Submitted:
05 May 2023
Posted:
08 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
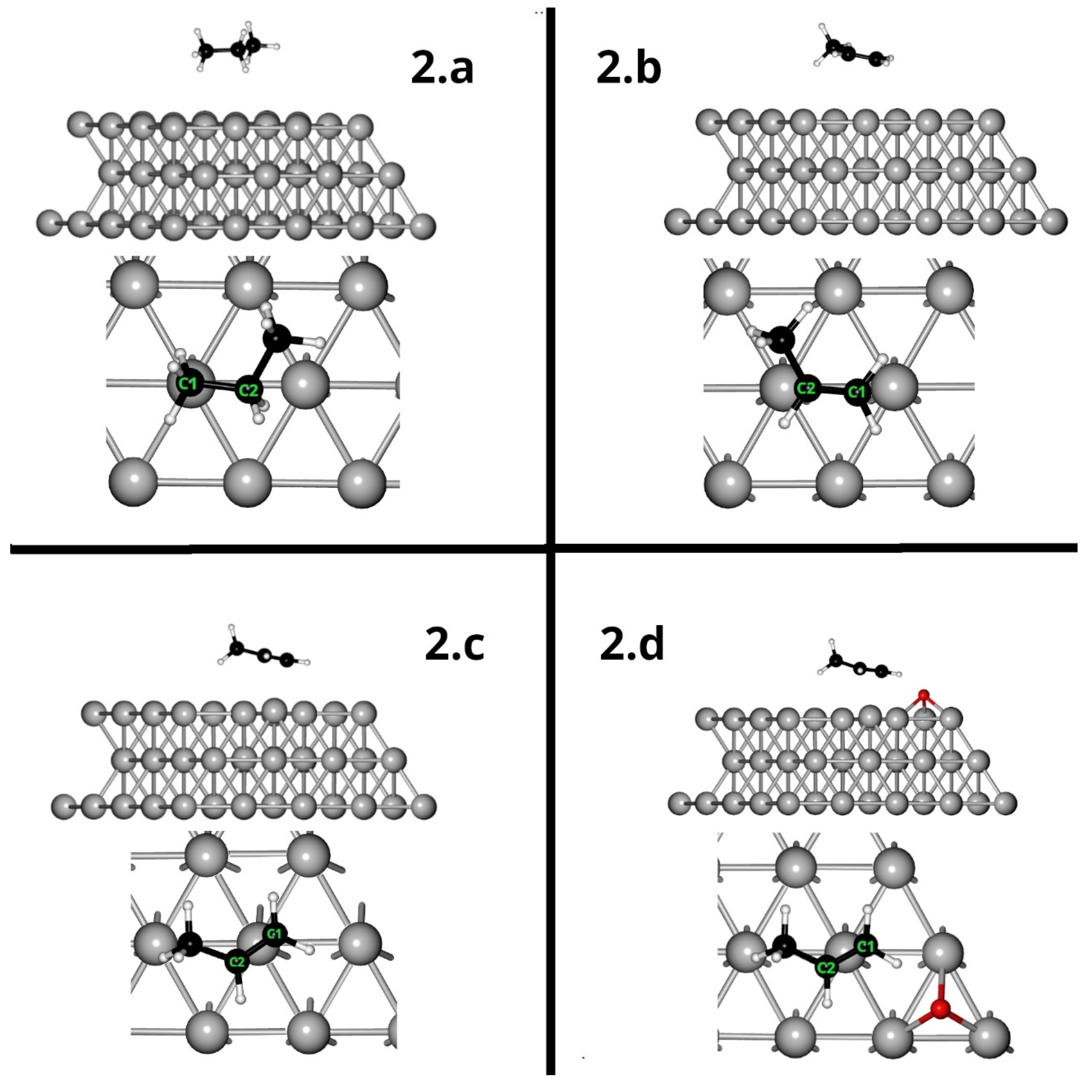
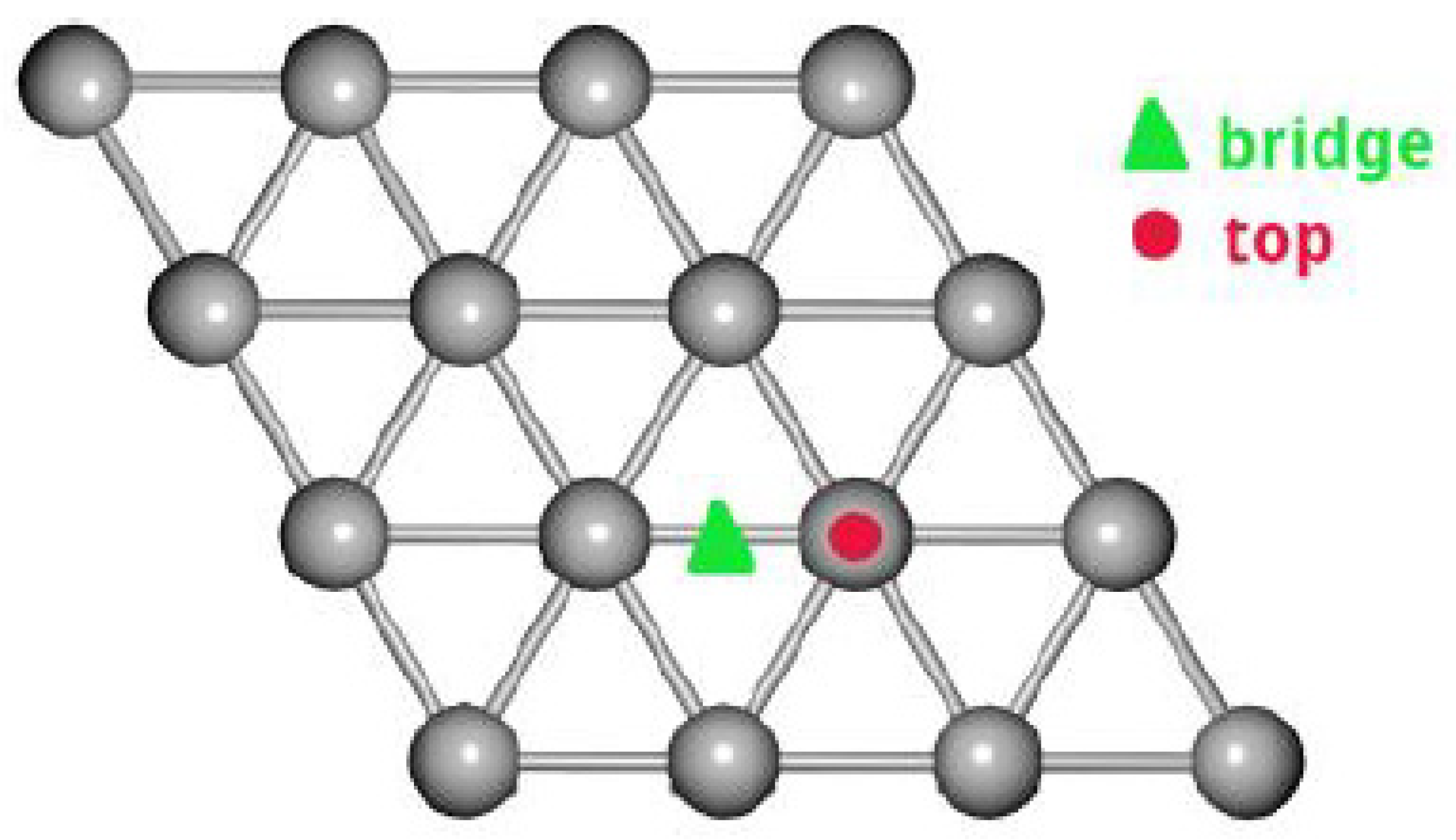
2.1. Adsorption on Ag (111)
2.2. Adsorption on Ag (111) in the Presence of Oxygen
4. Experimental
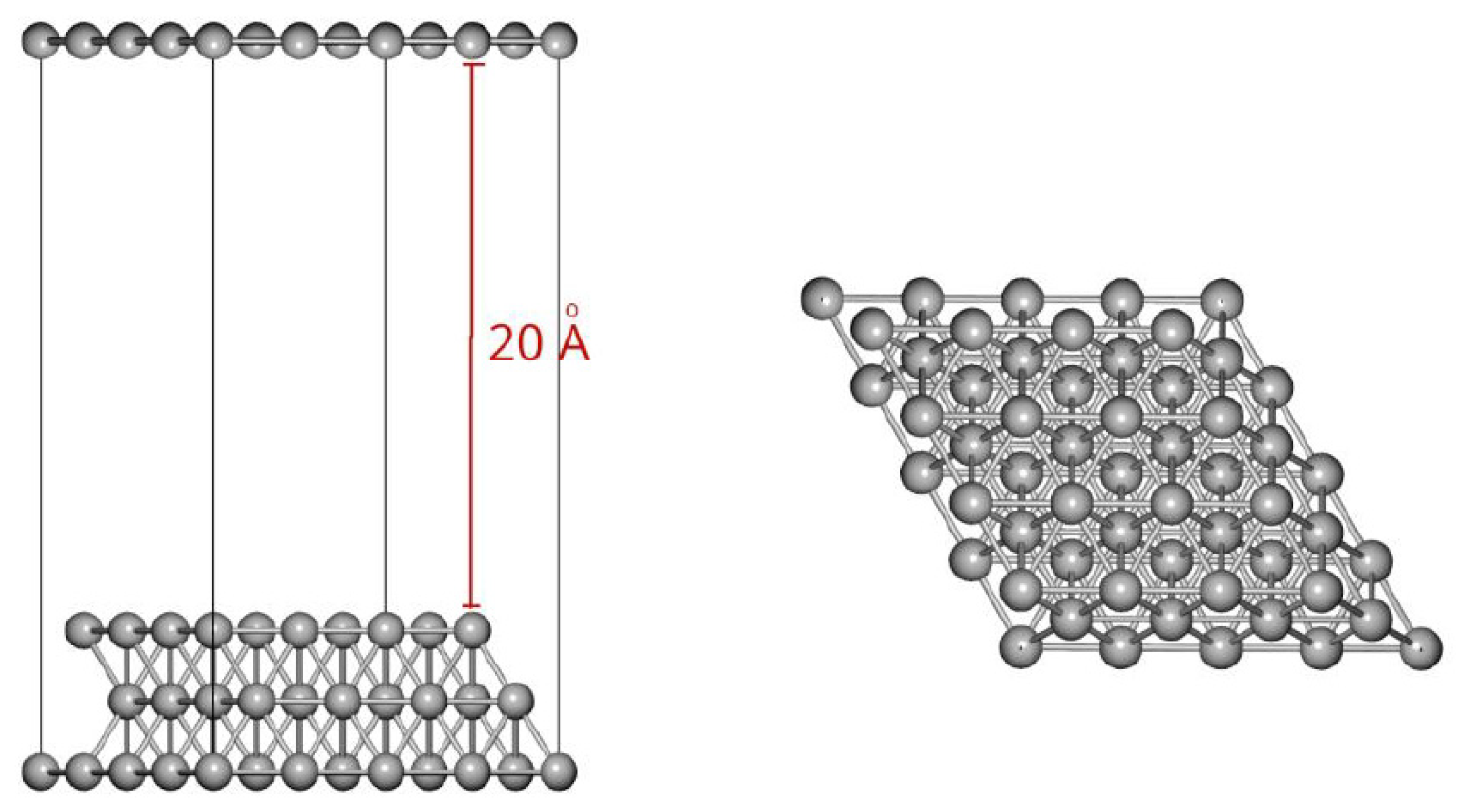
4.1. Computational Details
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Twigg, M.V. Progress and Future Challenges in Controlling Automotive Exhaust Gas Emissions. Applied Catalysis B: Environmental 2007, 70, 2–15. [CrossRef]
- Yang, A.-C.; Streibel, V.; Choksi, T.S.; Aljama, H.; Werghi, B.; Bare, S.R.; Sánchez-Carrera, R.S.; Schäfer, A.; Li, Y.; Abild-Pedersen, F.; et al. Insights and Comparison of Structure–Property Relationships in Propane and Propene Catalytic Combustion on Pd- and Pt-Based Catalysts. Journal of Catalysis 2021, 401, 89–101. [CrossRef]
- Choudhary, T.V.; Banerjee, S.; Choudhary, V.R. Catalysts for Combustion of Methane and Lower Alkanes. Applied Catalysis A: General 2002, 234, 1–23. [CrossRef]
- Shelef, M.; McCabe, R.W. Twenty-Five Years after Introduction of Automotive Catalysts: What Next? Catalysis Today 2000, 62, 35–50. [CrossRef]
- Stratakis, G.A.; Stamatelos, A.M. Thermogravimetric Analysis of Soot Emitted by a Modern Diesel Engine Run on Catalyst-Doped Fuel. Combustion and Flame 2003, 132, 157–169. [CrossRef]
- Liotta, L.F. Catalytic Oxidation of Volatile Organic Compounds on Supported Noble Metals. Applied Catalysis B: Environmental 2010, 100, 403–412. [CrossRef]
- Cortés-Reyes, M.; Herrera, C.; Larrubia, M.Á.; Alemany, L.J. Intrinsic Reactivity Analysis of Soot Removal in LNT-Catalysts. Applied Catalysis B: Environmental 2016, 193, 110–120. [CrossRef]
- Demoulin, O.; Le Clef, B.; Navez, M.; Ruiz, P. Combustion of Methane, Ethane and Propane and of Mixtures of Methane with Ethane or Propane on Pd/γ-Al2O3 Catalysts. Applied Catalysis A: General 2008, 344, 1–9. [CrossRef]
- Montaña, M.; Leguizamón Aparicio, M.S.; Ocsachoque, M.A.; Navas, M.B.; de C. L. Barros, I.; Rodriguez-Castellón, E.; Casella, M.L.; Lick, I.D. Zirconia-Supported Silver Nanoparticles for the Catalytic Combustion of Pollutants Originating from Mobile Sources. Catalysts 2019, 9, 297. [CrossRef]
- Avila, M.S.; Vignatti, C.I.; Apesteguía, C.R.; Garetto, T.F. Effect of Support on the Deep Oxidation of Propane and Propylene on Pt-Based Catalysts. Chemical Engineering Journal 2014, 241, 52–59. [CrossRef]
- Bratan, V.; Vasile, A.; Chesler, P.; Hornoiu, C. Insights into the Redox and Structural Properties of CoOx and MnOx: Fundamental Factors Affecting the Catalytic Performance in the Oxidation Process of VOCs. Catalysts 2022, 12, 1134. [CrossRef]
- Ertl, G. Reactions at Well-Defined Surfaces. Surface Science 1994, 299–300, 742–754. [CrossRef]
- Zhang, Z.; Jiang, Z.; Shangguan, W. Low-Temperature Catalysis for VOCs Removal in Technology and Application: A State-of-the-Art Review. Catalysis Today 2016, 264, 270–278. [CrossRef]
- Langmuir, I. Part II.—“Heterogeneous Reactions”. Chemical Reactions on Surfaces. Trans. Faraday Soc. 1922, 17, 607–620. [CrossRef]
- Huang, W.; Jiang, Z.; White, J.M. Distinct Oxidation Behaviors of π-Bonded and Di-σ-Bonded Propylene on Ag(111). Catalysis Today 2008, 131, 360–366. [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 2014, 114, 10613–10653. [CrossRef]
- Yang, M.-L.; Zhu, J.; Zhu, Y.-A.; Sui, Z.-J.; Yu, Y.-D.; Zhou, X.-G.; Chen, D. Tuning Selectivity and Stability in Propane Dehydrogenation by Shaping Pt Particles: A Combined Experimental and DFT Study. Journal of Molecular Catalysis A: Chemical 2014, 395, 329–336. [CrossRef]
- Arya, M.; Mirzaei, A.A.; Davarpanah, A.M.; Barakati, S.M.; Atashi, H.; Mohsenzadeh, A.; Bolton, K. DFT Studies of Hydrocarbon Combustion on Metal Surfaces. J Mol Model 2018, 24, 47. [CrossRef]
- Wang, X.; Rui, Z.; Ji, H. DFT Study of Formaldehyde Oxidation on Silver Cluster by Active Oxygen and Hydroxyl Groups: Mechanism Comparison and Synergistic Effect. Catalysis Today 2020, 347, 124–133. [CrossRef]
- Porter, W.N.; Lin, Z.; Chen, J.G. Experimental and Theoretical Studies of Reaction Pathways of Direct Propylene Epoxidation on Model Catalyst Surfaces. Surface Science Reports 2021, 76, 100524. [CrossRef]
- Burch, R.; Hayes, M.J. CH Bond Activation in Hydrocarbon Oxidation on Solid Catalysts. Journal of Molecular Catalysis A: Chemical 1995, 100, 13–33. [CrossRef]
- Tomita, A.; Miki, T.; Tai, Y. Effect of Water Treatment and Fe Doping on Pt Sintering and the Propane Oxidation Activity of Pt/Al2O3. Applied Catalysis A: General 2016, 522, 138–144. [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys.: Condens. Matter 2009, 21, 395502. [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced Capabilities for Materials Modelling with Quantum ESPRESSO. J. Phys.: Condens. Matter 2017, 29, 465901. [CrossRef]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burke, K. Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces. Phys. Rev. Lett. 2008, 100, 136406. [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [CrossRef]
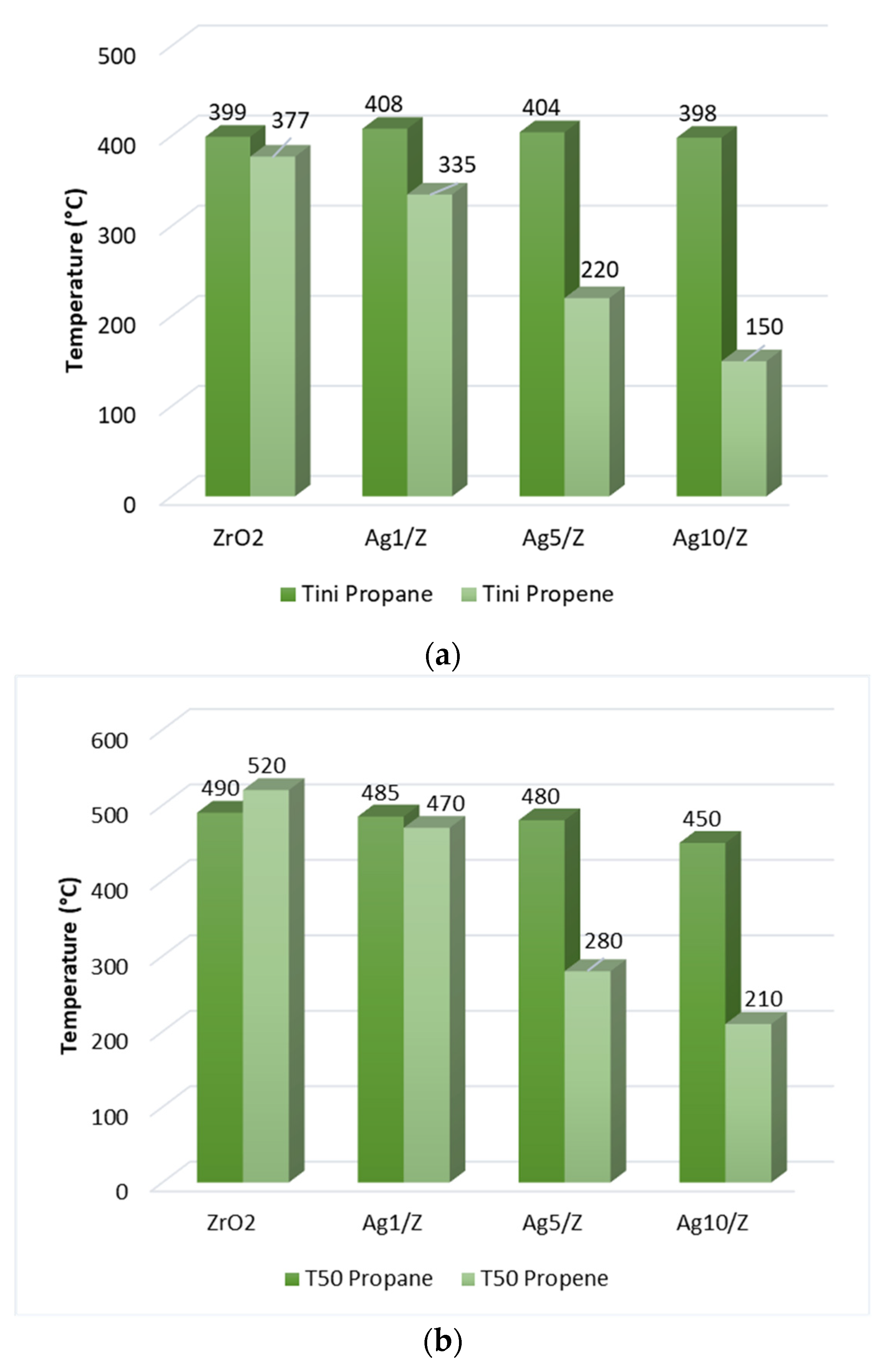
| Distances C1-Ag (Å) | Distances C2-Ag (Å) | Bond lentgh C1-C2 free (Å) | Bond lentgh C1-adsorbed C2 (Å) | Eads (eV) | |
|---|---|---|---|---|---|
| Propane | 3.57 | 3.87 | 1.52 | 1.52 | -0.14 |
| Propene bridge | 3.10 | 3.25 | 1.34 | 1.34 | -0.21 |
| Propene top | 2.58 | 2.74 | 1.34 | 1.35 | -0.77 |
| Propene O | 2.50 | 2.66 | 1.34 | 1.36 | -0.87 |
| Ag1/Z | Ag5/Z | Ag10/Z | |
|---|---|---|---|
| KE AgM4N45N45 | - | 357.5 | 357.9 |
| KE AgM5N45N45 | - | 352.5 (97)* 349.5 (3) |
352.8 (94) 349.4 (6) |
| Ag/Zr atomic ratio | 0.009 | 0.154 | 0.213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





