Submitted:
28 April 2023
Posted:
09 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
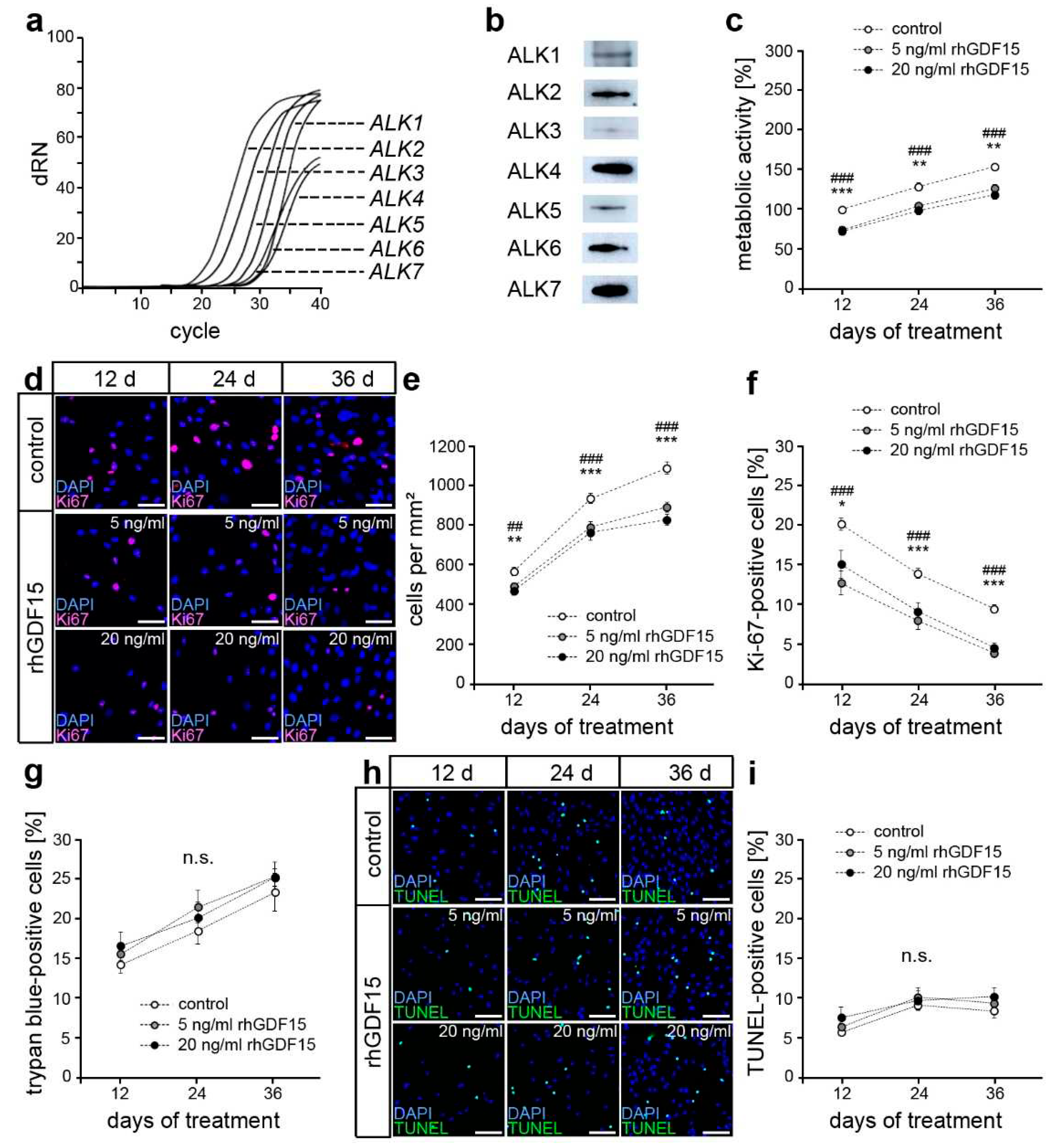
2.1. hPdLFs express potential receptors for GDF15
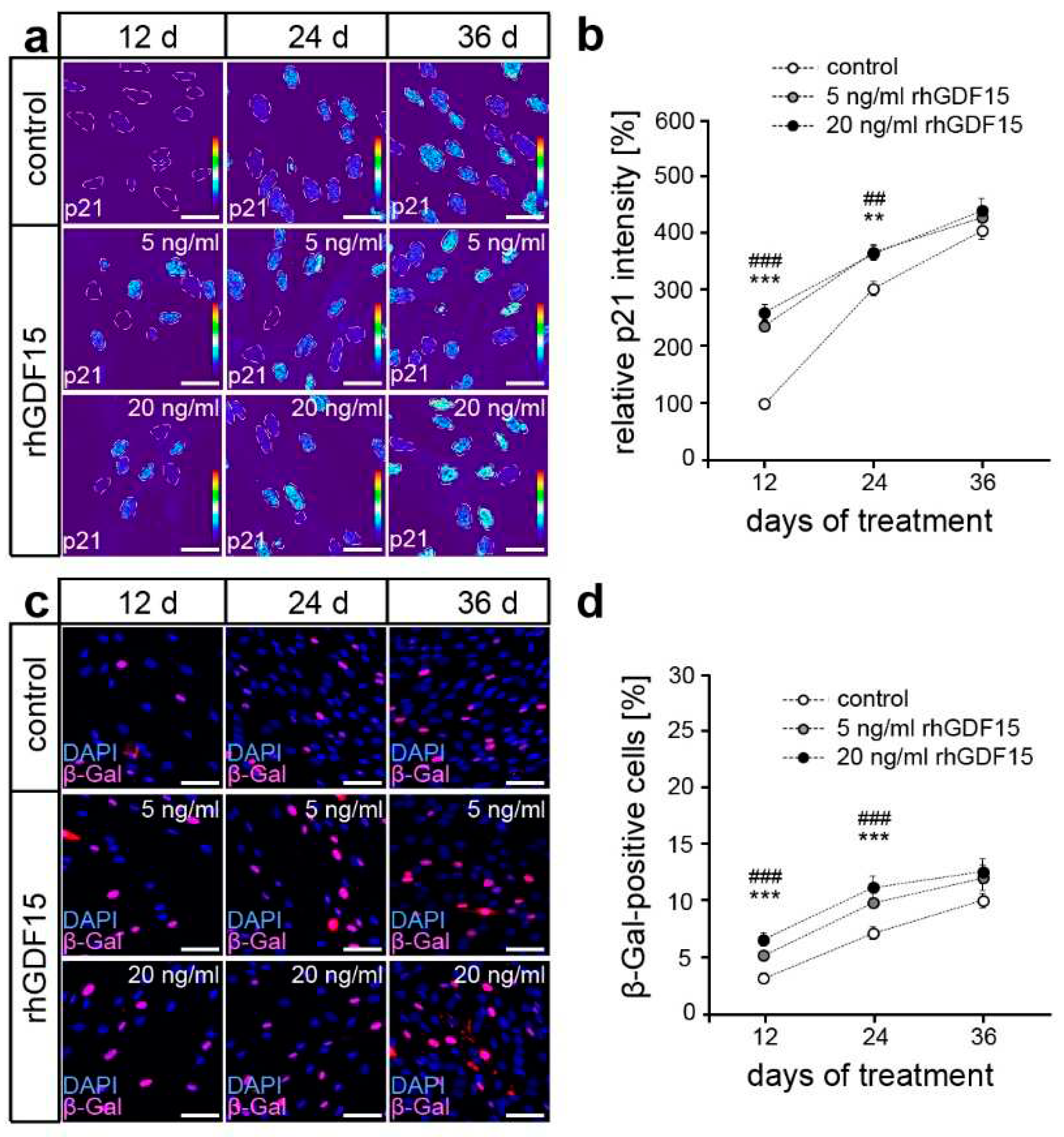
2.2. GDF15 exposure limits cell proliferation without affecting the survival of hPdLFs
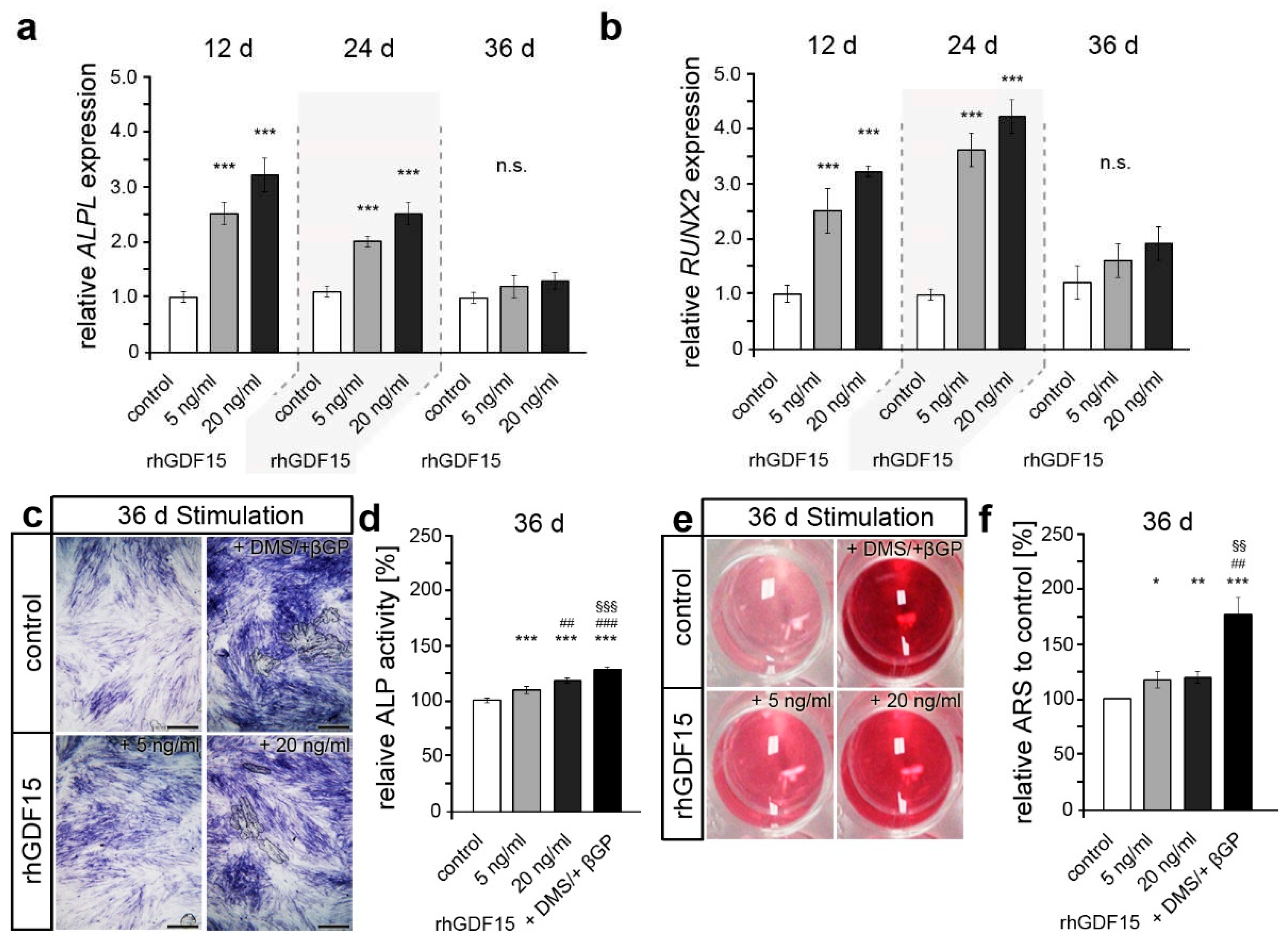
2.3. Long-term exposure to GDF15 promotes osteogenic cell fate of hPdLFs
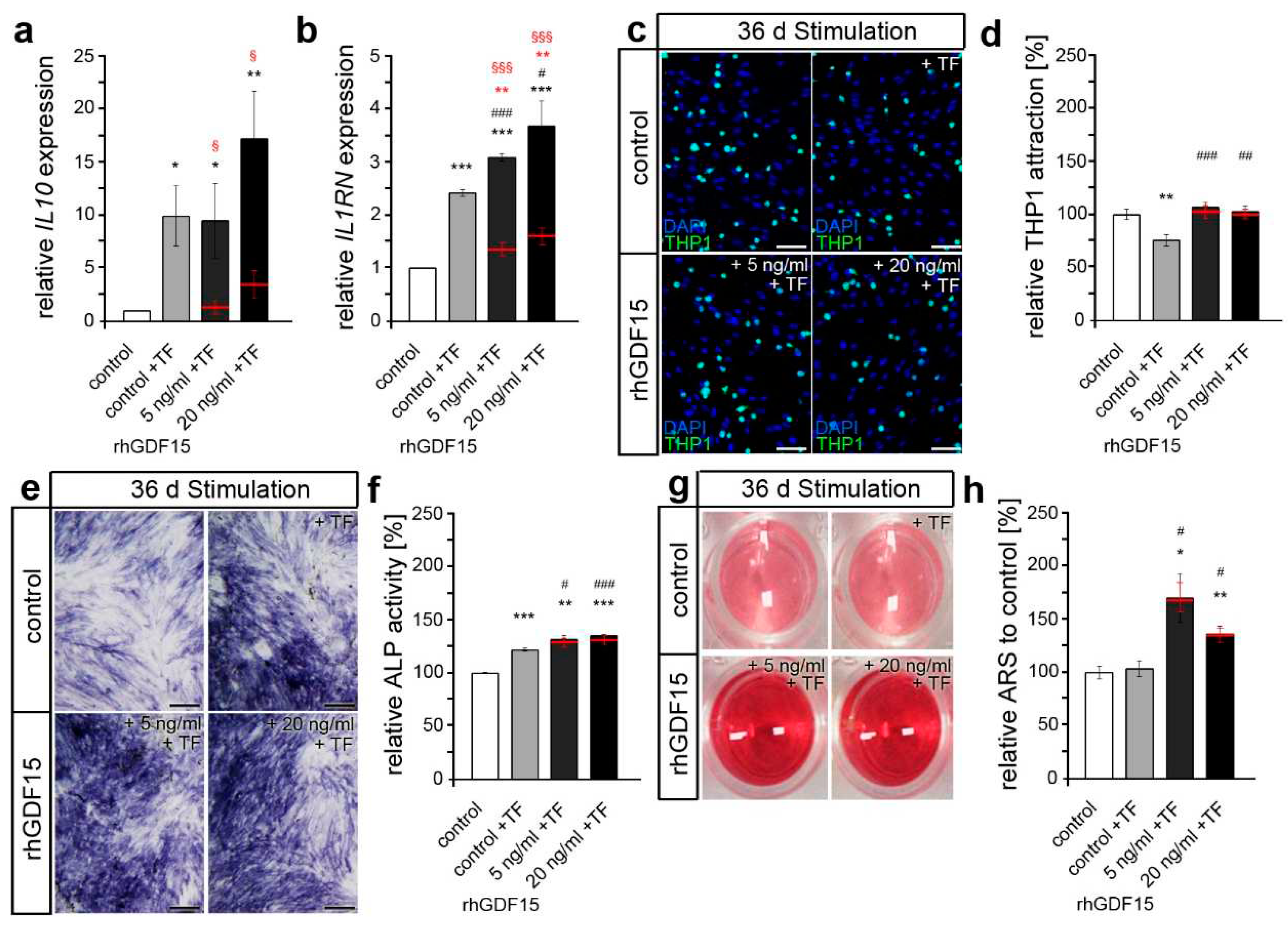
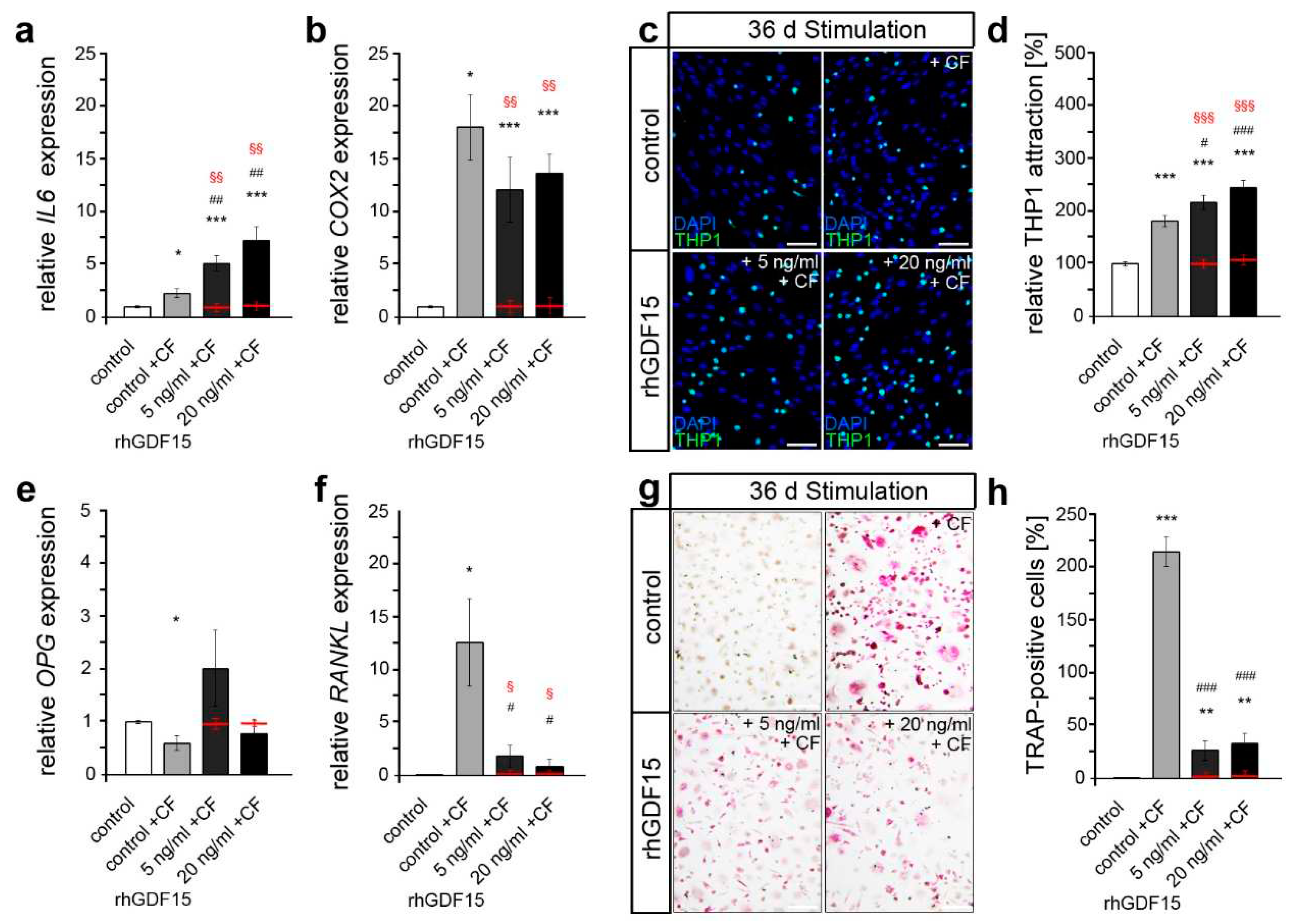
2.4. Preceding long-term exposure of hPdLFs to rhGDF15 affect their mechanoreactivity
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Stimulation with recombinant human GDF15 protein
4.3. Application of mechanical forces
4.4. RNA expression analysis
4.5. Protein preparation and expression analysis
4.6. Immunofluorescent Staining
4.7. MTT Assay
4.8. Cell death assays
4.9. Senescence assay
4.10. Alkaline phosphatase activity assay
4.11. Alizarin red staining
4.12. THP1 activation assay
4.13. Osteoclast activation assay and TRAP staining
4.14. Microscopy, Image Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navabi, N.; Farnudi, H.; Rafiei, H.; Arashlow, M.T., Orthodontic treatment and the oral health-related quality of life of patients. J Dent (Tehran) 2012, 9, 247-254 DOI.
- Sa-Pinto, A.C.; Rego, T.M.; Marques, L.S.; Martins, C.C.; Ramos-Jorge, M.L.; Ramos-Jorge, J., Association between malocclusion and dental caries in adolescents: A systematic review and meta-analysis. Eur. Arch. Paediatr. Dent. 2018, 19, 73-82. https://doi.org/10.1007/s40368-018-0333-0. [CrossRef]
- Alsulaiman, A.A.; Kaye, E.; Jones, J.; Cabral, H.; Leone, C.; Will, L.; Garcia, R., Incisor malalignment and the risk of periodontal disease progression. American journal of orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics 2018, 153, 512-522. https://doi.org/10.1016/j.ajodo.2017.08.015. [CrossRef]
- Yamaguchi, M.; Fukasawa, S., Is inflammation a friend or foe for orthodontic treatment?: Inflammation in orthodontically induced inflammatory root resorption and accelerating tooth movement. International journal of molecular sciences 2021, 22. https://doi.org/10.3390/ijms22052388. [CrossRef]
- Weltman, B.; Vig, K.W.; Fields, H.W.; Shanker, S.; Kaizar, E.E., Root resorption associated with orthodontic tooth movement: A systematic review. American journal of orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics 2010, 137, 462-476; discussion 412A. https://doi.org/10.1016/j.ajodo.2009.06.021. [CrossRef]
- Wishney, M., Potential risks of orthodontic therapy: A critical review and conceptual framework. Australian dental journal 2017, 62 Suppl 1, 86-96. https://doi.org/10.1111/adj.12486. [CrossRef]
- Theodorou, C.I.; Kuijpers-Jagtman, A.M.; Bronkhorst, E.M.; Wagener, F., Optimal force magnitude for bodily orthodontic tooth movement with fixed appliances: A systematic review. American journal of orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics 2019, 156, 582-592. https://doi.org/10.1016/j.ajodo.2019.05.011. [CrossRef]
- Talic, N.F., Adverse effects of orthodontic treatment: A clinical perspective. The Saudi dental journal 2011, 23, 55-59. https://doi.org/10.1016/j.sdentj.2011.01.003. [CrossRef]
- Parcianello, R.G.; Amerio, E.; Giner Tarrida, L.; Nart, J.; Flores Mir, C.; Puigdollers Perez, A., Local hormones and growth factors to enhance orthodontic tooth movement: A systematic review of animal studies. Orthodontics & craniofacial research 2022, 25, 281-303. https://doi.org/10.1111/ocr.12544. [CrossRef]
- Andrade Jr, I.; Taddei, S.R.A.; Souza, P.E.A., Inflammation and tooth movement: The role of cytokines, chemokines, and growth factors. Semin. Orthod. 2012, 18, 257-269. https://doi.org/10.1053/j.sodo.2012.06.004. [CrossRef]
- Nanci, A.; Bosshardt, D.D., Structure of periodontal tissues in health and disease. Periodontol 2000 2006, 40, 11-28. https://doi.org/10.1111/j.1600-0757.2005.00141.x. [CrossRef]
- Basdra, E.K.; Komposch, G., Osteoblast-like properties of human periodontal ligament cells: An in vitro analysis. Eur J Orthod 1997, 19, 615-621. https://doi.org/10.1093/ejo/19.6.615. [CrossRef]
- Somerman, M.J.; Young, M.F.; Foster, R.A.; Moehring, J.M.; Imm, G.; Sauk, J.J., Characteristics of human periodontal ligament cells in vitro. Archives of oral biology 1990, 35, 241-247. https://doi.org/10.1016/0003-9969(90)90062-f. [CrossRef]
- Arceo, N.; Sauk, J.J.; Moehring, J.; Foster, R.A.; Somerman, M.J., Human periodontal cells initiate mineral-like nodules in vitro. Journal of periodontology 1991, 62, 499-503. https://doi.org/10.1902/jop.1991.62.8.499. [CrossRef]
- Li, M.; Zhang, C.; Yang, Y., Effects of mechanical forces on osteogenesis and osteoclastogenesis in human periodontal ligament fibroblasts: A systematic review of in vitro studies. Bone & joint research 2019, 8, 19-31. https://doi.org/10.1302/2046-3758.81.BJR-2018-0060.R1. [CrossRef]
- Garlet, T.P.; Coelho, U.; Silva, J.S.; Garlet, G.P., Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. European journal of oral sciences 2007, 115, 355-362. https://doi.org/10.1111/j.1600-0722.2007.00469.x. [CrossRef]
- Howard, P.S.; Kucich, U.; Taliwal, R.; Korostoff, J.M., Mechanical forces alter extracellular matrix synthesis by human periodontal ligament fibroblasts. J Periodontal Res 1998, 33, 500-508. https://doi.org/10.1111/j.1600-0765.1998.tb02350.x. [CrossRef]
- Okamoto, K.; Nakashima, T.; Shinohara, M.; Negishi-Koga, T.; Komatsu, N.; Terashima, A.; Sawa, S.; Nitta, T.; Takayanagi, H., Osteoimmunology: The conceptual framework unifying the immune and skeletal systems. Physiol. Rev. 2017, 97, 1295-1349. https://doi.org/10.1152/physrev.00036.2016. [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S., Rankl/rank/opg pathway: A mechanism involved in exercise-induced bone remodeling. BioMed research international 2020, 2020, 6910312. https://doi.org/10.1155/2020/6910312. [CrossRef]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T., Rankl biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. https://doi.org/10.1186/s41232-019-0111-3. [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L., Osteoclast differentiation and activation. Nature 2003, 423, 337-342. https://doi.org/10.1038/nature01658. [CrossRef]
- Tyrovola, J.B.; Spyropoulos, M.N.; Makou, M.; Perrea, D., Root resorption and the opg/rankl/rank system: A mini review. Journal of oral science 2008, 50, 367-376. https://doi.org/10.2334/josnusd.50.367. [CrossRef]
- Zhang, L.; Ding, Y.; Rao, G.Z.; Miao, D., Effects of il-10 and glucose on expression of opg and rankl in human periodontal ligament fibroblasts. Braz. J. Med. Biol. Res. 2016, 49, e4324. https://doi.org/10.1590/1414-431X20154324. [CrossRef]
- Yan, T.; Xie, Y.; He, H.; Fan, W.; Huang, F., Role of nitric oxide in orthodontic tooth movement (review). International journal of molecular medicine 2021, 48. https://doi.org/10.3892/ijmm.2021.5001. [CrossRef]
- Tang, N.; Zhao, Z.; Zhang, L.; Yu, Q.; Li, J.; Xu, Z.; Li, X., Up-regulated osteogenic transcription factors during early response of human periodontal ligament stem cells to cyclic tensile strain. Arch. Med. Sci. 2012, 8, 422-430. https://doi.org/10.5114/aoms.2012.28810. [CrossRef]
- Shen, T.; Qiu, L.; Chang, H.; Yang, Y.; Jian, C.; Xiong, J.; Zhou, J.; Dong, S., Cyclic tension promotes osteogenic differentiation in human periodontal ligament stem cells. Int. J. Clin. Exp. Pathol. 2014, 7, 7872-7880 DOI.
- Li, Y.; Jacox, L.A.; Little, S.H.; Ko, C.C., Orthodontic tooth movement: The biology and clinical implications. The Kaohsiung journal of medical sciences 2018, 34, 207-214. https://doi.org/10.1016/j.kjms.2018.01.007. [CrossRef]
- Ullrich, N.; Schroder, A.; Jantsch, J.; Spanier, G.; Proff, P.; Kirschneck, C., The role of mechanotransduction versus hypoxia during simulated orthodontic compressive strain-an in vitro study of human periodontal ligament fibroblasts. International journal of oral science 2019, 11, 33. https://doi.org/10.1038/s41368-019-0066-x. [CrossRef]
- Vansant, L.; Cadenas De Llano-Perula, M.; Verdonck, A.; Willems, G., Expression of biological mediators during orthodontic tooth movement: A systematic review. Archives of oral biology 2018, 95, 170-186. https://doi.org/10.1016/j.archoralbio.2018.08.003. [CrossRef]
- Brooks, P.J.; Nilforoushan, D.; Manolson, M.F.; Simmons, C.A.; Gong, S.G., Molecular markers of early orthodontic tooth movement. The Angle orthodontist 2009, 79, 1108-1113. https://doi.org/10.2319/121508-638R.1. [CrossRef]
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K., et al., Mic-1, a novel macrophage inhibitory cytokine, is a divergent member of the tgf-beta superfamily. Proceedings of the National Academy of Sciences of the United States of America 1997, 94, 11514-11519. https://doi.org/10.1073/pnas.94.21.11514. [CrossRef]
- Assadi, A.; Zahabi, A.; Hart, R.A., Gdf15, an update of the physiological and pathological roles it plays: A review. Pflugers Arch. 2020, 472, 1535-1546. https://doi.org/10.1007/s00424-020-02459-1. [CrossRef]
- Symmank, J.; Zimmermann, S.; Goldschmitt, J.; Schiegnitz, E.; Wolf, M.; Wehrbein, H.; Jacobs, C., Mechanically-induced gdf15 secretion by periodontal ligament fibroblasts regulates osteogenic transcription. Scientific reports 2019, 9, 11516. https://doi.org/10.1038/s41598-019-47639-x. [CrossRef]
- Stemmler, A.; Symmank, J.; Steinmetz, J.; von Brandenstein, K.; Hennig, C.L.; Jacobs, C., Gdf15 supports the inflammatory response of pdl fibroblasts stimulated by p. Gingivalis lps and concurrent compression. International journal of molecular sciences 2021, 22. https://doi.org/10.3390/ijms222413608. [CrossRef]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jorgensen, S.B.; Steinberg, G.R., Gdf15: Emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nature reviews. Endocrinology 2021, 17, 592-607. https://doi.org/10.1038/s41574-021-00529-7. [CrossRef]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C., Insights into mechanisms of gdf15 and receptor gfral: Therapeutic targets. Trends in endocrinology and metabolism: TEM 2020, 31, 939-951. https://doi.org/10.1016/j.tem.2020.10.004. [CrossRef]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D., et al., Gfral is the receptor for gdf15 and the ligand promotes weight loss in mice and nonhuman primates. Nature medicine 2017, 23, 1150-1157. https://doi.org/10.1038/nm.4392. [CrossRef]
- Wu, Q.; Jiang, D.; Matsuda, J.L.; Ternyak, K.; Zhang, B.; Chu, H.W., Cigarette smoke induces human airway epithelial senescence via growth differentiation factor 15 production. Am. J. Respir. Cell Mol. Biol. 2016, 55, 429-438. https://doi.org/10.1165/rcmb.2015-0143OC. [CrossRef]
- Lin, W.; Zhang, W.W.; Lyu, N.; Cao, H.; Xu, W.D.; Zhang, Y.Q., Growth differentiation factor-15 produces analgesia by inhibiting tetrodotoxin-resistant nav1.8 sodium channel activity in rat primary sensory neurons. Neurosci. Bull. 2021, 37, 1289-1302. https://doi.org/10.1007/s12264-021-00709-5. [CrossRef]
- Artz, A.; Butz, S.; Vestweber, D., Gdf-15 inhibits integrin activation and mouse neutrophil recruitment through the alk-5/tgf-betarii heterodimer. Blood 2016, 128, 529-541. https://doi.org/10.1182/blood-2016-01-696617. [CrossRef]
- Min, K.W.; Liggett, J.L.; Silva, G.; Wu, W.W.; Wang, R.; Shen, R.F.; Eling, T.E.; Baek, S.J., Nag-1/gdf15 accumulates in the nucleus and modulates transcriptional regulation of the smad pathway. Oncogene 2016, 35, 377-388. https://doi.org/10.1038/onc.2015.95. [CrossRef]
- Lee, J.; Kim, I.; Yoo, E.; Baek, S.J., Competitive inhibition by nag-1/gdf-15 nls peptide enhances its anti-cancer activity. Biochemical and biophysical research communications 2019, 519, 29-34. https://doi.org/10.1016/j.bbrc.2019.08.090. [CrossRef]
- Wischhusen, J.; Melero, I.; Fridman, W.H., Growth/differentiation factor-15 (gdf-15): From biomarker to novel targetable immune checkpoint. Frontiers in immunology 2020, 11, 951. https://doi.org/10.3389/fimmu.2020.00951. [CrossRef]
- Liu, H.; Huang, Y.; Lyu, Y.; Dai, W.; Tong, Y.; Li, Y., Gdf15 as a biomarker of ageing. Experimental gerontology 2021, 146, 111228. https://doi.org/10.1016/j.exger.2021.111228. [CrossRef]
- Li, S.; Li, Q.; Zhu, Y.; Hu, W., Gdf15 induced by compressive force contributes to osteoclast differentiation in human periodontal ligament cells. Experimental cell research 2020, 387, 111745. https://doi.org/10.1016/j.yexcr.2019.111745. [CrossRef]
- Westhrin, M.; Moen, S.H.; Holien, T.; Mylin, A.K.; Heickendorff, L.; Olsen, O.E.; Sundan, A.; Turesson, I.; Gimsing, P.; Waage, A., et al., Growth differentiation factor 15 (gdf15) promotes osteoclast differentiation and inhibits osteoblast differentiation and high serum gdf15 levels are associated with multiple myeloma bone disease. Haematologica 2015, 100, e511-514. https://doi.org/10.3324/haematol.2015.124511. [CrossRef]
- Siddiqui, J.A.; Seshacharyulu, P.; Muniyan, S.; Pothuraju, R.; Khan, P.; Vengoji, R.; Chaudhary, S.; Maurya, S.K.; Lele, S.M.; Jain, M., et al., Gdf15 promotes prostate cancer bone metastasis and colonization through osteoblastic ccl2 and rankl activation. Bone research 2022, 10, 6. https://doi.org/10.1038/s41413-021-00178-6. [CrossRef]
- Park, H.; Kim, C.H.; Jeong, J.H.; Park, M.; Kim, K.S., Gdf15 contributes to radiation-induced senescence through the ros-mediated p16 pathway in human endothelial cells. Oncotarget 2016, 7, 9634-9644. https://doi.org/10.18632/oncotarget.7457. [CrossRef]
- Uchiyama, T.; Kawabata, H.; Miura, Y.; Yoshioka, S.; Iwasa, M.; Yao, H.; Sakamoto, S.; Fujimoto, M.; Haga, H.; Kadowaki, N., et al., The role of growth differentiation factor 15 in the pathogenesis of primary myelofibrosis. Cancer medicine 2015, 4, 1558-1572. https://doi.org/10.1002/cam4.502. [CrossRef]
- Langenbach, F.; Handschel, J., Effects of dexamethasone, ascorbic acid and beta-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell. Res. Ther. 2013, 4, 117. https://doi.org/10.1186/scrt328. [CrossRef]
- Long, P.; Hu, J.; Piesco, N.; Buckley, M.; Agarwal, S., Low magnitude of tensile strain inhibits il-1beta-dependent induction of pro-inflammatory cytokines and induces synthesis of il-10 in human periodontal ligament cells in vitro. Journal of dental research 2001, 80, 1416-1420. https://doi.org/10.1177/00220345010800050601. [CrossRef]
- Li, Z.H.; Si, Y.; Xu, G.; Chen, X.M.; Xiong, H.; Lai, L.; Zheng, Y.Q.; Zhang, Z.G., High-dose pma with rankl and mcsf induces thp1 cell differentiation into human functional osteoclasts in vitro. Molecular medicine reports 2017, 16, 8380-8384. https://doi.org/10.3892/mmr.2017.7625. [CrossRef]
- Asiry, M.A., Biological aspects of orthodontic tooth movement: A review of literature. Saudi J. Biol. Sci. 2018, 25, 1027-1032. https://doi.org/10.1016/j.sjbs.2018.03.008. [CrossRef]
- Sarkar, S.; Legere, S.; Haidl, I.; Marshall, J.; MacLeod, J.B.; Aguiar, C.; Lutchmedial, S.; Hassan, A.; Brunt, K.R.; Kienesberger, P., et al., Serum gdf15, a promising biomarker in obese patients undergoing heart surgery. Frontiers in cardiovascular medicine 2020, 7, 103. https://doi.org/10.3389/fcvm.2020.00103. [CrossRef]
- Kim, Y.I.; Shin, H.W.; Chun, Y.S.; Park, J.W., Cst3 and gdf15 ameliorate renal fibrosis by inhibiting fibroblast growth and activation. Biochemical and biophysical research communications 2018, 500, 288-295. https://doi.org/10.1016/j.bbrc.2018.04.061. [CrossRef]
- Kim, Y.I.; Shin, H.W.; Chun, Y.S.; Cho, C.H.; Koh, J.; Chung, D.H.; Park, J.W., Epithelial cell-derived cytokines cst3 and gdf15 as potential therapeutics for pulmonary fibrosis. Cell death & disease 2018, 9, 506. https://doi.org/10.1038/s41419-018-0530-0. [CrossRef]
- Guo, H.; Zhao, X.; Li, H.; Liu, K.; Jiang, H.; Zeng, X.; Chang, J.; Ma, C.; Fu, Z.; Lv, X., et al., Gdf15 promotes cardiac fibrosis and proliferation of cardiac fibroblasts via the mapk/erk1/2 pathway after irradiation in rats. Radiat. Res. 2021, 196, 183-191. https://doi.org/10.1667/RADE-20-00206.1. [CrossRef]
- Conte, M.; Giuliani, C.; Chiariello, A.; Iannuzzi, V.; Franceschi, C.; Salvioli, S., Gdf15, an emerging key player in human aging. Ageing research reviews 2022, 75, 101569. https://doi.org/10.1016/j.arr.2022.101569. [CrossRef]
- Li, S.; Ma, Y.M.; Zheng, P.S.; Zhang, P., Gdf15 promotes the proliferation of cervical cancer cells by phosphorylating akt1 and erk1/2 through the receptor erbb2. J. Exp. Clin. Cancer Res. 2018, 37, 80. https://doi.org/10.1186/s13046-018-0744-0. [CrossRef]
- Wu, M.; Chen, G.; Li, Y.P., Tgf-beta and bmp signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone research 2016, 4, 16009. https://doi.org/10.1038/boneres.2016.9. [CrossRef]
- Quinn, J.M.; Itoh, K.; Udagawa, N.; Hausler, K.; Yasuda, H.; Shima, N.; Mizuno, A.; Higashio, K.; Takahashi, N.; Suda, T., et al., Transforming growth factor beta affects osteoclast differentiation via direct and indirect actions. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2001, 16, 1787-1794. https://doi.org/10.1359/jbmr.2001.16.10.1787. [CrossRef]
- Wakchoure, S.; Swain, T.M.; Hentunen, T.A.; Bauskin, A.R.; Brown, D.A.; Breit, S.N.; Vuopala, K.S.; Harris, K.W.; Selander, K.S., Expression of macrophage inhibitory cytokine-1 in prostate cancer bone metastases induces osteoclast activation and weight loss. Prostate 2009, 69, 652-661. https://doi.org/10.1002/pros.20913. [CrossRef]
- Tsai, V.W.W.; Husaini, Y.; Sainsbury, A.; Brown, D.A.; Breit, S.N., The mic-1/gdf15-gfral pathway in energy homeostasis: Implications for obesity, cachexia, and other associated diseases. Cell metabolism 2018, 28, 353-368. https://doi.org/10.1016/j.cmet.2018.07.018. [CrossRef]
- Conte, M.; Ostan, R.; Fabbri, C.; Santoro, A.; Guidarelli, G.; Vitale, G.; Mari, D.; Sevini, F.; Capri, M.; Sandri, M., et al., Human aging and longevity are characterized by high levels of mitokines. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 600-607. https://doi.org/10.1093/gerona/gly153. [CrossRef]
- Andersson-Hall, U.; Svedin, P.; Mallard, C.; Blennow, K.; Zetterberg, H.; Holmang, A., Growth differentiation factor 15 increases in both cerebrospinal fluid and serum during pregnancy. PloS one 2021, 16, e0248980. https://doi.org/10.1371/journal.pone.0248980. [CrossRef]
- Klein, A.B.; Nicolaisen, T.S.; Ortenblad, N.; Gejl, K.D.; Jensen, R.; Fritzen, A.M.; Larsen, E.L.; Karstoft, K.; Poulsen, H.E.; Morville, T., et al., Pharmacological but not physiological gdf15 suppresses feeding and the motivation to exercise. Nature communications 2021, 12, 1041. https://doi.org/10.1038/s41467-021-21309-x. [CrossRef]
- Kim, Y.; Noren Hooten, N.; Evans, M.K., Crp stimulates gdf15 expression in endothelial cells through p53. Mediators of inflammation 2018, 2018, 8278039. https://doi.org/10.1155/2018/8278039. [CrossRef]
- Nishijima, Y.; Yamaguchi, M.; Kojima, T.; Aihara, N.; Nakajima, R.; Kasai, K., Levels of rankl and opg in gingival crevicular fluid during orthodontic tooth movement and effect of compression force on releases from periodontal ligament cells in vitro. Orthodontics & craniofacial research 2006, 9, 63-70. https://doi.org/10.1111/j.1601-6343.2006.00340.x. [CrossRef]
- Yamaguchi, M.; Aihara, N.; Kojima, T.; Kasai, K., Rankl increase in compressed periodontal ligament cells from root resorption. Journal of dental research 2006, 85, 751-756. https://doi.org/10.1177/154405910608500812. [CrossRef]
- Oshiro, T.; Shiotani, A.; Shibasaki, Y.; Sasaki, T., Osteoclast induction in periodontal tissue during experimental movement of incisors in osteoprotegerin-deficient mice. Anat. Rec. 2002, 266, 218-225. https://doi.org/10.1002/ar.10061. [CrossRef]
- Bottner, M.; Laaff, M.; Schechinger, B.; Rappold, G.; Unsicker, K.; Suter-Crazzolara, C., Characterization of the rat, mouse, and human genes of growth/differentiation factor-15/macrophage inhibiting cytokine-1 (gdf-15/mic-1). Gene 1999, 237, 105-111. https://doi.org/10.1016/s0378-1119(99)00309-1. [CrossRef]
- Kirschneck, C.; Batschkus, S.; Proff, P.; Kostler, J.; Spanier, G.; Schroder, A., Valid gene expression normalization by rt-qpcr in studies on hpdl fibroblasts with focus on orthodontic tooth movement and periodontitis. Scientific reports 2017, 7, 14751. https://doi.org/10.1038/s41598-017-15281-0. [CrossRef]
- Symmank, J.; Appel, S.; Bastian, J.A.; Knaup, I.; Marciniak, J.; Hennig, C.L.; Doding, A.; Schulze-Spate, U.; Jacobs, C.; Wolf, M., Hyperlipidemic conditions impact force-induced inflammatory response of human periodontal ligament fibroblasts concomitantly challenged with p. Gingivalis-lps. International journal of molecular sciences 2021, 22. https://doi.org/10.3390/ijms22116069. [CrossRef]
- Nazet, U.; Schroder, A.; Spanier, G.; Wolf, M.; Proff, P.; Kirschneck, C., Simplified method for applying static isotropic tensile strain in cell culture experiments with identification of valid rt-qpcr reference genes for pdl fibroblasts. Eur J Orthod 2020, 42, 359-370. https://doi.org/10.1093/ejo/cjz052. [CrossRef]
- Schuldt, L.; Reimann, M.; von Brandenstein, K.; Steinmetz, J.; Doding, A.; Schulze-Spate, U.; Jacobs, C.; Symmank, J., Palmitate-triggered cox2/pge2-related hyperinflammation in dual-stressed pdl fibroblasts is mediated by repressive h3k27 trimethylation. Cells 2022, 11. https://doi.org/10.3390/cells11060955. [CrossRef]
- Symmank, J.; Bayer, C.; Schmidt, C.; Hahn, A.; Pensold, D.; Zimmer-Bensch, G., Dnmt1 modulates interneuron morphology by regulating pak6 expression through crosstalk with histone modifications. Epigenetics 2018, 13, 536-556. https://doi.org/10.1080/15592294.2018.1475980. [CrossRef]
- Symmank, J.; Chorus, M.; Appel, S.; Marciniak, J.; Knaup, I.; Bastian, A.; Hennig, C.L.; Doding, A.; Schulze-Spate, U.; Jacobs, C., et al., Distinguish fatty acids impact survival, differentiation and cellular function of periodontal ligament fibroblasts. Scientific reports 2020, 10, 15706. https://doi.org/10.1038/s41598-020-72736-7. [CrossRef]
- Schuldt, L.; von Brandenstein, K.; Jacobs, C.; Symmank, J., Oleic acid-related anti-inflammatory effects in force-stressed pdl fibroblasts are mediated by h3 lysine acetylation associated with altered il10 expression. Epigenetics 2022, 1-13. https://doi.org/10.1080/15592294.2022.2090654. [CrossRef]
| Gene | Gene Symbol | NCBI Gene ID | Primer Sequence | Length |
|---|---|---|---|---|
| Activin A receptor like type 1 |
ACVRL1 (alias ALK1) |
94 | fw: GTGGAGTGTGTGGGAAAAGG rev: CATGTCTGAGGCGATGAAGC |
180 bp |
| Activin A receptor type 1 |
ACVR1 (alias ALK2) |
90 | fw: GCATTCCCAGAGCACCAATC rev: GGCCACTTCCCACAAAACAA |
166 bp |
| Activin A receptor type 1B |
ACVR1B (alias ALK4) |
91 | fw: TTAGTGCCCTCTGACCCTTC rev: ATCATCTTCCCCATCACCCG |
122 bp |
| Activin A receptor type 1C |
ACVR1C (alias ALK7) |
130399 | fw: TGTTGGTCTGGTTTACTGGGA rev: TGCTTCACAACTTTGCCACT |
181 bp |
| Alkaline Phosphatase | ALPL | 249 | fw: ACTGCAGACATTCTCAAA rev: GAGTGAGTGAGTGAGCA |
190 bp |
| Bone morphogenetic protein receptor type 1A | BMPR1A | 657 | fw: ACCACTTCCAGCCCTACATC rev: TTGACACACACAACCTCACG |
172 bp |
| Bone morphogenetic protein receptor type 1B |
BMPR1B (alias ALK6) |
658 | fw: GCATCAAGAAGTTACGCCCC rev: TGGGACTCTGACATTTTGGC |
160 bp |
| Interleukin 6 | IL6 | 3569 | fw: CATCCTCGACGGCATCTCAG rev: TCACCAGGCAAGTCTCCTCA |
164 bp |
| Interleukin 10 | IL10 | 3586 | fw: AGCCATGAGTGAGTTTGACA rev: AGAGCCCCAGATCCGATTTT |
141 bp |
| Interleukin 1 receptor antagonist | IL1RN | 3557 | fw: GATGTGCCTGTCCTGTGTCA rev: ACTCAAAACTGGTGGTGGGG |
146 bp |
| Mitochondrially encoded cytochrome c oxidase II |
MT-COX2 (alias COX2) |
4513 | fw: GATGATTGCCCGACTCCCTT rev: GGCCCTCGCTTATGATCTGT |
185 bp |
| Ribosomal protein L22 | RPL22 | 6146 | fw: TGATTGCACCCACCCTGTAG rev: GGTTCCCAGCTTTTCCGT TC |
98 bp |
| RUNX family transcription factor 2 | RUNX2 | 6146 | fw: CCCACGAATGCACTATCC rev: GGACATACCGAGGGACA |
120 bp |
| TATA-box binding protein |
TBP | 6908 | fw: CGGCTGTTTAACTTCGCTTCC rev: TGGGTTATCTTCACACGCCAAG |
86 bp |
| TNF receptor superfamily member 11b |
TNFRSF11B (alias OPG) |
4982 | fw: GAAGGGCGCTACCTTGA | 142 bp |
| rev: GCAAACTGTATTTCGCTC | ||||
| TNF Superfamily Member 11 |
TNFSF11 (alias RANKL) |
8600 | fw: ATCACAGCACATCAGAGCAGA rev: TCACTTTATGGGAACCAGATGGG |
160 bp |
| Transforming growth factor beta receptor 1 |
TGFBR1 (alias ALK5) |
7046 | fw: AAAACTTGCTCTGTCCACGG rev: TGCCAGTCCTAAGTCTGCAA |
157 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





