Submitted:
27 April 2023
Posted:
27 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Bacterial spores as mucosal vaccine delivery system
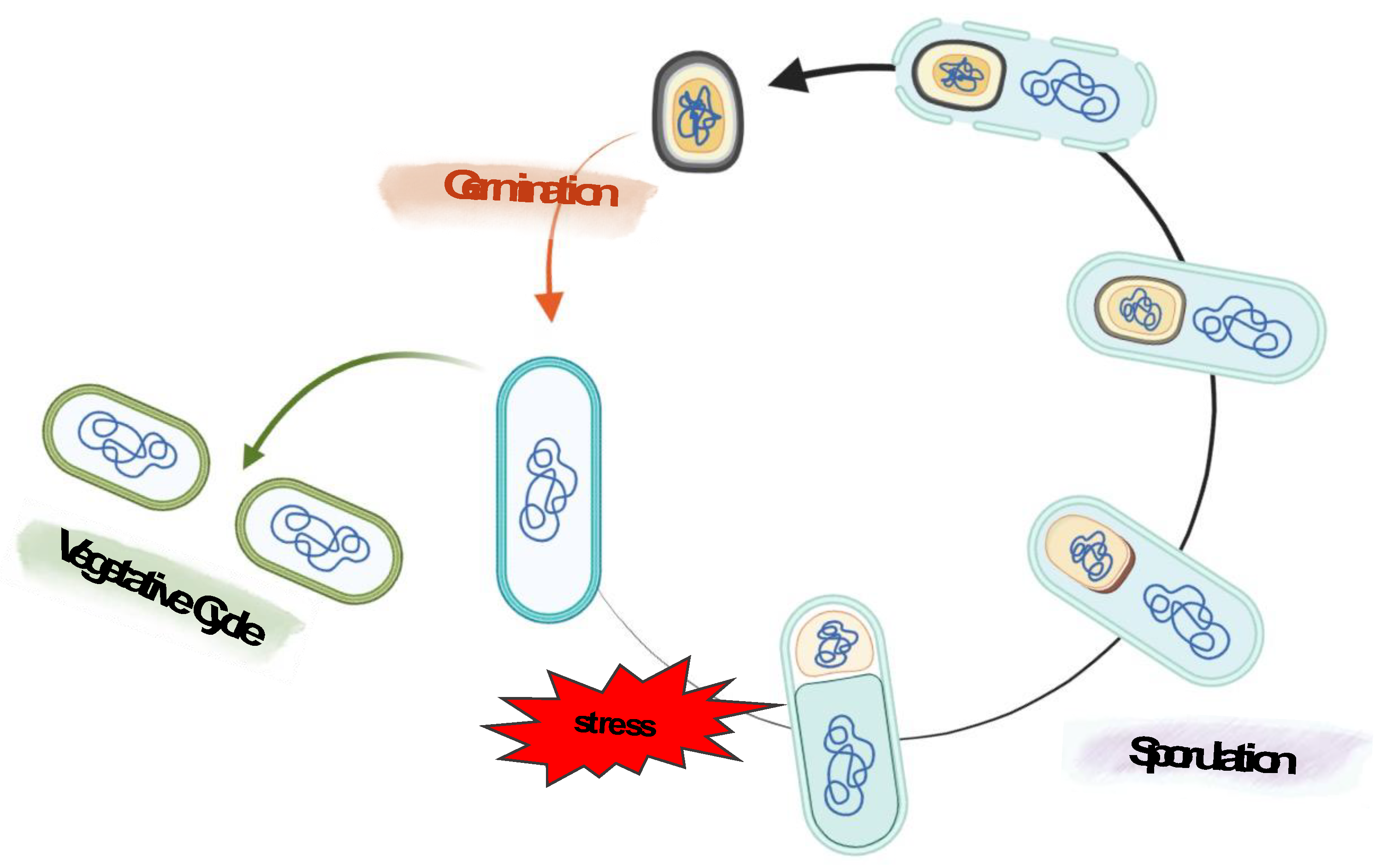
2.1. Bacterial spore, sporulation and intestinal life cycle
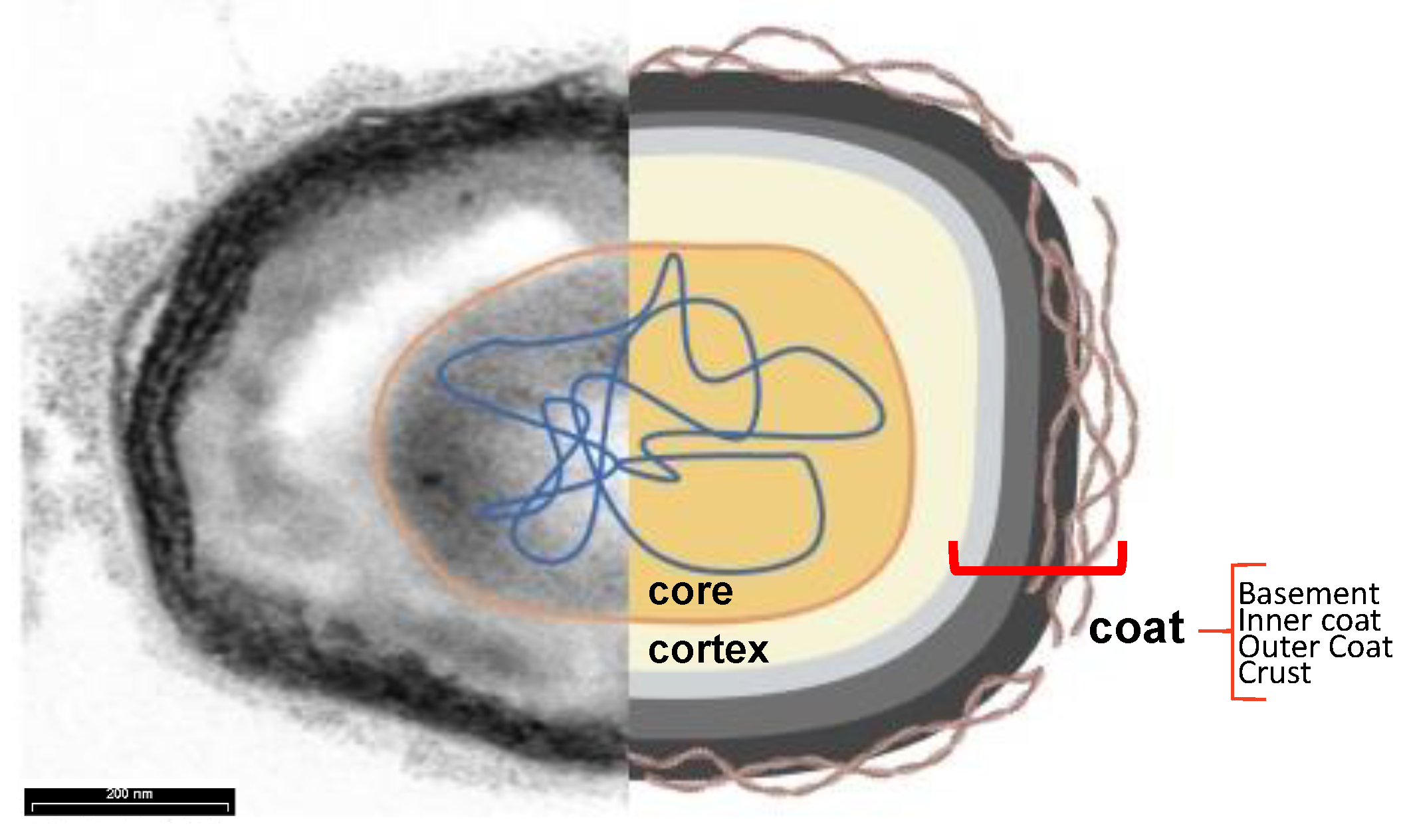
2.2. Spore surface
2.3. Bacterial spore as a recombinant vaccine platform
- (1)
- the extreme stability due to the well-documented resistance of the bacterial spore to high temperatures, acidic pH, and the presence of chemicals and enzymes [29,30,31,32]. Guaranteeing the high stability of the vaccine carrier system from production to administration to the patient is a crucial requirement of vaccine development. Bacillus spores are more stable than vegetative cells during the processing and storage of commercial preparations, making them a suitable candidate for vaccine formulations [62]. Moreover, stability at extreme temperatures is strongly required in the development of mucosal vaccines, mainly for those intended for use in developing countries, where poor distribution and storage conditions are the main limitations [3,7,60,61,62];
- (2)
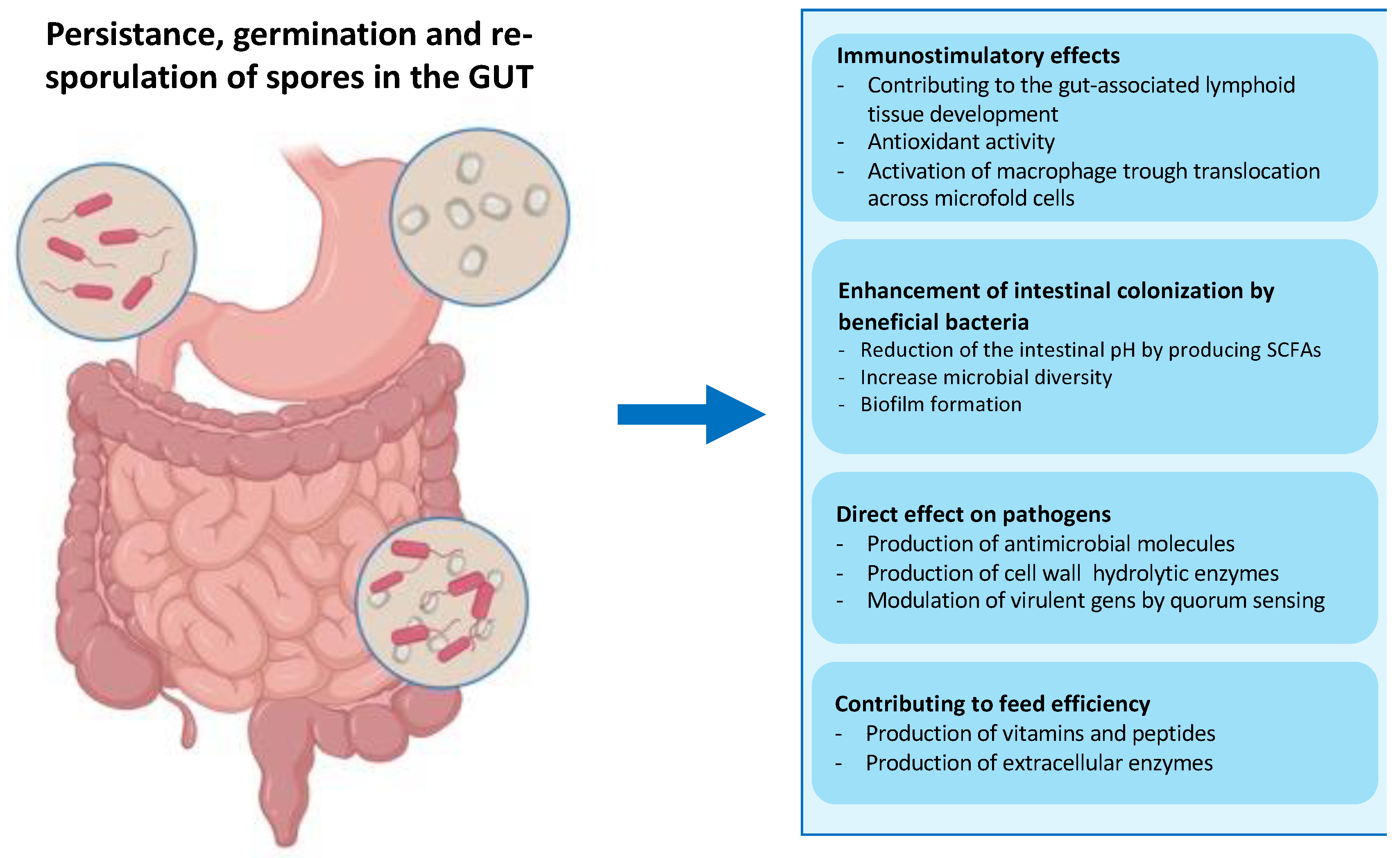
- the exceptional safety record of several spore-former species used worldwide in probiotic preparations for human and animal use as dietary supplements and growth promoters [41,42,63]. As mentioned above, several spore former species are part of the animal gut microbiota, which have a role in the development of the immune system, protection against intestinal pathogens, induction of cytoprotective responses, and of anti-oxidative stress responses in epithelial cells (Figure 4) [38,39,40,41,42]. The safety record is an essential requirement if the display system is intended for the delivery of antigens molecules to human mucosal surfaces;
- (3)
- All known coat proteins are synthesized in the mother compartment during sporulation [31,32,43]. Consequently, coat components and the antigens fused to them do not need to undergo a cell wall translocation step to expose the recombinant proteins externally, thus overcoming the size limitation often encountered with cell-based display systems [59,64];
- (4)
2.4. Spore-based vaccine design strategy
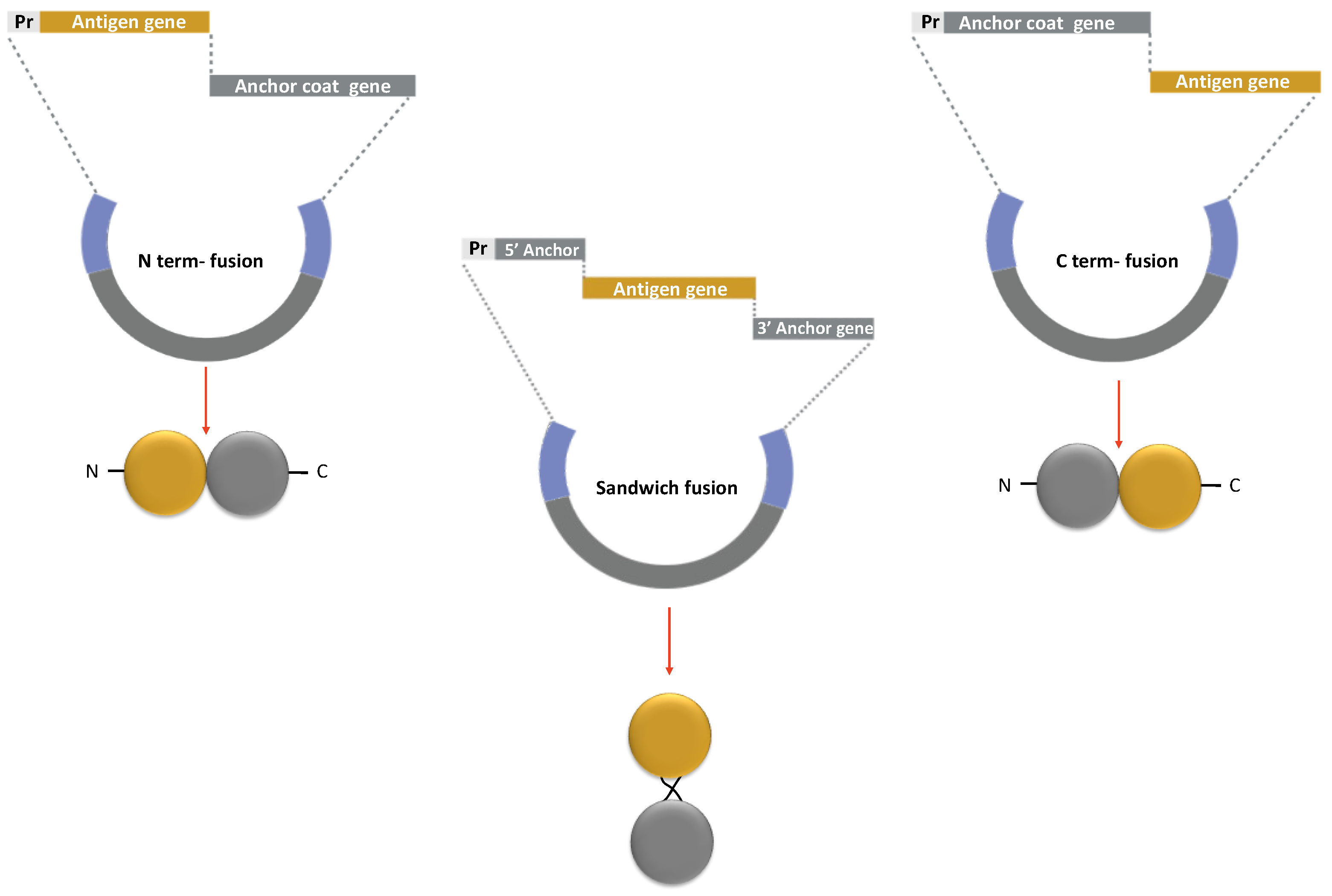
2.4.1. Anchor Proteins
| Anchor [Refs] | Target pathogen | Antigen | Fusion method | linker | Application |
| CotB | |||||
| [29] | Clostridium tetani | C-term fragment of the tetanus toxin, TTFC | C-term, N-term, sandwich | - | Oral vaccination for tetanus |
| [84] | Clostridium difficile | fagellin protein, FliD | C-terminal | GGGEA; AAKGGG | C. difficile oral vaccine |
| [75] | C-term repeat domains of toxins A and B, TcdA-TcdB | C-terminal | - | ||
| [74] | Clostridium perfringens | C-term of alpha toxin gene, Cpa247-370 fused to the GST gene | C-terminal | - | Oral and Nasal Vaccine against necrotic enteritis |
| [72,73] | Bacillus anthracis | anthrax protective antigen ,PA | C-terminal | - | Anthrax vaccine |
| [67] | Helicobacter acinonychis | urease subunit alpha, UreA | C-terminal | GGGEAA; AKGGG | Anti-Helicobacter vaccine |
| [88] | Helicobacter pylori | vacuolating cytotoxin A, CagA | C-terminal N- terminal | GGGGS | Anti-Helicobacter vaccine |
| [90] | Mycobacterium tuberculosis | immunodominant secretory antigen, MPT64 | C-terminal | - | Nasal Vaccine against tuberculosis |
| [91] | Streptococcus mutans | truncated P1 protein | N-terminal | NR | S. mutans vaccine |
| [76] | White spot syndrome virus | major envelope proteins, VP28 | C-terminal | - | Oral vaccine for shrimps |
| [92] | Influenza virus | ectodomain of influenza virus M2 protein | C-terminal | - | Oral Influenza vaccine |
| [93] | Adjuvant | human IL-2 | C-terminal | adiuvant to Helicobacter pylori vaccine | |
| CotC | |||||
| [70] | Clostridium tetani | C-term fragment of the tetanus toxin, TTFC | C-terminal N- terminal | - | Oral vaccination for tetanus |
| [75] | Clostrium difficile | C- term repeat domains of toxins A and B TcdA-TcdB | C-terminal | - | C. dìfficile oral vaccine |
| [70] | Escherichia coli | heat-labile enterotoxin B, LTB | C-terminal N- terminal | - | E. coli vaccine |
| [94] | Salmonella serovar pullorum | outer membrane protein (porin), OmpC | C-terminal | - | Salmonella vaccine |
| [72,73] | Bacillus anthracis | anthrax protective antigen, PA | C-terminal | - | Anthrax vaccine |
| [67] | Helicobacter acinonychis | urease subunit alpha, UreA | C-terminal | - | Anti-Helicobacter vaccine |
| [89] | Helicobacter pylori | urease subunit beta, UreB | C-terminal | - | Oral vaccine for H. pylori |
| [88] | vacuolating cytotoxin A, CagA | C-terminal N- terminal | GGGGS | ||
| [95] | cholera toxin B subunit, CTB and UreB | C-terminal | - | ||
| [78] | Clonorchis sinensis | tegumental protein 20.8 kD,TP20.8 | C-terminal | - | Liver flukes vaccine |
| [78] | tegumental protein 22.3 kDa, CsTP22.3 | C-terminal | - | ||
| [79] | cysteine proteases, CsCP | C-terminal | - | ||
| [80] | leucine aminopeptidase 2, CsLAP2 | C-terminal | - | ||
| [81] | enolase | C-terminal | - | ||
| [82] | paramyosin antigen, CsPmy | C-terminal | |||
| [83] | serpin, CsSer-3 | C-terminal | |||
| [96] | Schistosoma japonicum | 26 kDa full- length GST protein, SjGST | C-terminal | - | Liver flukes oral vaccine |
| [97] | grass carp reovirus | major outer capsid protein , VP4 | C-terminal | - | grass carp reovirus vaccine |
| [98] | White spot syndrome virus | major envelope proteins, VP28 and VP62 | C-terminal | - | Oral vaccine for shrimps |
| [99] | Bombyx mori | nucleopolyhedrovirus, GP64 | C-terminal | - | Bombyx mori vaccine |
| CotG | |||||
| [67] | Helicobacter acinonychis | urease subunit alpha, UreA | C-terminal | - | Anti-Helicobacter vaccine |
| [84] | Clostridium difficile | fagellin protein, FliD | C-terminal | GGGEAAAKGGG | Oral vaccine against C. difficile |
| [88] | Helicobacter pylori | vacuolating cytotoxin A, CagA | C-terminal N- terminal | GGGGS | Anti-Helicobacter vaccine |
| [87] | Transmissible gastroenteritis virus | transmissible gastroenteritis virus spike , TGEV-S | C-terminal | Transmissible gastroenteritis vaccine | |
| [85] | - | Streptavidin | C-terminal | GGGGS | - |
| CotZ | |||||
| [84] | Clostridium difficile | fagellin protein, FliD | C-terminal | GGGEAAAKGGG | Oral vaccine against C. difficile |
| [67] | Helicobacter acinonychis | urease subunit alpha, UreA | C-terminal | GGGGS | Anti-Helicobacter vaccine |
| [88] | Helicobacter pylori | vacuolating cytotoxin A, CagA | C-terminal N- terminal | GGGEAAAKGGG | Anti-Helicobacter vaccine |
| CgeA | |||||
| [88] | Helicobacter pylori | vacuolating cytotoxin A, CagA | C-terminal N- terminal | GGGEAAAKGGG | Anti-Helicobacter vaccine |
2.5. Optimizing antigen exposure on spore surface strategies.
2.5.1. Linker peptides to increase stability and flexibility of recombinant protein
2.5.2. Multi antigens spore-based mucosal vaccine
2.5.3. Non-recombinant display of antigens on the spore surface.
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mojgani, N.; Shahali, Y.; Dadar, M. Immune modulatory capacity of probiotic lactic acid bacteria and applications in vaccine development. Benef. Microbes 2020, 11, 213–226. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global task force on cholera control: overview of ending cholera — a global roadmap to 2030. 2018.
- Pavot, V.; Rochereau, N.; Genin, C.; Verrier, B.; Paul, S. New insights in mucosal vaccine development. Vaccine 2012, 30, 142–154. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jang, Y.-S. Antigen targeting to M cells for enhancing the efficacy of mucosal vaccines. Exp. Mol. Med. 2014, 46, e85–e85. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.J.; Curran, M.P. Live Attenuated Influenza Vaccine (FluMist®; Fluenz™). Drugs 2011, 71, 1591–1622. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.D.; Adhikari, A.; Yang, Y.; Kuschner, R.A.; Karasavvas, N.; Binn, L.N.; Walls, S.D.; Graf, P.C.F.; Myers, C.A.; Jarman, R.G.; et al. Live Oral Adenovirus Type 4 and Type 7 Vaccine Induces Durable Antibody Response. Vaccines 2020, 8, 411. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines — fortifying the frontiers. Nat. Rev. Immunol. 2021, 22, 236–250. [Google Scholar] [CrossRef]
- Tsakiri, M.; Naziris, N.; Demetzos, C. Innovative vaccine platforms against infectious diseases: Under the scope of the COVID-19 pandemic. Int. J. Pharm. 2021, 610, 121212–121212. [Google Scholar] [CrossRef]
- da Silva, A.J.; Zangirolami, T.C.; Novo-Mansur, M.T.M.; Giordano, R.d.C.; Martins, E.A.L. Live bacterial vaccine vectors: An overview. Braz. J. Microbiol. 2014, 45, 1117–1129. [Google Scholar] [CrossRef]
- Smith, G.L.; Mackett, M.; Moss, B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature 1983, 302, 490–495. [Google Scholar] [CrossRef]
- Moss, B.; Smith, G.L.; Gerin, J.L.; Purcell, R.H. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature 1984, 311, 67–69. [Google Scholar] [CrossRef]
- Draper, S.J.; Heeney, J.L. Viruses as vaccine vectors for infectious diseases and cancer. Nat. Rev. Microbiol. 2009, 8, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Jas, D.; Coupier, C.; Toulemonde, C.E.; Guigal, P.-M.; Poulet, H. Three-year duration of immunity in cats vaccinated with a canarypox-vectored recombinant rabies virus vaccine. Vaccine 2012, 30, 6991–6996. [Google Scholar] [CrossRef] [PubMed]
- Esaki, M.; Godoy, A.; Rosenberger, J.K.; Rosenberger, S.C.; Gardin, Y.; Yasuda, A.; Dorsey, K.M. Protection and Antibody Response Caused by Turkey Herpesvirus Vector Newcastle Disease Vaccine. Avian Dis. 2013, 57, 750–755. [Google Scholar] [CrossRef]
- FDA. First FDA-approved vaccine for the prevention of Ebola virus disease, marking a critical milestone in public health preparedness and response. 2020 Available at: https://www.fda.gov/news-events/press-announcements/first- fda-approved-vaccine-prevention.
- Afkhami, S.; D’agostino, M.R.; Zhang, A.; Stacey, H.D.; Marzok, A.; Kang, A.; Singh, R.; Bavananthasivam, J.; Ye, G.; Luo, X.; et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell 2022, 185, 896–915. [Google Scholar] [CrossRef]
- Peng, B.; Wang, L.R.; Gómez-Román, V.R.; Davis-Warren, A.; Montefiori, D.C.; Kalyanaraman, V.S.; Venzon, D.; Zhao, J.; Kan, E.; Rowell, T.J.; et al. Replicating Rather than Nonreplicating Adenovirus-Human Immunodeficiency Virus Recombinant Vaccines Are Better at Eliciting Potent Cellular Immunity and Priming High-Titer Antibodies. J. Virol. 2005, 79, 10200–10209. [Google Scholar] [CrossRef]
- Mielcarek, N.; Alonso, S.; Locht, C. Nasal vaccination using live bacterial vectors. Adv. Drug Deliv. Rev. 2001, 51, 55–69. [Google Scholar] [CrossRef]
- Detmer, A.; Glenting, J. Live bacterial vaccines – a review and identification of potential hazards. Microb. Cell Factories 2006, 5, 23–23. [Google Scholar] [CrossRef]
- Zhu, Q.; Berzofsky, J.A. Oral vaccines. Gut Microbes 2013, 4, 246–252. [Google Scholar] [CrossRef]
- Bastos, R.G.; Borsuk, S.; Seixas, F.K.; Dellagostin, O.A. 2009. Recom- binant Mycobacterium bovis BCG. Vaccine, 2009, 27, 6495–6503. [Google Scholar]
- Bruhn, K.W.; Craft, N.; Miller, J.F. Listeria as a vaccine vector. Microbes Infect. 2007, 9, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Curtiss, R.; Xin, W.; Li, Y. ; Kong, ; Wanda, S.Y.; Gunn, B.; et al. New technologies in using recombinant attenuated Salmonella vaccine vectors. Crit Rev Immunol, 2010, 30, 255–270. [Google Scholar]
- Roland, K.L.; A Tinge, S.; Killeen, K.P.; Kochi, S.K. Recent advances in the development of live, attenuated bacterial vectors. . 2005, 7, 62–72. [Google Scholar] [PubMed]
- Sundararaman, A. M.; Ray, P.V.; Ravindra, J.; Halami, P.M. Role of probiotics to combat viral infections with emphasison COVID-19. Appl Microbiol Biotechnol, 2020, 104, 8089–8104. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, T.; Hao, J.; Li, L.; Tian, M.; Jin, N.; Ren, L.; Li, C. A recombinant Lactobacillus plantarum strain expressing the spike protein of SARS-CoV-2. Int. J. Biol. Macromol. 2020, 160, 736–740. [Google Scholar] [CrossRef]
- Xu, J.; Ren, Z.; Cao, K.; Li, X.; Yang, J.; Luo, X.; Zhu, L.; Wang, X.; Ding, L.; Liang, J.; et al. Boosting Vaccine-Elicited Respiratory Mucosal and Systemic COVID-19 Immunity in Mice With the Oral Lactobacillus plantarum. Front. Nutr. 2021, 8, 789242. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.C.-C.; Liu, Y.; Wu, K.-C.; Choi, M.-C.; Ma, C.H.-Y.; Lin, J.; He, E.I.C.; Leung, D.Y.-M.; Sze, E.T.-P.; Hamied, Y.K.; et al. Retraction: Sung et al. Expression of SARS-CoV-2 Spike Protein Receptor Binding Domain on Recombinant B. subtilis on Spore Surface: A Potential COVID-19 Oral Vaccine Candidate. Vaccines 2022, 10, 2. Vaccines 2022, 10, 1852. [Google Scholar] [CrossRef]
- Isticato, R.; Cangiano, G.; Tran, H.T.; Ciabattini, A.; Medaglini, D.; Oggioni, M.R.; De Felice, M.; Pozzi, G.; Ricca, E. Surface Display of Recombinant Proteins on Bacillus subtilis Spores. J. Bacteriol. 2001, 183, 6294–6301. [Google Scholar] [CrossRef]
- Tan, I.S.; Ramamurthi, K.S. Spore formation in Bacillus subtilis. Environ. Microbiol. Rep. 2013, 6, 212–225. [Google Scholar] [CrossRef]
- Chastanet, A.; Vitkup, D.; Yuan, G.-C.; Norman, T.M.; Liu, J.S.; Losick, R.M. Broadly heterogeneous activation of the master regulator for sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. 2010, 107, 8486–8491. [Google Scholar] [CrossRef]
- McKenney, P.T.; Driks, A.; Eichenberger, P. The Bacillus subtilis endospore: assembly and functions of the multilayered coat. Nat. Rev. Microbiol. 2012, 11, 33–44. [Google Scholar] [CrossRef]
- Setlow, P. Germination of Spores of Bacillus Species: What We Know and Do Not Know. J. Bacteriol. 2014, 196, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P.; Christie, G. Bacterial Spore mRNA – What’s Up With That? Front. Microbiol. 2020, 11, 596092. [Google Scholar] [CrossRef] [PubMed]
- Swarge, B.; Abhyankar, W.; Jonker, M.; Hoefsloot, H.; Kramer, G.; Setlow, P.; Brul, S.; de Koning, L.J. Integrative Analysis of Proteome and Transcriptome Dynamics during Bacillus subtilis Spore Revival. mSphere 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus Endospores to Extreme Terrestrial and Extraterrestrial Environments. Microbiol. Mol. Biol. Rev. 2000, 64, 548–572. [Google Scholar] [CrossRef] [PubMed]
- Koopman, N.; Remijas, L.; Seppen, J.; Setlow, P.; Brul, S. Mechanisms and Applications of Bacterial Sporulation and Germination in the Intestine. Int. J. Mol. Sci. 2022, 23, 3405. [Google Scholar] [CrossRef] [PubMed]
- Casula, G.; Cutting, S.M. Bacillus Probiotics: Spore Germination in the Gastrointestinal Tract. Appl. Environ. Microbiol. 2002, 68, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Duc, L.H.; Hong, A.H.; Nguyen, Q.U.; Cutting, S.M. Intracellular fate and immunogenicity of B. subtilis spores. Vaccine, 2004, 22, 1873–1885. [Google Scholar] [CrossRef]
- Tam, N.K.M.; Uyen, N.Q.; Hong, H.A.; Duc, L.H.; Hoa, T.T.; Serra, C.R.; Henriques, A.O.; Cutting, S.M. The Intestinal Life Cycle of Bacillus subtilis and Close Relatives. J. Bacteriol. 2006, 188, 2692–2700. [Google Scholar] [CrossRef]
- Bernardeau, M.; Lehtinen, M.J.; Forssten, S.D.; Nurminen, P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Technol. 2017, 54, 2570–2584. [Google Scholar] [CrossRef]
- Elshaghabee, E.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef]
- McKenney, P.T.; Eichenberger, P. Dynamics of spore coat morphogenesis in Bacillus subtilis. Mol. Microbiol. 2011, 83, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Shuster, B.; Khemmani, M.; Abe, K.; Huang, X.; Nakaya, Y.; Maryn, N.; Buttar, S.; Gonzalez, A.N.; Driks, A.; Sato, T.; et al. Contributions of crust proteins to spore surface properties in Bacillus subtilis. Mol. Microbiol. 2018, 111, 825–843. [Google Scholar] [CrossRef]
- Cangiano, G.; Mazzone, A.; Baccigalupi, L.; Isticato, R.; Eichenberger, P.; De Felice, M.; Ricca, E. Direct and Indirect Control of Late Sporulation Genes by GerR of Bacillus subtilis. J. Bacteriol. 2010, 192, 3406–3413. [Google Scholar] [CrossRef] [PubMed]
- Ursem, R.; Swarge, B.; Abhyankar, W.R.; Buncherd, H.; de Koning, L.J.; Setlow, P.; Brul, S.; Kramer, G. Identification of Native Cross-Links in Bacillus subtilis Spore Coat Proteins. J. Proteome Res. 2021, 20, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Isticato, R.; Pelosi, A.; Zilhão, R.; Baccigalupi, L.; Henriques, A.O.; De Felice, M.; Ricca, E. CotC-CotU Heterodimerization during Assembly of the Bacillus subtilis Spore Coat. J. Bacteriol. 2008, 190, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Isticato, R.; Sirec, T.; Vecchione, S.; Crispino, A.; Saggese, A.; Baccigalupi, L.; Notomista, E.; Driks, A.; Ricca, E. The Direct Interaction between Two Morphogenetic Proteins Is Essential for Spore Coat Formation in Bacillus subtilis. PLOS ONE 2015, 10, e0141040. [Google Scholar] [CrossRef] [PubMed]
- Isticato, R.; Pelosi, A.; De Felice, M.; Ricca, E. CotE Binds to CotC and CotU and Mediates Their Interaction during Spore Coat Formation in Bacillus subtilis. J. Bacteriol. 2010, 192, 949–954. [Google Scholar] [CrossRef]
- Isticato, R.; Sirec, T.; Giglio, R.; Baccigalupi, L.; Rusciano, G.; Pesce, G.; Zito, G.; Sasso, A.; De Felice, M.; Ricca, E. Flexibility of the Prograamme of Spore Coat Formation in Bacillus subtilis: Bypass of CotE Requirement by Over-Production of CotH. PLOS ONE 2013, 8, e74949. [Google Scholar] [CrossRef]
- Cangiano, G.; Sirec, T.; Panarella, C.; Isticato, R.; Baccigalupi, L.; De Felice, M.; Ricca, E. The sps gene products affect germination, hydrophobicity and protein adsorption of Bacillus subtilis spores. Appl Environ Microbiol, 2014, 80, 7293–7302. [Google Scholar] [CrossRef]
- Ricca, E.; Baccigalupi, L.; Isticato, R. Spore-adsorption: Mechanism and applications of a non-recombinant display system. Biotechnol. Adv. 2020, 47, 107693. [Google Scholar] [CrossRef]
- Driks, A.; Eichenberger, P. The Spore Coat. Microbiol Spectr, 2016, 4(2).
- Pesce, G.; Rusciano, G.; Sasso, A.; Isticato, R.; Sirec, T.; Ricca, E. Surface charge and hydrodynamic coefficient measurements of Bacillus subtilis spore by optical tweezers. Colloids Surfaces B: Biointerfaces 2014, 116, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Dubois, T.; Krzewinski, F.; Yamakawa, N.; Lemy, C.; Hamiot, A.; Brunet, L.; Lacoste, A.-S.; Knirel, Y.; Guerardel, Y.; Faille, C. The sps Genes Encode an Original Legionaminic Acid Pathway Required for Crust Assembly in Bacillus subtilis. Mbio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.C. The Exosporium Layer of Bacterial Spores: a Connection to the Environment and the Infected Host. Microbiol. Mol. Biol. Rev. 2015, 79, 437–457. [Google Scholar] [CrossRef] [PubMed]
- Vittoria, M.; Saggese, A.; Barletta, G.D.G.; Castaldi, S.; Isticato, R.; Baccigalupi, L.; Ricca, E. Sporulation efficiency and spore quality in a human intestinal isolate of Bacillus cereus. Res. Microbiol. 2023, 174, 104030. [Google Scholar] [CrossRef] [PubMed]
- Sirec, T.; Cangiano, G.; Baccigalupi, L.; Ricca, E.; Isticato, R. The spore surface of intestinal isolates ofBacillus subtilis. FEMS Microbiol. Lett. 2014, 358, 194–201. [Google Scholar] [CrossRef]
- Isticato, R.; Ricca, E. Spore Surface Display. Microbiol. Spectr. 2014, 2, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yuan, H.; Du, J.; Liu, K.; Liu, H.; Wang, T. Progress in research and application development of surface display technology using Bacillus subtilis spores. Appl. Microbiol. Biotechnol. 2020, 104, 2319–2331. [Google Scholar] [CrossRef]
- Chen, H.; Ullah, J.; Jia, J. Progress in Bacillus subtilis Spore Surface Display Technology towards Environment, Vaccine Development, and Biocatalysis. Microb. Physiol. 2017, 27, 159–167. [Google Scholar] [CrossRef]
- Tang, Z.; Wu, Z.; Sun, H.; Zhao, L.; Shang, M.; Shi, M.; Jiang, H.; Lin, Z.; Zhou, X.; Li, X.; et al. The storage stability of Bacillus subtilis spore displaying cysteine protease of Clonorchis sinensis and its effect on improving the gut microbiota of mice. Appl. Microbiol. Biotechnol. 2021, 105, 2513–2526. [Google Scholar] [CrossRef]
- Lee, N.-K.; Kim, W.-S.; Paik, H.-D. Bacillus strains as human probiotics: characterization, safety, microbiome, and probiotic carrier. Food Sci. Biotechnol. 2019, 28, 1297–1305. [Google Scholar] [CrossRef]
- Knecht, L.D.; Pasini, P.; Daunert, S. Bacterial spores as platforms for bioanalytical and biomedical applications. Anal. Bioanal. Chem. 2011, 400, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Scheiblhofer, S.; Laimer, J.; Machado, Y.; Weiss, R.; Thalhamer, J. Influence of protein fold stability on immunogenicity and its implications for vaccine design. Expert Rev. Vaccines 2017, 16, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Turk, B. Targeting proteases: successes, failures and future prospects. Nat. Rev. Drug Discov. 2006, 5, 785–799. [Google Scholar] [CrossRef]
- Hinc, K.; Isticato, R.; Dembek, M.; Karczewska, J.; Iwanicki, A.; Peszyńska-Sularz, G.; De Felice, M.; Obuchowski, M.; Ricca, E. Expression and display of UreA of Helicobacter acinonychis on the surface of Bacillus subtilis spores. Microb. Cell Factories 2010, 9, 2–2. [Google Scholar] [CrossRef] [PubMed]
- Zilhão, R.; Serrano, M.; Isticato, R.; Ricca, E.; Moran, C.P.; Henriques, A.O. Interactions among CotB, CotG, and CotH during Assembly of the Bacillus subtilis Spore Coat. J. Bacteriol. 2004, 186, 1110–1119. [Google Scholar] [CrossRef]
- Giglio, R.; Fani, R.; Isticato, R.; De Felice, M.; Ricca, E.; Baccigalupi, L. Organization and Evolution of the cotG and cotH Genes of Bacillus subtilis. J. Bacteriol. 2011, 193, 6664–6673. [Google Scholar] [CrossRef]
- Mauriello, E.M.; Duc, L.H.; Isticato, R.; Cangiano, G.; Hong, H.A.; De Felice, M.; Ricca, E.; Cutting, S.M. Display of heterologous antigens on the Bacillus subtilis spore coat using CotC as a fusion partner. Vaccine 2004, 22, 1177–1187. [Google Scholar] [CrossRef]
- Ciabattini, A.; Parigi, R.; Isticato, R.; Oggioni, M.R.; Pozzi, G. Oral priming of mice by recombinant spores of Bacillus subtilis. Vaccine 2004, 22, 4139–4143. [Google Scholar] [CrossRef]
- Duc, L.H.; Hong, H.A.; Atkins, H.S.; Flick-Smith, H.C.; Durrani, Z.; Rijpkema, S.; Titball, R.W.; Cutting, S.M. Immunization against anthrax using Bacillus subtilis spores expressing the anthrax protective antigen. Vaccine 2007, 25, 346–355. [Google Scholar] [CrossRef]
- Oh, Y.; Kim, J.A.; Kim, C.-H.; Choi, S.-K.; Pan, J.-G. Bacillus subtilis spore vaccines displaying protective antigen induce functional antibodies and protective potency. BMC Veter- Res. 2020, 16, 1–10. [Google Scholar] [CrossRef]
- Hoang, T.H.; Hong, H.A.; Clark, G.C.; Titball, R.W.; Cutting, S.M. RecombinantBacillus subtilisExpressing theClostridium perfringensAlpha Toxoid Is a Candidate Orally Delivered Vaccine against Necrotic Enteritis. Infect. Immun. 2008, 76, 5257–5265. [Google Scholar] [CrossRef]
- Hong, H.A.; Hitri, K.; Hosseini, S.; Kotowicz, N.; Bryan, D.; Mawas, F.; Wilkinson, A.J.; van Broekhoven, A.; Kearsey, J.; Cutting, S.M. Mucosal Antibodies to the C Terminus of Toxin A Prevent Colonization of Clostridium difficile. Infect. Immun. 2017, 85, e01060–16. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Pham, C.K.; Pham, H.T.; Pham, H.L.; Dang, L.T.; Huynh, H.A.; Cutting, S.M.; Phan, T.-N. Bacillus subtilisspores expressing the VP28 antigen: a potential oral treatment to protectLitopenaeus vannameiagainst white spot syndrome. FEMS Microbiol. Lett. 2014, 358, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Isticato, R.; Di Mase, D.S.; Mauriello, E.M.; De Felice, M.; Ricca, E. Amino terminal fusion of heterologous proteins to CotC increases display efficiencies in the Bacillus subtilis spore system. BioTechniques 2007, 42, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xia, H.; Hu, X.; Huang, Y.; Li, Y.; Li, L.; Ma, C.; Chen, X.; Hu, F.; Xu, J.; et al. Oral administration of a Bacillus subtilis spore-based vaccine expressing Clonorchis sinensis tegumental protein 22.3kDa confers protection against Clonorchis sinensis. Vaccine 2008, 26, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Shang, M.; Chen, T.; Ren, P.; Sun, H.; Qu, H.; Lin, Z.; Zhou, L.; Yu, J.; Jiang, H.; et al. The immunological characteristics and probiotic function of recombinant Bacillus subtilis spore expressing Clonorchis sinensis cysteine protease. Parasites Vectors 2016, 9, 1–14. [Google Scholar] [CrossRef]
- Qu, H.; Xu, Y.; Sun, H.; Lin, J.; Yu, J.; Tang, Z.; Shen, J.; Liang, C.; Li, S.; Chen, W.; et al. Systemic and local mucosal immune responses induced by orally delivered Bacillus subtilis spore expressing leucine aminopeptidase 2 of Clonorchis sinensis. Parasitol. Res. 2014, 113, 3095–3103. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, T.; Xie, Z.; Liang, P.; Qu, H.; Shang, M.; Mao, Q.; Ning, D.; Tang, Z.; Shi, M.; et al. Oral delivery of Bacillus subtilis spore expressing enolase of Clonorchis sinensis in rat model: induce systemic and local mucosal immune responses and has no side effect on liver function. Parasitol. Res. 2015, 114, 2499–2505. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lin, Z.; Zhao, L.; Chen, T.; Shang, M.; Jiang, H.; Tang, Z.; Zhou, X.; Shi, M.; Zhou, L.; et al. Bacillus subtilis spore with surface display of paramyosin from Clonorchis sinensis potentializes a promising oral vaccine candidate. Parasites Vectors 2018, 11, 1–15. [Google Scholar] [CrossRef]
- Lin, Z.; Sun, H.; Ma, Y.; Zhou, X.; Jiang, H.; Wang, X.; Song, J.; Tang, Z.; Bian, Q.; Zhang, Z.; et al. Evaluation of immune response toBacillus subtilisspores expressingClonorchis sinensisserpin3. Parasitology 2020, 147, 1080–1087. [Google Scholar] [CrossRef]
- Negri, A.; Potocki, W.; Iwanicki, A.; Obuchowski, M.; Hinc, K. Expression and display of Clostridium difficile protein FliD on the surface of Bacillus subtilis spores. J. Med Microbiol. 2013, 62, 1379–1385. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, C.-S.; Kim, B.-G. Spore-displayed streptavidin: A live diagnostic tool in biotechnology. Biochem. Biophys. Res. Commun. 2005, 331, 210–214. [Google Scholar] [CrossRef]
- Kim., J.; Schumann, W. Kim. J.; Schumann, W. Display of proteins on Bacillus subtilis endospores. Cell Mol Life Sci, 2009, 66(19),3127–313.
- Mou, C.; Zhu, L.; Xing, X.; Lin, J.; Yang, Q. Immune responses induced by recombinant Bacillus subtilis expressing the spike protein of transmissible gastroenteritis virus in pigs. Antivir. Res. 2016, 131, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Iwanicki, A.; Piątek, I.; Stasiłojć, M.; Grela, A.; Łęga, T.; Obuchowski, M.; Hinc, K. A system of vectors for Bacillus subtilis spore surface display. Microb. Cell Factories 2014, 13, 30–30. [Google Scholar] [CrossRef] [PubMed]
- Stasiłojć, M.; Hinc, K.; Peszyńska-Sularz, G.; Obuchowski, M.; Iwanicki, A. Recombinant Bacillus subtilis Spores Elicit Th1/Th17-Polarized Immune Response in a Murine Model of Helicobacter pylori Vaccination. Mol. Biotechnol. 2015, 57, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Sibley, L.; Reljic, R.; Radford, D.S.; Huang, J.M.; Hong, H.A.; Cranenburgh, R.M.; et al. 2014. Recombinant Bacillus subtilis spores expressing MPT64 evaluated as a vaccine against tuberculosis in the murine model. FEMS Microbiol. Lett, 2014, 358, 170–179. [Google Scholar] [PubMed]
- Tavares, M.B.; Silva, B.M.; Cavalcante, R.C.; Souza, R.D.; Luiz, W.B.; Paccez, J.D.; Crowley, P.J.; Brady, L.J.; Ferreira, L.C.; Ferreira, R.C. Induction of neutralizing antibodies in mice immunized with an amino-terminal polypeptide ofStreptococcus mutansP1 protein produced by a recombinantBacillus subtilisstrain. FEMS Immunol. Med Microbiol. 2010, 59, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G. ; Miao,Y. ; Guo,Y.; Qiu, H.; Sun, S.; Kou, Z.; et al. Development of a heat-stable and orally delivered recombinant M2e-expressing B. subtilis spore-based influenza vaccine. Hum Vaccin Immunother, 2014, 10, 3649–3658. [Google Scholar]
- Hinc, K.; Stasiłojć, M.; Piątek, I.; Peszyńska-Sularz, G.; Isticato, R.; Ricca, E.; Obuchowski, M.; Iwanicki, A. Mucosal Adjuvant Activity of IL-2 Presenting Spores of Bacillus subtilis in a Murine Model of Helicobacter pylori Vaccination. PLOS ONE 2014, 9, e95187. [Google Scholar] [CrossRef]
- Dai, X.; Liu, M.; Pan, K.; Yang, J. Surface display of OmpC of Salmonella serovar Pullorum on Bacillus subtilis spores. PLOS ONE 2018, 13, e0191627. [Google Scholar] [CrossRef]
- Zhou, Z.; Gong, S.; Li, X.-M.; Yang, Y.; Guan, R.; Zhou, S.; Yao, S.; Xie, Y.; Ou, Z.; Zhao, J.; et al. Expression of Helicobacter pylori urease B on the surface of Bacillus subtilis spores. J. Med Microbiol. 2015, 64, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, X.; Wu, Z.; Xiong, S.; Zhou, Z.; Wang, X.; Xu, J.; Lu, F.; Yu, X. Immunogenicity of self-adjuvanticity oral vaccine candidate based on use of Bacillus subtilis spore displaying Schistosoma japonicum 26 KDa GST protein. Parasitol. Res. 2009, 105, 1643–1651. [Google Scholar] [CrossRef]
- Jiang, H.; Bian, Q.; Zeng, W.; Ren, P.; Sun, H.; Lin, Z.; Tang, Z.; Zhou, X.; Wang, Q.; Wang, Y.; et al. Oral delivery of Bacillus subtilis spores expressing grass carp reovirus VP4 protein produces protection against grass carp reovirus infection. Fish Shellfish. Immunol. 2018, 84, 768–780. [Google Scholar] [CrossRef]
- Valdez, A.; Yepiz-Plascencia, G.; Ricca, E.; Olmos, J. First Litopenaeus vannamei WSSV 100% oral vaccination protection using CotC::Vp26 fusion protein displayed on Bacillus subtilis spores surface. J. Appl. Microbiol. 2014, 117, 347–357. [Google Scholar] [CrossRef]
- Li, G.; Tang, Q.; Chen, H.; Yao, Q.; Ning, D.; Chen, K. Display of Bombyx mori Nucleopolyhedrovirus GP64 on the Bacillus subtilis Spore Coat. Curr. Microbiol. 2011, 62, 1368–1373. [Google Scholar] [CrossRef]
- Hinc, K.; Iwanicki, A.; Obuchowski, M. New stable anchor protein and peptide linker suitable for successful spore surface display in B. subtilis. Microb. Cell Factories 2013, 12, 22–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, G.; Zhang, C.; Xing, X.-H. A study on the effects of linker flexibility on acid phosphatase PhoC-GFP fusion protein using a novel linker library. Enzym. Microb. Technol. 2016, 83, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guérout-Fleury, A.-M.; Frandsen, N.; Stragier, P. Plasmids for ectopic integration in Bacillus subtilis. Gene 1996, 180, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Middleton, R.; Hofmeister, A. New shuttle vectors for ectopic insertion of genes into Bacillus subtilis. Plasmid 2004, 51, 238–245. [Google Scholar] [CrossRef]
- Huang, J.-M.; Hong, H.A.; Van Tong, H.; Hoang, T.H.; Brisson, A.; Cutting, S.M. Mucosal delivery of antigens using adsorption to bacterial spores. Vaccine 2010, 28, 1021–1030. [Google Scholar] [CrossRef]
- Bonavita, R.; Isticato, R.; Maurano, F.; Ricca, E.; Rossi, M. Mucosal immunity induced by gliadin-presenting spores of Bacillus subtilis in HLA-DQ8-transgenic mice. Immunol. Lett. 2015, 165, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Isticato, R.; Sirec, T.; Treppiccione, L.; Maurano, F.; De Felice, M.; Rossi, M.; Ricca, E. Non-recombinant display of the B subunit of the heat labile toxin of Escherichia coli on wild type and mutant spores of Bacillus subtilis. Microb. Cell Factories 2013, 12, 98–98. [Google Scholar] [CrossRef]
- Maia, A.R.; Reyes-Ramírez, R.; Pizarro-Guajardo, M.; Saggese, A.; Castro-Córdova, P.; Isticato, R.; Ricca, E.; Paredes-Sabja, D.; Baccigalupi, L. Induction of a Specific Humoral Immune Response by Nasal Delivery of Bcla2ctd of Clostridioides difficile. Int. J. Mol. Sci. 2020, 21, 1277. [Google Scholar] [CrossRef]
- Santos, F.D.S.; Mazzoli, A.; Maia, A.R.; Saggese, A.; Isticato, R.; Leite, F.; Iossa, S.; Ricca, E.; Baccigalupi, L. A probiotic treatment increases the immune response induced by the nasal delivery of spore-adsorbed TTFC. Microb. Cell Factories 2020, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, M.E.M.; Alves, K.C.S.; de Vasconcelos, M.G.S.; Pinto, T.S.; Glória, J.C.; Chaves, Y.O.; Neves, W.L.L.; Tarragô, A.M.; Neto, J.N.d.S.; Astolfi-Filho, S.; et al. Bacillus subtilis spores as delivery system for nasal Plasmodium falciparum circumsporozoite surface protein immunization in a murine model. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ricca, E.; Baccigalupi, L.; Cangiano, G.; De Felice, M.; Isticato, R. Mucosal vaccine delivery by non-recombinant spores of Bacillus subtilis. Microb. Cell Factories 2014, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pesce, G.; Rusciano, G.; Sasso, A.; Isticato, R.; Sirec, T.; Ricca, E. Surface charge and hydrodynamic coefficient measurements of Bacillus subtilis spore by optical tweezers. Colloids Surfaces B: Biointerfaces 2014, 116, 568–575. [Google Scholar] [CrossRef]
- Sirec, T.; Cangiano, G.; Baccigalupi, L.; Ricca, E.; Isticato, R. The spore surface of intestinal isolates ofBacillus subtilis. FEMS Microbiol. Lett. 2014, 358, 194–201. [Google Scholar] [CrossRef]
- Donadio, G.; Lanzilli, M.; Sirec, T.; Ricca, E.; Isticato, R. Localization of a red fluorescence protein adsorbed on wild type and mutant spores of Bacillus subtilis. Microb. Cell Factories 2016, 15, 1–12. [Google Scholar] [CrossRef]
- Lanzilli, M.; Donadio, G.; Fusco, F.A.; Sarcinelli, C.; Limauro, D.; Ricca, E.; Isticato, R. Display of the peroxiredoxin Bcp1 of Sulfolobus solfataricus on probiotic spores of Bacillus megaterium. New Biotechnol. 2018, 46, 38–44. [Google Scholar] [CrossRef]
- Bressuire-Isoard, C.; Bornard, I.; Henriques, A.O.; Carlin, F.; Broussolle, V. Sporulation Temperature Reveals a Requirement for CotE in the Assembly of both the Coat and Exosporium Layers of Bacillus cereus Spores. Appl. Environ. Microbiol. 2016, 82, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Isticato, R.; Lanzilli, M.; Petrillo, C.; Donadio, G.; Baccigalupi, L.; Ricca, E. Bacillus subtilis builds structurally and functionally different spores in response to the temperature of growth. Environ. Microbiol. 2019, 22, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, C.; Castaldi, S.; Lanzilli, M.; Saggese, A.; Donadio, G.; Baccigalupi, L.; Ricca, E.; Isticato, R. The temperature of growth and sporulation modulates the efficiency of spore-display in Bacillus subtilis. Microb. Cell Factories 2020, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




