1. Introduction
Taxane diterpenoids (taxoids), particularly paclitaxel, are plant secondary metabolites highly valued for anti-cancer activities [
1]. They are mostly obtained from different species of
Taxus spp. (yew) where they are accumulated in small quantities [
2]. Efforts have been made to produce taxoids in cell cultures as an alternative method to harvesting wild plants; however, there are only few successful examples of commercial paclitaxel production using cell suspensions [
3,
4]. The biotechnological approach to taxoid synthesis is hampered by low content of the target compounds in cell cultures as well as potential risks of synthesis shift in the course of the long-term in vitro cultivation [
5].
The problem of low metabolite content in cell cultures is frequently addressed by chemical elicitation, i.e., adding the specific compounds (stress-signaling molecules or precursors) to culture medium at the latter stages of culture growth which simulates the biosynthesis of secondary metabolites in cells [
2,
6,
7]. One of the most widely used elicitors is methyl jasmonate (MeJ), a non-specific stress signaling molecule which has ability to response to various stresses including herbivore and pathogen attack [
8]. The effects of MeJ and jasmonic acid in plants have been comprehensively studied [
9,
10,
11]; however, MeJ mode of action might be different in the in vitro grown cell cultures due to their specifics: dedifferentiation, the artificial growth conditions and the absence of the organismic control [
12,
13,
14]. MeJ has been used for elicitation of yew cell cultures since 1990s [
4,
15,
16]. However, there are evidences that MeJ effect may be species- and even genotype-specific and vary depending on culture conditions, possibly due to a cross-talk between MeJ and other growth regulators in culture medium [
17,
18,
19].
We previously reported that cell cultures of various yew species including
Taxus baccata, T. canadensis, T. wallichiana and two
T. × media hybrids, were able to synthesize both 13-oxygenated/hydroxylated (13-OH) taxoids (decinnamoyl-taxinine J, taxuspine F etc.) and 14-OH taxoids (7β-hydroxy-taxuyunnanin C, sinenxane C, taxuyunnanine C, 2α,5α,9α,10β,14β-pentaacetoxy-4(20), 11-taxadiene, and yunnanxane) [
20,
21]. Moreover, while 13-oxygenated/hydroxylated taxoids were detected in callus cell cultures of
T. × media [
21], cell cultures maintained for several years produced mainly 14-OH taxoids [
21,
22]. These results are of significant interest for commercial-scale cultivation which requires stable production of the desired secondary metabolites of known quality and quantity. However, little is known about the potential change of taxoid composition in the cell cultures during continuous maintenance and culture’s response to elicitation.
The aim of the present study was to investigate the effect of MeJ elicitation on growth and biosynthetic ability of the same cell culture of Taxus wallichiana at different ages (1.5- and 6 years after induction) during cultivation in flasks and pilot-scale bioreactors.
2. Materials and Methods
2.1. Plant material and culture conditions
Cell culture of
Taxus wallichiana Zucc., line Tw-bbg /B5-NB-pvp, used in this study was induced in January 2016 from a plant growing in the Minsk Botanical Garden of the National Academy of Sciences (Minsk, Belarus). Suspension culture was obtained from callus culture in June 2016 [
23]. The cell line was a finely aggregated suspension of light-yellow cells. The aggregates consisted of meristem-like and parenchyma-like cells, predominantly of round shape. Culture was maintained in 250-ml and 500-ml flasks filled with, respectively, 40 ml and 80 ml of liquid nutrient medium composed of B5 [
24] mineral salts, 1.0 g/L polyvinylpyrrolidone (PVP), 0.5 mg/L nicotinic acid, 0.1 mg/L thiamine and 0.1 mg/L pyridoxine vitamins, 2 mg/L α-naphthalene acetic acid (NAA) and 0.3 mg/L 6-benzylaminopurine (BAP). Cultures were grown on an orbital shaker (90 rpm) at 26 ± 0.5 °C and 75% relative air humidity in darkness. For subculturing, 1 ml or 2 ml packed volume of the cell suspension was transferred to a fresh medium every 28 days.
Bioreactor cultivation was performed in 20-L bubble-type glass bioreactors of our own design (Institute of Plant Physiology of RAS, Russia) with 15-L working volume and 75-L bioreactor Electrolux-El-75 (Sweden) with a working volume 50-L. All bioreactors were operated at a semi-continuous mode. Cultivation was performed at 26 ± 0.5 °C in darkness; air supplied varied from 0.1 to 1.0 v/vpm depending on the growth phase of the cell culture. Initial density of cell suspension for bioreactor cultivation was 2-3 g dry weight (DW) per L.
2.2. Elicitation of young and old culture with methyl jasmonate (MeJ)
Elicitation experiments were performed in two series: in 2017 (1.5 years after culture induction) and in 2021-2022 (5-6 years after culture induction).
The first experiments were done between May and July 2017. By this time, the culture was relatively «young» and had passed through 5 subculture cycles of callus and 12 cycles of suspension culture. In flask culture, MeJ at final concentration 100 µM (concentration was chosen based on literature data [
15]) was added to cell culture on day 21 of cultivation (the end of the exponential growth phase). In 20-L bioreactors, MeJ at final concentration 100 µM was added in the second cycle of cultivation at the end of the exponential growth phase (48th day of cultivation, 18th day from the beginning of the subculture cycle). The stock solution of MeJ (Sigma, USA) was prepared using methanol.
In 2021-2022, the second series of experiments was performed to compare the effect of MeJ on «young» and «old» cell cultures. By this time, the cell culture was 6-years-old and had passed through more than 70 subcultivation cycles. In this series of experiments, cultivation was performed in flasks and a 75-L bioreactor. MeJ was on day 19 in flasks and on day 17 in a bioreactor.
The influence of MeJ on cell culture growth and production of taxoid diterpenoids was assessed as described below.
2.3. Growth assessment
To assess the growth and physiological state of cell cultures in flasks and bioreactors, fresh weight (FW) and DW of cell biomass, and cell viability were recorded periodically during the cultivation cycle as described earlier [
23,
25]. For FW evaluation, 10–15 mL aliquots of cell suspension were pipetted on paper filters and the culture medium was removed under vacuum; cell biomass was washed three times by distilled water under vacuum and weighted. Dry weight was recorded after ai-drying of cell biomass to a constant weight at 60 °С.
Growth index, specific growth rate, doubling time, economic coefficient and productivity were calculated as previously described [
20,
23,
25].
Cell viability was determined by staining with 0.025% Evans blue [
23,
25] as the percentage of cell aggregates composed of colourless (living) cells. A minimum of 250 cell aggregates were examined in each of three replicates.
2.4. Analysis of taxoid diterpenoids in cell biomass
Taxoid diterpenoids were analyzed in dry cell biomass by UPLC-ESI-MS. Sample preparation for qualitative and quantitative analysis of taxoids was carried out according to previously published methods [
20,
21,
22].
UPLC-ESI-MS (structural identification of diterpenoids). Structural identification of the compounds was carried out using a previously published techniques [
20,
21,
22]. The 13- and 14-hydroxylated taxoids were identified based on comparison of their chromatographic behavior and mass spectra (positive ions) with the standard samples and the literature data [
21,
26,
27,
28], as well as the interpretation of their mass spectra [
20,
21,
22]. The following commercial reference samples of 13-hydroxylated taxoids were used: 7-xylosyl-10-deacetyltaxol, 10-deacetyltaxol, taxusin (ChromaDex, USA); cephalomannine, baccatin III, 10-deacetyl baccatin III, paclitaxel (Sigma Aldrich, USA), 13-acetyl-9-dihydrobaccatin III, taxinin M (TRC, Canada).
UPLC-ESI-MS (quantitative analysis). The analysis was carried out on an Agilent 1260 Infinity instrument (Agilent Technologies, USA) equipped with a mass selective detector (6100, Agilent Technologies, USA). Column: Poroshell 120 EC-C18 (100 mm × 3 mm, 2.7 µm, Agilent, USA). Column temperature 43°C, mobile phase flow rate 0.5 ml/min. Injection volume 0.5 µl. A 0.05% (v/v) solution of formic acid in water (solvent A) and acetonitrile (solvent B) were used as the mobile phase. Chromatographic separation was carried out in the gradient elution mode. During the analysis, the composition of the mobile phase changed as follows (B, % by volume): 0–1 min. – 41%, 1-3 min. – 41→55%, 3-11 min. - 55%, 11-13 min. – 55→85%, 13-17 min. – 85%. The analysis was carried out in the positive ion detection mode (m/z range 100–1300, fragmentor 70). Ionization source parameters: quadrupole temperature 100°С, carrier gas (nitrogen) temperature 250°С, nitrogen supply rate (spraying gas) 13 L/min, nitrogen pressure 2484 Torr, capillary voltage 4.0 kV. Quantitative determination of the content of individual taxoids was carried out by external calibration against standard samples of paclitaxel (Sigma, USA), 2α,5α,9α,10β,14β-pentaacetoxy-4(20), 11-taxadiene (previously isolated in our laboratory [
20]) or taxusin (ChromaDex, USA). Under the described analytical conditions, the relative standard deviation of the taxoid retention times did not exceed 1%. In the working concentration range (5.5-277.7 µg/ml, 3.6-71.4 µg/ml and 0.7-72.2 µg/ml for paclitaxel, 2α,5α,9α,10β,14β-pentaacetoxy-4(20), 11-taxadiene and taxusin, respectively), the taxoid calibration curves were approximated by straight lines with coefficients of determination (
R2) above 0.98. The relative standard deviation of taxoid peak areas did not exceed 10%.
2.5. Statistical analysis
Data on growth assessment and the analysis of taxoids are presented as the mean values with standard deviations recorded for the triplicates (3 flasks or 3 fixed-size samples of cell suspension collected from bioreactors) for each data point. STATISTICA10 software (StatSoft©, Moscow, Russia) was used for processing the data.
3. Results
3.1. Effect of MeJ on the growth and biosynthetic characteristics of the «young» (1.5-year-old) suspension cell culture of T. wallichiana in flasks and bioreactors
3.1.1. Effect of MeJ on the growth of the «young» suspension cell culture in flasks and bioreactors
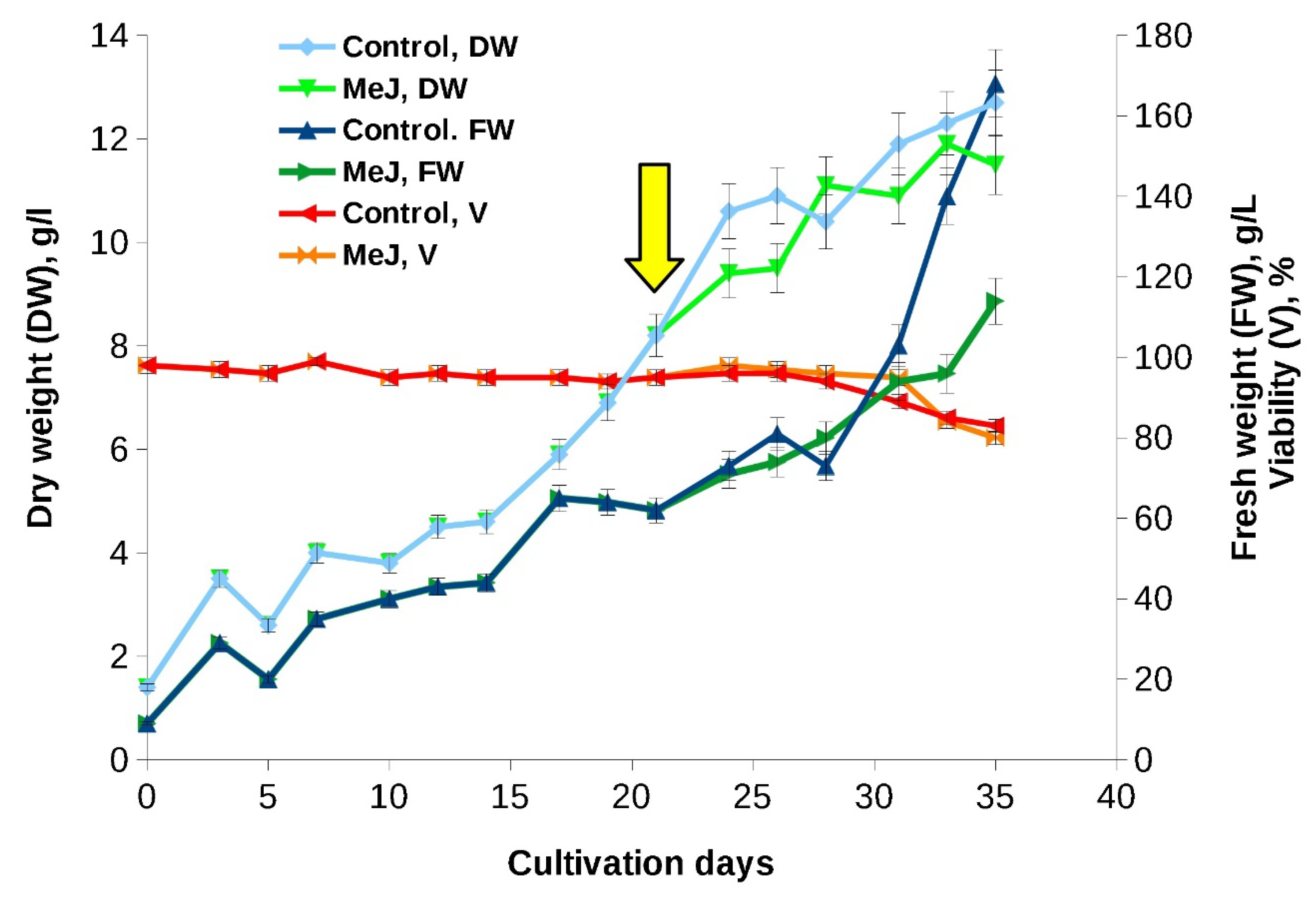
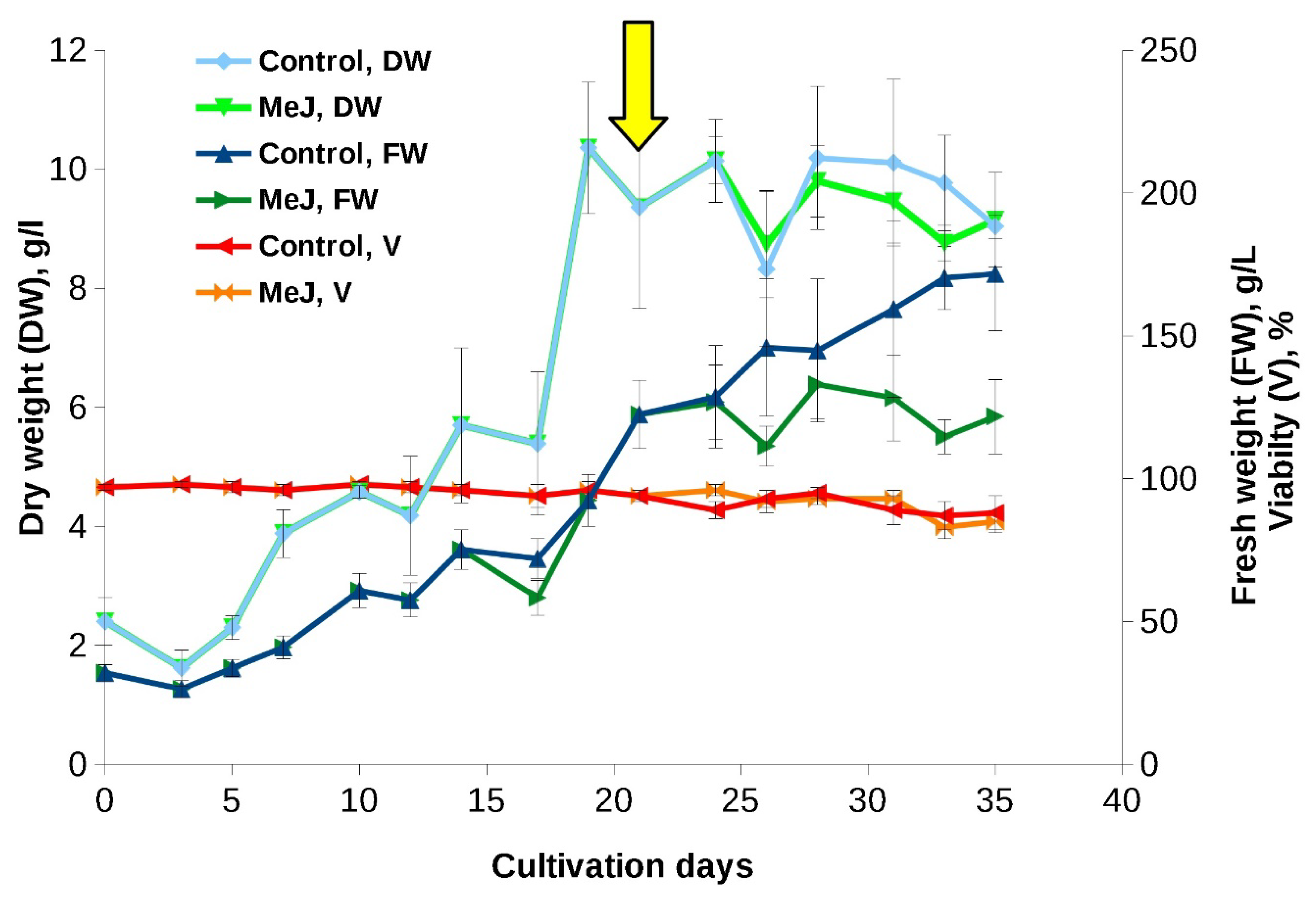
Growth curves and main growth parameters of «young» suspension cell culture of
T. wallichiana in flasks are shown in
Figure 1 and
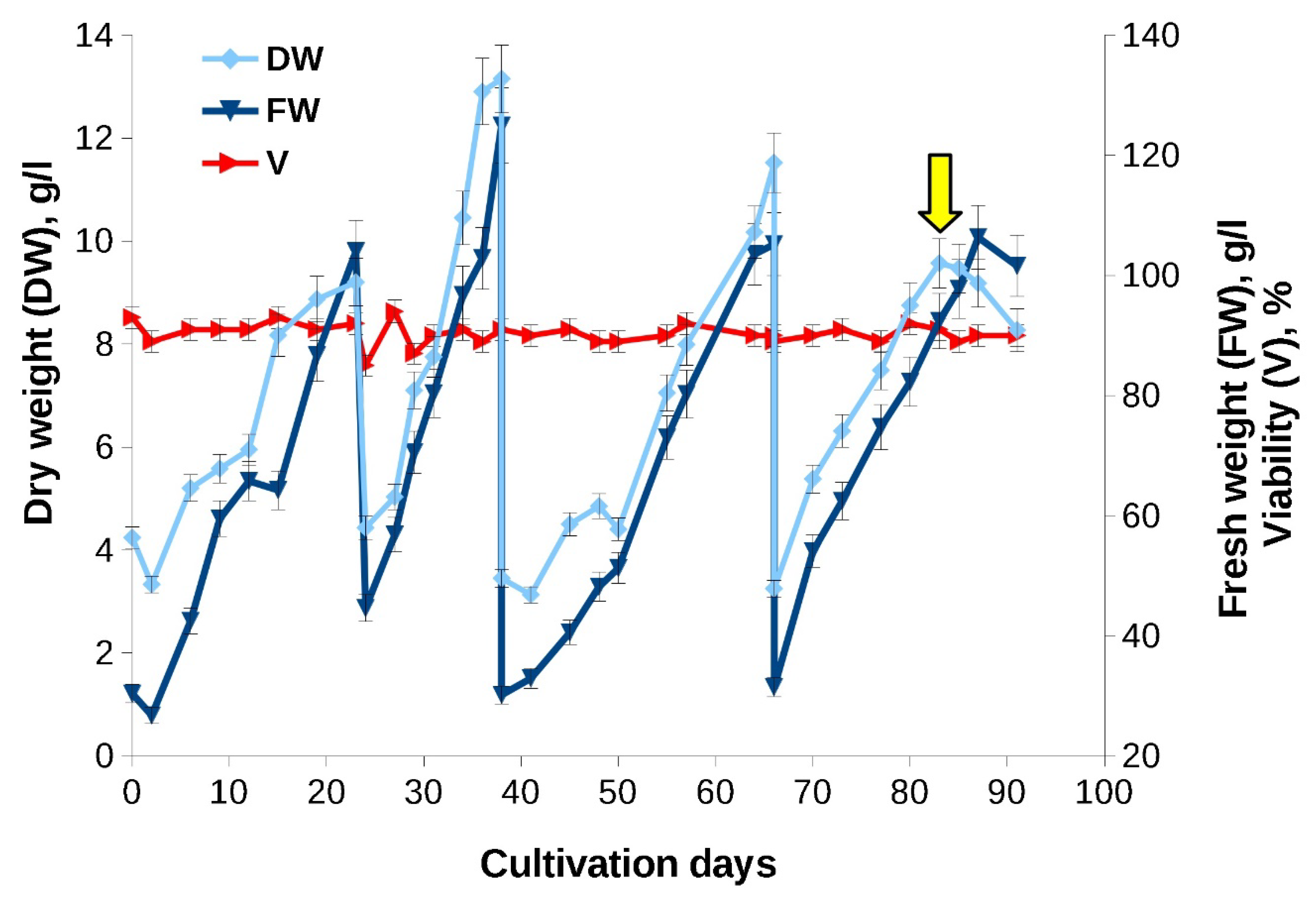
Table 1. The culture demonstrated rapid growth without a noticeable lag-phase. Growth curve had a standard S-shape with typical for plan cell cultures difference between FW and DW at the end of the subcultivation cycle, indicating a significant hydration of the cells. Cell viability remained high (95-97%) during 28 days of cultivation and decreased to 82-85% by the end of the subculture cycle (
Figure 1). In addition, during the subcultivation cycle, minor step-like fluctuations in both fresh and dry weights were recorded (
Figure 1).
The addition of MeJ (final concentration 100 had almost no effect on the growth characteristics and viability of the cell culture (
Figure 1) but resulted in a significant (almost 1.5-fold) reduction of cell hydration by the end of the subcultivation cycle.
Figure 1.
Growth curves of the «young» Taxus wallichiana suspension cell culture in flasks (experiment performed in 2017). The arrow indicates the timepoint of MeJ (final concentration 100 µM) addition to cell suspension. MeJ – culture elicited wih methyl jasmonate; DW – dry weight; FW – fresh weight; V – viability.
Figure 1.
Growth curves of the «young» Taxus wallichiana suspension cell culture in flasks (experiment performed in 2017). The arrow indicates the timepoint of MeJ (final concentration 100 µM) addition to cell suspension. MeJ – culture elicited wih methyl jasmonate; DW – dry weight; FW – fresh weight; V – viability.
Table 1.
Growth parameters of the «young» Taxus wallichiana suspension cell culture grown in flasks (experiment performed in 2017).
Table 1.
Growth parameters of the «young» Taxus wallichiana suspension cell culture grown in flasks (experiment performed in 2017).
| |
Growth parameter |
| I |
µ, day-1
|
τ, days |
Mmax, g/L |
Y |
P, g/(L×day) |
| Calculated on fresh weight basis |
16.9 |
0.22 |
3.1 |
17.3 |
n/a |
n/a |
| Calculated on dry weight basis |
10.2 |
0.21 |
3.3 |
12.7 |
0.33 |
0.39 |
| Calculated on cell number basis |
5.3 |
0.17 |
4.1 |
n/a |
n/a |
n/a |
Figure 2 and
Table 2 present growth curves and main growth parameters of the «young» suspension cell culture of
T. wallichiana grown in 20-L bioreactor.
Growth characteristics of the cell culture remained high during bioreactor cultivation. Minor decrease in the growth index observed compared to flasks may be due to high initial density of the cell culture. The addition of MeJ (final concentration 100 µM) had no effect on cell viability and maximum biomass accumulation during the same subculture cycle. Some increase in dry and fresh weight accumulation was observed in the next subculture cycle following MeJ treatment. Interestingly, MeJ treatment slightly reduced cell hydration at the end of the next subcultivation cycle (cycle 3 in
Figure 2) as reflected by lower difference between fresh and dry cell weights.
Figure 2.
Growth curves of a suspension culture of the «young» Taxus wallichiana suspension cell culture in 20-L bioreactor (experiment performed in 2017). The arrow indicates the timepoint of MeJ (final concentration 100 µM) addition to cell suspension. MeJ – culture elicited wih methyl jasmonate; DW – dry weight; FW – fresh weight; V – viability.
Figure 2.
Growth curves of a suspension culture of the «young» Taxus wallichiana suspension cell culture in 20-L bioreactor (experiment performed in 2017). The arrow indicates the timepoint of MeJ (final concentration 100 µM) addition to cell suspension. MeJ – culture elicited wih methyl jasmonate; DW – dry weight; FW – fresh weight; V – viability.
Table 2.
Growth parameters of the «young» Taxus wallichiana suspension cell culture grown in 20-L bioreactors bioreactor (experiment performed in 2017), calculated based on dry weight.
Table 2.
Growth parameters of the «young» Taxus wallichiana suspension cell culture grown in 20-L bioreactors bioreactor (experiment performed in 2017), calculated based on dry weight.
| Growth cycle |
Growth parameter |
| I |
µ, day-1 |
τ, days |
Mmax,g/L
|
Y |
P, g/(L×day) |
| 1 |
4.6 |
0.26 |
2.7 |
11.7 |
0.32 |
0.32 |
| 2 |
4.3 |
0.21 |
3.3 |
10.5 |
0.30 |
0.30 |
| 3 (MeJ elicitation) |
3.1 |
0.19 |
3.6 |
17.5 |
0.39 |
0.62 |
3.1.2. Effect of MeJ on accumulation of taxoid diterpenoids in the «young» suspension cell culture in flasks and bioreactors
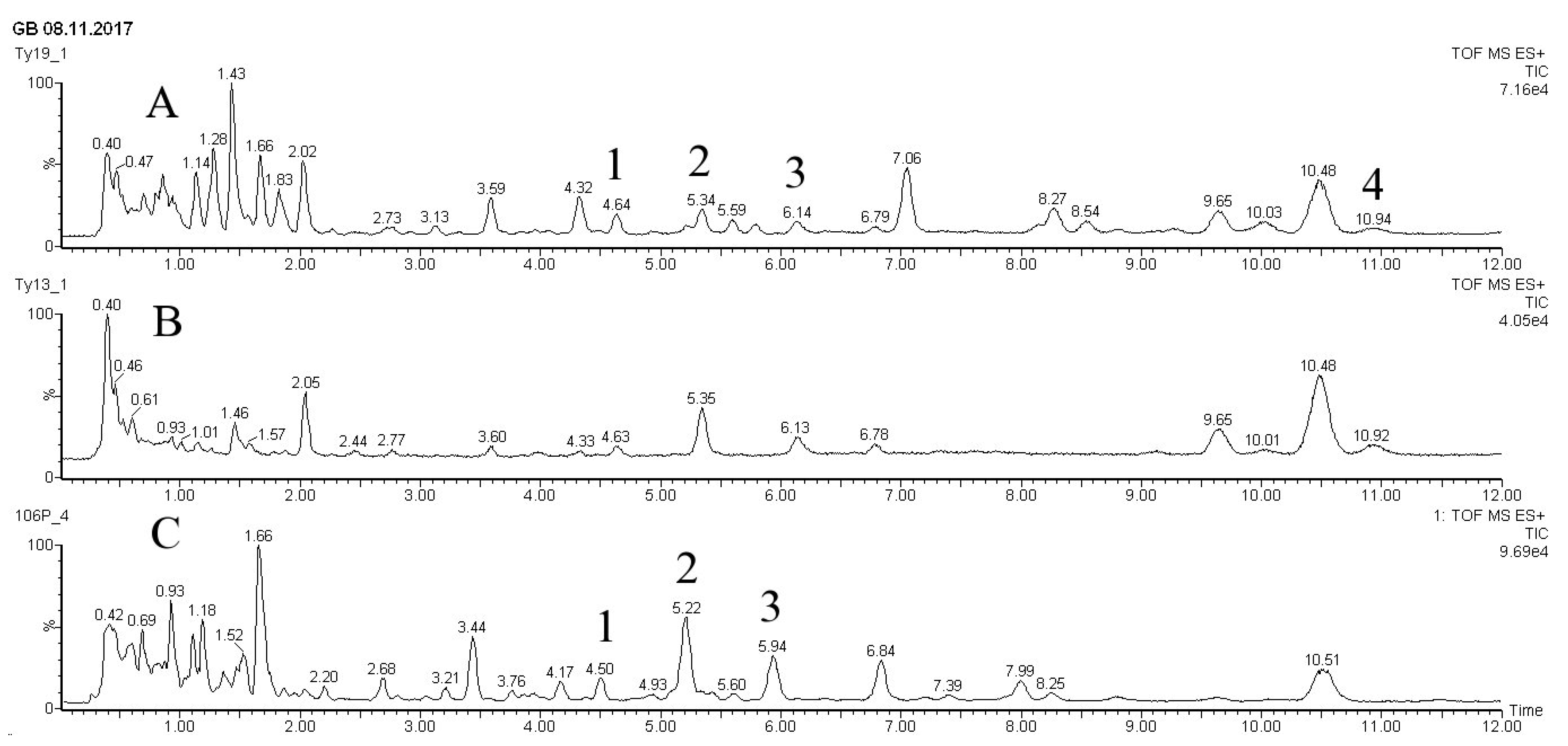
Chromatograms of taxoid diterpenoids detected in the dry biomass of the «young»
T. wallichiana cell suspension culture grown in flasks and bioreactors are presented in
Figure 3.
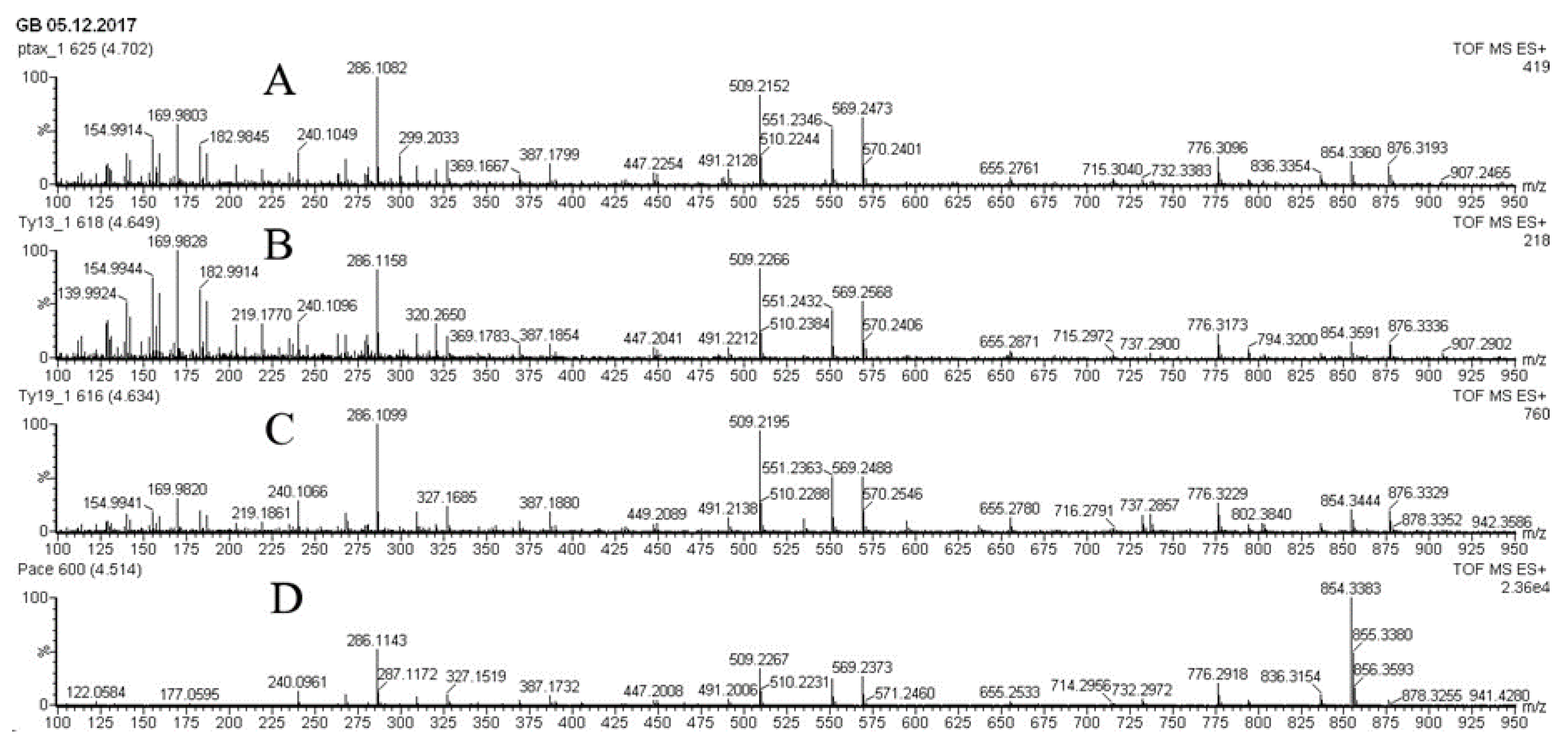
Figure 3.
UPLC-ESI-MS chromatograms (total ion current, positive ion mode) of methanolic extracts from biomass of Taxus wallichiana cell suspension culture. A - flasks, day 28, 7 days after MeJ elicitation (100 µM final concentration); B - flasks, 28 days, control without elicitation; C - 20-L bubble-type bioreactor, day 7 after MeJ elicitation. Peak numbers correspond to: 1 – paclitaxel; 2 – yunnanxane; 3 - taxuyunnanine C; 4 - sinenxan C. X – time, min; Y – detector signal, relative intensity, %.
Figure 3.
UPLC-ESI-MS chromatograms (total ion current, positive ion mode) of methanolic extracts from biomass of Taxus wallichiana cell suspension culture. A - flasks, day 28, 7 days after MeJ elicitation (100 µM final concentration); B - flasks, 28 days, control without elicitation; C - 20-L bubble-type bioreactor, day 7 after MeJ elicitation. Peak numbers correspond to: 1 – paclitaxel; 2 – yunnanxane; 3 - taxuyunnanine C; 4 - sinenxan C. X – time, min; Y – detector signal, relative intensity, %.
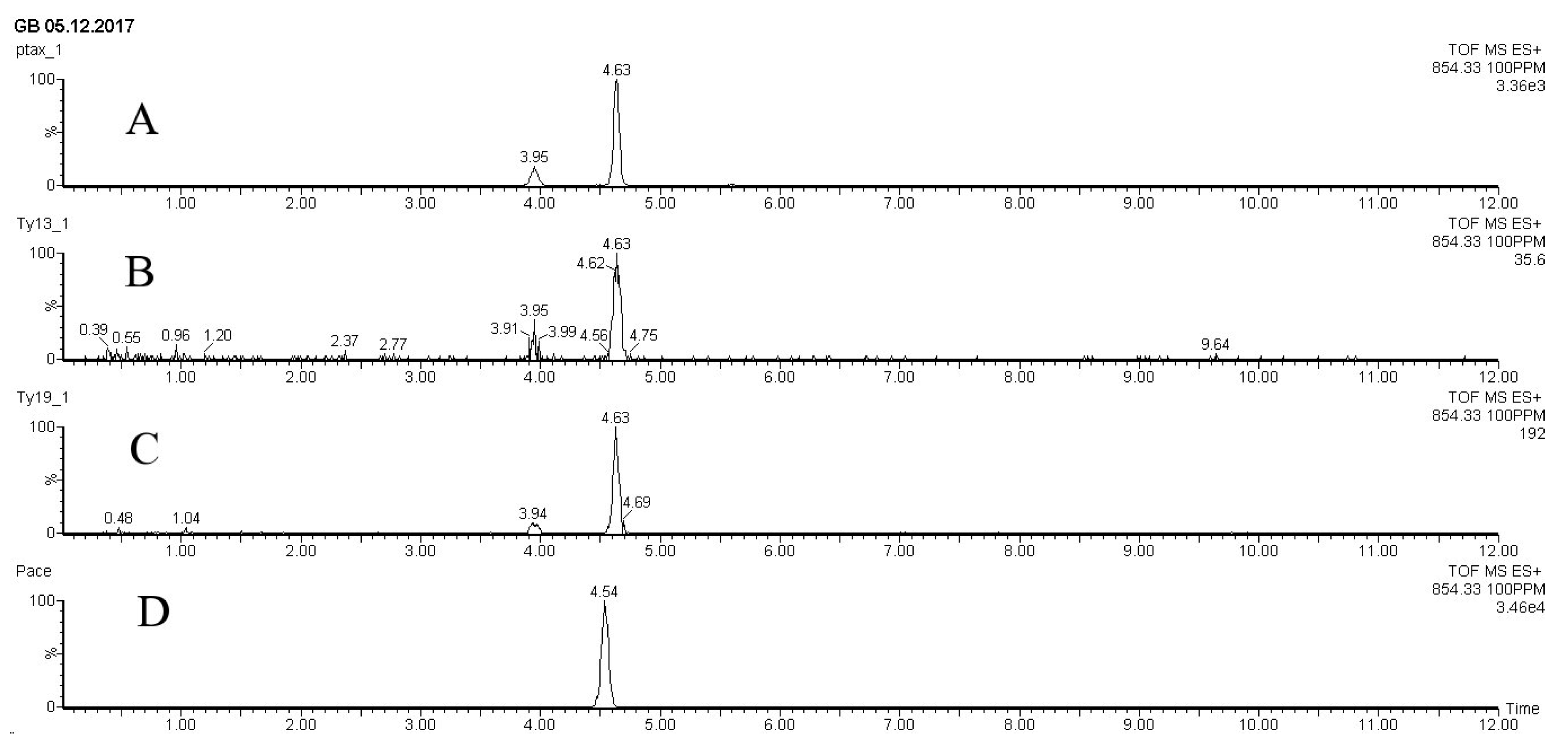
Figure 4.
UPLC-ESI-MS chromatograms (extracteed ion chromatograms for m/z 854.3 (corresponding to [M+H]+ for paclitaxel) of methanolic extracts from biomass of Taxus wallichiana cell suspension culture and solutions of standard samples of paclitaxel. A – sample of paclitexel isolated from the bark of T. cuspidata; B - methanolic extract from biomass of T. wallichiana cell suspension culture, flasks, 28 days, control without elicitation; C - methanolic extract from biomass of T. wallichiana cell suspension culture, flasks, day 28, 7 days after MeJ elicitation (100 µM final concentration); D – paclitaxel standard sample purchased from Sigma (USA). X – time, min; Y – detector signal, relative intensity, %.
Figure 4.
UPLC-ESI-MS chromatograms (extracteed ion chromatograms for m/z 854.3 (corresponding to [M+H]+ for paclitaxel) of methanolic extracts from biomass of Taxus wallichiana cell suspension culture and solutions of standard samples of paclitaxel. A – sample of paclitexel isolated from the bark of T. cuspidata; B - methanolic extract from biomass of T. wallichiana cell suspension culture, flasks, 28 days, control without elicitation; C - methanolic extract from biomass of T. wallichiana cell suspension culture, flasks, day 28, 7 days after MeJ elicitation (100 µM final concentration); D – paclitaxel standard sample purchased from Sigma (USA). X – time, min; Y – detector signal, relative intensity, %.
Figure 5.
MS spectra (positive ions) of the peaks corresponding to paclitaxel on UPLC-ESI-MS chromatograms of methanolic extracts from biomass of
Taxus wallichiana cell suspension culture and solutions of standard samples of paclitaxel (
Figure 4). A – sample of paclitexel isolated from the bark of
T. cuspidata; B - methanolic extract from biomass of
T. wallichiana cell suspension culture, flasks, 28 days, control without elicitation; C - methanolic extract from biomass of
T. wallichiana cell suspension culture, flasks, day 28, 7 days after MeJ elicitation (100 µM final concentration); D – paclitaxel standard sample purchased from Sigma (USA). X – m/z; Y – detector signal, relative intensity, %.
Figure 5.
MS spectra (positive ions) of the peaks corresponding to paclitaxel on UPLC-ESI-MS chromatograms of methanolic extracts from biomass of
Taxus wallichiana cell suspension culture and solutions of standard samples of paclitaxel (
Figure 4). A – sample of paclitexel isolated from the bark of
T. cuspidata; B - methanolic extract from biomass of
T. wallichiana cell suspension culture, flasks, 28 days, control without elicitation; C - methanolic extract from biomass of
T. wallichiana cell suspension culture, flasks, day 28, 7 days after MeJ elicitation (100 µM final concentration); D – paclitaxel standard sample purchased from Sigma (USA). X – m/z; Y – detector signal, relative intensity, %.
Biomass of suspension culture cells of
T. wallichiana contained diterpenoids of taxane series belonging to the structural type of taiwanxan (14-hydroxylated taxoids): sinenxane B, sinenxane C, taxuyunnanine C, 2α,5α,9α,10β,14β-pentaacetoxy-4(20), 11-taxadiene, yunnanxane, and isomer of 7-hydroxy-2,5,10,14-tetra-acetoxy taxadiene. Under standard conditions in flasks, the dominated compounds were 14-OH toxoids taxuyunnanine C and yunnanxane. C13-OH taxoids (paclitaxel) were also detected, but only in small amounts. The quantitative content of the main detected taxoids is shown in
Table 3.
After adding MeJ to the suspension cell culture, a significant change in the composition of taxoids was observed. As a result of MeJ action, paclitaxel content in cell biomass increased almost 10-times, from 0.02 mg/gDW to 0.19 mg/gDW. Seven days after MeJ treatment (28-31 days of cultivation), the paclitaxel content reached almost 0.02% DW, which is quite comparable with its content in intact yew plants [
29]. At the same time, the content of 14-OH taxoids (taxuyunnanine C and yunnanxane) changed insignificantly. The results are in agreement with the literature [
28,
30,
31] and indirectly support the assumption of different regulation of formation or accumulation of 13- and 14-hydroxylated taxoids in yew cells
in vitro.
The results of taxoid analysis in the biomass of
T. wallichiana cell suspension cultured in a 20-L bioreactor were comparable to those for flask culture (
Figure 3,
Table 3).
These results suggest that cell culture of
T. wallichiana grown in a bioreactor accumulated mainly 14-hydroxylated (taxuyunnanine C and yunnanxane), but their content is slightly lower compared to culture in flasks. Synthesis of paclitaxel in the cell culture was detected from the moment of adding MeJ (at 48th day). At the day 52, 4 days after adding MeJ, paclitaxel content reached 0.06 mg/gDW. Seven days after elicitation (at the 55th day of culturing) paclitaxel content was doubled at 0.11 mg/gDW, which was comparable to the level of this compound in intact plant of
T. wallichiana (
Table 3). It is important that the paclitaxel content increase occurred in the next cycle of cultivation following elicitation and reached 0.15 mg/gDW by the end of the experiment.
The presence of 14-hydroxylated taxoids (taxuyunnanine C and yunnanxane), which were synthesized both before- and after the addition of methyl jasmonate, was also recorded for the T. wallichiana cell culture growing in the bioreactor. The content of these compounds increased as a result of MeJ elicitation. For example, 7 days after elicitation (55th day of culture), the total content of 14-hydroxylated taxoids was 0.45 mg/gDW while on the 77 day of cultivation is was 0.70 mg/gDW.
Our results confirm the predominant content of 14-OH taxoids compared with other groups of taxoids, regardless of the cultivation system (flask or bioreactor). However, the composition and ratio of individual compounds were different from that in plant. In particular, baccatin III (13-OH taxoid) was not detected in T. wallichiana cell culture.
Table 3.
Taxoid content in the biomass of the «young» suspension culture of Taxus wallichiana grown in flasks and a 20-L bioreactor (experiment performed in 2017).
Table 3.
Taxoid content in the biomass of the «young» suspension culture of Taxus wallichiana grown in flasks and a 20-L bioreactor (experiment performed in 2017).
| Variant |
Days of culture |
Taxoid content, mg/gDW |
Yunnanxane 1
(14-OH-taxoid)
|
Taxuyunnanine C 1
(14-OH-taxoid)
|
Paclitaxel
(13-OH-taxoid)
|
| Flasks, control |
28 |
0.77 |
0.53 |
0.02 |
| 31 |
1.22 |
0.56 |
0.05 |
| Flasks, MeJ |
28 |
0.95 |
0.51 |
0.19 |
| 31 |
1.02 |
0.65 |
0.18 |
| 20-L bioreactor, control |
14 |
0.04 |
0.13 |
- |
| 28 |
0.12 |
0.02 |
- |
| 45 |
0.08 |
0.09 |
- |
| 20-L bioreactor, МеJ (48 days of culture) |
48 |
0.13 |
0.12 |
- |
| 52 |
0.15 |
0.13 |
0.06 |
| 55 |
0.27 |
0.23 |
0.11 |
| 77 |
0.36 |
0.34 |
0.15 |
| Tree bark2
|
|
- |
- |
0.13 |
3.2. Effect of MeJ on the growth and byosynthetic characeristics of the «old» (6-year-old) suspension cell culture of Taxus wallichiana in flasks and bioreactors
3.2.1. Effect of MeJ on the growth of the «old» suspension cell culture in flasks and bioreactors
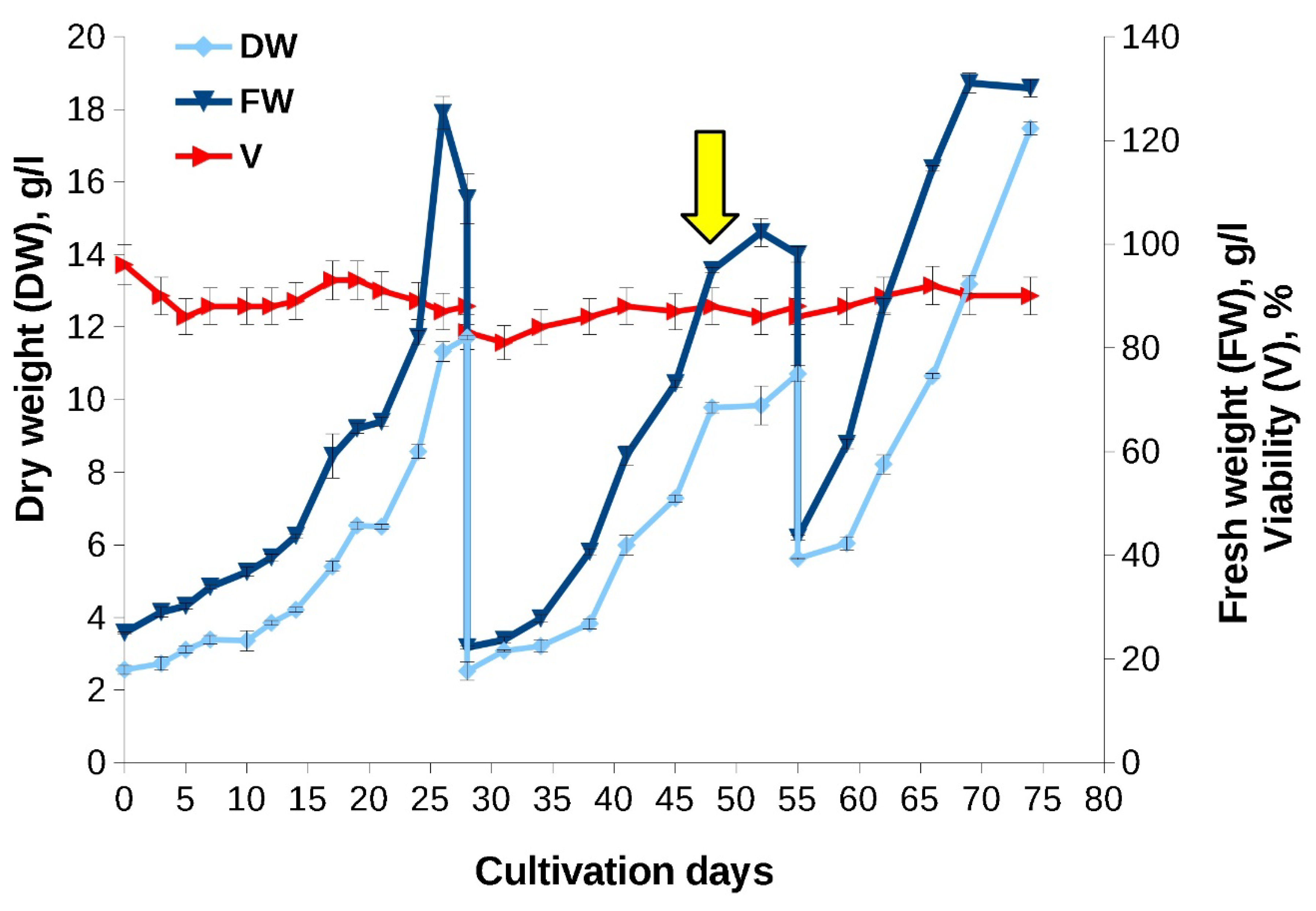
When a 6-year-old suspension cell culture was grown in flasks and bioreactors, its growth characteristics resembled those recorded for the «young» culture (
Figure 6,
Table 4). A minor decrease in the growth index compared to «young» culture observed may be due to a higher initial inoculum density. Interestingly, the culture preserved a step-wise growth curve observed for the «young» culture (
Figure 6) but, due to the increase of lag phase, the growth decrease was recorded at 2-5, 10-12 and 14-16 days of cultivation.
Similar to the experiments performed in 2017, the addition of MeJ (final concentration - 100 µM) had little effect on culture growth characteristics. Moreover, we observed the same effect of MeJ on reducing cellular hydration: in the control without elicitation, the ratio of FW to DW at the stationary growth stage was 1.4 - 1.5 times higher than in variant with elicitation which coincides with the results of the first experimental series performed in 2017 (
Figure 6).
Figure 6.
Growth curves of the «old» Taxus wallichiana cell suspension culture in flasks (experiment carried out in 2021 year). The arrow indicates the timepoint of MeJ (100 µM final concentration) addition to cell suspension. MeJ – culture elicited wih methyl jasmonate; DW – dry weight; FW – fresh weight; V – viability.
Figure 6.
Growth curves of the «old» Taxus wallichiana cell suspension culture in flasks (experiment carried out in 2021 year). The arrow indicates the timepoint of MeJ (100 µM final concentration) addition to cell suspension. MeJ – culture elicited wih methyl jasmonate; DW – dry weight; FW – fresh weight; V – viability.
Table 4.
Growth parameters of “old” Taxus wallichiana suspension cell culture grown in flasks (experiment carried out in 2021 year).
Table 4.
Growth parameters of “old” Taxus wallichiana suspension cell culture grown in flasks (experiment carried out in 2021 year).
| |
Growth parameter |
| I |
µ, day-1
|
τ, days |
Mmax, g/L |
Y |
P, g/(L×day) |
| Calculated on fresh weight basis |
4.4 |
0.27 |
2.6 |
168.2 |
|
|
| Calculated on dry weight basis |
4.9 |
0.25 |
2.8 |
10.5 |
0.29 |
0.44 |
«Old» cell suspension cultured in a bioreactor demonstrated good growth characteristics that were comparable to culture in flasks and bioreactor cultivation of «young» cell culture in 2017 (
Figure 7,
Table 5). Four cultivation cycles, 14-28 days each, were performed. During the first three cultivation cycles, the culture was adapting to the new cultivation conditions, so MeJ (final concentration 100 µM) was added in the fourth cultivation cycle on day 17, at the end of the exponential growth phase.
3.2.2. Effect of MeJ on accumulation of taxoid diterpenoids in the «old» suspension cell culture in flasks and bioreactors
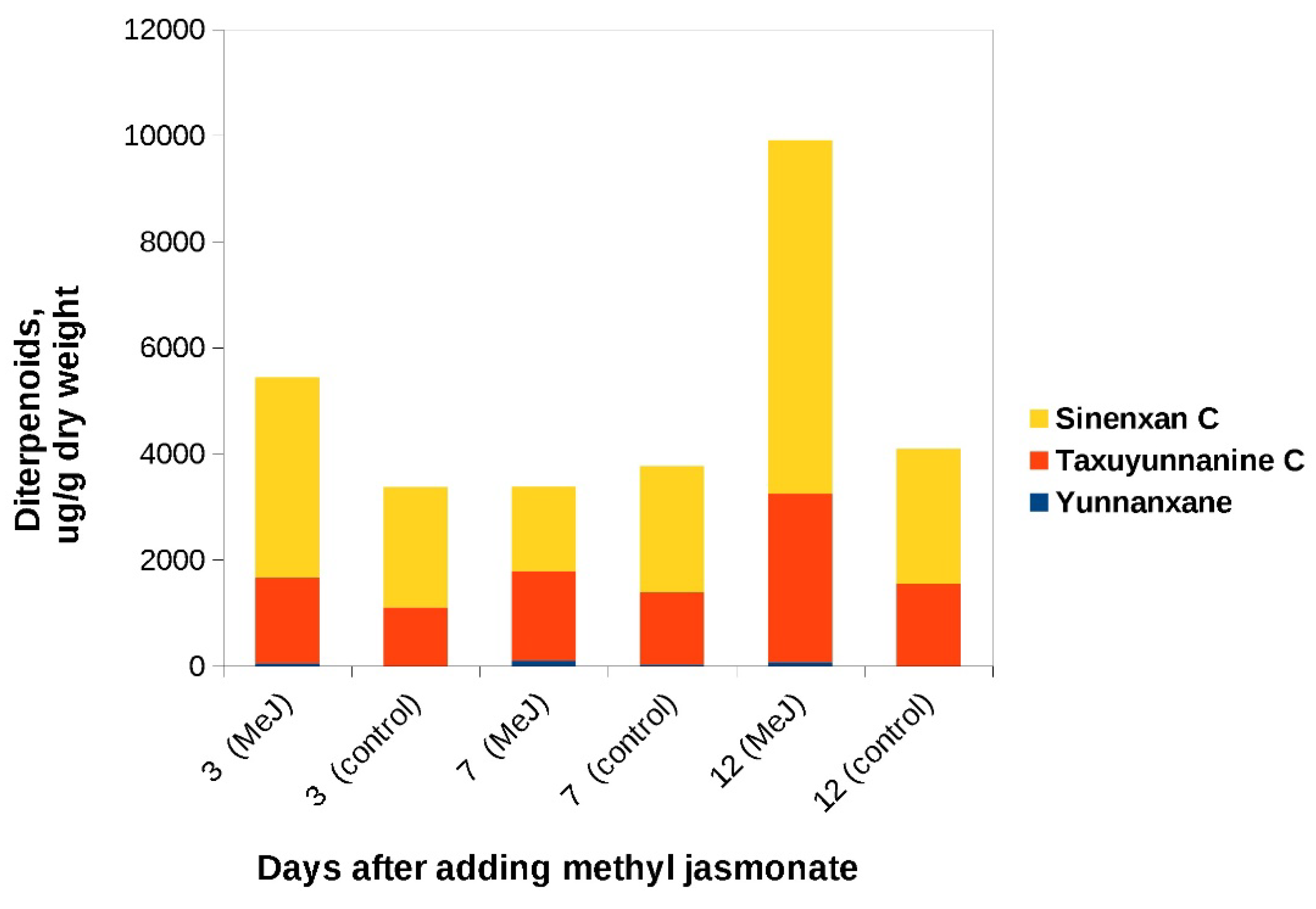
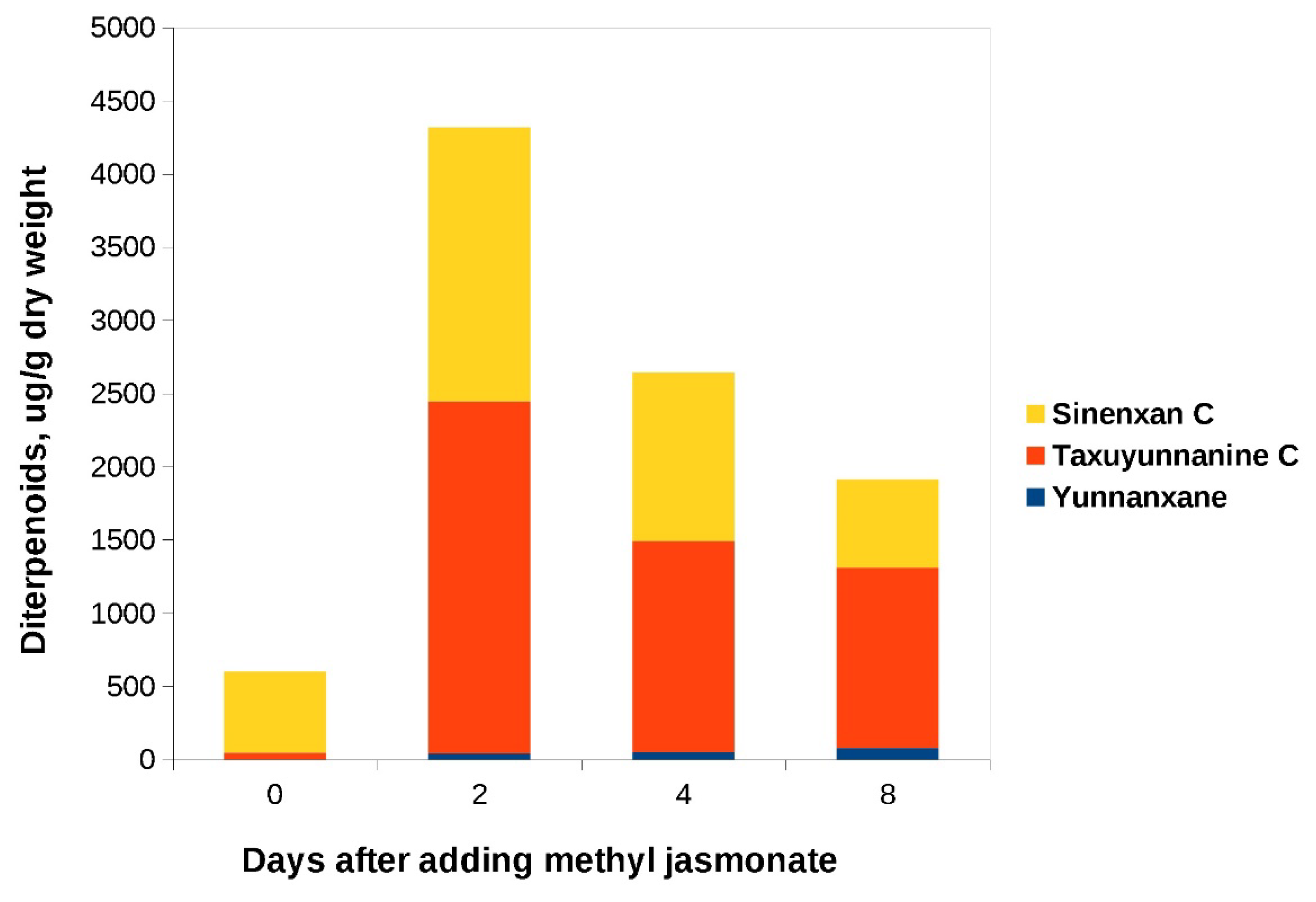
MeJ was added to the «old» culture during the phase of cell growth retardation: on day 19 of culture in flasks and on day 17 of bioreactor cultivation. In both flasks and bioreactor, C14-hydroxylated taxoids were found in significant amounts (Figs 8, 9). Synenxane C was the predominant compound regardless the addition of MeJ: its content varied by more than 10-times depending on cultivation system, from 0.55 mg/g DW to 6.66 mg/g DW. The content of yunnanxane ranged from zero to 0.1 mg/g DW. It is noteworthy that in the 2017 experiments, yunnanxane was quantitatively the predominant taxoid with the content reaching 1 mg/g DW while the “old” culture synthesized sinenxan C as the main compound. Elicitation with MeJ significantly increased yunnanxane content from 0-27 µg/g in the control culture to 40-100 µg/g DW in culture with elicitation.
Figure 8.
C14-hydroxylated taxoids content in cell biomass of the «old» suspension culture of Taxus wallichiana grown in flasks after addition of MeJ. Methyl jasmonate (100 µM final concentration) was added on the 19th day of cultivation. Sinenxan C, yunnanxane and taxuyunnanine C content were determined by the calibration curve for taxusin.
Figure 8.
C14-hydroxylated taxoids content in cell biomass of the «old» suspension culture of Taxus wallichiana grown in flasks after addition of MeJ. Methyl jasmonate (100 µM final concentration) was added on the 19th day of cultivation. Sinenxan C, yunnanxane and taxuyunnanine C content were determined by the calibration curve for taxusin.
Figure 9.
C14-hydroxylated taxoids content in cell biomass of the «old» suspension culture of Taxus wallichiana cultured in a 75-L bioreactor before (day 0) and after addition of MeJ. Methyl jasmonate (100 µM final concentration) was added on day 17 of culture cycle 4. . Sinenxan C, yunnanxane and taxuyunnanine C content were determined by the calibration curve for taxusin.
Figure 9.
C14-hydroxylated taxoids content in cell biomass of the «old» suspension culture of Taxus wallichiana cultured in a 75-L bioreactor before (day 0) and after addition of MeJ. Methyl jasmonate (100 µM final concentration) was added on day 17 of culture cycle 4. . Sinenxan C, yunnanxane and taxuyunnanine C content were determined by the calibration curve for taxusin.
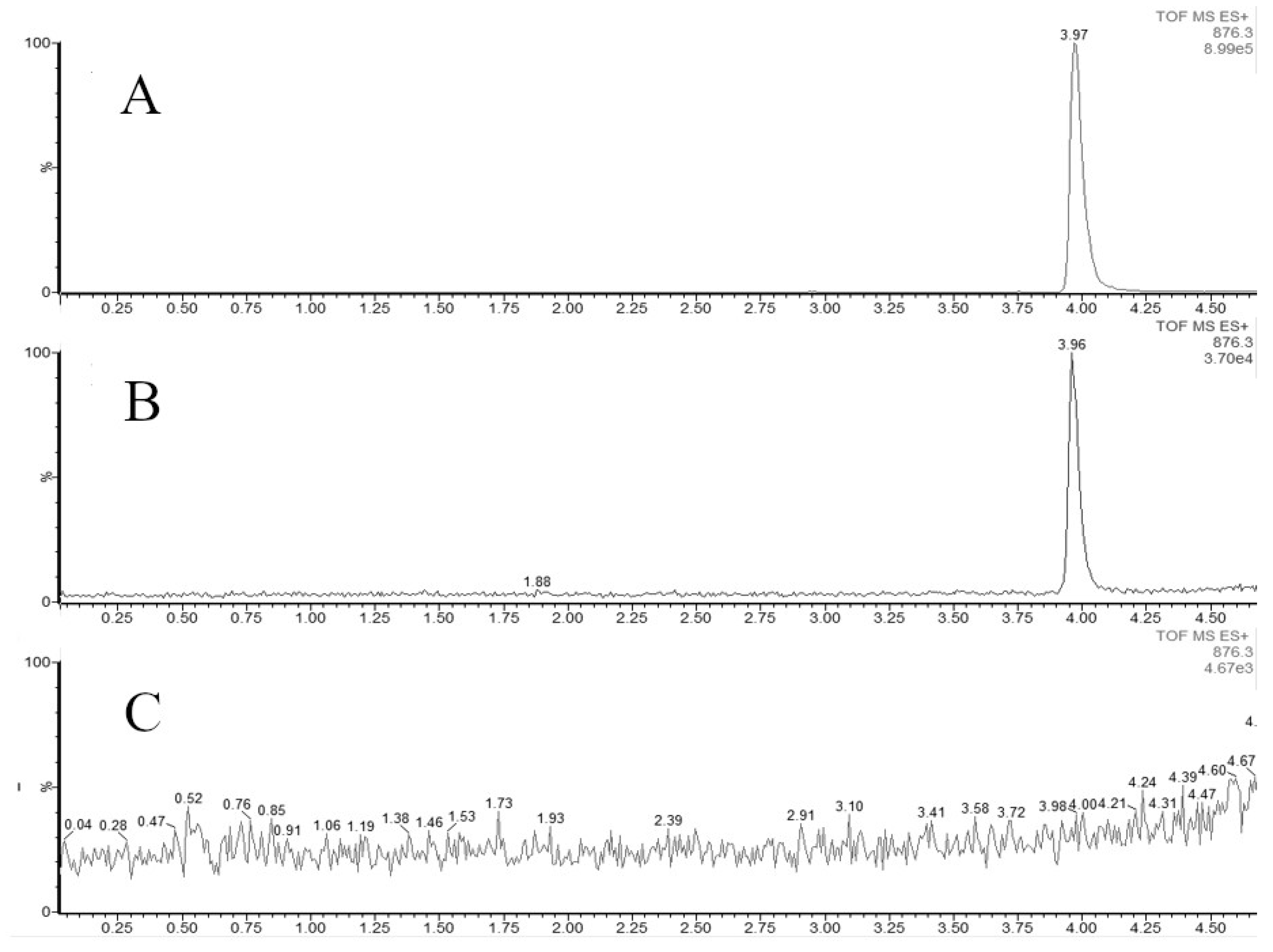
The effect of MeJ on the formation of C13-hydroxylated taxoids is expressed in the intensification of the accumulation of these diterpenoids in the cell culture biomass. However, the quantitative content of C13-hydroxylated taxoids does not exceed trace amounts. In experiments with cell culture in flasks, paclitaxel synthesis was observed only in trace amounts (less than 3.5 µg/gDW) on the 7th day after elicitation (
Figure 10). It is worth noting that, in addition to paclitaxel, other C13-oxygenated/hydroxylated taxoids appeared in trace amounts in culture: 10-deacetyltaxol, 13-acetyl-9-dihydrobaccatin III, baccatin III and cephalomannine. None of these compounds was detected in the control (not MeJ treated) culture in flasks.
When suspension was cultured in a bioreactor, addition of MeJ resulted in trace amounts of C13-oxygenated/hydroxylated taxoids, but their composition was different from that in flasks. Interestingly, trace amounts (less than 3.5 µg/gDW) of the C13-OH compound, 10-deacetylbaccatin III, were found in culture on day 17 of culture cycle 4, that is, before the addition of MeJ, and remained until the end of the cultivation cycle. This compound was only found in a bioreactor culture. At the same time, paclitaxel and baccatin III were not found until the 14th day of cultivation after the addition of methyl jasmonate.
Figure 10.
UPLC-ESI-MS chromatograms (extracteed ion chromatograms for m/z 876.3 (corresponding to [M+Na]+ for paclitaxel) of methanolic extracts from biomass of Taxus wallichiana cell suspension culture and solution of paclitaxel standard sample. A - paclitaxel standard sample purchased from Sigma (USA); B - methanolic extract from biomass of T. wallichiana cell suspension culture, flasks, day 28, 7 days after MeJ elicitation (100 µM final concentration); С - methanolic extract from biomass of T. wallichiana cell suspension culture, flasks, day 28, control without elicitation. X – time, min; Y – detector signal, relative intensity, %.
Figure 10.
UPLC-ESI-MS chromatograms (extracteed ion chromatograms for m/z 876.3 (corresponding to [M+Na]+ for paclitaxel) of methanolic extracts from biomass of Taxus wallichiana cell suspension culture and solution of paclitaxel standard sample. A - paclitaxel standard sample purchased from Sigma (USA); B - methanolic extract from biomass of T. wallichiana cell suspension culture, flasks, day 28, 7 days after MeJ elicitation (100 µM final concentration); С - methanolic extract from biomass of T. wallichiana cell suspension culture, flasks, day 28, control without elicitation. X – time, min; Y – detector signal, relative intensity, %.
4. Discussion
Plant secondary metabolites perform a vital role in plant signalling and response to environmental conditions including biotic and abiotic stresses, other plants, pollinators and predators [
9]. Taxoid diterpenoids are produced by a number of yew species as a part of their defence system against bark-colonizing fungi and oomycetes and are effective against the most widespread and important pathogens of conifers [
32,
33]. In medicine, these compounds are highly valued for their unique anti-tumor activities [
3]. Although multiple studies explored the use of yew cell culture for the production of taxoids, these efforts are still hampered by a low content of the target compounds, particularly paclitaxel, in the cell cultures compared to plants [
9].
Elicitation with MeJ is one of the most popular ways to stimulate the biosynthesis of the desired secondary metabolites in plant cell cultures. For example, it was effective in increasing the production of ecdysteroids in the cell cultures of various
Ajuga species [
34] and ginsenosides in the cell cultures of
Panax spp. [
35]. In 1996, Yukimune et al. first reported that elicitation with MeJ improved accumulation of paclitaxel and baccatin III (13-OH taxoids) in
Taxus spp. suspension cell cultures [
15]. Several
Taxus species were shown to be responsive to MeJ treatment. For example, MeJ induced production of paclitaxel in the suspension cultures of
T. cuspidata [
36] and
T. canadensis [
17]. In
T. canadensis, MeJ added to culture medium increased paclitaxel production to 48.3 mg/L and baccatin III to 53.6 mg/L compared to 0.4 mg/L of each of these compounds in control (non-elicited) cell culture [
37]. In the work by Ketchum et al. [
38], the greatest accumulation of paclitaxel in the cell cultures of
T. canadensis and
T. cuspidata occurred when MeJ was added to cultures at a final concentration of 200 μM on day 7 of the culture cycle. The concentration of paclitaxel increased in the extracellular (cell-free) medium to 23.4 mg/L per day within 7 days following elicitation. In cell culture of
T. baccata, addition of MeJ combined with lauryl alcohol stimulated a 2-fold increase in paclitaxel production without affecting culture growth characteristics [
39].
Similarly, in the present study, production of 13-OH taxoids including paclitaxel in «young» cell culture of T. wallichiana increased both in flasks and bioreactors following addition of MeJ, while culture growth and cell viability remained at a high level. Hence, the effect of MeJ on «young» cell culture of T. wallichiana was comparable to that in cell cultures of other yew species.
Other taxoids present in young cell culture of
T. wallichiana in this study were 14-OH taxoids yunnanxane and taxuyunnanine C. Our earlier study revealed that cell cultures of different
Taxus species and hybrids are able to produce 14-OH taxoids that accumulate in both cell biomass and culture medium [
20].
It is worth noting that nearly all studies referenced herein were performed using relatively young (recently induced) cell cultures. In this regard, it was interesting to compare taxoid synthesis and the effect of MeJ on the same cell culture after several years of maintenance by periodic subcultures.
The composition of taxoid diterpenoids in the cell culture of
T. wallichiana notably changed after 6 years of cultivation. In the old culture, sinenxane C and taxuyunnanine C became predominant compounds by contrast to young culture that produced mostly yunnanxane and taxuyunnanine C in comparable amounts. In addition, the old cell culture reacted differently to MeJ treatment. By contrast to young cell culture, in the 6-year-old cell culture, MeJ increased the production of 14-OH taxoids sinenxane C and taxuyunnanine C but did not lead to the appearance of 13-OH paclitaxel. These results are in agreement with literature findings. For example, McKee et al. [
19] reported that MeJ treatment of
T. cuspidata cell cultures only led to increasing in paclitaxel content if this compound was present in the culture before the elicitation. If paclitaxel was initially absent in the cell culture, MeJ could not induce its synthesis. MeJ differently affected growth and taxoid (taxol, 10-deacetylbaccatin III, baccatin III, 10-deacetyltaxol, cephalomannine) accumulation in cell culture of
T. globosa depending on growth regulator combination in culture medium and cell immobilization [
39]. Interestingly, in the present study, MeJ induced the appearance of paclitaxel in the «young» culture but had only minor effect on the content of 14-OH taxoids. By contrast, in the «old» culture, production of 14-OH taxoids was significantly affected by MeJ elicitation.
Several studies, including ours, demonstrated successful cultivation of taxoid-producing yew cell cultures in bioreactors of different volumes [
3,
21]. In the present study, the effect of MeJ was clearly related to culture age rather than a cultivation system (flasks or bioreactors). In both «young» and «old» cell suspensions, the response to MeJ observed in bioreactor cultures resembled those in flasks. Therefore, flask cultivation could be effectively used for the initial screening as well as elucidation of elicitation effects in yew cell cultures prior to scaling up the growth process to a more expensive bioreactor cultivation [
20].
The response of the cell culture to treatment with plant growth regulators and elicitors may be different from that in plants due to different physiology of plant cell culture as a population of undifferentiated cells lacking organismic control [
13]. Our findings suggest that the response to MeJ treatment in the same cell culture depends on many factors and may change in the course of cultivation. Hormonal signaling in dedifferentiated yew cells grown in vitro is different from that in plants and change with culture age. This might be a result of the high level of heterogeneity of cells in vitro and their constant auto-selection for proliferate intensity which leads to predominant formation of C14-OH taxoids versus C13-OH taxoids and modified cell response to exogenous MeJ treatment. These observations may have important implications for the commercial production of 13-OH taxoids, including paclitaxel, using the cell culture technology.
Author Contributions
Conceptualization, A.N., D.K.; methodology and investigation, E.D., E.G., D.K.; writing—original draft preparation, E.D., E.G., A.N., D.K.; writing—review and editing, E.D., E.G., D.K., A.N.; supervision, A.N.; funding acquisition, A.N., D.K. All authors have read and agreed to the published version of the manuscript.”.
Funding
The results were obtained within the state assignments of Ministry of Science and Higher Education of the Russian Federation theme No. 122042700045-3 (cell culture maintenance) and theme No. 122042600086-7 (bioreactor cultivation of the cell cultures). The commercial standards of 13-hydroxylated taxoids were purchased with the support of the Russian Foundation for Basic Research project no. 20-54-00014 Bel_a.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new datasets were generated during this study.
Acknowledgments
We are grateful to Dr. Elena Popova, K.A. Tymyryazev Institute of Plant Physiology of Russian Academy of Sciences, Moscow, Russia, for help and valuable comments during preparation of the manuscript.
References
- Howat, S.; Park, B.; Oh, I.S.; Jin, Y.-W.; Lee, E.-K.; Loake, G.J. Paclitaxel: biosynthesis, production and future prospects. New Biotechnol. 2014, 31, 242–245. [CrossRef]
- Cusido, R.M.; Onrubia, M.; Sabater-Jara, A.B.; Moyano, E.; Bonfill, M.; Goossens, A.; Pedreño, M.A.; Palazon, J. A rational approach to improving the biotechnological production of taxanes in plant cell cultures of Taxus spp.. Biotechnol. Adv. 2014, 32, 1157–1167. [CrossRef]
- Tabata, H. Production of Paclitaxel and the Related Taxanes by Cell Suspension Cultures of Taxus Species. Curr. Drug Targets 2006, 7, 453–461. [CrossRef]
- Malik, S.; Cusidó, R.M.; Mirjalili, M.H.; Moyano, E.; Palazón, J.; Bonfill, M. Production of the anticancer drug taxol in Taxus baccata suspension cultures: A review. Process. Biochem. 2011, 46, 23–34. [CrossRef]
- Onrubia, M.; Cusido, R.M.; Ramirez, K.; Hernandez-Vazquez, L.; Moyano, E.; Bonfill, M.; Palazon, J. Bioprocessing of plant in vitro systems for the mass production of pharmaceutically important metabolites: paclitaxel and its derivatives. Current Medicinal Chemistry 2013, 20(7), 880–891.
- Murthy, H. N.; Dandin, V.S.; Zhong, J.-J.; Paek, K.-Y. Strategies for enhanced production of plant secondary metabolites from cell and organ cultures. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.-Y.; Murthy, H.N.; Zhong, J.J., Eds, 2014, pp. 471–508.
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [CrossRef]
- E Farmer, E.; A Ryan, C. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves.. Proc. Natl. Acad. Sci. 1990, 87, 7713–7716. [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [CrossRef]
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697. [CrossRef]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [CrossRef]
- Gundlach, H.; Müller, M.J.; Kutchan, T.M.; Zenk, M.H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures.. Proc. Natl. Acad. Sci. 1992, 89, 2389–2393. [CrossRef]
- Nosov, A.M. Application of cell technologies for production of plant-derived bioactive substances of plant origin. 2012, 48, 609–624. [CrossRef]
- Cusido R.M.; Vidal, H.; Gallego, A.; Abdoli, M.; Palazon, J. Biotechnological production of taxanes: A molecular approach. Recent Advances in Pharmaceutical Sciences III, 2013: 91-107.
- Yukimune, Y.; Tabata, H.; Higashi, Y.; Hara, Y. Methyl jasmonate-induced overproduction of paclitaxel and baccatin III in Taxus cell suspension cultures. Nat. Biotechnol. 1996, 14, 1129–1132. [CrossRef]
- Exposito, O.; Syklowska-Baranek, K.; Moyano, E.; Onrubia, M.; Bonfill, M.; Palazon, J.; Cusido, R.M. Metabolic responses of Taxus media transformed cell cultures to the addition of methyl jasmonate. Biotechnol. Prog. 2010, 26, 1145–1153. [CrossRef]
- Senger, R.S.; Phisalaphong, M.; Karim, M.N.; Linden, J. C. Development of a culture sub-population induction model: signaling pathways synergy and taxanes production by Taxus canadensis. Biotechnology Progress 2006, 22(6), 1671–1682.
- Bonfill, M.; Bentebibel, S.; Moyano, E.; Palazón, J.; Cusidó, R.M.; Eibl, R.; Piñol, M.T. Paclitaxel and baccatin III production induced by methyl jasmonate in free and immobilized cells of Taxus baccata. Biol. Plant. 2007, 51, 647–652. [CrossRef]
- McKee, M.C.; Wilson, S.A.; Roberts, S.C. The Interface amongst Conserved and Specialized Pathways in Non-Paclitaxel and Paclitaxel Accumulating Taxus Cultures. Metabolites 2021, 11, 688. [CrossRef]
- Kochkin, D.V.; Demidova, E.V.; Globa, E.B.; Nosov, A.M. Profiling of Taxoid Compounds in Plant Cell Cultures of Different Species of Yew (Taxus spp.). Molecules 2023, 28, 2178. [CrossRef]
- Kochkin, D.V.; Globa, E.B.; Demidova, E.V.; Gaisinsky, V.V.; Galishev, B.A.; Kolotyrkina, N.G.; Kuznetsov, V.V.; Nosov, A.M. Occurrence of 14-hydroxylated taxoids in the plant in vitro cell cultures of different yew species (Taxus spp.). Dokl. Biochem. Biophys. 2017, 476, 337–339. [CrossRef]
- Kochkin, D.V.; Globa, E.B.; Demidova, E.V.; Gaisinsky, V.V.; Kuznetsov, V.V.; Nosov, A.M. Detection of Taxuyunnanin C in Suspension Cell Culture of Taxuscanadensis. Dokl. Biochem. Biophys. 2019, 485, 129–131. [CrossRef]
- Globa, E.B.; Demidova, E.V.; Gaysinskiy, V.V.; Kochkin, D.V. Obtainment and characterization of callus and suspension cell culture of Taxus wallichiana Zucc. Vestn. Severo-Vostochn. Fed. Univ. M.K. Ammosova 2018, 2, 18–25. (In Russian).
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [CrossRef]
- Globa, E.B.; Demidova E.V.; Turkin V.V.; Makarova S.S.; Nosov A.M. Callus and suspension culture introduction of four yew species: Taxus canadensis, T. baccata, T. cuspidata and T. media. Biotechnologia 2009, 3, 54–59.
- Madhusudanan, K.P.; Chattopadhyay, S.K.; Tripathi, V.K.; Sashidhara, K.V.; Kukreja, A.K.; Jain, S.P. LC-ESI-MS analysis of taxoids from the bark ofTaxus wallichiana. Biomed. Chromatogr. 2002, 16, 343–355. [CrossRef]
- Zhao, C.F.; Yu, L.J.; Li, L.Q.; Xiang, F. Simultaneous Identification and Determination of Major Taxoids from Extracts of Taxus chinensis Cell Cultures. 2007, 62, 1–10. [CrossRef]
- Song, G.H.; Zhao, C.F.; Zhang, M.; Fu, C.H.; Zhang, H.; Yu, L.J. Correlation analysis of the taxane core functional group modification, enzyme expression, and metabolite accumulation profiles under methyl jasmonate treatment. Biotechnol. Prog. 2013, 30, 269–280. [CrossRef]
- Baloglu, E.; Kingston, D.G.I. The taxane diterpenoids. Journal of Natural Products 1999, 62(10), 1448–1472.
- Menhard, B.; Eisenreich, W.; Hylands, P.J.; Bacher, A.; Zenk, M.H. Taxoids from Cell Cultures of Taxus Chinensis. Phytochemistry 1998, 49, 113–125. [CrossRef]
- Ketchum, R.E.; Rithner, C.D.; Qiu, D.; Kim, Y.S.; Williams, R.M.; Croteau, R.B. Taxus metabolomics: methyl jasmonate preferentially induces production of taxoids oxygenated at C-13 in Taxus x media cell cultures. Phytochemistry 2003, 62, 901–909. [CrossRef]
- Elmer, W.H.; Mattina, M.J.I.; MacEachern, G.J. Sensitivity of plant pathogenic fungi to taxane extracts from ornamental yews. Phytopathology 1994, 84, 1179-1185.
- Talbot, N.J. Plant Immunity: A Little Help from Fungal Friends. Curr. Biol. 2015, 25, R1074–R1076. [CrossRef]
- Cheng, D.M.; Yousef, G.G.; Grace, M.H.; Rogers, R.B.; Gorelick-Feldman, J.; Raskin, I.; Lila, M.A. In vitro production of metabolism-enhancing phytoecdysteroids from Ajuga turkestanica. Plant Cell, Tissue Organ Cult. (PCTOC) 2008, 93, 73–83. [CrossRef]
- Hu, F.-X.; Zhong, J.-J. Jasmonic acid mediates gene transcription of ginsenoside biosynthesis in cell cultures of Panax notoginseng treated with chemically synthesized 2-hydroxyethyl jasmonate. Process. Biochem. 2008, 43, 113–118. [CrossRef]
- Mirjalili, N.; Linden, J.C. Methyl Jasmonate Induced Production of Taxol in Suspension Cultures of Taxus cuspidata: Ethylene Interaction and Induction Models. Biotechnol. Prog. 1996, 12, 110–118. [CrossRef]
- Linden, J.C.; Phisalaphong, M. Oligosaccharides potentiate methyl jasmonate-induced production of paclitaxel in Taxus canadensis. Plant Sci. 2000, 158, 41–51. [CrossRef]
- Ketchum, R.E.; Gibson, D.M.; Croteau, R.B.; Shuler, M.L. The kinetics of taxoid accu-mulation in cell suspension cultures of Taxus following elicitation with methyljasmonate. Biotechnol Bioeng 1999, 62, 97–105.
- Ketchum, R.E.; Rithner, C.D.; Qiu, D.; Kim, Y.S.; Williams, R.M.; Croteau, R.B. Taxus metabolomics: methyl jasmonate preferentially induces production of taxoids oxygenated at C-13 in Taxus x media cell cultures. Phytochemistry 2003, 62, 901–909. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).