Submitted:
28 April 2023
Posted:
29 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
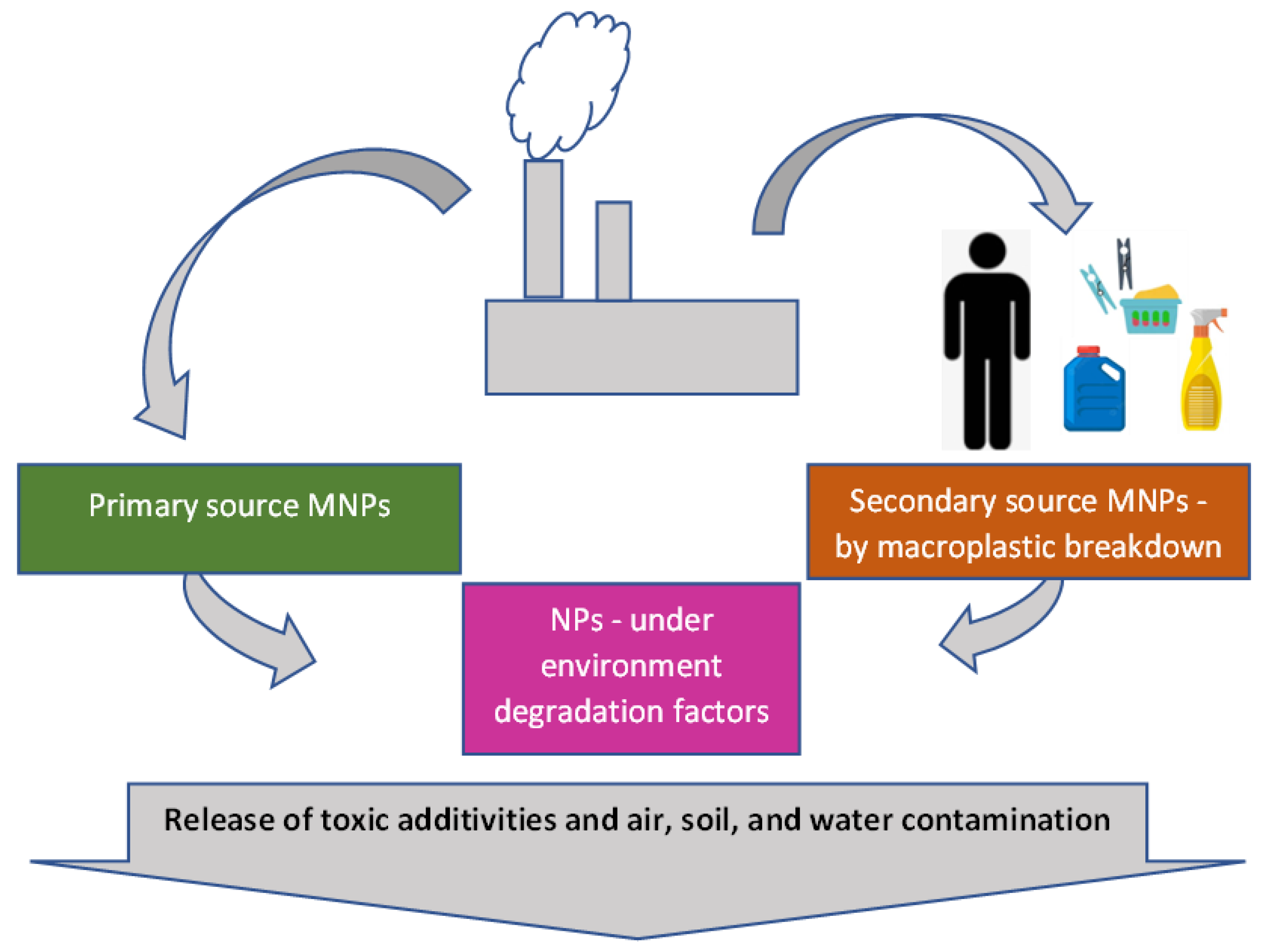
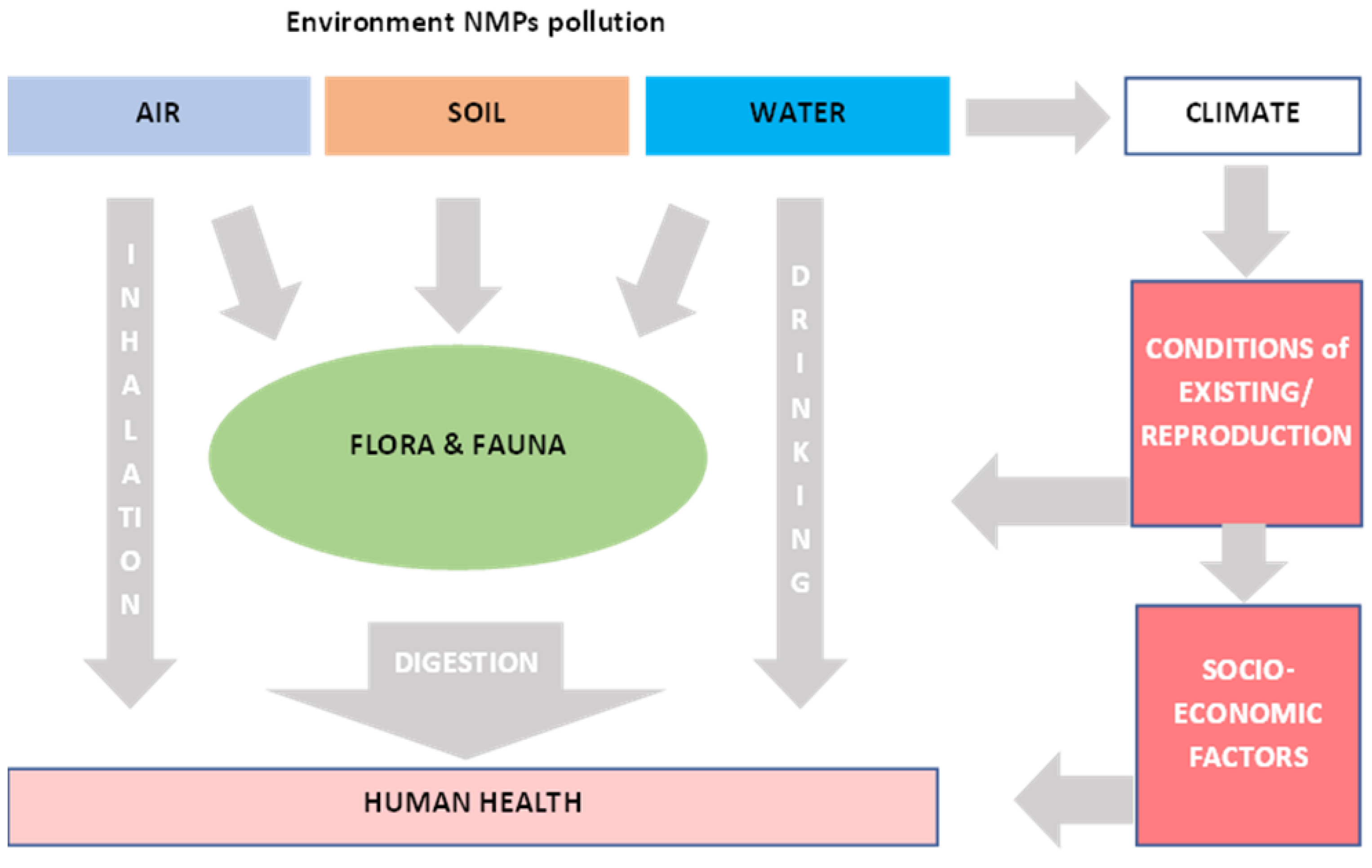
2. Sources and Fate of Atmospheric MNPs
2.1. Sources
2.2. Plastic Pollution
2.3. Atmospheric Microplastic: First Studies
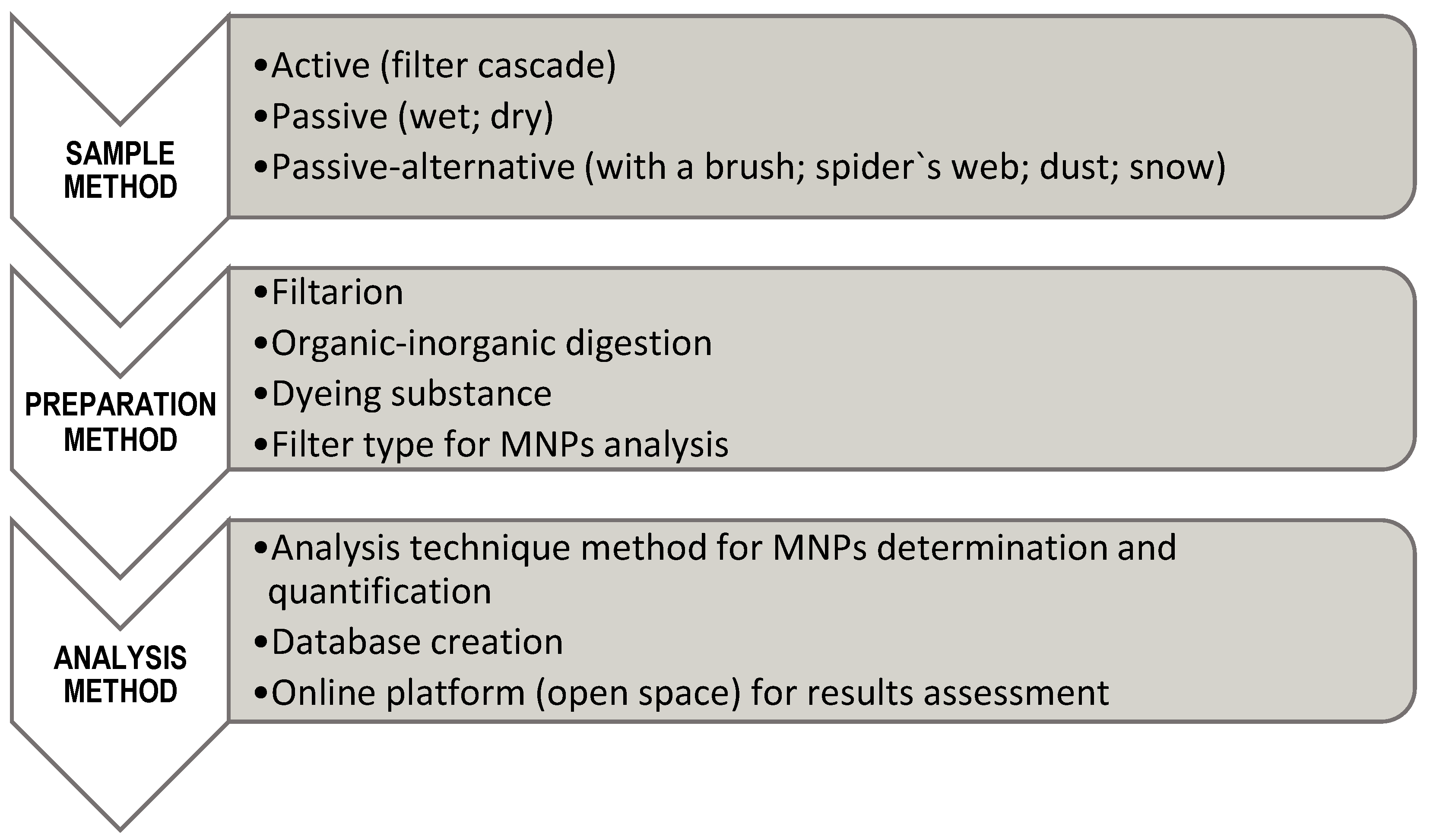
3. Sampling of Atmospheric MPs
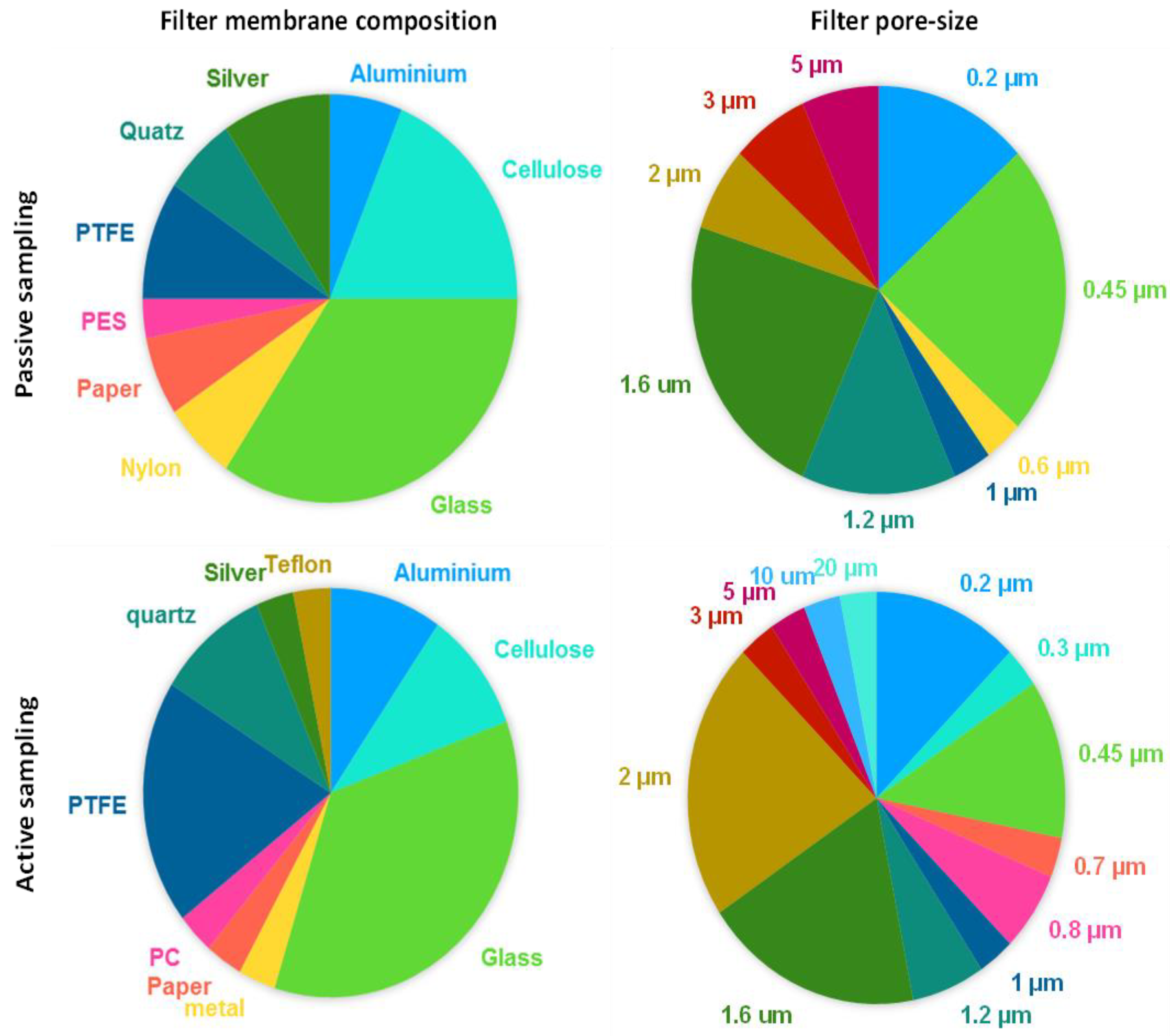
3.1. Sampling Methods
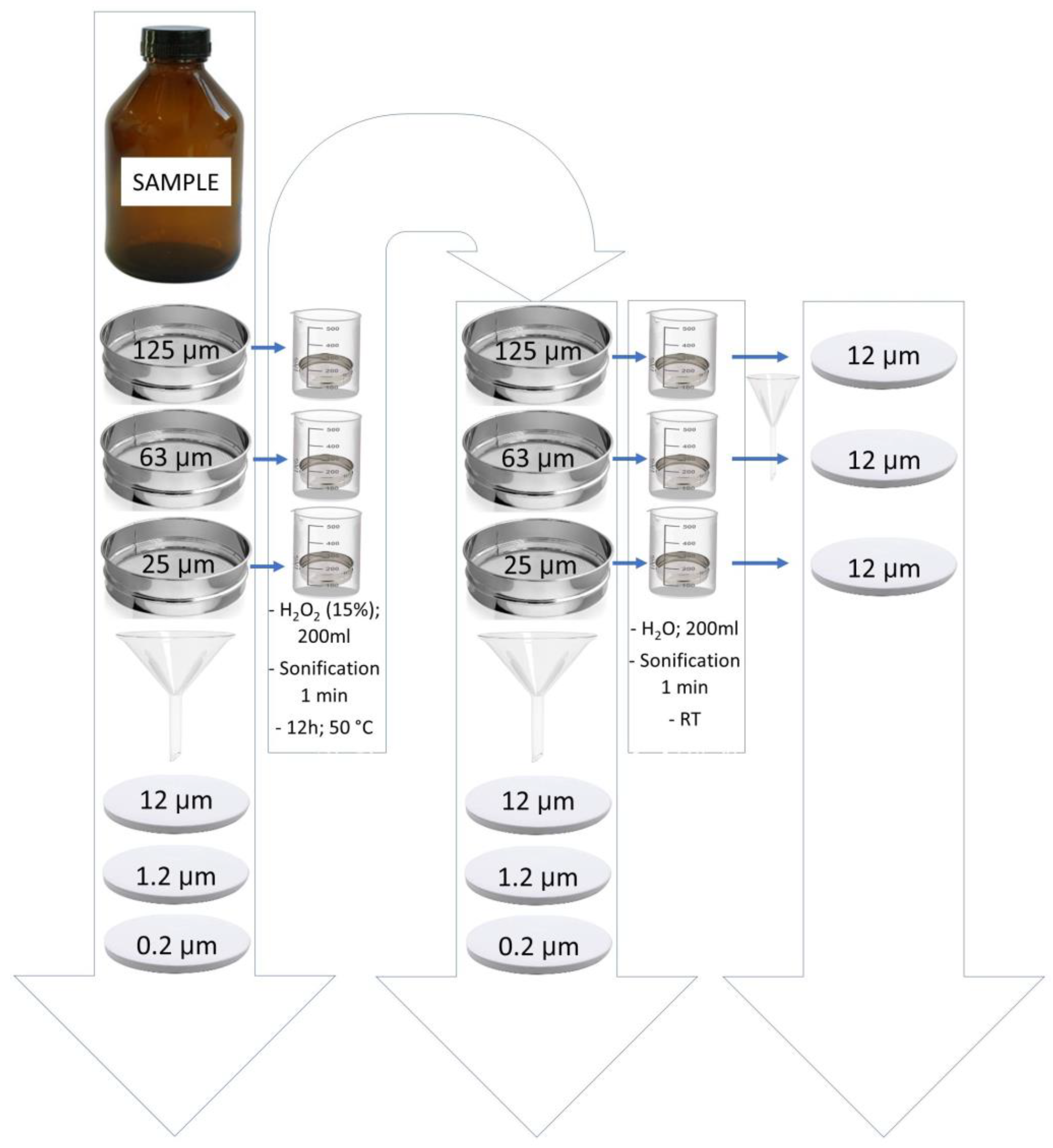
3.2. Sample Preparation
| Ref. | Sampling method | Filter type | Filter pore size μm | Sampling collect time | Digestion | Temperature/time | Sieving | |
|---|---|---|---|---|---|---|---|---|
| [34] | Passive | PTFE | 0.45 | 2018; 1 month | H2O2 ; 30% | RT / 7 days | --- | |
| [35] | Active/Passive | --- | --- | 2019; --- | --- | --- | --- | |
| [36] | Passive | Glass-fiber | 1.6 | 2019 - 2020; 3 - 48 days | --- | --- | --- | |
| [37] | Passive/Snow | CN; Glass-fiber | 0.45; 1.2 | 2019; 1 time | Fenton’s reagent |
45ºC / 2 - 3 hours | --- | |
| [38] | Passive | --- | --- | 2019 - 2020; 1 month | HF | --- | --- | |
| [39] | Active | Glass-fiber | 1.60 | 2017; 24 hours | --- | --- | --- | |
| [40] | Passive | CN; Glass-fiber | 12; 1.6 | 2018 - 2019; 24 hours | --- | --- | 30 | |
| [41] | Passive | Quartz-fiber | 1.6 | 2019 – 2020; --- | --- | --- | --- | |
| [42] | Passive | CN | 3 | --- | TWEEN | 20 (0.1%) | --- | |
| [43] | Active | CN | 5 | 2020; 48 hours | H2O2 ; 30% | 40ºC / 2 hours | 20 µm | |
| [44] | Passive | Silver-fiber | 0.45 | 2021; 24 hours | Washing with Ethanol | --- | ||
| [45] | Passive | PTFE | 0.45 | 2017 - 2019; 1week-1month |
--- | --- | ||
| [46] | Passive | PTFE | 0.45 | 2019; 30 min | H2O2 ; 30% | 55ºC / 24 hours | --- | |
| [47] | Passive | Glass-fiber | 1 | 2020, --- | Fenton’s reagent (FeSO4 + H2O2) |
--- | --- | |
| [48] | Passive | Nylon-fiber | 0.22 | 2021; 24 h | --- | --- | --- | |
| [49] | Active/Passive | Aluminum Oxide | 0.2 | 2018; 3 hours; 1 month | Fenton’s reagent (FeSO4 + H2O2); +Enzymatic digestion |
40ºC / 2 hours | 500 μm | |
| [50] | Active/Passive | Glass-fiber | 1.6 | 2020; 12 hours | --- | --- | --- | |
| [51] | Passive/Dust | Silver-fiber | 0.45 | --- | H2O2 ; 30% | 24 hours | --- | |
| [52] | Passive/Dust | Paper | 2 | 2019; --- | H2O2 ; 30% | RT /10 days | 5 mm | |
| [53] | Passive | --- | --- | 2020; 1 week | --- | --- | --- | |
| [54] | Active/Dust | Paper | --- | 2019; each 7 days | H2O2 ; 30% | RT / 8 days | 5 mm | |
| [55] | Passive | CN | 0.45 | 2018 - 2019; 96 hours | H2O2 ; 30% | 60ºC / 48 hours | 0.2-5 mm | |
| [56] | Active | PTFE | 2 | 2020; 24 hours | --- | --- | --- | |
| [57] | Active | Glass-fiber | 0.3 | 2019; 24 hours | --- | --- | --- | |
| [58] | Active | --- | --- | 2021; 6 hours | --- | --- | --- | |
| [59] | Active | Aluminum Oxide |
0.22 | 2020 - 2021; 4 hours | HCl; pH3 | 24 hours | --- | |
| [60] | Active/Passive | Quartz-fiber | 2.2 | - | H2O2 ; 30% | RT / 24 hours | --- | |
| [61] | Active | Glass-fiber | 1.6 | 2019 - 2020; 24 hours | --- | --- | --- | |
| [62] | Active/Passive | Glass-fiber | 3 | 2019; 12 - 24 hours | --- | --- | --- | |
| [63] | Passive | CN | 0.45 | ---; 22 - 40 days | H2O2 ; 30% | RT / 24 hours | --- | |
| [64] | Passive | MCE | 5 | 2019; 7 days | H2O2 ; 30% | 55ºC / 3 days | --- | |
| [65] | Passive | Glass-fiber | 1.2 | 2020; 6 days | --- | --- | --- | |
| [66] | Active | Glass-fiber; PTFE | 0.7; 0.45 | 2019; 2 - 3 days | H2O2 ; 30% | 70ºC / 1 hour | --- | |
| [67] | Active | PTFE | 2 | ---; 24 hours | H2O2 ; 30% | RT / 1 day | --- | |
| [68] | Passive/Dust | --- | --- | 2020 | --- | --- | 5-1mm | |
| [69] | Active | Glass-fiber | 1.6 | 2017; 24 hours | --- | --- | --- | |
| [70] | Active | Teflon; Silver-fiber |
0.2; 1.2 | 24 hours | --- | --- | --- | |
| [71] | Passive/Dust | Glass-fiber | 0.6 | 30 days | --- | --- | n/a | |
| [72] | Passive | Glass-fiber | 1.6 | 2017 - 2018; 1 - 8 days | --- | --- | --- | |
| [73] | Passive | Glass-fiber | 1.6 | 2018 - 2019; 1 year; 3 - 4 days |
Bioenzym SE/F + H2O2 |
40ºC / 48 hours | 1mm | |
| [74] | Passive/Dust | CN | 1.2 | 1 day | H2O2 ; 30% | --- | --- | |
| [75] | Active | Quartz-fiber; Glass-fiber | 2.2; 1.2 | 2020; 24 hours | H2O2 ; 15% | RT / 8 days | --- | |
| [76] | Active | PTFE | --- | 2019; --- | H2O2 ; 30% | --- | --- | |
| [77] | Active | Quartz-fiber; PTFE; Aluminum Oxide | 10; 0.45; 0.2 | 2018; 8 days | H2O2 ; 30% | 55ºC / 7 days | --- | |
| [78] | Active | Glass-fiber | 1 | 2020; 24 hours | --- | --- | --- | |
| [79] | Passive | PES | 0.45 | 2017 - 2019; 1 - 2 month | --- | --- | --- | |
| [80] | Active | Glass-fiber | 1.6 | 2019; 8 hours | --- | --- | --- | |
| [81] | Active | --- | --- | --- | --- | --- | --- | |
| [82] | Active | PTFE | 2.0 | 2017; 24 hours | --- | --- | --- | |
| [83] | Active/Dust | MCE | 0.8 | 2018; 6 - 8 hours | --- | --- | --- | |
| [84] | Passive | Glass-fiber | --- | 2018; --- | --- | --- | --- | |
| [85] | Active/Passive | Glass-fiber | 1.6 | 2018 – 2019; --- | H2O2 ; 30% +FeSO4(0.05 M) | --- | --- | |
| [86] | Passive/Snow | PTFE | 0.2 | 2017, --- | --- | --- | --- | |
| [87] | Passive | Glass-fiber | 1.6 | 2017 - 2018; 1 month | --- | --- | --- | |
| [88] | Active | PC | 0.8 | 2016; 12 - 24 hours | --- | --- | --- | |
| [89] | Active | Glass-fiber | 1.6 | 20219; 10 - 48 hours | --- | --- | --- | |
| [90] | Passive | Aluminum Oxide; Silver-fiber |
0.2; 1.2 | 2018; 3 - 4 days | --- | --- | --- | |
| [91] | Passive | Nylon-fiber | 100 | 2017; 1 minutes | H2O2 ; 30% | RT / 1 week | 75 μm | |
| [92] | Passive | --- | --- | 2010 -2014 | --- | --- | 150 μm | |
| [93] | Passive | Cellulose | 5 | 2019; 24 hours | --- | --- | --- | |
| [94] | Passive | Glass-fiber | 1.2 | 2017 - 2018 | --- | --- | 2 mm | |
| [95] | Active | Glass-fiber | 1.6 | 2018; 1 hour | --- | --- | --- | |
| [96] | Active | Glass-fiber | 1.6 | 2019; 1 hour | --- | --- | --- | |
| [97] | Active | Glass-fiber | 1.6 | 2018 - 2019; 4 - 24 hours | --- | --- | --- | |
| [98] | Active | Glass-fiber | 1.2 | 2019; 48 hours | H2O2 ; 15% | RT / 8 days | --- |
4. Sampling of Atmospheric MNPs
5. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Pardo, M.; Qiu, X.; Zimmermann, R.; Rudich, Y. Particulate Matter Toxicity Is Nrf2 and Mitochondria Dependent: The Roles of Metals and Polycyclic Aromatic Hydrocarbons. Chem. Res. Toxicol. 2020, 33, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, G.; Acharya, A.; Marahatha, R.; Modi, B.; Paudel, R.; Adhikari, A.; Raut, B.K.; Aryal, S.; Parajuli, N. Microplastics in Environment: Global Concern, Challenges, and Controlling Measures. Int. J. Environ. Sci. Technol. 2022. [CrossRef] [PubMed]
- Chen, G.; Li, Y.; Wang, J. Chapter Eight - Human health effects of airborne microplastics. Comprehensive Analytical Chemistry 2023, 100, 185–223. [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and Societal Benefits of Plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Uurasjärvi, E.; Hartikainen, S.; Setälä, O.; Lehtiniemi, M.; Koistinen, A. Microplastic concentrations, size distribution, and polymer types in the surface waters of a northern European lake. Water Environment Research. 2020, 92, 149–156. [Google Scholar] [CrossRef]
- Wiesinger,H. ; Wang, Z.; Hellweg, S. Deep Dive into Plastic Monomers, Additives, and Processing Aids. Environ. Sci. Technol. 2021, 55, 9339–9351. [Google Scholar] [CrossRef]
- Zimmermann, L.; Bartosova, Z.; Braun, K.; Oehlmann, J.; Völker, C.; Wagner, M. Plastic Products Leach Chemicals That Induce In Vitro Toxicity under Realistic Use Conditions. Environ. Sci. Technol. 2021, 55, 11814–11823. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Q.; Zhang, Q.; Zuo, C.; Shi, H. An Overview of Chemical Additives on (Micro)Plastic Fibers: Occurrence, Release, and Health Risks. Reviews of Environmental Contamination and Toxicology. 2022, 260, 22–10. [Google Scholar] [CrossRef]
- Pironti, C.; Notarstefano, V.; Ricciardi, M.; Motta, O.; Giorgini, E.; Montano, L. First Evidence of Microplastics in Human Urine, a Preliminary Study of Intake in the Human Body. Toxics 2023, 11, 40. [Google Scholar] [CrossRef]
- Pengfei Wu, P.; Lin, S.; Cao, G.; Wu, J.; Jin, H.; Wang, C.; Wonge, M.H.; Yanga, Z.; Cai, Z. Absorption, distribution, metabolism, excretion and toxicity of microplastics in the human body and health implications. J. Hazard Mater. 2022, 437, 129361. [Google Scholar]
- Coffin, S. The emergence of microplastics: charting the path from research to regulations. Environ. Sci.: Adv., 2023, 2, 356. [Google Scholar] [CrossRef]
- Andrade, J.M.; Ferreiro, B.; López-Mahía, P.; Muniategui-Lorenzo, S. Standardization of the Minimum Information for Publication of Infrared-Related Data When Microplastics Are Characterized. Mar. Pollut. Bull. 2020, 154. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A. Sources and Pathways of Microplastics to Habitats. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, 2015; pp. 229–244. ISBN 978-3-319-16510-3. [Google Scholar]
- Lamichhane, G.; Acharya, A.; Marahatha, R.; Modi, B.; Paudel, R.; Adhikari, A.; Raut, B.K.; Aryal, S.; Parajuli, N. Microplastics in Environment: Global Concern, Challenges, and Controlling Measures. Int. J. Environ. Sci. Technol. 2023, 20, 4673–4694. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Supporting Material: Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and Fragmentation of Plastic Debris in Global Environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and Importance of Microplastics in the Marine Environment. A Review of the Sources, Fate, Effects, and Potential Solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Martins, A.; Guilhermino, L. Transgenerational Effects and Recovery of Microplastics Exposure in Model Populations of the Freshwater Cladoceran Daphnia Magna Straus. Sci. Total Environ. 2018, 631–632, 421–428. [Google Scholar] [CrossRef]
- Liu, S.; Guo, J.; Liu, X.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. Detection of Various Microplastics in Placentas, Meconium, Infant Feces, Breastmilk and Infant Formula: A Pilot Prospective Study. Sci. Total Environ. 2023, 854, 158699. [Google Scholar] [CrossRef]
- Revell, L.E.; Kuma, P.; Le Ru, E.C.; Somerville, W.R.C.; Gaw, S. Direct radiative effects of airborne microplastics. Nature 2021, 598, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Michishita, S.; Gibble, C.; Tubbs, C.; Felton, R.; Gjeltema, J.; Lang, J.; Finkelstein, M. Microplastic in Northern Anchovies (Engraulis Mordax) and Common Murres (Uria Aalge) from the Monterey Bay, California USA - Insights into Prevalence, Composition, and Estrogenic Activity. Environ. Pollut. 2022, 120548. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of Microplastics in Human Lung Tissue Using $μ$FTIR Spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’Avino, S.; Gulotta, A.; et al. Raman Microspectroscopy Detection and Characterisation of Microplastics in Human Breastmilk. Polymers (Basel). 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.M.; Jantunen, L.; Bergmann, M.; Vorkamp, K.; Aherne, J.; Magnusson, K.; Herzke, D.; Granberg, M.; Hallanger, I.G.; Gomiero, A.; et al. Monitoring Microplastics in the Atmosphere and Cryosphere in the Circumpolar North: A Case for Multi-Compartment Monitoring. Arct. Sci. 2022, 11, 1–11. [Google Scholar] [CrossRef]
- Guo, J.-J.; Huang, X.-P.; Xiang, L.; Wang, Y.-Z.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Source, Migration and Toxicology of Microplastics in Soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef]
- Van der Meulen, M.D.; De Vriese, L.; Lee, J.; Maes, T. , Van Dalfsen, J.A.; Huvet, A.; Soudant, P.; Robbens, J.; Vethaak, A.D. Socio-economic impact of microplastics in the 2 Seas, Channel and France Manche Region: an initial risk assessment. MICRO Interreg project Iva. 2014. [Google Scholar] [CrossRef]
- Mofijur, M.; Ahmed, S.F.; Rahman, S.M.A.; Arafat Siddiki, S.K.Y.; Islam, A.B.M.S.; Shahabuddin, M.; Ong, H.C.; Mahlia, T.M.I.; Djavanroodi, F.; Show, P.L. Source, Distribution and Emerging Threat of Micro- and Nanoplastics to Marine Organism and Human Health: Socio-Economic Impact and Management Strategies. Environ. Res. 2021, 195, 110857. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic Contamination in an Urban Area: A Case Study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Cai, L.; Wang, J.; Peng, J.; Tan, Z.; Zhan, Z.; Tan, X.; Chen, Q. Characteristic of Microplastics in the Atmospheric Fallout from Dongguan City, China: Preliminary Research and First Evidence. Environ. Sci. Pollut. Res. 2017, 24, 24928–24935. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric Transport and Deposition of Microplastics in a Remote Mountain Catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Ferrero, L.; Scibetta, L.; Markuszewski, P.; Mazurkiewicz, M.; Drozdowska, V.; Makuch, P.; Jutrzenka-Trzebiatowska, P.; Zaleska-Medynska, A.; Andò, S.; Saliu, F.; et al. Airborne and Marine Microplastics from an Oceanographic Survey at the Baltic Sea: An Emerging Role of Air-Sea Interaction? Sci. Total Environ. 2022, 824, 153709. [Google Scholar] [CrossRef] [PubMed]
- Welsh, B.; Aherne, J.; Paterson, A.M.; Yao, H.; McConnell, C. Atmospheric Deposition of Anthropogenic Particles and Microplastics in South-Central Ontario, Canada. Sci. Total Environ. 2022, 835, 155426. [Google Scholar] [CrossRef]
- Aves, A.R.; Revell, L.E.; Gaw, S.; Ruffell, H.; Schuddeboom, A.; Wotherspoon, N.E.; Larue, M.; Mcdonald, A.J. First Evidence of Microplastics in Antarctic Snow. Cryosphere 2022, 16, 2127–2145. [Google Scholar] [CrossRef]
- Jarosz, K.; Janus, R.; Wądrzyk, M.; Wilczyńska-Michalik, W.; Natkański, P.; Michalik, M. Characteristic of Airborne Microplastic in the Atmospheric Deposition in Krakow (Southern Poland): A New Semi-Quantitative Approach by Means of the Py-Gc-Ms Technique. SSRN Electron. J. 2022. [CrossRef]
- Ding, J.; Sun, C.; He, C.; Zheng, L.; Dai, D.; Li, F. Atmospheric Microplastics in the Northwestern Pacific Ocean: Distribution, Source, and Deposition. Sci. Total Environ. 2022, 829, 154337. [Google Scholar] [CrossRef]
- Napper, I.; Parker-Jurda, N.; Wright, S.; Thompson, R. Examining the Release of Synthetic Microfibres to the Environment via Two Major Pathways: Atmospheric Deposition and Treated Wastewater Effluent. Sci. Total Environ. 2022, 154166. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; dos Santos Galvão, L.; Wiebeck, H.; Carvalho-Oliveira, R.; Mauad, T. Atmospheric Microplastic Fallout in Outdoor and Indoor Environments in São Paulo Megacity. Sci. Total Environ. 2022, 821, 153450. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Zhang, X.; Zhang, Y.; Gao, W.; Wang, R.; He, D. Air Conditioner Filters Become Sinks and Sources of Indoor Microplastics Fibers. Environ. Pollut. 2022, 292, 118465. [Google Scholar] [CrossRef]
- Choi, H.; Lee, I.; Kim, H.; Park, J.; Cho, S.; Oh, S.; Lee, M.; Kim, H. Comparison of Microplastic Characteristics in the Indoor and Outdoor Air of Urban Areas of South Korea. Water. Air. Soil Pollut. 2022, 233, 1–10. [Google Scholar] [CrossRef]
- Cui, J.; Chen, C.; Gan, Q.; Wang, T.; Li, W.; Zeng, W.; Xu, X.; Chen, G.; Wang, L.; Lu, Z.; et al. Indoor Microplastics and Bacteria in the Atmospheric Fallout in Urban Homes. Sci. Total Environ. 2022, 852, 158233. [Google Scholar] [CrossRef] [PubMed]
- Evangeliou, N.; Tichý, O.; Eckhardt, S.; Zwaaftink, C.G.; Brahney, J. Sources and Fate of Atmospheric Microplastics Revealed from Inverse and Dispersion Modelling: From Global Emissions to Deposition. J. Hazard. Mater. 2022, 432, 128585. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Liao, Z.; Ji, X.; Zhu, X.; Wang, Z.; Lu, C.; Shi, C.; Chen, Z.; Ge, L.; Zhang, M.; et al. Microplastic Ingestion from Atmospheric Deposition during Dining/Drinking Activities. J. Hazard. Mater. 2022, 432, 128674. [Google Scholar] [CrossRef] [PubMed]
- Goßmann, I.; Süßmuth, R.; Scholz-Böttcher, B.M. Plastic in the Air?! - Spider Webs as Spatial and Temporal Mirror for Microplastics Including Tire Wear Particles in Urban Air. Sci. Total Environ. 2022, 832, 155008. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Duan, Y.; Han, X.; Munyaneza, J.; Ma, J.; Xiu, G. Atmospheric Deposition of Microplastics in the Megalopolis (Shanghai) during Rainy Season: Characteristics, Influence Factors, and Source. Sci. Total Environ. 2022, 149501. [Google Scholar] [CrossRef] [PubMed]
- Kernchen, S.; Löder, M.G.J.; Fischer, F.; Fischer, D.; Moses, S.R.; Georgi, C.; Nölscher, A.C.; Held, A.; Laforsch, C. Airborne Microplastic Concentrations and Deposition across the Weser River Catchment. Sci. Total Environ. 2022, 818, 151812. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Zhu, L.; Liu, K.; Zong, C.; Wei, N.; Li, D. Enhanced Impacts Evaluation of Typhoon Sinlaku (2020) on Atmospheric Microplastics in South China Sea during the East Asian Summer Monsoon. Sci. Total Environ. 2022, 806, 150767. [Google Scholar] [CrossRef]
- Liu, P.; Shao, L.; Li, Y.; Jones, T.; Cao, Y.; Yang, C.-X.; Zhang, M.; Santosh, M.; Feng, X.; BéruBé, K. Microplastic Atmospheric Dustfall Pollution in Urban Environment: Evidence from the Types, Distribution, and Probable Sources in Beijing, China. Sci. Total Environ. 2022, 838, 155989. [Google Scholar] [CrossRef]
- Nematollahi, M.J.; Zarei, F.; Keshavarzi, B.; Zarei, M.; Moore, F.; Busquets, R.; Kelly, F.J. Microplastic Occurrence in Settled Indoor Dust in Schools. Sci. Total Environ. 2022, 807, 150984. [Google Scholar] [CrossRef]
- Ouyang, Z.; Mao, R.; Hu, E.; Xiao, C.; Yang, C.; Guo, X. The Indoor Exposure of Microplastics in Different Environments. Gondwana Res. 2022, 108, 193–199. [Google Scholar] [CrossRef]
- Pandey, D.; Banerjee, T.; Badola, N.; Chauhan, J.S. Evidences of Microplastics in Aerosols and Street Dust: A Case Study of Varanasi City, India. Environ. Sci. Pollut. Res. 2022. [CrossRef] [PubMed]
- Purwiyanto, A.I.S.; Prartono, T.; Riani, E.; Naulita, Y.; Cordova, M.R.; Koropitan, A.F. The Deposition of Atmospheric Microplastics in Jakarta-Indonesia: The Coastal Urban Area. Mar. Pollut. Bull. 2022, 174, 113195. [Google Scholar] [CrossRef] [PubMed]
- Shruti, V.C.; Kutralam-muniasamy, G.; Pérez-guevara, F.; Roy, P.D.; Martínez, I.E. Science of the Total Environment Occurrence and Characteristics of Atmospheric Microplastics in Mexico City. Sci. Total Environ. 2022, 847, 157601. [Google Scholar] [CrossRef] [PubMed]
- Syafina, Paramastri Rahmi Yudison, A.P.; Sembiring, E.; Irsyad, M.; Tomo, H.S. Identification of Fibrous Suspended Atmospheric Microplastics in Bandung Metropolitan Area, Indonesia. Chemosphere 2022, 100310. [CrossRef]
- Uddin, S.; Fowler, S.W.; Habibi, N.; Sajid, S.; Dupont, S.; Behbehani, M. Indoor Aerosol — Kuwait ’ s Baseline. Toxics 2022, 2–17. [Google Scholar]
- Xie, Y.; Li, Y.; Feng, Y.; Cheng, W.; Wang, Y. Inhalable Microplastics Prevails in Air: Exploring the Size Detection Limit. Environ. Int. 2022, 162. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Glamoclija, M.; Murphy, A.; Gao, Y. Characterization of Microplastics in Indoor and Ambient Air in Northern New. Environ. Res. 2022, 207, 112142. [Google Scholar] [CrossRef]
- Wang, F.; Lai, Z.; Peng, G.; Luo, L.; Liu, K.; Huang, X.; Xu, Y.; Shen, Q.; Li, D. Microplastic Abundance and Distribution in a Central Asian Desert. Sci. Total Environ. 2021, 800, 149529. [Google Scholar] [CrossRef]
- Ding, Y.; Zou, X.; Wang, C.; Feng, Z.; Wang, Y.; Fan, Q.; Chen, H. The Abundance and Characteristics of Atmospheric Microplastic Deposition in the Northwestern South China Sea in the Fall. Atmos. Environ. 2021, 253, 118389. [Google Scholar] [CrossRef]
- Huang, Y.; He, T.; Yan, M.; Yang, L.; Gong, H.; Wang, W.; Qing, X.; Wang, J. Atmospheric Transport and Deposition of Microplastics in a Subtropical Urban Environment. J. Hazard. Mater. 2021, 416, 126168. [Google Scholar] [CrossRef]
- Jenner, L.C.; Sadofsky, L.R.; Danopoulos, E.; Rotchell, J.M. Household Indoor Microplastics within the Humber Region (United Kingdom): Quantification and Chemical Characterisation of Particles Present. Atmos. Environ. 2021, 259, 118512. [Google Scholar] [CrossRef]
- Knobloch, E.; Ruffell, H.; Aves, A.; Pantos, O.; Gaw, S.; Revell, L.E. Comparison of Deposition Sampling Methods to Collect Airborne Microplastics in Christchurch, New Zealand. Water. Air. Soil Pollut. 2021, 232. [Google Scholar] [CrossRef]
- Liao, Z.; Ji, X.; Ma, Y.; Lv, B.; Huang, W.; Zhu, X.; Fang, M.; Wang, Q.; Wang, X.; Dahlgren, R.; et al. Airborne Microplastics in Indoor and Outdoor Environments of a Coastal City in Eastern China. J. Hazard. Mater. 2021, 417, 126007. [Google Scholar] [CrossRef] [PubMed]
- Narmadha, V.V.; Jose, J.; Patil, S.; Farooqui, M.O.; Srimuruganandam, B.; Saravanadevi, S.; Krishnamurthi, K. Assessment of Microplastics in Roadside Suspended Dust from Urban and Rural Environment of Nagpur, India. Int. J. Environ. Res. 2020, 14, 629–640. [Google Scholar] [CrossRef]
- O’Brien, S.; Okoffo, E.D.; Rauert, C.; O’Brien, J.W.; Ribeiro, F.; Burrows, S.D.; Toapanta, T.; Wang, X.; Thomas, K.V. Quantification of Selected Microplastics in Australian Urban Road Dust. J. Hazard. Mater. 2021, 416, 125811. [Google Scholar] [CrossRef]
- Peñalver, R.; Costa-Gómez, I.; Arroyo-Manzanares, N.; Moreno, J.M.; López-García, I.; Moreno-Grau, S.; Córdoba, M.H. Assessing the Level of Airborne Polystyrene Microplastics Using Thermogravimetry-Mass Spectrometry: Results for an Agricultural Area. Sci. Total Environ. 2021, 787. [Google Scholar] [CrossRef]
- Rahman, L.; Mallach, G.; Kulka, R.; Halappanavar, S. Microplastics and Nanoplastics Science: Collecting and Characterizing Airborne Microplastics in Fine Particulate Matter. Nanotoxicology 2021, 15, 1253–1278. [Google Scholar] [CrossRef]
- Soltani, N.S.; Taylor, M.P.; Wilson, S.P. Quantification and Exposure Assessment of Microplastics in Australian Indoor House Dust. Environ. Pollut. 2021, 283. [Google Scholar] [CrossRef]
- Szewc, K.; Graca, B.; Dołęga, A. Atmospheric Deposition of Microplastics in the Coastal Zone: Characteristics and Relationship with Meteorological Factors. Sci. Total Environ. 2021, 761. [Google Scholar] [CrossRef]
- Truong, T.N.S.; Strady, E.; Kieu-Le, T.C.; Tran, Q.V.; Le, T.M.T.; Thuong, Q.T. Microplastic in Atmospheric Fallouts of a Developing Southeast Asian Megacity under Tropical Climate. Chemosphere 2021, 272, 129874. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Liu, K.; Zhu, L.; Song, Z.; Li, D. Atmospheric Microplastic over the South China Sea and East Indian Ocean: Abundance, Distribution and Source. J. Hazard. Mater. 2020, 389. [Google Scholar] [CrossRef] [PubMed]
- Xumiao, L.; Prata, J.C.; Alves, J.R.; Duarte, A.C.; Rocha-Santos, T.; Cerqueira, M. Airborne Microplastics and Fibers in Indoor Residential Environments in Aveiro, Portugal. Environ. Adv. 2021, 6. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, W.; Fang, M.; Liao, Z.; Wang, Y.; Xu, L.; Mu, Q.; Shi, C.; Lu, C.; Deng, H.; et al. Airborne Microplastic Concentrations in Five Megacities of Northern and Southeast China. Environ. Sci. Technol. 2021, 55, 12871–12881. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; Allen, D.; Moss, K.; Le Roux, G.; Phoenix, V.R.; Sonke, J.E. Examination of the Ocean as a Source for Atmospheric Microplastics. PLoS One 2020, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Amato-Lourenço, L.F.; dos Santos Galvão, L.; de Weger, L.A.; Hiemstra, P.S.; Vijver, M.G.; Mauad, T. An Emerging Class of Air Pollutants: Potential Effects of Microplastics to Respiratory Human Health? Sci. Total Environ. 2020, 749. [Google Scholar] [CrossRef] [PubMed]
- Brahney, J.; Hallerud, M.; Heim, E.; Hahnenberger, M.; Sukumaran, S. Plastic Rain in Protected Areas of the United States. Science (80). 2020, 368, 1257–1260. [Google Scholar] [CrossRef]
- Gaston, E.; Woo, M.; Steele, C.; Sukumaran, S.; Anderson, S. Microplastics Differ Between Indoor and Outdoor Air Masses: Insights from Multiple Microscopy Methodologies. Appl. Spectrosc. 2020, 74, 1079–1098. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Edo, C.; Aguilera, Á.; Viúdez-Moreiras, D.; Pulido-Reyes, G.; González-Toril, E.; Osuna, S.; de Diego-Castilla, G.; Leganés, F.; Fernández-Piñas, F.; et al. Occurrence and Transport of Microplastics Sampled within and above the Planetary Boundary Layer. Sci. Total Environ. 2020, 761. [Google Scholar] [CrossRef]
- Levermore, J.M.; Smith, T.E.L.; Kelly, F.J.; Wright, S.L. Detection of Microplastics in Ambient Particulate Matter Using Raman Spectral Imaging and Chemometric Analysis. Anal. Chem. 2020, 92, 8732–8740. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Wei, N.; Song, Z.; Li, D. Accurate Quantification and Transport Estimation of Suspended Atmospheric Microplastics in Megacities: For Human Health. Environ. Int. 2019, 132. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Tang, C. A Review of Possible Pathways of Marine Microplastics Transport in the Ocean. Anthr. Coasts 2020, 3, 6–13. [Google Scholar] [CrossRef]
- Liu, S.; Guo, J.; Liu, X.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. Detection of Various Microplastics in Placentas, Meconium, Infant Feces, Breastmilk and Infant Formula: A Pilot Prospective Study. Sci. Total Environ. 2023, 854, 158699. [Google Scholar] [CrossRef] [PubMed]
- Materić, D.; Kasper-Giebl, A.; Kau, D.; Anten, M.; Greilinger, M.; Ludewig, E.; Van Sebille, E.; Röckmann, T.; Holzinger, R. Micro-and Nanoplastics in Alpine Snow: A New Method for Chemical Identification and (Semi)Quantification in the Nanogram Range. Environ. Sci. Technol. 2020, 54, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Roblin, B.; Ryan, M.; Vreugdenhil, A.; Aherne, J. Ambient Atmospheric Deposition of Anthropogenic Microfibers and Microplastics on the Western Periphery of Europe (Ireland). Environ. Sci. Technol. 2020, 54, 11100–11108. [Google Scholar] [CrossRef] [PubMed]
- Trainic, M.; Flores, J.M.; Pinkas, I.; Pedrotti, M.L.; Lombard, F.; Bourdin, G.; Gorsky, G.; Boss, E.; Rudich, Y.; Vardi, A.; et al. Airborne Microplastic Particles Detected in the Remote Marine Atmosphere. Commun. Earth Environ. 2020, 1, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Liu, K.; Zhu, L.; Song, Z.; Li, D. Atmospheric Microplastic over the South China Sea and East Indian Ocean: Abundance, Distribution and Source. J. Hazard. Mater. 2020, 389. [Google Scholar] [CrossRef]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric Microplastic Deposition in an Urban Environment and an Evaluation of Transport. Environ. Int. 2020, 136. [Google Scholar] [CrossRef] [PubMed]
- Yukioka, S.; Tanaka, S.; Nabetani, Y.; Suzuki, Y.; Ushijima, T.; Fujii, S.; Takada, H.; Van Tran, Q.; Singh, S. Occurrence and Characteristics of Microplastics in Surface Road Dust in Kusatsu (Japan), Da Nang (Vietnam), and Kathmandu (Nepal). Environ. Pollut. 2020, 256, 113447. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Du, F.; Cai, H.; Wang, G.; Shi, H. Microplastic Fallout in Different Indoor Environments. Environ. Sci. Technol. 2020, 54, 6530–6539. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Kannan, K. Microplastics in House Dust from 12 Countries and Associated Human Exposure. Environ. Int. 2020, 134, 105314. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Girão, A. V.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Identifying a Quick and Efficient Method of Removing Organic Matter without Damaging Microplastic Samples. Sci. Total Environ. 2019, 686, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Li, Y.; Jones, T.; Santosh, M.; Liu, P.; Zhang, M.; Xu, L.; Li, W.; Lu, J.; Yang, C.X.; et al. Airborne Microplastics: A Review of Current Perspectives and Environmental Implications. J. Clean. Prod. 2022, 347. [Google Scholar] [CrossRef]
- Habibi, N.; Uddin, S.; Fowler, S.W.; Behbehani, M. Microplastics in the Atmosphere: A Review. J. Environ. Expo. Assess. 2022. [CrossRef]
- Kang, P.; Ji, B.; Zhao, Y.; Wei, T. How Can We Trace Microplastics in Wastewater Treatment Plants: A Review of the Current Knowledge on Their Analysis Approaches. Sci. Total Environ. 2020, 745, 140943. [Google Scholar] [CrossRef]
- Cutroneo, L.; Reboa, A.; Geneselli, I.; Capello, M. Considerations on Salts Used for Density Separation in the Extraction of Microplastics from Sediments. Mar. Pollut. Bull. 2021, 166, 112216. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





