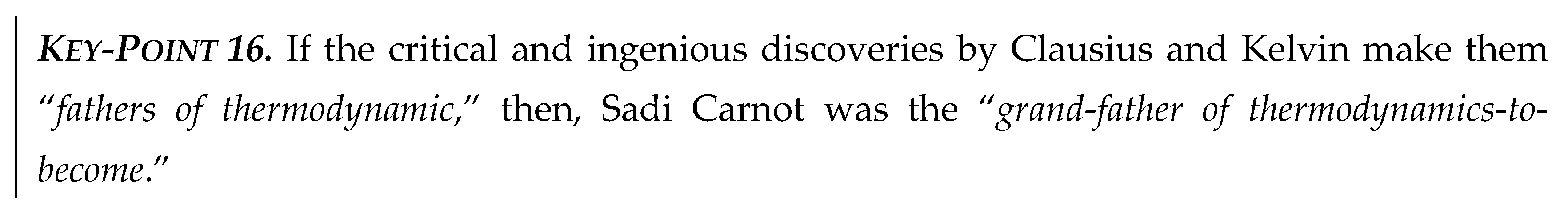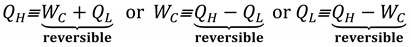1. Introduction
♦ ♦ ♦
“If your theory is found to be against the second law of thermodynamics, I give you no hope; there is nothing for it but to collapse in deepest humiliation.” – Arthur Eddington
Impasse: “Perhaps, after all, the wise man's attitude towards thermodynamics should be to have nothing to do with it. To deal with thermodynamics is to look for trouble.”
Anecdotal Laws of Thermodynamics (LT) [bracketed terms added]:♦[0LT]: You must play the game [equilibrium]. ♦[1LT]: You can't win [conservation]. ♦[2LT]: You can't break even [dissipation]. ♦[3LT]: You can't quit the game [0K impossible]. –Thermodynamics-WikiQuote
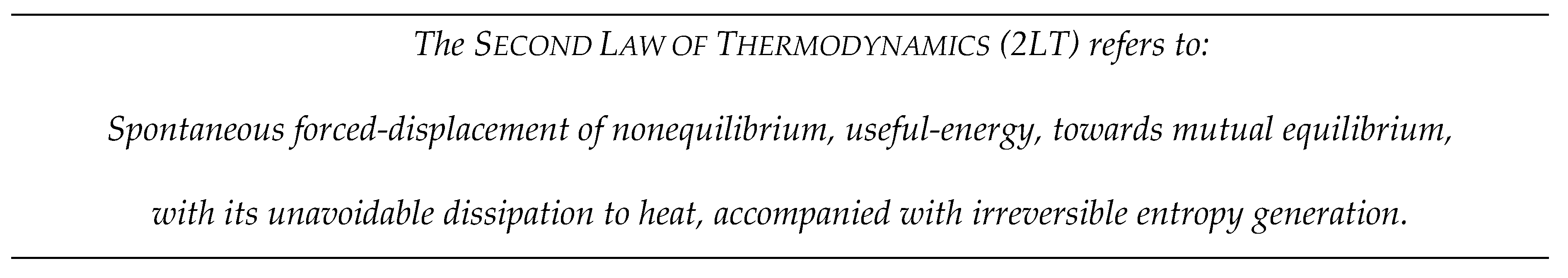
“The Second Law of thermodynamics can be challenged, but not violated - Entropy can be decreased, but not destroyed at any space or time scales. […] The self-forced tendency of displacing nonequilibrium useful-energy towards equilibrium, with its irreversible dissipation to heat, generates entropy, the latter is conserved in ideal, reversible processes, and there is no way to self-create useful-energy from within equilibrium alone, i.e., no way to destroy entropy.” –[2ndLaw.mkostic.com]
This treatise aims to present the lifelong endeavors and reflections, including additional, original reasoning and interpretations by this author, regarding the fundamental issues of
Thermodynamics and especially as related to the subtle
Second Law of Thermodynamics (2LT) [
1]. It is written for the
Special Issue of Entropy journal dedicated to this author’s 70
th Birthday [
2].
Science and technology have evolved over time on many scales and levels, so we now have the advantage to look at its historical developments more comprehensively and objectively than the pioneers. As Anthony Legget, a Nobel laureate, commented [
3], “Mathematical convenience versus physical insight […] that theorists are far too fond of fancy formalisms which are mathematically streamlined but whose connection with physics is at best at several removes […] heartfully agreed with Philippe Nozieres that ‘
only simple qualitative arguments can reveal the fundamental physics’.” In that regard, mostly physical and simple qualitative insights will be examined and emphasized here.
The goal has been to examine and scrutinize the ambiguous and challenging issues in thermal science and to present some novel contributions, with the hope to resolve a number of unsettled issues, and to encourage constructive criticism and collaboration for further progress. More specific and elaborate publications by this author and others are anticipated in the future.
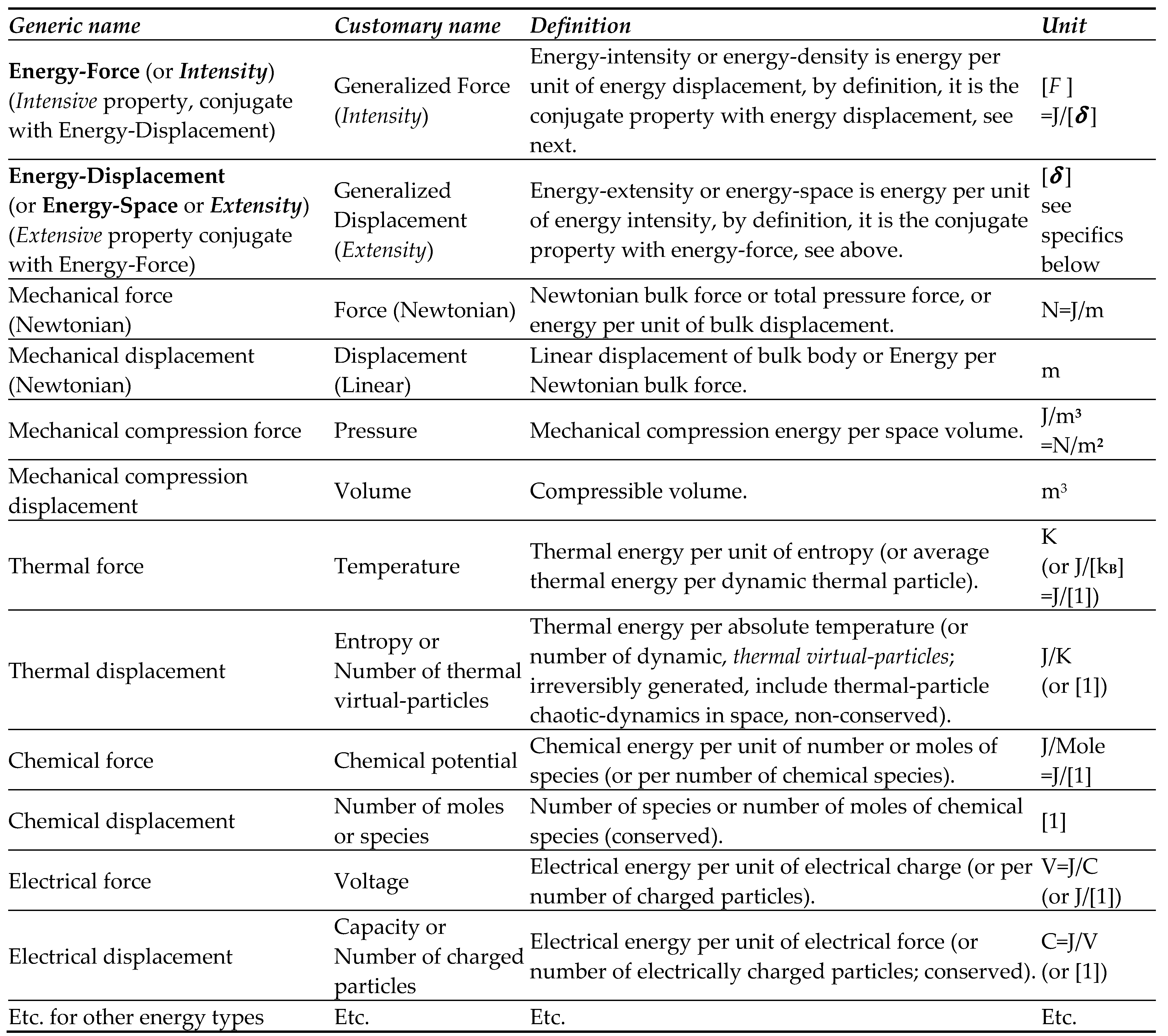
In addition to the original interpretations, the following, more specific and novel concepts are offered here: synergy of conjugate energy forcing-and-displacement; logical-proof of the fundamental laws; inevitable thermal-roughness concept; unfeasibility of entropy destruction; Carnot-Clausius heat-work equivalency; the impossibility of the 2LT violation at any space and time scale (for which ThD macro-properties are defined), without exception, among others.
The diverse and perplexing terminology and definitions (in different branches of science) contribute to further ambiguity and confusion, and sometimes misunderstanding. Due to the lack and inadequacy of specific scientific vocabulary, some thermodynamic terminology is emphasized and synergized here by uncommon connotations, by using “dashed-attributes” with the respective nouns, “quoting words” and similar, in order to emphasize thermodynamic-meaning, as distinction from the meaning of the common terminology. The selected assertions are emphasized throughout this treatise under “Key-Points” while questionable (deficient) statements and misrepresentations are underscored as “Challenge-Points” and “False-Points,” respectively.
Thermodynamics, as the science of energy and entropy, is the most fundamental discipline, and as such, it encompasses all existence in space and transformations in time, in nature. Due to the complexity of the diverse natural and artificial systems and processes, the fundamental laws often appear elusive and sometimes mystified. It is hoped that the logical reasoning presented here will contribute to improved comprehension of the fundamental concepts and related laws of thermodynamics and nature.
The fundamental Laws of Thermodynamics (LT) are the fundamental laws of nature, and they are considered to be axiomatic and experiential without proof, as never experienced otherwise, or as self-evident postulates. Due to the very complex micro- and macro-structures and their intricate interactions, it would be impossible to deterministically prove the Laws, but they could be reasoned logically, and their general validity inferred in principle, as it will be deduced here.
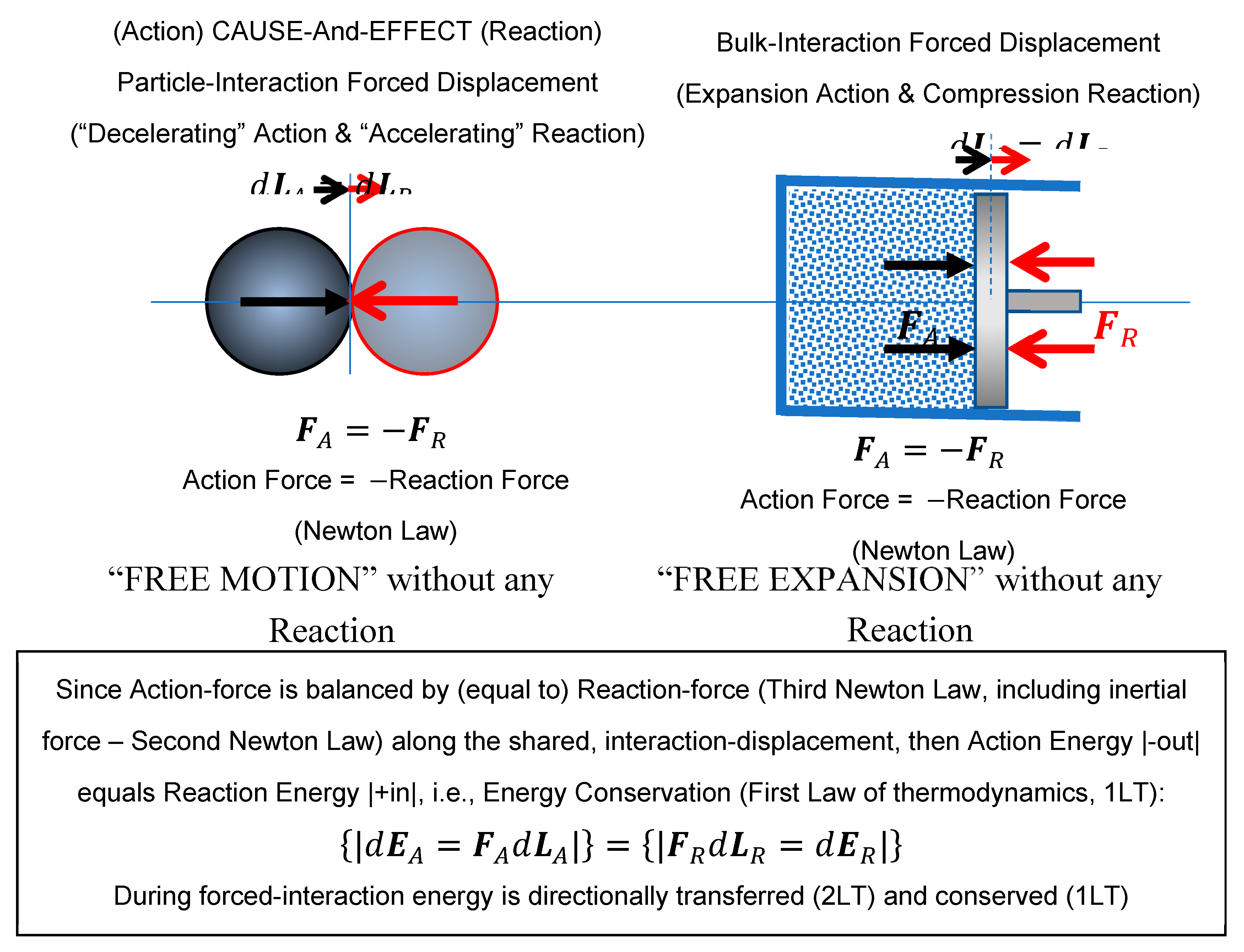
Since all existence is in principle mechanistic and physical, it will be demonstrated here that the LT are generalized extensions of the fundamental Newton’s Laws (NL) of mechanics. The First Law of Thermodynamics (1LT) is the generalized law of the conservation of energy, and the Second Law of Thermodynamics (2LT) describes the forcing tendency of nonequilibrium, useful-energy (or work-potential, WP) for its displacement and irreversible dissipation to heat with entropy generation, towards mutual equilibrium.
The content of this treatise is presented in several Sections. In the next Section
. 2, “
Energy forcing and displacement,” the related concepts are pondered. The “force or forcing” is nonequilibrium energy tendency to displace or redistribute (or to extend) from its higher to lower energy density (or
energy-intensity) towards mutual equilibrium with uniform properties. Then, the
Section 3, “
Reasoning logical-proof of the fundamental laws,” reveals the concept of energy displacement as the
mechanistic phenomenon in general, where the elementary particles (including “field-equivalent” particles) or bulk systems (consisting of elementary particles), mutually interact along shared displacement (with equal, respective action-reaction forces), thereby conserving energy during their interactive, mutual displacement. Then, in
Section 4, “
Ubiquity of thermal motion and heat, thermal roughness, and indestructability of entropy,” this author’s comprehension of related phenomena is further advanced by defining concept of “
thermal roughness” and reasoning impossibility of entropy destruction, among others. In
Section 5, “
Carnot maximum efficiency, reversible equivalency, and work potential,” the Sadi Carnot’s ground-breaking contributions of reversible processes and heat-engine cycles’ maximum-efficiency is put in historical and contemporary perspective, and it is argued that the Carnot’s contributions are among the
most important developments in natural sciences. In the succeeding
Section 6, named here “
Carnot-Clausius Heat-Work Equivalency” concept
, a notion of
true “heat-work interchangeability,” is articulated and named here, as an essential consequence of “
true” reversible equivalency. Lastly,
Section 7, “‛
No Hope’
for the Challengers of the Second Law of thermodynamics,” presents this author’s compelling arguments that “entropy can be reduced (locally, when heat is transferred out of a locality), but it cannot be destroyed by any means on any space or time scale of interest.” Entropy, as the “
final transformation” cannot be converted to anything else nor annihilated, but only transferred with heat and irreversibly generated with heat generation due to work dissipation, including Carnot “thermal work-potential” dissipation. Relevant conclusions are presented at the end.
Selected Abbreviations and Notes (in logical order for usage convenience):
ThD: Thermodynamics (Carnot’s concept of “maximum power [work] from heat” in 1824, name coined in 1854 by William Thompson, named Lord Kelvin).
ThM: Thermal motion (“vis-viva”: Lomonosov 1738, Count Rumford (Benjamin Thompson) 1798, Brown 1827, Clausius 1857, Maxwell & Boltzmann 1859).
ThT: Thermodynamic (absolute) temperature (1848 W. Thompson (Lord Kelvin): based on the Carnot concept via the IG derivation).
ThP; Thermal Particles are conserved physical particles, like atoms, molecules, electrons, and similar, that undergo thermal interactions via chaotic thermal-motion and collisions.
ThVP; Thermal Virtual-Particles [as named here] are non-conserved dynamic particles (as opposed to physical ThP) and they increase with entropy increase, i.e., with increase of thermal randomness of the physical-ThP.
NThVP: Number of Thermal Virtual-Particles, ThVP.
nth: Number of thermal moles is the number of ThVP per the Avogadro’s number, i.e., nth= NThVP/NA.
Self- or spontaneous is a self-driven process within the interacting systems, without any external forced-influence, i.e., without any “external compensation.”
Dissipation is any “frictional” conversion of work or work-potential (WP) into heat or thermal energy, with diminished remaining useful WP, resulting in irreversible energy degradation and commensurate entropy generation.
Irreversibility is “irreversible transformation”, or something permanently changed, without possibility to fully (or “truly”) reverse all interacting systems back by any means. It should not be confused with local change back to the original condition by “compensation” from elsewhere [
4].
Thermal-Roughness (and Thermal friction) [as named here] are the underlying cause and source of unavoidable irreversibility (2LT) since absolute-0K temperature is unfeasible (3LT), i.e., perpetual, real “smooth surface” is impossible due to perpetual and unavoidable, dynamic ThM of ThP.
Reversibility or
Reversible Equivalency is an
ideal concept, represented by
ideal processes without any energy degradation (with maximum possible efficiency or without irreversible dissipation) so that their output and input are
truly-equivalent and may self-reverse-back completing a cycle, or may perpetually repeat back-and-forth in any manner, therefore, effectively representing “
dynamic (quasi-) equilibrium” [
5].
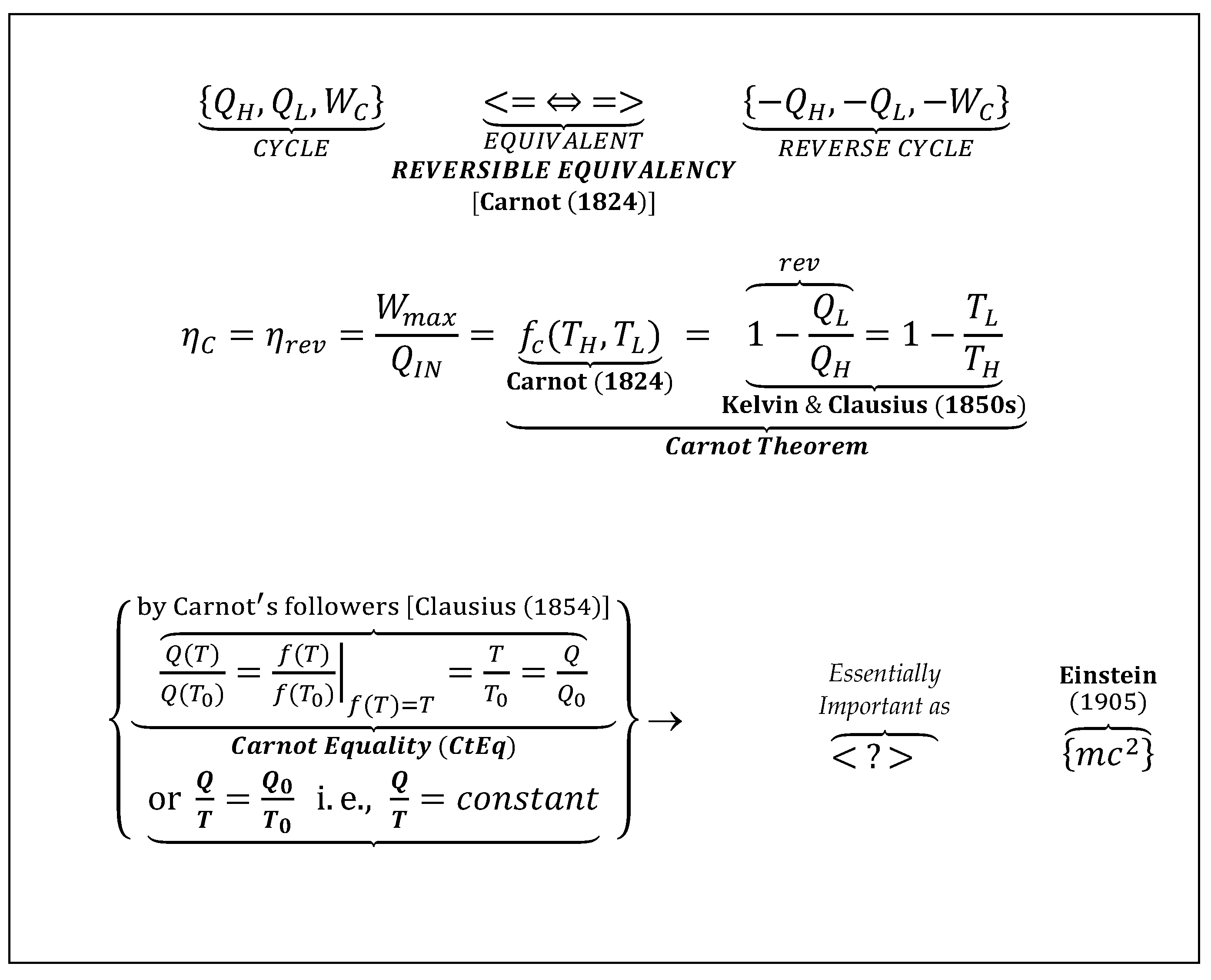
Carnot cycle is an ideal, reversible cycle (with maximum possible, 100% 2LT-efficiency) consisting of reversible heat transfers and isentropic work transfers, extracting maximum WP between the two reservoirs at high and low temperatures. Therefore, as the ‘work-extraction measuring-device’, its cyclic-efficiency is the measure of the WP between the two reservoirs only, i.e., it is dependent on the two temperatures only, and it is not dependent on its design or mode of operation (independent on the quasi-stationary cyclic path or any other, reversible stationary-process-path).
CtEf: Carnot Efficiency (a.k.a. Carnot Cycle Theorem) is the maximum possible, reversible cycle-efficiency interacting within the high-and-low temperature reservoirs (ηmax=ηC=WC/QH=[1-QL/QH]rev =FC(TH,TL)=1- TL/TH).
CtEq: Carnot Equality [as named here, to resound the integral Clausius Equality, being its precursor] is the heat-temperature ratio equality for reversible cycles between any two thermal reservoirs (QL/QH = TL/TH, or QH/TH =QL/TL=Qref/Tref =Q/T=constant).
CsEq: Clausius Equality of cyclic-integral for any reversible cycle (Cycle Integral[dQ/T]=0 is deduced from the Carnot Equality, CtEq).
CCHWE: Carnot-Clausius Heat-Work Equivalency,” [as emphasized and named here] is a generalized concept of heat-work interchangeability as an essential consequence of ‘true’ reversible equivalency.
WP: Work-potential or maximum-possible useful-energy of a nonequilibrium system with regard to its reference equilibrium (i.e., Carnot’s motive power of heat, sometimes work for short), or related free-energy, or exergy (where the surrounding reference is standardized).
IG: Ideal gas (PV/(t+C)=k Clapeyron in 1834; PV=nRT Renault in 1845).
1NL: First Newton Law of equilibrium motion or resting inertia.
2NL: Second Newton Law of forced change of momentum or acceleration.
3NL: Third Newton Law of action and equal reaction (duality of balanced forces and conservation of momentum).
0LT: Zeroth Law of Thermodynamics (temperature uniqueness of thermal equilibrium).
1LT: First Law of Thermodynamics or First Law for short (energy conservation; 1843 Joule, 1847 Helmholtz).
2LT: Second Law of Thermodynamics or Second Law for short (nonequilibrium useful-energy dissipation with entropy generation towards equilibrium; 1824 Carnot, 1850 Clausius, 1851 Thompson, 1854 Clausius theorem (dQ/T), 1865 Clausius entropy, 1874 Clausius formal statement of 2LT, 1867 Maxwell’s Demon, 1876 Gibbs free energy in Chemical ThD).
3LT: Third Law of Thermodynamics (impossibility of [thermal] emptiness [impossible absolute-0K nor to stop thermal motion]; 1906 Nernst).
4LT: Forth Law of Thermodynamics (impossibility of evolution forever, “growth without decay” is impossible; selective and self-reproductive evolution is extending inevitable irreversibility. NOTE the 4LT is evolving in many forms and it is still to-be-defined!).
PMM0 or PM: Perpetual-motion machine of the 0th kind (or “Perpetual [free] motion” in short) that violates the irreversible dissipation or friction (impossibility of free perpetual motion without dissipative resistance).
PMM1: Perpetual-motion machine of the 1st kind that violates the 1LT (impossibility of creating energy from nowhere).
PMM2: Perpetual-motion machine of the 2nd kind that violates the 2LT (impossibility of self-creating useful-energy or WP from within equilibrium).
PMM3: Perpetual-motion machine of the 3rd kind that violates the 3LT (impossibility of converting all heat to work since absolute-0K temperature is unachievable).
PMM4: Perpetual-motion machine of the 4th kind that violates the 4LT (impossibility of evolution forever without decay, or similar: NOTE the 4LT is evolving in many forms and it is still to-be-defined!).
4. Ubiquity of Thermal Motion and Heat, Thermal Roughness, and Indestructibility Of Entropy
4.1. Ubiquity of Thermal-Motion and Heat
The thermal-motion (ThM) and heat are ubiquitous since any work-potential (WP) transfer or storage (hence all processes) will be impeded and in part irreversibly dissipated to heat (in principle, increasing ThM and thermal energy, i.e., temperature and/or entropy) caused by different types of “dissipative-frictions,” ultimately instigated by “thermal roughness” (as reasoned, defined and named here) due to the existing, chaotic ThM of thermal particles (ThP). Furthermore, since the ThM cannot be ceased (i.e., zero absolute temperature is unattainable; the 3LT), the dissipative irreversibility is unavoidable in general (the 2LT), contributing to further ubiquity of heat and related thermal phenomena, for all processes without exception.

Entropy is elusive and sometimes puzzling with temperature since both tend to increase with heat generation and storage, and with heat transfer. However, more entropy, more thermal space more non-conserved, thermal virtual-particles, ThVP (as defined and named here), even if physical thermal particles, ThP, are conserved. The increase of ThM energy and its extensive randomness (entropy) is, in principle, complemented with higher average of ThM energy per physical thermal-particle (the higher temperature).
The ThM may be ideally intensified (i.e., thermal energy and temperature increased) by reversible work over conserved thermal-space (or conserved entropy), and in reverse when work is extracted (thermal energy and temperature are decreased), while thermal space (entropy) is also conserved (see the CCHWE in
Section 6 and elsewhere).
Thermal energy (or stored-heat or energy of ThM) is transferred (or displaced) as heat via ThM and thermal-collisions, from the ThP at a higher temperature on-average, to the ThP at a lower temperature on-average, by means of random “poking or jiggling” of ThP, across a real or imaginary boundary, without the need for physical ThP to be displaced across, similarly as the AC electrical energy is displaced (but in an orderly, wavy or cyclic manner, not like the chaotic thermal energy) from one electron to another, without need for electron displacement per se.
Temperature is thermal-intensity or thermal-force, i.e., particle-average thermal-motion energy per thermal particle, like atom, molecule, electron or similar.
Entropy is thermal displacement-space, defined as ThM energy per unit of its intensity (temperature), i.e., ‘thermal-space randomness’ of chaotically-moving thermal-particles, that is, “randomly traversed-space” by thermal particles, (randomness of both, space-directions and dynamic-motions), due to thermal collisions, and it may be represented by non-conserved “thermal virtual-particles, ThVP.”
We now define the number of
“thermal virtual-particles”
NThVP, which may be considered as the “
particle dimensionless entropy,” i.e.:
Then, we may define the number of “
thermal moles” nth, which may be considered as the “
molar dimensionless entropy,” i.e.:
Where, S is entropy; Uth is the internal thermal-energy; Ω is the number of the “possible thermal, microscopic states”; NA is the Avogadro’s number; kB is the particle Boltzmann constant; and Ru is the molar, universal gas constant.
The number of thermal virtual-particles, NThVP, is non-conserved, as opposed to conserved number of physical thermal-particles, NThP (like atoms, molecules, electrons, and similar). The former increases with entropy, i.e., with increase of thermal randomness.
Heat Q, and work W, are considered as “energies in-transfer," as process-quantities and not as properties, since after being stored within a system they “lose identities” and increase the system “internal energy (U or E), as if they are not distinguishable after being stored.
However, this author does not agree with such and similar accounts [
7] since the quality of such internal energies is not the same (i.e., different WPs and entropies; the 2LT). Namely, storing the same amounts of heat or work results in different system states, with different entropy, volume, etc., regardless that amount of total internal energies is the same (energy conserved, the 1LT; but not the same quality nor WP, the 2LT).
For example, if heat Q′ is stored at constant volume to a system at initial state (Ui, Vi, Si, ...), the final state will be (U′=Ui+Q′, V′=Vi, S′≠ Si, …), however, if the same amount of work W′′=Q′ is reversibly stored instead, the final state would be different (U′′=Ui+W′′=U′, V′′≠ V′, S′′=Si, …). If the processes are reversed back to the original initial state, it would be ideally possible to retrieve the original work W′′ from U′′ but that work could not be obtained from U′ (even though U′= U′′), which proves that the internal energies U′ and U′′ are not the same quality (not the same states, different WPs and exergies; the 2LT), regardless being the same quantity (same U-amounts; the 1LT).

Additional difficulties of distinguishing internal energy types are due to coupled dualities (conjugate multitude) of internal energy types. If work or/and heat are stored, even when
U is quantitatively the same, the other properties that characterize its quality and true equivalency (like
V, S, and others in more complex systems), are not the same. Furthermore, heat and work are interchangeable as demonstrated by the Carnot cycle and the
Carnot-Clausius Heat-Work Equivalency (CCHWE), as defined and named here (
Section 6). For example, the ideal gas energy of thermal motion,
NkBT, manifests also as mechanical compression energy
PV, as expressed by its equation of state:
PV≡ NkBT, see
Section 6.1.
4.2. Thermal Roughness and Thermal Friction
The “
Thermal Roughness” and “
Thermal Friction” are defined and named here as new concepts, as the underlying cause and source of inevitable irreversibility since absolute-0K temperature is unfeasible (3LT). No ideal systems nor frictionless processes are possible due to unavoidable thermal-motion (ThM) of thermal-particles (ThP). Even “superconductivity” at lower temperature must be at least infinitesimally irreversible since any energy flow must interact and be affected, regardless of the extent (even if infinitesimally), by the chaotic ThM collisions of the ThP, the latter being the cause and source of related
EM thermal radiation. It is impossible to have a perpetual “smooth boundary surface” due to unavoidable, dynamic and chaotic ThM of the ThP, see
Figure 2.
Figure 2.
Thermal Roughness and Thermal Friction are the underlying cause and source of unavoidable irreversibility (2LT) since absolute-0K temperature is unfeasible (3LT), i.e., perpetual “smooth surface” is impossible. No ideal systems nor frictionless processes are possible (even “superconductivity” must be infinitesimally irreversible) due to unavoidable interference with EM thermal radiation due to thermal motion and collisions.
Figure 2.
Thermal Roughness and Thermal Friction are the underlying cause and source of unavoidable irreversibility (2LT) since absolute-0K temperature is unfeasible (3LT), i.e., perpetual “smooth surface” is impossible. No ideal systems nor frictionless processes are possible (even “superconductivity” must be infinitesimally irreversible) due to unavoidable interference with EM thermal radiation due to thermal motion and collisions.
It is remarkable that all existing useful energy, quantified by the WP, the cause and source of process-forcing and energy displacement, dissipatedly convert to thermal motion (i.e., generated heat and entropy). In turn, the latter is the cause and source of the “thermal roughness” and thermal friction. Furthermore, all “other dissipations” are ultimately caused by the underlying “thermal friction”, by dynamic “thermal roughness” due to chaotic ThM (random fluctuations of ThP).
4.3. Indestructibility of Entropy
All transformations or processes are caused by the nonequilibrium work-potential (WP) and are accompanied by energy forced-displacement, either as work (in an orderly way) or as heat (via chaotic ThM and thermal collisions, including the “Carnot WP of heat,” see
Section 5).
Ideally, in limit, the heat and work could be displaced or transferred in reversible processes without any dissipative loss of the WP, like in ideal Carnot cyclic processes. Heat is transferred at infinitesimal temperature difference so that entropy transfer from a thermal source is the same as into a thermal sink (either the thermal reservoirs or the system); therefore, the entropy is conserved. Similarly, the adiabatic, reversible work transfers are ideal processes without any dissipation of WP to heat, i.e., they are not associated with entropy and therefore isentropic.
However, all real processes are caused and accompanied by displacement and dissipation of WP. If no WP to displace, there would be no process-forcing (no process would be possible) as in a self-sustained, perpetual equilibrium. The dissipation of WP is not an “annihilation loss” per se, but its conversion and degradation of work to heat, accompanied with entropy generation, the latter commensurate with WP dissipation per relevant absolute temperature, often figuratively named as “work-loss” or degradation of useful energy.
Irreversibility,
I, is the “irreversible loss” of the WP, or more accurately the work dissipative conversion to generated-heat,
I=WLOSS= Wdiss= Qgen,). The work dissipation is directly related to the entropy generation,
Sgen, at relevant reference, absolute temperature,
Tref, (the Gouy-Stodola correlation, Equation (5)). The work dissipation and related entropy generation are the two sides of the same coin (“half empty vs. half full”), i.e.,
In ideal, thermodynamically reversible processes the WP and entropy are “re-organized” and conserved, while in real, irreversible processes the WP, as cause and source of forced energy displacement, is transferred and expended (diminished), due to its dissipation (i.e., dissipative conversion) to heat with entropy generation.
A nonequilibrium (i.e., its WP) may be increased only by forcing on the expense of another WP, as a necessary WP-source. During such forced interactions the WP in ideal reversible processes would be reorganized and conserved (1LT & 2LT), or, in part, it would irreversibly dissipate to heat, i.e., the WP would be irreversibly diminished (2LT) — however, the totality of energy (WP and generated-heat) would be conserved (1LT, again). Therefore, there is no way to self-create nonequilibrium work-potential against the natural forcing towards equilibrium. The former would be contradiction of the latter.
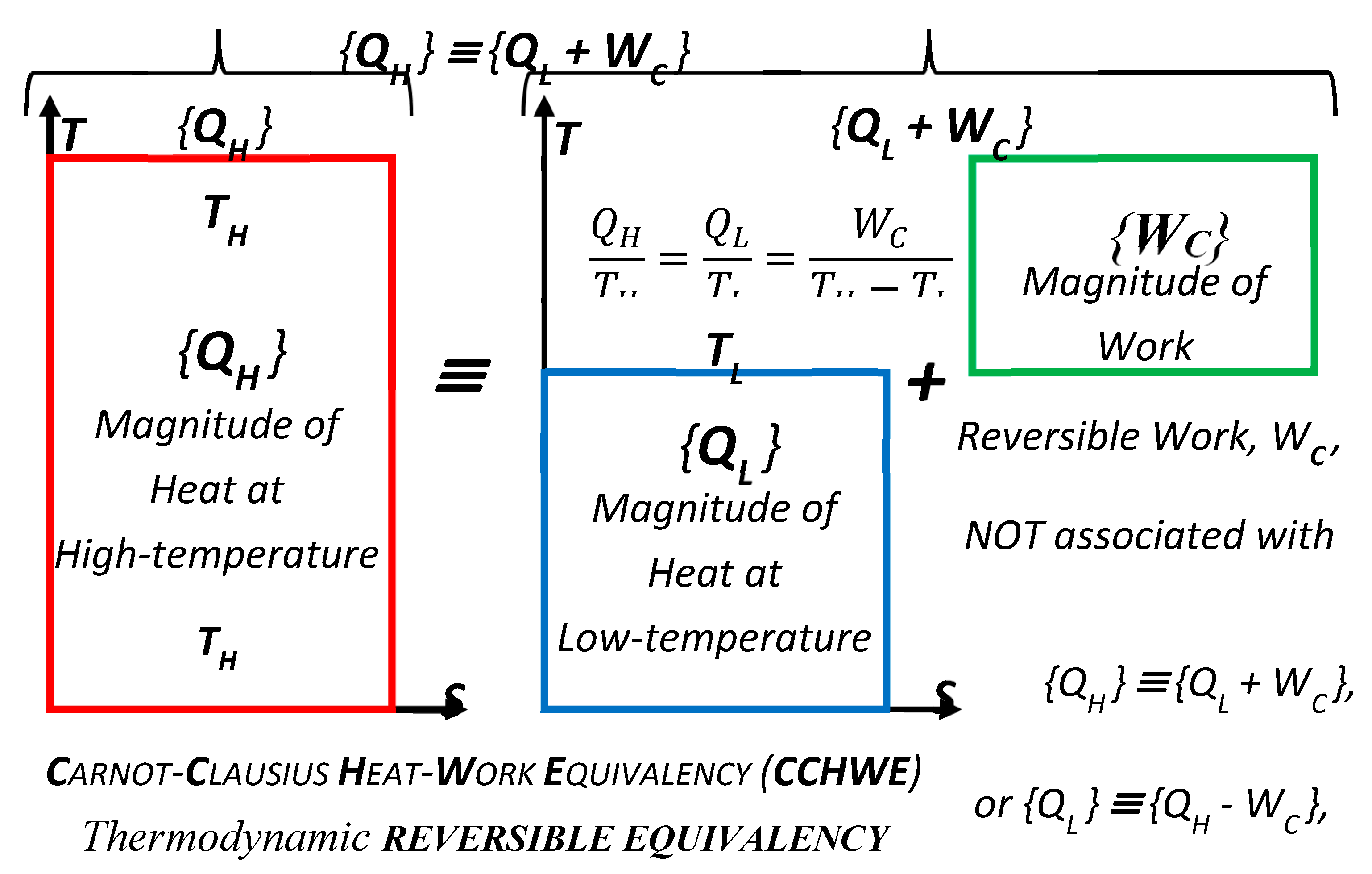
6. Carnot-Clausius Heat-Work Equivalency (CCHWE) Concept
Carnot-Clausius Heat-Work Equivalency (
CCHWE), as named and ‘highlighted’ here, establishes
interchangeability and equivalency between
heat and work., see
Figure 7. It is based on the early work of Carnot (1824) [
8], that “all reversible processes and cycles have equal and maximum efficiency for the given thermal reservoirs’ temperatures, regardless of device and mode of operation,” and among others, including Thomson (Kelvin) and Clausius’ meticulous work, around 1850’s [
11,
12].
Clausius “struggled,” in his “
Mechanical Theory of Heat” [
12] (Ch. IV:
Principle of the Equivalence of Transformations), to fully decern the Carnot’s postulates, and to finalize his ‘
transformations’, i.e., “when heat is reversibly transferring from high temperature and in part releasing [converting to] work, and in part transferring to heat at low temperature.”
Clausius’ reasoning was ingenious like Carnot’s, with debatable particulars, but with accurate final deductions of the “transformations′ equivalence-values,” with the f(t)=1/T integration factor, that resulted in the definition of new property, the entropy (the ‘missing transformation’) and definition of the quantitative correlation of the Second Law of Thermodynamics (2LT), including the Clausius Equality (CsEq) for reversible cycles, and Clausius Inequality for all real, irreversible cycles, the latter include entropy generation caused by work dissipation to heat, due to irreversibilities of different kinds.
Figure 7.
Carnot-Clausius Heat-Work Equivalency (CCHWE) [as named here], established interchangeability and equivalency between ‘Heat-and-Work’, based on the early work of Carnot (1824), that all reversible processes and cycles have equal and maximum efficiency, and among others, Kelvin and Clausius’ meticulous work, around 1850’s, that finalized the Thermodynamic temperature, Reversible cycle efficiency, Carnot Equality, Clausius (In)Equality, Entropy, and generalized the Second Law of Thermodynamics [2LT].
Figure 7.
Carnot-Clausius Heat-Work Equivalency (CCHWE) [as named here], established interchangeability and equivalency between ‘Heat-and-Work’, based on the early work of Carnot (1824), that all reversible processes and cycles have equal and maximum efficiency, and among others, Kelvin and Clausius’ meticulous work, around 1850’s, that finalized the Thermodynamic temperature, Reversible cycle efficiency, Carnot Equality, Clausius (In)Equality, Entropy, and generalized the Second Law of Thermodynamics [2LT].
Due to reversible equivalency, as originally devised by Carnot [
8] (see
Key-Pt. 16 and Equation (8)), elaborated by Clausius [
12], and re-interpreted in the
Section 5 (Equation (8) and
Figure 6), it is evident that heat and work are interchangeable and truly (reversibly) equivalent as follows:
The above correlations are much more important than they appear at first, since they represent the “heat-work reversible equivalency” in general, for all reversible steady-state processes not only for cycles (see
Figure 7). Namely, heat
QH at high temperature
TH is equivalent with sum of heat
QL at lower temperature
TL and Carnot work
WC, Equation ((9) Left); or any other relevant rearrangement, Equation ((9) Center or Right), along with the reversible
Carnot Equality, as formalized and named here,
Figure 3 and Equation (10).
It is important to emphasize again that the CCHWE is not only valid for reversible cycles, but in general. The cycles are ‘used’ to demonstrate maximum efficiency, but the latter is independent on the cycle design and mode of operation, therefore not dependent on the cycle per se, as implicitly postulated by Carnot, “maximum work is obtained by any reversible cycle, independent form the medium used or mode of operation, is dependent only on the temperatures of the two heat reservoirs [hence, not dependent on the cycle but the reservoirs’ properties/temperatures only].”


The CCHWE is demonstrated by Carnot cycle (and other power cycles along with the reverse-refrigeration cycles), where the Carnot cyclic-work (
WC) and rejected heat at lower temperature (
QL) are obtained from heat at higher temperature (
QH) alone; and in-reverse, where the utilized work in refrigeration reverse-cycles (
WC) is added (in)to a heat at low temperature (
QL), resulting in the heat at higher temperature (
QH) alone, see
Figure 7.
The above correlations, Equations (9) and (10), the latter in simple arithmetic form for constant temperature of the thermal reservoirs, will require proper integration for thermal systems with variable temperatures, the way the Carnot Equality (named and highlighted here, see Sectison 5.2), is generalized with the integral form in the Clausius Equality.
6.1. Ideal Gas Equation of State and CCHWE Confirmation
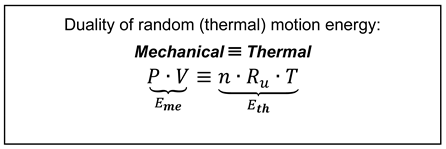
Ideal gas equation of state: Ideal gas (IG) is composed of many randomly moving, hypothetical massive point-particles that undergo elastic collisions but without any other particle interactions. Regardless of its simple structure, the IG is a good approximation of the behavior of many real gases in many applications. The IG energy consists of random- or thermal-motion (ThM) of its particles. It may be expressed as its thermal energy [EIG ≡ EThM]=Eth=N(kBT)=nRuT, where, N is number of particles, kB [J/K] is the Boltzmann constant (or energy-temperature conversion factor), and T is particle-average absolute temperature, i.e., (kBT) is the particle average energy, n is number of moles, and Ru is the universal, molar gas constant.
All systems allow storage and transfer of ubiquitous thermal energy (since the ThM cannot be averted, even absolute-0K cannot be achieved, the 3LT); however, the rigid systems do not allow storage of mechanical compression energy like gases. More complex systems with relevant structures may allow storage and transfer of other energy types; like electrical charging, magnetization, chemical or nuclear reactions, and similar.
The ThM of IG particles along with temperature also exhibit the pressure on any hypothetical or real boundary surface and, therefore, its energy may also be represented as mechanical (pressure) energy:
[EIG ≡ EThM ]=Eme=PV, where
P is mechanical pressure (defined as relevant energy per unit of volume), and
V volume of IG. Therefore, we may express the
IG equation of state (i.e., the constitutive correlation of its mechanical and thermal properties), as the equivalence (
“≡”) of the two forms of the same energy (Equation (11)).
However, due to duality of the ThM, the P & T are conjugate-and-interrelated via the IG equation of state, Equation (11). Similarly, real gases (including steam; called simple [thermo-] compressible substances) allow thermal and mechanical energy storage and transfer, and manifest duality and interchangeability of heat and work, as formulated and named here, see next.
Carnot-Clausius Heat-Work Equivalency (CCHWE) confirmation: The CCHWE will be demonstrated and confirmed using IG for its simplicity. Furthermore, since the correlations for the reversible equivalency are, in principle, valid in general, regardless of intermediary, working system or mode of its operation (any reversible process or cyclic path are equivalent), the results obtained with IG will be, in principle, valid in general.
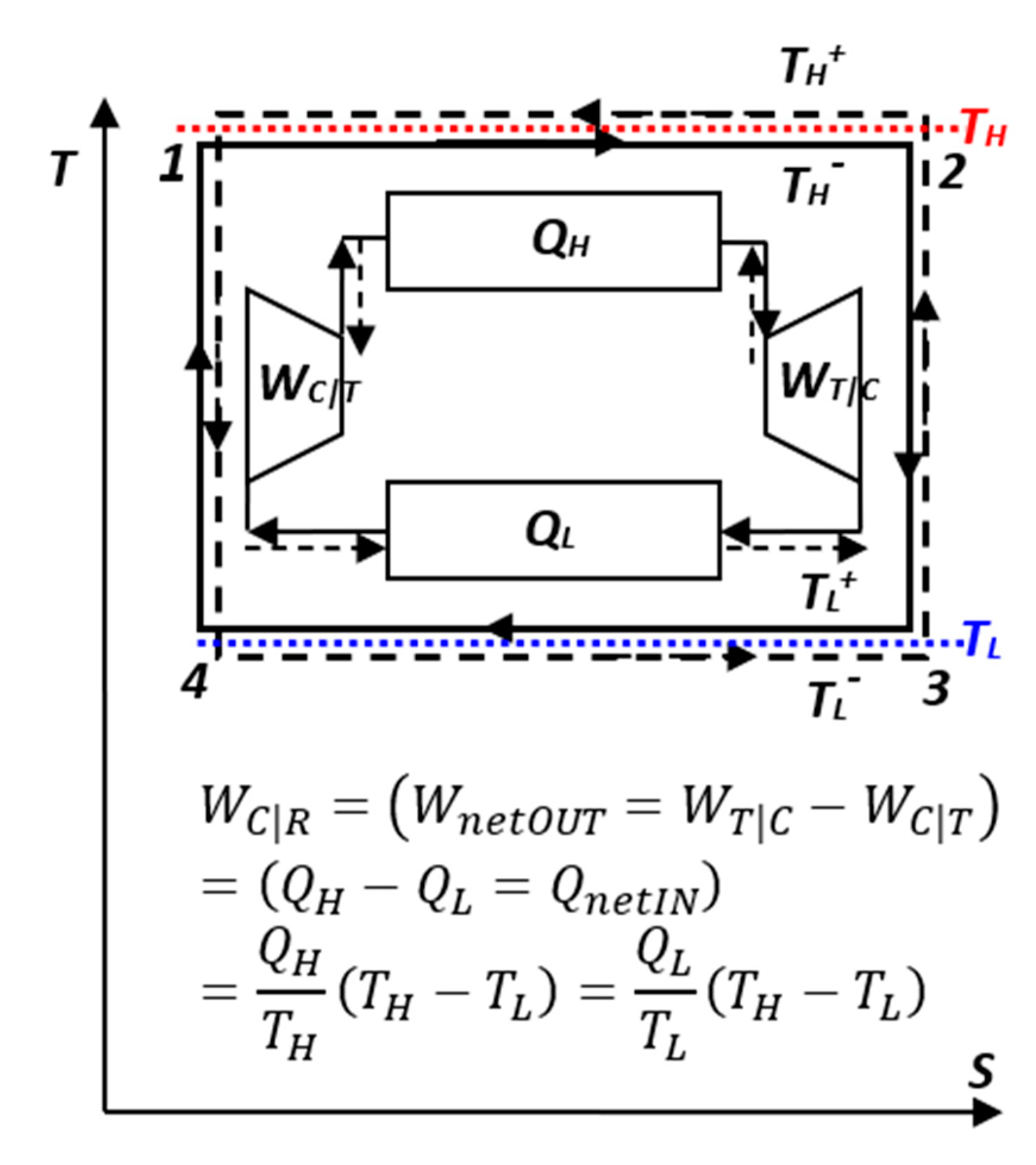
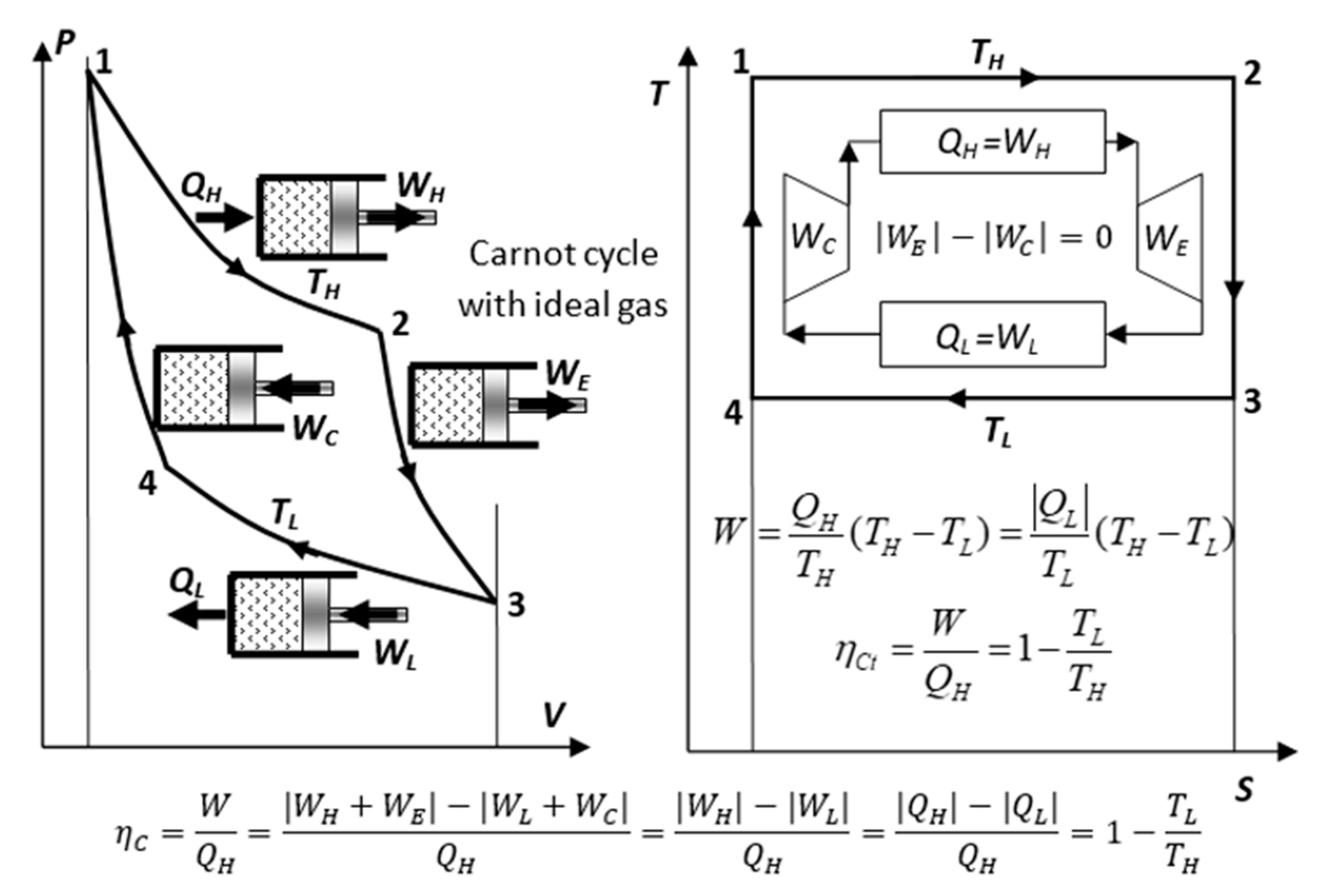
As stated before, the reversible Carnot cycle (
Figure 4 and
Figure 8) is the “measuring yardstick” of related equivalencies of all relevant input and output quantities, see next.
Figure 8.
Carnot cycle with ideal-gas: Isothermal expansion and compression’s works result in cycle net-work out, while adiabatic expansion and compression’s works cancel out, but they change temperatures required for reversible heat transfer.
Figure 8.
Carnot cycle with ideal-gas: Isothermal expansion and compression’s works result in cycle net-work out, while adiabatic expansion and compression’s works cancel out, but they change temperatures required for reversible heat transfer.
The reversible Carnot cycle also comprises isothermal processes where heat is entirely (100%) converted to work (QH=WH) while increasing volume and entropy (process 1-2), or in reverse, where work is entirely (100%) converted to heat (WL=QL) while decreasing volume and entropy (process 3-4). Note, that the isothermal ideal-gas heating is accompanied with the expansion work-out equal to heat-in, WH=QH), while the quantity of its internal energy is unchanged. However, its quality is degraded (part of its work potential replaced with heat), as manifested by the increase of entropy (i.e., U=constant, but decrease of the WP and free energy G=U-TS)!
Furthermore, the Carnot cycle comprises reversible adiabatic processes where internal energy (stored heat in IG) is entirely (100%) converted to work, -Cv(TL -TH)=W23, by lowering temperature and increasing volume (process 2-3), or in reverse, where work is entirely (100%) reversibly converted to internal energy (stored heat in IG), W41 =Cv(TH -TL), by increasing temperature and decreasing volume (process 4-1).
Note that the reversible, adiabatic works have equal magnitudes (|
W23|=|
W41|) and cancel out for the whole cycle (they have the main purpose to change temperature levels for reversible heat transfer to be possible). Consequently, the net cycle work is the result of the isothermal works’ difference due to the respective temperature difference,
W=|WH|-|WL|=(
TH -
TL)|
DS|, while exchanging the same entropy in-and-out, |
DS12|=|
DS34|=|
DS|, so that entropy cancels out, enabling the completion of the cycle (note that the isentropic works do not contribute to the entropy balance). For more details and all specific equations, see
Table 1 in [10, p.344].
7. “No Hope” for the Challengers of the Second Law of Thermodynamics
“
It is hard to believe that a serious scientist nowadays, who truly comprehends the Second Law and its essence, would challenge it based on incomplete and elusive facts […] However, sometimes, even highly accomplished scientists in their fields do not fully realize the essence of the Second Law of thermodynamics [
10,
13,
14,
15,
16,
17,
18].”
As already stated, this treatise [
1], written for a special occasion [
2], is presenting this author’s lifelong endeavors and reflections [
6,
7,
9,
10,
13,
14,
15,
16,
17,
18], including additional, original reasoning and interpretations, regarding the fundamental issues of thermodynamics, and especially as related to the subtle Second Law of thermodynamics (2LT), as well as to put certain physical and philosophical concepts in historical and contemporary perspective.
In this section, a number of related issues are presented and emphasized with several
Key-Points, including a
Deceptive Example (
Section 7.1), “Three
Primary-deception structures” of the 2LT, classified by this author (
Section 7.2), and critical discussions on the two selected publications by avid challengers of the 2LT, one recent publication, challenging the 2LT [
19], and another, self-claimed as a ‘landmark paper’, experimentally challenging the validity of the 2LT [
20], see
Section 7.3 below.
A small, but adventurous and stanch group of creative scientists and inventors-to-be, named here as “2LT Challengers,” are ‘bravely’ challenging the 2LT universal validity, often based on a fact that they have been successful in achieving a perpetual non-equilibrium (with limited work-potential, WP, but without perpetual work production), using innovative and creative methods and processes, and hoping to utilize it to ‘somehow’ perpetually produce work (useful-energy) from within the environment alone, as a single thermodynamic reservoir in equilibrium.
However, nobody has been successful to achieve spontaneous and sustained conversion (stationary or cyclic) of the surroundings’ (thermal) energy to useful work, nor to provide reliable evidence (comprehensive energy and entropy ‘accounting’) of achieving a sustainable, overall process efficiency higher than the Carnot's maximum possible (which is zero from a single thermal reservoir only).


7.1. “Perpetual-Motion Watch” Deceptive Example
We may “wishfully hypothesize” miraculous processes to achieve impossible outcomes by overlooking elusive but critically important phenomena. We will present here one trivial example that may appear to be a “perpetual-motion watch”, in order to demonstrate, that without full knowledge what is inside a ‘black-box’, it may deceive some to “jump to unjustified conclusions” without due comprehension.
If a watch is running for years without supplying any energy from outside, it may appear without knowing what is inside the watch, that it somehow runs by self-creating energy (PMM1) or that it somehow produces useful-energy from the surroundings as a single thermal reservoir (PMM2, self-creating WP), see
Figure 9, with pictured a real watch with 5-10 years battery life.
It would be wrong to hypothesize that the useful work is perpetually supplied by heat from the surroundings alone (a violation of the 2LT), or that the work is somehow miraculously generated from nowhere (a violation of the 1LT).
Figure 9.
“Perpetual-motion-like” watch with 5-10 years battery life. As if its battery lasts forever. We could mistakenly hypothesize (as if we have proved experimentally), that it works without using energy (PMM1, 1LT violation), or it consumes energy from the surroundings’ thermal reservoir alone (PMM2, 2LT violation).
Figure 9.
“Perpetual-motion-like” watch with 5-10 years battery life. As if its battery lasts forever. We could mistakenly hypothesize (as if we have proved experimentally), that it works without using energy (PMM1, 1LT violation), or it consumes energy from the surroundings’ thermal reservoir alone (PMM2, 2LT violation).
Similarly, many other subtle electro-chemical and other interactions at different time and space scales may allude to violation of the fundamental laws, especially for near-reversible processes with negligible frictional and other dissipations, resembling “perpetually self-running watch” on
Figure 9, running for years without any supply of energy from the outside. Some near-ideal processes, with apparent perpetual-motions, may appear to work without energy consumption or be ‘mistakenly hypothesized’ to somehow, spontaneously produce work from surrounding equilibrium only.
Since the 1LT of energy conservation appears more intuitive and the 2LT is more elusive, the inventors-to-be or even some seasoned researchers may mistakenly hypothesize the spectacular inventions that violate the 2LT. We should be very careful to avoid premature and sensational hypotheses based on inadequate experiments and incomplete analyses.
7.2. Three “Primary Deception Structures” of Hypothetical Violation of the Second Law of Thermodynamics
(1)
First-deception structures (or “
dynamic [quasi-] equilibrium”) is about confusing the notion of “
perpetual free-motion (or free-oscillation)” without load (without extracting useful-energy but self-sustaining unavoidable dissipation), with notion of “
perpetual motion machine” of perpetually producing useful-work without due WP source. It was named “
dynamic [quasi-] equilibrium” and it has been discussed in more detail by this author in ([
16,
17] (Appendix 1)) and elsewhere.
(2)
Second-deception structures (or “
structural [quasi-] equilibrium”) is about creation of “systems with perpetual non-equilibrium properties” with transient, limited WP (like non-uniform temperature or pressure, or EM charge, and similar), to be somehow miraculously utilized for “perpetual self-creation of useful-work” from within an equilibrium-surroundings alone, thus without due, perpetual WP source [
20], see
Section 7.3. The former, a perpetual “non-equilibrium system state,” with limited WP energy, is not at all the same as a “self-creation of perpetual work” from within an equilibrium, or having a more efficient perpetual-cycle than the Carnot cycle. It would be against the forcing-direction of energy-displacement, from higher to lower energy-density (it would be a PPM2); and it would defy the very existence of equilibrium (it would be the equilibrium’s “contradiction impossibility”). It is called “
structural [quasi-] equilibrium” and it has been discussed in more detail by this author in [
14,
15,
16,
17,
18] and elsewhere.
(3)
Third-deception structures (or “
persistent-currents quasi-equilibrium”) is about certain “persistent currents” phenomena with self-perpetual, like
“dissipate-and-reverse” processes (like the Brownian motion, and the Meisner effect, as a process-reverse to [(micro) irreversible] dissipation of the “superconducting persistent currents” or non-superconducting “persistent currents in metal rings” [
19], and similar), that appear to perpetually micro-dissipate-and-reverse WP within their locality in equilibrium, as if they “quasi-reversibly dissipate-to-themselves” or self-create WP for its own dissipation; and thus, as if they violate universal validity of the 2LT, regardless of not producing any useful energy.
The challengers argue that such phenomena, in principle, ‘disapprove’ the 2LT universal validity, and could potentially, with future innovations be used as PMM2 to produce useful-energy while violating the 2LT [19, 20, with large number of references therewith]. However, the existence of such structures in quasi-equilibrium, since they do not produce perpetual work from within an equilibrium, is not justification for valid violation of the 2LT.


Therefore, the quasi-static (or quasi-equilibrium)
phase-change of real systems in equilibrium with its surroundings are, ideally reversible processes and could perpetually be reversed back-and-forth with infinitesimal change of respective intensive properties, i.e., they are
virtually irreversible within their self-sustained, perpetual
virtual equilibrium. Similarly, the
persistent-currents equilibrium (as the
Third-deception structures) may be
virtually irreversible (or
near-reversible) within their self-sustained and perpetual,
virtual [quasi-] structural-equilibrium. Such structures do not self-produce perpetual work nor perpetually destroy entropy, and therefore do not violate the 2LT, as speculated in [
19,
20].

Therefore, spontaneous displacement of energy from lower to higher energy density, in opposite direction from natural forcing and self-creation of non-equilibrium, would be like forcing in one direction with acceleration in opposite direction. Such wishful thinking would be the natural contradiction impossibility. It would negate stable equilibrium existence and will imply self-creation of WP with entropy destruction. Consequently, a non-equilibrium, the source of WP and forcing, cannot be generated (contradiction impossibility of unavoidable dissipation), but only could be transferred and ideally conserved, while in realty a WP will tend to dissipate to heat (within complex microstructure and fluctuating micro-processes), towards a mutual macro equilibrium.
As the fundamental laws of nature and thermodynamics are expended from simple systems in physics and chemistry, to different space and time scales and to much more complex systems in biology, life and intelligent processes, there are more challenges to be comprehended and understood. There is a need to better discern the fundamental concepts at different scales and complex systems, like diverse and more complex and self-sustained “structural equilibriums,” without net-fluxes at scale of interest, but with non-uniform concentration-potentials under coupled force fields; e.g., hydrostatic pressure and adiabatic temperature distributions in gravity field, charge and species concentration distributions in electromagnetic or chemical fields, as well as, to differentiate between transient and “near stationary” processes under influence of known and stealthy boundary and field conditions.
7.3. “Experimental Test of a Thermodynamic Paradox” Demystified
Professor Sheehan, an avid 2LT challenger, who organized several 2LT conferences and wrote extensively regarding the validity and violations of the 2LT, was claiming in their landmark paper [20, also in references therewith] that, “There are now roughly three dozen theoretical proposals for its violation in the mainstream scientific literature, more than half of which have resisted resolution as of this date. There are also experiments which purport to violate the 2LT, and not all of these have been discounted. My experiments in particular, published in Found. Physics in 2014 [20], have not been disproved or shown to be in error in any meaningful way.”
It is reasoned and argued here that the claims in the landmark paper by Sheehan
et al. [
20], are misplaced and over-stretching. Even if ‘
more than half” of the challenges “
have resisted resolution as of today” the other half have been disavowed and none has been verified to date. Especially problematic are incomplete, misleading, and biased experimental results, as if the challengers are not comprehending or ‘conveniently ignoring’ the very fundamentals and essence of the 2LT.
Specifically, the last two concluding sentences of the Sheehan’s et al. paper [
20], were, “In summary, Duncan’s temperature difference has been experimentally measured via differential hydrogen dissociation on tungsten and rhenium surfaces under high temperature blackbody cavity conditions. We know of no credible way to reconcile these results with standard interpretations of the second law.” The claim of ‘black-body cavity conditions’ is questionable since it should be of much larger size than the devices inside. But, even if within a black-body cavity, the existence of stationary nonuniform properties (nonuniform temperatures, etc.) will not violate the 2LT of thermodynamics.
The last sentence of the paper is rather speculative, since the paper results describe a non-homogeneous, structural equilibrium established after externally imposed non-equilibrium (by heating the container tube to a very high temperatures). However, the 2LT, as classically stated for simple compressible substances for heat-work interactions only (where temperature is uniform at equilibrium). In general, the 2LT describes process conditions during spontaneous directional displacement of mass-energy (cyclic or stationary extraction of work), accompanied with irreversible generation (production) of entropy due to partial dissipation of work potential to thermal heat, which was not tested at all by the reported experiments, but only hypothetical and wishful claims stated. After all, before the 2LT-violation claims are stated, the reliable criteria for the 2LT-violation, including proper definition and evaluation of entropy balances (very important), should be established based on full comprehension of the fundamental Laws of nature.
Even more problematic is the Authors’ claim that their experiments “point to physics beyond the traditional understanding of the second law,” to justify their belief regarding the possibility of the 2LT violation, without due clarification and justification. The experiments relate to a special system with non-uniform temperature distribution due to dissociation/recombination, but do not represent a black-body cavity, and especially do not relate to the essence of the 2LT verification nor violation, as detailed below:
Most of the fundamental formulations of the phenomenological, classical thermodynamics (called “
standard thermodynamics” in the last sentence in
Section 2 in the paper [
20]), are reasoned and derived for the “simple compressible thermodynamic system,” the latter structure allows for heat and mechanical work interactions and storage only, but not other interactions, as well as for the ideal, black body cavities, with uniform thermodynamic properties in equilibrium (uniform temperatures and pressures in such simple material structures and systems). The experimental system described in the paper is not nearly closed to the ideal black-body cavity, and the described, dissociation/recombination interactions between heterogeneous devices within a controlled isothermal tube (of the same order of magnitude size as the devices inside), are much more complex than simple thermo-mechanical interaction of simple compressible system in ideal black-body cavity.
The stationary quasi-equilibriums (with non-uniform properties) are abundant in nature, and do not violate the 2LT at all. For example, hydrostatic pressure distribution in a container, or adiabatic atmospheric temperature distribution, or non-uniform distribution of other properties in a stationary equilibrium, in gravity, electromagnetic or chemical fields, like the presented results. I called the above a “structural equilibrium” (sustainable equilibrium but with non-uniform properties), as opposed to ideal thermodynamic equilibrium (with uniform properties) between the simple compressible systems with boundary heat and work interactions only, immune from any other structure or field interactions. This is one of several other problems of the paper’s judgments and conclusions.
-
The statements in
Section 6. Discussion in the paper [
20], “Within the traditional understanding of the second law, stationary temperature differentials such as those reported should not be possible.” This statement is arbitrary and not justified, see also comments above. Likewise, “Second, the temperature differences in DP experiments generated Seebeck voltages that can drive currents—and did, through their thermocouple gauges—thus, were capable of performing work like a heat engine.” This is pure speculation, since we do not know what kind of stationary process will re-establish if a heat engine (HE) or electrical load is interfaced to utilize temperature or Seebeck voltage differences within the described system and devices.
A simple question arises: why the Authors have not experimentally verified their hypothesis, if a stationary work extraction would be possible from within an environment in equilibrium? Such straightforward experiments could and should have been performed to check out experimentally such a critical hypothesis. Based on classical thermodynamics, which allows transient processes, after an initial non-equilibrium is externally imposed (as in the paper experiments), the appropriate, stationary structural equilibrium with properties-gradients will establish as in the paper, but stationary process with perpetual work extraction outside of an equilibrium is not possible, without external perpetual work source.
The last two concluding sentences of the paper were, “In summary, Duncan’s temperature difference has been experimentally measured via differential hydrogen dissociation on tungsten and rhenium surfaces under high temperature blackbody cavity conditions. We know of no credible way to reconcile these results with standard interpretations of the second law.” The assumptions and conclusions are misleading and unjustified, as specifically described above.
The Authors [
19,
20] (and a number of other “
Challengers” of the 2LT) often misinterpret the fundamental laws, present elusive hypotheses, and perform incomplete, biased experiments, always short of simple confirmation of their 2LT violation claims. The Authors’ implication that with creative devices, “
Challengers’ Demons” (like
Maxwell Demon; see
Ref. [
17]), it is possible to imbed them to a macro-equilibrium environment and extract stationary ‘useful work’, are philosophically and scientifically unsound. Such a magic and wishful ‘demons’, if possible and inserted as a ‘black box’ in a system or environment at equilibrium, to create a steady-state (stationary) work-extracting process from within such equilibrium, would be in opposite direction of existing natural forces, and also be a ‘catastrophically unstable’ processes with a potential to ‘syphon’ all existing mass-energy in an infinitesimal-size singularity with infinite mass-energy potential, a super black-hole-like. If it were ever possible, we would not exist ‘as we know it’ here and now!
8. Conclusions
As already stated, this comprehensive treatise [
1], written for a special occasion [
2], presents this author’s lifelong endeavors and reflections [
6,
7,
9,
10,
13,
14,
15,
16,
17,
18], including original reasoning and re-interpretations, regarding the fundamental issues of thermodynamics, and especially as related to the subtle Second Law of thermodynamics (2LT), as well as to put certain physical and philosophical concepts in historical and contemporary perspective, see
Section 1 (
Introduction, which ends with the
Selected Abbreviations and Notes). The main content of this treatise was presented in the following Sections:
In
Section 2, “
Energy forcing and displacement,” the related concepts have been pondered. The “force or forcing” is nonequilibrium energy tendency to displace or redistribute (or to extend) from its higher to lower energy density (or energy-intensity) towards mutual equilibrium with uniform properties. Typical, energy
intensive and
extensive conjugate-properties (
energy-force and
energy-displacement) were presented in
Table 1. All but thermal energy-displacements are conserved, while
thermal-displacement (
entropy or number of
thermal virtual-particles,
NThVP, as defined and named here) is irreversibly generated due to dissipation of all other energy types to heat.
Then, in
Section 3, “
Reasoning logical-proof of the fundamental laws,” the concept of energy forced-displacement as the mechanistic phenomenon in general was reaffirmed. The elementary particles (including “field-equivalent” particles) or bulk systems (consisting of elementary particles), mutually interact along shared displacement (with equal, respective action-reaction forces), thereby conserving energy during their interactive, mutual displacement. Since all existence is in principle mechanistic and physical, it was demonstrated here that the
Laws of thermodynamics (LT) are generalized extensions of the fundamental Newton’s Laws (NL) of mechanics. The First Law of thermodynamics (1LT) is the generalized law of the conservation of energy, and the Second Law of thermodynamics (2LT) describes the forcing tendency of nonequilibrium, useful-energy (or work-potential, WP) for its displacement and irreversible dissipation to heat with entropy generation, towards mutual equilibrium.
In
Section 4, “
Ubiquity of thermal motion and heat, thermal roughness, and indestructability of entropy”, this author’s comprehension of related phenomena has been further advanced by defining a new concept of “
thermal roughness” and reasoning impossibility of entropy destruction, among others. Entropy, as the “final transformation” cannot be converted to anything else nor annihilated, but only transferred with heat and irreversibly generated with heat generation due to work dissipation, including Carnot “thermal work-potential” dissipation.
The “thermal roughness” and related “thermal friction” were defined and named here as new concepts, as the underlying cause and source of inevitable irreversibility since absolute-0K temperature is unfeasible (3LT). Since all real, irreversible processes generate heat and entropy due to unavoidable dissipation of work and/or WP to heat (ultimately instigated by the “thermal roughness” as elaborated and named here), and all ideal, reversible processes conserve entropy, then, there is no other processes left to miraculously generate WP without a due WP-source. Furthermore, no “imaginary process” could destroy (or annihilate) entropy, since it would be a ‘self-reversal of dissipation’ impossibility and contradiction-impossibility against the natural forcing — it would imply self-generation of nonequilibrium (and its WP); therefore, rendering a logical proof of indestructibility of entropy (the 2LT).
In the following,
Section 5, “
Carnot maximum efficiency, Reversible equivalency, and Work potential” the Sadi Carnot’s ground-breaking contributions of reversible processes and heat-engine cycle maximum-efficiency was put in historical and contemporary perspective. Furthermore, it has been argued that the
Carnot’s contributions are among the most important developments in natural sciences.
The proof by “contradiction-impossibility” of an established fact is, by definition, the logical proof of the stated fact. If a contradiction of a fact is possible then that fact would be void and impossible. It is illogical, absurd, and impossible to have it both, “the one-way and the opposite-way.” For example, if heat self-transfers from higher to lower temperature, it would be “contradiction-impossibility” to self-transfer in the opposite direction. All reversible processes (including cyclic processes) under the same conditions must have equal and maximum efficiency, as demonstrated by relevant “contradiction impossibility.”
As a matter of fact, the reversible processes and cycles were a priori “specified” as ideal, with maximum possible efficiency, with a priory 100% “2LT reversible-efficiency,” not dependent on their design or mode of operation (independent on their quasi-stationary cyclic path or any other, reversible stationary-process-path). Actually, as the ideal ‘work-extraction measuring-devices’, all reversible processes and cycles determine, not their efficiency per se, but in fact, they determine the WP (as % or ratio efficiency with reference to relevant total energy) of an energy-source system with another reference system (like the two thermal reservoirs with the Carnot cycle, so their WP-ratio being dependent on their temperatures only). The Carnot’s Equality (CtEq), Q/T=constant, the well-known correlation, the precursor for the famous Clausius Equality (CsEq), CI(dQ/T)=0 (the cyclic-integral for variable-temperature reversible-cycles), was specifically named here ‘as such’ by this author in Sadi Carnot’s honor and to resample the CsEq name.
In the succeeding
Section 6, named here “
Carnot-Clausius Heat-Work Equivalency (CCHWE) concept”, a notion of ‘true’
heat-work interchangeability has been enlightened and named here, as an essential consequence of thermodynamic
reversible equivalency.
The correlations,
QH ≡
WC +
QL and
QH/
TH=
QL/
TL=
WC/(
TH -
TL), Equations ((9) & (10)), are much more important than they appear at first, since they represent the “
heat-work reversible equivalency and interchangeability” in general, for all reversible steady-state processes not only for cycles (see
Figure 7). Namely, heat
QH at high temperature
TH is equivalent with sum of heat
QL at lower temperature
TL and Carnot work
WC.
The energy of thermal-motion (ThM) of ideal-gas (IG) particles, EThM=Eth=N(kBT)=nRuT, along with temperature also exhibit the pressure on any hypothetical or real boundary surface and, therefore, its energy may also be represented as mechanical (pressure) energy: EThM =Eme=PV. Therefore, we may express the IG equation of state (i.e., the constitutive correlation of its mechanical and thermal properties), as the equivalence (“≡”) of the two forms of the same energy, PV ≡ nRuT, thus rendering its logical proof.
Thermal-transformers were named and discussed by this author in 2004 [
6], revisited later [
10], and reiterated here in
Section 6. It could be functioning as an ideal, “
reversible thermal-transformer.” Namely, the reversible heat transfer from higher
TH to lower
TL temperature with
W, Carnot cycle work output; or in reverse, the reversible heat transfer from lower
TL to higher
TH temperature with
W, Carnot cycle work input. Likewise, the real
thermal-transformers, power and refrigeration cycles (including heat-pump cycles), also transfer heat from any to any temperature level, except for reduced efficiency due to unavoidable dissipation of WP to generated heat and entropy (Equations (5) & (7)).
Lastly, in
Section 7, “‛
No Hope’
for the Challengers of the Second Law of thermodynamics,” this author’s compelling arguments were presented, that “entropy can be reduced (locally, when heat is transferred out of a locality), but it cannot be destroyed by any means on any space or time scale of interest. A
“Perpetual-Motion Watch” was presented as a trivial,
deceptive example. Furthermore, three
primary-deception structures (PDS), of hypothetical violation of the 2LT were classified by this author:
First-PDS (or “
dynamic [quasi-] equilibrium”),
Second-PDS (or “
structural [quasi-] equilibrium”), and new,
Third-PDS (or “
persistent-currents quasi-equilibrium”). And, lastly, critical discussions on the two selected publications by avid challengers of the 2LT, one recent publication challenging the 2LT [
19], and another, self-claimed as a ‘landmark paper’, experimentally challenging the validity of the 2LT [
20], were presented.
The Challengers misinterpret the fundamental laws, present elusive hypotheses, and perform incomplete and misleading, biased experiments, always short of straightforward confirmation of their 2LT violation claims. That is why all resolved, Challengers’ paradoxes and misleading violations of the 2LT to date, have been resolved in the favor of the 2LT and never against. We are still to witness a single, still open Second Law violation, to be verified and utilized.
The violation of the 2LT should be the last and not the first hypothesis to justify an unsolved phenomenon. It appears that the Challengers are misusing the elusive ‘Entropy Law’ (2LT): "Whoever uses the term 'entropy' in a discussion always wins since no one knows what entropy really is, so in a debate one always has the advantage" [As lamented by John von Neumann]. It would be more probable to assume that such structures are infinitesimally irreversible (or near-reversible) and very-slowly approaching true equilibrium while negligibly exhausting its own WP, or even hypothesize that they may be driven by negligibly-stealthy, yet-to-be-discovered “cold fusion” like energy within atomic nucleus, or some stealthy WP from within or from the surroundings.
This treatise is concluded with the following:
Furthermore, the time and spatial integrals of micro quantities must result in macro quantities, for the conservation laws to be valid. Therefore, claiming violation of the 2LT on micro-scale or special processes is questionable and due to lack of full comprehension of the 2LT, or due to lack of proper ‘tooling’ (conceptual, analytical, numerical, or experimental limitations), or sometimes may be due to a desire for unjustified attention.
In reality, all processes must be at least infinitesimally irreversible, including underlining processes at equilibrium, the latter being an ideal state. The underlying mass-energy structures and processes within the ‘finest micro scales’ are more complex and undetected at our present state of tooling and mental comprehension. However, their integral manifestation at macroscopic level, are more realistically observable and reliable, thus being the ultimate ‘check-and-balance’ of microscopic and quantum hypotheses.
The fundamental physical laws are independent from any system structure or scale, and they should take primacy over any special analysis based on approximations and limitations of modeling of system, its properties, and processes; and especially if based on ‘thought experiments’ [
17]. After all, micro- and sub-micro simulations and experimental analyses are also based on the fundamental Laws, and therefore, they cannot be used to negate those fundamental Laws. As the fundamental laws of nature and thermodynamics are expanded from simple systems in physics and chemistry, to different space and time scales and to much more complex systems in biology, life and intelligent processes, there are more challenges to be comprehended and understood [
16].