1. Introduction
Nonlinear optical (NLO) materials have received much attention due to their application in various optoelectronic devices [
1,
2]. For example, strong saturable absorption can be used for Q-switching and mode-locking techniques [
3,
4]. Reverse saturable absorption based optical limiting is considered to be the ideal mechanism for the protection from high intensity laser [
5]. The development of fluorescent materials with strong multiphoton absorption (MPA) are desirable for
in vivo imaging of deep tissue and optical limiting [
6,
7]. The NLO materials with large nonlinear refraction but weak nonlinear absorption can be used in all-optical switching and optical communications [
8,
9].
Thus far, various kinds of NLO materials have been reported, including organic molecules [
10,
11], semiconductor nanocrystals [
12], metal nanocrystals [
13], organic-inorganic hybrid materials [
14] and 2D materials [
15]. Among them, organic molecules have a lot of advantages, such as tunable NLO properties through the design of molecular structures, excellent biocompatibility and low fabrication cost [
16,
17]. Compared with the molecules with visible absorption, near-infrared (NIR) ones have important applications in communication, biological and security [
18,
19]. For example, the NIR molecules with strong MPA have deep penetration and high resolution in fluorescence imaging [
20,
21]. In addition, due to the resonance effect, NIR molecules usually have strong nonlinear refraction effects in the communication band, making them to be promising in all-optical switching [
22,
23].
Boron-dipyrromethene (BODIPY) belongs to a family of organoboron compounds, which displays good photostability and excellent NLO properties [
24,
25,
26]. As various BODIPY derivatives, Aza-BODIPY derivatives possess superior optical properties, including red-shifted spectra and high molar extinction coefficients [
27,
28]. Interestingly, through rational molecular design, the absorption and emission spectra of Aza-BODIPY derivatives can shift towards NIR-II biological window (1000-1350 nm), which are extremely attractive for various applications [
29]. Up to now, there are only several papers reporting the NLO properties of Aza-BODIPY derivatives. For example, Chang et al. determined the values of two-photon absorption (2PA) and excited-state absorption (ESA) cross-sections of four Aza-BODIPY derivatives [
27]. Liu et al. theoretically investigated 2PA of functionalized Aza-BODIPY derivatives at telecommunication wavelengths [
30]. It was also confirmed that Aza-BODIPY derivatives can be used as potential optical limiter [
31,
32]. However, the study on the NLO properties of Aza-BODIPY derivatives is still very insufficient. For example, there is no literature on the nonlinear refraction and saturation absorption effects of these dyes, which is not conducive to the expansion of relevant applications, or to the understanding of the relationship between NLO properties and molecular structures and excitation wavelength. Therefore, there is an obvious need for further investigation on the influences of the molecular structures and excitation wavelength on the NLO properties of Aza-BODIPY dyes.
In this work, we reported the NLO properties of two Aza-BODIPY derivatives, which are abbreviated as BDP-1 and BDP-2, in which two electron-donating groups 4-(N, N-dimethylamino) phenyl and 1-ethyl-1,2,3,4-tetrahydro- quinoline were connected to the 3 and 5 positions of Aza-BODIPY cores. Actually, Bai et al. reported these two derivatives exhibit effective NIR emission and strong intramolecular charge transfer, implying they may have significant NLO effects [
33]. Therefore, we conducted further research on their NLO properties. The experimental results show that both two derivatives exhibit strong saturable absorption at 800 nm. Meanwhile, benefitting from the stronger electron donating ability, Aza-BODIPY-2 possesses larger nonlinear refractive index at 1300 nm and effective two-photon brightness in the wavelength range of 1200 to 1600 nm.
2. Results and Discussion
2.1. The UV-visible absorption and emission spectra
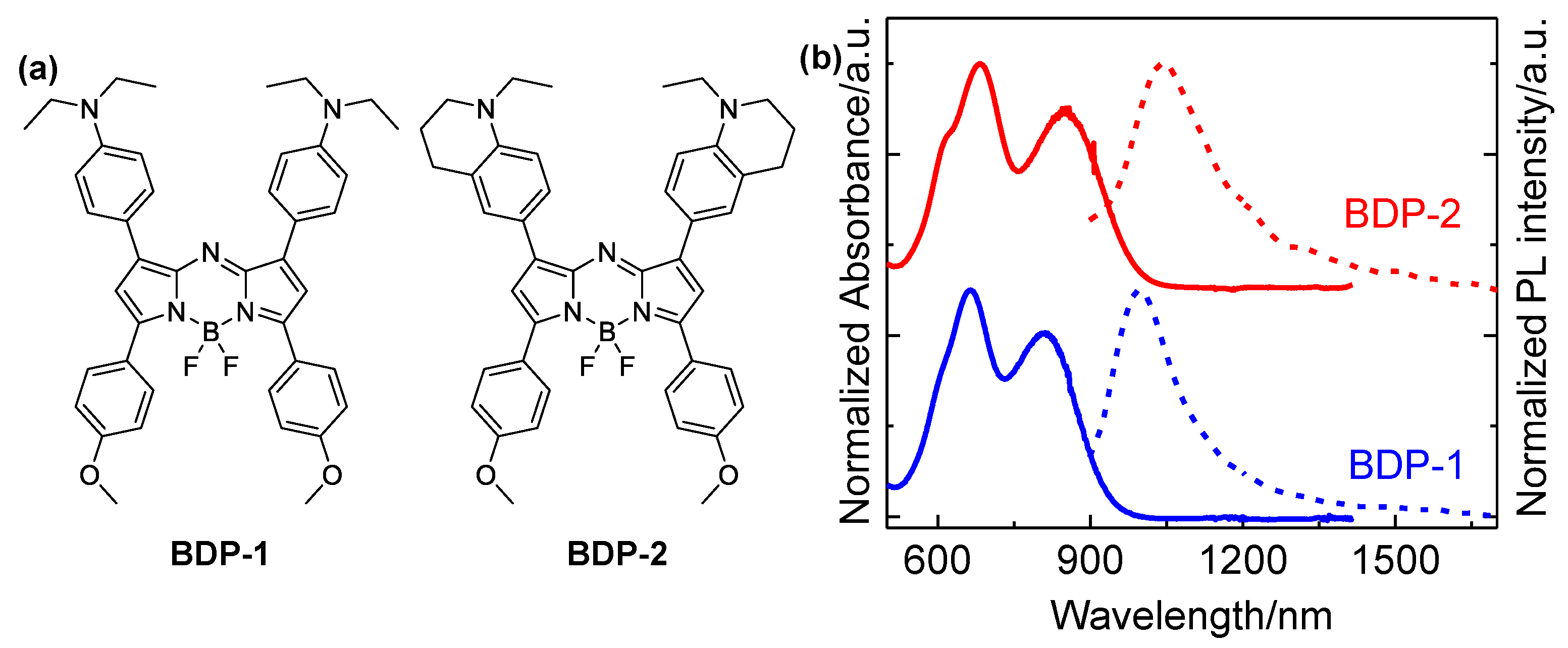
Figure 1a shows the structures of two Aza-BODIPY derivatives studied in this work, while their absorption and emission spectra are depicted in
Figure 1b. It can be seen that both two samples exhibit two main absorption bands, which are peaking at 690 and 800 nm for BDP-1, and 700 and 860 nm for BDP-2, respectively. Excited by the pulses at 350 nm, the fluorescence emission spectra of two derivatives are peaking at 1000 and 1050 nm, respectively. The photoluminescence quantum yields (PLQYs) were 0.9% and 0.6%, respectively. Importantly, the derivatives emit in the NIR-II biological window. Compared with fluorescence imaging in the NIR-I biological window (650 - 950 nm), NIR-II fluorescence imaging can afford higher spatial resolution, deeper penetration depth into a living body [
34]. In addition, two Aza-BODIPY derivatives have large Stokes shifts,
i.e, 187 nm (53476 cm
-1) vs 194 nm (51546 cm
-1), which is an additional advantage for fluorescence imaging in organisms. It was also found that the absorption and emission peaks of BDP-2 shift toward longer wavelengths compared to BDP-1, as a result of the stronger electron-donating ability of 1-ethyl-1,2,3,4-tetrahydroquinoline group in the former [
35,
36].
2.2. Ultrafast dynamics
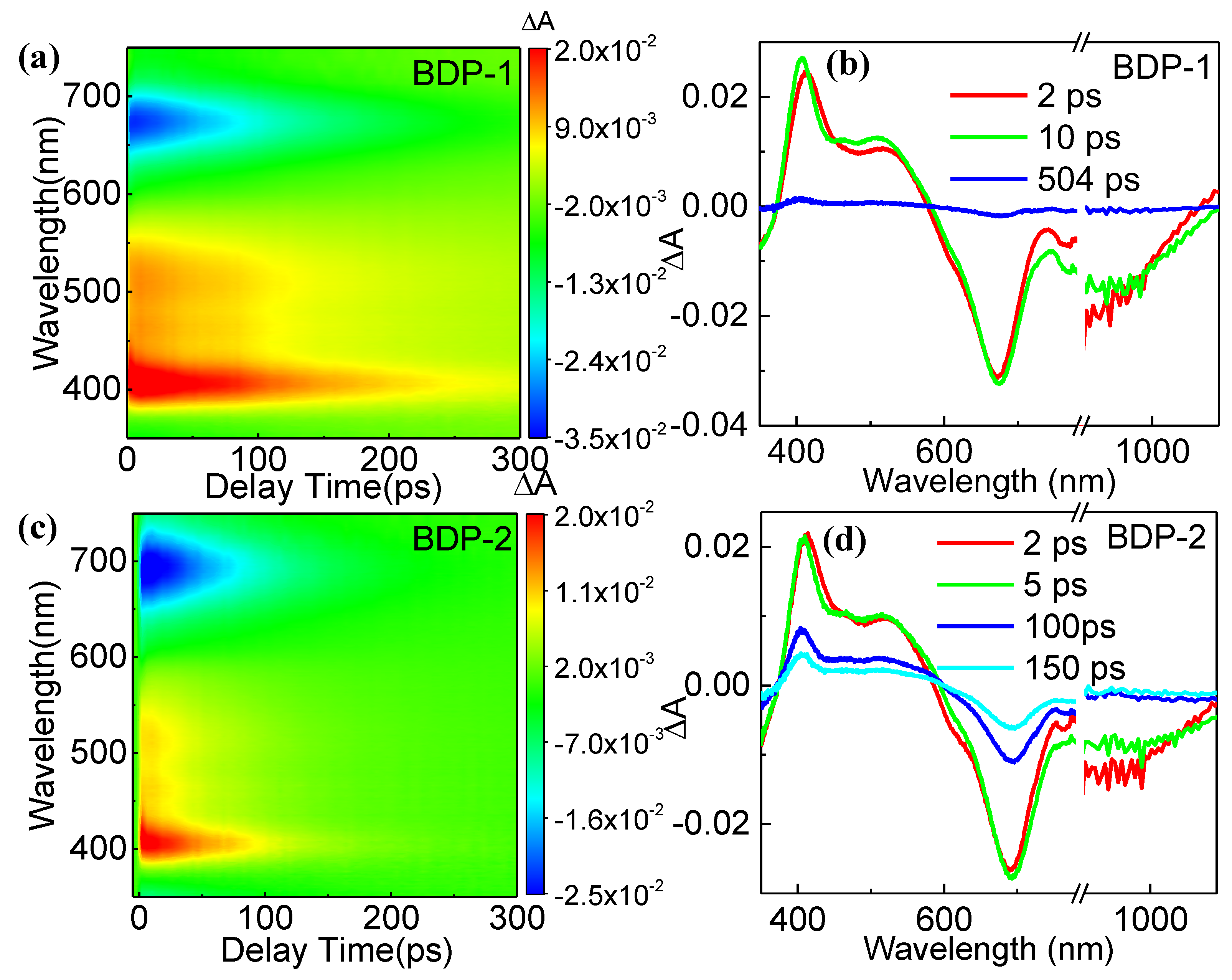
Next, we investigated the carrier dynamics of two derivatives.
Figure 2a and
Figure 2c shows the 2D color images of femtosecond-transient absorption (fs-TA) spectra of two derivatives under the excitation wavelength of 800 nm, while their corresponding spectra at selected delay times are depicted in
Figure 2b and
Figure 2d, respectively. It can be seen that two derivatives exhibit similar signals, in which positive signals appear within the range of 400 - 580 nm, caused by the ESA. Meanwhile, negative signals were observed within the range of 600 - 950 nm. According to the absorption spectrum, we believe these negative signals arise from the ground-state bleaching effect. Two derivatives exhibit negative signals in the wavelength range above 1000 nm, corresponding to the PL spectra. Thus, these negative signals are caused by the stimulated emission. The ESA peaks of two derivatives in the range of 400 - 580 nm exhibit a slight blue shift with increasing time. Possibly, it is caused by the solvent effect in that the polarization degrees of molecules under an excited state will be enhanced [
37]. BDP-2 shows a more significant blue shift (10 nm) than that (7 nm) of BDP-1, indicating a higher polarization degree of excitation state that may induce a more significant NLO effect in the former.
2.3. NLO properties at different excitation wavelengths
2.3.1. Resonant NLO properties at 800 nm
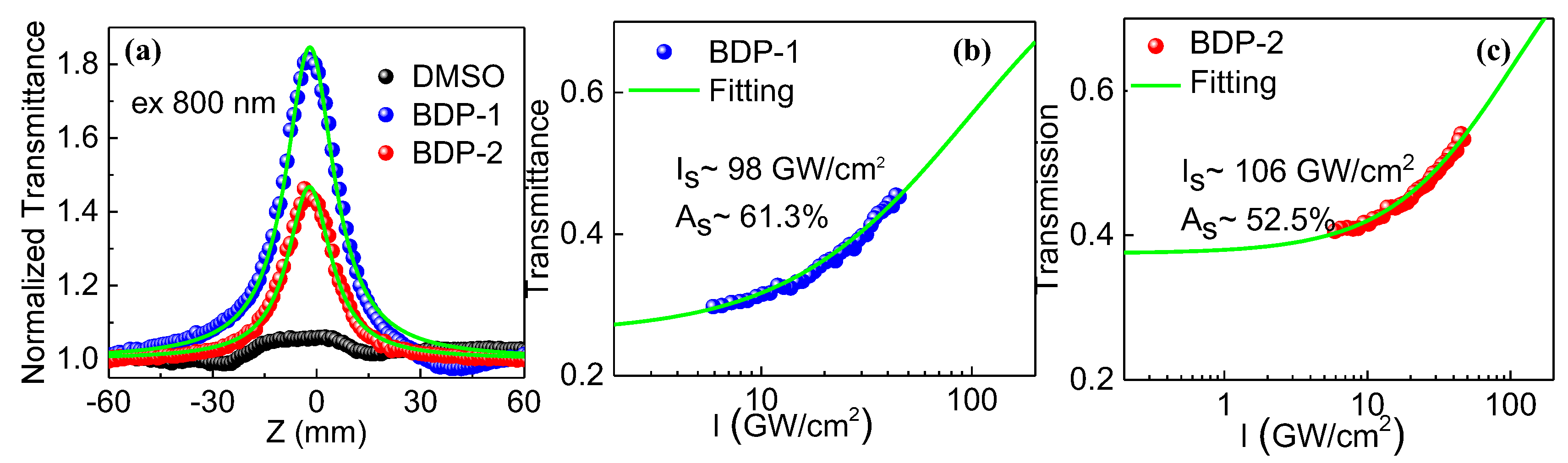
Because two derivatives possess strong linear absorption below 860 nm, it was expected that strong nonlinear absorption effect may occur within the absorption band. Then, using femtosecond pulses of 800 nm (1000 Hz, 100 fs) as an excitation light source, we measured the open aperture Z-scan data of the DMSO solutions of two derivatives with a concentration of around 1.3 × 10
-3 M (
Figure 3a). The incident light intensity was around 76.4 GW/cm
2. Under the same incident light intensity, derivatives exhibit strong saturable absorption effect, while no obvious signals were observed in the DMSO solvent.
In the open-aperture experiment, the simplified expression formula of normalized transmittance can be described as follows [
38]:
where β (in unit of cm/GW) is the nonlinear absorption coefficients. I
0 (in unit of GW/cm
2) is the light intensity at the focal point (z=0). L
eff (in unit of cm) is the effective length of the sample, which is expressed as L
eff=(1-e
-αL)/L, with the linear absorption coefficient α and the physical thickness L. z
0=kω
0/2 is the diffraction length, in which k=2π/λ is the wave number and ω
0 is the spot radius at the focal point. By fitting the experimental data with Equation (1), the nonlinear absorption coefficients (β) of BDP-1 and BDP-2 in DMSO solutions can be determined as -0.30 and -0.16 cm/GW. Considering the NLO properties of sample solutions were influenced by the concentration, it will be more objective to compare the intrinsic NLO parameters among different samples according to following equation [
39]:
in which
x (solution) is the total NLO parameters of the sample solution, including nonlinear absorption coefficient, nonlinear refractive index.
f is the volume fraction of the derivatives relative to DMSO.
x (solvent) is the NLO parameters of DMSO.
x (intrinsic) is the intrinsic NLO parameters of the derivatives. As a result, the intrinsic nonlinear coefficients of BDP-1 and BDP-2 were determined, i.e., β (intrinsic) -1.48 × 10
4 and -8.26 × 10
3 cm/GW, respectively. Considering the 4-(N, N-dimethylamino) phenyl group has weaker electron-donating ability than the 1-ethyl-1,2,3,4-tetrahydroquinoline groups, the larger β (intrinsic) of BDP-1 should be caused by its stronger absorption at 800 nm.
To determine the saturable absorption intensity (I
s) and modulation depth (A
S) of two derivatives, which are two important parameters for the Q-switching or mode-locking of fiber lasers, we measured their transmittances under different excitation intensities, as shown in
Figure 3b and
Figure 3c. It can be seen that with the increase of incident light intensity their transmittances gradually increase, indicating a saturable absorption effect, which is consistent with the Z-scan data. I
s and A
S of two derivatives were estimated,
i.e., 98 GW/cm
2 and 61.3% for BDP-1 and 106 GW/cm
2 and 52.5% for BDP-2, according to the following equation [
40]:
where I is the input intensity; A
S is the modulation depth; I
s is the saturable absorption; n
s is the nonsaturable loss. In addition, BDP-1 has larger modulation depth and smaller saturable absorption intensity, implying this derivative is more promising for the application in Q-switching or mode-locking of fiber lasers. In order to facilitate comparison, the NLO parameters at 800 nm for two derivatives were compared in
Table 1.
2.3.2. Nonresonant NLO properties at 1300 nm
Considering two derivatives have negligible absorption at 1300 nm, it is interesting to investigate their nonlinear refraction effect and 2PA. From the closed-aperture Z-scan experimental data divided by corresponding open-aperture ones, as shown in
Figure 4a, it can be seen that the solutions of two derivatives exhibit a self-defocusing effect, while the DMSO solvent exhibits a self-focusing effect. The experimental data in
Figure 4a can be theoretically fitted using following formula [
41]:
where ΔΦ
0(t)=kΔnL
eff is the nonlinear phase shift at the focal point and Δn=n
2I
0. n
2 is the nonlinear refractive index, while x=z/z
0 is the ratio of the sample position z to the diffraction length z
0. As a result, the nonlinear refractive indices of BDP-1 and BDP-2 in derivative solutions, and pure DMSO solvent were determined to as -3.36 × 10
-7, -4.48 × 10
-7 and 7.71 × 10
-7 cm
2/GW. Although the solution concentrations of two derivatives were much low, their nonlinear refractive indices were only several times smaller than that of carbon disulfide (n
2 ~ 3.0 × 10
-6 cm
2/GW). With the influence of DMSO solvent subtracted according to Equation (3), the intrinsic nonlinear refractive indices of two derivatives were estimated, i.e., n
2 (intrinsic) - 7.60 × 10
-3 cm
2/GW for BDP-1 and -7.85 × 10
-3 cm
2/GW for BDP-2, respectively. Furthermore, they are even higher than those of organic molecules reported in a lot of NLO materials, including Zn-terpyridine polymer (~ 1.1 × 10
-4 cm
2/GW at 765 nm) [
42] and crystalline nickel-p-benzenedicarboxylic acid MOF (Ni-MOF)(~ - 8.9 × 10
-7 cm
2/GW at 1550 nm) [
14].
The open aperture Z-scan data of two derivatives under the excitation of femtosecond pulses of 1300 nm (an excitation light intensity of 337 GW/cm
2) are shown in
Figure 4b. It can be seen that the signal of solvent can be ignored, and two samples have the smallest transmittances at the focal point, which is caused by 2PA. According to the Equation (1), the 2PA coefficients of BDP-1 and BDP-2 were estimated as 3.35 × 10
-3 and 4.19 × 10
-3 cm/GW, respectively. In addition, with the help of Equation (2), the intrinsic 2PA coefficients of two derivatives were determined as 165 and 213 cm GW
-1. 2PA cross-section (σ
2) that is in unit of GM, with 1 GM= 10
-50 cm
4 s
-1 photon
-1, can be determined according to the following equation [
38],
where h is the Planck's constant in unit of J·s; υ is the incident light frequency in unit of 1/s, N
A is Avogadro’s number and C
0 is concentration in unit of M/L.
As a result, 2PA cross-sections of BDP-1 and BDP-2 can be estimated as 59 and 77 GM at 800 nm, respectively, as summarized in
Table 2. It can be concluded that BDP-2 exhibits larger 2PA cross-section compared to that of BDP-1, as a result of stronger electron-donating ability of the 1-ethyl-1,2,3,4-tetrahydroquinoline groups [
43].
To realize the application in all-optical switching, the NLO materials should have strong nonlinear refraction but weak nonlinear absorption. To realize such an application, two Stegeman’s figures of merit should satisfy following conditions [
44,
45]:
Since linear absorption of two derivatives are negligible at 1300 nm, their W values are larger than 1. In addition, based on the experimental data, T is estimated as 1.06 for BDP-1 and 0.652 for BDP-2. Therefore, BDP-2 may have potential application in all-optical device applications.
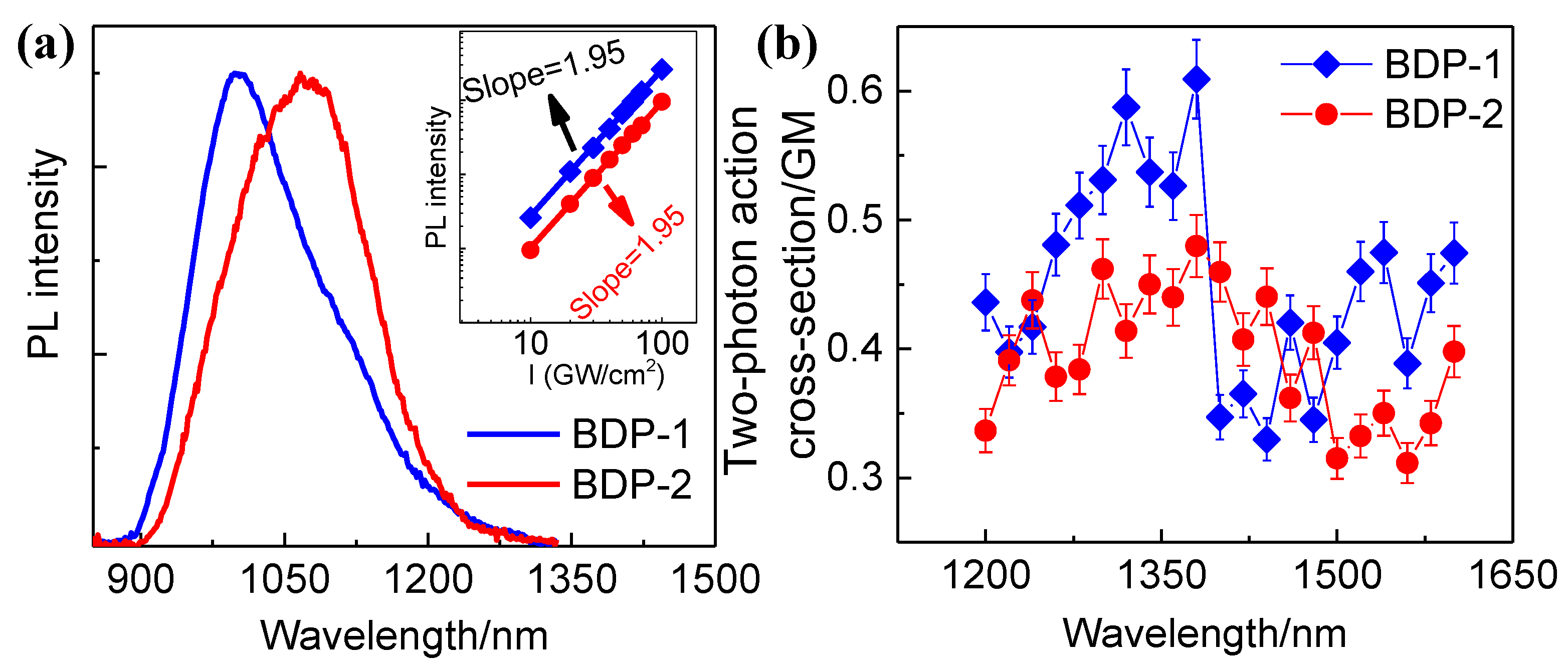
Meanwhile, in order to avoid the influence of linear absorption, we measured the PL spectra of two derivatives under 1400 nm excitation, which are peaking at 1000 and 1060 nm, respectively, as shown in
Figure 5a. The spectra are similar to those under single-photon excitation, indicating that the same emission states are involved in two cases. The inset shows the relationship between the PL intensity and excitation power. The slopes around 2 for the best-fitting straight lines on logarithmic scales indicate the occurrence of 2PA. We repeated Z-scan measurements at different wavelengths and obtained their 2PA cross-sections within the range of 1200 - 1600 nm. The molecular probes with large 2PA action cross-sections (the production of a 2PA cross section and PLQY), which is also called two-photon fluorescence brightness, will be beneficial for the two-photon bioimaging. Therefore, 2PA action cross-sections of two derivatives at different wavelengths are calculated and presented in
Figure 5b. The moderate 2PA action cross-sections of BDP-2 and PL emission in NIR-II window make it to be promising in deep-tissue bioimaging.
3. Experimental Section
3.1. Synthesis of Aza-BODIPY derivatives
The detailed synthesis procedures were described in Ref. 34. Briefly, BDP-1 or BDP-2 were designed by introducing 4-(N, N-dimethylamino) phenyl or 1-ethyl-1,2,3,4-tetrahydroquinolinyl into the 3 and 5 positions of aza-BODIPY core as electron-donating groups, respectively. In addition, the electron-rich 4-anisoly was incorporated to the 1 and 7 positions of electron-withdrawing aza-BODIPY core. As a result, both BDP-1 and BDP-2 possess a D-A-D structure. Consequently, a metallic brown solid (yield: 90%) was obtained for BDP-1, while a metallic brown solid (yield: 90%) was obtained for BDP-2.
3.2. Linear optical measurements
The UV-visible absorption spectra of the Aza-BODIPY derivatives were measured using an ultraviolet-visible-NIR spectrophotometer (Lambda 950). Meanwhile, PL spectra and PLQYs were determined with a FluoroSENS fluorescence spectrometer equipped with a 150 W xenon lamp and an integrating sphere accessory. During the measurements, the samples were dissolved in DMSO, and the solutions obtained with a concentration of 1.3 × 10-5 M were filled into 1 cm cuvettes.
3.3. fs-TA spectrum
During the measurements, the pump pulses at 800 nm were generated with an optical parametric amplifier system (Spectra-physics Spitfire Ace) that was combined with TOPAS. Meanwhile, the probe pulses in the wavelength ranges of 350 – 750 nm or 950 – 1200 nm were generated by a YAG crystal or CaF2 crystal, respectively. The sample solutions were filled in quartz liquid cells with a thickness of 1 mm. To eliminate any contribution from coherent artifacts, the linear polarization of the pump pulse was adjusted to be perpendicular to that of the probe pulse. Pump-induced changes of transmission (Δ𝑇/𝑇) of the probe beam were monitored using a monochromator/photomultiplier configuration with lock-in detection.
3.4. NLO characterizations
The NLO parameters of two derivatives at 800 nm and 1200-1600 nm were determined using Z-scan technique [
41]. Because of the ultrashort pulse width (100 fs) and low repetition frequency (1000 Hz) of the excitation light, the thermal effect (occurring within the ns range) can be neglected in the measurements of NLO parameters [
46,
47]. Before conducting the measurement, the CS
2 was used as reference to calibrate the Z-scan system, and the obtained n
2 value of CS
2 (~4.53 × 10
-6 cm
2/GW) is consistent with that in previous literature [
48], confirming the reliability of our Z-scan experiment. Generally, n
2 and β can be obtained by the measurement of the normalized transmittance for the closed and open aperture versus sample position. If there is nonlinear absorption existing in the samples, the closed transmittance is affected by both n
2 and β. The determination of n
2 is less straight-forward from the closed aperture Z-scan curve. It is necessary to separate the effect of nonlinear absorption by dividing closed-aperture Z-scan experimental data by corresponding open-aperture ones. For the I-scan measurements, the samples were fixed at the focal point, and the incident light intensity was continuously changed by computer-controlled electric attenuator. For the measurements of multiphoton excited PL spectra, the femtosecond pulses with wavelengths from 1200 to 1600 nm were used as the excitation source and the signals were vertically collected with a compact spectrometer (NIRQuest 512).
4. Conclusions
In this work, we studied the NLO properties of two Aza-BODIPY derivatives, with their PL emission wavelengths in NIR-II window. The experimental results indicate that two derivatives possess strong saturable absorption and large modulation depth under the excitation at 800 nm, while they exhibit strong nonlinear refraction at 1300 nm. Interestingly, two derivatives have moderate 2PA action cross-sections in the wavelength range of 1200-1600 nm. The excellent NLO properties of the Aza-BODIPY derivatives implied they are promising for various application, including Q-switching or mode-locking of pulse lasers, all-optical switching, deep tissue bioimaging in NIR-II window.
Author Contributions
Conceptualization, T.H.; methodology, validation, formal analysis, C.R. and S.X.; investigation, C.R., S.X. and Y.C.; resources, T.H.; data curation, writing—original draft preparation, C.R. and S.X.; writing—review and editing, and visualization, C.R. and T.H.; supervision, X.L., J.H. and T.H.; project administration, T.H.; funding acquisition, C.R. and T.H.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Guangdong Basic and Applied Basic Research Foundation (2022A1515011246), the Science and Technology Planning Project of Shenzhen Municipality (JCYJ20210324094414039), and the China Postdoctoral Science Foundation (2021TQ0214).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nguyen, V.-N.; Ha, J.; Cho, M.; Li, H.; Swamy, K.M.K.; Yoon, J. Recent developments ofBODIPY-based colorimetric and fluorescent probes for the detection of reactive oxygen/nitrogen species and cancer diagnosis. Coord. Chem. Rev. 2021, 439, 213936. [Google Scholar] [CrossRef]
- Ghanavatkar, C.W.; Mishra, V.R.; Sekar, N. Review of NLOphoric azo dyes – Developments in hyperpolarizabilities in last two decades. Dyes Pigm. 2021, 191, 109367. [Google Scholar] [CrossRef]
- Wang, W.; Yue, W.; Liu, Z.; Shi, T.; Du, J.; Leng, Y.; Wei, R.; Ye, Y.; Liu, C.; Liu, X.; et al. Ultrafast Nonlinear Optical Response in Plasmonic 2D Molybdenum Oxide Nanosheets for Mode-Locked Pulse Generation. Adv. Opt. Mater. 2018, 6, 1700948. [Google Scholar] [CrossRef]
- Zhang, C.; Ouyang, H.; Miao, R.; Sui, Y.; Hao, H.; Tang, Y.; You, J.; Zheng, X.; Xu, Z.; Cheng, X.a.; et al. Anisotropic Nonlinear Optical Properties of a SnSe Flake and a Novel Perspective for the Application of All-Optical Switching. Adv. Opt. Mater. 2019, 7, 1900631. [Google Scholar] [CrossRef]
- Tian, Y.-B.; Li, Q.-H.; Wang, Z.; Gu, Z.-G.; Zhang, J. Coordination-Induced Symmetry Breaking on Metal-Porphyrinic Framework Thin Films for Enhanced Nonlinear Optical Limiting. Nano Lett. 2023, 23, 3062–3069. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Yuan, Y.; Wan, Y.; Li, J.; Song, Y.; Chen, W.-C.; Zhao, D.; Chi, Y.; Li, M.; Lee, C.-S.; et al. Near-Infrared Thermally Activated Delayed Fluorescence Nanoparticle: A Metal-Free Photosensitizer for Two-Photon-Activated Photodynamic Therapy at the Cell and Small Animal Levels. Small 2022, 18, 2106215. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Okawachi, Y.; Griffith, A.G.; Picqué, N.; Lipson, M.; Gaeta, A.L. Silicon-chip-based mid-infrared dual-comb spectroscopy. Nat. Commun. 2018, 9, 1869. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, C.-L.; Kong, F.; Mao, J.-G. High-Performance Second-Harmonic-Generation (SHG) Materials: New Developments and New Strategies. Acc. Chem. Res. 2021, 54, 2775–2783. [Google Scholar] [CrossRef] [PubMed]
- Beharry, A.A.; Woolley, G.A. Azobenzene photoswitches for biomolecules. Chem. Soc. Rev. 2011, 40, 4422–4437. [Google Scholar] [CrossRef]
- Ren, C.; Xiao, S.; Li, J.; Ma, L.; Chen, R.; Ye, C.; Gao, Y.; Su, C.; He, T. Large Nonlinear Optical Activity of a Near-infrared-absorbing Bithiophene-based Polymer with a Head-to-head Linkage. Chemistry – An Asian Journal 2021, 16, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Achelle, S.; Verbitskiy, E.V.; Fecková, M.; Bureš, F.; Barsella, A.; Robin-le Guen, F. V-Shaped Methylpyrimidinium Chromophores for Nonlinear Optics. ChemPlusChem 2021, 86, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Hu, D.; Cui, Y.; Chen, P.; Xu, X.; Cheng, J.; He, T. Ag-doped InP/ZnS quantum dots for type-I photosensitizers. Chem. Commun. 2023, 59, 2311–2314. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ren, C.; Qiu, X.; Lin, X.; Chen, R.; Yin, C.; He, T. Ultrafast optical nonlinearity of blue-emitting perovskite nanocrystals. Photonics Res. 2018, 6, 554–559. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, L.; Liu, S.; Zhang, Y.; He, Z.; Li, W.; Zhang, F.; Shi, Y.; Lü, W.; Li, Y.; et al. Ultrathin Metal–Organic Framework: An Emerging Broadband Nonlinear Optical Material for Ultrafast Photonics. Adv. Opt. Mater. 2018, 6, 1800561. [Google Scholar] [CrossRef]
- Yu, J.; Kuang, X.; Li, J.; Zhong, J.; Zeng, C.; Cao, L.; Liu, Z.; Zeng, Z.; Luo, Z.; He, T.; et al. Giant nonlinear optical activity in two-dimensional palladium diselenide. Nat. Commun. 2021, 12, 1083. [Google Scholar] [CrossRef]
- Wen, K.; Tan, H.; Peng, Q.; Chen, H.; Ma, H.; Wang, L.; Peng, A.; Shi, Q.; Cai, X.; Huang, H. Achieving Efficient NIR-II Type-I Photosensitizers for Photodynamic/Photothermal Therapy upon Regulating Chalcogen Elements. Adv. Mater. 2022, 34, 2108146. [Google Scholar] [CrossRef]
- Liu, J.; Ouyang, C.; Huo, F.; He, W.; Cao, A. Progress in the enhancement of electro-optic coefficients and orientation stability for organic second-order nonlinear optical materials. Dyes Pigm. 2020, 181, 108509. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Liu, J.; Chen, C.; Liu, B. NIR-II Light Activated Photosensitizer with Aggregation-Induced Emission for Precise and Efficient Two-Photon Photodynamic Cancer Cell Ablation. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Yu, P.; Yan, K.; Wang, S.; Yao, C.; Lei, Z.; Tang, Y.; Zhang, F. NIR-II Dyad-Doped Ratiometric Nanosensor with Enhanced Spectral Fidelity in Biological Media for In Vivo Biosensing. Nano Lett. 2022, 22, 9732–9740. [Google Scholar] [CrossRef]
- Liu, P.; Mu, X.; Zhang, X.-D.; Ming, D. The Near-Infrared-II Fluorophores and Advanced Microscopy Technologies Development and Application in Bioimaging. Bioconjugate Chem. 2020, 31, 260–275. [Google Scholar] [CrossRef]
- Guo, X.; Tang, B.; Wu, Q.; Bu, W.; Zhang, F.; Yu, C.; Jiao, L.; Hao, E. Engineering BODIPY-based near-infrared nanoparticles with large Stokes shifts and aggregation-induced emission characteristics for organelle specific bioimaging. J. Mater. Chem. B 2022, 10, 5612–5623. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-W.; Jiang, X.-M.; Zeng, H.-Y.; Guo, G.-C. [ABa2Cl][Ga4S8] (A = Rb, Cs): Wide-Spectrum Nonlinear Optical Materials Obtained by Polycation-Substitution-Induced Nonlinear Optical (NLO)-Functional Motif Ordering. J. Am. Chem. Soc. 2020, 142, 10641–10645. [Google Scholar] [CrossRef]
- Kang, D.; Li, R.; Cao, S.; Sun, M. Nonlinear optical microscopies: physical principle and applications. Appl. Spectrosc. Rev. 2021, 56, 52–66. [Google Scholar] [CrossRef]
- Li, K.; Duan, X.; Jiang, Z.; Ding, D.; Chen, Y.; Zhang, G.-Q.; Liu, Z. J-aggregates of meso-[2.2]paracyclophanyl-BODIPY dye for NIR-II imaging. Nat. Commun. 2021, 12, 2376. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Deng, X.; Hu, W.; Li, J.; Miao, X.; Xiao, S.; Liu, H.; Fan, Q.; Wang, K.; He, T. A near-infrared I emissive dye: toward the application of saturable absorber and multiphoton fluorescence microscopy in the deep-tissue imaging window. Chem. Commun. 2019, 55, 5111–5114. [Google Scholar] [CrossRef]
- Chang, H.-J.; Bondar, M.V.; Munera, N.; David, S.; Maury, O.; Berginc, G.; Le Guennic, B.; Jacquemin, D.; Andraud, C.; Hagan, D.J.; et al. Femtosecond Spectroscopy and Nonlinear Optical Properties of aza-BODIPY Derivatives in Solution. Chem.-Eur. J. 2022, 28, e202104072. [Google Scholar] [CrossRef] [PubMed]
- Merkes, J.M.; Lammers, T.; Kancherla, R.; Rueping, M.; Kiessling, F.; Banala, S. Tuning Optical Properties of BODIPY Dyes by Pyrrole Conjugation for Photoacoustic Imaging. Adv. Opt. Mater. 2020, 8, 1902115. [Google Scholar] [CrossRef]
- Gurubasavaraj, P.M.; Sajjan, V.P.; Muñoz-Flores, B.M.; Jiménez Pérez, V.M.; Hosmane, N.S. Recent Advances in BODIPY Compounds: Synthetic Methods, Optical and Nonlinear Optical Properties, and Their Medical Applications. Molecules 2022, 27, 1877. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, T.; Sun, C.; Meng, Y.; Xiao, L. Synthesis of aza-BODIPY dyes bearing the naphthyl groups at 1,7-positions and application for singlet oxygen generation. Chin. Chem. Lett. 2019, 30, 1055–1058. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Li, K.; Sun, X.; Wu, Z.; Ren, A.; Feng, J. New insights into two-photon absorption properties of functionalized aza-BODIPY dyes at telecommunication wavelengths: a theoretical study. Phys. Chem. Chem. Phys. 2013, 15, 4666–4676. [Google Scholar] [CrossRef]
- David, S.; Chang, H.-J.; Lopes, C.; Brännlund, C.; Le Guennic, B.; Berginc, G.; Van Stryland, E.; Bondar, M.V.; Hagan, D.; Jacquemin, D.; et al. Benzothiadiazole-Substituted Aza-BODIPY Dyes: Two-Photon Absorption Enhancement for Improved Optical Limiting Performances in the Short-Wave IR Range. Chem.-Eur. J. 2021, 27, 3517–3525. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Chateau, D.; Chang, H.-J.; Karlsson, L.H.; Bondar, M.V.; Lopes, C.; Le Guennic, B.; Jacquemin, D.; Berginc, G.; Maury, O.; et al. High-Performance Optical Power Limiting Filters at Telecommunication Wavelengths: When Aza-BODIPY Dyes Bond to Sol–Gel Materials. J. Phys. Chem. C 2020, 124, 24344–24350. [Google Scholar] [CrossRef]
- Bai, L.; Sun, P.; Liu, Y.; Zhang, H.; Hu, W.; Zhang, W.; Liu, Z.; Fan, Q.; Li, L.; Huang, W. Novel aza-BODIPY based small molecular NIR-II fluorophores for in vivo imaging. Chem. Commun. 2019, 55, 10920–10923. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.F.; Tao, H.; Hu, W.; Pan, Y.; Fan, Q.; Huang, W. Elucidating the excited-state dynamics behavior in near-infrared Bodipy dye and aggregates toward biophotonics. Sci. China Chem. 2020, 63, 1075–1081. [Google Scholar] [CrossRef]
- Tekin, S.; Küçüköz, B.; Yılmaz, H.; Sevinç, G.; Hayvalı, M.; Gul Yaglioglu, H.; Elmali, A. Enhancement of two photon absorption properties by charge transfer in newly synthesized aza-boron-dipyrromethene compounds containing triphenylamine, 4-ethynyl-N,N-dimethylaniline and methoxy moieties. J. Photochem. Photobiol., A 2013, 256, 23–28. [Google Scholar] [CrossRef]
- Karatay, A.; Yılmaz, H.; Yildiz, E.A.; Sevinç, G.; Hayvali, M.; Boyacioglu, B.; Unver, H.; Elmali, A. Two-photon absorption and triplet excited state quenching of near-IR region aza-BODIPY photosensitizers via a triphenylamine moiety despite heavy bromine atoms. Phys. Chem. Chem. Phys. 2022, 24, 25495–25505. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Castner, E.W. Intermolecular Dynamics of Substituted Benzene and Cyclohexane Liquids, Studied by Femtosecond Nonlinear-Optical Polarization Spectroscopy. The Journal of Physical Chemistry 1996, 100, 3330–3343. [Google Scholar] [CrossRef]
- Mushtaq, A.; Kushavah, D.; Ghosh, S.; Pal, S.K. Nonlinear optical properties of benzylamine lead(II) bromide perovskite microdisks in femtosecond regime. Appl. Phys. Lett. 2019, 114, 051902. [Google Scholar] [CrossRef]
- He, G.S.; Zhu, J.; Baev, A.; Samoć, M.; Frattarelli, D.L.; Watanabe, N.; Facchetti, A.; Ågren, H.; Marks, T.J.; Prasad, P.N. Twisted π-System Chromophores for All-Optical Switching. J. Am. Chem. Soc. 2011, 133, 6675–6680. [Google Scholar] [CrossRef]
- Dong, N.; Li, Y.; Zhang, S.; McEvoy, N.; Gatensby, R.; Duesberg, G.S.; Wang, J. Saturation of Two-Photon Absorption in Layered Transition Metal Dichalcogenides: Experiment and Theory. ACS Photonics 2018, 5, 1558–1565. [Google Scholar] [CrossRef]
- Sheikbahae, M.; Said, A.A.; Wei, T.H.; Hagan, D.J.; Vanstryland, E.W. Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 1990, 26, 760–769. [Google Scholar] [CrossRef]
- He, T.; Too, P.C.; Chen, R.; Chiba, S.; Sun, H. Concise Synthesis and Two-Photon-Excited Deep-Blue Emission of 1,8-Diazapyrenes. Chem.-Asian J. 2012, 7, 2090–2095. [Google Scholar] [CrossRef]
- Yager, K.G.; Barrett, C.J. Novel photo-switching using azobenzene functional materials. J. Photochem. Photobiol., A 2006, 182, 250–261. [Google Scholar] [CrossRef]
- Mizrahi, V.; DeLong, K.W.; Stegeman, G.I.; Saifi, M.A.; Andrejco, M.J. Two-photon absorption as a limitation to all-optical switching. Opt. Lett. 1989, 14, 1140–1142. [Google Scholar] [CrossRef]
- Zhao, P.; Reichert, M.; Hagan, D.J.; Van Stryland, E.W. Dispersion of nondegenerate nonlinear refraction in semiconductors. Opt. Express 2016, 24, 24907–24920. [Google Scholar] [CrossRef] [PubMed]
- Kovsh, D.I.; Hagan, D.J.; Stryland, E.W.V. Numerical modeling of thermal refraction in liquids in the transient regime. Opt. Express 1999, 4, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, K.; Lyu, B.; Zhang, J.; Wang, Y.; Wang, P.; Xiao, S.; Gao, Y.; He, J. Enhanced Nonlinear Optical Response of Rectangular MoS2 and MoS2/TiO2 in Dispersion and Film. J. Phys. Chem. C 2016, 120, 18243–18248. [Google Scholar] [CrossRef]
- Ganeev, R.A.; Ryasnyansky, A.I.; Baba, M.; Suzuki, M.; Ishizawa, N.; Turu, M.; Sakakibara, S.; Kuroda, H. Nonlinear refraction in CS2. Appl. Phys. B 2004, 78, 433–438. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).