. Highin vitroactivity of gold and silver nanoparticles from Solanum mammosum L. against SARS-CoV-2 surrogate Phi6 and viral model PhiX174. Nanotechnology 2023, 34. [Google Scholar] [CrossRef]
- Loutfy, S.A.; Abdel-Salam, A.I.; Moatasim, Y.; Gomaa, M.R.; Abdel Fattah, N.F.; Emam, M.H.; Ali, F.; ElShehaby, H.A.; Ragab, E.A.; Alam El-Din, H.M.; et al. Antiviral activity of chitosan nanoparticles encapsulating silymarin (Sil-CNPs) against SARS-CoV-2 (in silico and in vitro study). RSC Adv 2022, 12, 15775–15786. [Google Scholar] [CrossRef]
- Saadh, M.J.; Aldalaen, S.M. Inhibitory effects of epigallocatechin gallate (EGCG) combined with zinc sulfate and silver nanoparticles on avian influenza A virus subtype H5N1. Eur Rev Med Pharmacol Sci 2021, 25, 2630–2636. [Google Scholar] [CrossRef]
- Saadh, M.J.; Aggag, M.M.; Alboghdadly, A.; Kharshid, A.M.; Aldalaen, S.M.; Abdelrazek, M.A. Silver nanoparticles with epigallocatechingallate and zinc sulphate significantly inhibits avian influenza A virus H9N2. Microb Pathog 2021, 158, 105071. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane handbook for systematic reviews of interventions; John Wiley & Sons, 2019. [Google Scholar]
| Reference | Local | Model and Patients | Intervention | Outcomes | Adverse effects |
|---|---|---|---|---|---|
| COVID-19 | |||||
| [36] | Iran | Placebo-controlled randomized, double-blind clinical trial with 108 COVID-19 outpatients, 55 male; 53 female; ≥30y. | 10mL of violet syrup (Viola odorata L. aqueous extract) thrice daily for 7 days. | Patients who received violet syrup ameliorated faster and had lower mean severity scores regarding cough, myalgia, headache, and diarrhea. It shows that violet syrup effectively controlled prevalent COVID-19 manifestations. | No serious adverse events were reported during the trial. |
| [37] | Iran | Placebo-controlled randomized, single-blind clinical trial with 178 COVID-19 outpatients, 120 male; 58 female; ≤60y. | 200mL of pomegranate juice thrice daily and 1.5g of SUMAC (composed of tannins, flavonoids, anthocyanins, isoflavones, terpenoids, and diterpenes) twice daily. Time of intervention was not elucidated. | Patents who received pomegranate and SUMAC interventions had significant decreases in fever, weakness, cough, chills, smell and taste disorders, diarrhea, shortness of breath, abdominal pain, and vomiting compared with those patients who did not undergo this treatment. | Not reported. |
| [38] | Iran | Placebo-controlled randomized, double-blind clinical trial with 50 mild to moderate COVID-19 patients, 34 male; 16 female; ≤80y. | Covexir (Ferula foetida oleo-gum) twice daily for 7 days. | Covexir inhibited cough and diminished the severity of anorexia, sense of taste, anosmia, and myalgia between the intervention and placebo groups. | No adverse events were reported during the trial. |
| [39] | Iran | Placebo-controlled randomized triple-blind clinical trial with 116 mild to moderate COVID-19, 57 men; 59 females; 20-70y. | 7.5mL of Zufa syrup (Nepeta bracteata, Ziziphus jujube, Glycyrrhizaglabra, Ficuscarica, Cordia myxa, Papaver somniferum, Fennel, Adiantumcapillus veneris, Viola, Viper’s-buglosses, Lavender, and Iris) every 4 hours for 10 days. | There were no significant differences between placebo and intervention groups in cough, dyspnea, anxiety, anorexia, insomnia, myalgia, and oxygen saturation decline occurrence. | No serious adverse events were reported during the trial. |
| [40] | Iran | Multicenter open-labeled, randomized, controlled clinical trial with 358 mild to moderate COVID-19 patients, 197 male; 161 female; ≤75y. | Patients received a polyherbal decoction (1 sachet of the following per day respecting the order Matricaria chamomilla L., Zataria multiflora Boiss., Glycyrrhiza glabra L., Ziziphus jujuba Mill., Ficus carica L., Urtica dioica L., Althaea officinalis L., and Nepeta bracteata Benth) every 8 hours and two herbal capsules (Rheum palmatum L. rizhome, Glycyrrhiza glabra root, Punica granatum L. fruit peel, and Rheum palmatum for capsule 1 and Nigella sativa L. for capsule 2) every 12 hours for 7 days. | Patients who received the intervention had significantly lower dyspnea periods, as well as accelerated clinical improvement of dry cough, headache, muscle pain, vertigo chills, fatigue, anorexia, sputum cough, and runny nose. | Gastrointestinal adverse effects like nausea and diarrhea were observed. |
| [41] | India | Placebo-controlled randomized, double-blind pilot clinical trial with 95 patients who had no or mild symptoms of covid-19 and were positive on RT-PCR, 77 males; 18 females; 15- 80 y. | 1g of Giloy, 2 g of Swasari Ras, 0.5 g each of Ashwagandha, and Tulsi Ghanvati were given orally to the patients in the treatment group twice per day for 7 days. | Ayurvedic treatment can expedite virological clearance, help rapid recovery, and reduce the risk of viral dissemination and inflammation markers (suggesting less severity of SARS-CoV-2 infection in the treated group). | There were no side effects. |
| [42] | USA | Controlled Clinical Trial with 114 multiply exposed adults, 60 female, 40 males; ≥30y. | Patients in the treatment group received a daily dose of OTC for 20 weeks, while the control group did not receive any placebo as they refused the study regimen. | Just under 4% of the compliant test group presented flu-like symptoms, but none of the test group was COVID-positive; whereas 20% of the non-compliant control group presented flu-like symptoms, three-quarters of whom (15%) overall the control group) were COVID-positive. | Not reported. |
| Influenza | |||||
| [43] | China | Randomized, a double-blinded, placebo-controlled study with 150 healthy community-dwelling Chinese elderly, 75 male and 75 female; 65–70 y. |
The treated group received a single-dose sachet containing 13.7 g/day Lacto-Wolfberry (wolfberry fruit (530 mg/gram), bovine skimmed milk (290 mg/gram), and maltodextrin, 180 mg/g). The placebo group received the same sachet but with a skimmed bovine formulation of milk, maltodextrin, sucrose, and colorants / 92 d. | The treated subjects showed significative higher immunoglobulin G levels in postvaccination and seroconversion (between days 30 and 90, compared with the placebo group). | No serious adverse events were reported during the trial. |
| [44] | USA | Randomized, double-blind, placebo-controlled with 120 healthy men (55) and women (65), 21-50 y with BMI 18 - 30 kg/m2. | Each patient consumed four capsules/day of either AGE (2.56 g per day) or a placebo for 90 days. | The use of aged garlic extract enhanced immune cell function (possibly responsible for the reduction of cold and flu severity. | Not reported. |
| [45] | Japan | Open-labeled, randomized controlled trial with 33 patients, 14 male; 14 female; 20–64y, presented within 48 h of onset of flu symptoms, including fever, and were positive by quick diagnostic test kit for influenza virus antigens from nasal swabs. | Patients were randomized into 3 groups to receive Maoto orally at 2.5 g TID, or Oseltamivir orally 75 mg BID, or Zanamivir by inhalation of 20 mg BID for 5 days. | The administration of oral maoto granules to healthy adults with seasonal influenza was well tolerated and associated with equivalent clinical and virological efficacy to neuraminidase inhibitors. | One patient in the maoto group and one in the oseltamivir group showed a mildly elevated serum aminotransferase level after treatment. |
| [46] | Korea | Randomized, double-blinded, placebo-controlled trial in 100 healthy volunteers, 38 male; 62 female, 30 - 70 y. | The treatment group received concentrated red ginseng 1.0 g 3 times a day (3.0g/day)/ 12 w. Placebo was similar in taste and appearance but with no principal ingredients. | KRG is effective in protecting subjects against ARI, and may decrease the duration and scores of ARI symptoms. | There were no specific clinical and laboratory side effects |
| [48] | USA | A randomized, double-blind, placebo-controlled trial with 69 healthy young adult smokers and nonsmokers, 26 female; 25 male; 18–40 y. | Smokers and nonsmokers ingested one daily dose 200g of BSH or placebo (ASH) for 4 days. On Day 0 they received standard vaccine dose of LAIV intranasally. | In smokers, short-term intake of BSH appears to significantly reduce some markers of inflammation, such as IL-6, and reduce the amount of the influenza virus. | No patients reported intolerable taste or side effects. |
| [47] | USA | Randomized, double-blinded, placebo-controlled study with 42 healthy volunteers, 19 female; 10 male; 25- 28y. | Subjects received BSH or placebo (ASH) for 4 consecutive days. A daily portion of BSH shake was about 200g. On day 0 they received a standard vaccine dose of LAIV intranasally. |
BSH increases virus-induced peripheral blood NK cell granzyme B production, an effect that may be important for enhanced antiviral defense responses. | No subject reported intolerable taste or side effects. |
| [49] | China | Randomized clinical trial with 120 subjects who have mild Influenza A (H1N1). Including 62 males and 58 females, 14-65 y. | The treated group received chima qingwen decoction 2 times a day for 5 days (children received half the dose). The antiviral group took oral oseltamivir every 75 mg (50 mg children), 2 times a day, one course of 5 days. | The overall effective rate was 93.3%. A combination therapy (Chinese and Western medicine) is effective for mild cases of influenza A (H1N1). | No adverse events occurred. |
| [50] | USA | Randomized, Double-Blind, Placebo-Controlled Trial with Eighty-seven patients, ≥4, with less than 48 hours of at least two moderate-severity symptoms of influenza and positive polymerase chain reaction influenza test, 49 males; 38 females. | Participants from age 5 to 12 y received placebo or 15 mL (5.7 g) elderberry extract orally 2xd for 5 d; those > 12 years received 15 mL 4xd for 5 d. Patients were permitted to choose to also receive the standard dosage of oseltamivir. | No evidence was found that the elderberry benefits the duration or severity of the flu. | Dry mouth, constipation, rash, and bad taste. There were no significant differences between the elderberry and placebo. |
| [51] | Belgium, The Netherlands, and France | Randomized, open-label, proof-of-concept trial with 88 critically ill influenza patients, 41 male; 32 female, ≥18y. | Participants submitted to the prophylaxis arm received the first dose of POS prophylaxis within 48 h of admission to the ICU, starting with a loading dose of 300 mg 2xd on day 1, followed by a 1xd of 300mg from day 2 onwards for 7 days. The other group received the standard of care only. | The higher-than-expected incidence of early IAPA precluded any definitive conclusions about POS prophylaxis. After 48 hours, still, 11% of patients developed IAPA. | Not reported. |
| Study | Question focus | Appropriate randomization | Allocation blinding | Double-blind | Losses (<20%) |
Prognostics or demographic characteristics |
Outcomes | Intention to treat analysis | Sample calculation | Adequate follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| [36] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| [37] | Yes | No | Yes | No | Yes | No | Yes | No | Yes | NR |
| [38] | Yes | No | Yes | Yes | No | Yes | Yes | No | No | Yes |
| [39] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| [40] | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| [41] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N | No | Yes |
| [42] | Yes | No | No | No | Yes | NR | Yes | No | Yes | Yes |
| [43] | Yes | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | Yes |
| [44] | Yes | Yes | Yes | Yes | Yes | NR | Yes | Yes | NR | Yes |
| [45] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| [46] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NR | Yes |
| [48] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| [47] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| [49] | Yes | NR | NR | NR | NR | Yes | Yes | NR | Yes | Yes |
| [50] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| [51] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Bioactive compound | Molecular structure | Antiviral function (s) | Reference |
|---|---|---|---|
| Bioactive compounds of medicinal plants that affect mainly SARS-CoV-2 | |||
| Casticin | 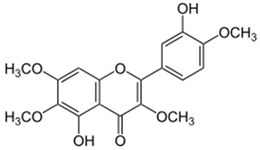 |
|
[79] |
| Emodin | 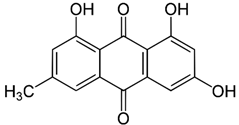 |
|
[73,74,89] |
| Dieckol | 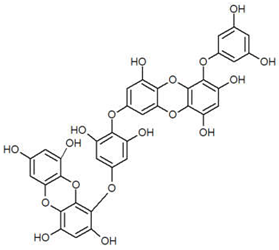 |
|
[78] |
| Curcumin | 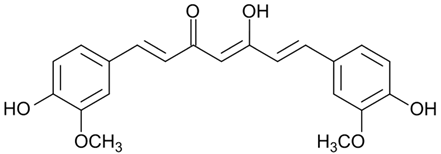 |
|
[74,90] |
| Myricetin | 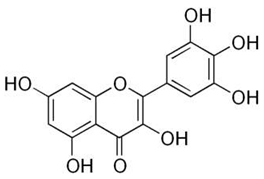 |
|
[73,86] |
| Hirsutenone | 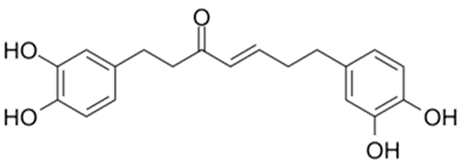 |
|
[78] |
| Scutellarein | 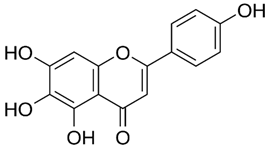 |
|
[73,86] |
| Quercetin | 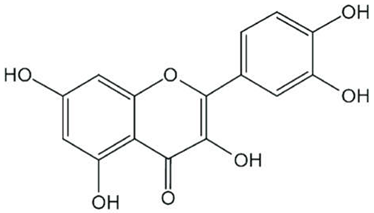 |
|
[73,74,90,91,92] |
| Sulforaphane | 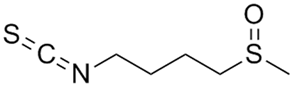 |
|
[73] |
| Resveratrol | 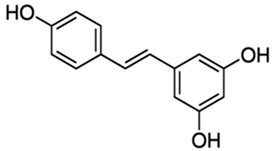 |
|
[90] |
| Ginsenoside | 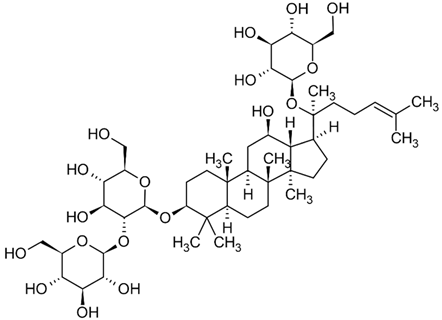 |
|
[90] |
| Kaempferol | 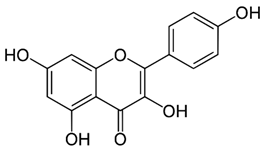 |
|
[73,92] |
| Caffeic acid | 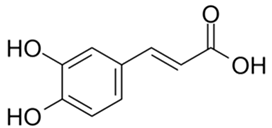 |
|
[73,74] |
| Desmethoxyreserpine | --- |
|
[74,93] |
| Luteolin | 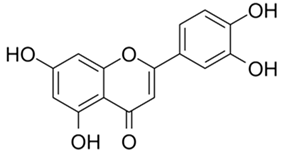 |
|
[73,74,89,92] |
| Betulinic acid | 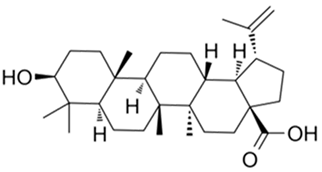 |
|
[74] |
| Xanthoangelol E | 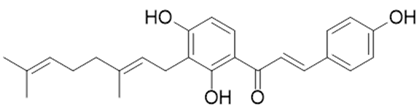 |
|
[94] |
| Hyoscyamine | 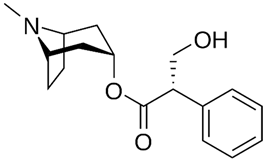 |
|
[79] |
| Cryptotanshinone | 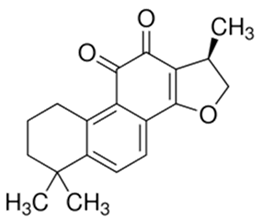 |
|
[73,74] |
| Allicin | 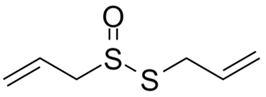 |
|
[79,90] |
| Dihydrotanshinone – 1 | 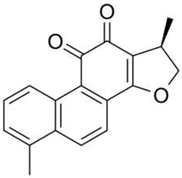 |
|
[74,78,93] |
| Tartaric acid | 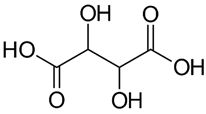 |
|
[79] |
| Andrographolide | 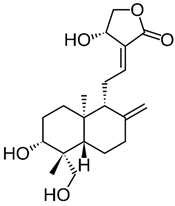 |
|
[76] |
| Amentoflavone | 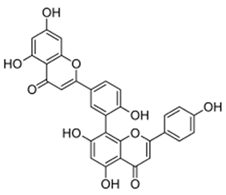 |
|
[78] |
| Nicotianamine | 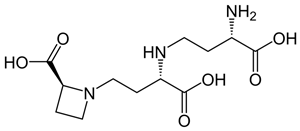 |
|
[89] |
| Glycyrrhizin | 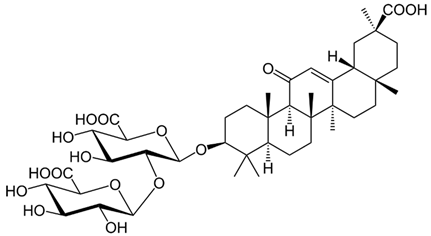 |
|
[89] |
| Bilobetin | 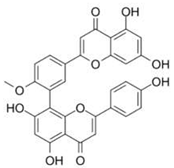 |
|
[95] |
| Sciadopitysin | 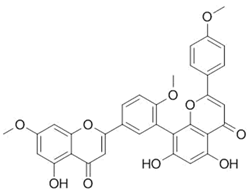 |
|
[95] |
| Bioactive compounds of medicinal plants that affect mainly influenza. | |||
| Allicin | 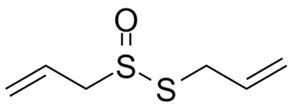 |
|
[79,90,96] |
| Eucalyptol | 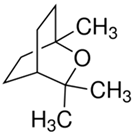 |
|
[71,97] |
| Pentagalloylglucose(polyphenol) | 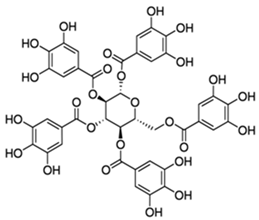 |
|
[70] |
| Coumarin | 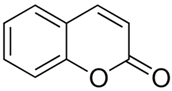 |
|
[70] |
| Ellagic acid | 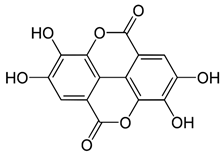 |
|
[79] |
| Chlorogenic acid | 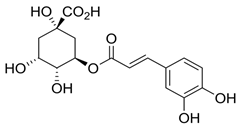 |
|
[96,98,99] |
| Isoquercetin | 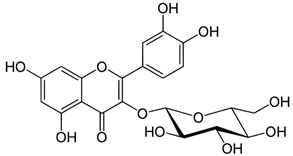 |
|
[96] |
| Luteolin | 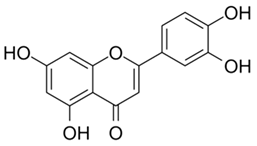 |
|
[96,100,101] |
| Glycyrrhizin | 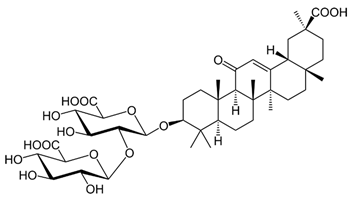 |
|
[102] |
| Glycyrrhizin acid | 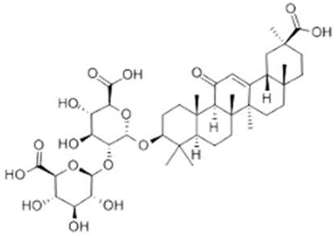 |
|
[102] |
| Aloin | 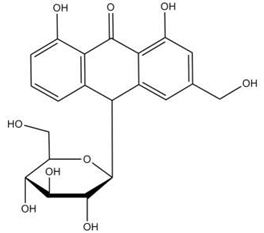 |
|
[103] |
| Quercetin-3-sophoroside | 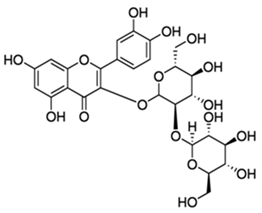 |
|
[96,100] |
| 4-Methoxycinnamaldehyde | 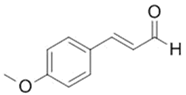 |
|
[103] |
| Punicalagin | 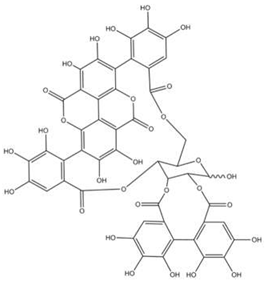 |
|
[104] |
| Agathisflavone | 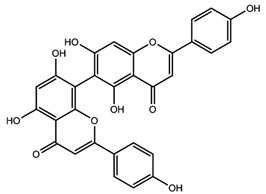 |
|
[96] |
| Guggulsterone | 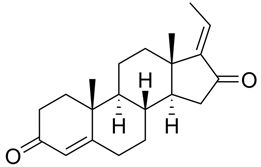 |
|
[103] |
| Chinonin | 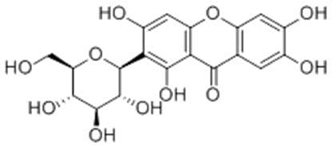 |
|
[105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





