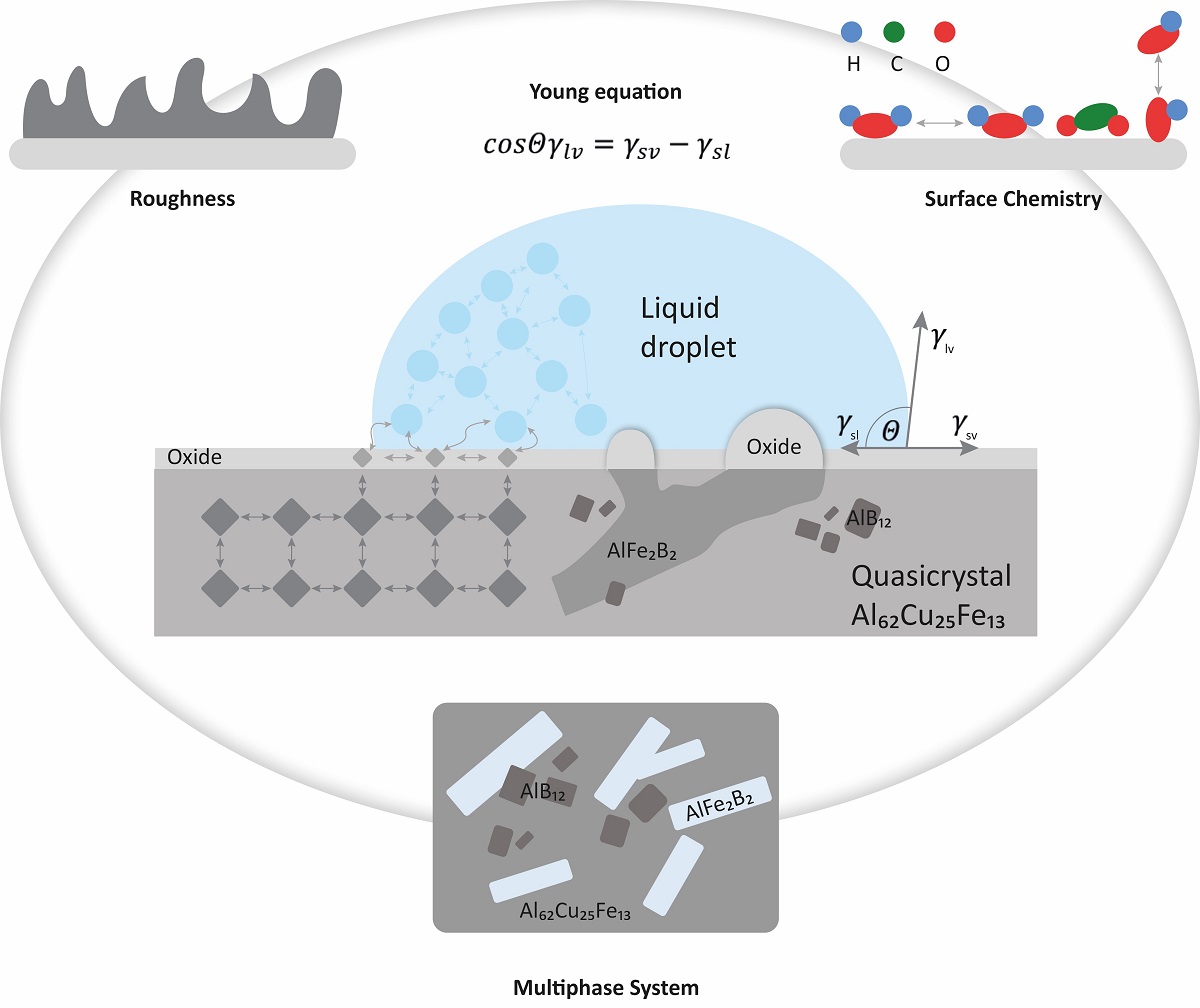
Introduction
The discovery of quasicrystals in melt-spun Al-Mn ribbons by Shechtman et al. in 1982 [
1] triggered a revolution in crystallography and changed our understanding of the structure of solids. Just a few years later, the existence of a thermodynamically stable quasicrystal was reported in the Al-Cu-Fe system by Tsai et al. [
2]. A stable quasicrystal can have several interesting properties, such as a high hardness, a low coefficient of friction, a low surface energy and a high oxidation and corrosion resistance [
3,
4]. These properties are appropriate for technological applications such as low-stick surfaces or mechanical devices with reduced friction [
5]. Therefore, it is necessary to assess the surface energy of a quasicrystal material that comes into contact with an antagonist like water or a lubricant in low-stick applications [
6] or a solid such as hard steel in mechanical applications [
7]. Among the methods used for surface-energy investigations, the sessile droplet technique is convenient for laboratory experiments. The measurements yield a contact angle
between the droplet of the test liquid and the surface of interest, neglecting the equilibrium pressure (
) of the adsorbed vapour from the liquid. The Young equation defines the angle
at equilibrium between the solid, the liquid, and its vapour:
where
,
and
are the solid-vapour, liquid-vapour, solid-liquid interfacial tensions [
8].
At thermodynamic equilibrium, the solid-vapour interfacial tension
is usually taken as the solid surface energy
. Similarly, the interfacial tension
is the liquid surface energy
. The authentic surface energy is determined as the surface excess free energy per unit area of a particular crystal facet and is a physical property of the material. The state of the solid surface can be quantified through the surface energy [
9]. It determines the equilibrium shape of the crystal and the adhesion energy at the interfaces with other materials; it plays an essential role in roughening, faceting, crystal growth and in the nucleation kinetics of precipitates [
10]. The surface energy can be computed [
9] for periodic crystals, for example, some elementary metals:
=1200 mJ/m
2,
=2200 mJ/m
2,
=1800 mJ/m
2. Unfortunately, computational methods are restricted to relatively simple compounds and do not apply to complex metallic alloys such as quasicrystals or their approximants with giant unit cells of up to thousands of atoms. Due to the absence of a periodic lattice for the surface energy of a quasicrystal we have to rely on indirect techniques, for instance, friction experiments in a vacuum [
11] or thin-film growth [
12]. The typical surface energy
of a quasicrystal is in the range 600–800 mJ/m
2 for aluminium-based alloys [
13], i.e., significantly less than the surface energy of the individual constituents [
9]. Theory suggests a reduction in the surface energy on quasicrystals as a consequence of the icosahedral symmetry and the formation of a deep pseudo-gap [
13] or the excitation of phason modes [
14]. In addition, freshly polished and dried samples have contact angles with water in the range 100–120°, much larger than the contact angle observed for aluminium, the main component of the alloy. This implies a sensitivity to the density of itinerant electrons in the bulk of the quasicrystal, beneath its superficial native oxide. Below 10–12 nm of oxide thickness, it has been shown to vary in inverse proportion to the thickness of the oxide [
13], suggesting a coupling between the electronic distribution on the liquid molecules (e.g., the electric dipole of the water molecule), on the one hand, and the topology of the quasicrystal’s Fermi surface on the other [
15]. The same trend was observed with water deposited on Al-based quasicrystalline coatings [
16,
17,
18,
19] and films [
20], or with quasicrystal-reinforced composites [
21], or even with liquid metals on Al-Co approximants [
22].
These quasicrystals, however, are always covered by a native oxide layer when exposed to the atmosphere. The amorphous alumina layer formed after a very brief time is a few nanometres thick [
23]. Annealing above room temperature, typically at 500 °C, leads to a significant growth of the oxide layer and causes the partition of the chemical species below the layer, due to the migration of Al atoms to feed the growth of the oxide [
24]. These studies were performed with laboratory samples made of pure QC phase. Technological applications require the preparation of the material under industrial conditions, using raw materials with an acceptable economic cost, containing impurities, and following an industrial protocol that does not necessarily provide a perfect quasicrystalline material [
23]. For instance, commercially available precursor quasicrystalline powders can be applied to prepare surface coatings with plasma spraying. These coatings need to be additionally heat treated after the deposition to eliminate undesirable crystalline phases that endanger the corrosion resistance of the coating and provoke the formation of the QC phases [
25]. The main property affecting this behaviour is the surface energy. However, as the material is covered by an oxide layer, we probe not the surface energy of the metallic material, but the surface energy of the material containing the oxide layer. To avoid confusion between these two entities, we coin a particular word to label the surface energy probed on top of the oxide layer: "surfenergy”. The surfenergy is a few tens of mJ/m
2 and it should not be confused with the surface energy of bare metallic materials found underneath the oxide, which is in the range of several hundred mJ/m
2 or more. In reality, the surfaces have physical (e.g., roughness) and chemical (e.g., functional groups) heterogeneities. The influences of various surface heterogeneities were studied by Wenzel [
26] and Good [
27] (for physical heterogeneity) or Cassie [
28] (for chemical heterogeneity). Oxidation behaviour has an influence on the physical properties of the interface, especially at elevated temperatures, and it is relevant for technological applications and determines the viability of valuable materials [
13,
23,
29].
This article reviews the surface behaviour of a sample made from atomised powder by sintering at a temperature close to the peritectic reaction, leading to quasicrystal growth. We study the equilibrium shape of the droplets of two different liquids, which are placed in contact with the surface of a quasicrystalline material based on Al, Cu, Fe and B. The goal is to investigate the oxidation behaviour of the quasicrystalline sample in the air and to determine to what extent the constituent species take part in the formation of the oxide layer.
Discussion
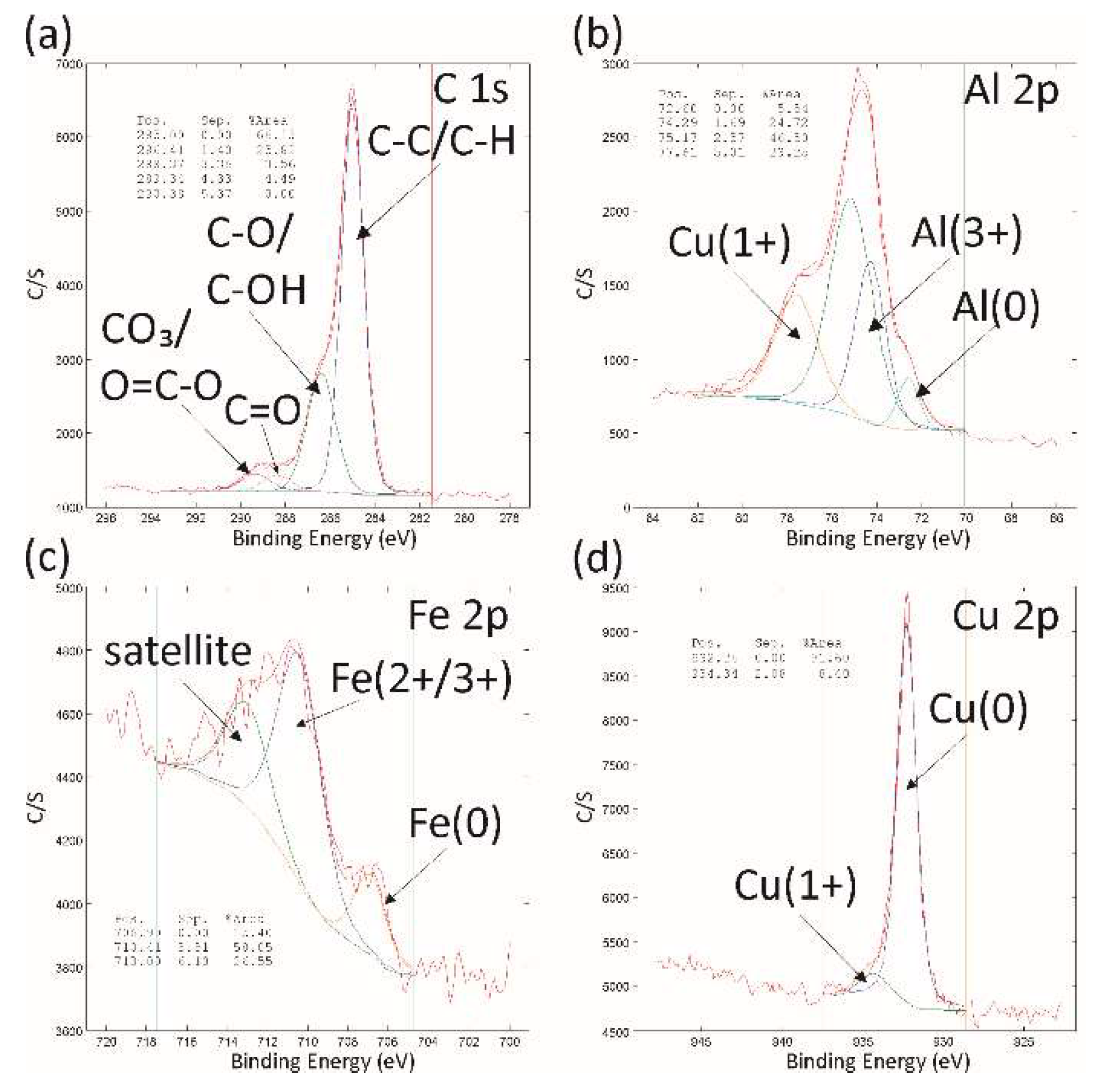
The surface oxidation is responsible for the samples’ properties, as proven by the XPS analysis, which revealed a diverse chemistry of various carbon-based hydrogen and dangling-oxide bonds on the top-most layer of the aged QC surface. In particular, the revealed oxidation states of elements deduced form the surface XPS spectra were in accordance with previous studies for comparable QC systems of Al-Fe-Cu-Cr [
29,
38], Al-Fe-Cu [
39] and Al-Fe-Cu-B [
40]. The measured XPS depth profile revealed an enrichment of the oxide and the aluminium, accompanied by a depletion of the iron and copper in the top surface layer. From this we concluded that the Al-oxide layer serves as a passivation agent, hindering any further oxidation in the interior of the QC bulk, as previously reported [
39]. The electron-diffraction analysis confirmed the presence of the i-phase with a 2-fold symmetry pattern, in agreement with the literature [
41], coexisting with an orthorhombic, needle-like AlFe
2B
2 phase and the AlB
12 phase with a globular morphology. The same was observed by Brien et al. [
30] for a sintered Al-Fe-Cu-B alloy. The TEM cross-sectional analysis of the i-phase revealed a smooth, 10-nm-thick, amorphous, oxide Al-rich layer after the longest ageing time. After annealing at 500 °C for 1 h, the initially amorphous, oxide layer on top of the i-phase was converted into an aluminium-oxide-rich nanocrystalline film with an average thickness of 20 nm. The needle-like AlFe
2B
2 phase grew in a more complex oxide assembly. The corresponding oxide thickness after annealing for 1 h at 500 °C increased to 50–60 nm, as confirmed by the AFM measurements. The transformation due to annealing was accompanied by a dramatic change in the topography of the surface, which was quantified by a 44 % greater roughness in comparison to that of the aged sample. The general composition of the oxide film on the matrix phase is aluminium (33 at.%) and oxygen (66 at.%). The secondary, AlFe
2B
2 phase consists of iron (27 at.%), oxygen (65 at.%) and a very small amount of aluminium (6 at.%), while the B content is unknown. As confirmed by the EDXS measurements, the matrix i-phase Al
62Cu
25Fe
13 and its surface oxide did not exhibit any detectable morphology changes. In contrast, the oxidation of the secondary AlFe
2B
2 phase implies morphological changes after ageing and even more drastically after annealing. This complex oxide grows in different forms, either in terms of sharp needles or dome-like objects, depending on the annealing times.
The amount of boron trapped in the quasicrystalline sample is an additional technological optimization parameter, where the surface roughness and the resulting surfenergy can be, to some extent, controlled by the amount of B-doping, without significantly altering the dominant matrix QC phase. The oxidation behaviour for both cases, during ageing and annealing, exposes different trends for surface oxidation, which is directly related to the underlying crystal phase. However, the average oxide film thickness remains significantly lower for the aged samples when compared to their annealed counterparts. It can be assumed that the surface energy is also influenced by the subsurface chemistry and not just the formation of the oxide layer and the resulting surface morphology.
The surface roughness and the oxide population influence the surfenergy. The tendency of the QC-treated surfaces to have a hydrophobic behaviour can be, in the simplest terms, explained by their increased surface roughness in the nm range. However, it is known that the hydrophobic behaviour can be controlled by the surface roughness, as previously shown in reference [
26]. As a rule of thumb, the level of hydrophobicity grows with the roughness, which is affected by the shape and size of the oxide forms. On the other hand, there is a relation between the oxides and the two components of the surfenergy.
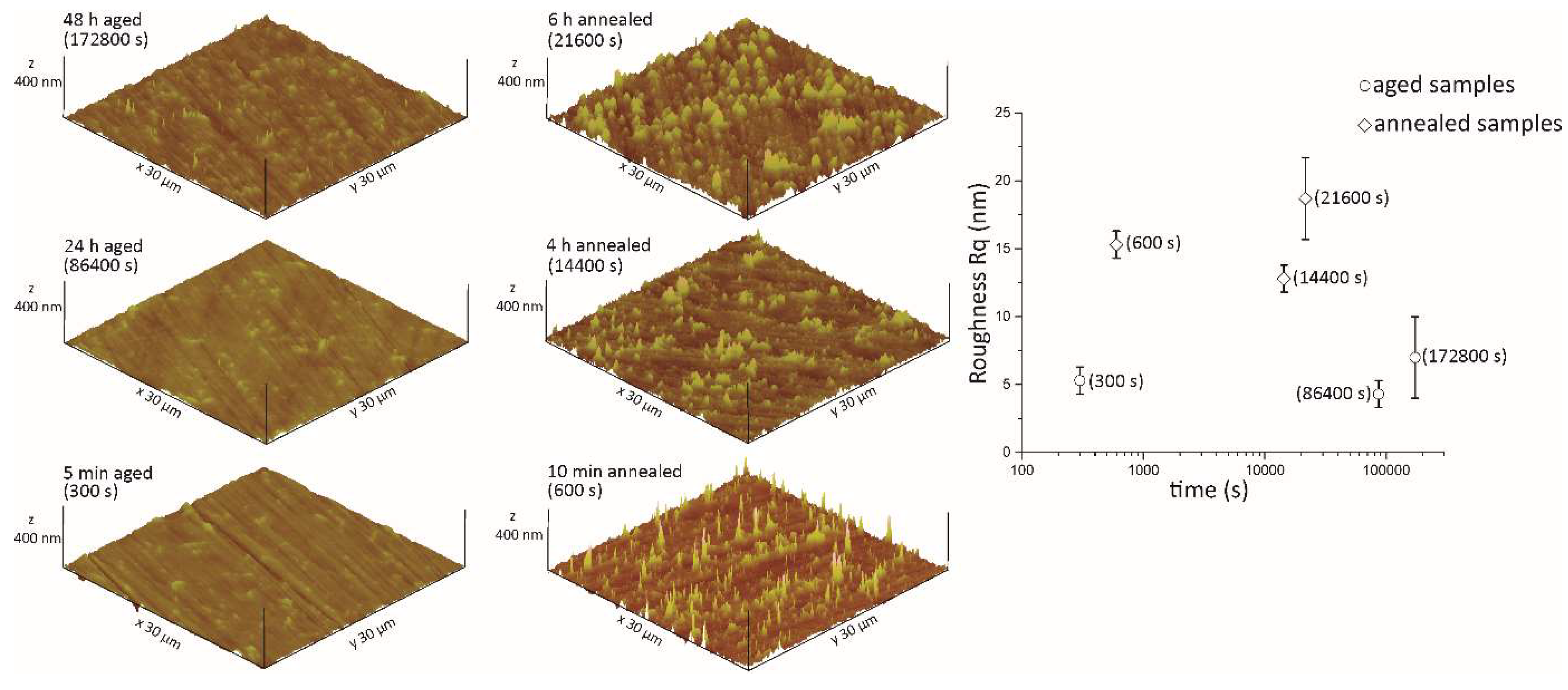
The changes in roughness are mostly associated with nanocrystalline oxides above the AlFe2B2 phase at various time intervals. Namely, for shorter annealing times, the oxide phase is mainly in the form of sharp needles forming above the surface. In contrast, for the longest annealing times, the formation of various oxides becomes largely delocalized in a uniform, dome-shaped oxide layer, populating the sample’s surface.
The presence of needles and domes is reflected in an enhanced surface roughness and energy. The latter phenomenon can be explained at the atomistic level by means of charge accumulation at the tips [
42]. A high charge density due to a specific morphology, in our case in the oxides above the secondary phase, is associated with an increased surface energy in comparison with a flat sample, in agreement with previous literature reports (Dubois [
23]).
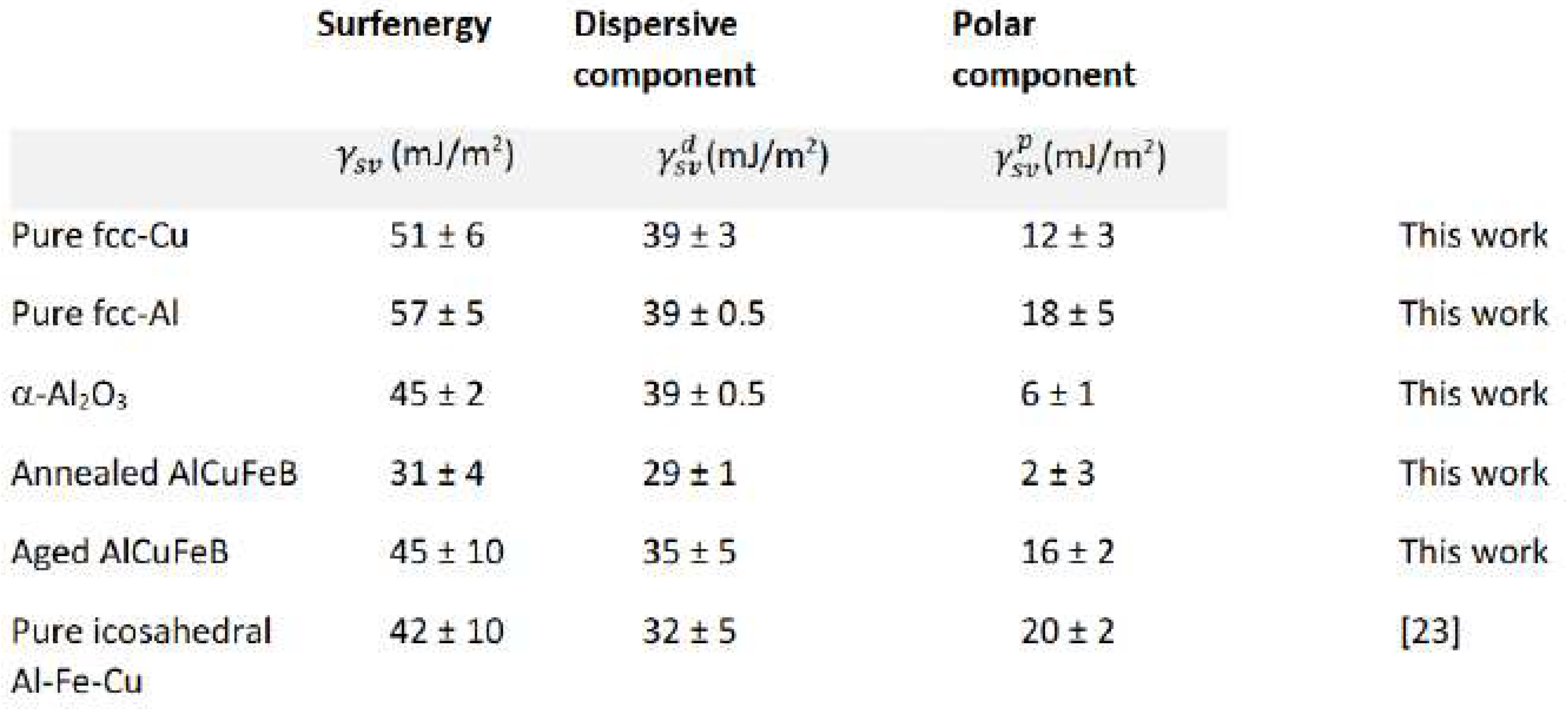
The experimental setup makes it possible to assess the surfenergy with its two main constituents, i.e., the dispersive and polar surfenergy components. To better understand the behaviour of the surfenergy components, a droplet experiment was performed on different materials, see
Table 2. The results revealed a significant difference between our material and the pure aluminium, the main component of the quasicrystalline sample. The dispersive components match to within 8 %, because it is less sensitive to the structure, morphology and chemical composition. However, there is a significant difference in the polar components, ascribed to the influence of the processing techniques. As highlighted in the following text, in strong contrast to these reference materials, the quasicrystalline sample does exhibit a much lower polar component under suitable preparation conditions. We assigned these differences to a coexistence with the majority i-phase of a small amount of a needle-like AlFe
2B
2 phase that promotes the formation of specific oxides on the sample surface.
The surface morphology has direct and indirect influences on the behaviour of the liquid droplets. The indirect influence is related to the distribution of electric charges and, consequently, to the energy of the surface, whereas the shape of the droplet is directly affected by the surface landscape.
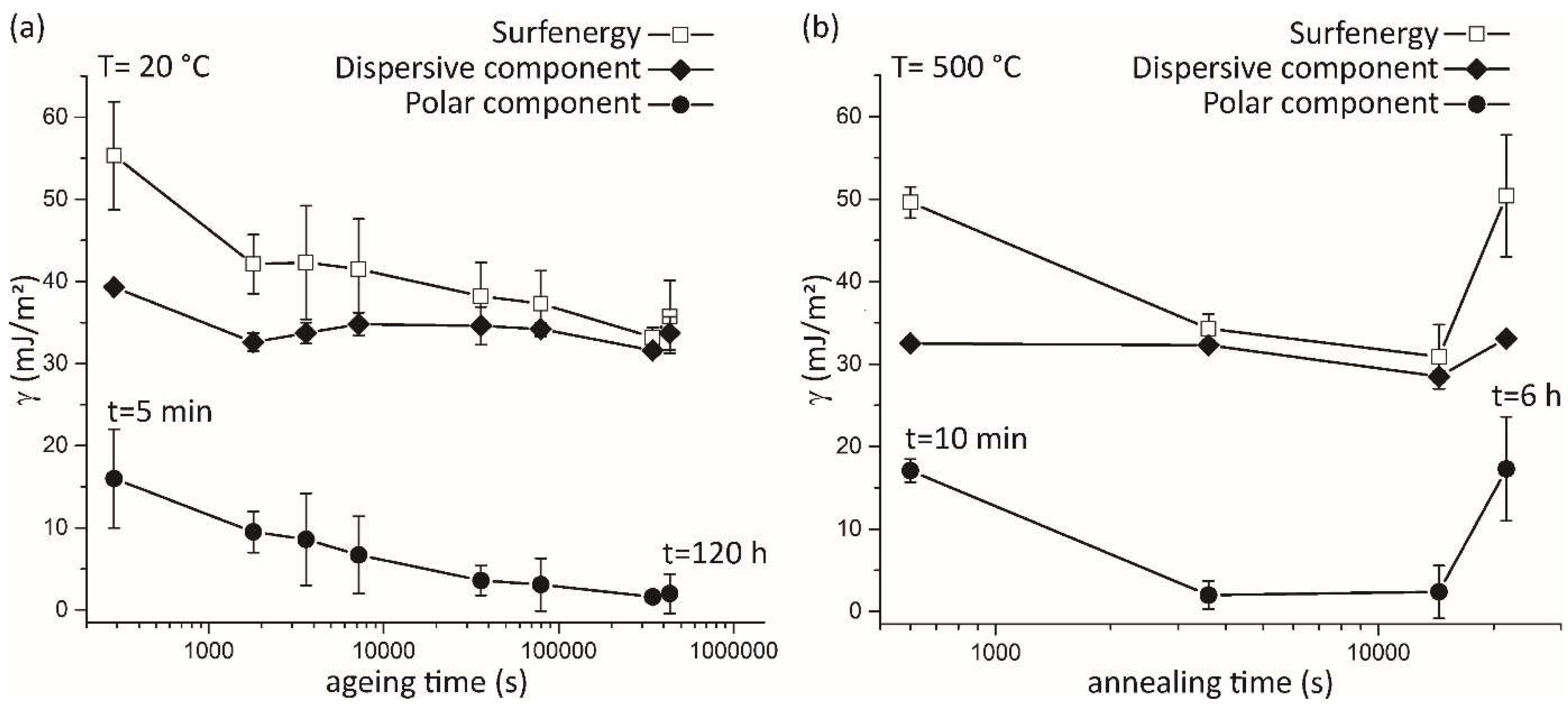
Conceptually, both polar and dispersive components can contribute to the overall surfenergy. However, as shown in this study, an oxide film thickness of a minimum 5–10 nm completely masks the QC contribution of the dispersive component, which can be attributed solely to the amorphous oxide layer. Therefore, the dispersive component remains time independent, regardless of the oxide-layer thickness. The only noticeable drop in the dispersive component occurs at an early stage of ageing, when the oxide-layer thickness is sufficiently low, below 5 nm. It is worth mentioning that the droplet experiment for the surfenergy was performed about 5 min after polishing, whereas the XPS measurements were carried out at least 15 min later, preventing us from obtaining an insight into the surface chemistry before complete oxidation. Extremely low values of the dispersive contribution to the QC surface energy, close to the data for polymers (Teflon) in previous studies [
13], were obtained by applying a high vacuum, so preventing surface oxidation. Those experiments are of limited technological importance for atmospheric/ambient conditions. Therefore, it is very desirable to prevent excessive oxide-layer growth and to retain the surface influence of the QC electronic states. This can be achieved by means of tuning the sample-processing parameters. The goal is to find a balance between the polar and the dispersive component, since the former is decreased with the ageing/annealing times. The rapid decay of the polar component can be explained by the liquid-molecule reorientation and the surface-charges redistribution due to the electrostatic forces, leading to an energy minimization. This is reflected in the relaxation of the respective atomic positions and the lowering of the surfenergy. The restriction is maintaining the pseudo gap in the electronic structure, which is characteristic for QC materials, and apparently responsible for desirable frictional and wetting properties [
43].
Since the majority of the oxide layer is formed above the B-containing phase, it might be important to control the content of boron. The amount of boron trapped in the quasicrystalline sample opens an additional technological optimization parameter, directly influencing the roughness without changing the matrix phase. Utilizing the investigated material for future technologies should be focused on the processing optimization and the boron content related to the interplay between the polar and the dispersive component of the surfenergy.
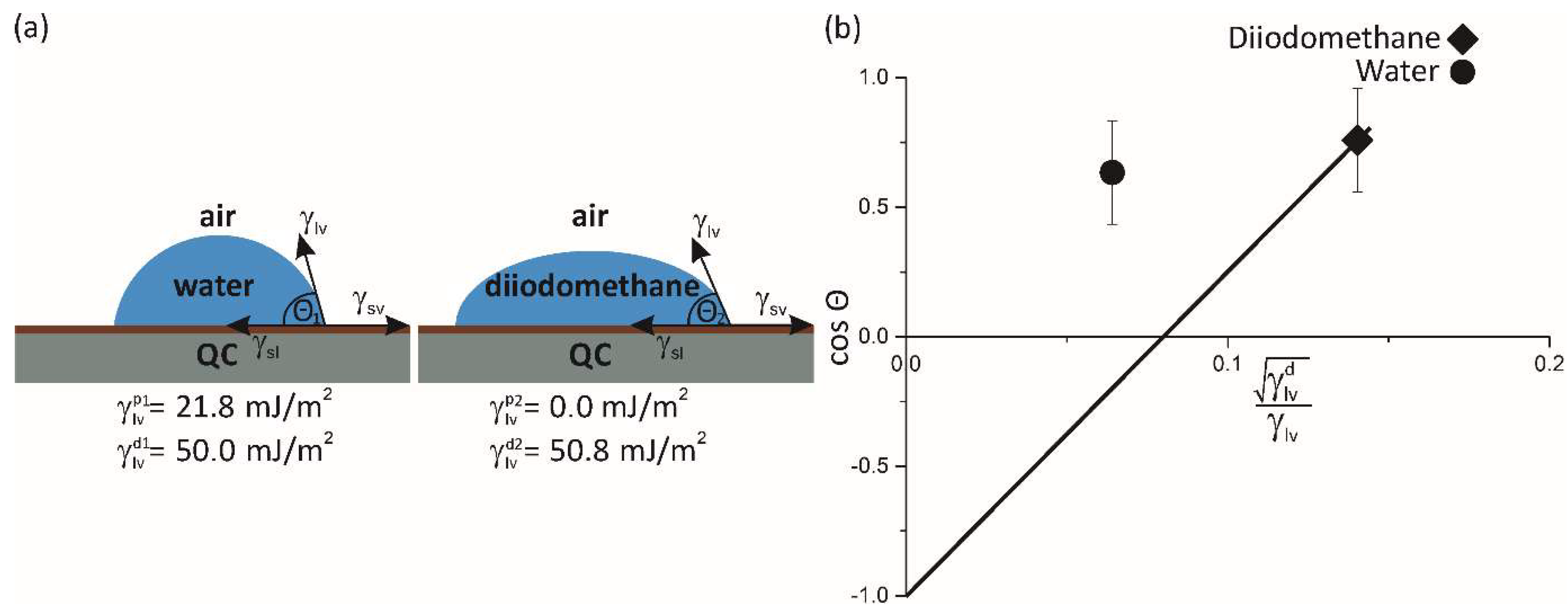
Figure 1.
Sketch of a sessile drop, vapour/liquid/solid system. The contact angle is shown (). (a) the shape of a water droplet as a polar liquid on a QC surface, and the shape of a diiodomethane droplet as an apolar liquid on a QC surface with associated components. (b) The plot of cos versus parameter for pure water (labelled by a full circle) and diiodomethane (labelled by a diamond) for our quasicrystalline sample.
Figure 1.
Sketch of a sessile drop, vapour/liquid/solid system. The contact angle is shown (). (a) the shape of a water droplet as a polar liquid on a QC surface, and the shape of a diiodomethane droplet as an apolar liquid on a QC surface with associated components. (b) The plot of cos versus parameter for pure water (labelled by a full circle) and diiodomethane (labelled by a diamond) for our quasicrystalline sample.
Figure 2.
(a) Evolution of the dispersive (solid diamonds) and polar (full circles) components of the total QC surfenergy (open squares) during the ageing experiment at room temperature for different times on a logarithmic scale. Symbols are connected by solid lines to guide the eyes. (b) Variation of the dispersive and polar components of the surfenergy for different annealing times at 500 °C.
Figure 2.
(a) Evolution of the dispersive (solid diamonds) and polar (full circles) components of the total QC surfenergy (open squares) during the ageing experiment at room temperature for different times on a logarithmic scale. Symbols are connected by solid lines to guide the eyes. (b) Variation of the dispersive and polar components of the surfenergy for different annealing times at 500 °C.
Figure 3.
XPS spectra C 1s, Al 2p/Cu 3p, Fe 2p and Cu 2p with indicated oxidation states measured on the sample surface after ageing for 0.5h.
Figure 3.
XPS spectra C 1s, Al 2p/Cu 3p, Fe 2p and Cu 2p with indicated oxidation states measured on the sample surface after ageing for 0.5h.
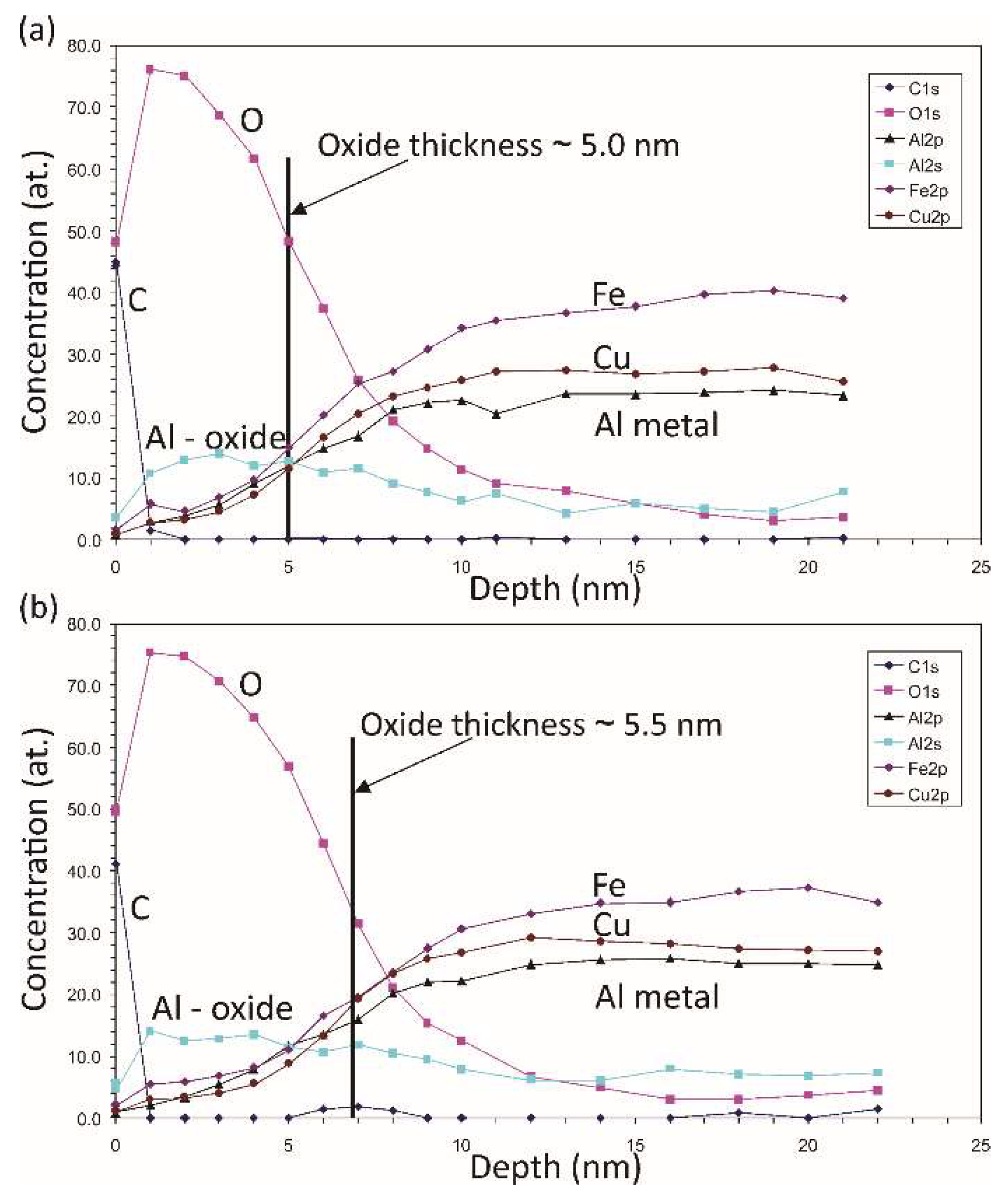
Figure 4.
XPS depth profiles of elements measured on the sample after ageing in air at room temperature (a) for 0.5 h and (b) after 22 h.
Figure 4.
XPS depth profiles of elements measured on the sample after ageing in air at room temperature (a) for 0.5 h and (b) after 22 h.
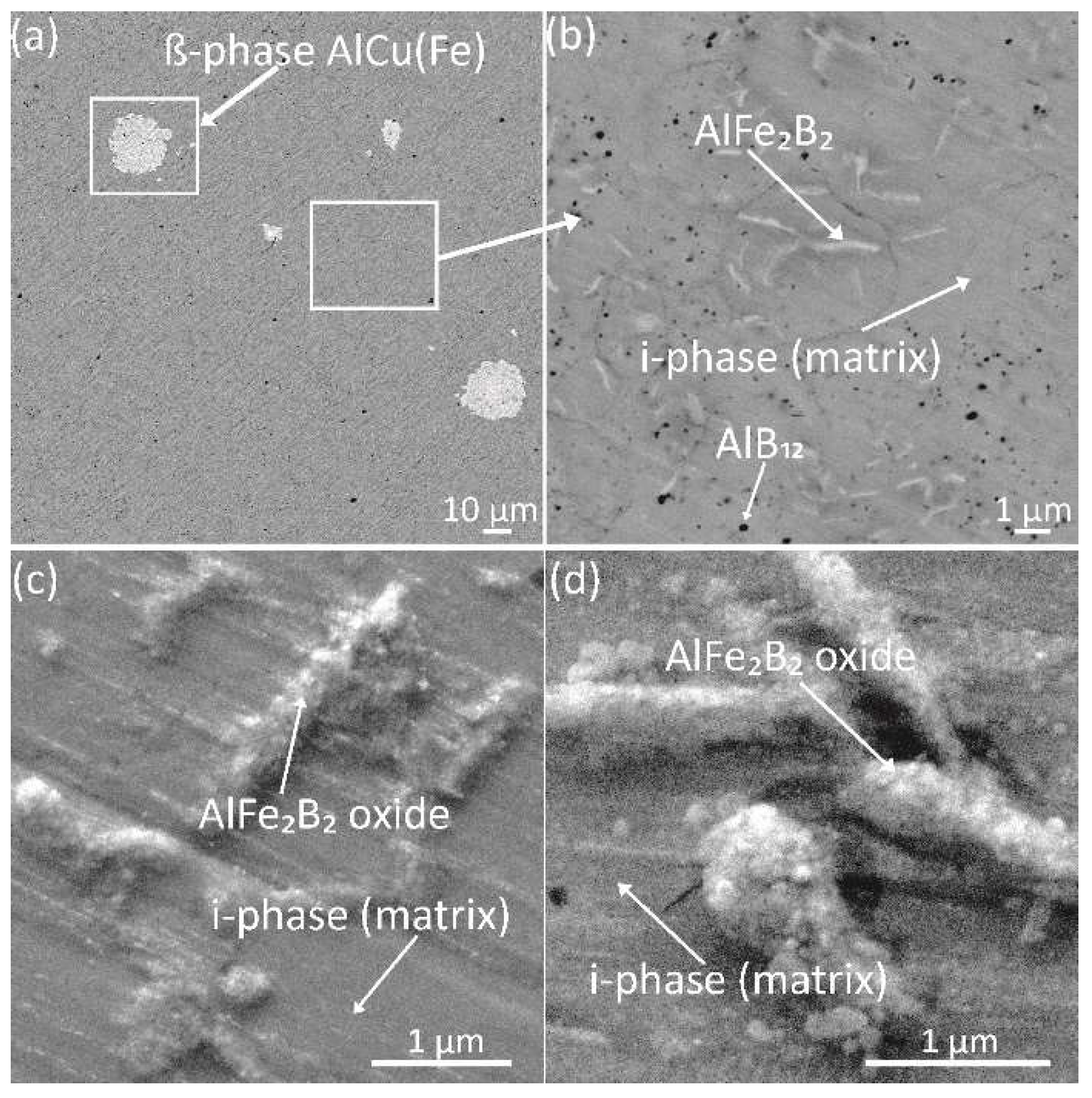
Figure 5.
SEM images of the Al59Cu25Fe13B3 sample when aged and annealed for different times, (a) overall microstructure (b) characteristic higher-magnification micrograph of the phases, (c) and (d) high-magnification micrographs show the growth of the oxide observed on top of the AlFe2B2 phase after annealing for 1 h and 6 h at 500 °C, respectively.
Figure 5.
SEM images of the Al59Cu25Fe13B3 sample when aged and annealed for different times, (a) overall microstructure (b) characteristic higher-magnification micrograph of the phases, (c) and (d) high-magnification micrographs show the growth of the oxide observed on top of the AlFe2B2 phase after annealing for 1 h and 6 h at 500 °C, respectively.
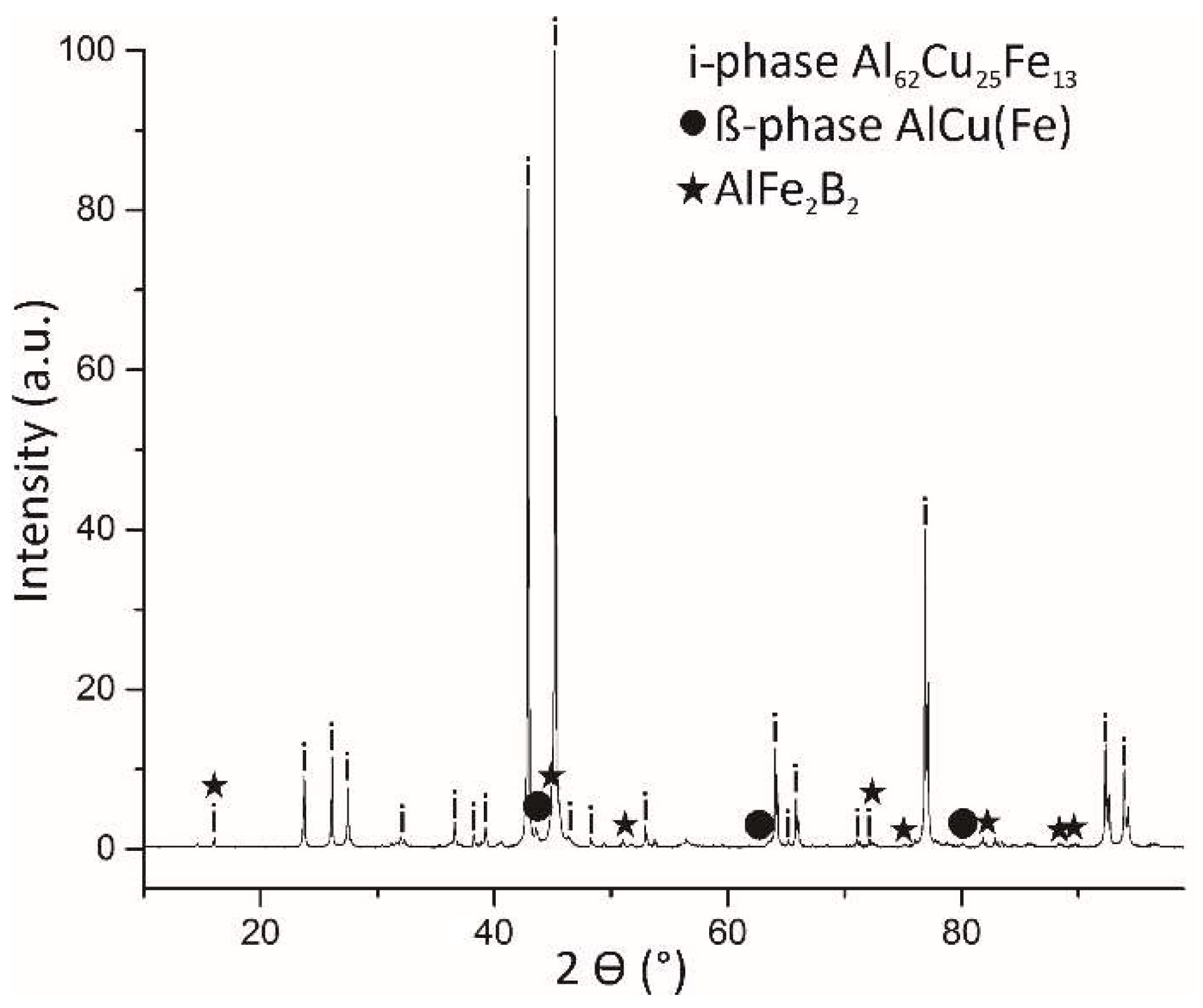
Figure 6.
PXRD pattern of the quasicrystalline material with nominal composition of Al59Cu25Fe13B3 (at.%). The highest-intensity peaks belong to the icosahedral phase (i-phase). The other minority phases are β-AlFe(Cu) and AlFe2B2.
Figure 6.
PXRD pattern of the quasicrystalline material with nominal composition of Al59Cu25Fe13B3 (at.%). The highest-intensity peaks belong to the icosahedral phase (i-phase). The other minority phases are β-AlFe(Cu) and AlFe2B2.
Figure 7.
Surface topography and roughness (Rq) of the quasicrystalline material with a nominal composition of the Al59Cu25Fe13B3 (at.%).
Figure 7.
Surface topography and roughness (Rq) of the quasicrystalline material with a nominal composition of the Al59Cu25Fe13B3 (at.%).
Figure 8.
TEM images of the QC annealed at 500 °C for 1 h in air. (a) Overall image with the presented phases in the bulk, (b) High-magnification image of the oxides on the surface labelled as 1 and 2 with associated FFT pattern of the oxide layer. The insets show the electron-diffraction patterns of the elongated ternary AlFe2B2 phase (up) and the i-phase along the 2-fold axis (down). (c) shows the EDXS mapping of the high-magnification oxide layer above the AlFe2B2 phase and the i-phase.
Figure 8.
TEM images of the QC annealed at 500 °C for 1 h in air. (a) Overall image with the presented phases in the bulk, (b) High-magnification image of the oxides on the surface labelled as 1 and 2 with associated FFT pattern of the oxide layer. The insets show the electron-diffraction patterns of the elongated ternary AlFe2B2 phase (up) and the i-phase along the 2-fold axis (down). (c) shows the EDXS mapping of the high-magnification oxide layer above the AlFe2B2 phase and the i-phase.
Table 1.
Liquid-vapour interfacial tensions, decomposed into the dispersive and polar components for water and diiodomethane [
33].
Table 1.
Liquid-vapour interfacial tensions, decomposed into the dispersive and polar components for water and diiodomethane [
33].
| |
Liquid-vapour interfacial tension |
Dispersive component |
Polar component |
| |
(mJ/m2) |
(mJ/m2) |
(mJ/m2) |
Purified
milli Q water
|
72.8 |
21.8 |
51.0 |
| Diiodomethane (CH2I2) |
50.8 |
50.8 |
0 |
Table 2.
Surfenergy components measured on samples relevant to the present study, pure copper and aluminium metals, sintered α-Al2O3 alumina, and the Al-Cu-Fe-B in comparison to data from the literature.
Table 2.
Surfenergy components measured on samples relevant to the present study, pure copper and aluminium metals, sintered α-Al2O3 alumina, and the Al-Cu-Fe-B in comparison to data from the literature.
Table 3.
Chemical composition (in at.%) of phases inside the quasicrystalline sample with a nominal composition of Al59Cu25Fe13B3.
Table 3.
Chemical composition (in at.%) of phases inside the quasicrystalline sample with a nominal composition of Al59Cu25Fe13B3.
| Phase assigned from PXRD |
Shape of the phase |
Size of the phase
(µm) |
Detected Composition with EDXS (at.%) |
| i-phase Al62Cu25Fe13
|
Spherical granules |
1–5 |
Al62Cu25Fe13
|
| AlFe2B2
|
Needles |
2 x 1 |
Al22Fe24Cu3B51
|
| Nodule of β-phase AlCu(Fe) |
flower morphology |
10 |
Al52Fe8Cu36Cr3Ni1
|
| AlB12
|
globules |
≥1 |
Al6B94
|