Submitted:
19 April 2023
Posted:
20 April 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
- capability of the cGAN to correct CBCT (scatter reduction and HU remapping) when applied to small FOV;
- consistency of the proton dosimetry computed on corrected CBCT with respect to the original planning CT.
2. Materials and Methods
2.1. Dataset Description
2.1.1. CBCT simulation
2.2. CBCT-to-CT Correction
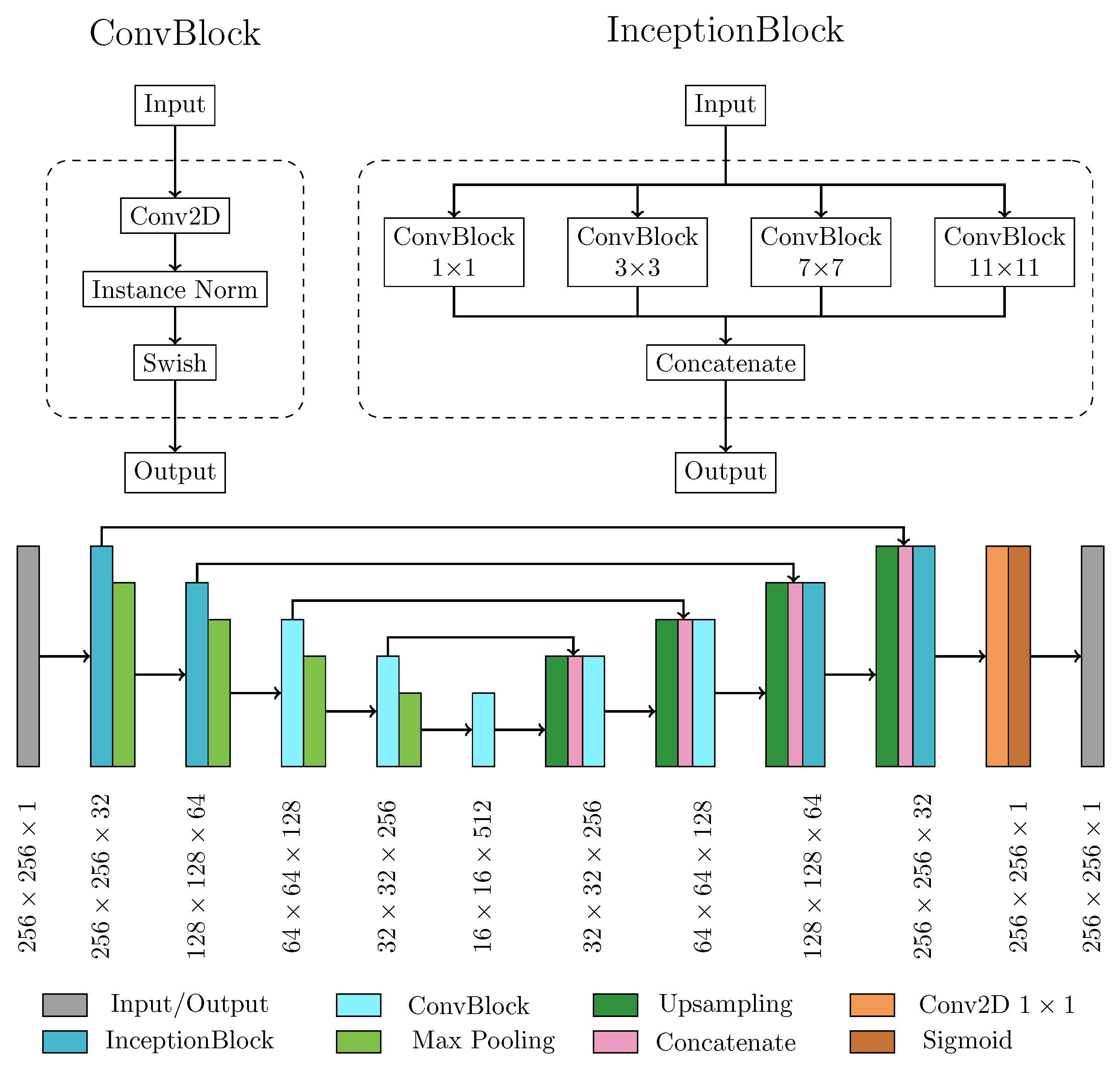
2.2.1. Neural Network Architecture and Main Processing Layers
2.2.2. Model Training
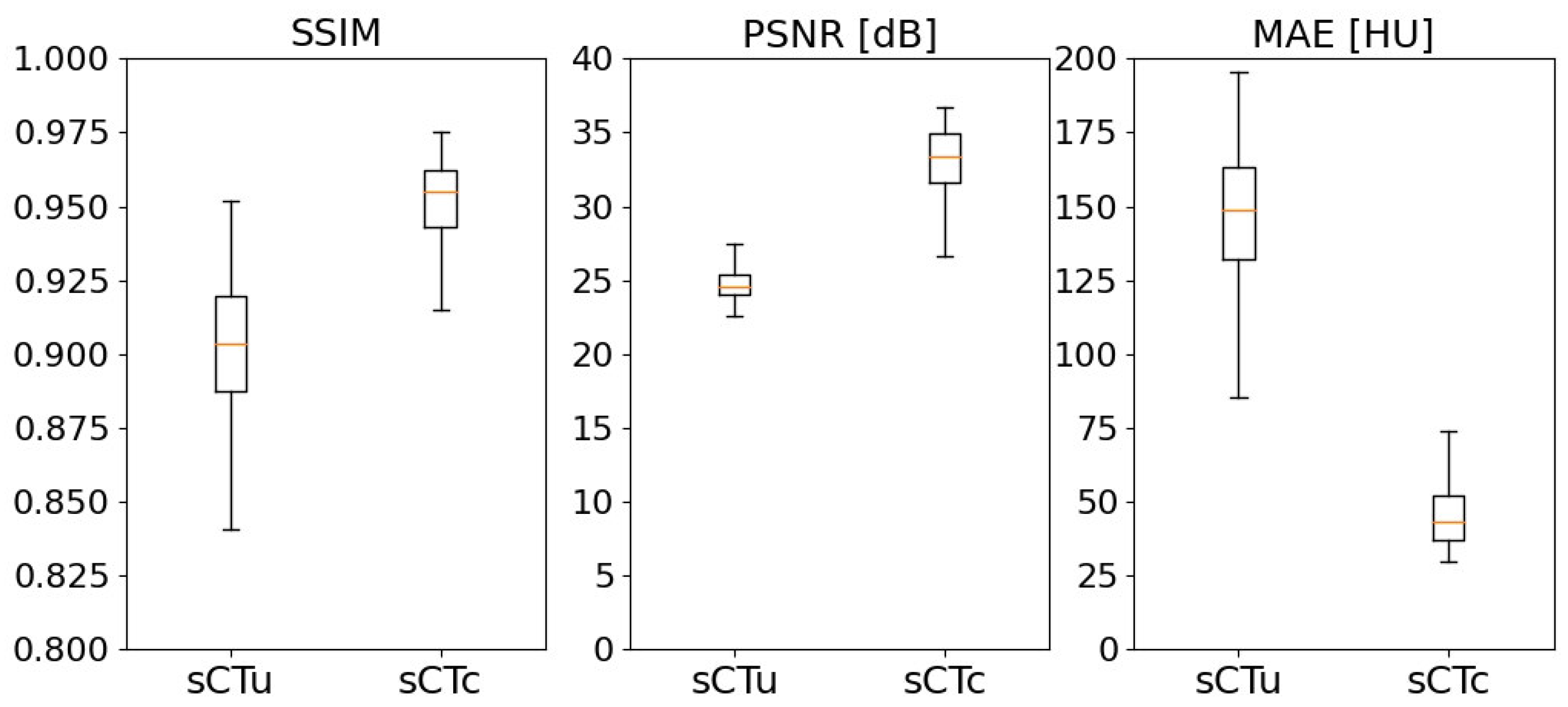
2.2.3. Performance Metrics for Model Evaluation
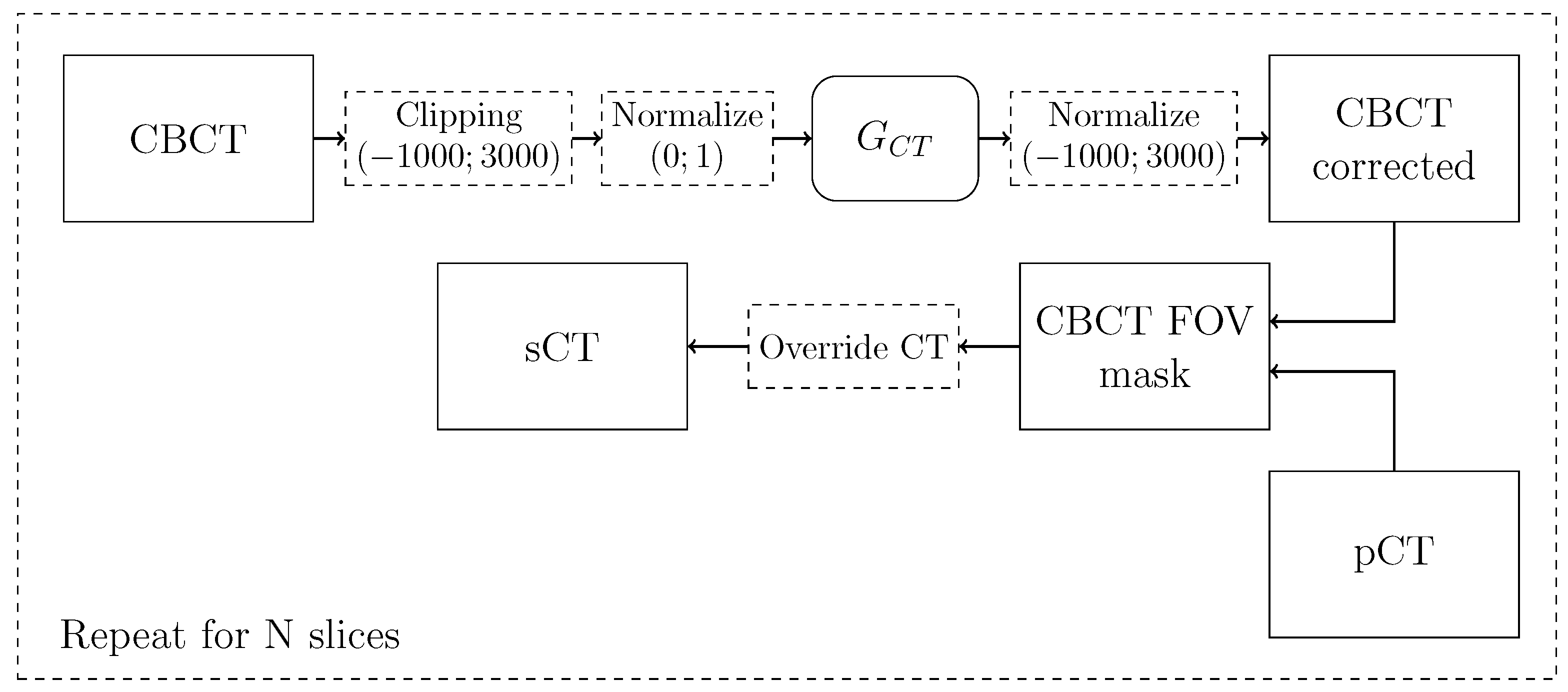
2.2.4. Synthetic CT Generation Pipeline
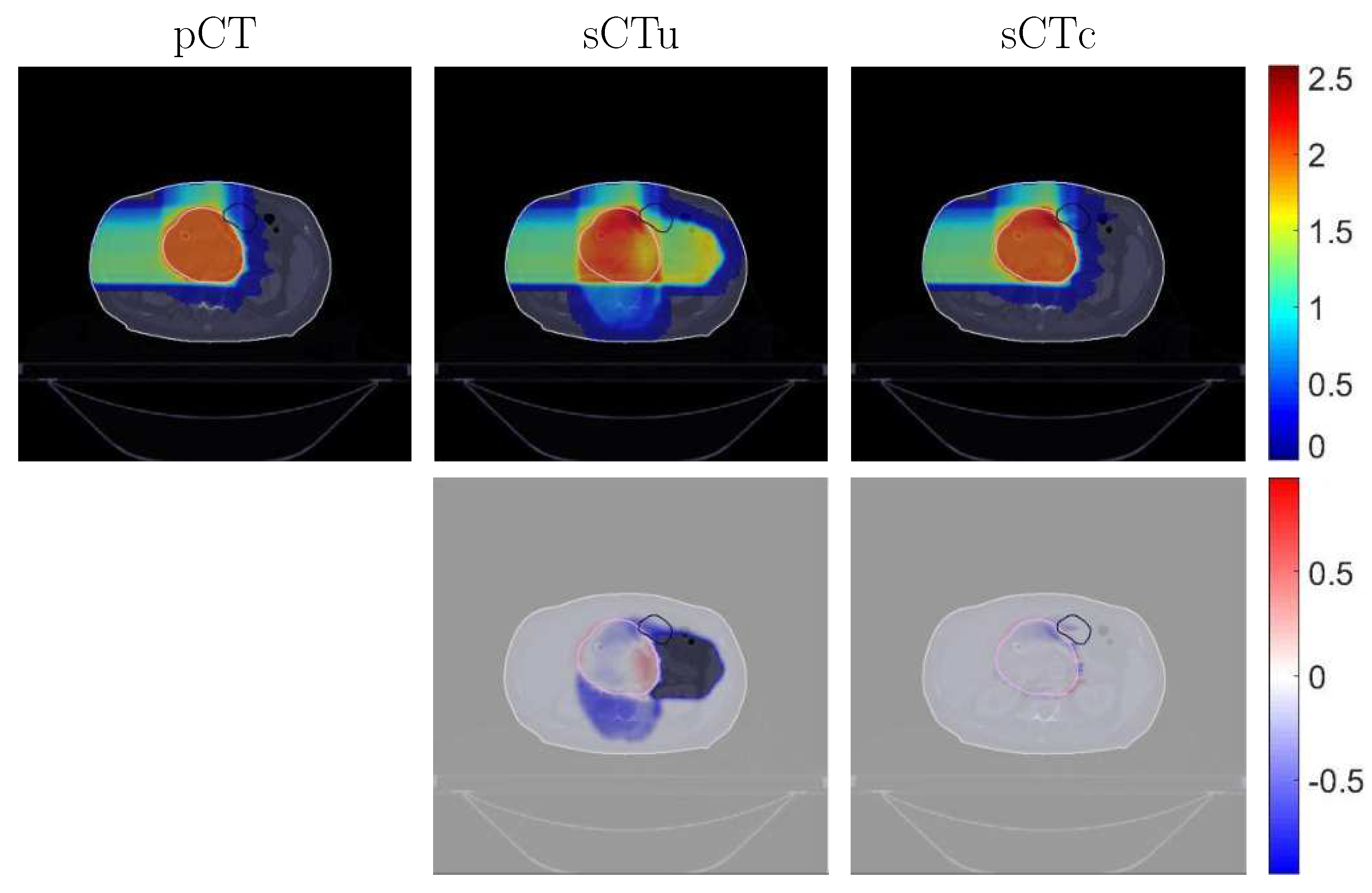
2.3. Dosimetric Analysis
2.3.1. Proton-Based Treatment Planning
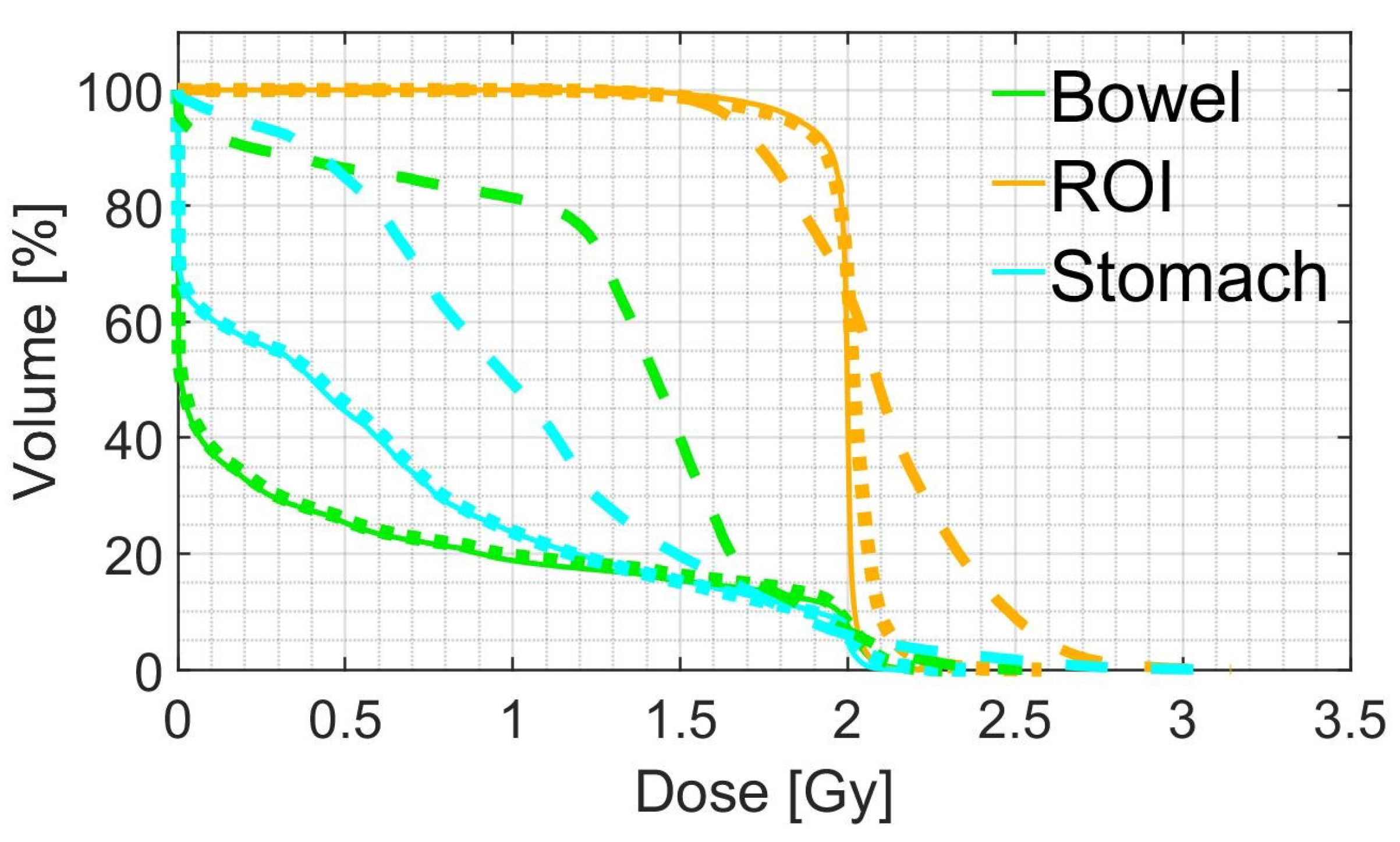
2.3.2. Dose Evaluation
3. Results
3.1. cGAN Model Evaluation
3.2. Treatment Planning Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBCT | Cone-Beam Computed Tomography |
| cGAN | cycle-consistent Generative Adversarial Network |
| CNN | Convolutional Neural Network |
| CT | Computed Tomography |
| D | Discriminator CBCT |
| D | Discriminator CT |
| DPR | Dose Difference Pass Rate |
| DVH | Dose–Volume Histogram |
| FOV | Field of View |
| G | Generator CBCT |
| G | Generator CT |
| GPR | Gamma Pass Rate |
| IQR | Interquartile Range |
| MAE | Mean Absolute Error |
| MC | Monte Carlo |
| OAR | Organ at Risk |
| pCT | planning CT |
| PSNR | Peak Signal-to-Noise Ratio |
| ROI | Region of Interest |
| sCT | synthetic CT |
| sCTc | corrected sCT |
| sCTu | uncorrected sCT |
| SSIM | Structural Similarity Index Measure |
References
- Bortfeld, T. IMRT: A review and preview. Physics in Medicine and Biology 2006, 51, R363–R379. [CrossRef]
- Joseph, P.M.; Spital, R.D. The effects of scatter in x-ray computed tomography. Medical Physics 1982, 9, 464–472. [CrossRef]
- Schulze, R.; Heil, U.; Groβ, D.; Bruellmann, D.D.; Dranischnikow, E.; Schwanecke, U.; Schoemer, E. Artefacts in CBCT: A review. Dentomaxillofacial Radiology 2011, 40, 265–273. [CrossRef]
- Kurz, C.; Kamp, F.; Park, Y.K.; Zöllner, C.; Rit, S.; Hansen, D.; Podesta, M.; Sharp, G.C.; Li, M.; Reiner, M.; et al. Investigating deformable image registration and scatter correction for CBCT-based dose calculation in adaptive IMPT. Medical Physics 2016, 43, 5635–5646. [CrossRef]
- Thing, R.S.; Bernchou, U.; Mainegra-Hing, E.; Hansen, O.; Brink, C. Hounsfield unit recovery in clinical cone beam CT images of the thorax acquired for image guided radiation therapy. Physics in Medicine and Biology 2016, 61, 5781–5802. [CrossRef]
- Giacometti, V.; Hounsell, A.R.; McGarry, C.K. A review of dose calculation approaches with cone beam CT in photon and proton therapy. Physica Medica 2020, 76, 243–276. [CrossRef]
- Sun, M.; Star-Lack, J.M. Improved scatter correction using adaptive scatter kernel superposition. Physics in Medicine and Biology 2010, 55, 6695–6720. [CrossRef]
- Sisniega, A.; Zbijewski, W.; Badal, A.; Kyprianou, I.S.; Stayman, J.W.; Vaquero, J.J.; Siewerdsen, J.H. Monte Carlo study of the effects of system geometry and antiscatter grids on cone-beam CT scatter distributions. Medical Physics 2013, 40, 051915. [CrossRef]
- Stankovic, U.; Ploeger, L.S.; van Herk, M.; Sonke, J.J. Optimal combination of anti-scatter grids and software correction for CBCT imaging. Medical Physics 2017, 44, 4437–4451. [CrossRef]
- Rusanov, B.; Hassan, G.M.; Reynolds, M.; Sabet, M.; Kendrick, J.; Rowshanfarzad, P.; Ebert, M. Deep learning methods for enhancing cone-beam CT image quality toward adaptive radiation therapy: A systematic review. Medical Physics 2022, 49, 6019–6054. [CrossRef]
- Kida, S.; Nakamoto, T.; Nakano, M.; Nawa, K.; Haga, A.; Kotoku, J.; Yamashita, H.; Nakagawa, K. Cone Beam Computed Tomography image quality improvement using a deep convolutional neural network. Cureus 2018. [CrossRef]
- Jiang, Y.; Yang, C.; Yang, P.; Hu, X.; Luo, C.; Xue, Y.; Xu, L.; Hu, X.; Zhang, L.; Wang, J.; et al. Scatter correction of cone-beam CT using a deep residual convolution neural network (DRCNN). Physics in Medicine & Biology 2019, 64, 145003. [CrossRef]
- Landry, G.; Hansen, D.; Kamp, F.; Li, M.; Hoyle, B.; Weller, J.; Parodi, K.; Belka, C.; Kurz, C. Comparing Unet training with three different datasets to correct CBCT images for prostate radiotherapy dose calculations. Physics in Medicine & Biology 2019, 64, 035011. [CrossRef]
- Chen, L.; Liang, X.; Shen, C.; Jiang, S.; Wang, J. Synthetic CT generation from CBCT images via deep learning. Medical Physics 2020, 47, 1115–1125. [CrossRef]
- Rossi, M.; Belotti, G.; Paganelli, C.; Pella, A.; Barcellini, A.; Cerveri, P.; Baroni, G. Image-based shading correction for narrow-FOV truncated pelvic CBCT with deep convolutional neural networks and transfer learning. Medical physics 2021, 48, 7112–7126. [CrossRef]
- Kida, S.; Kaji, S.; Nawa, K.; Imae, T.; Nakamoto, T.; Ozaki, S.; Ohta, T.; Nozawa, Y.; Nakagawa, K. Visual enhancement of Cone-beam CT by use of CycleGAN. Medical Physics 2020, 47, 998–1010. [CrossRef]
- Eckl, M.; Hoppen, L.; Sarria, G.R.; Boda-Heggemann, J.; Simeonova-Chergou, A.; Steil, V.; Giordano, F.A.; Fleckenstein, J. Evaluation of a cycle-generative adversarial network-based cone-beam CT to synthetic CT conversion algorithm for adaptive radiation therapy. Physica Medica 2020, 80, 308–316. [CrossRef]
- Dong, G.; Zhang, C.; Liang, X.; Deng, L.; Zhu, Y.; Zhu, X.; Zhou, X.; Song, L.; Zhao, X.; Xie, Y. A Deep Unsupervised Learning Model for Artifact Correction of Pelvis Cone-Beam CT. Frontiers in Oncology 2021, 11. [CrossRef]
- Sun, H.; Fan, R.; Li, C.; Lu, Z.; Xie, K.; Ni, X.; Yang, J. Imaging Study of Pseudo-CT Synthesized From Cone-Beam CT Based on 3D CycleGAN in Radiotherapy. Frontiers in Oncology 2021, 11. [CrossRef]
- Uh, J.; Wang, C.; Acharya, S.; Krasin, M.J.; ho Hua, C. Training a deep neural network coping with diversities in abdominal and pelvic images of children and young adults for CBCT-based adaptive proton therapy. Radiotherapy and Oncology 2021, 160, 250–258. [CrossRef]
- Zhao, J.; Chen, Z.; Wang, J.; Xia, F.; Peng, J.; Hu, Y.; Hu, W.; Zhang, Z. MV CBCT-Based Synthetic CT Generation Using a Deep Learning Method for Rectal Cancer Adaptive Radiotherapy. Frontiers in Oncology 2021, 11. [CrossRef]
- Xie, S.; Liang, Y.; Yang, T.; Song, Z. Contextual loss based artifact removal method on CBCT image. Journal of Applied Clinical Medical Physics 2020, 21, 166–177. [CrossRef]
- Wu, W.; Qu, J.; Cai, J.; Yang, R. Multiresolution residual deep neural network for improving pelvic CBCT image quality. Medical Physics 2022, 49, 1522–1534. [CrossRef]
- Rossi, M.; Cerveri, P. Comparison of Supervised and Unsupervised Approaches for the Generation of Synthetic CT from Cone-Beam CT. Diagnostics (Basel, Switzerland) 2021, 11. [CrossRef]
- Zhang, Y.; Yue, N.; Su, M.Y.; Liu, B.; Ding, Y.; Zhou, Y.; Wang, H.; Kuang, Y.; Nie, K. Improving CBCT quality to CT level using deep learning with generative adversarial network. Medical Physics 2021, 48, 2816–2826. [CrossRef]
- Landry, G.; Hua, C.h. Current state and future applications of radiological image guidance for particle therapy. Medical Physics 2018, 45, e1086–e1095. [CrossRef]
- Lu, W.; Yan, H.; Zhou, L.; Cervino, L.; Jiang, S.; Jia, X. TU-G-141-05: Limited Field-Of-View Cone-Beam CT Reconstruction for Adaptive Radiotherapy. Medical Physics 2013, 40, 457–457. [CrossRef]
- Clackdoyle, R.; Defrise, M. Tomographic Reconstruction in the 21st Century. IEEE Signal Processing Magazine 2010, 27, 60–80. [CrossRef]
- Fattori, G.; Riboldi, M.; Pella, A.; Peroni, M.; Cerveri, P.; Desplanques, M.; Fontana, G.; Tagaste, B.; Valvo, F.; Orecchia, R.; et al. Image guided particle therapy in CNAO room 2: Implementation and clinical validation. Physica Medica 2015, 31, 9–15. [CrossRef]
- Hong, J.; Reyngold, M.; Crane, C.; Cuaron, J.; Hajj, C.; Mann, J.; Zinovoy, M.; Yorke, E.; LoCastro, E.; Apte, A.P.; et al. Breath-hold CT and cone-beam CT images with expert manual organ-at-risk segmentations from radiation treatments of locally advanced pancreatic cancer (Pancreatic-CT-CBCT-SEG), 2021. [CrossRef]
- Poludniowski, G.; Evans, P.M.; Hansen, V.N.; Webb, S. An efficient Monte Carlo-based algorithm for scatter correction in keV cone-beam CT. Physics in Medicine and Biology 2009, 54, 3847–3864. [CrossRef]
- Jan, S.; Santin, G.; Strul, D.; Staelens, S.; Assié, K.; Autret, D.; Avner, S.; Barbier, R.; Bardiès, M.; Bloomfield, P.M.; et al. GATE: A simulation toolkit for PET and SPECT. Physics in Medicine and Biology 2004, 49, 4543–4561. [CrossRef]
- Poludniowski, G.; Omar, A.; Bujila, R.; Andreo, P. Technical Note: SpekPy v2.0—a software toolkit for modeling x-ray tube spectra. Medical Physics 2021, 48, 3630–3637. [CrossRef]
- Rit, S.; Oliva, M.V.; Brousmiche, S.; Labarbe, R.; Sarrut, D.; Sharp, G.C. The Reconstruction Toolkit (RTK), an open-source cone-beam CT reconstruction toolkit based on the Insight Toolkit (ITK). Journal of Physics: Conference Series 2014, 489, 012079. [CrossRef]
- Zhu, J.Y.; Park, T.; Isola, P.; Efros, A.A. Unpaired Image-to-Image Translation Using Cycle-Consistent Adversarial Networks. In Proceedings of the 2017 IEEE International Conference on Computer Vision (ICCV). IEEE, 2017. [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation, 2015. [CrossRef]
- Isola, P.; Zhu, J.Y.; Zhou, T.; Efros, A.A. Image-to-Image Translation with Conditional Adversarial Networks. In Proceedings of the 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). IEEE, 2017. [CrossRef]
- Chollet, F.; et al. Keras. https://keras.io, 2015.
- Abadi, M.; Agarwal, A.; Barham, P.; Brevdo, E.; Chen, Z.; Citro, C.; Corrado, G.S.; Davis, A.; Dean, J.; Devin, M.; et al. TensorFlow: Large-Scale Machine Learning on Heterogeneous Systems, 2015. Software available from tensorflow.org.
- Spadea, M.F.; Maspero, M.; Zaffino, P.; Seco, J. Deep learning based synthetic-CT generation in radiotherapy and PET: A review. Medical Physics 2021, 48, 6537–6566. [CrossRef]
- Hore, A.; Ziou, D. Image quality metrics: PSNR vs. SSIM. In Proceedings of the 2010 20th International Conference on Pattern Recognition. IEEE, 2010. [CrossRef]
- Wang, Z.; Bovik, A.C.; Sheikh, H.R.; Simoncelli, E.P. Image quality assessment: From error visibility to structural similarity. IEEE Transactions on Image Processing 2004, 13, 600–612. [CrossRef]
- Wieser, H.P.; Cisternas, E.; Wahl, N.; Ulrich, S.; Stadler, A.; Mescher, H.; Müller, L.R.; Klinge, T.; Gabrys, H.; Burigo, L.; et al. Development of the open-source dose calculation and optimization toolkit matRad. Medical Physics 2017, 44, 2556–2568. [CrossRef]
- Dreher, C.; Habermehl, D.; Ecker, S.; Brons, S.; El-Shafie, R.; Jäkel, O.; Debus, J.; Combs, S.E. Optimization of carbon ion and proton treatment plans using the raster-scanning technique for patients with unresectable pancreatic cancer. Radiation Oncology 2015, 10. [CrossRef]
- Hansen, D.C.; Landry, G.; Kamp, F.; Li, M.; Belka, C.; Parodi, K.; Kurz, C. ScatterNet: A convolutional neural network for cone-beam CT intensity correction. Medical Physics 2018, 45, 4916–4926. [CrossRef]
- Thummerer, A.; Oria, C.S.; Zaffino, P.; Meijers, A.; Marmitt, G.G.; Wijsman, R.; Seco, J.; Langendijk, J.A.; Knopf, A.C.; Spadea, M.F.; et al. Clinical suitability of deep learning based synthetic CTs for adaptive proton therapy of lung cancer. Medical Physics 2021, 48, 7673–7684. [CrossRef]
- Kurz, C.; Maspero, M.; Savenije, M.H.F.; Landry, G.; Kamp, F.; Pinto, M.; Li, M.; Parodi, K.; Belka, C.; van den Berg, C.A.T. CBCT correction using a cycle-consistent generative adversarial network and unpaired training to enable photon and proton dose calculation. Physics in Medicine & Biology 2019, 64, 225004. [CrossRef]
- Miften, M.; Olch, A.; Mihailidis, D.; Moran, J.; Pawlicki, T.; Molineu, A.; Li, H.; Wijesooriya, K.; Shi, J.; Xia, P.; et al. Tolerance limits and methodologies for IMRT measurement-based verification QA: Recommendations of AAPM Task Group No. 218. Medical Physics 2018, 45. [CrossRef]
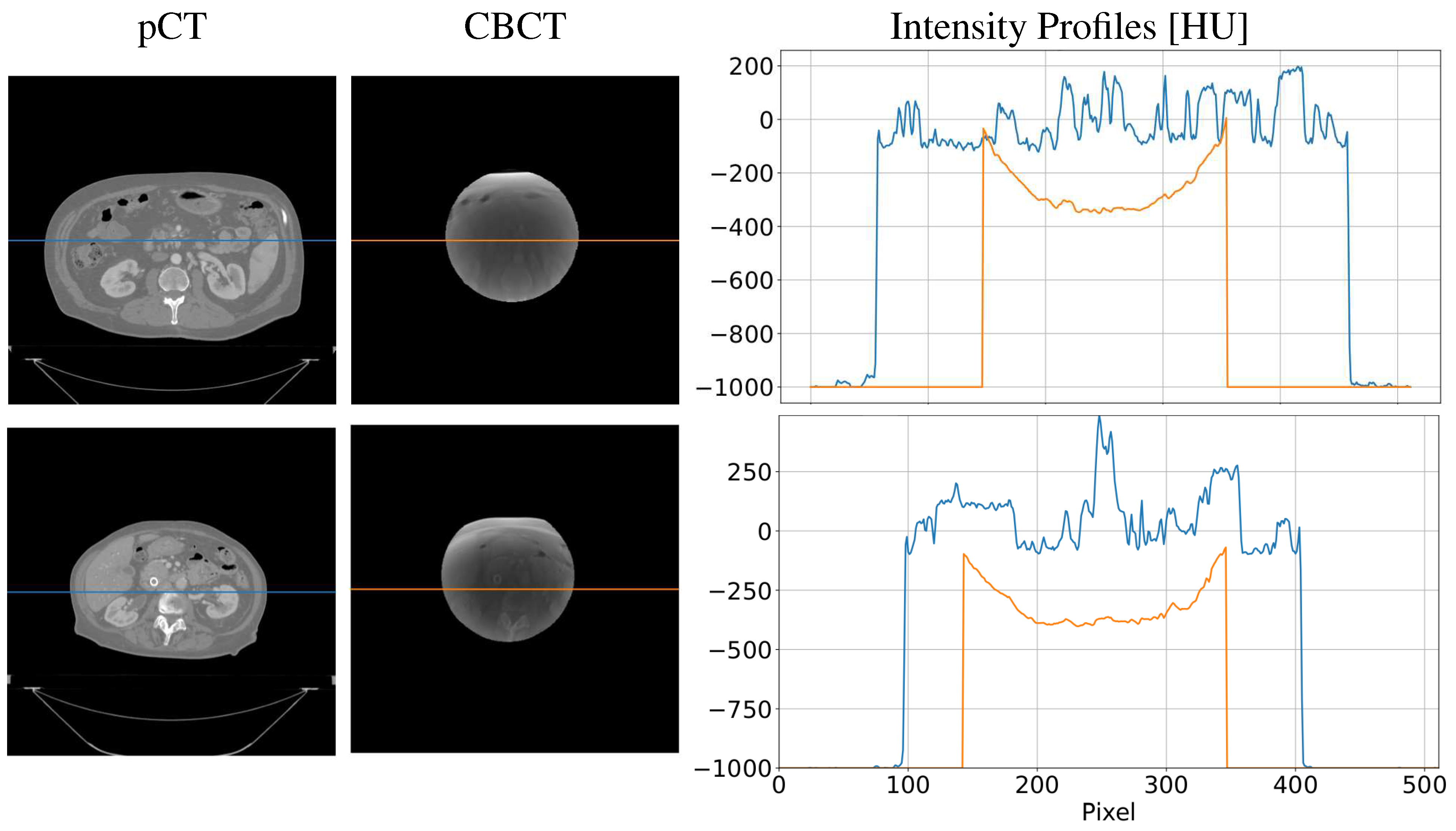
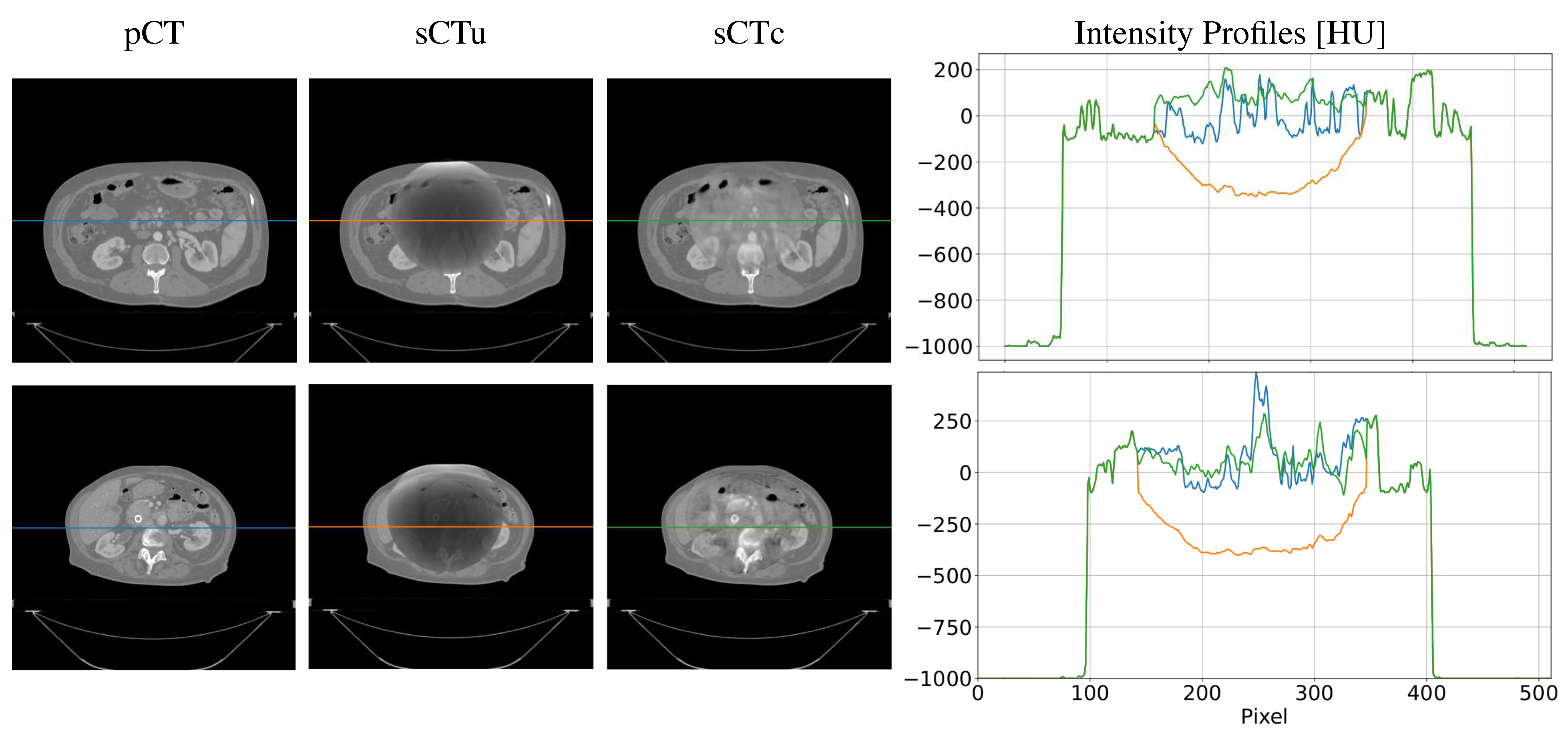
| Gamma Criterion | sCTu | sCTc |
|---|---|---|
| 1%/1 mm | 44.68 (6.91) | 74.37 (4.63) |
| 2%/2 mm | 51.72 (8.15) | 87.30 (6.32) |
| 3%/2 mm | 53.78 (8.53) | 90.26 (5.70) |
| 3%/3 mm | 57.57 (7.49) | 92.82 (5.94) |
| Mean dose | D5 | D95 | ||
|---|---|---|---|---|
| pCT | 1.98 (0.01) | 2.04 (0.01) | 1.86 (0.07) | |
| ROI | sCTu | 2.10 (0.09) | 2.61 (0.28) | 1.64 (0.10) |
| sCTc | 1.93 (0.08) | 2.09 (0.05) | 1.54 (0.28) | |
| pCT | 0.47 (0.44) | 2.01 (0.04) | 0.00 (0.00) | |
| Bowel | sCTu | 0.93 (0.52) | 2.06 (0.31) | 0.00 (0.00) |
| sCTc | 0.48 (0.40) | 2.00 (0.13) | 0.00 (0.00) | |
| pCT | 0.65 (0.27) | 2.01 (0.03) | 0.00 (0.00) | |
| Stomach | sCTu | 0.97 (0.35) | 2.14 (0.24) | 0.00 (0.12) |
| sCTc | 0.64 (0.29) | 2.02 (0.11) | 0.00 (0.00) |
| Work | Model | Anatomic Site | axial FOV [mm] | Patient cohort | GPR 2%/2 mm |
|---|---|---|---|---|---|
| Hansen et al. [45] | Unet | Pelvis | 410 | 30 | 53% |
| Landry et al. [13] | Unet | Pelvis | 410 | 42 | 85% |
| Thummerer et al. [46] | UNet | Thorax | 500 | 33 | 90.7% |
| Kurz et al. [47] | cGAN | Pelvis | 550 | 33 | 96% |
| Uh et al. [20] | cGAN | Abdomen/Pelvis | 530 | 50 | 98.5% |
| This work | cGAN | Pelvis | 204 | 40 | 87.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





