1. Introduction
For the first time in history, humankind is confronted with a major crisis related to climate change, energy demands, and power sources. The continuous human population growth together with the carbon-based energy economy has led to a striking production of greenhouse gasses. The dominant greenhouse gas, carbon dioxide (CO
2), has increased by 149% since the pre-industrial period, reaching values higher than 410 parts per million in 2020 according to the World Meteorological Organization (WMO) Greenhouse Gas Bulletin [
1]. The rise of carbon dioxide emissions, due to anthropogenic activity, has led policymakers, the scientific community and global industry to focus on effective methods of controlling and managing processes associated with CO
2 emissions.
The direction of geologic sequestration of CO
2 involves risks, such as inadequate underground volumes for the necessary CO
2 quantities to reach net negative emissions [
2]. In addition, the risk of leakage, the induced seismicity, and the cost of CO
2 capture from the stationary power plants [
3,
4] are additional factors that lead the community to study alternative routes of CO
2 utilization, mainly to the transformation of CO
2 into value added chemicals and high energy fuels, mainly through the reaction of CO
2 with hydrogen [
5,
6,
7,
8].
Therefore, the production of useful fuels from the CO
2 hydrogenation reaction (such as methane, syngas, methanol, ethanol, formic acid, etc. [
5,
9,
10]), has evolved to an important research area, including the challenge associated with it like physical (sintering, fouling, and attrition) and chemical deactivation (poisoning or vapor-solid reaction) of the catalyst [
10,
11], the origin of the necessary for the reaction hydrogen [
10], as well as the reactor operating conditions [
7].
The Reverse Water- Gas Shift reaction (RWGS) is an important reaction which may occur over a hydrogenation catalyst when co-feeding of CO
2 and H
2:
The above reaction, leading to syngas production, is mildly endothermic and favored at high temperatures. The inability of utilizing active RWGS catalysts at low temperatures due to the thermodynamic limitations (CO production is predicted at temperatures higher than 700 °C) [
12] poses a problem. Two are the main popular mechanisms suggested in the literature for the RWGS reaction which comprehensively depend on whether the dissociated H
2 species are involved in the formation of the carbon-containing intermediates [
13]. The first one (surface redox mechanism) involves the oxidation of the catalyst through the dissociative adsorption of the CO
2 to carbonyl (CO
ads) and O
ads followed by the desorption of CO and catalyst reduction by dissociatively adsorbed hydrogen (H
ads) to form H
2O. The second mechanism (associative mechanism) proposes that the adsorbed on the catalytic surface CO
2 reacts directly with dissociatively adsorbed hydrogen (H
ads) to form intermediate species, such as formate (HCOO), carboxyl (COOH), and bicarbonate (HCO
3−), which is then decomposed to CO and H
2O [
14].
Further to the RWGS reaction, CO
2 hydrogenation is a synthesis reaction leading to the formation of a range of hydrocarbons or/and alcohols according to equation (2):
Reaction (2) is usually exothermic, favored at lower temperatures and the derived products depend on the reaction conditions (temperature, pressure, etc.) as well as the type of the used catalyst.
CO
2 reduction to valuable fuels via heterogeneous catalytic processes has been thoroughly studied using various active metals and supports. Noble metals, including Ru, Rh, Pt, and Pd [
5,
15,
16], as well as inexpensive metals such as Ni, Cu, and Co[
17,
18,
19,
20], deposited on various supported materials, have been thoroughly discussed in the literature for their catalytic and electrocatalytic performance in terms of activity and selectivity in a plethora of reactors such as fixed-bed, single-chamber, monolithic-type and SOFC type reactors [
19,
21,
22,
23,
24,
25,
26,
27]. In this respect, the Electrochemical Promotion of Catalysis (EPOC) can be utilized to increase the overall CO
2 conversion to valuable products. EPOC has been used extensively in the past for the modification of metal catalyst work function and thus resulting in notable activity and selectivity change over various catalytic reactions [
28,
29,
30]. The utilization of electronegative or electropositive promoters/supports in conjunction with current or interfacial potential application leads to the desired catalytic activity and selectivity modification [
31].
EPOC has been applied in numerous studies for the CO
2 hydrogenation reaction [
20,
21,
23,
24,
25,
32,
33,
34,
35] both in laboratory scale single chambers as well as in semi-pilot reactors. In both cases, there are design drawbacks mainly associated with the reactor/cell configuration and the wiring of the electrodes with the external energy source (i.e. potentiostat/galvanostat). Thus, application of EPOC on an industrial scale is limited. A potential solution to the aforementioned problems will be the simultaneous production of the necessary power for the electrochemical promotion of a desired reaction in the reactor itself.
A fuel cell type configuration, where the chemical energy of a fuel is directly converted into electricity, could play such a role. Solid oxide fuel cell (SOFC) is one of the most attractive cell types due to their low-cost solid materials, long-term operation, and economic maintenance [
36]. Moreover, green hydrogen utilization via fuel cells (i.e. hydrogen produced by clean energy derived from renewable energy sources [
37]) meets the zero-emission of many global and national associations targets set for 2050. Although the use of a solid oxide fuel cell reactor for the investigation of EPOC has a lot of benefits, only a limited number of studies have been reported [
21,
22,
27].
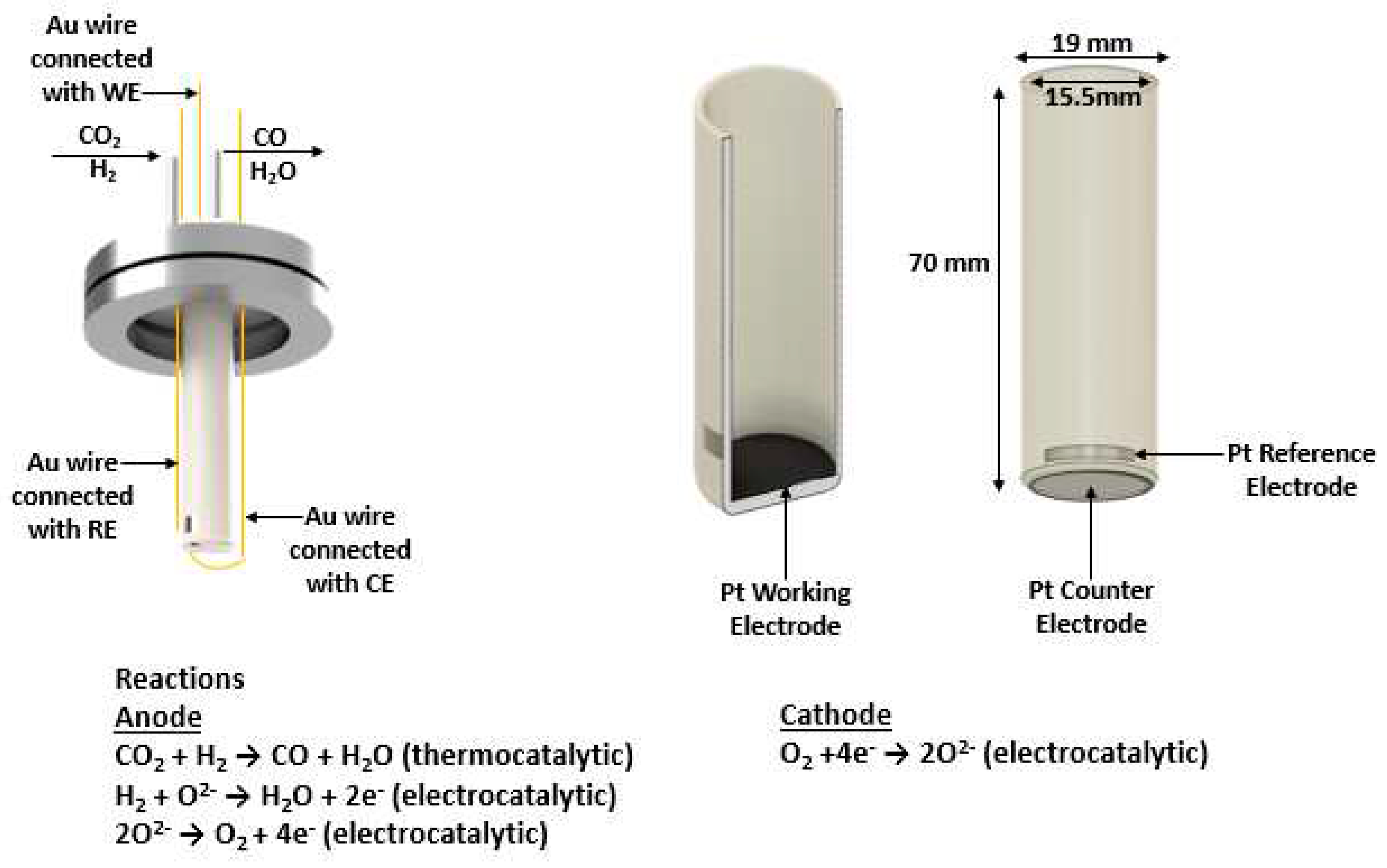
Herein, we introduce a robust low-temperature tubular SOFC type reactor where the principles of EPOC are being applied (
Figure 1). The CO
2 hydrogenation reaction, occurring on the anodic catalytic surface area, is electrochemically promoted either using power generated by the fuel cell (galvanic mode) or using an external power by a potentiostat/ galvanostat (electrolytic mode). It is worth noting that although the most efficient operating temperature of SOFCs often ranges between 800- 1000
oC [
38], the SOFC temperature operation of the present study is remarkably limited to lower temperatures. This is directly connected with the necessary for electrochemical promotion power values which are feasible even at low temperatures (<500
oC).
3. Results
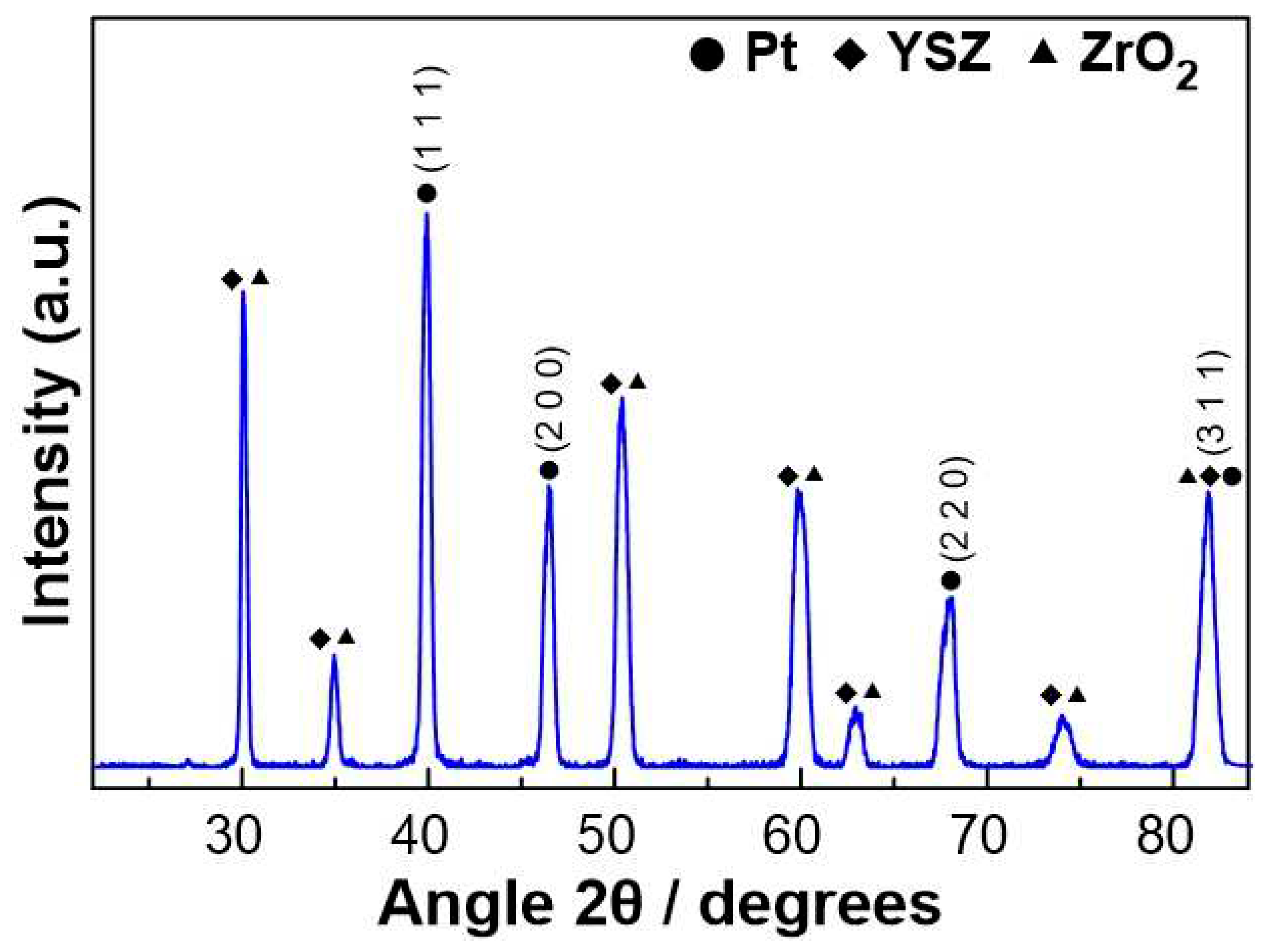
The characterization of the fresh (prior to experiments) Pt catalyst, after the reduction step, was carried out by XRD, in order to obtain information about the crystallinity of the platinum catalytic film. As shown in
Figure 2, well-defined reflections were revealed, at angles 2θ= 39.9, 46.5, 67.8, and 81.5
o, which correspond to the metallic Pt phase. The remaining peaks shown in
Figure 2 were attributed to the YSZ solid electrolyte.
The average size of the Pt crystallites was estimated using Scherrer’s [
40] equation:
where L is the average size of the ordered (crystalline) domains, which can be less than or equal to the particle size (nm), λ is the wavelength of the incident beam (nm), β is the width of the diffraction peak at half its height (rad), θ is the angle between the incident beam and the reflecting plane (Bragg angle) and K is a dimensionless constant with a value of 0.9. Based on the peaks observed at the angles 2θ= 39.9, 46.5, 67.8, and 81.5
o, the size of the Pt crystallites was estimated equal to 14.3±1.8 nm.
XPS measurements were conducted on the fresh reduced sample and after 25 hours of operation under oxidizing (i.e. P
CO2: P
H2 =4:1) conditions.
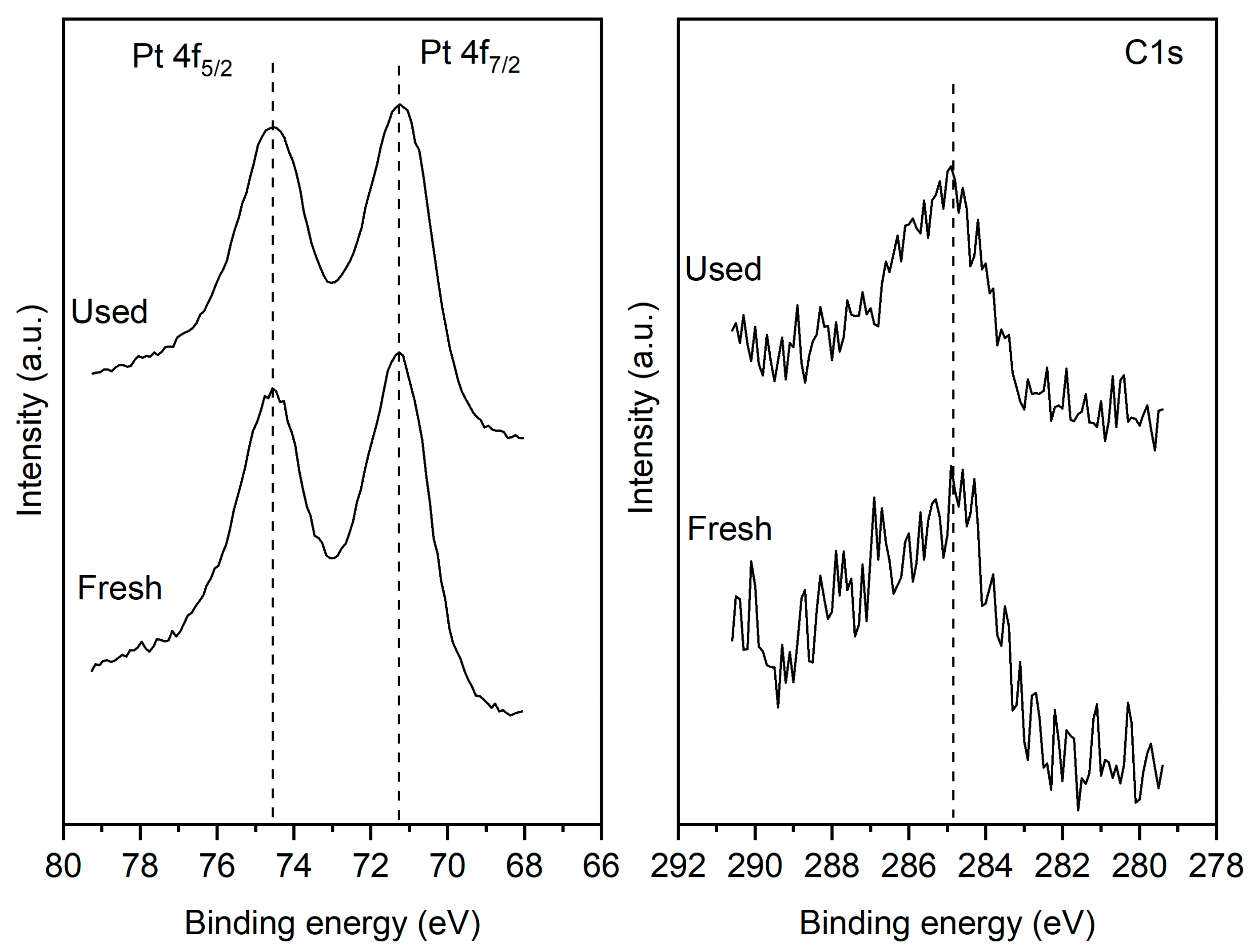
Figure 3(a) shows that for both the fresh and used samples the Pt 4f
7/2 peak is centered at 71.2 eV which is characteristic of metallic Pt. The results clearly indicate that after the reaction the catalyst film retains its metallic state. More importantly, the reaction does not lead to any increase in the carbon content of the surface. The C1s signal shown in
Figure 3(b) indicates the presence of adventitious surface carbon on both the fresh and used samples. Crucially the carbon content of the surface does not increase for the post reaction sample. It is worth noting that the XPS measurement shows only trace quantities of Cl
2p suggesting that the sample treatment has removed most of the chlorine present in the precursors which were used to prepare the films.
The steady-state effect of the total gas mixture flow rate on the catalytic rate and CO
2 conversion at 400
oC is shown in
Figure 4. The catalytic rate of CO production initially increases and for a wide range between 200 and 400 cm
3/min reaches a plateau. Thus, mass transfer limitations are negligible for gas flow rates higher than 200 cm
3/min. This value was selected for the rest of the experiments.
The performance of the SOFC type reactor was evaluated both under fuel cell operation, i.e. using H
2 as the unique reactant, and dual operation, i.e. during co-feeding of H
2 and CO
2 at the anode of the reactor.
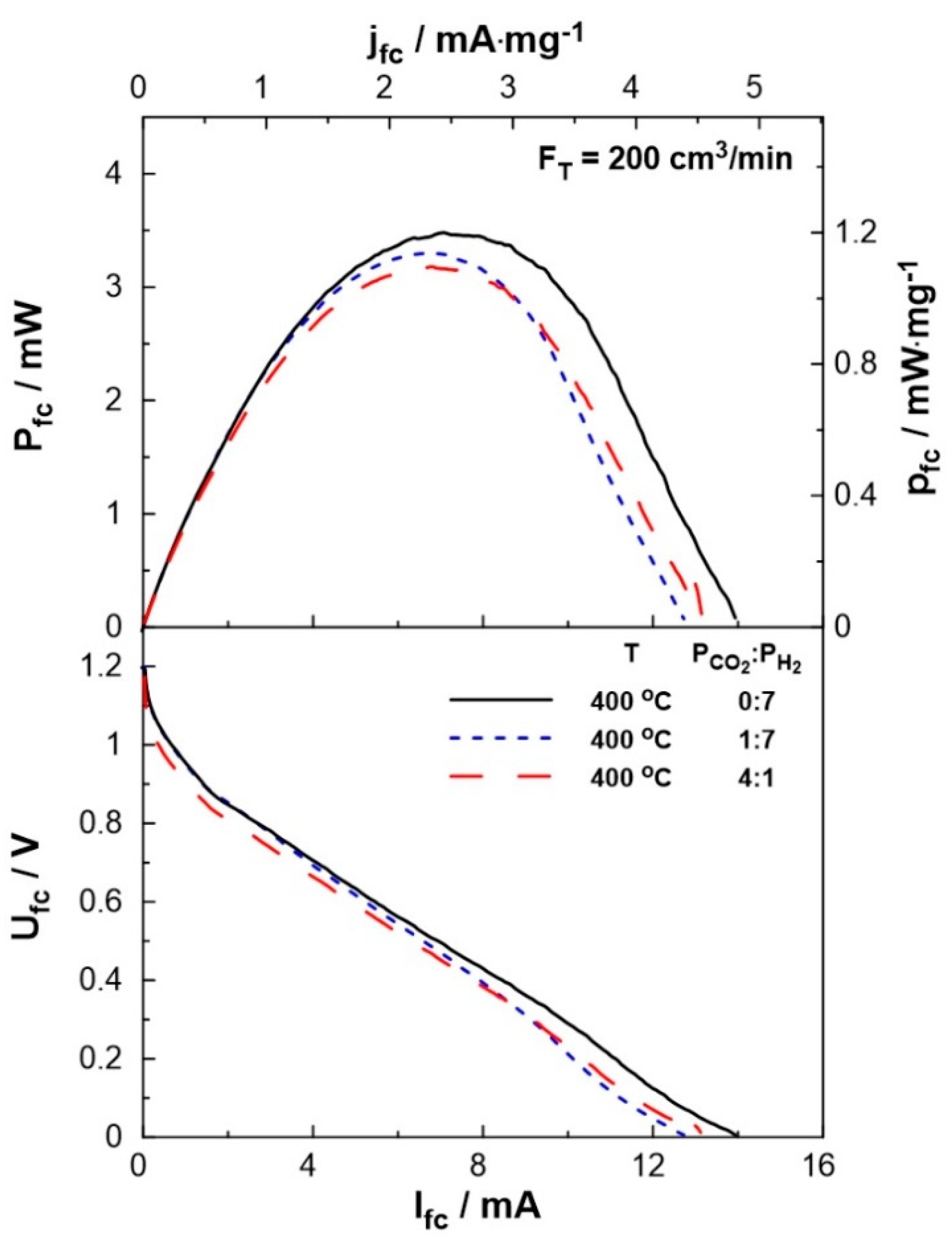
Figure 5 shows the effect of current on the cell potential and power output. The experiments were carried out at a constant temperature of T=400
oC and various reactant ratios (P
CO2:P
H2 = 0:7, 1:7, 4:1). The maximum current (I
fc= 14 mA), as well as the maximum power output (P
fc= 3.5 mW), were observed under fuel cell operation. Under dual operation, the presence of CO
2 on the anode leads to a small decrease in the performance of the fuel cell, which, is less than 15%, even at high partial pressures of CO
2 (4 kPa). It is worth mentioning, that although the observed current values are lower than those produced in conventional fuel cells (as expected, due to the low operation temperature), they are within the same range as those applied in EPOC experiments [
41,
42] and can be utilized for the electrochemical promotion of the catalytic reaction.
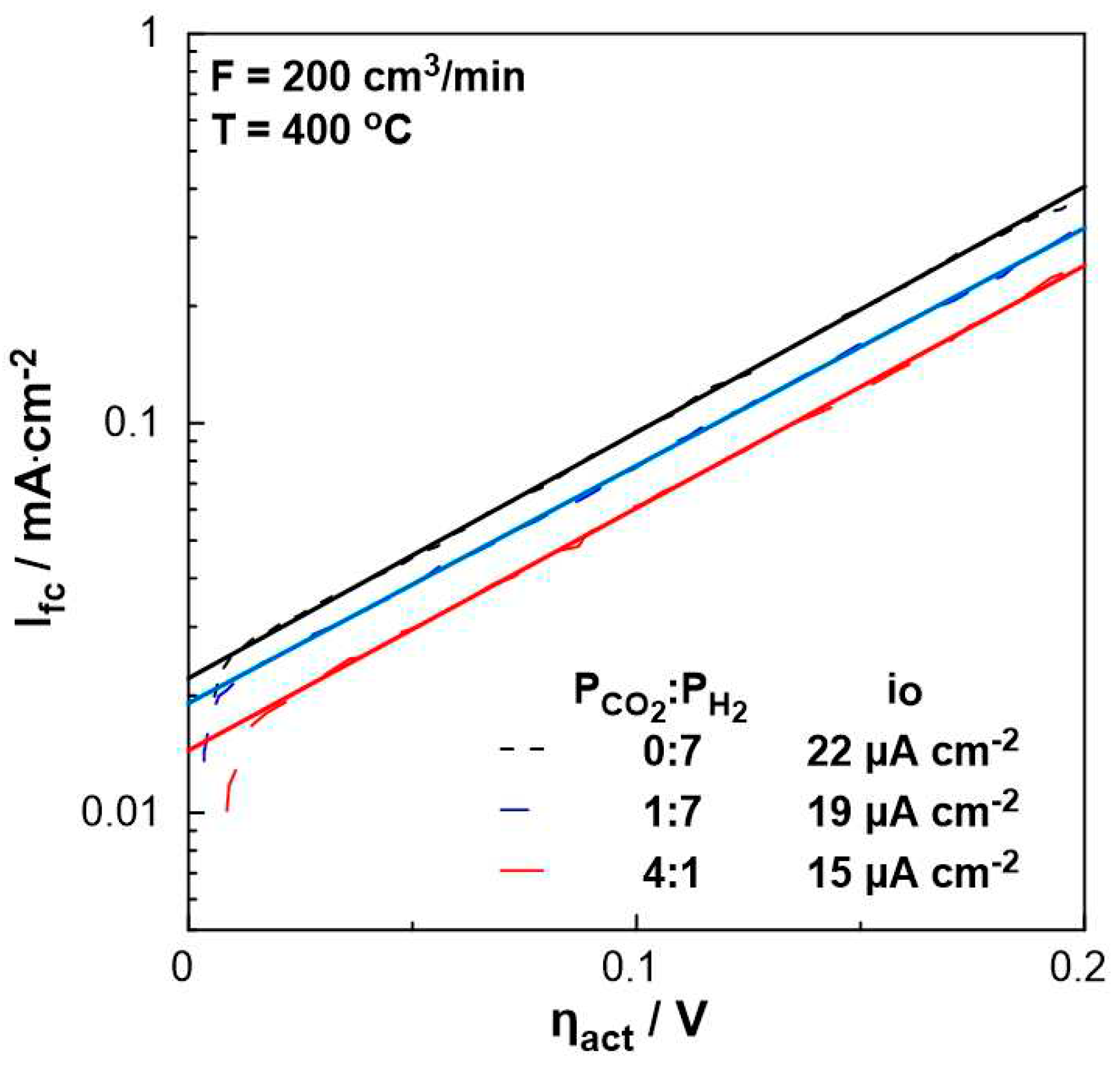
In order to evaluate the polarizability of the catalyst (working electrode), which is associated with its capability for electrochemical promotion, the exchange current density, i
0, and charge transfer coefficient, α, were estimated under different P
CO2:P
H2 ratio feeding conditions.
Figure 6 shows the current density produced by the fuel cell as a function of the activation overpotential (Tafel diagram) for the region of activation polarization (U
fc > 1V in
Figure 5). The intercept of the solid lines corresponds to the exchange current density, i
o, which varies with feed gas mixture ratio. More specifically, in the absence of CO
2, the exchange current density is higher than that obtained under strongly reducing conditions (P
CO2:P
H2= 1:7), whereas it is the lowest under oxidizing conditions. Thus, the stronger the reducing conditions, the more polarizable the metal-solid electrolyte interface. Moreover, the charge transfer coefficient, α, which is equal to the slope of the solid lines in
Figure 6, remains constant (α=0.4), indicating that the mechanism of the electrochemical reactions does not change by the ratio of reactants.
Figure 7 depicts the thermocatalytic and electropromoted rates of CO production and CO
2 consumption and conversion at a temperature range between 260 and 460
oC under both reducing (P
CO2: P
H2 =1:7) and oxidizing conditions (P
CO2: P
H2 =4:1). One can observe that the catalytic rate of CO production increases with temperature, which is consistent with the thermodynamic predictions since the RWGS reaction is slightly endothermic, whereas, for temperatures above 380
oC, this increase is almost monotonous in both oxidizing and reducing conditions. It is also observed that under reducing conditions, CO production begins at a lower temperature and the catalytic rate is slightly higher compared to the oxidιzing conditions, which is in agreement with thermodynamic analysis [
43]. The thermocatalytic rate was monitored under open circuit (o.c.) conditions, in which no electrochemical reactions occur, the current is zero and the open circuit potential was close to the theoretical value of a hydrogen fuel cell predicted by Nernst’s equation (U~ -1.2V). On the other hand, by short-circuiting the anodic and cathodic electrode using an external cable (U = 0V), the electrons produced by the oxidation of H
2, flow through the circuit creating a condition similar to a positive polarization via external devices (e.g. potentiostat) in EPOC studies [
21,
23,
41,
42]. In this case, however, the necessary polarization energy is derived directly from the parallel operation of the fuel cell, and the migration of oxygen ions from the cathode to the anode/catalyst, leads to a Pt-catalyst work function increase. As a result, the bond between the catalyst and the electron acceptor (in this case, CO
2) weakens leading to a CO production decrease. The observed electrophilic behavior of CO production agrees with previous EPOC studies [
19,
22,
23]. In order to study the behavior of the catalytic reaction under oxygen ion removal conditions from the catalyst (anode) to the counter electrode, negative potential (U =-2V) was applied using a potentiostat/ galvanostat. The resulted decrease in the catalyst work function caused the strengthening of the Pt- CO
2 bond and thus, the increase of the catalytic rate both under reducing and oxidizing conditions (Figure 7).
An estimation of the activation energy of the reaction across the various conditions utilized can be accomplished via Arrhenius plots. Based on the calculated values (shown in Figure 7), it appears that the presence of oxygen ions on the catalytic surface (U=0V) affects the activation energy of the reaction which takes notably increased values under both oxidizing and reducing conditions.
For the quantification of the electrochemical promotion of the CO production rate, shown in Figure 7, the rate enhancement ratio, ρ, defined by (4) [
41,
42] was utilized:
where r is the electropromoted catalytic rate (U = -2V or U = 0V) and r
o is the unpromoted rate (i.e., the open-circuit catalytic rate).
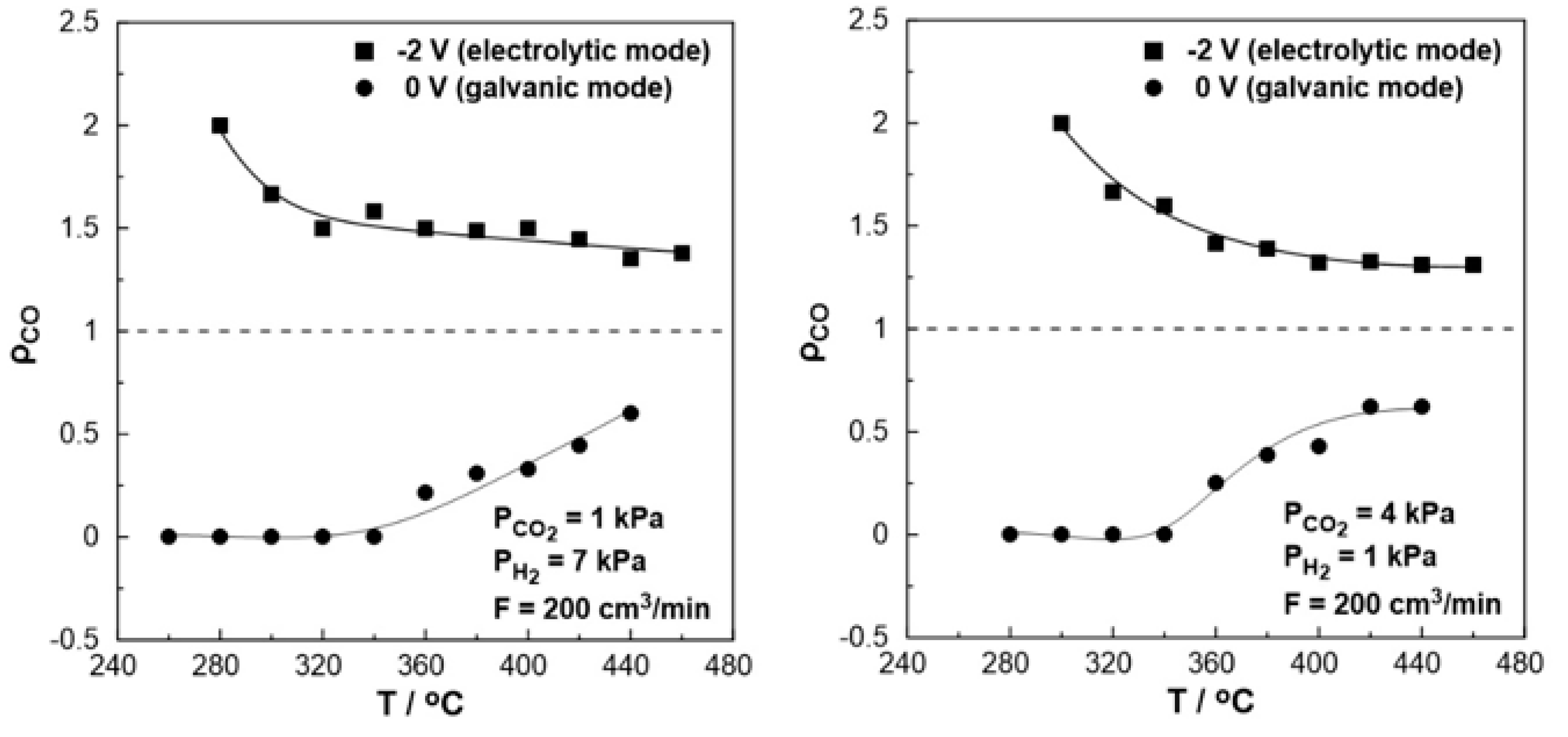
The effect of temperature on the rate enhancement ratio of CO under both reducing (left) and oxidizing (right) conditions is illustrated in
Figure 8. Under short circuit conditions (U = 0V, galvanic mode) at lower temperatures (<340
oC), the observed rate enhancement ratio values are always less than one while approach to zero, indicating the elimination of the produced CO. On the other hand, the rate enhancement ratio for the RWGS reaction tends to be higher under negative polarization, in agreement with the electrophilic behavior of the reaction. Values up to 2 were observed at 280
oC under reducing and 300
oC under oxidizing conditions. At higher temperatures (>380
oC), the ρ value reaches a plateau with an increase of 40-50% (ρ=1.4-1.5) under reducing and 30% (ρ=1.3) under oxidizing conditions even at 460
oC compared to the open circuit conditions.
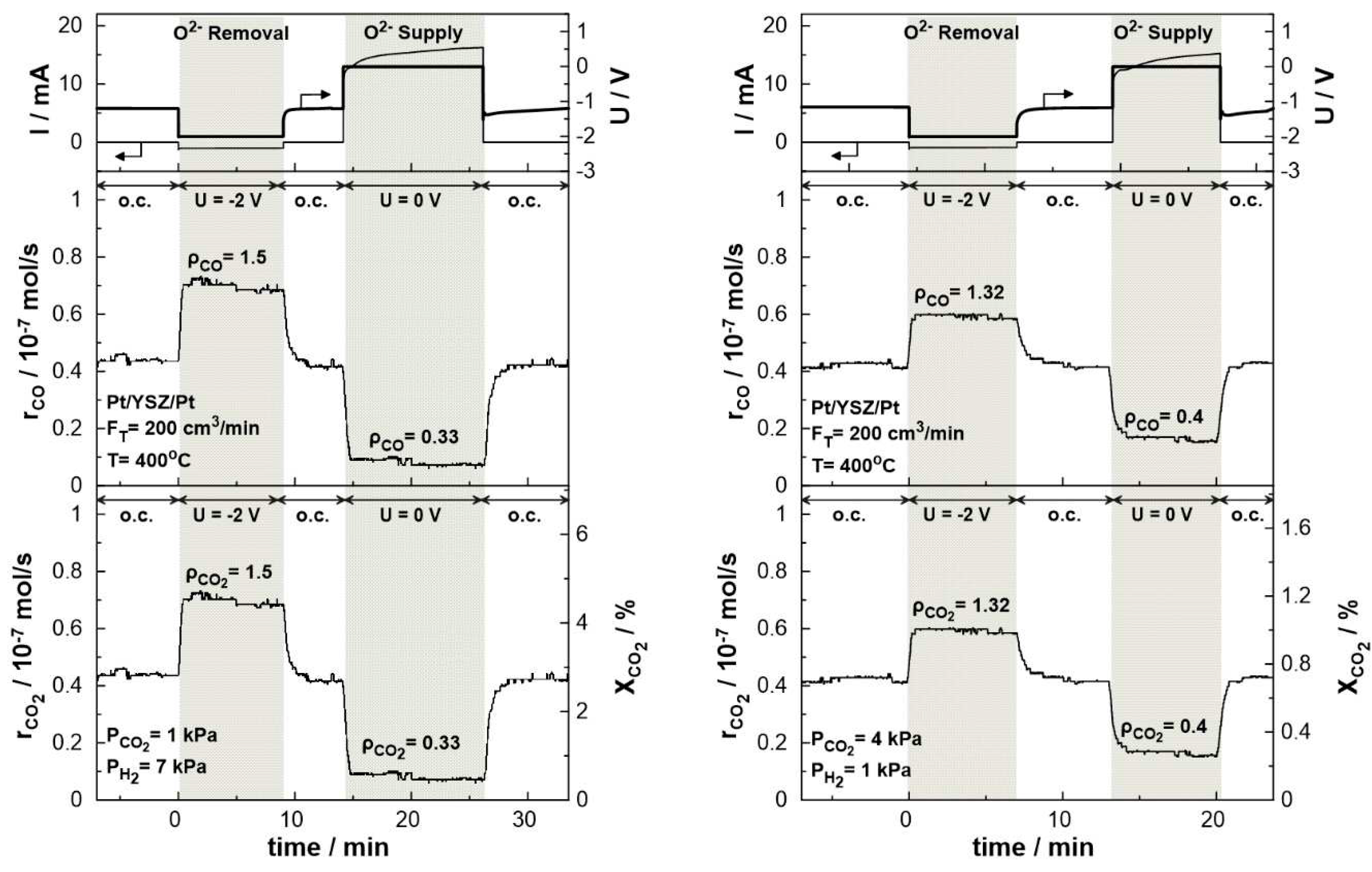
Figure 9 displays the transient effect of negative polarization (U = -2V) and short-circuit conditions (U = 0) on the catalytic rate of CO production and CO
2 consumption and conversion. The experiments were carried out under both reducing (P
CO2: P
H2 =1:7) and oxidizing (P
CO2: P
H2 =4:1) conditions at 400
oC and a total gas flow rate of 200cm
3/min. The current response after the potential application is also shown in the same figure. Under electrolytic mode, i.e. negative potential (U
WR=-2V), the removal of O
2- from the catalytic surface results in the enhancement of the production rate of CO, due to the electrophilic behavior of the RWGS reaction. The CO
2 conversion commences under open circuit conditions at a value of 2.8 % with a carbon monoxide production rate of approximately 0.45 × 10
− 7 mol/sec. Upon constant application of a negative potential (U = -2V), a new steady-state is achieved, with a corresponding increase in the rate of CO production. The rate enhancement ratio, ρ, for the CO production reaches the value of 1.5, denoting a significant 50% increase in the overall carbon monoxide production rate. In the promoted state, the CO
2 conversion is approximately 4.5 %. On the other hand, under galvanic mode (U = 0V), the migration of oxygen ions to the catalytic surface leads to an enhancement of the Pt-H bond resulting in the decrease of the CO production rate to 0.1 × 10
− 7 mol/sec with a rate enhancement ratio of 0.33. Both effects of negative polarization and short-circuit conditions are fully reversible, as the rates of CO production and CO
2 consumption return to their initial values upon interruption of the applied potential. Similar behavior was observed under oxidizing conditions. Negative polarization results in CO production increases by a factor of 1.32 (ρ
CO=1.32), while under short-circuit conditions, the production rate drops by a factor of 0.4 (ρ
CO= 0.4) compared to the thermocatalytic production of CO at the same temperature.
Figure 10 displays the steady-state effect of catalyst potential, U, on the CO production rate, CO
2 consumption rate and constant temperature of T=380
oC both under reducing and oxidizing conditions. Based on the results shown in
Figure 10, the rate of CO formation increases monotonically with decreasing catalyst potential. The negative potential has a greater effect on the catalytic rate of CO (strong increase).
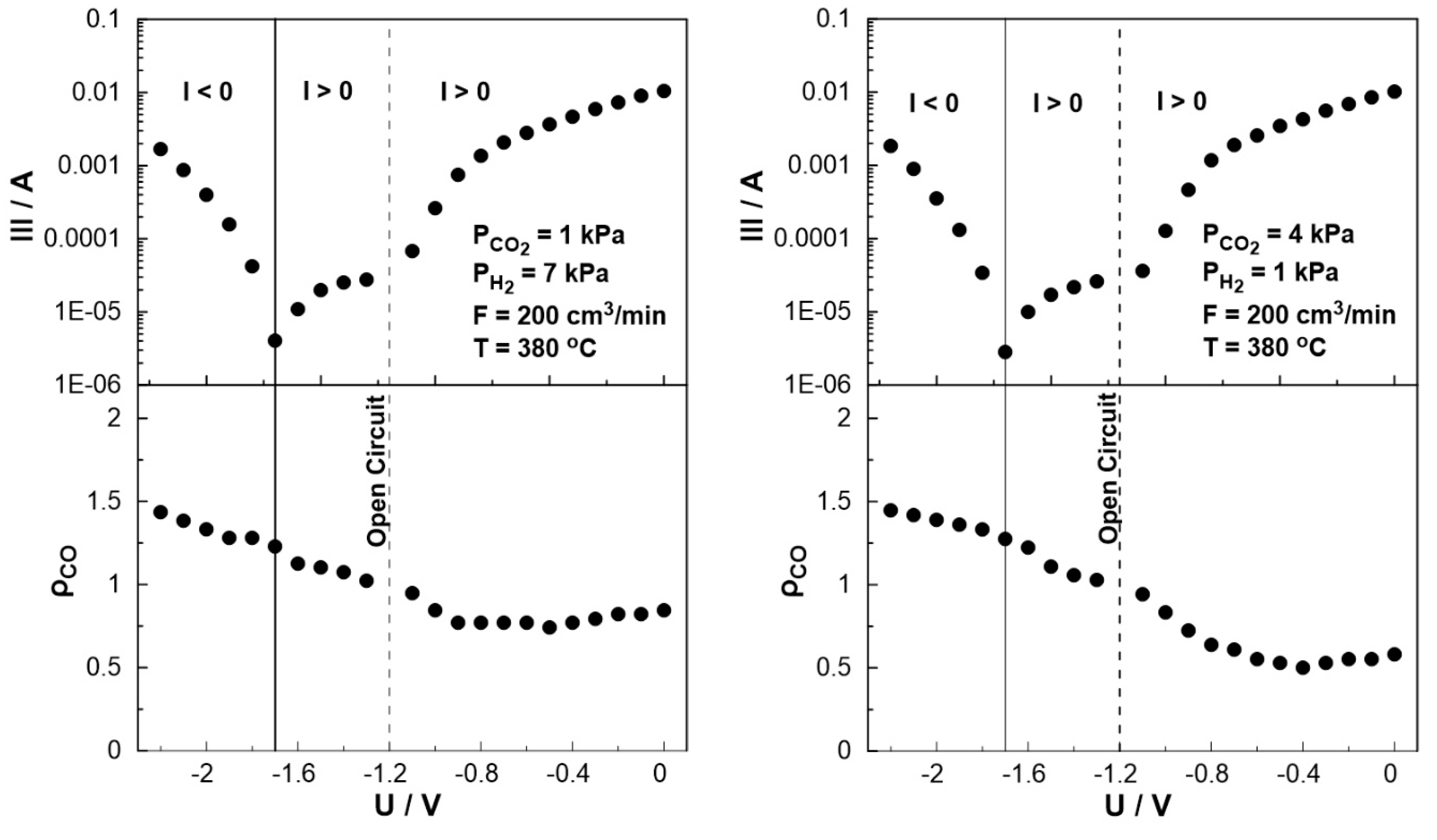
The effect of catalyst potential, U, both on the current (Tafel plot) and the rate enhancement ratio of carbon monoxide, ρ
CO, at constant temperature is illustrated in
Figure 11. As the potential shifts from the open circuit value (U = -1.2V) towards zero, i.e. operating under galvanic mode (I>0), the rate enhancement ratio of CO decreases in agreement with the observed behavior in
Figure 8.
As the potential approaches zero, the rate enhancement ratio of CO reaches a plateau. It is worth noting that the current, in this particular case, exhibits a positive trend that aligns with the anticipated behavior of a standard Tafel plot. On the other hand, as the catalyst potential increases towards higher absolute values in comparison to the open circuit potential (-1.7 < U < -1.2), the current demonstrates a deviation from the typical Tafel plot behavior. More specifically, although one would expect to observe negative currents under these conditions, positive currents were measured for applied potentials as low as -1.7 V. This observation indicates a shift in the value of the open circuit potential, U
O.C., which can be associated with the conducting simultaneous electrochemical and thermochemical reactions at the anodic electrode. In fact, the enhanced rate of CO production (ρ
CO>1) affects both the coverage of species on the electrode surface and the fraction of the water vapor on the reactant mixture. The concentration of the produced water at the anode (both electrochemically and catalytically) could play a key role in the shift of the open circuit potential, which can also be confirmed from Nernst’s equation for the reversible open circuit potential, since all experiments were initially begun under dry reactant conditions. This is not happening in conventional SOFC studies [
44,
45,
46,
47,
48] in which the anode stream usually contains a significant and constant amount of water (to ensure a specific open circuit value). It is worth noting that the above behavior was observed both under oxidizing and reducing conditions.
Figure 1.
Schematic representation of the tubular SOFC reactor. Cross section of the YSZ tube with the Pt working electrode (anode), the external view of the YSZ tube with the Pt reference and Pt counter (cathode) electrodes, and the Au wire connections.
Figure 1.
Schematic representation of the tubular SOFC reactor. Cross section of the YSZ tube with the Pt working electrode (anode), the external view of the YSZ tube with the Pt reference and Pt counter (cathode) electrodes, and the Au wire connections.
Figure 2.
XRD diagram of the Pt catalytic film deposited on YSZ after reduction treatment.
Figure 2.
XRD diagram of the Pt catalytic film deposited on YSZ after reduction treatment.
Figure 3.
Pt 4f (left) and C1s (right) spectra of the fresh and used sample.
Figure 3.
Pt 4f (left) and C1s (right) spectra of the fresh and used sample.
Figure 4.
Effect of the total gas flow rate, FT, on CO formation catalytic rate (top), on CO2 reduction rate (bottom left), and CO2 conversion (bottom right), under open circuit conditions (Ufc=1 V). PH2=7 kPa, PCO2=1 kPa, T=400 oC.
Figure 4.
Effect of the total gas flow rate, FT, on CO formation catalytic rate (top), on CO2 reduction rate (bottom left), and CO2 conversion (bottom right), under open circuit conditions (Ufc=1 V). PH2=7 kPa, PCO2=1 kPa, T=400 oC.
Figure 5.
SOFC current-power, Ifc-Pfc, (top) and current-potential, Ifc-Ufc, (bottom) curves for different PCO2:PH2 (=0:7, 1:7, 4:1) ratio. Solid curves correspond to single operation of the cell while dashed curves correspond to dual operation (see text). FT = 200 cm3 /min, T=400 oC.
Figure 5.
SOFC current-power, Ifc-Pfc, (top) and current-potential, Ifc-Ufc, (bottom) curves for different PCO2:PH2 (=0:7, 1:7, 4:1) ratio. Solid curves correspond to single operation of the cell while dashed curves correspond to dual operation (see text). FT = 200 cm3 /min, T=400 oC.
Figure 6.
Logarithm of the current density, Ifc, as a function of the overpotential, ηact, for different PCO2:PH2 (=0:7, 1:7, 4:1) ratios. FT = 200 cm3 /min, T=400 oC.
Figure 6.
Logarithm of the current density, Ifc, as a function of the overpotential, ηact, for different PCO2:PH2 (=0:7, 1:7, 4:1) ratios. FT = 200 cm3 /min, T=400 oC.
Figure 8.
Effect of temperature under steady-state conditions on the rate enhancement ratio, ρ. Left: PH2= 7 kPa and PCO2= 1 kPa. Right: PH2= 1 kPa and PCO2= 2 kPa. FT = 200 cm3 /min.
Figure 8.
Effect of temperature under steady-state conditions on the rate enhancement ratio, ρ. Left: PH2= 7 kPa and PCO2= 1 kPa. Right: PH2= 1 kPa and PCO2= 2 kPa. FT = 200 cm3 /min.
Figure 9.
Transient effect of constant potential (U=-2V under negative polarization, U =0 V under short circuit conditions, and U =-1.2 V under open circuit conditions) on current (top), catalytic rates of CO production (middle) and CO2 consumption (bottom left) and CO2 conversion (bottom right). Left: PH2= 7 kPa and PCO2= 1 kPa. Right: PH2= 1 kPa and PCO2= 2 kPa. FT = 200 cm3 /min. T = 400 ◦C.
Figure 9.
Transient effect of constant potential (U=-2V under negative polarization, U =0 V under short circuit conditions, and U =-1.2 V under open circuit conditions) on current (top), catalytic rates of CO production (middle) and CO2 consumption (bottom left) and CO2 conversion (bottom right). Left: PH2= 7 kPa and PCO2= 1 kPa. Right: PH2= 1 kPa and PCO2= 2 kPa. FT = 200 cm3 /min. T = 400 ◦C.
Figure 10.
Steady-state effect of catalyst potential, U, on the catalytic rate of CO formation (top) and CO2 consumption (bottom left axis) and CO2 conversion (bottom right axis). Left: PH2= 7 kPa and PCO2= 1 kPa. Right: PH2= 1 kPa and PCO2= 2 kPa. FT = 200 cm3 /min. T = 380 ◦C.
Figure 10.
Steady-state effect of catalyst potential, U, on the catalytic rate of CO formation (top) and CO2 consumption (bottom left axis) and CO2 conversion (bottom right axis). Left: PH2= 7 kPa and PCO2= 1 kPa. Right: PH2= 1 kPa and PCO2= 2 kPa. FT = 200 cm3 /min. T = 380 ◦C.
Figure 11.
Steady-state effect of catalyst potential, U, on the rate enhancement ratio of CO formation (bottom) and current (Tafel plot, top) Left: PH2= 7 kPa and PCO2= 1 kPa. Right: PH2= 1 kPa and PCO2= 2 kPa. FT = 200 cm3 /min. T = 380 ◦C.
Figure 11.
Steady-state effect of catalyst potential, U, on the rate enhancement ratio of CO formation (bottom) and current (Tafel plot, top) Left: PH2= 7 kPa and PCO2= 1 kPa. Right: PH2= 1 kPa and PCO2= 2 kPa. FT = 200 cm3 /min. T = 380 ◦C.