2. Methods
This study is part of a research project approved by the Health Research Authority discussed at the Montpellier University Hospital. Patient consent was obtained after the assigned IRB approval number (IRB-MTP_2022_07_202201173) in accordance with the research guidance. This study complies with the Declaration of Helsinki.
2.1. Data Extraction
Comprehensive data extraction retrospectively collected from 3 cardiac surgery centers (Centre Cardiologique du Nord, Saint-Denis France; University Hospital Henri Mondor, Creteil, France and DISC Department, University of Genoa) were included in a central cardiac database which was analyzed retrospectively by the statistician at Weill Cornell Medicine–New York. Presbyterian Medical Center. The registry retrospectively collated demographic information, as well as pre-and postoperative clinical information, including mortality, for cardiac surgery procedures in patients who developed TAAAD from 2008 to 2021. In summary, data entered locally by surgeons were validated at every single center by the database managers before being loaded into a single database. In this step, further validation was performed and reports on missing data were generated for the primary variables (e.g., EuroSCORE risk factors, patient identifiers, and outcome data). The data was then checked again and forwarded to the statistician for cleaning and analysis. Duplicate records were removed, transcriptional discrepancies recoded, and clinical and temporal conflicts resolved. Missing data were resolved during the data transfer validation stages from the 3 individual centers.
2.2. Patients and endpoints
The database included a total of 601 patients with their baseline, demographic, and follow-up data that were examined. The inclusion criteria for this study comprised patients with TAAAD or intramural hematoma involving the ascending aorta. Patients were > 18 years of age, the onset of symptoms within 7 days of surgery, indication for primary surgical repair of acute type A aortic dissection, and any other major cardiac surgical procedure concurrent with surgery for TAAAD and retrograde TAAAD with lesion primarily located in the descending aorta. Exclusion criteria consisted of individuals aged < 18 years, onset of symptoms > 7 days from surgery, a previous procedure for TAAAD, concomitant endocarditis, and TAAAD following blunt or penetrating chest trauma.
The primary endpoint of the study consisted of operative mortality defined as 30-day and in-hospital mortality. Secondary endpoints were stroke or cerebrovascular accident resulting in permanent neurologic deficit, spinal cord injury (SCI; paraplegia, paraparesis), respiratory failure requiring tracheostomy, need for new dialysis, intensive care unit (ICU) stay, composite of major adverse events (MAE) including operative mortality, cerebrovascular accidents, need for dialysis, or need for tracheostomy and late survival.
The primary and secondary endpoints were assessed by means of the segment of the aorta replaced, conservative versus extensive surgery, and the impairment of the patient's clinical condition at hospital admission requiring surgery. The urgency of the procedure has been classified into five categories established on the increasing severity of hemodynamic instability and the need and timing of cardiopulmonary resuscitation. So, we considered the following status: urgent, emergency grade 1, emergency grade 2, salvage grade 1, and salvage grade [
12].
2.3. Surgical procedure
Median sternotomy was performed in all cases for access. The surgical procedure regarding the preferred site for cannulation was as per the surgeon’s preference which in the majority of patients was performed in the innominate artery, right femoral artery, axillary artery, or central aortic lumen. The degree of systemic cooling once the patients were positioned for surgery and cardiopulmonary bypass management was also surgeon-specific. Antegrade cardioplegia was used primarily althoguh retrograde cardioplegia was used in patients with concomitant aortic insufficiency or extensive aortic arch repairs were planned. The ascending aorta excision was extended up to the sinotubular junction. The false lumen of the aortic root was cleared of thrombus to permit visualisation of the defect. The anatomy of the root was inspected alongside the aortic valve leaflets. The intima was re-approximated to the adventitia. The commissures were routinely resuspended using 4-0 or 5-0 sutures reinforced with a Teflon pledget over each commissure. Biologic glue neo-media was routinely performed during reconstruction while the use of felt was dictated by the individual surgeons’ habits. The proximal anastomosis was performed using a 4-0 or 5-0 polypropylene suture which concomitantly secured the intima to the adventitia. To achieve an uninterrupted external ring of felt reinforcement, the use of felt neo-media or an overlay of horizontal felt-mattress sutures was positioned circumferentially and dictated by the surgeon. The replacement of the aortic root using a composite valve graft or valve-sparing root reimplantation was recommended in patients with sinus of Valsalva exceeding 4.5 cm in diameter on computed tomography imaging, in the presence of connective tissue disorders, or when the intimal tear involves the sinus. Patients with normal-sized aortic roots but poor-quality valve leaflets underwent concomitant aortic valve replacement with the use of conventional xenograft or mechanical prosthesis favored.
Total arch replacement procedures (TARP) were performed using deep hypothermic circulatory arrest and with either antegrade or retrograde cerebral perfusion, and systemic cooling (19°C to 25°C, as per surgeon). Symmetric brain cooling and warming were monitored through the use of near-infrared spectroscopy. The technique of cerebral protection, type of cannulation, and technique of perfusion, were at the discretion of the surgeon. In the majority of patients, antegrade cerebral protection was delivered using the endoluminal technique or direct cannulation of the right axillary artery, innominate trunk, or common carotid. The flow rate injected was 800-1,000 mL/min at 28°C or 36°C while maintaining systemic pressure between 40 and 60 mmHg. In the remaining 19.5% of cases undergoing arch repair, the procedure was performed using retrograde cerebral perfusion during deep hypothermic circulatory arrest. Cerebroplegia was administered by a cannula inserted into the superior vena cava and delivered at 200-350 mL/min at 18°C with central venous pressure maintained between 25 and 35 mmHg.
1- and 4-branch prostheses have been preferred in patients undergoing TARP. This comprised of resection of all the aortic tissue up to the left common carotid artery (total hemiarch) or reimplantation of the innominate trunk only (partial hemi-arch). TARP that required large vessel reimplantation was instead addressed in patients with a large arch aneurysm or extensive intimal lesion within the arch. The surgical option to perform arch debranching and selective vessel implantation has been preferred in patients with connective tissue disorders or significant dislocation of the great vessels. Patients who needed a frozen elephant trunk (FET) procedure underwent either insular replantation or selective debranching/implantation of vessels. Antegrade cardiopulmonary bypass was reinstituted using a reperfusion branch of the graft. Systemic warming was performed while preserving a temperature gradient of 10°C between the blood and the core temperature during hemostasis. The remaining anastomoses were completed and reinforced according to the previously described technique and the cardiopulmonary bypass was stopped when the core body temperature reached 36°C.
2.4. Statistical analysis
Descriptive Statistics – Categorical variables were compared with Pearson’s χ2 test or Fisher’s exact test as appropriate, and continuous variables were compared with Mann-Whitney U test after checking for normality. Pre/intra/post-operative variables were compared across subgroups of our population sample, to eminently discern their association with the extent of aortic replacement (i.e., “ascending only”, “+ root”, “+ arch”, “+arch & root”) and with the urgency status at presentation (i.e., “urgent”, “emergency 1”, “emergency 2”, “salvage 1”, “salvage 2”) [
11]. Survival was assessed with Kaplan Meier curves log-rank tests use to compare the groups. For all statistical analyses, a p-value <0.05 was accepted as significant without adjustment for multiple testing.
Risk Adjustment – Risk factors for mortality, both during index hospitalization and during follow-up, according to published evidence and clinical experience were evaluated first with univariate regression. Predictors presenting an association of p < 0.2 with in-hospital or follow-up mortality were retained respectively for the multivariable logistic and the Cox regression models. Multi-collinearity among independent variables was assessed with variance inflation factor analysis, and excluded by a value ≤ 3.
Statistical software - R studio, with the packages ‘dplyr, ggfortify, ggplot2, ranger, survival, survminer” was utilized for the statistical elaboration.
4. Discussion
Although the ideal repair strategy for TAAAD should incur the lowest operative mortality for the index procedure alongside minimal risk of late reoperation; however, the severity of the patient's clinical condition is a parameter that can direct the optimal surgical option. The achievement of the goal of reduced operative mortality in patients presenting with an additional surgical complexity can be hindered by an increase in operating times and by the secondary risk of prolonged periods of organ and cardiac ischemia. These conditions may greatly affect acceptable operational mortality in those patients whose hospitalization occurs in salvage 1 and 2 clinical status.
Given the evidence gathered in multicenter studies, this concept seems to guide the surgical option of many surgeons. The report of the Society of Thoracic Surgeons from 2014 to 2016, indicates the operative mortality of extended TAR procedures was 26.9% compared to 16.3% for hemiarch repair [
12,
13]. Likewise, complete resection of the intimal tear and prosthetic replacement of the ascending aorta with or without hemiarch were still the most commonly performed procedures for type A aortic dissections as reported in large series of patients [
14,
15,
16,
17]. There are no unanimous results from the literature. Proponents of conservative repair argue that the limited involvement of arch repair was associated with lower operative mortality thereby restoring the functional integrity of the aorta.[
18,
19] Advocates for extensive surgery in addition to ascending aortic replacement cite the adverse consequences of the persistent false recirculating lumen and argue that in patients with impaired optimal organ perfusion, more extensive repair may prevent a progressive state of adverse multiorgan ischemia and reduce the risk of reoperation [
20,
21,
22]. A meta-analysis compared proximal repair to extended arch repair reporting that the former had a reduced risk of early mortality (relative risk, 0.69) albeit showing a higher risk of distal reoperations (relative risk, 3. 14) [
23]. The conflicting results were even more evident in single-center studies. Rylski and colleagues [
18] observed an increase in operative mortality with the progressive extension of surgical repair involving all or part of the aortic arch (ascending only, 9.8%; hemiarch, 21.6%; and TAR, 28.6%). Kim and colleagues instead reported results that do not match previous results when comparing patients undergoing TAR to those who received hemiarch repair (13.4% vs 9.7%). However, they observed a significantly higher incidence of permanent neurologic deficit in the TAR group than in those where the procedure was limited to the ascending aorta alone with extension to the hemiarch (22.7% vs 6.3%) [
24].
A large body of available literature reports disparities in the rates observed in several studies with neutral findings for TAAAD repair. Di Eusanio and colleagues [
25] compared patients receiving conservative repair with those undergoing total arch replacement procedures and observed that both the incidence of operative mortality (24.1% vs 22.6%) and that of cerebro-vascular accident with permanent neurological deficit (9.1% vs 7.5%) were quite similar. Likewise, Zhang and colleagues [
26] reported similar results comparing conservative hemiarch repair with frozen elephant trunk, and the difference in mortality ranged from 5.4% to 5.7% while permanent neurologic deficit ranged from 1.4% to 2.3%. Of note that the evidence of reduced operative mortality consistently below 10% stands out among advocates when comparing a more conservative surgical approach or aggressive techniques such as TARP, antegrade stent grafting, or FET. In fact, this low mortality seems to be the prerogative of a few centers.
In this analysis, it was observed that to keep down the operative mortality without incurring the risk of reoperation and not compromise late survival, a conservative approach was used for TAAAD repair. We report a lower number of operations with a more extensive approach involving the aortic root and aortic arch which involved low-risk patients, however, the limited extent of the operation does not expose them to greater risk of future reoperations. The cohort of patients who received a conservative procedure consisted of older subjects with multiple comorbidities, such as pulmonary disease, extracardiac arteriopathy, poor mobility, and emergent status. This group received either ascending aortic replacement associated with root sparing and aortic valve resuspension (40.9%), or receiving associated hemiarch replacement (24.4%). Patients for whom a more extensive approach was used were younger with a greater risk for downstream reoperations, they revealed a higher incidence of bicuspid aortic valve, a slightly higher incidence of recent myocardial infarction (P= 0.34), and more severe aortic regurgitation (P < 0.01). The choice to minimalize the procedure of aortic repair was dictated to their stable clinical condition at hospitalization for TAAAD associated with the manifestation of more limited comorbidities. This cohort of patients received an urgent procedure and they underwent surgery on a working day following hospitalization or later during the index hospitalization [
12]. In these paucisymptomatic subjects, hemodynamic parameters were steady with no evidence of clinical signs of malperfusion and/or aortic rupture. So, we are convinced that this status allowed them to better tolerate the longer cross-clamp and bypass times required to complete larger repairs. The total arch replacement was performed in patients with a tear localized to the aortic arch, in those where the rupture occurred in the presence of a large arch aneurysm, or in subjects who experienced bicuspid aortic valve disease. We report only 17.5% who received aortic root replacement. The choice to perform this procedure was dictated by the extension of the tear in Valsalva sinus, by the severe dilatation of the aortic root, or in the case of bicuspid aortic valve. A considerable proportion of these patients had severe aortic regurgitation and were referred to the surgeon for emergency procedure 2 surgery, which was performed immediately after hospital admission or in consideration of the worsening of the patient's clinical condition. These patients revealed hemodynamic instability despite the use of inotropes and/or malperfusion, however, they did not require cardiopulmonary resuscitation. However, caution was dictated for patients disclosing root or arch aneurysms of more moderate size, the choice of a conservative approach was preferred especially in those of advanced age or in those with extensive comorbidities or hemodynamic instability.
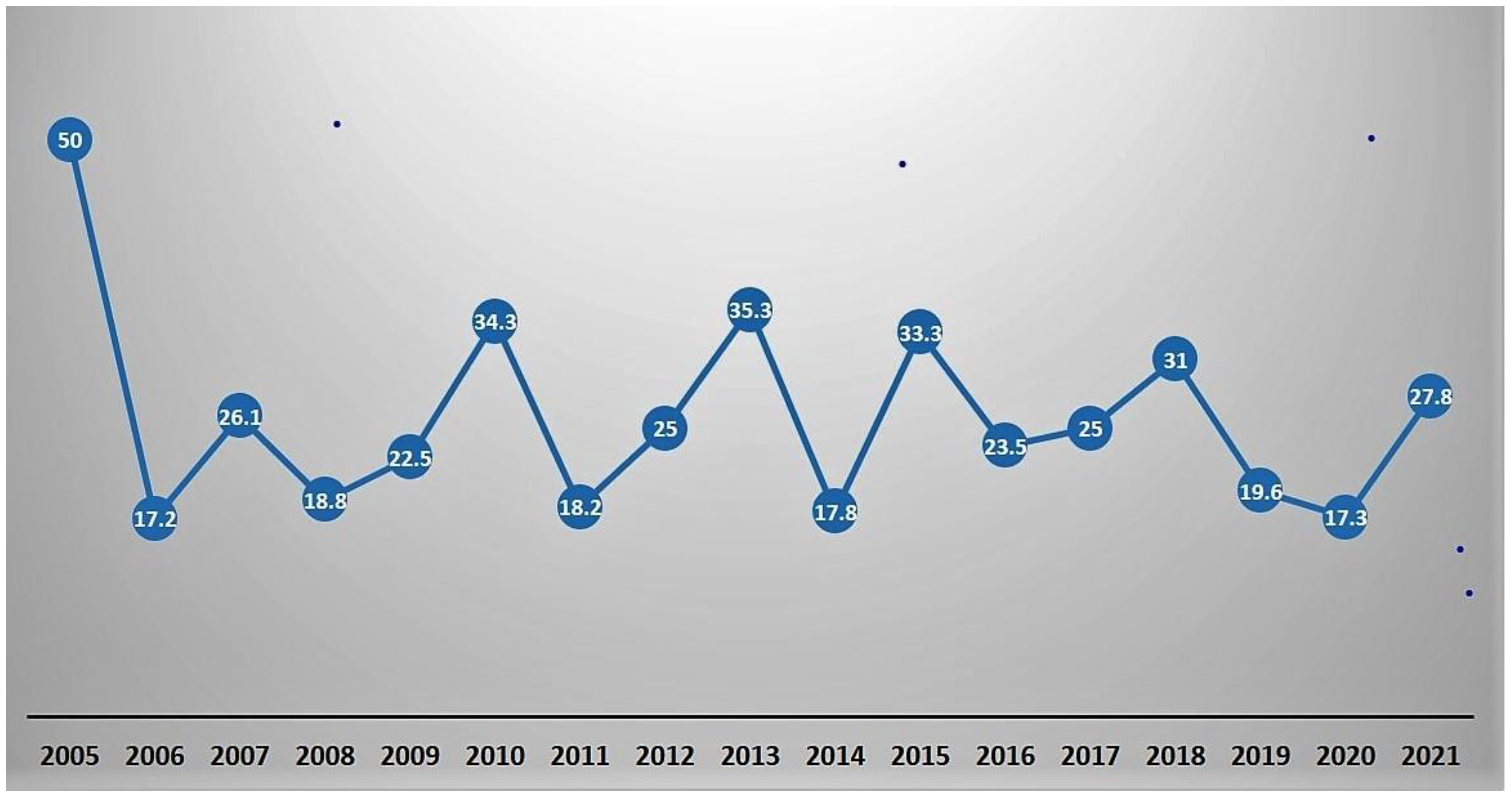
We noted that the strategy used to manage aortic repair in TAAAD did not produce differences in operative mortality between groups (P=0.84). The raw operative mortality rate was 24,3% with a decrease over time from 34,3 % in 2010 to 27,8% in 2021 (
Figure 4), albeit a substantial correlation with the severity of clinical conditions at hospital admission was noted. We observed a significant difference in operative mortality with respect to the urgency of the required procedure based on the patient's clinical condition (from 15.8% in the urgent group to 60% in the salvage 2 group). However, the operative mortality was comparable to those reported in other national and international registries. The crude mortality rate reported in UK nationwide dataset for type A acute aortic dissection (UK National Adult Cardiac Surgical Audit) was 17.8% decreasing over time from 22% in 2009 to 15% in 2018 [
2]. Previous reports from IRAD revealed a decrease in surgical mortality from 25% to 18%. This reduction meant that over time, the in-hospital mortality rate of TAAAD patients dropped significantly from 31% to 22% [
15]. In the last report from IRAD that assembled information from 2952 subjects with type A aortic dissection enrolled from 28 referral centers in North America, Europe, Asia, and Australia, the overall mortality rate for TAAAD in hospital admission was 5.8% at 48 hours. Non-surgery cohort TAAAD revealed a mortality rate of 0.5% per hour (23.7% at 48 hours) which decreased the mortality rate to 4.4% in the surgery cohort at 48 hours [
5]. Results from the NORCAAD, a collaborative registry of eight academic cardiothoracic centers in Denmark, Finland, Iceland, and Sweden mortality, recorded an in-hospital mortality rate of 16% [
3]. Likewise, the GERAADA collected data on 2137 recipients’ surgical procedure for TAAAD enrolled in 50 centers in Germany, Austria, Switzerland, and Luxembourg from July 2006 to June 2017 scoring overall 30-day mortality of 16,9 % [
3,
18]. However conflicting results in mean operative mortality have been reported in single-center studies that pointed out consistent inequality of numerical percentage [
9,
10,
11] in respect of registry studies [
1,
2,
3,
4,
5]. For example recently, Lau and colleagues [
20] observed a mean operative mortality of 5.6% with no substantial difference between patients who received conservative repair involving root-sparing or hemiarch surgery versus those undergoing a more aggressive surgery with an extended repair involving root replacement and/or a full aortic arch.
In the present analysis, similar results were also reported with respect to individual complications. The incidence of permanent and temporary neurological damage was equally distributed in both groups supporting our preferred cerebral protection strategy by adopting normothermic cerebral perfusion and ensuring a very brief deep hypothermic circulatory arrest for the low body. Although there was a relatively high incidence of preoperative malperfusion in both groups (24.9% vs 23.6%; P=0.78), we observed that permanent sequelae were rare and were in part due to our favored primary aortic repair approach supported by rapid restoration of true lumen blood flow, preferentially through cannulation of the innominate artery into the central true lumen. Composite MAE outcomes (37.7% vs 44.2%; P=0.14) were higher in the "extensive" group and were probably dictated by the reflex effect more dependent on the preoperative comorbidity than by the surgical procedure. In univariable analyses, extensive surgery including root or arch replacement, cerebral perfusion, and recent myocardial infarction was not a predictor of operative mortality, while malperfusion, creatine, and increase of cardiac biomarkers reach statistical significance. Instead, for both groups, multivariate logistic regression showed that age, arterial lactate level, intubated/sedated status at hospitalization as well as the degree of severity determined by emergency or salvage status were predictive of higher operative mortality. Although patients receiving the extensive surgical approach incurred the expected longer operative times, careful preoperative selection allowed them to contain this additional risk and lead to a gratifying early and late survival rate. Survival at 1 year (72.8% vs 70.0%), 5 years (68.6% vs 61.3%) and 10 years (53.4% vs AR < 10) were similar in both groups (P = 0.56).
There is a wide variety of procedures to manage aortic arch repair in the context of TAAAD, including ascending aortic replacement extended to hemiarch replacement, antegrade stent grafting, and more complex surgical solutions such as TARP, or TARP with FET. In our analysis, we report that hemiarch replacement is a durable technique with low remote reoperation if it is addressed to selected patients who do not present with arch aneurysmal disease or bicusoid aortic valve. In the present study, the results reported after arch repair suggest that in patients with BAV or arch aneurysm disease, TARP can be performed safely with no difference in operative mortality, especially in younger patients with few comorbidities (P= 0.37). Although both large databases [
13] and single-center results have shown an increase in mortality [
19] and permanent neurological deficit [
25] in patients undergoing extensive arch repair, however, we have observed that adequate patient selection patient, cerebral protection, and meticulous attention to surgical hemostasis have a favorable impact by reducing major morbidity and mortality to levels comparable with those associated with less complex repairs.
Total arch replacement associated with the frozen elephant trunk procedure is another arch repair strategy that deserves reflection. Centers of excellence, strong proponents of this remediation strategy, have reported impressive results. Sun and colleagues performed this procedure in 148 patients with TAAAD associated with arch injury, arch aneurysm, or with Marfan syndrome, reporting exceptional results. They observed no differences in in-hospital mortality (4.7% vs 6.1%; P=0.74), SCI (1.4% vs 0; P = 0.1), or stroke (2.7% vs 1.5%; P = 0.1) with respect to the replacement of the hemiarch. Furthermore, the risk of false lumen thrombosis was considerably reduced, improved, and fewer reoperations were reported in the FET group (1 vs 4 patients; P = 0.03). However, despite improved aortic remodeling, there was no difference in late mortality.[
8] Shrestha and colleagues have routinely used the frozen elephant trunk procedure in patients with TAAAD as well. Operative mortality was 13%, similar to the operative mortality reported with many other procedures. However, in this series, a stroke rate of 15% and an incidence of spinal cord injury of 5% emerge which are worrying, particularly if 12% of patients discharged from the hospital after surgery still needed aortic reoperations downstream [
29]. Deserving of attention is spinal cord injury which has been highlighted as a particularly devastating complication associated with the FET procedure. A large meta-analysis based on a higher number of TAAAD patients undergoing FET revealed an aggregated SCI rate of 4.7%, which increased significantly more when a stent longer than 15 cm was implanted or when in which coverage of the aorta was extending to T8 or lower segments [
23]. Poon and colleagues evaluated the results of a database consisting of 507 patients receiving arch repair and analyzed the database reporting that FET had a higher risk of spinal cord injury versus conventional TARP conventional procedure (1.5% vs 3.9%; P= 0.03) [
30].
In this series, we report similar rates of SCI in recipients of a conservative and extended approach (3.3% vs 5.8%; P=0.22) and in patients hospitalized with more or less severe clinical conditions (2.6% urgency vs 5,6% emergency 1 and emergency 2; P=0.35). Furthermore, for the TARP associated with the FET, we have described a procedure dictated to a very short time of circulation arrest of the lower part of the body which is kept at temperatures that never drop below 28°C. The true lumen is rapidly perfused after making the distal anastomosis followed by the execution of the proximal aortic and cerebral arterial trunk anastomoses [
28]. Gambardella and colleagues observed that spinal cord injury after elective and open thoracoabdominal aorta aneurysm repair for chronic type A dissection is a rare complication [
31]. The indication for a frozen elephant trunk procedure is questionable considering the limited percentage of patients requiring a late distal reoperation [
8,
19,
20] and therefore caution is warranted in recommending the use of routine frozen elephant trunk. Nevertheless, it would be advantageous to evaluate a select group of patients receiving the FET procedure whom we expect to be at high risk for distal reoperation.
Patients with TAAAD referred for root replacement undergo a more complex operation demanding notably longer bypass and cross-clamp times than those receiving root replacement. The fragility characteristic of the dissected aortic tissue could constitute an additional risk factor for the reimplantation of the coronary button and its manipulation could be dangerous. Furthermore, these patients have a higher risk of both postoperative bleeding and ischemia which can be life-threatening. However, in centers of high experience and volume of cases treated it has been observed that a selection of patients can mitigate the greater risks dictated by the surgical strategy in the cohort of TAAAD patients requiring elective aortic root surgery [
31]. In this series, 21.5% of patients, who tended to have more severe BAV and aortic regurgitation, underwent root replacement via a biological or mechanical composite graft or valve sparring aortic replacement. We did not observe an increase in operative mortality compared with those receiving a root-sparing procedure (19% versus 24.3%; P = 0.37). It is deserving to observe, however, that root-sparing surgery is performed in most patients with TAAAD even when moderate aortic regurgitation is present. In our series, 56.6% of patients in the root preservation group had mild or moderate AR while severe AR (44.3%) was treated in 90 of 129 patients who underwent root replacement. Importantly, in the majority of patients, the valvular dysfunction was primarily due to the collapse of 1 or more commissures of the aortic valve rather than related to true native valvular disease. This group of patients presenting with severe AR also had aortic root dilatation. We believe that the scrupulous resuspension of the commissures plays a key role in restoring the correct geometry of the aortic root thus allowing to re-establish the diameter of the sinotubular junction and guaranteeing the resolution of severe aortic regurgitation with the continence of the aortic valve. We perform an aortic suture corresponding exactly to the upper limit of the commissures recording a complete resolution of the AR in the group of patients receiving conservative surgery and we have observed that this technique is safe and durable. A word of caution is directed to choosing composite conduits toward valve sparring root replacement procedure as the best definitive treatment option for root pathology in the context of TAAAD. We perform the valve-sparing root replacement procedure in selected cases (1.3%) that include young subjects with intact aortic flap tissue. Sievers and colleagues reported higher rates of patients undergoing valve-sparing root replacement revealing excellent short- and medium-term outcomes [
10].
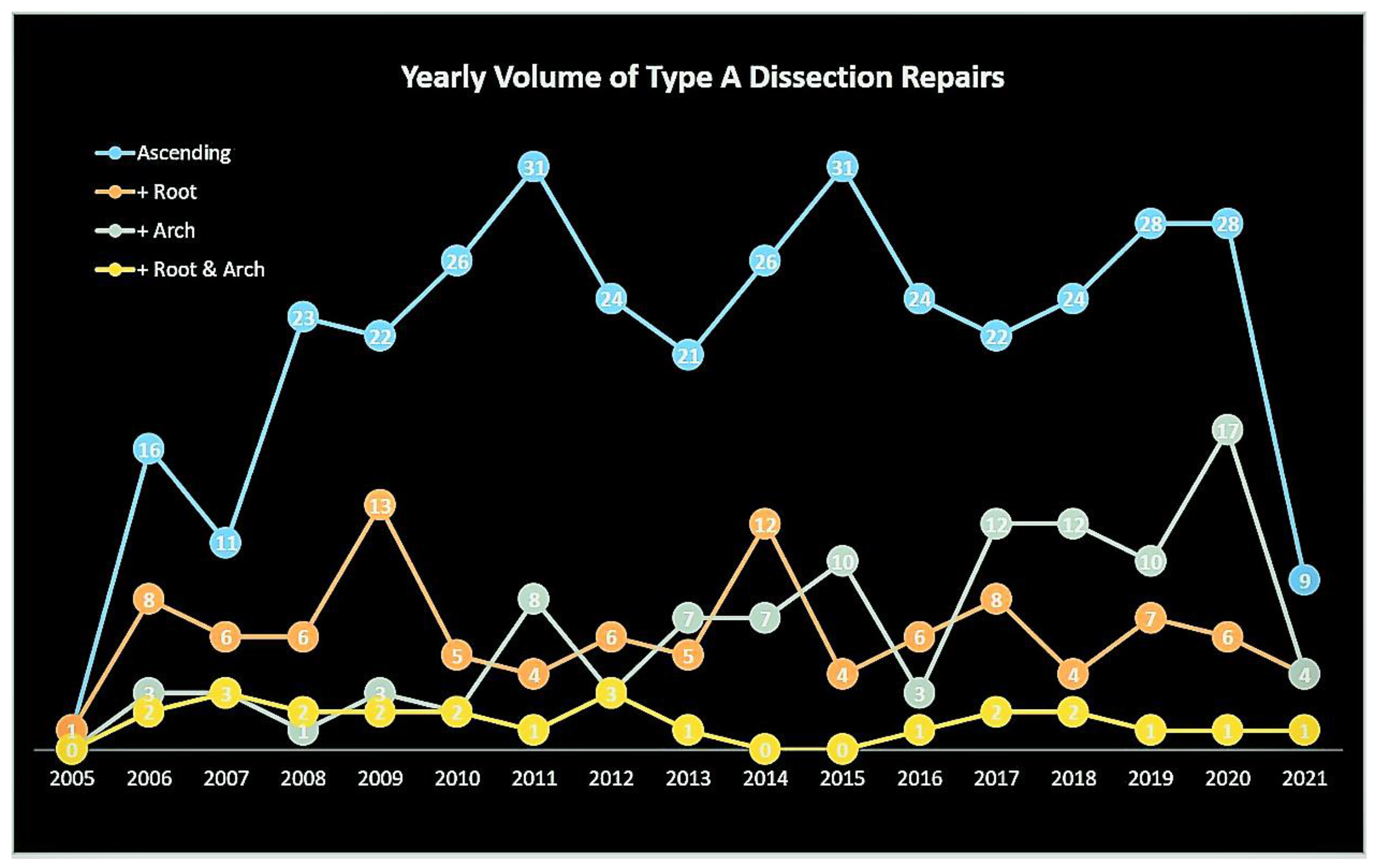
Figure 1.
Title – Yearly volume of type A aortic dissection repairs. Caption – Yearly volume of type A aortic dissection repairs. The curves are color-coded according to the aortic segment replaced.
Figure 1.
Title – Yearly volume of type A aortic dissection repairs. Caption – Yearly volume of type A aortic dissection repairs. The curves are color-coded according to the aortic segment replaced.
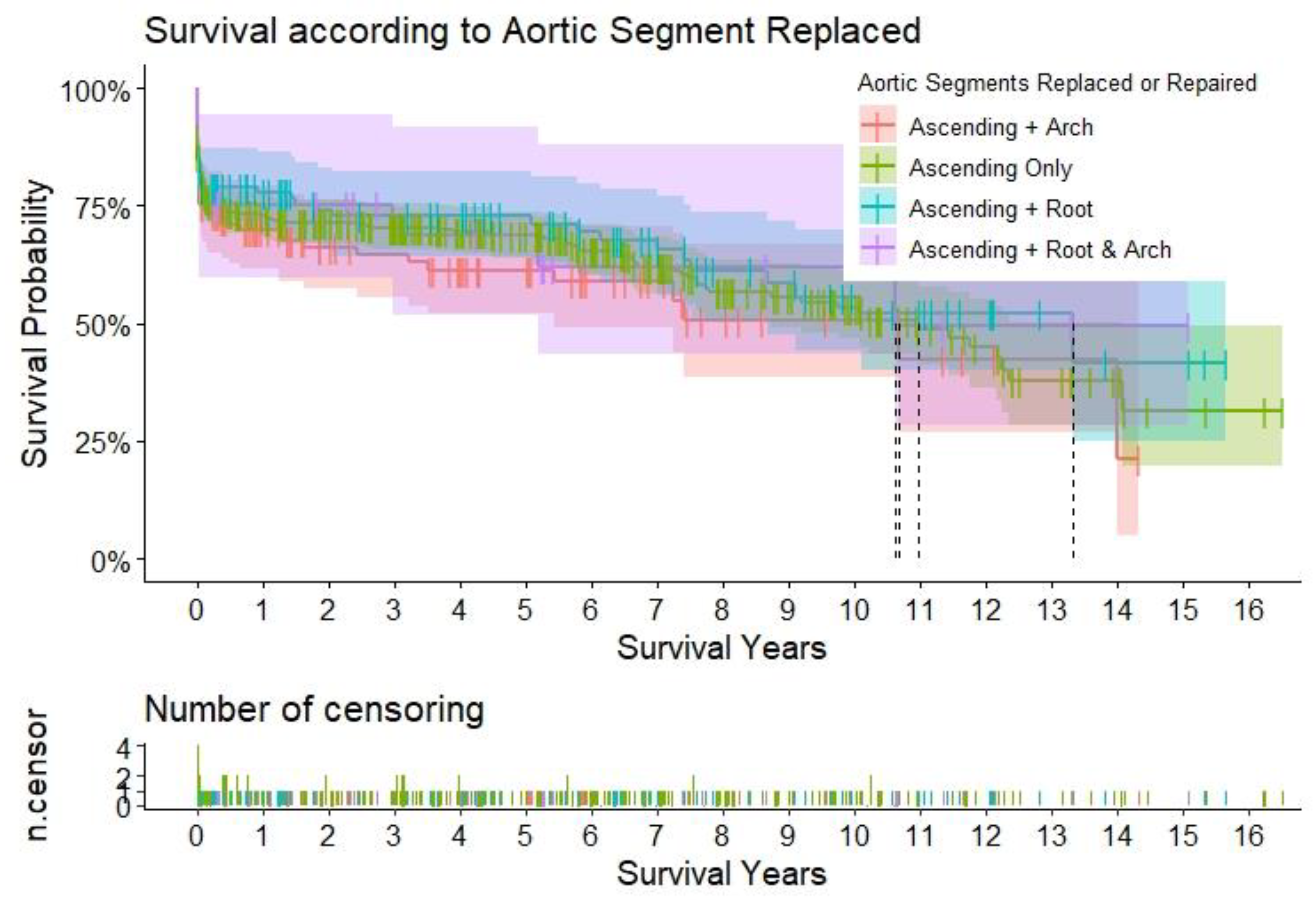
Figure 2.
Title – Survival according to the aortic segments replaced. Caption – Kaplan-Meier curves to assess survival after type A aortic dissection repair. The curves are color-coded according to the aortic segment replaced, and the relative shaded areas represent the 95% confidence interval. The censored patients are represented by the short vertical lines along the survival curves. The dotted black lines represent the estimated median survival, which can only be calculated if the survival has dropped < 50% for the relative subgroup at the end of the study period.
Figure 2.
Title – Survival according to the aortic segments replaced. Caption – Kaplan-Meier curves to assess survival after type A aortic dissection repair. The curves are color-coded according to the aortic segment replaced, and the relative shaded areas represent the 95% confidence interval. The censored patients are represented by the short vertical lines along the survival curves. The dotted black lines represent the estimated median survival, which can only be calculated if the survival has dropped < 50% for the relative subgroup at the end of the study period.
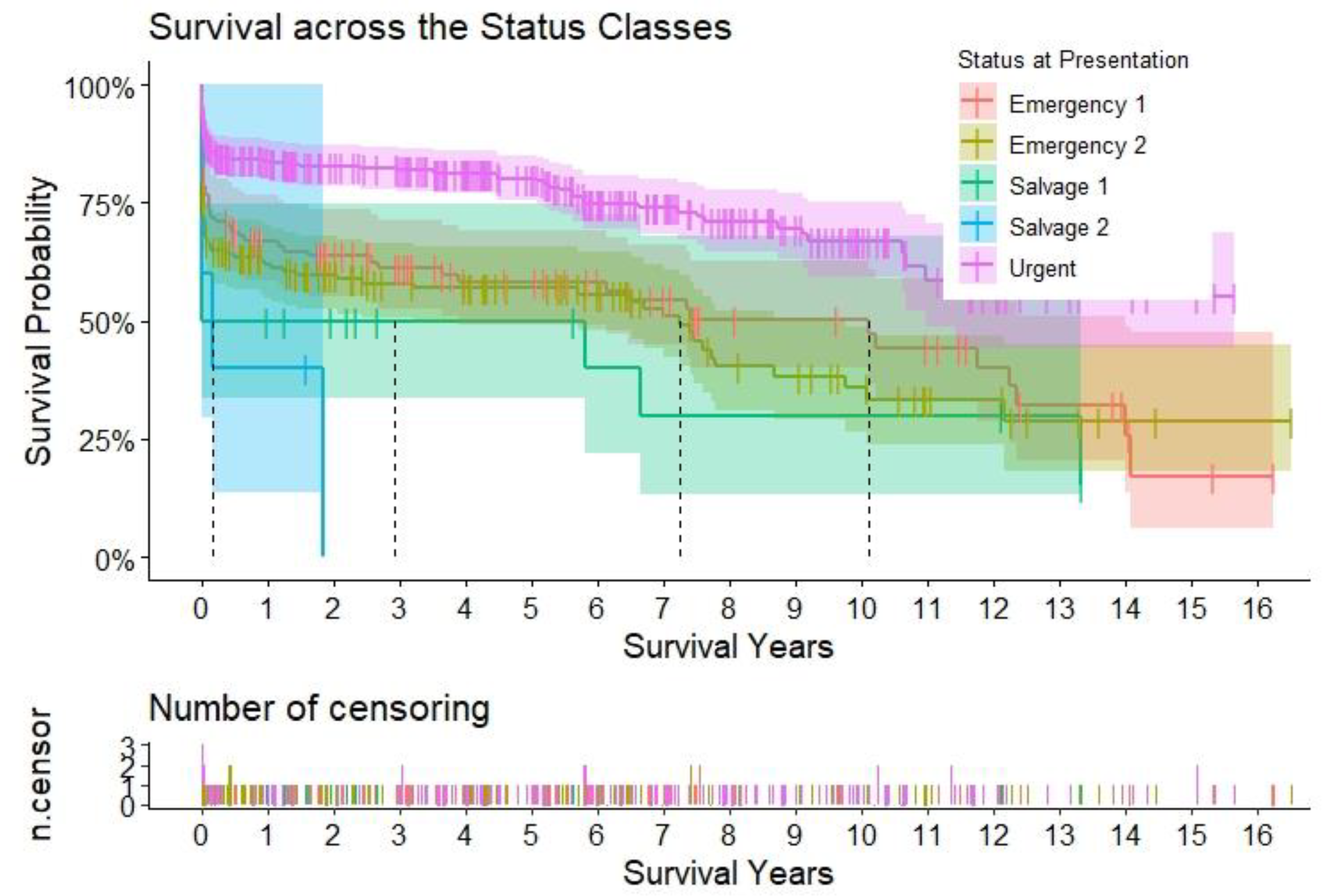
Figure 3.
Title – Survival according to urgency status. Caption – Kaplan-Meier curves to assess survival after type A aortic dissection repair. The curves are color-coded according to the urgency status at presentation, and the relative shaded areas represent the 95% confidence interval. The censored patients are represented by the short vertical lines along the survival curves. The dotted black lines represent the estimated median survival, which can only be calculated if the survival has dropped < 50% for the relative subgroup at the end.
Figure 3.
Title – Survival according to urgency status. Caption – Kaplan-Meier curves to assess survival after type A aortic dissection repair. The curves are color-coded according to the urgency status at presentation, and the relative shaded areas represent the 95% confidence interval. The censored patients are represented by the short vertical lines along the survival curves. The dotted black lines represent the estimated median survival, which can only be calculated if the survival has dropped < 50% for the relative subgroup at the end.
Figure 4.
Title: Operative mortality during study period. Caption- The line graph shows the operative mortality summed as a percentage of cases done over the years (2005-2021).
Figure 4.
Title: Operative mortality during study period. Caption- The line graph shows the operative mortality summed as a percentage of cases done over the years (2005-2021).
Table 1.
Title – Pre/Intra/Post-operative variables in the conservative vs extensive surgery groups. Caption - Pre/Intra/Post-operative variables after type A aortic dissection repair. Abbreviations and acronyms: BMI= body mass index; ICU= intensive care unit; IQR= interquartile range; Major adverse events= composite of in-hospital mortality and stroke, spinal cord injury, tracheostomy, and hemodialysis.
Table 1.
Title – Pre/Intra/Post-operative variables in the conservative vs extensive surgery groups. Caption - Pre/Intra/Post-operative variables after type A aortic dissection repair. Abbreviations and acronyms: BMI= body mass index; ICU= intensive care unit; IQR= interquartile range; Major adverse events= composite of in-hospital mortality and stroke, spinal cord injury, tracheostomy, and hemodialysis.
| Table 1. pRE/INTRA/POST-OPERATIVE VARIABLES IN THE Overall Sample. |
|---|
| Demographics |
|
Malperfusion – n (%) |
147 (24.5) |
|
64.4 (20.1) |
|
80 (13.3) |
|
25.8 (5.2) |
|
12 (2.0) |
|
180 (30.0) |
|
61 (10.1) |
| Biochemestry |
|
|
33 (5.5) |
|
88.4 (29.1) |
|
32 (5.3) |
|
121.0 (28.5) |
|
|
|
220.0 (194.5) |
Aortic Segments Replaced |
|
|
2.2 (2.3) |
❖ Ascending only – n (%) ❖ Hemi-Arch – n (%) |
246 (62.6)
147 (37.4) |
|
150 (25.0) |
|
105 (17.5) |
| Comorbidities and Presentation |
|
|
103 (17.5) |
|
36 (6.0) |
|
|
|
32 (5.3) |
|
|
|
33 (5.5) |
Type of Root Procedure |
|
|
21 (3.5) |
|
121 (20.1) |
|
49 (8.2) |
|
5 (0.8) |
|
3 (0.5) |
|
3 (0.5) |
|
19 (3.2) |
Type of Arch Procedure |
|
|
26 (4.3) |
|
|
| Status |
|
|
52 (8.7) |
|
304 (50.6) |
|
51 (8.5) |
|
107 (17.8) |
Type of Cerebroplegia |
|
|
161 (26.8) |
|
248 (41.3) |
|
24 (4.0) |
|
117 (19.5) |
|
5 (0.8) |
|
|
| Aortic Valve |
|
|
76 (12.6) |
|
12 (2.0) |
|
25 (4.2) |
|
|
|
27 (4.5) |
|
203 (33.8) |
|
63 (10.5) |
|
185 (30.8) |
|
146 (24.3) |
|
95 (15.8) |
|
240 (39.9) |
|
117 (19.5) |
|
9.0 (17.0) |
Table 2.
Pre/Intra/Post-operative variables after type A aortic dissection repair, according to the aortic segment replaced. Abbreviations and acronyms: BMI= body mass index; Cr= creatinine; Hb= hemoglobin; ICU= intensive care unit; IQR= interquartile range; lactate= arterial lactate; Major adverse events= composite of in-hospital mortality and stroke, spinal cord injury, tracheostomy, and hemodialysis; PLT= platelets count; enzymes= cardiac enzymes.
Table 2.
Pre/Intra/Post-operative variables after type A aortic dissection repair, according to the aortic segment replaced. Abbreviations and acronyms: BMI= body mass index; Cr= creatinine; Hb= hemoglobin; ICU= intensive care unit; IQR= interquartile range; lactate= arterial lactate; Major adverse events= composite of in-hospital mortality and stroke, spinal cord injury, tracheostomy, and hemodialysis; PLT= platelets count; enzymes= cardiac enzymes.
| Table II. Pre/Intra/Post-operative variables according to aortic segment replaced. |
|---|
| No. of patients |
Ascending Only
No 246 |
+ Root
No 105 |
+ Arch
No 250 |
+ Root & Arch
No 24 |
P Value |
| Demographic Characteristics |
|
|
|
|
|
|
67.4 (20.6) |
61.7 (19.8) |
61.1 (16.5) |
59.7 (11.4) |
< 0.01 |
|
25.9 (5.4) |
25.6 (4.3) |
25.6 (4.5) |
25.4 (4.0) |
0.64 |
|
128 (34.9) |
23 (21.9) |
25 (23.8) |
4 (16.7) |
< 0.01 |
| Biochemestry |
|
|
|
|
|
|
88.5 (32.4) |
88.0 (19.9) |
88.4 (33.0) |
81.0 (7.0) |
0.50 |
|
122.0 (28.5) |
122.0 (31.0) |
120.0 (28.0) |
112.5 (29.5) |
0.16 |
|
211.0 (166.0) |
250.0 (182.5) |
199.0 (224.0) |
281.0 (186.2) |
0.05 |
|
2.2 (2.4) |
2.1 (2.0) |
2.0 (2.4) |
2.5 (1.9) |
0.80 |
|
90 (24.5) |
81 (77.1) |
32 (30.5) |
20 (83.3) |
0.41 |
| Comorbidities and Presentation |
|
|
|
|
|
|
21 (5.7) |
6 (5.7) |
9 (8.6) |
0 (0.0) |
0.42 |
|
19 (5.2) |
7 (6.7) |
5 (4.8) |
1 (4.2) |
0.91 |
|
25 (6.8) |
5 (4.8) |
1 (1.0) |
2 (8.3) |
0.12 |
|
18 (4.9) |
1 (1.0) |
2 (1.9) |
0 (0.0) |
0.12 |
|
37 (10.1) |
7 (6.7) |
5 (4.8) |
0 (0.0) |
0.12 |
|
3 (0.8) |
0 (0.0) |
0 (0.0) |
0 (0.) |
0.59 |
|
9 (2.5) |
6 (5.7) |
3 (2.9) |
1 (4.2) |
0.40 |
|
14 (3.8) |
6 5.7) |
6 (5.7) |
0 (0.0) |
0.51 |
|
100 (27.2) |
32 (30.5) |
37 (35.2) |
10 (41.7) |
0.23 |
| Status |
|
|
|
|
0.06 |
|
77 (21.0) |
18 (17.1) |
12 (11.4) |
0 (0.0) |
|
|
98 (26.7) |
29 (27.6) |
30 (28.6) |
4 (16.7) |
|
|
12 (3.3) |
6 (5.7) |
6 (5.7) |
0 (0.0) |
|
|
4 (1.1) |
1 (1.0) |
0 (0.0) |
0 (0.0) |
|
|
176 (48.0) |
51 (48.6) |
57 (54.3) |
20 (83.3) |
|
| Aortic Valve |
|
|
|
|
|
|
4 (1.1) |
6 (5.7) |
1 (1.0) |
1 (4.2) |
0.02 |
|
|
|
|
|
< 0.01 |
|
135 (36.9) |
10 (9.5) |
57 (54.3) |
1 (4.2) |
|
|
147 (40.2) |
8 (7.6) |
29 (27.6) |
1 (4.2) |
|
|
59 (16.1) |
17 (16.2) |
12 (11.4) |
7 (29.2) |
|
|
25 (6.8) |
70 (66.7) |
7 (6.7) |
15 (62.5) |
|
| Malperfusion |
89 (24.3) |
27 (25.7) |
27 (25.7) |
4 (16.7) |
0.81 |
|
47 (12.8) |
15 (14.3) |
16 (15.2) |
2 (8.3) |
|
|
7 (1.9) |
3 (2.9) |
2 (1.9) |
0 (0.0) |
|
|
37 (10.1) |
11 (10.5) |
12 (11.4) |
1 (4.2) |
|
|
25 (6.8) |
4 (3.8) |
3 (2.9) |
1 (4.2) |
|
|
18 (4.9) |
5 (4.8) |
7 (6.7) |
2 (8.3) |
|
| Outcomes |
|
|
|
|
|
|
43 (11.7) |
8 (7.6) |
23 (21.9) |
2 (8.3) |
0.01 |
|
12 (3.3) |
6 (5.7) |
6 (5.7) |
1 (4.2) |
0.57 |
|
16 (4.4) |
6 (5.7) |
5 (4.8) |
0 (0.0) |
0.67 |
|
33 (9.0) |
11 (10.5) |
15 (14.3) |
4 (16.7) |
0.35 |
|
89 (24.3) |
20 (19.0) |
31 (29.5) |
6 (25.0) |
0.37 |
|
137 (37.3) |
40 (38.1) |
53 (50.5) |
10 (41.7) |
0.11 |
|
7.0 (15.0) |
10.0 (22.0) |
11.0 (17.0) |
15.5 (13.2) |
< 0.01 |
Table 3.
Pre/Intra/Post-operative variables after type A aortic dissection repair, comparing a conservative (i.e., ascending ± hemiarch replacement) vs an extensive (i.e., + aortic root or total aortic arch replacement) surgical approach. Abbreviations and acronyms: BMI= body mass index; Cr= creatinine; Hb= hemoglobin; ICU= intensive care unit; IQR= interquartile range; lactate= arterial lactate; Major adverse events= composite of in-hospital mortality and stroke, spinal cord injury, tracheostomy, and hemodialysis; PLT= platelets count; enzymes= cardiac enzymes.
Table 3.
Pre/Intra/Post-operative variables after type A aortic dissection repair, comparing a conservative (i.e., ascending ± hemiarch replacement) vs an extensive (i.e., + aortic root or total aortic arch replacement) surgical approach. Abbreviations and acronyms: BMI= body mass index; Cr= creatinine; Hb= hemoglobin; ICU= intensive care unit; IQR= interquartile range; lactate= arterial lactate; Major adverse events= composite of in-hospital mortality and stroke, spinal cord injury, tracheostomy, and hemodialysis; PLT= platelets count; enzymes= cardiac enzymes.
| Table III Pre/Intra/Post-operative variables |
|
|
|
| No. of patients |
Conservative
No 393 |
Extensive
No 208 |
P Value |
| Demographic Characteristics |
|
|
|
|
66.9 (20.4) |
61.1 (17.4) |
< 0.01 |
|
25.9 (5.6) |
25.7 (4.3) |
0.53 |
|
137 (34.9) |
43 (20.7) |
< 0.01 |
| Biochemestry |
|
|
|
|
88.4 (31.6) |
88.0 (25.7) |
0.86 |
|
122.0 (29.0) |
119.0 (27.0) |
0.22 |
|
212.5 (168.0) |
230.0 (204.7) |
0.12 |
|
2.2 (2.3) |
2.3 (2.2) |
0.85 |
|
95 (24.2) |
55 (26.4) |
0.61 |
| Comorbidities and Presentation |
|
|
|
|
22 (5.6) |
14 (6.7) |
0.71 |
|
7 (1.8) |
7 (3.4) |
0.35 |
|
26 (6.6) |
7 (3.4) |
0.14 |
|
18 (4.6) |
3 (1.4) |
0.08 |
|
39 (9.9) |
10 (4.8) |
0.04 |
|
3 (0.8) |
0 (0.0) |
0.59 |
|
10 (2.5) |
9 (4.3) |
0.34 |
|
14 (3.6) |
12 (5.8) |
0.29 |
|
107 (27.2) |
72 (34.6) |
0.07 |
| Status |
|
|
0.10 |
|
79 (20.1) |
28 (13.5) |
|
|
108 (27.5) |
53 (25.5) |
|
|
12 (3.1) |
12 (5.8) |
|
|
4 (1.0) |
1 (0.5) |
|
|
190 (48.3) |
114 (54.8) |
|
| Aortic Valve |
|
|
|
|
4 (1.0) |
8 (3.8) |
0.04 |
|
|
|
< 0.01 |
|
143 (36.5) |
60 (28.8) |
|
|
153 (39.0) |
32 (15.4) |
|
|
69 (17.6) |
26 (12.5) |
|
|
27 (6.9) |
90 (43.3) |
|
| Malperfusion |
98 (24.9) |
49 (23.6) |
0.78 |
|
52 (13.2) |
28 (13.5) |
|
|
8 (2.0) |
4 (1.9) |
|
|
41 (10.4) |
20 (9.6) |
|
|
26 (6.6) |
7 (3.4) |
|
|
22 (5.6) |
10 (4.8) |
|
| Outcomes |
|
|
|
|
48 (24.9) |
28 (23.6) |
0.75 |
|
13 (3.3) |
12 (5.8) |
0.22 |
|
18 (4.6) |
9 (4.3) |
1 |
|
37 (9.4) |
26 (12.5) |
0.30 |
|
97 (24.7) |
49 (23.6) |
0.84 |
|
148 (37.7) |
92 (44.2) |
0.14 |
|
8.0 (15.0) |
11.0 (22.0) |
0.03 |
Table 4.
Pre/Intra/Post-operative variables after type A aortic dissection repair, according to urgency status at presentation. Abbreviations and acronyms: BMI= body mass index; Cr= creatinine; Hb= hemoglobin; ICU= intensive care unit; IQR= interquartile range; lactate= arterial lactate; Major adverse events= composite of in-hospital mortality and stroke, spinal cord injury, tracheostomy, and hemodialysis; PLT= platelets count; enzymes= cardiac enzymes.
Table 4.
Pre/Intra/Post-operative variables after type A aortic dissection repair, according to urgency status at presentation. Abbreviations and acronyms: BMI= body mass index; Cr= creatinine; Hb= hemoglobin; ICU= intensive care unit; IQR= interquartile range; lactate= arterial lactate; Major adverse events= composite of in-hospital mortality and stroke, spinal cord injury, tracheostomy, and hemodialysis; PLT= platelets count; enzymes= cardiac enzymes.
| PRE/INTRA/POST-OPERATIVE VARIABLES ACCORDING TO URGENCY STATUS AT PRESENTATION |
|---|
| Variables |
Urgent
No 304 |
Emergency 1
No 107 |
Emergency 2
No 161 |
Salvage 1
No 24 |
Salvage 2
No 5 |
P value |
| Demographic Characteristics |
|
|
|
|
|
|
|
64.2 (18.2) |
64.0 (21.2) |
63.4 (21.2) |
67.1 (13.1) |
73.2 (13.2) |
0.41 |
|
25.8 (5.4) |
26.8 (5.0) |
25.7 (4.7) |
25.3 (3.2) |
24.2 (2.1) |
0.41 |
|
104 (34.2) |
26 (24.3) |
43 (26.7) |
6 (25.0) |
1 (20.0) |
0.23 |
| Biochemical Analysis |
|
|
|
|
|
|
|
82.0 (23.0) |
94.1 (40.2) |
96.0 (40.5) |
88.0 (27.0) |
145.0 (34.5) |
< 0.01 |
|
116.0 (25.0) |
130.0 (26.0) |
125.5 (28.0) |
114.0 (18.0) |
99.0 (28.0) |
< 0.01 |
|
254.0 (187.0) |
198.0 (67.5) |
207.5 (182.2) |
166.0 (208.0) |
128.0 (23.0) |
< 0.01 |
|
2.5 (2.5) |
1.2 (1.3) |
2.3 (2.0) |
2.3 (4.5) |
3.3 (6.0) |
< 0.01 |
|
82 (27.0) |
14 (13.1) |
41 (25.5) |
12 (50.0) |
1 (20.0) |
< 0.01 |
| Patients Status Before Surgery |
|
|
|
|
|
|
|
23 (7.6) |
4 (3.7) |
7 (4.3) |
2 (8.3) |
0 (0.0) |
0.46 |
|
13 (4.3) |
6 (5.6) |
11 (6.8) |
1 (4.2) |
1 (20.0) |
0.46 |
|
17 (5.6) |
8 (7.5) |
6 (3.7) |
1 (4.2) |
1 (20.) |
0.42 |
|
6 (2.0) |
8 (7.5) |
6 (3.7) |
1 (4.2) |
0 (0.0) |
0.12 |
|
33 (10.9) |
5 (4.7) |
9 (5.6) |
2 (8.3) |
0 (0.0) |
0.16 |
|
2 (0.7) |
0 (0.0) |
1 (0.6) |
0 (0.0) |
0 (0.0) |
0.93 |
|
7 (2.3) |
3 (2.8) |
7 (4.3) |
2 (8.3) |
0 (0.0) |
0.44 |
|
0 (0.0) |
0 (0.0) |
0 (0.0) |
21 (87.5) |
5 (100) |
< 0.01 |
|
95 (31.2) |
15 (14.0) |
59 (36.6) |
9 (37.5) |
1 (20.0) |
< 0.01 |
| Aortic Valve |
|
|
|
|
|
|
|
3 (1.0) |
4 (3.7) |
5 (3.1) |
0 (0.0) |
0 (0.0) |
0.30 |
|
|
|
|
|
|
0.14 |
|
110 (36.2) |
27 (25.2) |
55 (34.4) |
10 (41.7) |
1 (20.0) |
|
|
96 (31.6) |
36 (33.6) |
47 (29.4) |
5 (20.8) |
1 (20.0) |
|
|
36 (11.8) |
27 (25.2) |
27 16.9) |
3(12.5) |
2 (40.) |
|
|
62 (20.4) |
17 (15.9) |
31 (19.4) |
6 (25.0) |
1 (20.0) |
|
| Malperfusion |
0 (0.0) |
0 (0.0) |
128 (79.5) |
16 (66.7) |
3 (60.0) |
< 0.01 |
|
0 (0.0) |
0 (0.0) |
66 (41.0) |
12 (50.0) |
2 (40.0) |
|
|
0 (0.0) |
0 (0.0) |
8 (5.0) |
4 (16.7) |
0 (0.0) |
|
|
0 (0.0) |
0 (0.0) |
55 (34.2) |
4 (16.7) |
2 (40.0) |
|
|
0 (0.0) |
0 (0.0) |
25 (15.5) |
5 (20.8) |
3 (60.0) |
|
|
0 (0.0) |
0 (0.0) |
30 (18.6) |
2 (8.3) |
0 (0.0) |
|
| Aortic Segments Replaced |
|
|
|
|
|
0.06 |
|
176 (57.9) |
77 (72.0) |
98 (60.9) |
12 (50.0) |
4 (40.0) |
|
|
51 (16.8) |
18 (16.8) |
29 (18.0) |
6 (25.0) |
1 (20.0) |
|
|
57 (18.8) |
12 (11.2) |
30 (18.6) |
6 (25.0) |
0 (0.0) |
|
|
20 (6.6) |
0 (0.0) |
4 (2.5) |
0 (0.0) |
0 (0.0) |
|
| Adverse Events |
|
|
|
|
|
|
|
41 (13.5) |
11 (10.3) |
18 (11.2) |
6 (25.0) |
0 (0.0) |
0.28 |
|
8 (2.6) |
6 (5.6) |
9 (5.6) |
2 (8.3) |
0 (0.0) |
0.35 |
|
16 (5.3) |
3 (2.8) |
8 (5.0) |
0 (0.0) |
0 (0.0) |
0.63 |
|
24 (7.9) |
6 (5.6) |
30 (18.6) |
3 (12.5) |
0 (0.0) |
< 0.01 |
|
48 (15.8) |
27 (25.2) |
56 (34.8) |
12 (50.0) |
3 (60.0) |
< 0.01 |
|
100 (32.9) |
39 (36.4) |
82 (50.9) |
16 (66.7) |
3 (60.0) |
< 0.01 |
|
11.0 (20.0) |
5.0 (10.0) |
8.0 (16.0) |
3 (13.5) |
1.0 (14.0) |
< 0.01 |
Table 5.
Title – Survival after type A aortic dissection. Caption – Survival after type A aortic dissection repair in the overall sample, and across subgroups according to the aortic segment replaced and according to urgency status at presentation. Abbreviations and acronyms: SE= standard error.
Table 5.
Title – Survival after type A aortic dissection. Caption – Survival after type A aortic dissection repair in the overall sample, and across subgroups according to the aortic segment replaced and according to urgency status at presentation. Abbreviations and acronyms: SE= standard error.
| SURVIVAL - OVERALL SAMPLE |
|---|
| Elapsed Time |
At Risk - No
|
Events - No
|
Survival - % |
SE - % |
|
|
| 1-Year |
370 |
157 |
73.3 |
1.8 |
|
|
| 5-Year |
225 |
22 |
68.2 |
2 |
|
|
| 10-Year |
69 |
33 |
53.5 |
2.8 |
|
|
| SURVIVAL ACCORDING TO AORTIC SEGMENTS REPAIRED |
| Elapsed Time |
Ascending |
+ Root |
+ Arch |
+ Root & Arch |
P value |
|
| 1-Year - % ± SE |
72.8 ± 2.4 |
80.0 ± 4.0 |
70.0 ± 4.5 |
75.0 ± 8.8 |
0.56 |
|
| 5-Year - ± SE |
68.6 ± 2.5 |
72.7 ± 4.5 |
61.3 ± 5.2 |
68.7 ± 10.1 |
|
| 10-Year - % ± SE |
53.4 ± 3.6 |
55.6 ± 6.4 |
At Risk < 10 |
At Risk < 10 |
|
| < 0.01 |
| Elapsed Time |
Urgent |
Emergency 1 |
Emergency 2 |
Salvage 1 |
Salvage 2 |
P value |
| 1-Year - % ± SE |
84.0 ± 2.1 |
66.9 ± 4.6 |
62.1 ± 3.8 |
50 ± 10.2 |
40.0 ± 21.9 |
|
| 5-Year - % ± SE |
80.2 ± 2.4 |
58.3 ± 5.0 |
56.9 ± 4.0 |
50 ± 10.2 |
At Risk < 10 |
| 10-Year - % ± SE |
66.8 ± 3.9 |
50.2 ± 5.8 |
35.9 ± 5.6 |
At Risk < 10 |
At Risk < 10 |