1. Introduction
Chlamydia trachomatis (CT) is an obligate intracellular human pathogen known to cause the blinding eye disease trachoma, and sexually transmitted infections and their sequelae such as prostatitis, epididymitis, pelvic inflammatory disease, ectopic pregnancy, and infertility, particularly when left untreated with antibiotics [1-3].
CT proliferation in host epithelial cells is characterized by two distinct developmental stages: the infectious, extracellular form called elementary body (EB) and the intracellular, replicative form termed reticulate body (RB). The EB, which is 0.2-0.6 μm in diameter, has an inner and outer membrane, and variable periplasmic space. They are round, highly electron-dense, and metabolically inactive [
4]. The RB is larger, with a diameter of 0.6-1.5 μm, more pleomorphic, and metabolically active [
5]. Infection is initiated when the EB attaches to a susceptible epithelial cell. EBs enter cells by phagocytosis, pinocytosis (in non-clathrin coated pits), or receptor-mediated endocytosis (in clathrin-coated pits, particularly in polarized epithelial cells) [
3]. These mechanisms provide flexibility for chlamydiae in different mucosal environments to attach to and enter epithelial cells that carry vastly different physiological functions [
3]. Because lysosomal fusion is inhibited by unknown mechanisms, EBs are able to reside in a protected membrane-bound vesicle called an inclusion [
6]. Within host epithelial cells, CT appears to survive through its ability to inhibit fusion between the entry vacuoles and host cell lysosomes [
2,
7]. Several hours after internalization, the EBs differentiate into non-infectious reticulate bodies (RBs) that proliferate within a vacuole termed chlamydial inclusion. Within 24 hours, large inclusion bodies with up to a thousand bacteria may appear. After approximately 2 days of infection, the RBs differentiate back to EBs, the EBs are released from the infected cell, and a new cycle of infection can begin. The entire process can occur with the infected epithelial cell having minimal or insignificant damage, elucidating why chlamydial infections tend to be chronic and why the associated diseases and complications are immune-mediated [
3].
Dendritic cells (DCs) play a key role in eliciting the cellular immune response by presenting chlamydial proteins to T lymphocytes of the cell-mediated immune system [
8]. In vivo, the importance of DC is likely related to their dynamic presence in mucosal tissues, their motility and ability to transport antigens from the mucosal epithelium to the draining mucosal inductive sites [9-11], and their efficient processing and presentation of antigens [
8]. In microbial infections, DCs limit bacterial ingestion by entering the maturation program, downregulating phagocytosis, and enhancing migratory activity [
12].
Experimental in vitro infections of DC with CT have mainly focused on anti-chlamydial immune responses and have not described in detail the developmental cycle of CT in DC. This study was conducted with the aim of identifying whether DC can be infected with CT and whether the bacteria can survive and multiply within DC. Previous findings by Matyszak
et al. [
13] showed that CT do not readily proliferate in DC. Datta
et al. [
8] demonstrated that the host-pathogen interaction in chlamydia infection is serovar specific, with serovars Ba and D predominantly suffering degradation within DCs. Previous studies have also shown that once DCs are stimulated by bacterial products, such as lipopolysaccharide (LPS), they downregulate bacterial ingestion and undergo maturation [
12,
14], thus, no increase in chlamydial RBs between 4 to 48 h after infection should occur if CT were not capable of reproducing within DC. However, in the present study, fluorescence microscopy revealed that infected DC can support the growth of CT serovar F. Chlamydial elementary and reticulate bodies (RBs) were detected and RB was shown to increase in size and number over the 48-h infection. Intracellular EB was detected at the latest time point (48 h), indicating a transformation of RB to EB, but the infectivity of such progeny was not determined. Together with future investigations on the ultrastructure and viability of EB and RB within DC, the results gathered here will provide new insights into the possible participation of DCs in sustaining chronic or latent infection, or even dissemination of infection to other sites of infection.
2. Materials and Methods
2.1. Bacterial strain and cultivation
Chlamydia trachomatis serovar F, VR 346 (American Type Culture Collection, Manassas, VA) was cultivated in HeLa 229 cell cultures maintained in an antibiotic-free medium consisting of RPMI 1640 with GlutamaxTM (Invitrogen Corporation, UK) and 10% fetal calf serum (Invitrogen Corporation, UK), at 37 °C in a humidified 5% CO2 atmosphere.
HeLa 229 (ATCC, Manassas, VA) cell cultures were maintained in RPMI 1640 (with GlutamaxTM) medium, supplemented with 10% fetal calf serum, and 1% antibiotics consisting of penicillin (100 units/mL), streptomycin (100 μg/mL), and amphotericin B (0.25 μg/mL) (Invitrogen Corp., UK) at 37 °C in a humidified 5% CO2 atmosphere. For maintenance of stock cultures and use in CT cultivation, HeLa cultures were split on average of 1:4, every 3-5 days.
HeLa cell cultures were infected with 0.5 mL of CT (resuspended in RPMI) per tissue culture flask and then subjected to centrifugation (Eppendorf Centrifuge 5810 R) at 2000 rpm for 30 min and incubation at 37 °C in 5% CO2. After incubation of 5 days, CT was harvested from trypsinised HeLa cells using the following steps: (1) ultrasonication (Branson Ultrasonicator) for 5 minutes, (2) centrifugation at 800 rpm for 6 minutes, and (3) centrifugation of collected supernatant (from #2) at 4000 rpm for 15 minutes. CT harvests were either aliquoted for freezing in liquid nitrogen or used directly to infect fresh HeLa or human dendritic cell cultures.
2.2. Quantitative analysis of Chlamydia trachomatis (CT) concentration
The concentration of CT used in the infection of human dendritic and HeLa cell cultures was determined by spectrophotometry and direct microscopic count. The optical density of CT (diluted in phosphate-buffered saline) was measured at wavelengths 400, 500, and 600 nm before each infection using the Beckman DU® 640 Spectrophotometer. A direct microscopic count on an area of 25 x 106 mm2 on the cytoslide was also performed to determine the bacterial count in the CT dilutions.
2.3. In-vitro generation of human dendritic cells
Peripheral blood mononuclear cells were isolated from various donors of buffy coats (Australian Red Cross Blood Service, Perth) by density gradient centrifugation with Ficoll Paque (Pharmacia Biotech). The resulting peripheral blood mononuclear cells were resuspended in RPMI 1640 with GlutamaxTM medium, supplemented with 10% fetal calf serum and 1% antibiotics (penicillin, 100 units/mL; streptomycin, 100 μg/mL; and amphotericin B, 0.25 μg/mL). The peripheral blood mononuclear suspensions were then dispensed in tissue culture flasks and incubated at 37 °C in 5% CO2 for monocytes to adhere. After 1-h incubation, nonadherent cells were removed by washing with phosphate buffered saline (PBS). Adherent cells were then grown in RPMI 1640 with GlutamaxTM medium containing 5% fetal calf serum and 1% antibiotics. To obtain dendritic cells, cytokines granulocyte-macrophage colony stimulating factor (GM-CSF) and interleukin-4 (IL-4), obtained from R&D Systems, Inc., were added at 10 μl/mL.
2.4. Infection of dendritic cells with Chlamydia trachomatis
Dendritic cells (DCs), at day 5 of culture, were collected and co-incubated with CT for 45 minutes at 37 °C and distributed to tissue culture flasks with RPMI and 5% fetal calf serum. To monitor developmental events of Chlamydial infection in dendritic cells, infected DCs were fixed with 4% paraformaldehyde in PBS at 0, 4, 24, and 48 h post-infection. A second experimental setting, where DCs were infected with different concentrations of CT (1, 0.5, 0.25, and 0.125), was established to test the influence of CT concentration on DC infection. Infected cells from this experimental setting were fixed with 4% paraformaldehyde in PBS at 24 h post-infection.
2.5. Immunocytochemistry
Infected and uninfected (control) DC samples were prepared in two sets for the purpose of doing separate staining for CT elementary bodies and reticulate bodies.
In preparation for staining, control and infected DCs were centrifuged in the Shandon cytospin (80 μl sample/slide), permeabilized with Triton X-100 and washed in PBS. To detect elementary bodies, a three-step method was done. Specimens were incubated in primary goat polyclonal anti-Chlamydia trachomatis (Fitzgerald) at 1:25 (30 minutes), and then in biotinylated anti-goat antibody (Vector Laboratories Inc., CA) at 1:50 (30 minutes). The biotin was visualized by reacting with streptavidin-fluorescein (Amersham Pharmacia Biotech, UK) at 1:50 (30 minutes). To detect reticulate bodies, samples were incubated in primary rabbit polyclonal anti-Chlamydia (Fitzgerald) at 1:100 (30 minutes) and probed with a goat anti-rabbit antibody-Alexa Fluor® 488 conjugate (Molecular Probes) at 1:50 (30 minutes). Specimens were washed twice in PBS in between the incubations. Specimens were co-stained with DAPI at 1:100 in the last step of each staining to detect the nuclei. Mounting was done with Aquaperm (Shandon). To test the specificity of the primary antibodies, a negative control (where the primary antibody was omitted) was used.
2.6. Microscopy and Imaging
Specimens were examined with the Leica DMR Microscope in the Monster + Leica microscope (upright) station at the Image Acquisition and Analysis Facility (University of Western Australia). Examination and image photography (MTI Dage camera) were done with 1000x magnification. Image-pro software was used for image editing.
3. Results
3.1. Detection of Elementary and Reticulate Bodies of Chlamydia trachomatis in Dendritic Cells
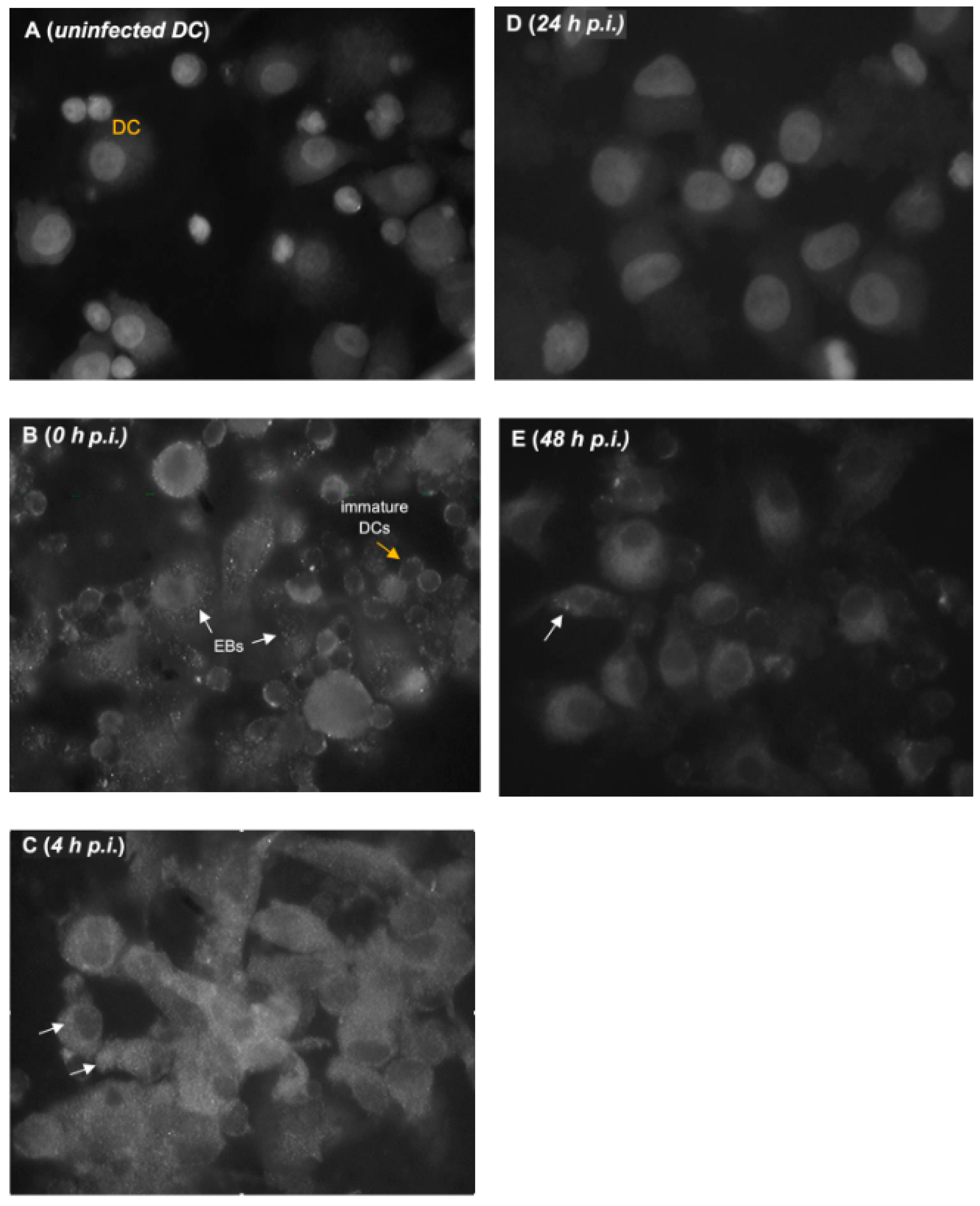
Figure 1 shows representative results of CT infection in DCs, showing elementary bodies (EB) in DCs at various time points. At 0 h when DCs were mostly round and immature, EBs were extracellular and hardly detected in the cell cytoplasm (
Figure 1B). In host cells, EB typically attaches to the cell surfaces for entry at this time point. As described by Matyszak
et al. [
13], the entry of CT into DC is mediated by the attachment of CT to heparan sulfates. In contrast to the DCs observed at 0 h, DCs exposed to live EBs at 4 h post-infection acquired a mature DC morphology (
Figure 1C). Intracellular EBs were visible while extracellular EBs not taken up by DCs were still evident. Of note, is the absence of EBs at 24 h (
Figure 1D). This confirms the differentiation of EBs into RBs that are typically proliferating at 24 h (
Figure 2D). Intracellular EBs were again detected at 48 h, which may suggest that RB can convert back to EB in DC (
Figure 2E). This observation, however, is true for only a few DCs in the culture. This may also indicate that infectious progeny can be produced within DCs. To determine infectivity of these EB, repassaging of cells on fresh DC cultures will be necessary. No release of EB from DC was obvious and no extracellular EB was detected at this time point. Negative staining in the control (uninfected) DCs confirmed that staining for EB in infected cells was specific (
Figure 1A).
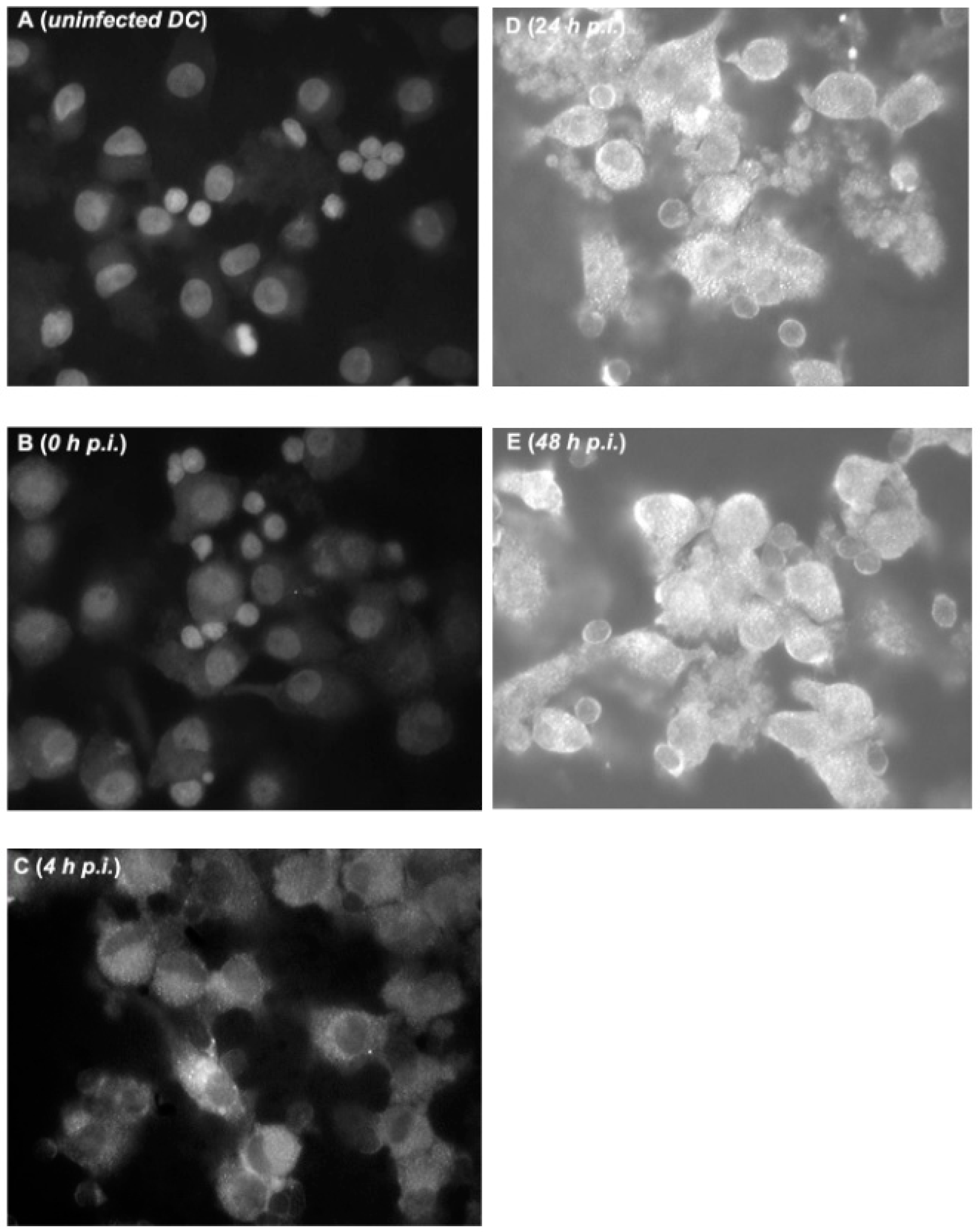
Figure 2 (A-E) shows the gradual increase in the number and size of reticulate bodies (RB) over the 48-h cycle in DCs.
Figure 2C shows representative results of obvious RB in DCs as early as 4 h. This is consistent with results in host cells where, as early as 2 h after infection, EB begins to differentiate into RB, although no increase in size is yet apparent [
15].
Figure 2C to 2E show that from 4 to 48 h, RB multiplied within DCs as demonstrated by an increased number, size, intensity, and number of positive spots. In the CT developmental cycle, RB reaches a mature size by 12 h post-infection [
15]. It is also known that the inclusion expands to accommodate the RB’s increase in size and number. In this study, the peak of labeling was at 24 h where all DCs stained positive for RB (
Figure 2D), with RB particles almost occupying the entire cytoplasm of each cell. At 48 h, RBs were still largely evident in almost all cells (
Figure 2E). However, many RB detected at this time point did not revert to EB as shown in
Figure 1E.
3.2. Dendritic Cell Infection with Different Concentrations of Chlamydia trachomatis
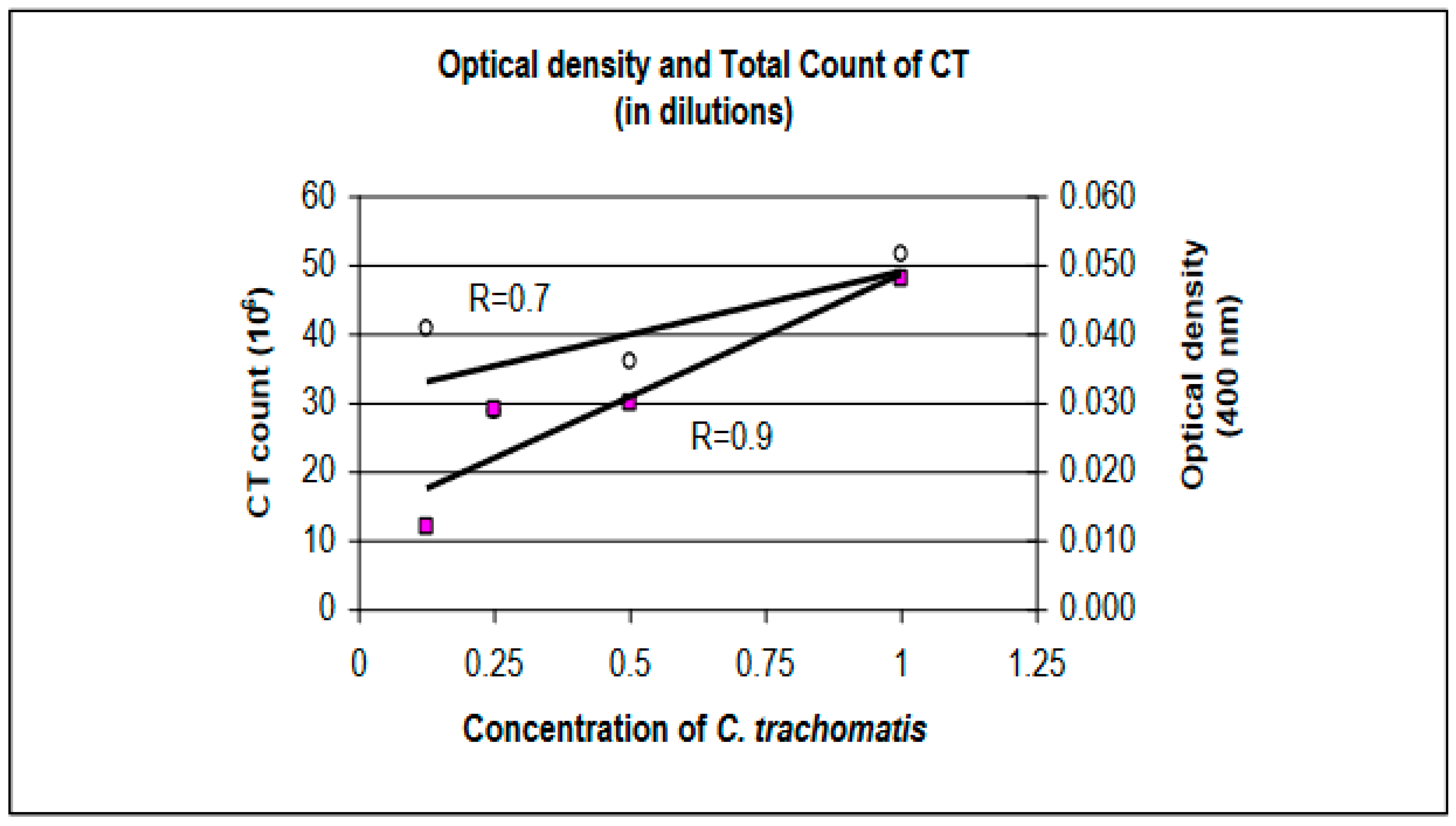
With decreasing concentrations of CT, a corresponding decrease in OD was measured (
Table 1). However, results from the direct count did not correlate with those from optical density (
Figure 3). With decreasing CT concentrations, the number of CT (n) in each dilution was expected to drop but an increase in the total count from the 0.125 concentration was observed (n higher than in 0.5 concentration). A possible cause for this increase may be pipetting errors in loading CT into the cytofunnel (e.g. loading more than 80 μl). It may also be due to insufficient vortexing of the dilution that allowed clumped or CT aggregate to be loaded into the cytofunnel and fixed onto the cytoslide.
4. Discussion
Most of the in vitro models developed for Chlamydiae infection involve professional phagocytic cells such as monocytes and macrophages [
16,
17]. The fate of Chlamydiae in these cells has been found to be dependent on the bacterial strain, and particularly among
Chlamydia trachomatis (CT), differed between the trachoma and LGV biovars [
18]. Most chlamydial strains that successfully replicate within phagocytes have the means of impairing host defenses, and this often involves inhibition of phagolysosomal fusion. A few studies have demonstrated that CT also infect and grow in some human and mouse dendritic cells (DCs) [13, 19-22]. However, DCs have been reported to support the survival but not the replication of microbes [
21].
Dendritic cells (DCs) play an important role in initiating and regulating immune responses for protective immunity against
Chlamydia infection as well as the immunopathology that can develop following natural infection. DCs are very efficient in capturing the bacteria, processing them into antigenic peptides, and presenting them to T cells, which then initiate an immune response [
8,
23]. DCs also produce cytokines and chemokines that activate other immune cells, such as T cells and B cells, to help clear the infection. However, their function may also be subverted as part of the life cycle of a pathogen [
24]. The role of DCs in the immunopathological inflammatory processes of
Chlamydia trachomatis (CT) infections has been investigated but little is known about the growth characteristics and survival of the different biovariants of CT in DCs. Among the three biovariants of CT, trachoma (serovars A-C), urogenital (serovars D-K), and lymphogranuloma venereum (L1-L3), only serovars B, D, E, L2 have been investigated in
in vitro models of CT infection. It was therefore the aim of the study to initially determine whether CT serovar F can infect and persist in human DCs.
Here we report that human dendritic cells (DCs) derived from peripheral blood mononuclear cells are susceptible to in vitro
Chlamydia trachomatis (CT) infection. When DCs were generated in the presence of GM-CSF and IL-4, infected with CT at the immature stage (day 5 culture), and incubated with CT for up to 48 h, CT survived and multiplied within the DCs as demonstrated by immunofluorescent staining for elementary and reticulate bodies of CT. The average optical density of CT used to infect the DC cultures was 0.020 units at 400 nm. Although DCs are not considered professional phagocytes, they are capable of considerable phagocyctic activity. Human DCs have been reported to phagocytose
B. burgdorferi [
25],
M. tuberculosis,
L. monocytogenes, influenza virus, measles virus, the protozoans
T. cruzi and
L. donovani, and the fungus
H. capsulatum [
26]. Several organisms have also been reported to survive and multiply intracellularly within DCs. These include
T. cruzi, within human DCs, and
L. monocytogenes,
B. bronchiseptica,
S. enterica subsp. enterica, within murine DC line CB1 [
26]. In murine systems, CT has been described to be internalized and killed by DC [
27]. After a 1-day incubation, no intact bacteria were detected. In contrast, Matyszak
et al. [
13] showed that in human DCs, CT persists in vacuoles that do not fuse with lysosomes. These vacuoles did not develop into characteristic inclusion bodies. In addition, CT taken up by immature DCs did not readily proliferate. Their FACS analysis showed that after uptake of CT or activation of DC, the rate of phagocytosis was dramatically decreased.
In the present study, CT was shown to invade immature DCs. However, the observation of proliferation of reticulate bodies within DCs was inconsistent with those previously described. It should be noted that a different strain (L2 serovar) was used in Matyzsak
et al. study [
13]. This demonstrates that DC susceptibility varies according to different CT strains.
It is well-recognized that DCs are activated by inflammatory stimuli including bacterial products like LPS. It is also known that the rate of phagocytosis is decreased upon uptake of CT or activation of DC. Therefore, there should be a decrease rather than an increase in the number and intensity of chlamydial RBs from 4 hours (when they are internalized in limited number within DC) up to 48 hours, if RBs were killed or were not capable of multiplying. Aside from proliferation, growth in the size of RB particles was evident. This may suggest that CT remains metabolically active within DC. The presence of RBs at 48 h post infection are consistent with the studies of Matyszak
et al. [
13] and Gervassi
et al. [
19] that reported CT inclusions in distinct vacuoles 48 h post in vitro infection.
Despite the limited information gathered from fluorescence microscopy, it could be noted that CT development in DCs generally follows the pattern of development in epithelial host cells [
16]. For example, there is evident development of EB to RB and multiplication of RB, with characteristic increase in size of particles and number. However, it is not clear whether RB can differentiate back into EB in DCs. Very few intracellular EBs (relative to the number of RBs) were detected at 48 hrs and this may indicate control mechanisms in DCs that inhibit differentiation of RB to EB. More studies are needed to determine whether there are control mechanisms and whether DCs can limit CT infections by inhibiting the production and release of infectious EBs. Whether DCs support the long-term survival of Chlamydia and whether the surviving bacteria could be a reservoir for long-term Chlamydia infection is unknown.
5. Conclusions
The in vitro model of CT infection in DC in the present study demonstrated that human monocyte-derived immature DCs can be infected with CT and can allow the intracellular multiplication of CT. With the evidence presented here, CT uptake by immature DCs, and proliferation of CT in DC, it would be significant to further investigate the DC response to CT infection. The evidence on whether invasion of DCs by CT results in either cell death or dissemination and persistent infection will help explain the role of DC in CT pathogenesis. The influence of CT infection on DC function should also be investigated, whether infection leads to impaired immune responses, inefficient bacterial clearance and/or promotion of bacterial spread as described for other organisms [28-29]. A comprehensive study of the interaction between CT and DC could provide the basis for the development of effective vaccines against CT infections.
Author Contributions
Conceptualization, Z.M. and L.F.; methodology, Z.M. and L.F.; formal analysis, Z.M.; investigation, Z.M.; resources, L.F.; data collection, Z.M.; writing—original draft preparation, Z.M.; writing—review and editing, Z.M.; visualization, Z.M. and L.F.; supervision, L.F.; funding acquisition, L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the use of anonymous blood samples from the Australian Red Cross Blood Centre in Perth, WA. The donors agreed through a questionnaire of the Blood Centre that their blood may be used for research purposes.
Data Availability Statement
Not applicable.
Acknowledgments
This research was supported by the Australian Development Scholarships and assisted by the School of Anatomy and Human Biology, University of Western Australia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dessus-Babus, S.; Knight, S.T.; Wyrick, P.B. Chlamydial infection of polarized HeLa cells induces PMN chemotaxis but the cytokine profile varies between disseminating and non-disseminating strains. Cell. Microbiol. 2000, 2, 317–329. [Google Scholar] [CrossRef]
- Brockett, M.R.; Liechti, G.W. Persistence alters the interaction between Chlamydia trachomatis and its host cell. Infect. Immun. 2021, 89, e00685–20. [Google Scholar] [CrossRef]
- Wyrick, P.B. Intracellular survival by Chlamydia. Cell. Microbiol. 2000, 2, 275–282. [Google Scholar] [CrossRef]
- Everett, K.D.; Bush, R.M.; Andersen, A.A. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 1999, 49 Pt 2, 415–40. [Google Scholar] [CrossRef] [PubMed]
- Fields, K.A.; Hackstadt, T. The chlamydial inclusion: escape from the endocytic pathway. Annu. Rev. Cell Dev. Biol. 2002, 18, 221–45. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Batteiger, R. Chlamydia Diseases. In Principles and Practice of Infectious Diseases, 5th ed.; Mandell, G., Bennett, J., Dolin, R., Eds.; Churchill Livingstone: Pennsylvania, USA, 2000; Volume 2, pp. 1986–2004. [Google Scholar]
- Del Balzo, D.; Capmany, A.; Cebrian, I.; Damiani, M.T. Chlamydia trachomatis Infection Impairs MHC-I Intracellular Trafficking and Antigen Cross-Presentation by Dendritic Cells. Front. Immunol. 2021, 12, 5662096. [Google Scholar] [CrossRef]
- Datta, B.; Njau, F.; Thalmann, J.; Haller, H.; Wagner, A.D. Differential infection outcome of Chlamydia trachomatis in human blood monocytes and monocyte-derived dendritic cells. BMC Microbiol. 2014, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- Neutra, M.R.; Pringault, E.; Kraehenbuhl, J.P. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu. Rev. Immuno. 1996, 14, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Chlamydia Biology/Immunology. Available online: http://www.chlamydiae.com (accessed on 29 July 2021).
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11 (Suppl 4), S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M.; Granucci, F.; Citterio, S.; Foli, M.; Ricciardi-Castagnoli, P. Coordinated events during bacteria-induced dendritic cell maturation. Immunol. Today 1999, 20, 200–203. [Google Scholar] [CrossRef]
- Matyszak, M.K.; Young, J.L.; Gaston, J.S. Uptake and processing of Chlamydia trachomatis by human dendritic cells. Eur. J. Immunol. 2002, 32, 742–51. [Google Scholar] [CrossRef]
- Xiao, Q.; Xia, Y. Insights into dendritic cell maturation during infection with application of advanced imaging techniques. Front. Cell. Infect. Microbiol. 2023, 13, 1140765. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.; Dooley, C.; Fischer, E.; Scidmore, M.; Fields, K.; Hackstadt, T. Three temporal classes of gene expression during the Chlamydia trachomatis development cycle. Mol. Microbiol. 2000, 37, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Beagley, K.W.; Huston, W.M.; Hansbro, P.M.; Timms, P. Chlamydial infection of immune cells: altered function and implications for disease. Crit. Rev. Immunol. 2009, 29, 275–305. [Google Scholar] [CrossRef] [PubMed]
- Herweg, J.; Rudel, T. Interaction of Chlamydiae with human macrophages. FEBS J. 2016, 283, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Airenne, S.; Surcel, H.; Alakarppa, H.; Laitinen, K.; Paavonen, J.; Saikku, P.; Laurila, A. Chlamydia pneumoniae infection in Human Monocytes. Infect. Immun. 1999, 67, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Gervassi, A.; Alderson, M.R.; Suchland, R.; Maisonneuve, J.F.; Grabstein, K.H.; Probst, P. Differential regulation of inflammatory cytokine secretion by human dendritic cells upon Chlamydia trachomatis infection. Infect. Immun. 2004, 72, 7231–9. [Google Scholar] [CrossRef]
- Wittkop, U.; Krausse-Opatz, B.; Gust, T.C.; Kirsch, T.; Hollweg, G.; Kohler, L.; Zenke, M.; Gérard, H.C.; Hudson, A.P.; Zeidler, H.; Wagner, A.D. Fate of Chlamydophila pneumoniae in human monocyte-derived dendritic cells: long lasting infection. Microb. Pathog. 2006, 40, 101–9. [Google Scholar] [CrossRef]
- Rey-Ladino, J.; Jiang, X.; Gabel, B.R.; Shen, C.; Brunham, R.C. Survival of Chlamydia muridarum within dendritic cells. Infect. Immun. 2007, 75, 3707–14. [Google Scholar] [CrossRef]
- Kaiko, G.E. , Phipps, S.; Hickey, D.K.; Lam, C.E.; Hansbro, P.M.; Foster, P.S.; Beagley, K.W. Chlamydia muridarum infection subverts dendritic cell function to promote Th2 immunity and airways hyperreactivity. J. Immunol. 2008, 180, 2225–32. [Google Scholar]
- Mellman, I.; Steinman, R.M. Dendritic cells: specialized and regulated antigen processing machines. Cell 2001, 106, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Stockwin, L.; Mcgonagle, D.; Martin, I.; Blair, E. Dendritic cells: immunological sentinels with a central role in health and disease. Immunol. Cell Biol. 2000, 78, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Filgueira, L.; Nestle, F.; Rittig, M.; Joller, H.; Groscurth, P. Human Dendritic Cells Phagocytose and Process Borrelia burgdorferi. J. Immunol. 1996, 157, 2998–3005. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.; Holly, A. Candida albicans is phagocytosed, killed, and processed for antigen presentation by human dendritic cells. Infect. Immun. 2001, 69, 6813–6822. [Google Scholar] [CrossRef]
- Ojcius, D.M.; Bravo De Alba, Y.; Kanellopoulos, J.M.; Hawkins, R.A.; Kelly, K.A.; Rank, R.G.; Dautry-Varsat, A. Internalization of Chlamydia by dendritic cells and stimulation of Chlamydia-specific T cells. J. Immunol. 1998, 160, 1297–303. [Google Scholar] [CrossRef]
- Guzman, C.; Rohde, M.; Chakraborty, T.; Domann, E.; Hudel, M.; Wheland, J.; Timmis, K. Interaction of Listeria monocytogenes with mouse dendritic cells. Infect. Immun. 1995, 63, 3665–3673. [Google Scholar] [CrossRef]
- Sewell, A.; Price, D. Dendritic cells and transmission of HIV-1. Trends Immunol. 2001, 22, 173–175. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).