Submitted:
04 May 2023
Posted:
05 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
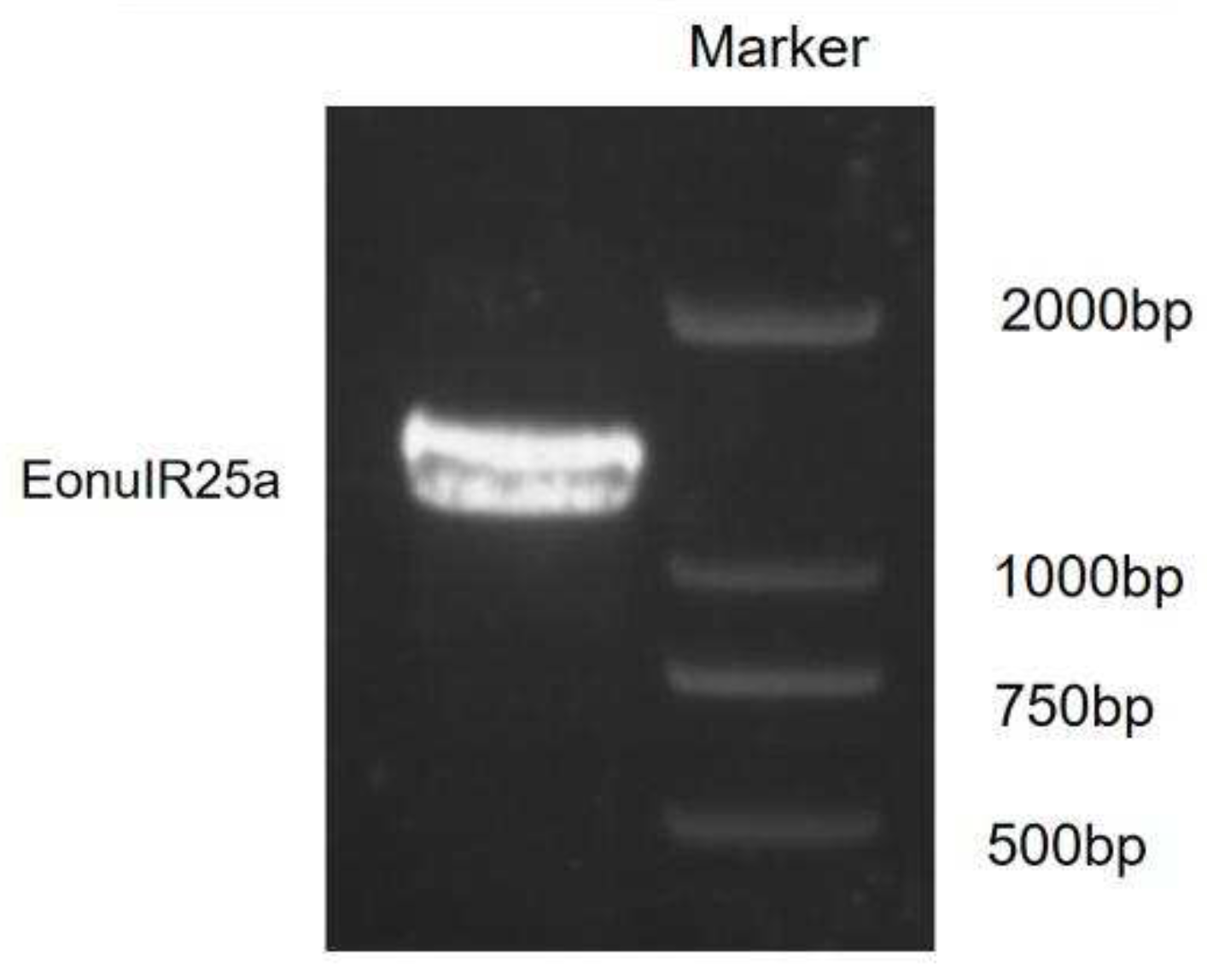
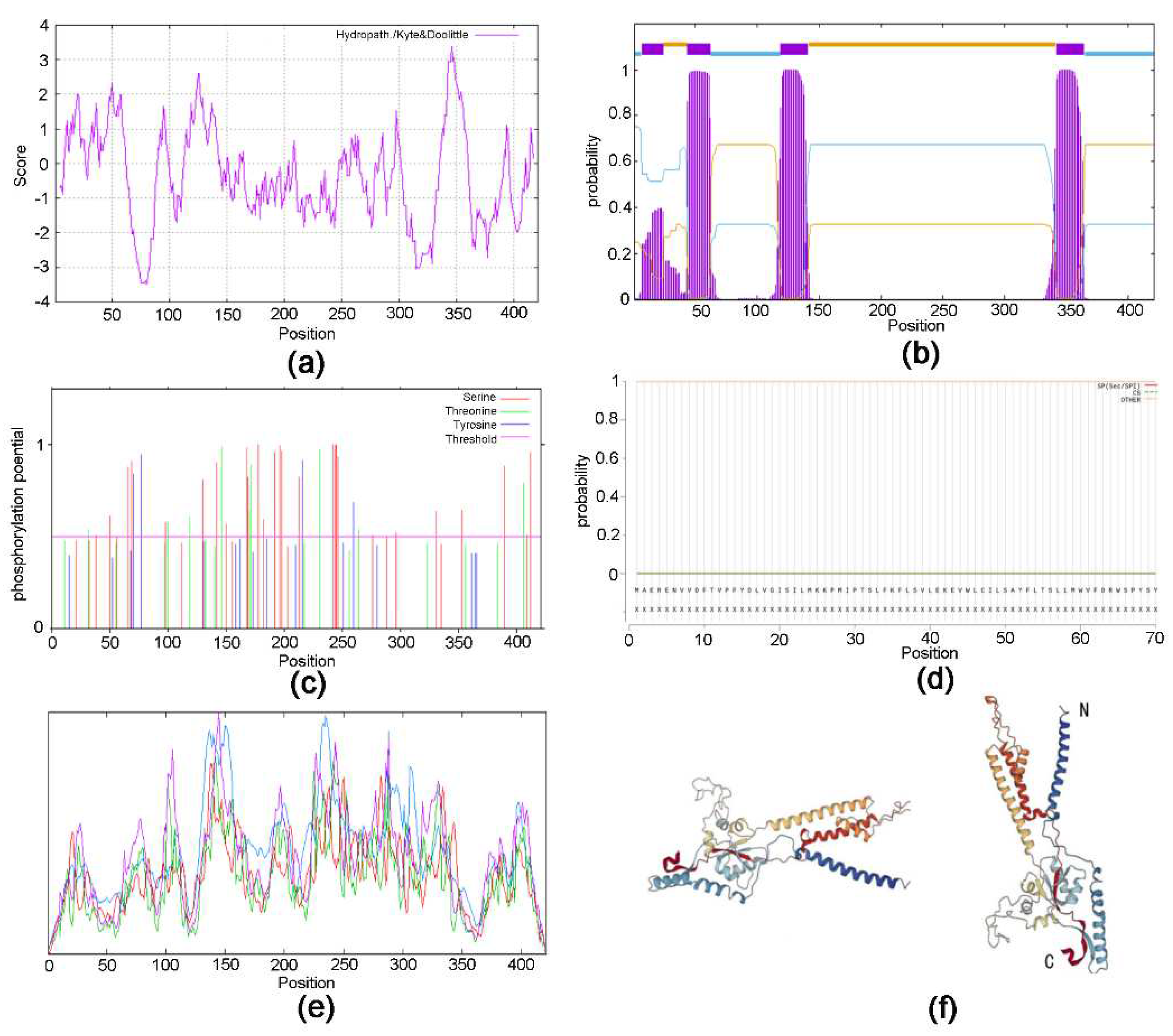
2.1. Sequence analysis of the EonuIR25a gene
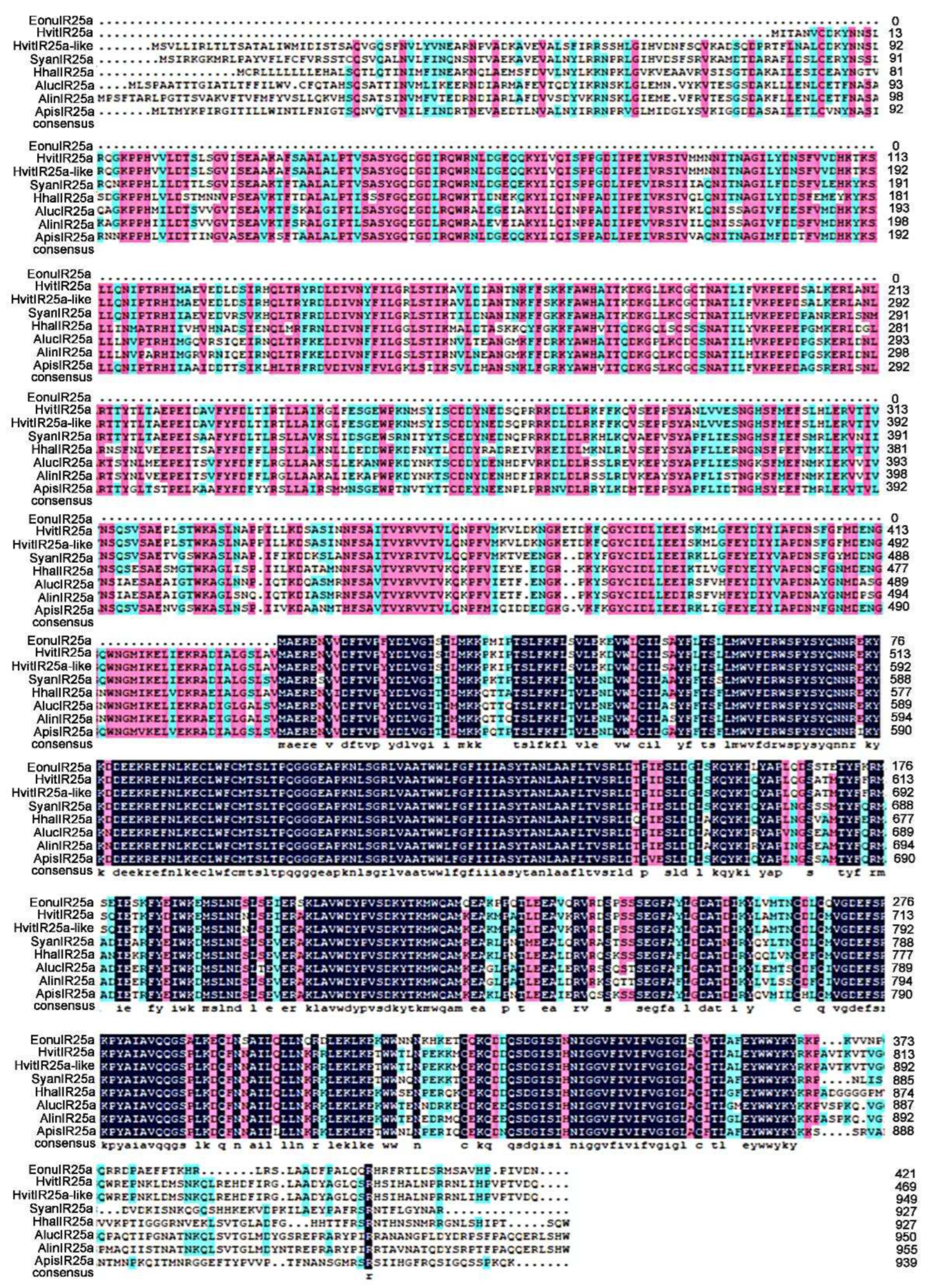
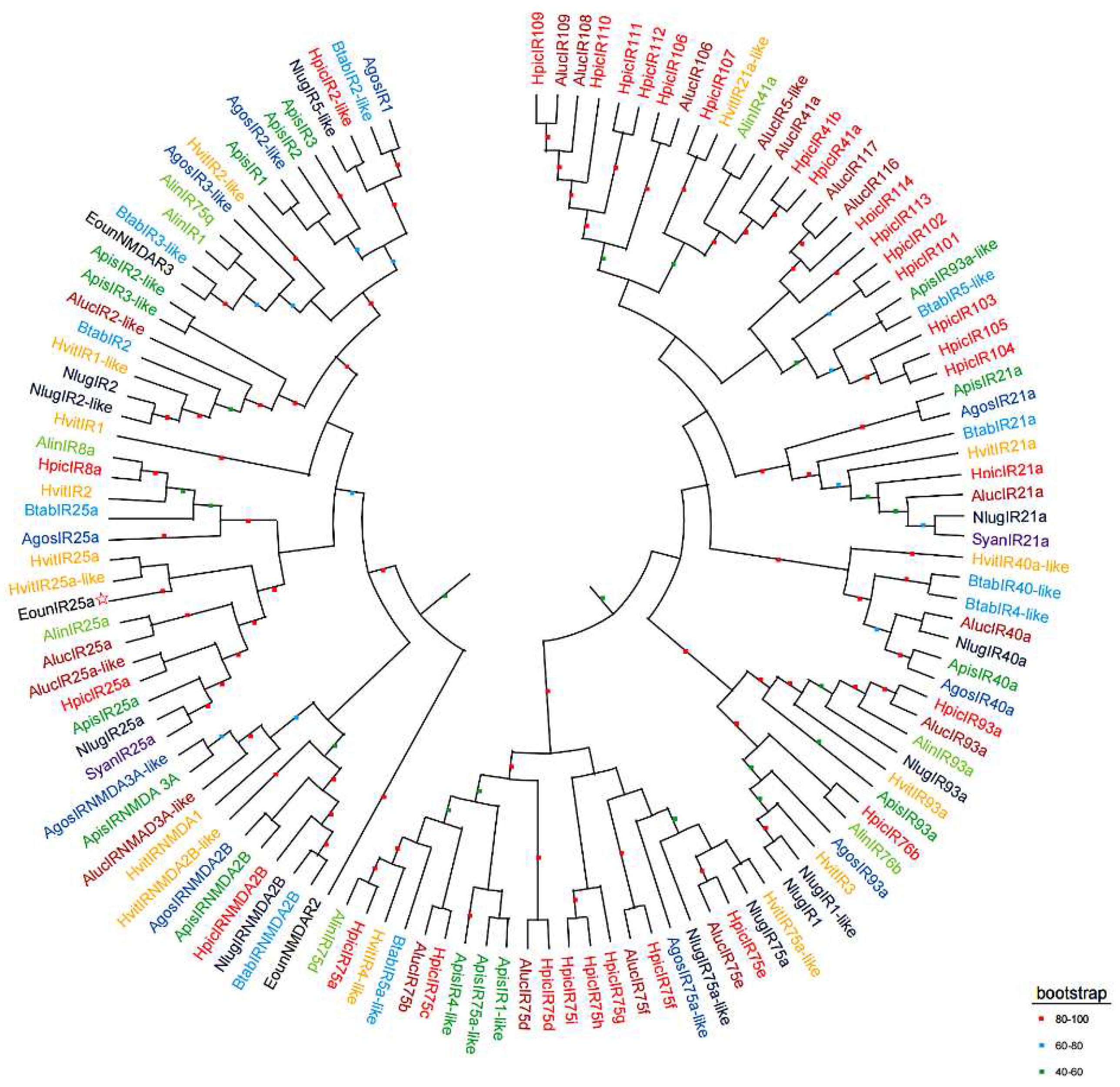
2.2. Multiple sequence alignment and phylogenetic tree analysis
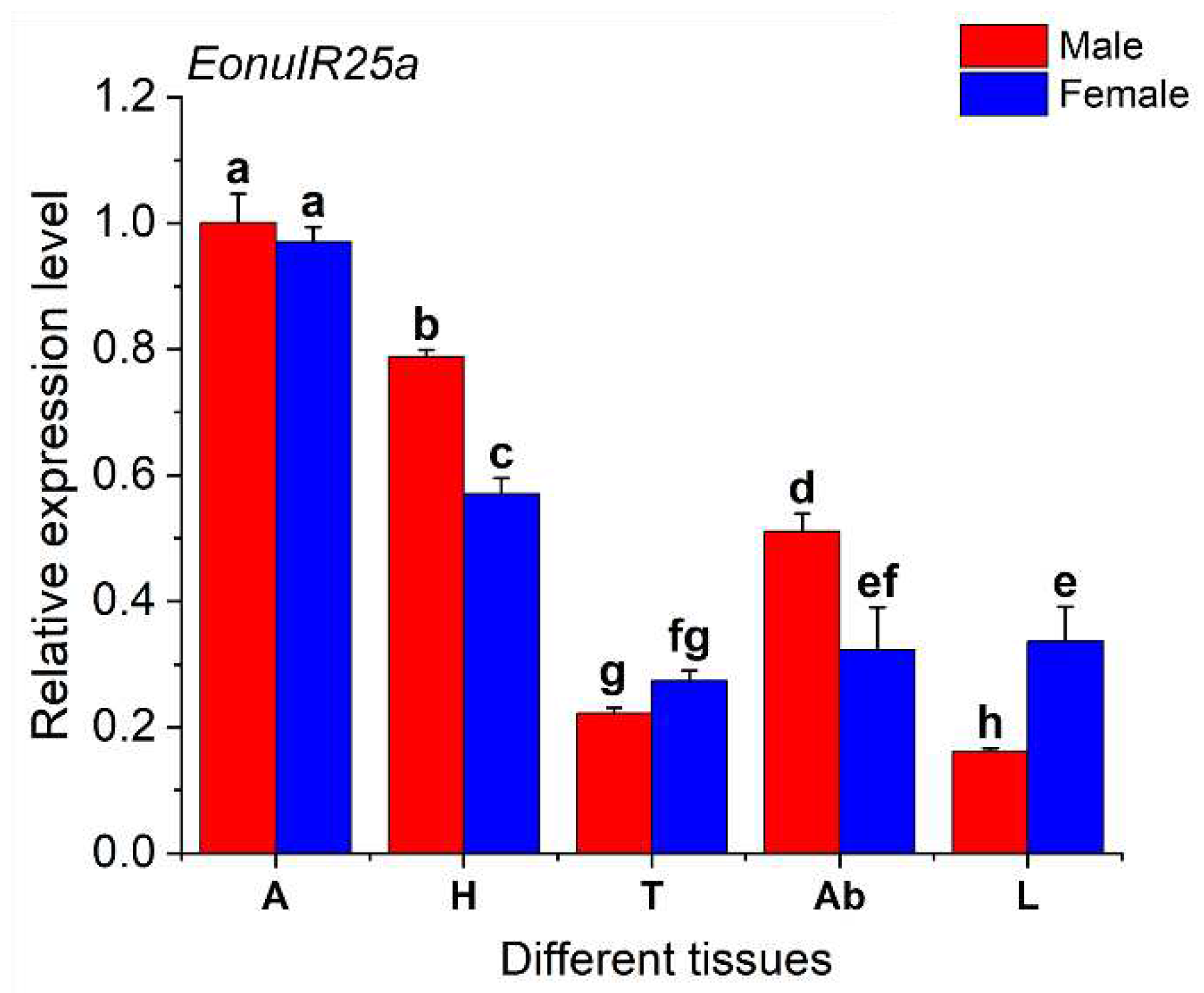
2.3. Tissue expression analysis
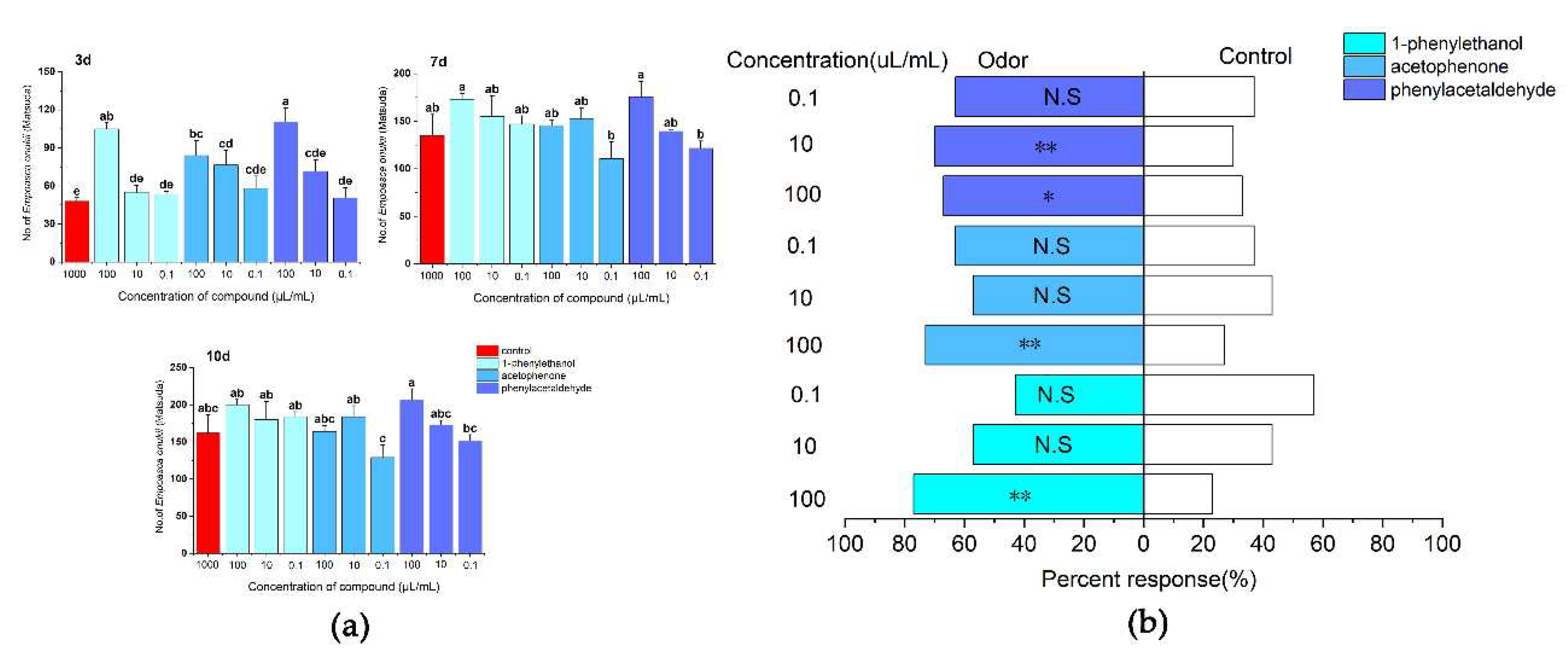
2.4. Correspondence of the compounds by E. onukii
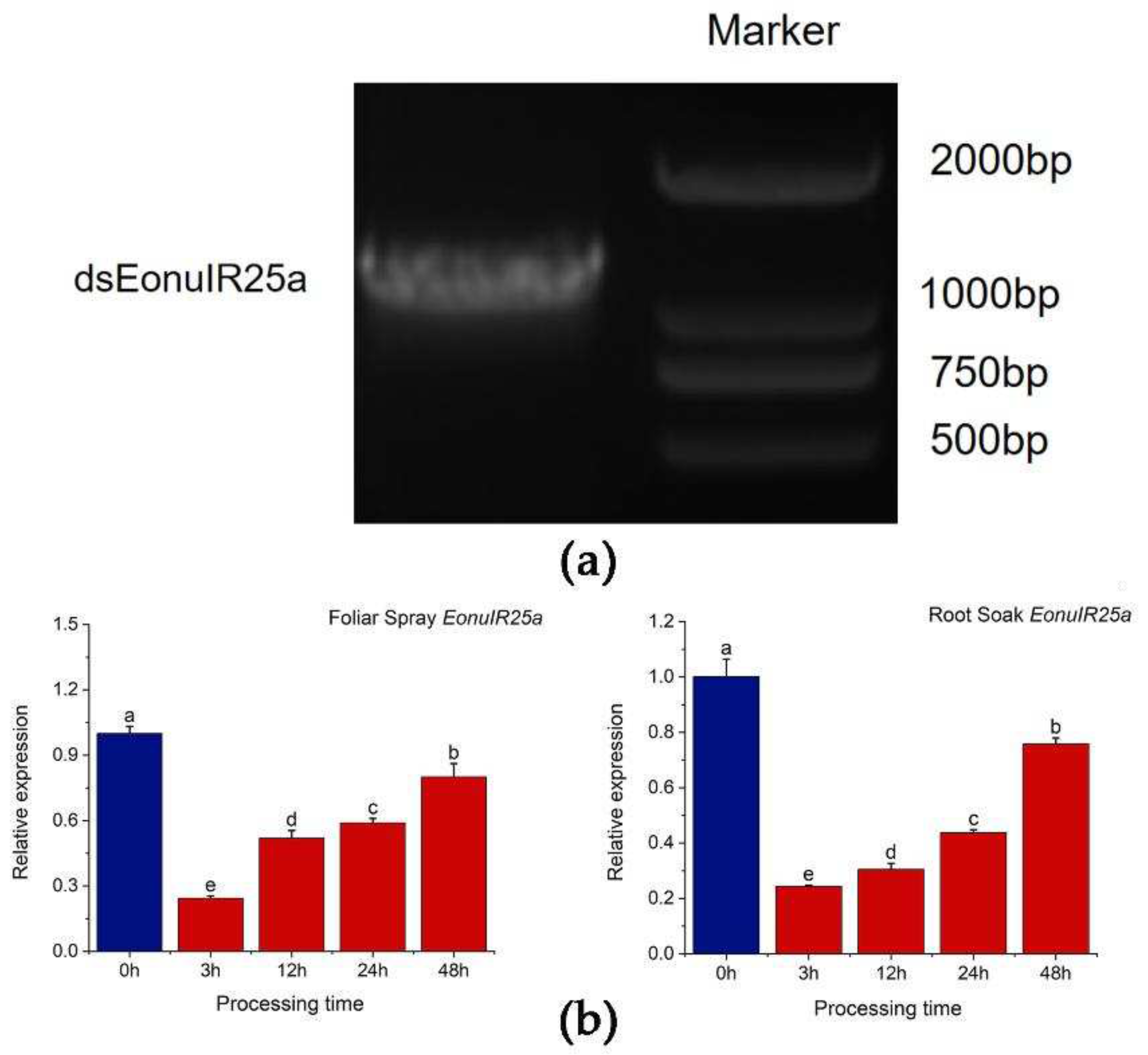
2.5. RNA synthesis and analysis of interference efficiency
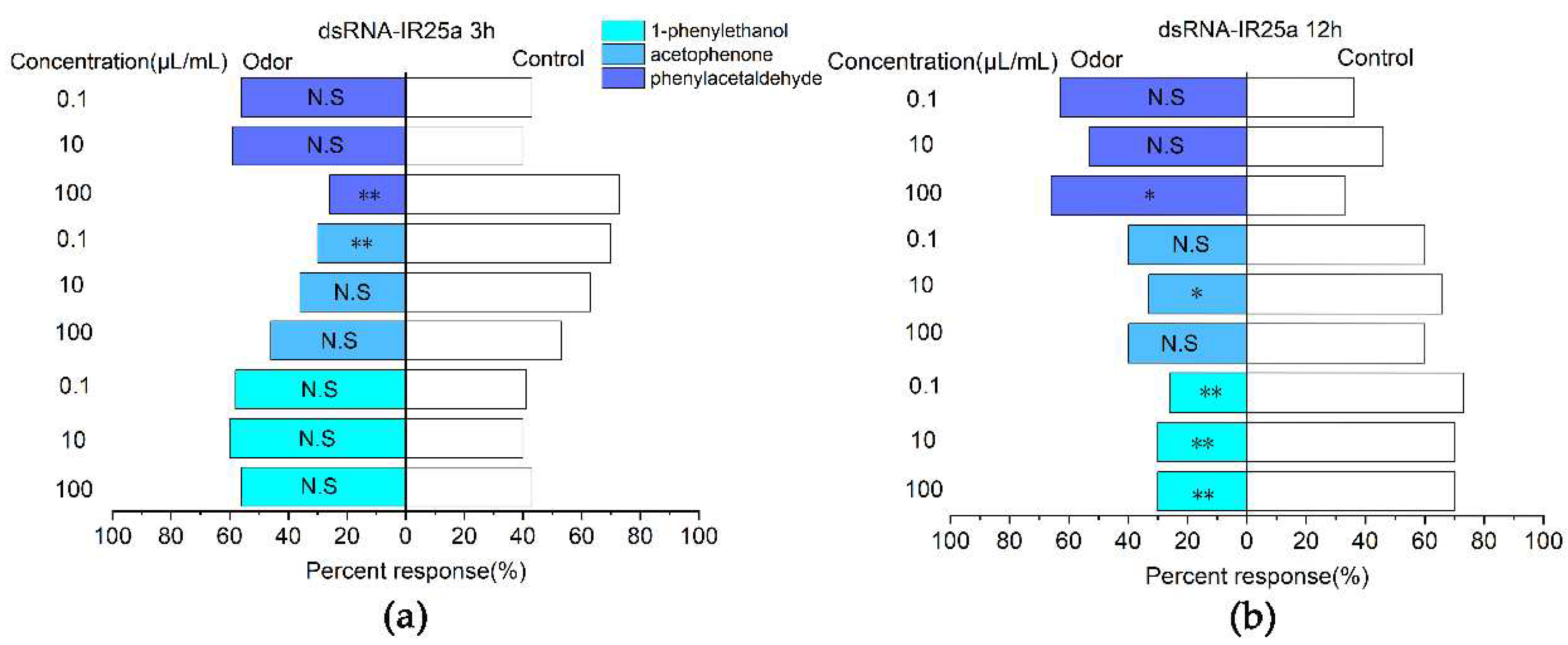
2.6. Changes in the response of E. onukii to compounds after silencing EonuIR25a
3. Discussion
4. Materials and methods
4.1. Insect culture
4.2. Total RNA isolation and RT-PCR
4.3. Cloning and nucleotide sequencing
4.4. Bioinformatics analysis
4.5. Phylogenetic analysis
4.5. Expression in Several Tissues Using qRT-PCR
4.6. Field experiment
4.7. Olfactometer Bioassays
4.8. dsRNA Synthesis
4.9. Feeding method to interfere with EnouIR25a in E. onukii
4.10. Data Statistics and Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Silva Pinto, M. Tea: a new perspective on health benefits. Food. Res. Int. 2013, 53, 558-567. [CrossRef]
- Ye, G. Y.; Xiao, Q.; Chen, M.; Chen, X. X.; Yuan, Z. Y.; Stanley, D. W.; Hu, C. Tea: biological control of insect and mite pests in China. Biol. Control. 2014, 68, 73-91. [CrossRef]
- Xin, Z. J.; Li, X. W.; Bian, L.; Sun, X. L. Tea green leafhopper, Empoasca vitis, chooses suitable host plants by detecting the emission level of (3Z)-hexenyl acetate. B. Entomol. Res. 2017, 107, 77-84. [CrossRef]
- Silva, L. A brief history of biochemical genetics’ 50 years and a reflection about past and present research directions. Biochem. Genet. 2018, 56, 1-6. [CrossRef]
- Gadenne, C.; Barrozo, R. B.; Anton, S. Plasticity in insect olfaction: To smell or not to Smell? Annu. Rev. Entomol. 2016, 61, 317-333. [CrossRef]
- Wang, Z. H.; Yang, F.; Sun, A.; Song, J. Y .; Shan, S.; Zhang, Y. J.; Wang S. N. Expressional and functional comparisons of five clustered odorant binding proteins in the brown marmorated stink bug Halyomirpha halys. Int. J. Biol. Macromol. 2022, 209, 1352-1358. [CrossRef]
- Conchou, L.; Lucas, P.; Meslin, C.; Proffit, M.; Staudt, M.; Renou, M. Insect odorscapes: from plant volatiles to natural olfactory scenes. Front. Physiol. 2019, 10, 972. [CrossRef]
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger. J. Access to the odor world: olfactory receptors and their role for signal transduction in insects. Cell. Mol. Life. Sci. 2018, 75, 485-508. [CrossRef]
- Robertson, H. M. Molecular evolution of the major arthropod chemoreceptor gene families. Annu. Rev. Entomol. 2019, 64, 227-242. [CrossRef]
- Leal, W. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373-391. [CrossRef]
- Pelosi, P .; Iovinella, I.; Zhu, J.; Wang, G. R.; Dani, F. R. Beyond chemoreception: Diverse tasks of soluble olfactory proteins in insects. Biol. Rev. 2018, 93, 184-200. [CrossRef]
- Benton, R.; Vannice, K. S; Gomez-Diaz, C.; Vosshall, L. B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009, 136, 149-162. [CrossRef]
- Mayer, M. L.; Armstrong, N. Structure and function of glutamate receptor ion channels. Annu. Rev. Physiol. 2004, 66, 161-181. [CrossRef]
- Armstrong, N.; Sun, Y.; Chen, G. Q.; Gouaux, E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998, 395, 913-917. [CrossRef]
- Kuner, T.; Seeburg, P. H.; Guy, H. R. A common architecture for K+ channels and ionotropic glutamate receptors?Trends. Neurosci. 2003, 26, 27-32. [CrossRef]
- Croset, V.; Rytz, R.; Cummins, S. F.; Budd, A.; Brawand, D.; Kaessmann, H.; Gibson, T. J.; Benton, R. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS. Genet. 2010, 6, e1001064. [CrossRef]
- Chen, C. H.; Buhl, E.; Xu, M.; Croset, V.; Rees, J. S.; Lilley, K. S.; Benton, R.; Hodge, J. J. L.; Stanewsky, R. Drosophila Ionotropic Receptor 25a mediates circadian clock resetting by temperature.Nature. 2015, 527, 516-520. [CrossRef]
- Lee, Y.; Poudel, S.; Kim, Y.; Thakur, D.; Montell, C. Calcium taste avoidance in Drosophila. Neuron. 2018, 97, 67-74. [CrossRef]
- Steck, K.; Walker, S. J.; Itskov, P. M.; Baltazar, C.; Moreira, J. M.; Ribeiro, C. Internal amino acid state modulates yeast taste neurons to support protein homeostasis in Drosophila. Elife. 2018, 7, e31625. [CrossRef]
- Sánchez-Alcañiz, J. A.; Silbering, A. F.; Croset, V.; Zappia, G.; Sivasubramaniam, A. K.; Abuin, L.; Sahai, S. Y.; Münch, D.; Steck, K.; Auer, T. O.; Cruchet, S.; Neagu-Maier G. Larisa.; Sprecher, S. G.; Ribeiro, C.; Yapici, N.; Benton R. An expression atlas of variant ionotropic glutamate receptors identifies a molecular basis of carbonation sensing. Nat. Commun. 2018, 9(1), 4252. [CrossRef]
- Wang, S. N.; Peng, Y.; Lu, Z.Y.; Dhiloo, K. H.; Zheng, Y.; Shan, S.; Li, R. J.; Zhang, Y. J.; Guo, Y. Y. Cloning and expression profile of ionotropic receptors in the parasitoid wasp Microplitis mediator (Hymenoptera: Braconidae). J. Insect. Physiol. 2016, 90, 27-35. [CrossRef]
- Rimal, S.; Lee, Y. The multidimensional ionotropic receptors of Drosophila melanogaster. Insect. Mol. Biol. 2018, 27, 1-7. [CrossRef]
- Knecht, Z. A.; Silbering, A. F.; Ni, L.; Klein, M.; Budelli, G.; Abuin, L.; Ferrer, A. J.; Samuel, A. D. T. Distinct combinations of variant ionotropic glutamate receptors mediate thermosensation and hygrosensation in Drosophila. Elife. 2016, 5, e17879. [CrossRef]
- Raji, J. I.; Melo, N.; Castillo, J. S.; Gonzalez, S.; Saldana, V.; Stensmyr, M. C.; DeGennaro, M. Aedes aegypti mosquitoes detect acidic volatiles found in human odor using the IR8a pathway. Curr. Biol. 2019, 29, 1253-1262. [CrossRef]
- Liu, C.; Jason, Pitts, R., Bohbot, J. D., Jones, P. L., Wang, G., Zwiebel, L. J. Distinct olfactory signaling mechanisms in the malaria vector mosquito Anopheles gambiae. PLoS. Biol. 2010, 8, 27-28. [CrossRef]
- Tang, R.; Jiang, N. J.; Ning, C., Li, C.; Huang, L. Q.; Wang, C. Z. The olfactory reception of acetic acid and ionotropic receptors in the Oriental armyworm, Mythimna separata Walker. Insect. Biochem. Mol. Biol. 2020, 118, 103312. [CrossRef]
- Zhang, J.; Bisch-Knaden, S.; Fandino, R. A.; Yan, S.; Obiero, G. F.; Grosse-Wilde, E.; Hansson, B. S.; Knaden, M. The olfactory coreceptor IR8a coverns larval fecesmediated competition avoidance in a hawkmoth. PNAS. 2019, 116(43), 21828-21833. [CrossRef]
- Pitts, R. J.; Derryberry, S. L.; Zhang, Z. W.; Zwiebel, L. J. Variant ionotropic receptors in the malaria vector mosquito Anopheles gambiae tuned to amines and carboxvlic acids. Sci. Rep. 2017, 7, 40297. [CrossRef]
- Ahn, J. E.; Chen, Y.; Amrein, H. Molecular basis of fatty acid taste in Drosophila. Elife. 2017, 6, e30115. [CrossRef]
- Frank, D. D.; Enjin, A.; Jouandet, G. C.; Zaharieva, E. E.; Para, A., Stensmyr, M. C., Gallio, M. Early integration of temperature and humidity stimuli in the Drosophila brain. Curr. Biol. 2017, 27, 2381-2388. [CrossRef]
- Budelli, G., Ni, L., Berciu, C., van Giesen, L., Knecht, Z. A., Chang, E. C., Kaminski, B.; Silbering, A. F.; Samuel , A.; Klein, Mason.; Benton R.; Nicastro D.; Garrity P. A. Ionotropic receptors specify the morphogenesis of phasic sensors controlling rapid thermal preference in Drosophila. Neuron. 2019, 101, 738-747. [CrossRef]
- Ni, L. The structure and function of ionotropic receptors in Drosophila. Front. Mol. Neurosci. 2021, 13, 638839. [CrossRef]
- Rytz, R.; Croset, V.; Benton, R. Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect. Biochem. Molec. 2013, 43(9): 888-897. [CrossRef]
- Benton, R.; Vannice, K. S.; Gomez-Diaz C.; Vosshall, L. B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell, 2009, 136: 149-162. [CrossRef]
- Wang, T. T.; Hao, Y. J.; He, Z. B.; Met, T.; Chen, B. Characteristics and classification position of the ionotropic receptor genes IR8a and IR25a in four vector mosquito species of medical importance. Acta. Entomol. Sin. 2017, 60(4), 379-388. [CrossRef]
- Guo, M.; Krieger, J.; Grosse-Wilde, E.; Missbach, C.; Zhang, L.; Breer, H. Variant ionotropic receptors are expressed in olfactory sensory neurons of coeloconic sensilla on the antenna of the desert locust (Schistocerca gregaria). Int. J. Biol. Sci. 2014, 10(1), 1-14. [CrossRef]
- Ganguly, A.; Pang, L.; Duong, V. K.; Lee, A.; Schoniger, H.; Varady, E.; Dahanukar, A. A molecular and cellular context-dependent role for Ir76b in detection of amino acid taste. Cell. Rep. 2017, 18(3), 737-750. [CrossRef]
- Ai, M.; Min, S.; Grosjean, Y.; Leblanc, C.; Bell, R.; Benton, R.; Suh, G. S. B. Acid sensing by the Drosophila olfactory system. Nature. 2010, 468(7324), 691-695. [CrossRef]
- Min, S.; Ai, M.; Shin, S. A.; Suh, G. S. Dedicated olfactory neurons mediating attraction behavior to ammonia and amines in Drosophila. PNAS. 2013, 110(14), E1321-E1329. [CrossRef]
- Hussain, A.; Zhang, M.; Ucpunar, H. K.; Syensson, T.; Quillery, E.; Gompel, N.; Ignell, Rickard.; Grunwald Kadow, I. C. Ionotropic chemosensory receptors mediate the taste and smell of polyamines. PLoS. Biol. 2016, 14(5), e1002454. [CrossRef]
- Chen, Y.; Amrein, H. Ionotropic receptors mediate Drosophila oviposition preference through sour gustatory receptor neurons. Curr. Biol. 2017, 27(18), 2741-2750. [CrossRef]
- Ai, M.; Blais, S.; Park, J. Y.; Min, S.; Neubert, T. A.; Suh, G. S. Ionotropic glutamate receptors IR64a and IR8a form a functional odorant receptor complex in vivo in Drosophila. J. Neurosci. 2013, 33(26): 10741-10749. [CrossRef]
- Gregg, P. C.; Del Socorro, A. P.; Hawes, A. J.; Binns, M. R. Developing bisexual attract-and-kill for polyphagous insects: ecological rationale versus pragmatics. J. Chem. Ecol. 2016, 42, 666-675. [CrossRef]
- Di, C.; Ning, C.; Huang, L. Q.; Wang, C. Z. Design of larval chemical atractants based on odorant response spectra of odorantreceptors in the cotton bollworm. Insect . Biochem. Mol. Biol. 2017, 84, 48-62. [CrossRef]
- Shepherd, W. P.; Sullivan, B. T . Southern pine beetle, Dendroctonus frontalis, antennal and behavioral responses to nonhost leaf and bark volatiles. J. Chem. Ecol. 2013, 39(4), 481-493. [CrossRef]
- Wang, C.; Li, G. N.; Miao, C. J.; Zhao, M.; Wang, B.; Guo, X. R. Nonanal modulates oviposition preference in female Helicoverpa assulta (Lepidoptera: Noctuidae) via the activation of peripheral neurons. Pest. Manag. Sci. 2020, 76(9), 3159-3167. [CrossRef]
- Magsi, F. H.; Luo, Z. X.; Zhao, Y. J.; Li, Z. Q.; Cai, X. M.; Bian, L.; Chen Z. M. Electrophysiological and behavioral responses of Dasychira baibarana (Lepidoptera: Lymantriidae) to tea plant volatiles. Environ. Entomol. 2021, 50(3), 589-598. [CrossRef]
- Hou, X. Q.; Zhang, D. D.; Powell, D.; Wang, H. L.; Andersson, M. N.; Löfstedt, C. Ionotropic receptors in the turnip moth Agrotis segetum respond to repellent medium-chain fatty acids. BMC. Biol. 2022, 20(1), 1-19. [CrossRef]
- Schutter, K. D.; Taning, C. N. T .; Daele, L. V.; Van Damme, E. J. M.; Dubruel, P.; Smagghe, G. RNAi-based biocontrol products: market status, regulatory aspects, and risk assessment. Front. Insect. Sci. 2022, 1, 818037. [CrossRef]
- Christiaens, O.; Whyard, S.; Vélez, A. M.; Smagghe, G. Double-stranded RNA technology to control insect pests: current status and challenges. Front. Plant. Sci. 2020, 11, 451. [CrossRef]
- Souza, D.; Christensen, S. A.; Wu, K.; Buss, L.; Kleckner, K.; Darrisaw, C.; Shirk, P. D.; Siegfried, B. D. RNAi-induced knockdown of white gene in the southern green stink bug (Nezara viridula L.). Sci. Rep. 2022, 12(1), 10396. [CrossRef]
- Joga, M. R.; Zotti, M.; Smagghe, G.; Christiaens, O. RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: what we know so far. Front. Physiol. 2016, 7, 553. [CrossRef]
- Kunte, N.; Mcgraw, E.; Bell, S.; Held, D.; Avila, L. Prospects, challenges and current status of RNAi through insect feeding. Pest. Manag. Sci. 2020, 76(1), 26-41. [CrossRef]
- Andrade, E. C.; Hunter, W. B. RNAi feeding bioassay: development of a non-transgenic approach to control Asian citrus psyllid and other hemipterans. Entomol. Exp. Appl. 2017, 162(3), 389-396. [CrossRef]
- Yan, S.; Qian, J.; Cai, C.; Ma, Z. Z.; Li, J. H.; Yin, M. Z.; Ren, B.; Shen, J. Spray method application of transdermal dsRNA delivery system for efficient gene silencing and pest control on soybean aphid Aphis glycines. J. Pest .Sci. 2020, 93: 449-459. [CrossRef]
- Li, H.; Bowling, A.J.; Gandra, P.; Rangasamy, M.; Pence, H. E.; McEwan, R. E.; Khajuria, C.; Siegfried, B. D.; Narva, K. E. Narva Systemic RNAi in western corn rootworm, Diabrotica virgifera virgifera, does not involve transitive pathways. Insect. Sci. 2018, 25, 45-56. [CrossRef]
- Khajuria, C.; Velez, A. M.; Rangasamy, M.; Wang, H. C.; Fishilevich, E.; Frey, M. L. Parental RNA interference of genes involved in embryonic development of the western corn rootworm, Diabrotica virgifera virgifera LeConte. Insect. Biochem. Mol. Biol. 2015, 63, 54-62. [CrossRef]
- Ramaseshadri, P.; Segers, G.; Flannagan, R.; Wiggins, E.; Clinton, W.; Ilagan, O.; McNulty, B.; Clark, T.; Bolognesi, R. Physiological and cellular responses caused by RNAi-mediated suppression of Snf7 orthologue in western corn rootworm (Diabrotica virgifera virgifera) larvae. PLoS. One. 2013, 8(1), e54270. [CrossRef]
- Zhu, K.Y.; Palli, S. R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293-311. [CrossRef]
- Cui, Y. Y.; Wang, J. G.; Liu, Q. Y.; Li, D. M.; Zhang, W.; Liu, X. B.; Wang, J.; Song, X. P.;·Yao, F.; Wu, H. X.; Zhao, N. Identification and expression of potential olfactory-related genes related to Niemann–Pick C2 protein and ionotropic receptors in Haemaphysalis longicornis. Exp. Appl. Acarol. 2022, 87(4), 337-350. [CrossRef]
- Zhang, Y.; Yang, B. Y.; Yu, J.; Pang, B. P.; Wang, G. R. Expression profiles and functional prediction of ionotropic receptors in Asian corn borer, Ostrinia furnacalis (Lepidoptera: Crambidae). J. Integr. Agr. 2022, 21(2), 474-485. [CrossRef]
- Wicher, D.; Miazzi, F. Functional properties of insect olfactory receptors: ionotropic receptors and odorant receptors. Cell. Tissue. Res. 2021, 383: 7-19. [CrossRef]
- 61. Yin, N. N.; Nuo, S. M.; Xiao, H. Y.; Zhao, Y. J.; Zhu, J.Y.; Liu, N. Y. The ionotropic receptor gene family in Lepidoptera and Trichoptera: Annotation, evolutionary and functional perspectives. Genomics. 2021, 113(1), 601-612. [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731-2739. [CrossRef]
- Livak, K. J.; Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001, 25(4), 402-408. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




