Submitted:
18 April 2023
Posted:
19 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
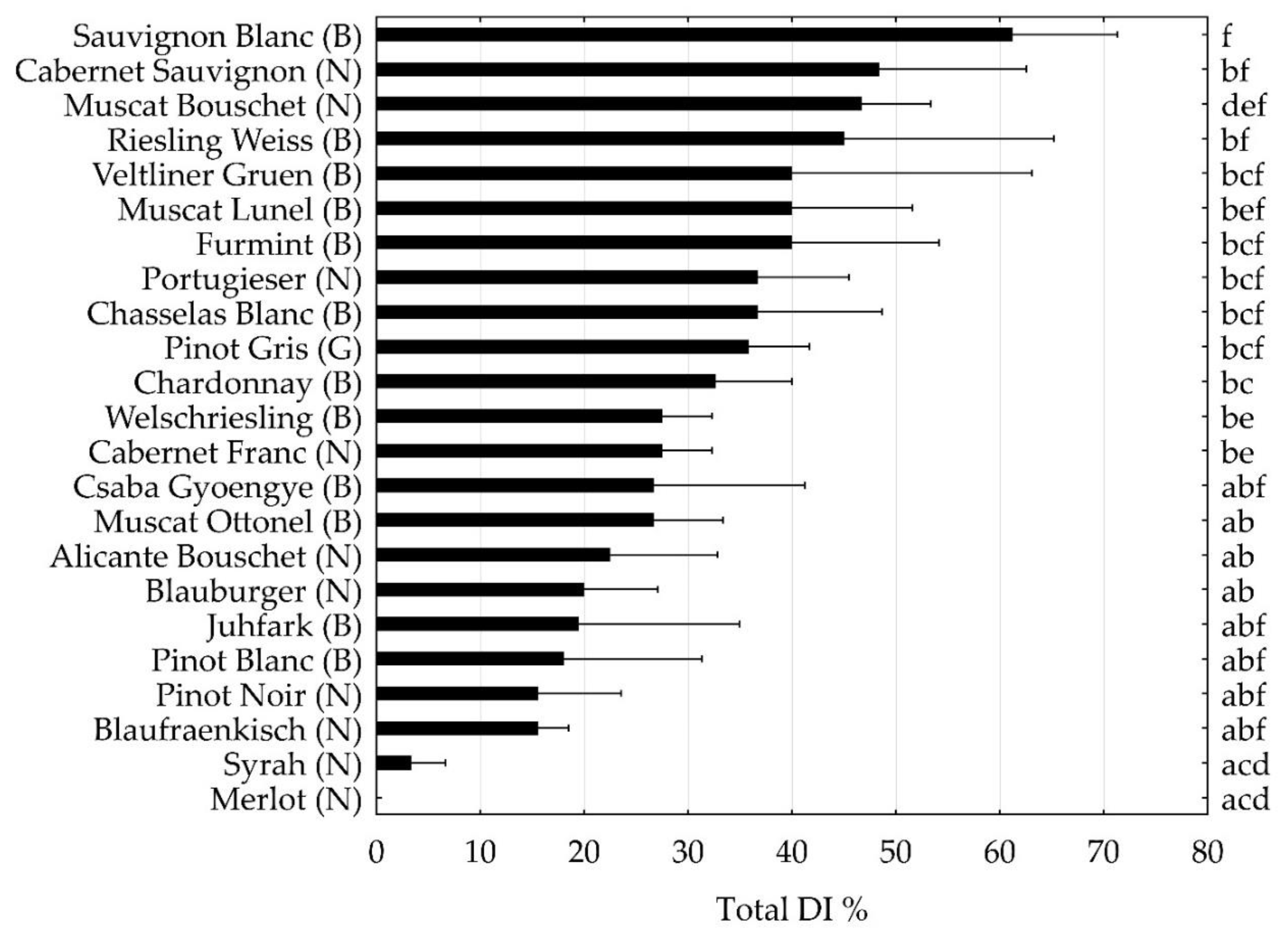
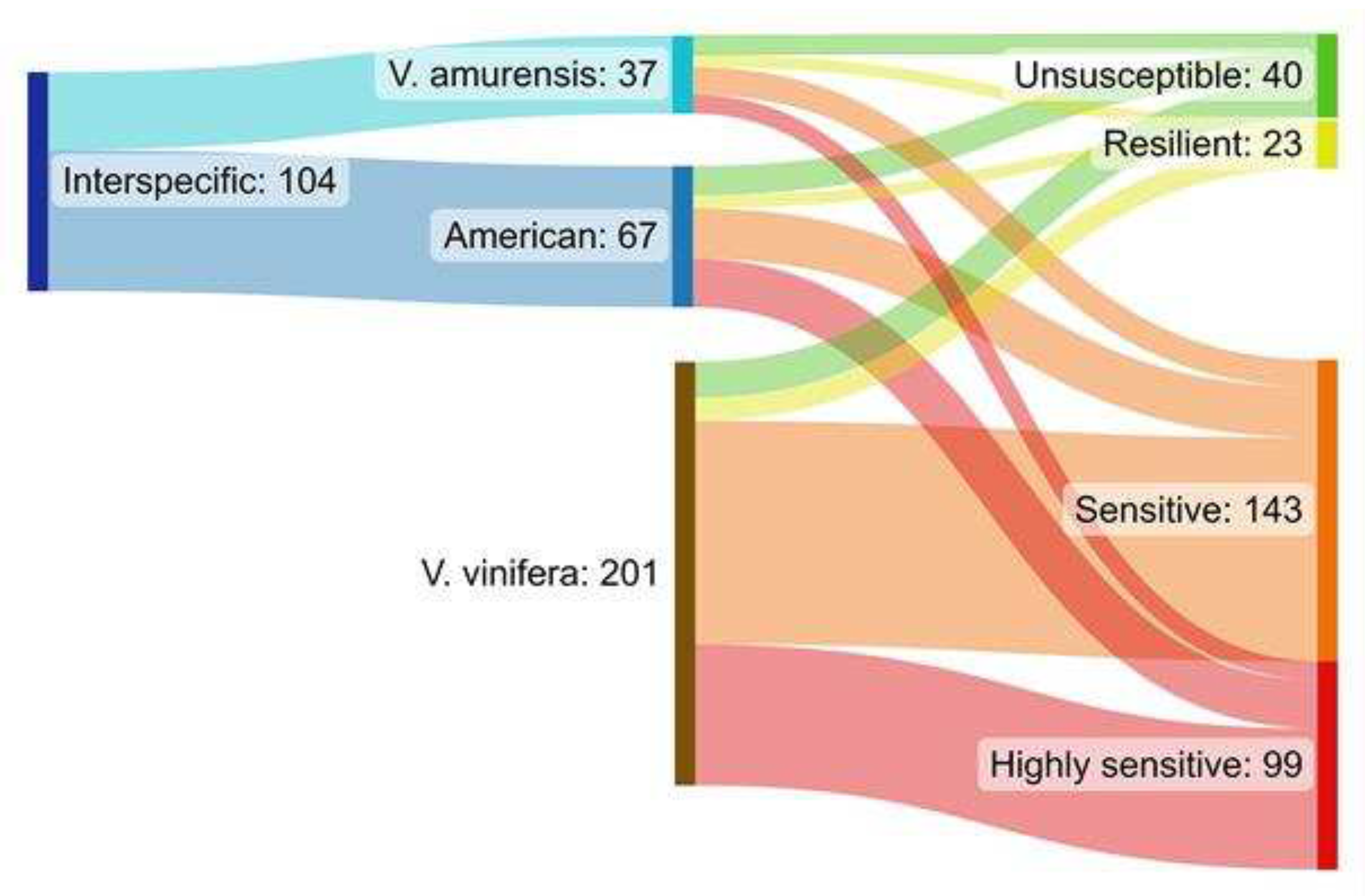
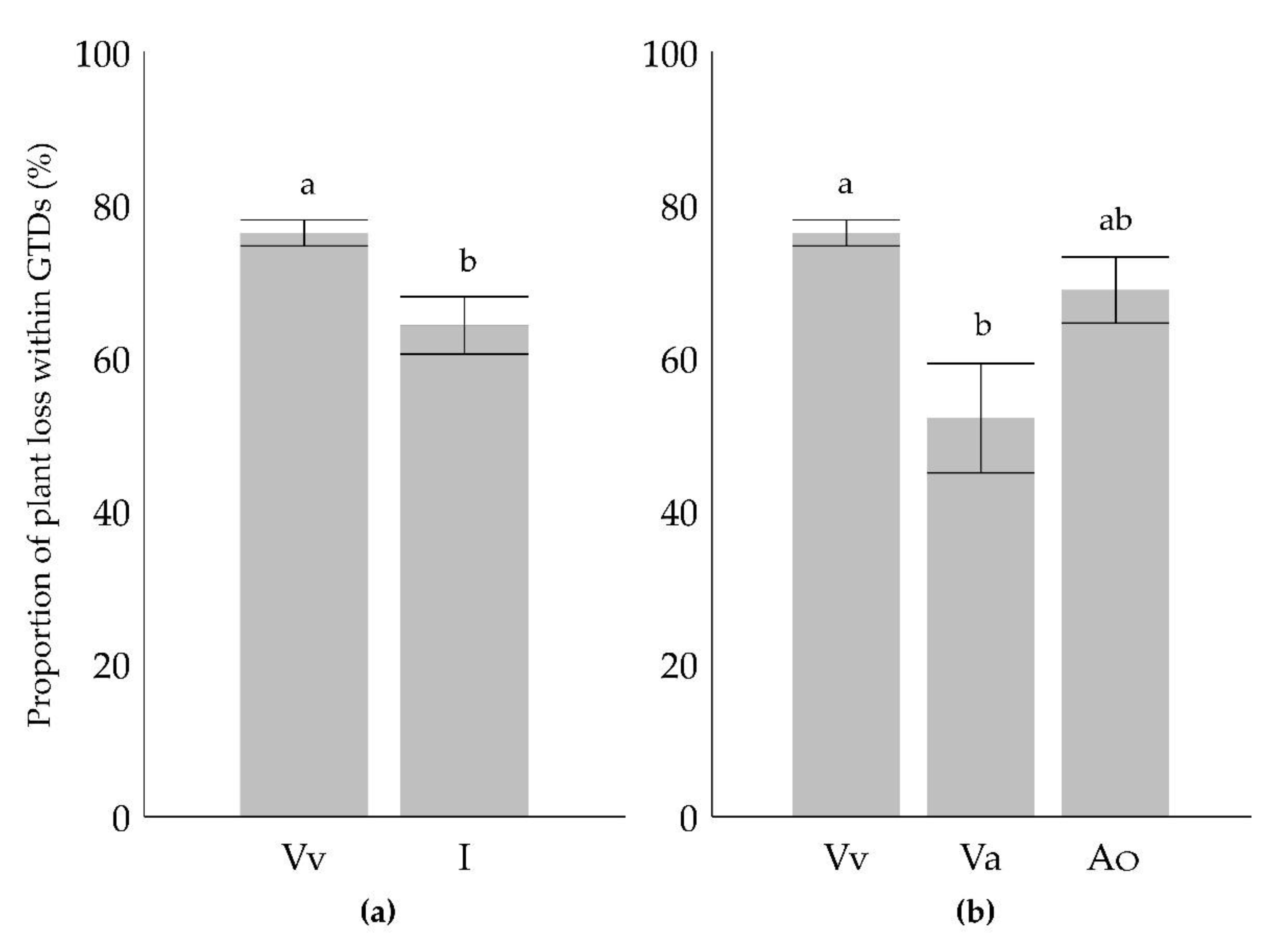
2. Results
3. Discussion
4. Materials and Methods
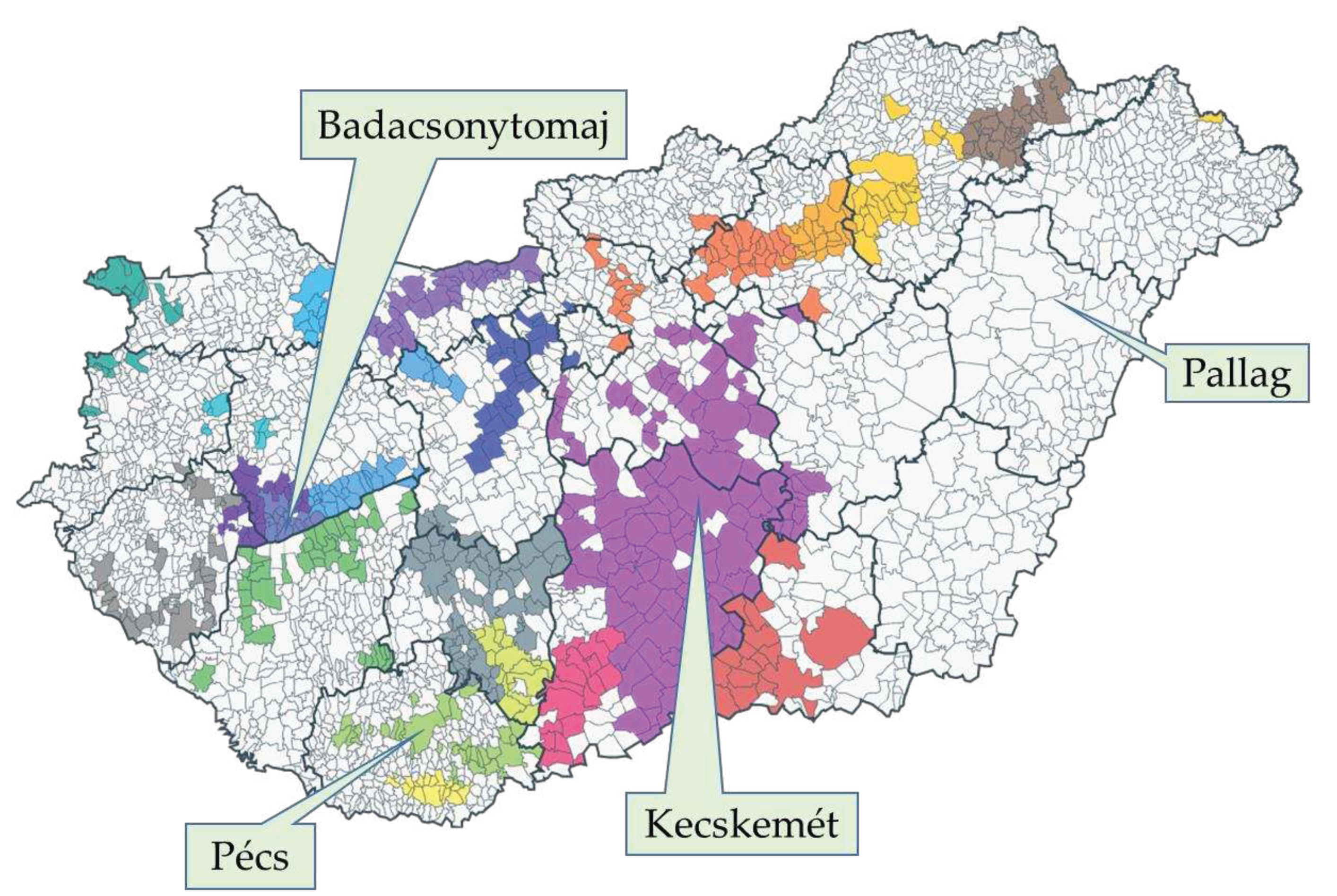
4.1. Survey sites and cultivars
| Badacsonytomaj | Kecskemét | Pallag | Pécs | |
|---|---|---|---|---|
| Soil | erubase soil | sand | acidic sand | Brown earth |
| Relief | mountain slope (top-valley row direction, terrace cultivation) | lowland | lowland | mountain slope (terrace cultivation) |
| Cultivation type | grafted | own rooted | own rooted | grafted |
| Climate | submediterranean with dry, warm summer | continental | continental | submediterranean with dry, warm summer |
| Relative climate sector1 | IIIc | Ib | Ia | IIIb |
| Average temperature fluctuation (°C) | 21-22 | 23-24.5 | 23-24 | 21-22 |
| Annual precipitation (mm) | 600-800 | 500-550 | 550-700 | 600-800 |
| Annual sunshine duration (h) | 1950-2050 | 2000-2150 | 1900-2050 | 2000-2100 |
4.2. Data analysis
4.2.1. Susceptibility analysis
4.2.2. Sensitivity categories and analysis
4.3. Statistical analysis and software background
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hofstetter, V.; Buyck, B.; Croll, D.; Viret, O.; Couloux, A.; Gindro, K. What if esca disease of grapevine were not a fungal disease? Fungal Divers. 2012, 54, 51–67. [Google Scholar] [CrossRef]
- De la Fuente, M.; Gramaje, D.; Armengol, J.; Fontaine, F.; Smart, R.; Nagy, Z.A.; Borgo, M.; Rego, C.; Corio-Costet, M.-F. ;. Grapevine Trunk Diseases. A Review; OIV Publications: Paris, France, 2016; ISBN 979-10-91799-60-7. https://hal.science/hal-01604038/. (accessed on 28 03 2023); ISBN 979-10-91799-60-7. [Google Scholar]
- Pollastro, S.; Dongiovanni, C.; Abbatecola, A.; Faretra, F. Observations on the fungi associated with esca and on spatial distribution of esca symptomatic plants in Apulian (Italy) vineyards. Phytopathol. Mediterr. 2000, 39, 206 210. Available online: http://www.jstor.org/stable/26456547 accessed on 28 March 2023.
- Kovács, C.; Balling, P.; Bihari, Z.; Nagy, A.; Sándor, E. Incidence of grapevine trunk diseases is influenced by soil, topology and vineyard age, but not by Diplodia seriata infection rate in the Tokaj Wine Region, Hungary. Phytoparasitica 2017, 45, 21–32. [Google Scholar] [CrossRef]
- Martín, M.T.; Cobos, R. Identification of fungi associated with grapevine decline in Castilla y León (Spain). Phytopathol. Mediterr. 2007, 46, 18–25, http://www.jstor.org/stable/26463267. [Google Scholar]
- Úrbez-Torres, J.R.; Haag, P.; Bowen, P.A.; O’Gorman, D.T. Grapevine Trunk Diseases in British Columbia: Incidence and characterization of the fungal pathogens associated with esca and Petri diseases of grapevine. Plant Dis. 2014, 98, 469 482. [Google Scholar] [CrossRef] [PubMed]
- Surico, G.; Marchi, G.; Braccini, P.; Mugnai, L. Epidemiology of esca in some vineyards in Tuscany (Italy). Phytopathol. Mediterr. 2000, 39, 190–205. [Google Scholar] [CrossRef]
- Calzarano, F.; Di Marco, Wood, S. Discoloration and decay in grapevines with esca proper and their relationship with foliar symptoms. Phytopathol. Mediterr. 2007, 46, 96–101. [Google Scholar] [CrossRef]
- Lehoczky, J. Black dead arm disease of grapevine caused by Bortyosphaeria stevensii infection. Acta Phytopathol. Hun. 1974, 9, 319–327. [Google Scholar]
- Marchi, G.; Peduto, F.; Mugnai, L.; Di Marco, S.; Calzarano, F.; Surico, G. Some observations on the relationship of manifest and hidden esca to rainfall. Phytopathol. Mediterr. 2006, 45, 117–126, http://www.jstor.org/stable/26463242. (accessed on 28 03 2023). [Google Scholar] [CrossRef]
- Lecomte, P.; Darrieutort, G.; Dewasme, C.; Blancard, D.; Goutouly, J.P.; Rey, P.; Guérin-Dubrana, L. Impact of biotic and abiotic factors on the development of Esca decline disease. Integrated protection and production in viticultureIOBC/ wprs Bulletin 2009, 2011, 67. 171–180, https://iobc-wprs.org/product/impact-of-biotic-and-abiotic-factors-on-the-development-of-esca-decline-disease/. (accessed on 28 03 2023). [Google Scholar]
- Jakab, M.K.; Werner, J.; Csikászné Krizsics, A. Az évjáratok hatása a faszöveti betegségek tüneteinek jelentkezésére különböző szőlőfajtákon.G. for Agric. 2016, 20, 39-43. Available online: https://journal.uni-mate.hu/index.php/gfa/issue/view/433/138 (accessed on 1 April 2023).
- Gramaje, D.; Úrbez-Torres, J.R.; Sosnowski, M.R. Managing Grapevine Trunk diseases With Respect to Etiology and Epidemiology: Current Strategies and Future Prospects. Plant Dis. 2018, 102, 1, 1239, 31. [Google Scholar] [CrossRef]
- Surico, G.; Mugnai, L.; Marchi, G. Older and more recent observations on esca: a critical overview. Phytopathol. Mediterr. 2006, 45, 68–86. [Google Scholar] [CrossRef]
- Dubos, B. 2002 Le syndrome de l'Esca. In Editions Féret, Maladies cryptogamiques de lavigne, 2° ed., Bordeaux: 127-136. cited in: Dewasme, C.; Mary, S.; Darrieutort, G.; Roby, J.P.;Gambetta, G.A. (2022). Long-Term Esca Monitoring Reveals Disease Impacts on Fruit Yield and Wine Quality. Plant Dis., 106(12), 3076–3082. [CrossRef]
- Fussler, L.; Kobès, N.; Bertrand, F.; Maumy, M.; Grosman, M.; Savary, S. A characterization of grapevine trunk diseases in France from data generated by the National Grapevine Wood Disease Survey. Phytopathology 2008, 98, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Songy, A.; Fernandez, O.; Clément, C.; Larignon, P.; Fontaine, F. Grapevine trunk diseases under thermal and water stresses. Planta 2019, 249, 1655–1679. [Google Scholar] [CrossRef] [PubMed]
- Dewasme, C.; Mary, S.; Darrieutort, G.; Roby, J.-P.; Gambetta, G.A. Long-Term Esca Monitoring Reveals Disease Impacts on Fruit Yield and Wine Quality. Plant Disease 2022, 106(12), 3076–3082. [Google Scholar] [CrossRef] [PubMed]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine Trunk Diseases: A Review of Fifteen Years of Trials for Their Control with Chemicals and Biocontrol Agents. Plant Dis. 2018, 102(7), 1189–1217. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, M.; Ayres, M.; Wicks, T.; McCarthy, M. In search of resistance to grapevine trunk diseases. Wine Vitic. J. 2013, 28(4), 55–58. Available online: https://winetitles.com.au/wvj/articles/wine-viticulture-journal-volume-28-no-4-2013/in-search-of-resistance-to-grapevine-trunk-diseases/.
- Guan, X.; Essakhi, S.; Laloue, H.; Nick, P.; Bertsch, C.; Chong, J. Mining new resources for grape resistance against Botryosphaeriaceae: a focus on Vitis vinifera subsp. sylvestris. Plant Pathol. 2016, 65, 273–284. [Google Scholar] [CrossRef]
- Martínez-Diz, M.P.; Díaz-Losada, E.; Barajas, E.; Ruano-Rosa, D.; Andrés-Sodupe, M.; Gramaje, D. Screening of Spanish Vitis vinifera germplasm for resistance to Phaeomoniella chlamydospora. Sci. Hortic. 2019, 246, 104–109. [Google Scholar] [CrossRef]
- Reynolds, A.G. 2015 Grapevine breeding in France – a historical perspective. In: Woodhead Publishing Series in Food Science, Tecgnology and Nutrition, Grapevine Breeding Programs for the Wine Industry, Reynolds, A.G. Ed.; Woodhead Publishing, 2015; 65-76. [CrossRef]
- Kozma, P. A szőlő és termesztése I. Akadémiai kiadó: Budapest, Hungary; 2000; Volume 91, p. 319. ISBN 9630577208. [Google Scholar]
- Buonassisi, D.; Colombo, M.; Migliaro, D.; Dolzani, C.; Peressotti, E.; Mizzotti, C.; Velasco, R.; Masiero, S.; Perazzolli, M.; Vezzulli, S. Breeding for grapevine downy mildew resistance: a review of “omics” approaches. Euphytica 2017, 213(5), 103. [Google Scholar] [CrossRef]
- Armijo, G.; Schlechter, R.; Agurto, M.; Munoz, D.; Nunez, C.; Arce-Johnson, P. Grapevine pathogenic microorganisms: understanding infection strategies and host response scenarios. Front. Plant Sci. 2016, 7, 382. [Google Scholar] [CrossRef]
- Merdinoglu, D.; Schneider, C.; Prado, E.; Wiedemann-Merdinoglu, S.; Mestre, P. Breeding for durable resistance to downy and powdery mildew in grapevine. OENO One 2018, 52, 203–209. [Google Scholar] [CrossRef]
- Villano, C.; Aversano, R. Towards grapevine (Vitis vinifera L.) mildews resistance: molecular defence mechanisms and new breeding technologies. Italus Hortus 2020, 27, 1–17. [Google Scholar] [CrossRef]
- Blasi, P.; Blanc, S.; Wiedemann-Merdinoglu, S.; Prado, E.; Rühl, E.H.; Mestre, P.; Merdinoglu, D. Construction of a reference linkage map of Vitis amurensis and genetic mapping of Rpv8, a locus conferring resistance to grapevine downy mildew. Theor. Appl. Genet. 2011, 123(1), 43–53. [CrossRef]
- Kuczmog, A.; Galambos, A.; Horváth, S.; Mátai, A.; Kozma, P.; Szegedi, E.; Putnoky, P. Mapping of crown gall resistance locus Rcg1 in grapevine. Theor. Appl. Genet. 2012, 125(7), 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Schwander, F.; Eibach, R.; Fechter, I.; Hausmann, L.; Zyprian, E.; Topfer, R. Rpv10: a new locus from the Asian Vitis gene pool for pyramiding downy mildew resistance loci in grapevine. Theor. Appl. Genet. 2012, 124, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, H. Review: Research progress in amur grape, Vitis amurensis Rupr. Can. J. Plant. Sci. 2013, 93(4), 565–575. [Google Scholar] [CrossRef]
- Venuti, S.; Copetti, D.; Foria, S.; Falginella, L.; Hoffmann, S.; Bellin, D.; Di Gaspero, G. Historical introgression of the downy mildew resistance gene Rpv12 from the Asian species Vitis amurensis into grapevine varieties. PLoS ONE 2013, 8, e61228. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Wu, W.; Lai, G.; Li, R.; Peng, Y.; Yang, B.; Wang, B.; Yin. ; L.; Qu, J.; Song, S.; Lu, J. Identifying Plasmopara viticola resistance loci in grapevine (Vitis amurensis) via genotyping-bysequencing-based QTL mapping. Plant Physiol. Biochem. 2020, 154, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Kozma, P.; Dula, T. Inheritance of resistance to downy mildew and powdery mildew of hybrid family Muscadinia x V. vinifera x V. amurensis x Franco-American hybrid. Acta Hortic., 2003, 603, 457–63. [Google Scholar] [CrossRef]
- Foria, S.; Magris, G.; Jurman, I.; Schwope, R.; De Candido, M.; De Luca, M.; Ivanišević, D.; Morgante, M.; Di Gaspero, G. Extent of wild–to–crop interspecific introgression in grapevine (Vitis vinifera) as a consequence of resistance breeding and implications for the crop species definition. Hort. Res. 2022, 9, uhab010. [Google Scholar] [CrossRef]
- Myles, S.; Boyko, A.R.; Owens, C.L.; Brown, P.J.; Grassi, F.; Aradhya, M.K.; Prins, B.; Reynolds, A.; Chia, J.M.; Ware, D.; Bustamante, C.; Buckler, E.S. Genetic structure and domestication history of the grape. P. Natl. A. Sci. 2011, 108(9), 3530–3535. [Google Scholar] [CrossRef]
- Grassi, F.; De Lorenzis, G. Back to the Origins: Background and Perspectives of Grapevine Domestication. Int. J. Mol. Sci. 2021, 22, 4518. [Google Scholar] [CrossRef]
- Dubos, B. Mise au point sur les maladies de dépéréssiment dans la vignoble francais. Le Progrés Agriculture et Viticulture 1987, 104, 135–140, cited in: Sosnowski, M.R.; Ayres, M.R.; McCarthy, M.G; Scott, E.S. Winegrape cultivars (Vitis vinifera) vary in susceptibility to the grapevine trunk pathogens Eutypa lata and Diplodia seriata. Aust. J. Grape Wine. R. 2022 28, 166-174. [Google Scholar] [CrossRef]
- Carter, M.V. . The status of Eutypa lata as pathogen. Monograph- Phytopathological Paper N0.32 1991 International Mycological Institute, Surrey, UK. https://www.cabdirect.org/cabdirect/abstract/19912304635.
- Borgo, M.; Pegoraro, G.; Sartori, E. usceptibility of grape varieties to esca disease. In BIO Web Conf.; Proceedings of the 39th World Congress of Vine and Wine, Bento Goncalves, Brazil, 24-28. 10.2016. [CrossRef]
- Murolo, S.; Romanazzi, G. Efects of grapevine cultivar, rootstock and clone on esca disease. Australas. Plant Pathol. 2014, 43, 215–221. [Google Scholar] [CrossRef]
- Sosnowski, M.; Ayres, M.; McCarthy, M. Pests and diseases: investigating the potential for resistance to grapevine trunk diseases. Wine Vitic. J. 2016, 31, 41. [Google Scholar] [CrossRef]
- Travadon, R.; Rolshausen, P.E.; Gubler, W.D.; Cadle-Davidson, L.; Baumgartner, K. Susceptibility of cultivated and wild Vitis spp. to wood infection by fungal trunk pathogens. Plant Dis. 2013, 97, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Billones-Baaijens, R.; Jones, E.E.; Ridgway, H.J.; Jaspers, M.V. Susceptiblity of common rootstock and scion varieties of grapevines to Botryosphaeriaceae species. Australas. Plant Path. 2014, 43, 25–31. [Google Scholar] [CrossRef]
- Foglia, R.; Landi, L.; Romanazzi, G. Analyses of Xylem Vessel Size on Grapevine Cultivars and Relationship with Incidence of Esca Disease, a Threat to Grape Quality. Appl. Sci. 2022, 12, 1177. [Google Scholar] [CrossRef]
- Feliciano, A.; Eskalen, A.; Gubler, W.D. Diferential susceptibility of three grapevine cultivars to Phaeomoniella chlamydospora in California. Phytopathol. Mediterr. 2004, 43, 66–69. [Google Scholar]
- Cardot, C.; Mappa, G.; La Camera, S.; Gaillard, C.; Vriet, C.; Lecomte, P.; Ferrari, G.; Coutos-Thévenot, P. Comparison of the molecular responses of tolerant, susceptible and highly susceptible grapevine cultivars during interaction with the pathogenic fungus Eutypa lata. Front. Plant Sci. 2019, 10, 991. [Google Scholar] [CrossRef]
- Péros, J.P.; Berger, G. A rapid method to assess the aggressiveness of Eutypa lata isolates and the susceptibility of grapevine cultivars to Eutypa Dieback. Agronomie 1994, 14, 515–523. [Google Scholar] [CrossRef]
- Quaglia, M.; Covarelli, L.; Zazzerini, A. Epidemiological survey on esca disease in Umbria, central Italy. Phytopathol. Mediterr. 2009, 48, 84–91. [Google Scholar] [CrossRef]
- Maul, E.; .Töpfer, R. (2023) Vitis International Variety Catalogue - www.vivc.de –. (accessed on 2 April 2023).
- Sankeymatic online diagram builder. Avaliable online: https://sankeymatic.com (accessed on (2023.01.07.).
- Claverie, M.; Notaro, M.; Fontaine, F.; Wéry, J. Current knowledge on grapevine trunk diseases with complex etiology: A systemic approach. Phytopathol. Med. 2020, 59, 29–53. [Google Scholar] [CrossRef]
- Moret, F.; Lemaître-Guillier, C.; Grosjean, C.; Clément, G.; Coelho, C.; Negrel, J.; Jacquens, L.; Morvan, G.; Mouille, G.; Trouvelot, S.; Fontaine, F.; Adrian, M. Clone-dependent expression of esca disease revealed by leaf metabolite analysis. Front. Plant Sci. 2019, 9, 1960. [Google Scholar] [CrossRef] [PubMed]
- Serra, S.; Ligios, V.; Schianchi, N.; Prota, V.A.; Deidda, A.; Scanu, B. Incidence of grapevine trunk diseases on four cultivars in Sardinia, Southern Italy. Vitis 2021, 60, 35–42. [Google Scholar] [CrossRef]
- Bruez, E.; Lecomte, P.; Grosman, J.; Doublet, B.; Bertsch, C.; Fontaine, F.; Ugaglia, A.; Teissedre, P.-L.; Da Costa, J.-P.; GuerinDubra, L. Overview of grapevine trunk diseases in France in the 2000s. Phytopathol. Mediterr. 2013, 52, 262–275. [Google Scholar]
- Andolfi, A.; Mugnai, L.; Luque, J.; Surico, G.; Cimmino, A.; Evidente, A. Phytotoxins produced by fungi associated with grapevine trunk diseases. Toxins. 2011, 3, 1569–1605. [Google Scholar] [CrossRef] [PubMed]
- Bortolami, G.; Gambetta, G.A.; Cassan, C.; Dayer, S.; Farolfi, E.; Ferrer, N.; Gibon, Y.; Jolivet, J.; Lecomte, P.; Delmas, C.E.L. Grapevines under drought do not express esca leaf symptoms. Proc. Natl. Acad. Sci. USA 2021, 118, e2112825118. [Google Scholar] [CrossRef] [PubMed]
- Pouzoulet, J.; Scudiero, E.; Schiavon, M.; Rolshausen, P.E. Xylem vessel diameter affects the compartmentalization of the vascular pathogen Phaeomoniella chlamydospora in grapevine. Front. Plant. Sci. 2017; 8, 1442. [Google Scholar] [CrossRef]
- Pouzoulet, C.A.; Pivovaroff, A.L.; Santiago, L.S.; Rolshausen, P.E. Can vessel dimension explain tolerance toward fungal vascular wilt diseases in woody plants? Lessons from Dutch elm disease and esca disease in grapevine. Front. Plant. Sci. 2014, 5, 253. [Google Scholar] [CrossRef] [PubMed]
- Csótó, A.; Balling, P.; Nagy, A.; Sándor, E. The Role of Cultivar Susceptibility and Vineyard Age in GTD: Examples from theCarpathian Basin. Acta Agrar. Debr. 2020, 2, 57–63. [Google Scholar] [CrossRef]
- Rolshausen, P.E.; Greve, L.C.; Labavitch, J.M.; Mahoney, N.E.; Molyneux, R.J.; Gubler, W.D. Pathogenesis of Eutypa lata in grapevine: identification of virulence factors and biochemical characterization of cordon dieback. Biochemistry and Cell Biology. Phytopathology 2007, 98, 222–229. [Google Scholar] [CrossRef]
- Smith, M.S.; Centinari, M. Young grapevines exhibit interspecific differences in hydraulic response to freeze stress but not in recovery. Planta 2019, 250, 495–505. [Google Scholar] [CrossRef]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (black measles) and brown wood-streaking: Two old and elusive diseases of grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.L.; Rodriguez-Zaccaro, F.D.; Lee, T.F.; Valdovinos, J.; Toschi, H.S.; Martinez, J.A.; Pratt, R.B. Grapevine xylem development, architecture, and function. In Functional and Ecological Xylem Anatomy; Hacke, U., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 133–162. [Google Scholar]
- Guo, X.W.; Fu, W.H.; Wang, G.J. Studies on cold hardiness of grapevine roots. Vitis 1987, 26, 161–171. [Google Scholar] [CrossRef]
- DeKrey, D.H.; Klodd, A.E.; Clark, M.D.; Blanchette, R.A. Grapevine trunk diseases of cold-hardy varieties grown in Northern Midwest vineyards coincide with canker fungi and winter injury. PLoS One 2022, 17, e0269555. [Google Scholar] [CrossRef] [PubMed]
- Csótó, A.; Balling, P.; Rakonczás, N.; Kovács, C.; Nagy, A.; Sándor, E. The effect of extreme weather conditions on the incidence and spreading of grapevine trunk diseases. In: 16th Congress of the Mediterranean Phytopathological Union (2022) pp. 60-60. , 1 p. https://cyprusconferences.org/mpu2022/wp-content/uploads/2022/04/MPU-2022_ABSTRACTS-ALL-13_04_2022-2.pdf.
- Zhao, Y.; Wang, Z.X.; Yang, Y.M.; Liu, H.S.; Shi, G.L.; Ai, J. Analysis of the cold tolerance and physiological response differences of amur grape (Vitis amurensis) germplasms during overwintering. Sci. Hortic. 2020, 259, 108760. [Google Scholar] [CrossRef]
- Xin, H.P.; Zhu, W.; Wang, L.; Xiang, Y.; Fang, L.; Li, J.; Sun, X.; Wang, N.; Londo, J.P.; Li, S. Genome wide transcriptional profile analysis of Vitis amurensis and Vitis vinifera in response to cold stress. PLoS One 2013, 8, e58740. [Google Scholar] [CrossRef]
- Zhang, J.X.; Wu, X.C.; Niu, R.X.; Liu, Y.; Liu, N.; Xu, W.; Wang, Y. Cold-resistance evaluation in 25 wild grape species. Vitis 2012, 51, 153–160. [Google Scholar] [CrossRef]
- Wang, Y.; Xin, H.; Fan, P.; Zhang, J.; Liu, Y.; Dong, Y.; Wang, Z.; Yang, Y.; Zhang, Q.; Ming, R.; Zhong, G.Y.; Li, S.; Liang, Z. The genome of Shanputao (Vitis amurensis) provides a new insight into cold tolerance of grapevine. T. P. J. 2021, 105, 1495–1506. [Google Scholar] [CrossRef]
- Chai, F.; Liu, W.; Xiang, Y.; Meng, X.; Sun, X.; Cheng, C.; Liu, G.; Duan, L.; Xin, H.; Li, S. Comparative metabolic profiling of Vitis amurensis and Vitis vinifera during cold acclimation. Hortic. Res. 2019, 6, 8. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Liu, S.Y.; Sun, X.; Fang, Y. Oenological potential and health benefits of Chinese non-Vitis vinifera species: An opportunity to the revalorization and to breed new varieties. Food Res. Int. 2020, 137, 109443. [Google Scholar] [CrossRef]
- Ciaffi, M.; Paolacci, A.R.; Paolocci, M.; Alicandri, E.; Bigini, V.; Badiani, M.; Muganu, M. Transcriptional regulation of stilbene synthases in grapevine germplasm differentially susceptible to downy mildew. BMC Plant Biol. 2019, 19, 404. [Google Scholar] [CrossRef]
- Clark, M.D. Development of cold climate grapes in the Upper Midwestern US: The Pioneering Work of Elmer Swenson. In Plant Breeding Reviews; Goldman, I., Ed.; Wiley: Hoboken, NJ, USA, 2020; Volume 43, pp. 31–60. [Google Scholar] [CrossRef]
- Viret, O.; Spring, J.-L.; Gindro, K. Stilbenes: biomarkers of grapevine resistance to fungal diseases OENO One 2018, 52, 235-240. https://doi.org/10.20870/oeno-one.2018.52.3.2033. 52,. [CrossRef]
- Hong, L.; Huan, L.; Yinshan, G.; Satoru, K.; Yuhui, Z.; Guangli, S.; Xiuwu, G. QTLs and candidate genes for downy mildew resistance conferred by interspecific grape (V. vinifera L.×V. amurensis Rupr.) crossing. Sci. Hortic. 2019, 244, 200–207. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Yin, L.; Lu, J. The mode of host resistance to Plasmopara viticola infection of grapevines. Phytopathology 2012, 102, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Xinlong, L.; Jiao, W.; Ling, Y.; Yali, Z.; Junjie, Q.; Jiang, L. Comparative transcriptome analysis reveals defense-related genes and pathways against downy mildew in Vitis amurensis grapevine. Plant Physiol. Bioch. 2015, 95, 1–14. [Google Scholar] [CrossRef]
- Pretorius, I.S.; Høj, P.B. Grape and wine biotechnology: challenges, opportunities and potential benefits. Aust. J. Grape Wine R. 2005, 11, 83–108. [Google Scholar] [CrossRef]
- Barba, P.; Lillis, J.; Luce,R. S.; Travadon, R.; Osier, M.; Baumgartner, K.; Wilcox, W.F.; Reisch, B.I.; Cadle-Davidson, L. Two dominant loci determine resistance to Phomopsis cane lesions in F1 families of hybrid grapevines. Theor. Appl. Genet. 2018, 131, 1173–1189. [Google Scholar] [CrossRef]
- Travadon, R.; Baumgartner, K.; Rolshausen, P.E.; Gubler, W.D.; Sosnowski, M.R.; Lecomte, P.; Halleen, F.; Peros, J.P. Genetic structure of the fungal grapevine pathogen Eutypa lata from four continents. Plant Pathol. 2012, 61, 85–95. [Google Scholar] [CrossRef]
- Töpfer, R.; Hausmann, L.; Harst, M.; Maul, E.; Zyprian, E.; Eibach, R. New horizons for grapevine breeding. In Methods in Temperate Fruit Breeding; Flachowsky, H., Hanke, M.V., Eds.; Vegetable and cereal science and biotechnology; Global Science Books Ltd., UK: Kagawa, Japan, 2011; pp. 79–100, http://www.globalsciencebooks.info/Online/GSBOnline/images/2011/FVCSB_5(SI1)/FVCSB_5(SI1)79-100o.pdf. (accessed on 07 04 2023); ISBN 978-4-903313-75-7. [Google Scholar]
- Bartholy, J.; Weidinger, T. 2010 Magyarország éghajlati képe. In: Pannon Enciklopédia - Magyarország földje. Ed.: Karátson, D. Urbis, Budapest, Hungary, 2010; pp. 240-241. ISBN: 978-963-9706-68-2.
- Rakonczás, N. Production data of wine grape gene bank (Vitis spp.) of University of Debrecen, East Hungary. I. J. H. S. 2019, 25, 32–36. [Google Scholar] [CrossRef]
- KSH: Borvidékek. Available online: https://www.ksh.hu/docs/hun/teruleti/egyeb_egysegek/borvidekek.pdf (accessed on 8 April 2023).
- Patocskai, Z.; Vidéki, R.; Szépligeti, M.; Bidlo, A.; Kovács; G. Talajviszonyok a Szent György-hegyen. Talajvédelem special issue, 2008, 639-644. http://publicatio.bibl.u-szeged.hu/5647/7/talajtani%20vandorgyules2008.pdf.
- Fehér, O.; Füleky, G.; Madarász, B.; Kertész, A. Hét vulkáni kőzeten kialakult talajszelvény morfológiai és diagnosztikai jellemzői a hazai genetikai talajosztályozás és a WRB (World Reference Base for Soil Resources, 1998) szerint (Morphological and diagnostic properties of seven volcanic soil profiles according to the Hungarian Soil Classification and the World Reference Base for Soil Resources (WRB, 1998). ) Agrokémia és Talajtan 2011, 60, 131–148. [Google Scholar] [CrossRef]
- Díaz, G.A.; Latorre, B.A. Efficacy of paste and liquid fungicide formulations to protect pruning wounds against pathogens associated with grapevine trunk diseases in Chile. Crop Prot. 2013, 2013 46, 106–112. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Leavitt, G.M.; Guerrero, J.C.; Guevara, J.; Gubler, W.D. Identification and Pathogenicity of Lasiodiplodia theobromae and Diplodia seriata, the Causal Agents of Bot Canker Disease of Grapevines in Mexico. Plant Dis. 2008, 92, 519–529. [Google Scholar] [CrossRef]
- Berman, H.B. , "Statistics and Probability", Available online:. Available online: https://stattrek.com/. (accessed on 7 April 2023).


| Cultivars1 | GTDs | Inoculation test/Disease incidence survey3 | References | ||
|---|---|---|---|---|---|
| Tolerance | Disease2 | ||||
| White | Chardonnay | high | BD, Eutypa | Test | [44] |
| medium | BD | Survey | [43] | ||
| medium | Esca | Survey | [41] | ||
| Pinot Gris | high | BD, Eutypa | Survey | [43] | |
| medium | Esca | Survey | [10] | ||
| Riesling | high | Eutypa | Survey | [43] | |
| medium | BD | Test | [45] | ||
| medium/low | Esca | Test | [44] | ||
| Sauvignon Blanc | high | BD | Test | [45] | |
| medium | Eutypa | Test and Survey | [43] | ||
| low | BD | Test and Survey | [43] | ||
| low | Esca | Survey | [42,46] | ||
| Semillon | high | BD, Eutypa | Test and Survey | [43] | |
| low | Esca | Survey | [10] | ||
| Thompson seedless | high | Esca | Test | [44,47] | |
| medium/low | Eutypa | Test | [44] | ||
| low | BD, Eutypa | Test | [44,47] | ||
| Ugni Blanc | medium/high | BD | Survey | [43] | |
| low | Eutypa | Test | [48] | ||
| low | Esca, Eutypa | Test | [49] | ||
| Survey | [41,43] | ||||
| Welshriesling | high | BD, Eutypa | Test and Survey | [43] | |
| Survey | [41] | ||||
| low | Esca | Survey | [7] | ||
| Red | Cabernet Franc | medium/high | Eutypa | Test | [44] |
| medium | BD | Test | [44] | ||
| low | Esca | Test and Survey | [43] | ||
| Cabernet Sauvignon | high | BD | Test | [45] | |
| low | Eutypa | Test | [48] | ||
| low | Esca, Eutypa | Survey | [41,46,50 | ||
| BD | Survey | [43] | |||
| Grenache | high | Esca, Eutypa | Survey | [43] | |
| high | Esca | Test | [47] | ||
| BD | medium/high | Survey | [43] | ||
| Merlot | high | Eutypa | Test | [44,48] | |
| medium/high | BD | Test | [44] | ||
| medium | Esca | Survey | [42,50] | ||
| Pinot Noir | high | Esca | Survey | [41] | |
| Eutypa, Esca | Test and Survey | [43] | |||
| medium | BD | Test and Survey | [43] | ||
| Sangiovese | high | BD, Esca, Eutypa | Test and Survey | [43] | |
| medium | Esca | Survey | [41] | ||
| Syrah | high | Esca | Survey | [41] | |
| low | BD, Eutypa | Test | [21,44] | ||
| Test and Survey | [43] | ||||
| Hybrid(V. labrusca hybrid) | Concord (Vitis labrusca hybrid) |
high | BD, Esca, Eutypa | Test | [44] |
| Location | No. Cultivars | GTDs | |
|---|---|---|---|
| Total DI % (±SE) | Ratio of dead plants (% ±SE) | ||
| Badacsonytomaj | 90 | 44.58(±2.62) c | 74.63(±3.14) a |
| Kecskemét | 130 | 28.05(±2.19) a | 76.49(±3.11) a |
| Pallag | 166 | 37.05(±2.16) b | 73.78(±3.10) a |
| Pécs | 151 | 28.41(±1.92) a | 69.94(±3.23) a |
| Total | 537 | 33.70(±1.13) | 73.56(±1.59) |
| Ancestors in parent or grandparent level | Categorization I. | Categorization II. |
|---|---|---|
| Vitis vinifera | Vitis vinifera (Vv) | Vitis vinifera (Vv) |
| Occurrence of American species 1 | Interspecific (I) | American origin (Ao) |
| Occurrence of Vitis amurensis | Interspecific (I) | Vitis amurensis origin (Va) |
| Sensitivity categories | GTDs symptoms | ||
|---|---|---|---|
| Two groups | Four groups | apoplexy (dead plant) | leaf symptoms and fresh dieback |
| More sensitive | Highly sensitive (HS) | exclusively | - |
| Sensitive (S) | present | present | |
| Less sensitive | Resilient (R) | - | exclusively |
| Unsusceptible (U) | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





