Submitted:
10 May 2023
Posted:
10 May 2023
Read the latest preprint version here
Abstract
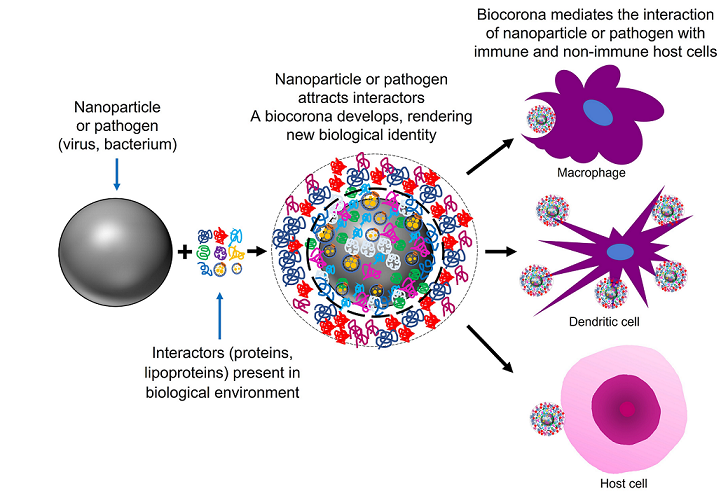
Keywords:
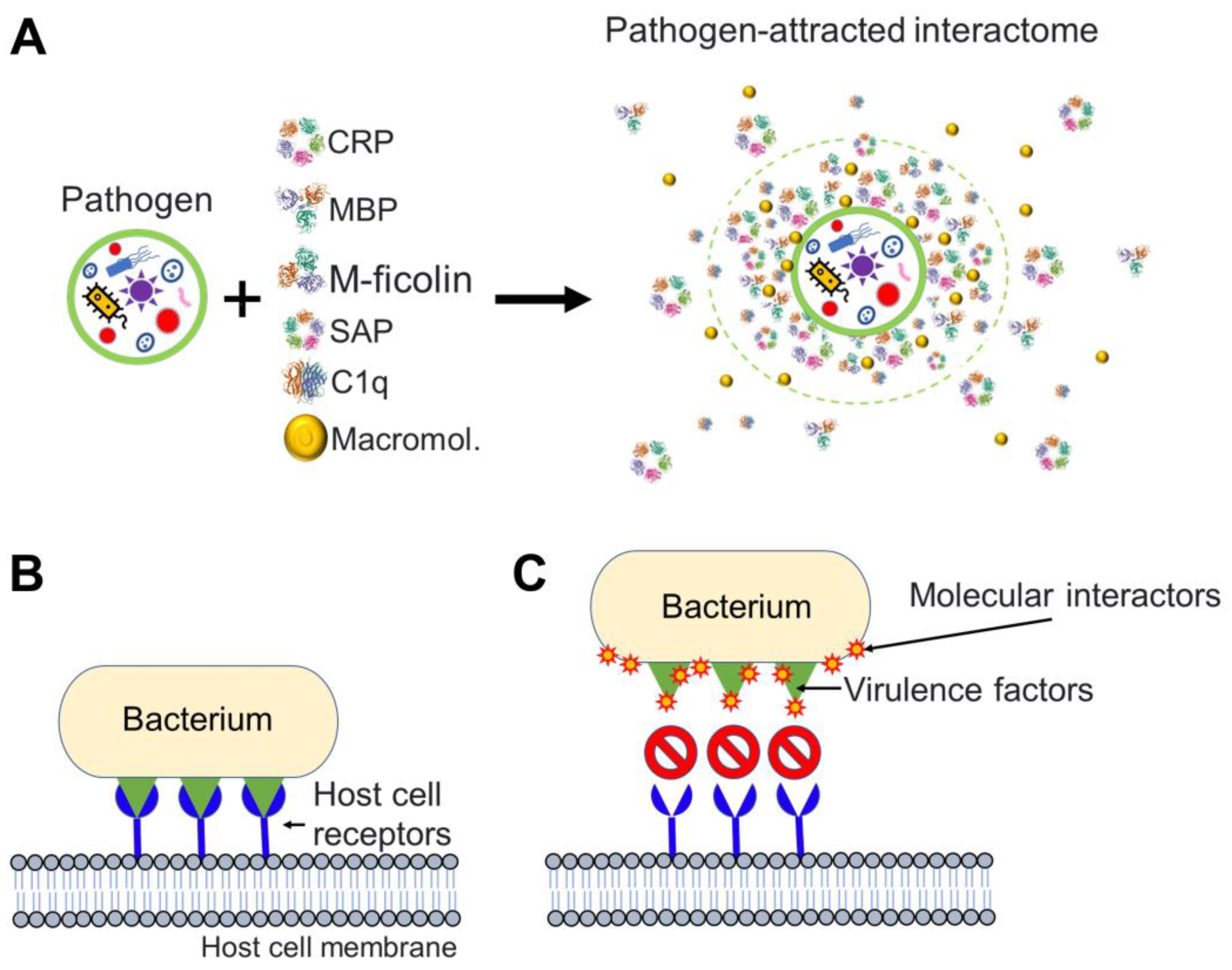
1. Introduction
2.1. Artificial nanoparticles and their ‘acquired protein coronas’
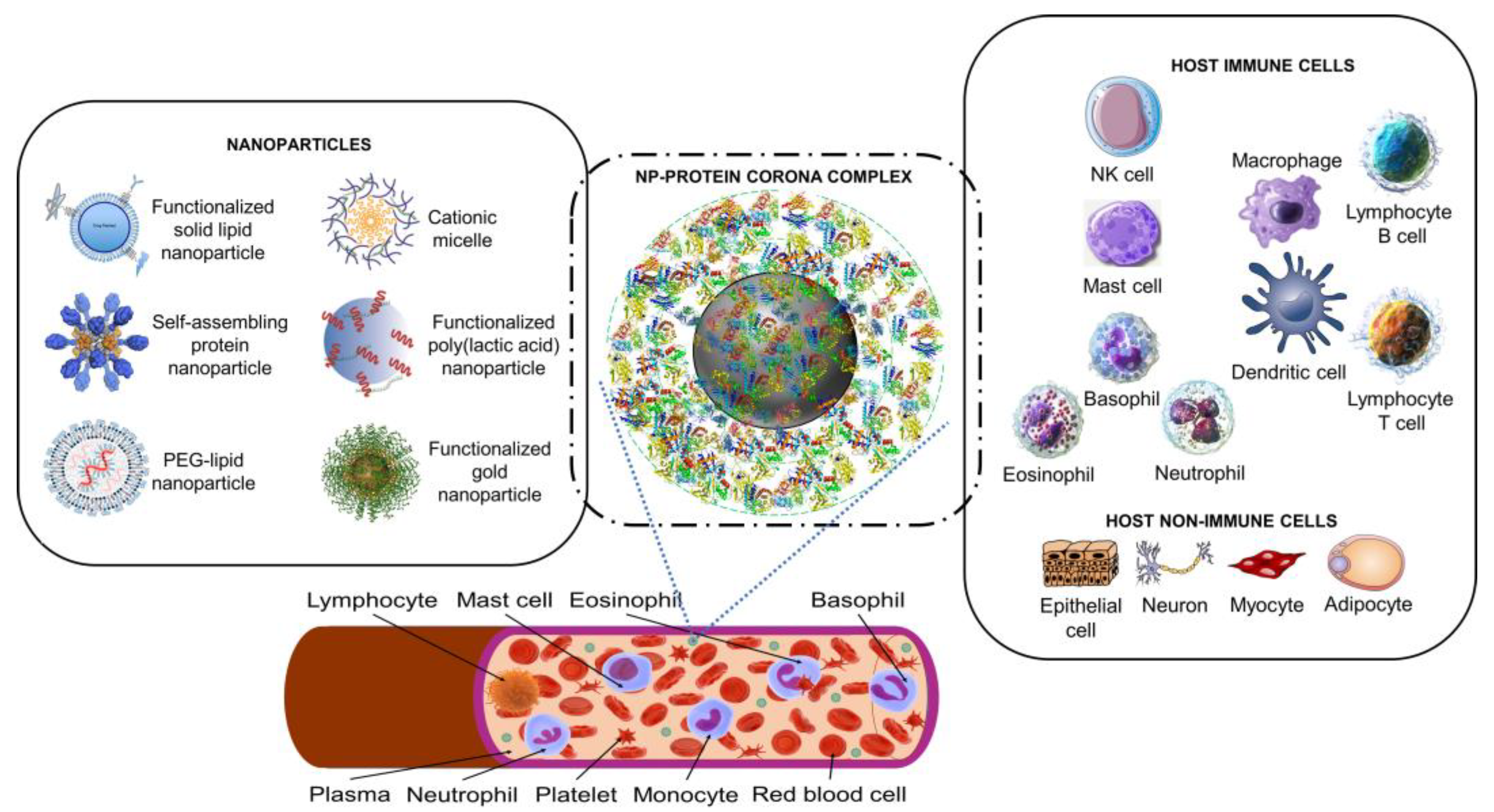
2.2. Nanoparticles can acquire biocoronas: Do viruses also acquire a biocorona?
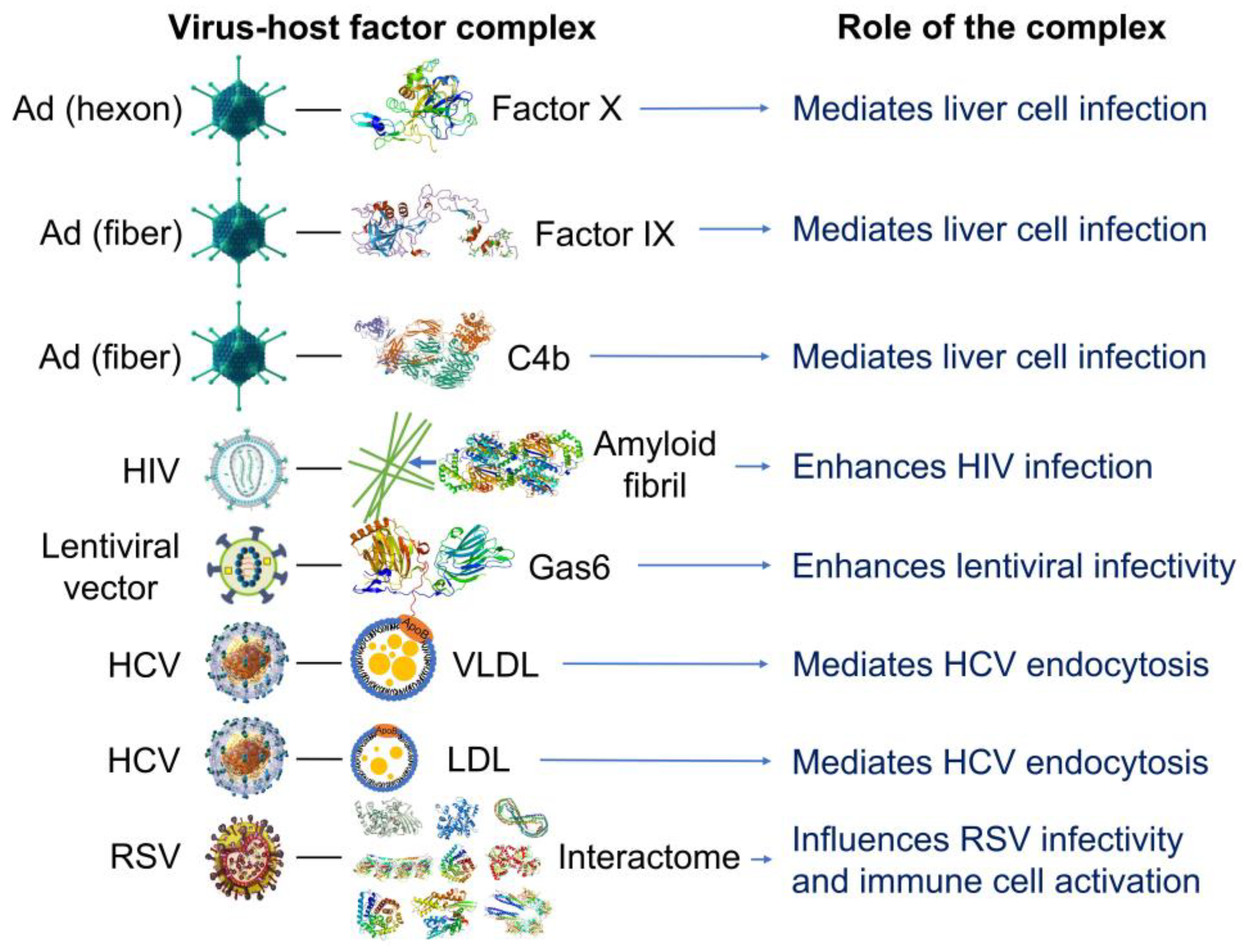
2.3. The discovery that cortisol and dexamethasone can bind to multiple sites on SARS-CoV-2 S1 suggests that glucocorticoids could be components of viral biocoronas with potential to alter the affinity of S1 for the human host receptor, ACE2
2.4. Nanoparticles and many different viruses can acquire biocoronas: Do pathogenic bacteria and other microorganisms acquire a biocorona?
3. Conclusions
| NPsc | Synthesis material | Use | Top 10 most-abundant constituents | Ref. |
|---|---|---|---|---|
| Liposomes | HSPC, DSPG, Chol | Drug delivery (e.g., liposomal amphotericin B) | Blood coagulation: coagulation factor XIII B chain, coagulation factor XIII A chain, fibrinogen beta chain, fibrinogen gamma chain, alpha-1-antitrypsin 1-3 Adaptive immunity: Ig kappa chain C region, fibrinogen beta chain Complement pathway: C4b-binding protein Others: Serum albumin, actin (cytoplasmic 2), fibronectin |
[81] |
| Lysolipid-containing TSL | DPPC, MSPC, HSPC, DSPE-PEG2000, Chol, Dox | Drug delivery (e.g., cancer therapy) |
Acute phase: Alpha-2-macroglobulin Lipid transport: Apolipoprotein C-III, apolipoprotein E Others: Beta-globin (A8DUK0 [+2]), Beta-globin (A8DUK4), Beta-2-globin (fragment), Beta-globin OS, Alpha-globin 1 (Q91V88 [+2]), Alpha-globin A8DUV1, Ig mu chain region |
[34] |
| PEGylated cationic liposomes | DOTAP, DC-Chol, DOPC, DOPE, and DOPE-PEG 2000 |
Potential vehicle to target cancer cells | Adaptive immunity: Ig kappa chain C region, Ig mu chain C region, Ig lambda-2 chain C regions Complement pathway: complement C3, complement C1q subcomponent subunit A, complement C1q subcomponent subunit B, complement C4-B Lipid transport: Apolipoprotein C-III, apolipoprotein E, Others: Serum albumin |
[82] |
| Silica NPs modified with surface NH2 | Silicon dioxide | Targeting drug delivery | Blood coagulation: Coagulation factor V Complement pathway: complement C3 Complement alternate pathway: Complement factor H, complement C1r subcomponent Lipid transport: Apolipoprotein B100, apolipoprotein A Others: Fibronectin, gelsolin, thrombospondin, inter α trypsin inhibitor heavy chain H4 |
[24] |
| Negatively charged hydrophilic silica NPs | Silicon dioxide | Studies on nano-bio interfaces | Blood coagulation: Plasminogen Lipid transport: Apolipoprotein A-I Others: serum albumin, hemoglobin fetal subunit beta, hemoglubin subunit alpha, alpha-1 antiproteinase, tetranectin, alpha-2-HS-glycoprotein, beta-2-glycoprotein 1, serotransferrin |
[83] |
| Silica NPs bioconjugated with PEG and transferrin | Silicon dioxide, PEG, and transferrin | Active targeting | Adaptive immunity: Immunoglobulin kappa constant, immunoglobulin heavy constant mu Complement pathway: Immunoglobulin lambda-like polypeptide 5, complement C3 Lectin complement pathway: Ficolin-3 Lipid transport: Apolipoprotein A-I Others: albumin, actin cytoplasmic 1, hemoglobin subunit beta, serotransferrin |
[84] |
| Carbon nanotubes (ssDNA-SWCNTs) | Bioimaging, molecular sensing, delivery | Blood coagulation: Histidine-rich glycoprotein, kininogen-1, prothrombin Adaptive immunity: Ig heavy constant gamma Immunity: Haptoglobin Complement pathway: Clusterin, complement C3 Complement alternate pathway: Complement factor H, complement C1r subcomponent Lipid transport: Aapolipoprotein A-I Cell adhesion: Vitronectin Others: A disintegrin and metalloproteinase with thrombospondin motifs 12 |
[85] | |
| Riboflavin-coated SPIONs | Cores made of iron oxides (e.g., magnetite or maghemite) | Theranostic applications | Complement pathway: Complement C4 (fragments) Complement alternate pathway: Complement factor H Lipid transport: Apolipoprotein E, apolipoprotein A-I Others: Hemoglobin fetal subunit beta, hemoglubin subunit alpha, serum albumin, peptidyl-prolyl cis-trans isomerase A, tetranectin, α-2-HS-glycoprotein |
[86] |
| Carboxylated polystyrene-NPs | Polystyrene, surface carboxyl groups | Drug delivery and diagnostic fields | Blood coagulation: Fibrinogen, histidine-rich glycoprotein, kininogen-1, plasma kallikrein Adaptive immunity: Immunoglobulin Complement pathway: Complement components, clusterin Lipid transport: Apolipoproteins Cell adhesion: Vitronectin Others: serum albumin, trypsin inhibitor heavy chains, beta-2-glycoprotein 1 |
[87] |
| TiO2 NPs | Titanium dioxide | Nanoparticle toxicity studies | Host-virus interaction: Moesin, annexin A2, keratin (type II cytoskeletal 8) Autophagy: Ras-related protein Rab-8A Others: Pulmonary surfactant-associated protein A1, actin (cytoplasmic 1), L-lactate dehydrogenase A-like 6A, alpha-actinin-4, POTE ankyrin domain family member E, serum albumin |
[88] |
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, H.; Kawai, T.; Akira, S. Pathogen Recognition by the Innate Immune System. Int Rev Immunol 2011, 30, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A.; Medzhitov, R. Innate Immune Recognition. Annu Rev Immunol 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, C. Innate Immune Recognition: Mechanisms and Pathways. Immunol Rev 2000, 173, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, C. Innate Immunity. N Engl J Med 2000, 343, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Paludan, S.R.; Pradeu, T.; Masters, S.L.; Mogensen, T.H. Constitutive Immune Mechanisms: Mediators of Host Defence and Immune Regulation. Nature Reviews Immunology 2020 21:3 2020, 21, 137–150. [Google Scholar] [CrossRef]
- Hardy, E.; Fernandez-Patron, C. Could Endogenous Glucocorticoids Influence SARS-CoV-2 Infectivity? Cells 2022, 11. [Google Scholar] [CrossRef]
- Module 3: Characteristics of Particles - Particle Size Categories | Basic Concepts in Environmental Sciences | APTI | USEPA. Available online: https://web.archive.org/web/20101203205130/http://www.epa.gov/apti/bces/module3/category/category.htm (accessed on 10 April 2023).
- Vert, M.; Doi, Y.; Hellwich, K.H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for Biorelated Polymers and Applications (IUPAC Recommendations 2012). Pure and Applied Chemistry 2012, 84, 377–410. [Google Scholar] [CrossRef]
- ISO/TS 80004-2:2015 - Nanotechnologies — Vocabulary — Part 2: Nano-Objects. Available online: https://www.iso.org/standard/54440.html (accessed on 10 April 2023).
- Fadeel, B. Understanding the Immunological Interactions of Engineered Nanomaterials: Role of the Bio-Corona. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2022, 14. [Google Scholar] [CrossRef]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted Drug Delivery Strategies for Precision Medicines. Nat Rev Mater 2021, 6, 351–370. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nature Reviews Drug Discovery 2020 20:2 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular Coronas Provide the Biological Identity of Nanosized Materials. Nat Nanotechnol 2012, 7, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid Formation of Plasma Protein Corona Critically Affects Nanoparticle Pathophysiology. Nat Nanotechnol 2013, 8, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding Biophysicochemical Interactions at the Nano-Bio Interface. Nat Mater 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Champion, J.A.; Pustulka, S.M.; Ling, K.; Pish, S.L. Protein Nanoparticle Charge and Hydrophobicity Govern Protein Corona and Macrophage Uptake. ACS Appl Mater Interfaces 2020, 12, 48284–48295. [Google Scholar] [CrossRef]
- Ren, J.; Andrikopoulos, N.; Velonia, K.; Tang, H.; Cai, R.; Ding, F.; Ke, P.C.; Chen, C. Chemical and Biophysical Signatures of the Protein Corona in Nanomedicine. J Am Chem Soc 2022, 144, 9184–9205. [Google Scholar] [CrossRef] [PubMed]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time Evolution of the Nanoparticle Protein Corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef] [PubMed]
- Docter, D.; Strieth, S.; Westmeier, D.; Hayden, O.; Gao, M.; Knauer, S.K.; Stauber, R.H. No King without a Crown--Impact of the Nanomaterial-Protein Corona on Nanobiomedicine. Nanomedicine (Lond) 2015, 10, 503–519. [Google Scholar] [CrossRef]
- Corbo, C.; Molinaro, R.; Parodi, A.; Toledano Furman, N.E.; Salvatore, F.; Tasciotti, E. The Impact of Nanoparticle Protein Corona on Cytotoxicity, Immunotoxicity and Target Drug Delivery. Nanomedicine (Lond) 2016, 11, 81–100. [Google Scholar] [CrossRef]
- Ke, P.C.; Lin, S.; Parak, W.J.; Davis, T.P.; Caruso, F. A Decade of the Protein Corona. ACS Nano 2017, 11, 11773–11776. [Google Scholar] [CrossRef]
- Dilliard, S.A.; Siegwart, D.J. Passive, Active and Endogenous Organ-Targeted Lipid and Polymer Nanoparticles for Delivery of Genetic Drugs. Nat Rev Mater 2023, 8, 282. [Google Scholar] [CrossRef]
- Berardi, A.; Baldelli Bombelli, F. Oral Delivery of Nanoparticles - Let’s Not Forget about the Protein Corona. Expert Opin Drug Deliv 2019, 16, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Lee, B.J. Protein Corona: A New Approach for Nanomedicine Design. Int J Nanomedicine 2017, 12, 3137–3151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Zhou, Y.; Sun, J.; Wang, X.; Jia, Y.; Ge, K.; Yan, Y.; Dawson, K.A.; Guo, S.; Zhang, J.; et al. The Yin and Yang of the Protein Corona on the Delivery Journey of Nanoparticles. Nano Res 2023, 16, 715–734. [Google Scholar] [CrossRef] [PubMed]
- Papini, E.; Tavano, R.; Mancin, F. Opsonins and Dysopsonins of Nanoparticles: Facts, Concepts, and Methodological Guidelines. Front Immunol 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Moein Moghimi, S.; Patel, H.M. Serum Opsonins and Phagocytosis of Saturated and Unsaturated Phospholipid Liposomes. BBA - Biomembranes 1989, 984, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Tavano, R.; Gabrielli, L.; Lubian, E.; Fedeli, C.; Visentin, S.; Polverino De Laureto, P.; Arrigoni, G.; Geffner-Smith, A.; Chen, F.; Simberg, D.; et al. C1q-Mediated Complement Activation and C3 Opsonization Trigger Recognition of Stealth Poly(2-Methyl-2-Oxazoline)-Coated Silica Nanoparticles by Human Phagocytes. ACS Nano 2018, 12, 5834–5847. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, M.; Bianchi, M.; Sherry, B.; Sama, A.; Tracey, K.J. Fetuin (Alpha2-HS-Glycoprotein) Opsonizes Cationic Macrophagedeactivating Molecules. Proc Natl Acad Sci U S A 1998, 95, 14429–14434. [Google Scholar] [CrossRef]
- Abarca-Cabrera, L.; Fraga-García, P.; Berensmeier, S. Bio-Nano Interactions: Binding Proteins, Polysaccharides, Lipids and Nucleic Acids onto Magnetic Nanoparticles. Biomater Res 2021, 25. [Google Scholar] [CrossRef]
- Abarca-Cabrera, L.; Xu, L.; Berensmeier, S.; Fraga-García, P. Competition at the Bio-Nano Interface: A Protein, a Polysaccharide, and a Fatty Acid Adsorb onto Magnetic Nanoparticles. ACS Appl Bio Mater 2023, 6, 146–156. [Google Scholar] [CrossRef]
- Docter, D.; Westmeier, D.; Markiewicz, M.; Stolte, S.; Knauer, S.K.; Stauber, R.H. The Nanoparticle Biomolecule Corona: Lessons Learned - Challenge Accepted? Chem Soc Rev 2015, 44, 6094–6121. [Google Scholar] [CrossRef]
- Lynch, I.; Cedervall, T.; Lundqvist, M.; Cabaleiro-Lago, C.; Linse, S.; Dawson, K.A. The Nanoparticle-Protein Complex as a Biological Entity; a Complex Fluids and Surface Science Challenge for the 21st Century. Adv Colloid Interface Sci 2007, 134–135, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmady, Z.S.; Hadjidemetriou, M.; Gubbins, J.; Kostarelos, K. Formation of Protein Corona in Vivo Affects Drug Release from Temperature-Sensitive Liposomes. J Control Release 2018, 276, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Farrera, C.; Fadeel, B. It Takes Two to Tango: Understanding the Interactions between Engineered Nanomaterials and the Immune System. European Journal of Pharmaceutics and Biopharmaceutics 2015, 95, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Fasoli, E. Protein Corona: Dr. Jekyll and Mr. Hyde of Nanomedicine. Biotechnol Appl Biochem 2021, 68, 1139–1152. [Google Scholar] [CrossRef]
- Pellett, P.E.; Mitra, S.; Holland, T.C. Basics of Virology. Handb Clin Neurol 2014, 123, 45. [Google Scholar] [CrossRef] [PubMed]
- Michen, B.; Graule, T. Isoelectric Points of Viruses. J Appl Microbiol 2010, 109, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Heffron, J.; Mayer, B.K. Virus Isoelectric Point Estimation: Theories and Methods. Appl Environ Microbiol 2021, 87, 1–17. [Google Scholar] [CrossRef]
- Wilson, S.S.; Wiens, M.E.; Smith, J.G. Antiviral Mechanisms of Human Defensins. J Mol Biol 2013, 425, 4965–4980. [Google Scholar] [CrossRef]
- Hazrati, E.; Galen, B.; Lu, W.; Wang, W.; Ouyang, Y.; Keller, M.J.; Lehrer, R.I.; Herold, B.C. Human Alpha- and Beta-Defensins Block Multiple Steps in Herpes Simplex Virus Infection. J Immunol 2006, 177, 8658–8666. [Google Scholar] [CrossRef]
- Rapista, A.; Ding, J.; Benito, B.; Lo, Y.T.; Neiditch, M.B.; Lu, W.; Chang, T.L. Human Defensins 5 and 6 Enhance HIV-1 Infectivity through Promoting HIV Attachment. Retrovirology 2011, 8. [Google Scholar] [CrossRef]
- Ezzat, K.; Pernemalm, M.; Pålsson, S.; Roberts, T.C.; Järver, P.; Dondalska, A.; Bestas, B.; Sobkowiak, M.J.; Levänen, B.; Sköld, M.; et al. The Viral Protein Corona Directs Viral Pathogenesis and Amyloid Aggregation. Nat Commun 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zeng, L.; Yao, L.; Wang, Z.; Yang, X.; Shi, J.; Hu, L.; Liu, Q.; Chen, C.; Xia, T.; et al. Inherited and Acquired Corona of Coronavirus in the Host: Inspiration from the Biomolecular Corona of Nanoparticles. Nano Today 2021, 39, 101161. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Flamholz, A.; Phillips, R.; Milo, R. SARS-CoV-2 (COVID-19) by the Numbers. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat Rev Immunol 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Ye, D.; Liu, Q. Review of the 2019 Novel Coronavirus (SARS-CoV-2) Based on Current Evidence. Int J Antimicrob Agents 2020, 55. [Google Scholar] [CrossRef] [PubMed]
- Stahn, C.; Buttgereit, F. Genomic and Nongenomic Effects of Glucocorticoids. Nat Clin Pract Rheumatol 2008, 4, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P.; Kino, T. Glucocorticoid Action Networks and Complex Psychiatric and/or Somatic Disorders. Stress 2007, 10, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Taves, M.D.; Gomez-Sanchez, C.E.; Soma, K.K. Extra-Adrenal Glucocorticoids and Mineralocorticoids: Evidence for Local Synthesis, Regulation, and Function. Am J Physiol Endocrinol Metab 2011, 301. [Google Scholar] [CrossRef]
- Shimba, A.; Ikuta, K. Control of Immunity by Glucocorticoids in Health and Disease. Semin Immunopathol 2020, 42, 669–680. [Google Scholar] [CrossRef]
- Shimba, A.; Ikuta, K. Immune-Enhancing Effects of Glucocorticoids in Response to Day-Night Cycles and Stress. Int Immunol 2020, 32, 703–708. [Google Scholar] [CrossRef]
- Sarker, H.; Panigrahi, R.; Hardy, E.; Glover, J.N.M.; Elahi, S.; Fernandez-Patron, C. Glucocorticoids Bind to SARS-CoV-2 S1 at Multiple Sites Causing Cooperative Inhibition of SARS-CoV-2 S1 Interaction With ACE2. Front Immunol 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.R.; Perlman, S. Coronaviruses: An Overview of Their Replication and Pathogenesis. Methods Mol Biol 2015, 1282, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ziebuhr, J. Molecular Biology of Severe Acute Respiratory Syndrome Coronavirus. Curr Opin Microbiol 2004, 7, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Bosch, B.J.; Zee, R. van der; Haan, C.A.M. de; Rottier, P.J.M. The Coronavirus Spike Protein Is a Class I Virus Fusion Protein: Structural and Functional Characterization of the Fusion Core Complex. J Virol 2003, 77, 8801. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Sheng, Y.; Wang, M.; Ni, S.; Ding, H.; Ma, Y. Protein Corona Critically Affects the Bio-Behaviors of SARS-CoV-2. 2021. [Google Scholar]
- Iles, J.; Zmuidinaite, R.; Sadee, C.; Gardiner, A.; Lacey, J.; Harding, S.; Ule, J.; Roblett, D.; Heeney, J.; Baxendale, H.; et al. SARS-CoV-2 Spike Protein Binding of Glycated Serum Albumin-Its Potential Role in the Pathogenesis of the COVID-19 Clinical Syndromes and Bias towards Individuals with Pre-Diabetes/Type 2 Diabetes and Metabolic Diseases. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.N. The Battle with the Host over Microbial Size. Curr Opin Microbiol 2013, 16, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Sohlenkamp, C.; Geiger, O. Bacterial Membrane Lipids: Diversity in Structures and Pathways. FEMS Microbiol Rev 2016, 40, 133–159. [Google Scholar] [CrossRef]
- Bastos, P.; Trindade, F.; da Costa, J.; Ferreira, R.; Vitorino, R. Human Antimicrobial Peptides in Bodily Fluids: Current Knowledge and Therapeutic Perspectives in the Postantibiotic Era. Med Res Rev 2018, 38, 101–146. [Google Scholar] [CrossRef]
- Bechinger, B.; Gorr, S.U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J Dent Res 2017, 96, 254–260. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.C.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q.Y. The Antimicrobial Peptides and Their Potential Clinical Applications. Am J Transl Res 2019, 11, 3919. [Google Scholar] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front Microbiol 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Giangaspero, A.; Sandri, L.; Tossi, A. Amphipathic Alpha Helical Antimicrobial Peptides. Eur J Biochem 2001, 268, 5589–5600. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduction and Targeted Therapy 2021 6:1 2021, 6, 1–24. [Google Scholar] [CrossRef]
- Howard, M.; Farrar, C.A.; Sacks, S.H. Structural and Functional Diversity of Collectins and Ficolins and Their Relationship to Disease. Semin Immunopathol 2018, 40, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Hirschfield, G.M. C-Reactive Protein: A Critical Update. J Clin Invest 2003, 111, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Booth, D.R.; Hutchinson, W.L.; Gallimore, J.R.; Collins, P.M.; Hohenester, E. Amyloid P Component. A Critical Review. http://dx.doi.org/10.3109/13506129709003838 2009, 4, 274–295. [Google Scholar] [CrossRef]
- Clos, T.W. Du Pentraxins: Structure, Function, and Role in Inflammation. ISRN Inflamm 2013, 2013, 1–22. [Google Scholar] [CrossRef]
- Schwalbe, R.A.; Nelsestuen, G.L.; Dahlbáck, B.; Coe, J.E. Pentraxin Family of Proteins Interact Specifically with Phosphorylcholine and/or Phosphorylethanolamine. Biochemistry 1992, 31, 4907–4915. [Google Scholar] [CrossRef]
- Dommett, R.M.; Klein, N.; Turner, M.W. Mannose-Binding Lectin in Innate Immunity: Past, Present and Future. Tissue Antigens 2006, 68, 193–209. [Google Scholar] [CrossRef]
- Kingma, P.; Jobe, A.H. The Surfactant System. Kendig’s Disorders of the Respiratory Tract in Children. 2019, 57–63.e2. [Google Scholar] [CrossRef]
- Bidula, S.; Sexton, D.W.; Schelenz, S. Ficolins and the Recognition of Pathogenic Microorganisms: An Overview of the Innate Immune Response and Contribution of Single Nucleotide Polymorphisms. J Immunol Res 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.B.M. Complement Component C1q: Historical Perspective of a Functionally Versatile, and Structurally Unusual, Serum Protein. Front Immunol 2018, 9, 764. [Google Scholar] [CrossRef]
- Kishore, U.; Ghai, R.; Greenhough, T.J.; Shrive, A.K.; Bonifati, D.M.; Gadjeva, M.G.; Waters, P.; Kojouharova, M.S.; Chakraborty, T.; Agrawal, A. Structural and Functional Anatomy of the Globular Domain of Complement Protein C1q. Immunol Lett 2004, 95, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Complement. Immunology for Pharmacy 2012, 87–96. [CrossRef]
- Mantovani, A.; Garlanda, C. Humoral Innate Immunity and Acute-Phase Proteins. N Engl J Med 2023, 388, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Calvert, M.B.; Jumde, V.R.; Titz, A. Pathoblockers or Antivirulence Drugs as a New Option for the Treatment of Bacterial Infections. Beilstein Journal of Organic Chemistry 2018, 14, 2607. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, E.; Koch, G.; Wagner, R.M.; Fekete, A.; Stengel, S.T.; Schneider, J.; Mielich-Süss, B.; Geibel, S.; Markert, S.M.; Stigloher, C.; et al. Membrane Microdomain Disassembly Inhibits MRSA Antibiotic Resistance. Cell 2017, 171, 1354–1367.e20. [Google Scholar] [CrossRef]
- Amici, A.; Caracciolo, G.; Digiacomo, L.; Gambini, V.; Marchini, C.; Tilio, M.; Capriotti, A.L.; Colapicchioni, V.; Matassa, R.; Familiari, G.; et al. In Vivo Protein Corona Patterns of Lipid Nanoparticles. RSC Adv 2017, 7, 1137–1145. [Google Scholar] [CrossRef]
- Pozzi, D.; Caracciolo, G.; Capriotti, A.L.; Cavaliere, C.; Piovesana, S.; Colapicchioni, V.; Palchetti, S.; Riccioli, A.; Laganà, A. A Proteomics-Based Methodology to Investigate the Protein Corona Effect for Targeted Drug Delivery. Mol Biosyst 2014, 10, 2815–2819. [Google Scholar] [CrossRef]
- Mohammad-Beigi, H.; Hayashi, Y.; Zeuthen, C.M.; Eskandari, H.; Scavenius, C.; Juul-Madsen, K.; Vorup-Jensen, T.; Enghild, J.J.; Sutherland, D.S. Mapping and Identification of Soft Corona Proteins at Nanoparticles and Their Impact on Cellular Association. Nat Commun 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Solorio-Rodríguez, A.; Escamilla-Rivera, V.; Uribe-Ramírez, M.; Chagolla, A.; Winkler, R.; García-Cuellar, C.M.; De Vizcaya-Ruiz, A. A Comparison of the Human and Mouse Protein Corona Profiles of Functionalized SiO2 Nanocarriers. Nanoscale 2017, 9, 13651–13660. [Google Scholar] [CrossRef] [PubMed]
- Pinals, R.L.; Yang, D.; Rosenberg, D.J.; Chaudhary, T.; Crothers, A.R.; Iavarone, A.T.; Hammel, M.; Landry, M.P. Quantitative Protein Corona Composition and Dynamics on Carbon Nanotubes in Biological Environments. Angew Chem Int Ed Engl 2020, 59, 23668–23677. [Google Scholar] [CrossRef] [PubMed]
- Mekseriwattana, W.; Thiangtrongjit, T.; Reamtong, O.; Wongtrakoongate, P.; Katewongsa, K.P. Proteomic Analysis Reveals Distinct Protein Corona Compositions of Citrate- and Riboflavin-Coated SPIONs. ACS Omega 2022, 7, 37589–37599. [Google Scholar] [CrossRef] [PubMed]
- Faserl, K.; Chetwynd, A.J.; Lynch, I.; Thorn, J.A.; Lindner, H.H. Corona Isolation Method Matters: Capillary Electrophoresis Mass Spectrometry Based Comparison of Protein Corona Compositions Following On-Particle versus In-Solution or In-Gel Digestion. Nanomaterials (Basel) 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, H.; Mackay, R.M.; Elgy, C.; Morgan, C.; Griffiths, M.; Clark, H.; Skipp, P.; Madsen, J. Nanoparticles in the Lung and Their Protein Corona: The Few Proteins That Count. Nanotoxicology 2016, 10, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Francia, V.; Yang, K.; Deville, S.; Reker-Smit, C.; Nelissen, I.; Salvati, A. Corona Composition Can Affect the Mechanisms Cells Use to Internalize Nanoparticles. ACS Nano 2019, 13, 11107–11121. [Google Scholar] [CrossRef] [PubMed]
- Chéneau, C.; Kremer, E.J. Adenovirus-Extracellular Protein Interactions and Their Impact on Innate Immune Responses by Human Mononuclear Phagocytes. Viruses 2020, 12. [Google Scholar] [CrossRef]
- Bottermann, M.; Foss, S.; Caddy, S.L.; Clift, D.; van Tienen, L.M.; Vaysburd, M.; Cruickshank, J.; O’Connell, K.; Clark, J.; Mayes, K.; et al. Complement C4 Prevents Viral Infection through Capsid Inactivation. Cell Host Microbe 2019, 25, 617–629.e7. [Google Scholar] [CrossRef]
- Doronin, K.; Flatt, J.W.; Di Paolo, N.C.; Khare, R.; Kalyuzhniy, O.; Acchione, M.; Sumida, J.P.; Ohto, U.; Shimizu, T.; Akashi-Takamura, S.; et al. Coagulation Factor X Activates Innate Immunity to Human Species C Adenovirus. Science 2012, 338, 795–798. [Google Scholar] [CrossRef]
- Waddington, S.; McVey, J.; Bhella, D.; Cell, A.P.-. 2008, undefined Adenovirus Serotype 5 Hexon Mediates Liver Gene Transfer. Elsevier.
- Castellano, L.M.; Shorter, J. The Surprising Role of Amyloid Fibrils in HIV Infection. Biology (Basel) 2012, 1, 58–80. [Google Scholar] [CrossRef] [PubMed]
- Morizono, K.; Xie, Y.; Olafsen, T.; Lee, B.; Dasgupta, A.; Wu, A.M.; Chen, I.S.Y. The Soluble Serum Protein Gas6 Bridges Virion Envelope Phosphatidylserine to the TAM Receptor Tyrosine Kinase Axl to Mediate Viral Entry. Cell Host Microbe 2011, 9, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Wrensch, F.; Crouchet, E.; Ligat, G.; Zeisel, M.B.; Keck, Z.Y.; Foung, S.K.H.; Schuster, C.; Baumert, T.F. Hepatitis C Virus (HCV)-Apolipoprotein Interactions and Immune Evasion and Their Impact on HCV Vaccine Design. Front Immunol 2018, 9, 1436. [Google Scholar] [CrossRef] [PubMed]
- Croll, T.I.; Andersen, G.R. Re-Evaluation of Low-Resolution Crystal Structures via Interactive Molecular-Dynamics Flexible Fitting (IMDFF): A Case Study in Complement C4. Acta Crystallogr D Struct Biol 2016, 72, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Kirchdoerfer, R.N.; Wang, N.; Pallesen, J.; Wrapp, D.; Turner, H.L.; Cottrell, C.A.; Corbett, K.S.; Graham, B.S.; McLellan, J.S.; Ward, A.B. Stabilized Coronavirus Spikes Are Resistant to Conformational Changes Induced by Receptor Recognition or Proteolysis. Sci Rep 2018, 8. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Shrive, A.K.; Cheetham, G.M.T.; Holden, D.; Myles, D.A.A.; Turnell, W.G.; Volanakis, J.E.; Pepys, M.B.; Bloomer, A.C.; Greenhough, T.J. Three Dimensional Structure of Human C-Reactive Protein. Nat Struct Biol 1996, 3, 346–354. [Google Scholar] [CrossRef]
- Weis, W.I.; Drickamer, K. Trimeric Structure of a C-Type Mannose-Binding Protein. Structure 1994, 2, 1227–1240. [Google Scholar] [CrossRef]
- Tanio, M.; Kondo, S.; Sugio, S.; Kohno, T. Trivalent Recognition Unit of Innate Immunity System: Crystal Structure of Trimeric Human M-Ficolin Fibrinogen-like Domain. J Biol Chem 2007, 282, 3889–3895. [Google Scholar] [CrossRef]
- Emsley, J.; White, H.E.; O’Hara, B.P.; Oliva, G.; Srinivasan, N.; Tickle, I.J.; Blundell, T.L.; Pepys, M.B.; Wood, S.P. Structure of Pentameric Human Serum Amyloid P Component. Nature 1994 367:6461 1994, 367, 338–345. [Google Scholar] [CrossRef]
- Gaboriaud, C.; Juanhuix, J.; Gruez, A.; Lacroix, M.; Darnault, C.; Pignol, D.; Verger, D.; Fontecilla-Camps, J.C.; Arlaud, G.J. The Crystal Structure of the Globular Head of Complement Protein C1q Provides a Basis for Its Versatile Recognition Properties. Journal of Biological Chemistry 2003, 278, 46974–46982. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





